Abstract
We report results of studies of the conversion of adenosylcobyric acid (AdoCby) to adenosylcobinamide-phosphate, the last step of the de novo corrin ring biosynthetic branch of the adenosylcobalamin (coenzyme B12) pathway of Salmonella enterica serovar Typhimurium LT2. Previous reports have implicated the CbiB protein in this step of the pathway. Hydropathy analysis predicted that CbiB would be an integral membrane protein. We used a computer-generated topology model of the primary sequence of CbiB to guide the construction of CbiB-LacZ and CbiB-PhoA protein fusions, which were used to explore the general topology of CbiB in the cell membrane. A refined model of CbiB as an integral membrane protein is presented. In vivo analyses of the effect of single-amino-acid changes showed that periplasm- and cytosol-exposed residues are critical for CbiB function. Results of in vivo studies also show that ethanolamine-phosphate (EA-P) is a substrate of CbiB, but l-Thr-P is not, and that CbiB likely activates AdoCby by phosphorylation. The latter observation leads us to suggest that CbiB is a synthetase not a synthase enzyme. Results from mass spectrometry and bioassay experiments indicate that serovar Typhimurium synthesizes norcobalamin (cobalamin lacking the methyl group at C176) when EA-P is the substrate of CbiB.
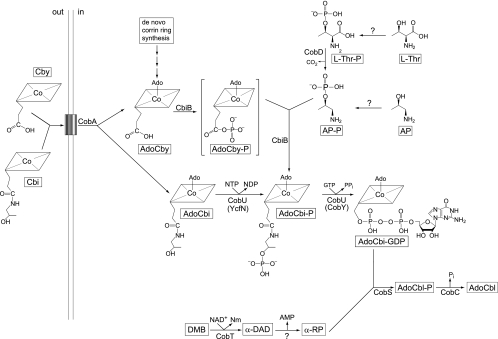
Synthesis of coenzyme B12 (adenosylcobalamin [AdoCbl]) requires a vast amount of genetic information, and although it is essential for many organisms, only bacteria and archaea synthesize it (reviewed in references 6, 30, and 39). Salmonella enterica serovar Typhimurium (hereafter referred to as serovar Typhimurium) synthesizes the corrin ring of AdoCbl de novo only under anaerobic conditions. This bacterium, however, salvages incomplete corrinoids (e.g., cobinamide [Cbi] and cobyric acid [Cby]) from its environment under aerobic or anaerobic conditions (19, 41). Upon entry into the cell via the corrinoid-specific transport system (11, 16, 38), Cbi and Cby are adenosylated by the ATP:Co(I)rrinoid adenosyltransferase (CobA, EC 2.5.1.17) enzyme, yielding AdoCbi or AdoCby (14, 33). These precursors enter the late steps of the nucleotide loop assembly pathway at different points (Fig. 1). AdoCbi is converted to AdoCbi-GDP in two steps, catalyzed by the CobU enzyme (NTP:AdoCbi kinase, EC 2.7.7.62; GTP:AdoCbi-P guanylyltransferase, EC 2.7.1.156) (24, 37). The kinase activity of CobU is required for Cbi salvaging but not for de novo synthesis of AdoCbl (7, 36). However, in the absence of CobU kinase activity, poor but reproducible salvaging of Cbi is observed due to the nonspecific activity of the YcfN protein (26), a known thiamine kinase enzyme (23). On the other hand, AdoCby is condensed with 1-amino-propanoyl-2-phosphate (AP-P) into AdoCbi-phosphate (AdoCbi-P) by the putative AdoCbi-P synthase (CbiB, EC 6.3.1.10) enzyme (29, 41). The CbiB-catalyzed reaction is considered the last step in de novo corrin ring biosynthesis (7). Although genetic data have been reported in support of the idea that AdoCbi-P synthase activity is associated with the CbiB protein, in vitro studies of this activity are lacking. There is no evidence reported in the literature to support the annotation of CbiB as a “synthase,” that is, an enzyme that does not require energy input other than that provided by its substrates to drive the reaction.
FIG. 1.
Coenzyme B12 biosynthesis in serovar Typhimurium. Shown are the intermediates, substrates, and enzymes of the pathway. α-DAD, alpha-5,6-dimethylbenzimidazole adenine dinucleotide; Nm, nicotinamide; α-RP, alpharibazole-5′-phosphate; AdoCbl-P, adenosylcobalamin phosphate; CobA, ATP:Co(I)rrinoid adenosyltransferase; CbiB, putative AdoCbi-P synthetase; CobD, l-Thr O-3-phosphate decarboxylase; CobT, NaMN:5,6-dimethylbenzimidazole phosphoribosyltransferase; CobU, AdoCbi kinase and guanylyltransferase; CobS, cobalamin phosphate synthase; CobC, cobalamin-phosphate phosphatase; YcfN, thiamine kinase (AdoCbi kinase); CobY, nucleotidyltransferase from M. mazei. The box represents the corrinoid transport system (BtuBFCD). AdoCbyP (in brackets) is a proposed intermediate.
The CobU enzyme converts AdoCbi-P to AdoCbi-GDP, which is condensed with α-ribazole-5′-P by the AdoCbl-5′-P synthase (CobS, EC 2.7.8.26) to yield AdoCbl-5′-P. The last step of AdoCbl biosynthetic pathway is catalyzed by the CobC phosphatase (EC 3.1.3.73) (42).
Here we report the results of genetic and biochemical studies of the last step in de novo corrin ring biosynthesis in serovar Typhimurium. We present evidence that CbiB is localized to the cell membrane; a topological model for CbiB is presented. Data support the conclusion that AdoCbi-P is the product of the CbiB reaction and that the conversion of AdoCby to AdoCbi-P likely proceeds via an AdoCby-P intermediate, strongly suggesting that CbiB is a synthetase not a synthase. We also present results of in vivo and in vitro experiments that show that CbiB uses alternative substrates to AP-P to synthesize different, physiologically active cobamides.
MATERIALS AND METHODS
Bacterial strains and culture media.
Strains and plasmids used in this work are described in Table 1. All serovar Typhimurium strains used in these studies carry a null allele of the metE gene. The metE mutation inactivates the cobamide-independent methionine synthase enzyme (28), thus making cell growth dependent on the activity of the Cbl-dependent methionine synthase (MetH) enzyme (12, 15, 35). No-carbon essential (NCE) medium (2) was used to grow cells under chemically defined conditions. When the following supplements were added to the medium, they were added at the concentrations indicated: glucose, 11 mM; glycerol, 22 mM; MgSO4, 1 mM; corrinoids (Cby, Cbi, Cbl, and other corrinoids), 10 nM; l-threonine (l-Thr), l-Thr-phosphate (l-Thr-P), and ethanolamine-O-phosphate (EA-P), 1 mM; 1-amino-2-propanol (AP), 10 mM; 5,6-dimethylbenzimidazole (DMB), 0.3 mM; and trace minerals, 10 ml/liter (1). All corrinoids were added in their cyano forms. Chemicals used in this work were commercially available, high-purity compounds. Cobinamide dicyanide [(CN)2Cbi], cyanocobalamin (CNCbl), EA-P, l-Thr, and l-Thr-P were purchased from Sigma; DMB was purchased from Aldrich; and cyanocobyric acid (CNCby) was a gift from Paul Renz (Universität-Hohenheim, Stuttgart, Germany). Nutrient broth (NB; Difco Laboratories) (0.8% wt/vol) containing NaCl (85 mM) was used as rich medium to culture serovar Typhimurium strains. CNCby and (CN)2Cbi were purified by high-performance liquid chromatography (HPLC) using protocols described previously (4), with the following modifications of system I: a Waters HPLC system equipped with an Alltima (Alltech) HP C18 HL 5-μm column (150 by 4.6 mm) equilibrated and developed at a flow rate of 1 ml min−1.
TABLE 1.
Strains and plasmidsa
| Strain or plasmid | Relevant genotype | Reference/source |
|---|---|---|
| S. enterica serovar Typhimurium strain LT2 derivatives | ||
| TR6583 (formerly SA2929) | metE205 ara-9 | K. Sanderson via J. Roth |
| JE9534 | ΔmetE ara-9 cbiB1344::cat+ | |
| JE9535 | ΔmetE ara-9 ΔcbiB1345c | |
| Derivatives of TR6583 | ||
| JE2216 | cobD1302::Tn10d(cat+)b | Laboratory collection |
| JE4107 | zeb6400::Tn10d(cat+)b | Laboratory collection |
| JE6045 | cobD1302::Tn10d(cat+)/pT7-7b | Laboratory collection |
| JE8126 | cbiB1308::cat+ | |
| JE8185 | ΔcbiB1309 | |
| JE8214 | cobS1312::cat+ | |
| JE8268 | ΔcobU1315 ΔycfN112 | Laboratory collection |
| JE8311 | ΔcobU1315 ΔycfN112/pSU39 | Laboratory collection |
| JE8312 | ΔcobU1315 ΔycfN112/pCOBY38 | Laboratory collection |
| JE8836 | cobD1302::Tn10d(cat+)/pCBIB4b | |
| JE9266 | zeb64500::Tn10d(cat+) cbiB1260/pT7-7 | |
| JE9268 | zeb64500::Tn10d(cat+) cbiB1261/pT7-7 | |
| JE9283 | zeb64500::Tn10d(cat+) cbiB1265/pT7-7 | |
| JE9290 | ΔcbiB1309/pCBIB4 | |
| JE9291 | ΔcbiB1309/pT7-7 | |
| JE9515 | cbiB1308::cat+ ΔcobU1315 ΔycfN112 | |
| JE9709 | JE9515/pCBIB4/pCOBY38 | |
| JE9710 | JE9515/pCBIB4/pSU39 | |
| JE9713 | JE9515/pCBIB34/pCOBY38 | |
| JE9714 | JE9515/pCBIB34/pSU39 | |
| JE9715 | JE9515/pCBIB36/pCOBY38 | |
| JE9716 | JE9515/pCBIB36/pSU39 | |
| JE9717 | JE9515/pCBIB35/pCOBY38 | |
| JE9718 | JE9515/pCBIB35/pSU39 | |
| JE9719 | JE9515/pCBIB37/pCOBY38 | |
| JE9720 | JE9515/pCBIB37/pSU39 | |
| JE9721 | JE9515/pCBIB39/pCOBY38 | |
| JE9722 | JE9515/pCBIB39/pSU39 | |
| JE9723 | JE9515/pCBIB38/pCOBY38 | |
| JE9724 | JE9515/pCBIB38/pSU39 | |
| JE9725 | JE9515/pCBIB40/pCOBY38 | |
| JE9726 | JE9515/pCBIB40/pSU39 | |
| E. coli strains | ||
| CC118 | araD139 Δ(ara leu)7697 ΔlacX74 phoAΔ20 galE galK thi rpsE rpoB argE(Am) recA1 | 22 |
| XL10Gold | Tetr Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac Hte [F′ proAB lacIZDM15 Tn10 (Tetr) Amy Cmr] | Stratagene |
| DH5α | φ80Δlac(lacZ)M15 recA1 endA1 gyrA96 thi-1 hsdR17 (rK− mK+) supE44 relA1 deoR Δ(lacZYA-argF)U169 | Laboratory collection |
| JE7306 | DH5α/pCBIB13 | |
| JE7307 | XL10Gold/pCBIB18 | |
| JE7308 | DH5α/pCBIB15 | |
| JE9437 | XL10Gold/pCBIB12 | |
| JE9438 | XL10Gold/pCBIB16 | |
| JE9439 | XL10Gold/pCBIB14 | |
| JE9790 | DH5α/pCBIB17 | |
| JE9791 | DH5α/pCBIB19 | |
| JE9792 | DH5α/pCBIB20 | |
| Derivatives of CC118 | ||
| JE9033 | CC118/pCBIB33 (loop VI, phoA) | |
| JE9034 | CC118/pCBIB28 (region IV, phoA) | |
| JE9035 | CC118/pCBIB32 (loop V-VI, phoA) | |
| JE9538 | CC118/pCBIB21 (region I, lacZ) | |
| JE9638 | CC118/pCBIB29 (region IV, lacZ) | |
| JE9639 | CC118/pCBIB31 (loop IV-V, lacZ) | |
| JE9641 | CC118/pCBIB25 (region III, phoA) | |
| JE9775 | CC118/pCBIB22 (region II, phoA) | |
| JE9776 | CC118/pCBIB24 (region II, lacZ) | |
| JE9777 | CC118/pCBIB26 (region III, lacZ) | |
| S. enterica serovar Typhimurium strains | ||
| JE9778 | CC118/pCBIB30 (loop IV-V, phoA) | |
| JE9779 | CC118/pCBIB4 | |
| Plasmids | ||
| pSU39 | Cloning vector; kan+ | |
| pT7-7 | Cloning vector; bla+ | 34 |
| pCOBY38 | cobY+ (M. mazei) in pSU39 Kan+ | Laboratory collection |
| pCBIB4 | cbiB+ in pT7-7 bla+ | Laboratory collection |
| pCH40 | bla::phoA | 17 |
| pSKS107 | lacZYA+bla+ | 8 |
| Derivatives of pT7-7 | ||
| pCBIB12 | cbiB1346 (PstI site at cbiB nucleotide no. 70) | |
| pCBIB13 | cbiB1347 (PstI site at cbiB nucleotide no. 106) | |
| pCBIB14 | cbiB1348 (PstI site at cbiB nucleotide no.169) | |
| pCBIB15 | cbiB1349 (PstI site at cbiB nucleotide no. 229) | |
| pCBIB17 | cbiB1351 (PstI site at cbiB nucleotide no. 403) | |
| pCBIB18 | cbiB1352 (PstI site at cbiB nucleotide no. 574) | |
| pCBIB19 | cbiB1353 (PstI site at cbiB nucleotide no. 778) | |
| pCBIB20 | cbiB1354 (PstI site at cbiB nucleotide no. 940) | |
| pCBIB21 | cbiB-lacZ (last CbiB aa P23) | |
| pCBIB22 | cbiB-phoA (last CbiB aa F35) | |
| pCBIB24 | cbiB-lacZ (last CbiB aa F35) | |
| pCBIB25 | cbiB-phoA (last CbiB aa V56) | |
| pCBIB26 | cbiB-lacZ (last CbiB aa A76) | |
| pCBIB28 | cbiB-phoA (last CbiB aa Q136) | |
| pCBIB29 | cbiB-lacZ (last CbiB aa Q136) | |
| pCBIB30 | cbiB-phoA (last CbiB aa Y191) | |
| pCBIB31 | cbiB-lacZ (last CbiB aa Y191) | |
| pCBIB32 | cbiB-phoA (last CbiB aa G259) | |
| pCBIB33 | cbiB-phoA (last CbiB aa C313) | |
| pCBIB34 | cbiB1355 (encodes CbiBH24A) | |
| pCBIB35 | cbiB1356 (encodes CbiBR121A) | |
| pCBIB36 | cbiB1357 (encodes CbiBE150A) | |
| pCBIB37 | cbiB1358 (encodes CbiBT179I) | |
| pCBIB38 | cbiB1359 (encodes CbiBD181N) | |
| pCBIB39 | cbiB1360 (encodes CbiBG195D) | |
| pCBIB40 | cbiB1361 (encodes CbiBL262A) |
Strains and plasmids were constructed during the course of this work unless stated otherwise. aa, amino acid.
Strain is described in reference 13.
The cbiB1345 gene encodes CbiBW126Z Δ127-319.
Recombinant DNA techniques. Construction of plasmids encoding CbiB-LacZ and CbiB-PhoA fusion proteins.
The protocol used for the construction of Cbi-LacZ and CbiB-PhoA fusions was described previously (21). The resulting plasmids encoded CbiB-PhoA or CbiB-LacZ fusion proteins in which the PhoA or LacZ proteins were preceded by lengths of CbiB sequences that varied from 23 to 313 residues (Tables 1 and 2).
TABLE 2.
PstI restriction sites and primersa
| Plasmid | PstI site location (nucleotide no. from the start of cbiB) | Primers |
|---|---|---|
| pCBIB12 | 70 | 5′-GGCCCCTGCAGGTACGCTGGATAGG-3′ |
| 5′-CAGCGTACCTGCAGGGGCCAGTGTTG-3′ | ||
| pCBIB13 | 106 | 5′-TTTAATTACGTTTCTGCAGCGTATTGTGCG-3′ |
| 5′-CGCACAATACGCTGCAGAAACGTAATTAAA-3′ | ||
| pCBIB14 | 169 | 5′-GGCGTACTGCAGGTTGTGGTTGTCG-3′ |
| 5′-CCACAACCTGCAGTACGCCGCCGCC-3′ | ||
| pCBIB15 | 229 | 5′-TACTGGCGCTGGCGCTGCAGATTCACCCCTGGTT-3′ |
| 5′-AACCAGGGGTGAATCTGCAGCGCCAGCGCCAGTA-3′ | ||
| pCBIB17 | 403 | 5′-GATACGTCGCAACTGCAGCCAGCGCAGATC-3′ |
| 5′-GATCTGCGCTGGCTGCAGTTGCGACGTATC-3′ | ||
| pCBIB18 | 574 | 5′-CAAACATGAAAAATACCTGCAGATTGGTATGGTCAGCG-3′ |
| 5′-CGCTGACCATACCAATCTGCAGGTATTTTTCATGTTTG-3′ | ||
| pCBIB19 | 778 | 5′-GCCGGCGCCTTAGGACTGCAGCTCGGTGGCCCA-3′ |
| 5′-TGGGCCACCGAGCTGCAGTCCTAAGGCGCCGGC-3′ | ||
| pCBIB20 | 940 | 5′-TGCGGCGCGGTGCCTGCAGTCTGGCGTGG-3′ |
| 5′-GGCCACGCCAGACTGCAGGCACCGCGCCG-3′ |
PstI restriction sites are shown in bold type.
Construction of plasmids containing cbiB point mutations.
A QuikChange XL site-directed mutagenesis kit (Stratagene) was used to construct plasmids containing cbiB single mutations, using plasmid pCBIB4 as the DNA template. A list of primers used is provided in Table 3. All plasmids were sequenced as described above to verify the presence of the mutations.
TABLE 3.
Mutagenic primers used to introduce the indicated changes in CbiBa
| Plasmid or chromosome | Residue changed | Primers |
|---|---|---|
| Plasmid | ||
| pCBIB34 | H24A | 5′-GGCCCGCTCCGGTACGCTGGATAGG-3′ |
| 5′-GTACCGGAGCGGGCCAGTGTTGAGG-3′ | ||
| pCBIB35 | R121A | 5′-CGGAAAGCGCAATAAAACTCTCCTG-3′ |
| 5′-GCGCTTTCCGCAAGGTCGTTCTTCC-3′ | ||
| pCBIB36 | E150A | 5′-GTTGCAGCAAACACCGTTGACGGC-3′ |
| 5′-GGTGTTTGCTGCAACCGTTTCCACC-3′ | ||
| pCBIB37 | T179I | 5′-GCCGTCAATATCCTGGATTCAATGG-3′ |
| 5′-CCAGGATATTGACGGCTTTGTAGG-3′ | ||
| pCBIB38 | D181N | 5′-CAATACCCTGAATTCAATGGTGGG-3′ |
| 5′-CATTGAATTCAGGGTATTGACGGC-3′ | ||
| pCBIB39 | G195D | 5′-CGATTGATATGGTCAGCGCCCGTATG-3′ |
| GCTGACCATATCAATCGCCCGGTAT-3′ | ||
| pCBIB40 | L262A | 5′-AGGAATCCAGTGCGGTGGCCCAAAT-3′ |
| 5′-GGCCACCGCACTGGATTCCTAAGGC-3′ | ||
| Chromosome | ||
| JE9535 | W126Z (Δ127-319) | 5′-CGACCGTTACGGAAGAACGACCTTGCGGAAAGCCGA |
| ATAAAACTCTCCTGAGTGTAGGCTGGAGCTGCTTC-3′ | ||
| 5′-TCAGGCCACGCCAGATAACCCGCACCGCGC | ||
| CGCAATTAACAGCGCCAGCGCCATATGAATATCCTCCTTAG-3′ | ||
| JE8185 | ΔcbiB | 5′-ATGACGATTCTTGCCTGGTGTATCGCCTGGGTGCTG |
| GATTTTATCATCGGCGTGTAGGCTGGAGCTGCTTC-3′ | ||
| 5′-TCAGGCCACGCCAGATAACCCGCACCGCGCCG | ||
| CAATTAACAGCGCCAGCGCCATATGAATATCCTCCTTAG-3′ |
Nucleotides in bold type indicate base pairs changed to encode the desired residue. Underlined nucleotides are part of the cbiB gene sequence.
Genetic techniques. Construction of serovar Typhimurium mutant strains. (i) Hydroxylamine chemical transposon mutagenesis.
Transductional crosses were performed using the mutant phage P22 HT105/1 int-201 (31, 32) as the donor, and transductants were screened for phage sensitivity as described previously (9). Transductions employed as the donor a hydroxylamine-mutagenized lysate of phage P22 (2-log kill) grown on strain JE4107 [zeb6400::Tn10d(cat+), linked to the cbi genes], and serovar Typhimurium strain JE6583 (Table 1, cbi+) was used as the recipient. Chloramphenicol-resistant (Cmr) colonies were selected and screened for Cbi-dependent growth on NCE medium supplemented with glucose and CNCby. Confirmation that the mutations affected cbiB function was obtained by complementation analysis using a plasmid carrying a wild-type cbiB+ allele (41). The location of the mutations within cbiB+ was confirmed by PCR amplification of the gene, followed by DNA sequencing using BigDye (ABI PRISM) protocols (Biotechnology Center, University of Wisconsin—Madison, Madison, WI).
(ii) The cbiB::cat+ ΔcobU ΔycfN strain.
This strain was constructed by transduction, where a P22 phage lysate of strain JE8126 (cbiB1308::cat+) was used as the donor and strain JE8268 (ΔcobU1315 ΔycfN112) was used as the recipient. Cmr colonies were selected on NB containing Cm and screened for their inability to grow on Cbi on minimal NCE medium supplemented with glycerol, MgSO4, trace minerals, and DMB; growth was restored by Cbl.
(iii) Construction of nonpolar cbiB deletion strains.
In-frame deletions of the cbiB gene were constructed and verified using protocols described previously (5, 10). Mutagenic primers used for this purpose (Integrated DNA Technologies) are listed in Table 3.
Assessment of Cby salvaging.
Plasmids were introduced into serovar Typhimurium by electroporation as described previously (25). Strains were grown to full density (2 × 109 CFU/ml) in NB supplemented with ampicillin (100 μg/ml) to ensure plasmid retention. Minimal NCE medium (198 μl) supplemented with glucose or glycerol, MgSO4, trace minerals, and Cby and DMB, l-Thr, l-Thr-P, or EA-P was inoculated with 4 × 106 CFU (2 μl) of a full-density NB culture. Growth at 37°C with continuous shaking (19 Hz) was monitored using an ELx808 UltraMicroplate reader (BioTek Instruments).
Cbi salvaging in the presence of higher levels of CbiB protein.
Serovar Typhimurium strains JE6583, JE9515, JE8311, JE8312, JE9709, and JE9710 (Table 1) were grown as described above. Growth behavior was assessed using the 96-well format approach described above, using minimal NCE medium (198 μl) supplemented with glycerol, MgSO4, trace minerals, Cbi, and DMB.
Growth behavior as a function of available corrinoids.
Strain JE8214 (cobS) was grown to full density in NB. Minimal NCE medium (198 μl) supplemented with glycerol, MgSO4, trace minerals, DMB, and HPLC-purified corrinoid was inoculated with 4 × 106 CFU (2 μl) of a full-density NB culture; growth behavior was monitored as described above. Corrinoid concentration was determined using a SpectraMax Plus spectrophotometer (Molecular Devices) with path length correction, in which the CNCbl extinction coefficient at 550 nm (ɛ550) = 8,700 M−1 cm−1.
Biochemical techniques. Corrinoid extractions.
Two liters of minimal NCE medium supplemented with glycerol, MgSO4, trace minerals, CNCby, and DMB was inoculated with 20 ml of an overnight culture (2 × 109 CFU/ml) of strain JE8836 [cobD1302::Tn10d(cat+)/pCBIB4 cbiB+] grown in NB (containing 100 μg ml−1 ampicillin). Cultures were grown at 37°C with shaking at 180 rpm for 24 h. The same growth conditions were used for cultures supplemented with EA-P and l-Thr-P. Cells were harvested, cell paste was processed, and corrinoids were extracted as described previously (42).
Reverse-phase (RP) HPLC analysis of corrinoids.
Corrinoids present in the samples were derivatized to their cyano forms by adding 50 μl of 100 mM KCN (5 μmol; final volume, 300 μl), followed by irradiation with a 60-W incandescent light at a distance of approximately 6 cm on ice for 15 min. Samples were filtered on Spin-X centrifuge filters (Corning). Corrinoids were resolved using a Beckman-Coulter HPLC system equipped with an Alltima (Alltech) HP C18 HL 5-μm column (150 by 4.6 mm) developed with a modification of the mobile-phase system reported elsewhere (4) at a flow rate of 1 ml min−1. The column was equilibrated with a 98% A/2% B buffer system (see below). Column development started 2 min after sample injection. A linear gradient was applied until the buffer system reached 75% A/25% B. A second 15-min linear gradient was used to develop the column to 65% A/35% B, and a third 35-min linear gradient was applied until a final buffer composition of 100% B was reached. The solvents used were as follows: buffer A (100 mM potassium phosphate buffer [pH 6.5]-10 mM KCN); buffer B (100 mM potassium phosphate buffer [pH 8.0]-acetonitrile [1:1, vol/vol]). Corrinoid elution from the column was detected with a Beckman-Coulter photodiode array detector. Authentic CNCby, (CN)2Cbi, and CNCbl were used as standards.
In vitro AdoCbi-P synthase (CbiB) activity assay.
The reaction mixtures contained AdoCby (10 μmol), GTP (0.02 μmol), EA-P (10 μmol), MgSO4 (2 μmol), Tris (Tris-HCl) buffer (pH 8 at 37°C; 10 μmol), and cell extract from the JE9290 (pcbiB+) or the JE9291 (pT7-7, vector-only control; 100 μg) strain grown under conditions where CbiB activity was required for growth. The final reaction mixture volume was 200 μl. Cell extracts were obtained by sonication on ice for 2 min at 66% duty in Tris-HCl buffer (50 mM [pH 8] at 37°C) containing dithiothreitol (10 mM) and the protease inhibitor phenylmethylsulfonyl fluoride (1 mM). Alternatively, cell disruption was achieved by using 1× BugBuster reagent (Novagen) in Tris-HCl (50 mM [pH 7.5] at room temperature) or N-cyclohexyl-2-aminoethanesulfonic acid buffer (50 mM [pH 9] at room temperature) containing dithiothreitol (10 mM) and phenylmethylsulfonyl fluoride (1 mM). The cell extracts obtained using BugBuster reagent were dialyzed against 2 liters of buffer without reagent for 5 h at 4°C. The reaction mixtures were incubated at 37°C for 2 and 24 h and were stopped by the addition of 50 μl of KCN (0.1 M), followed by heating at 80°C for 10 min. This treatment converted all corrinoids to their cyano forms.
All reactions were performed under dim light to prevent cleavage of the C—Co bond between the adenosyl moiety and the cobalt ion in the corrin ring. The CbiB reaction mixture was coupled to the guanylyltransferase reaction mixture by the addition of 10 μg of homogeneous CobU protein after 30 min of incubation. The CbiB-CobU reaction was coupled to the CobS reaction mixture by the addition of α-ribazole-5′-phosphate and CobS-enriched cell extracts (10 μg) (42).
Because AP-P was not commercially available, homogenous l-Thr-P decarboxylase enzyme (CobD) (5 μg) and l-Thr-P (10 μmol) were added to the CbiB-CobU-CobS reaction mixture instead of EA-P to synthesize Cbl. The reaction product was isolated by (RP) HPLC as described above and its identity established by UV-visible spectroscopy and mass spectrometry.
Translational fusion assays.
β-Galactosidase and alkaline phosphatase activities were determined as described previously (21).
Mass spectrometry.
Atomic mass values of HPLC-purified corrinoids were determined as described previously (42).
RESULTS
CbiB is an integral membrane protein.
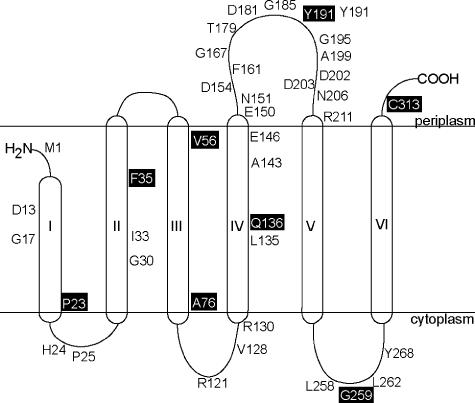
To probe the topology of CbiB, we constructed 11 CbiB-PhoA and CbiB-LacZ translational fusions. The construction of these fusion proteins was based on a hypothetical topology model obtained by amino acid sequence hydropathy analysis using TMpred software (18). We used a sensitive fluorometric method to quantify the number of molecules of alkaline phosphatase (PhoA) or β-galactosidase (LacZ) per cell (21). Background measurements were determined in strain JE9779 (Escherichia coli CC118 harboring plasmid pCBIB4 cbiB+). Only 0.2 active molecules of β-galactosidase and 2 active molecules of alkaline phosphatase were detected per cell of strain JE9779.
Based on the hypothetical topology model, residues P23, F35, Q136, and G259 were predicted to localize to the cytosol; residues A76, Y191, and C313 would localize to the periplasm; and residue V56 would be buried in the membrane. Figure 2 shows the membrane topology model for CbiB, supported by data obtained in these studies (Table 4) in combination with data obtained from TMpred analysis. Localization of residues in which either PhoA or LacZ fusion was not available was based on the hypothetical model and the constraints imposed by the length of the membrane-spanning region. Data for fusions CbiB35-LacZ, CbiB35-PhoA, CbiB136-LacZ, and CbiB136-PhoA suggested that residues F35 and Q136 might be buried in the membrane and could not be localized to the cytosol as predicted. A low or undetectable level of β-galactosidase activity was measured in cells containing the CbiB76-LacZ or CbiB23-LacZ fusion, respectively, suggesting that residues A76 and P23 were not exposed to the cytosol and were probably buried in the membrane. Levels of alkaline phosphatase activity measured in cells containing CbiB56-PhoA, CbiB191-PhoA, CbiB259-PhoA, and CbiB313-PhoA suggested that only residues Y191 and C313 were localized to the periplasm, while residue V56 was probably buried in the membrane and residue G259 was exposed to the cytoplasm. The PhoA data confirmed that residues Y191 and C313 were exposed to the periplasm, whereas residue V56 might be buried in the membrane.
FIG. 2.
Refined membrane topology model of the CbiB protein. The topology model was generated using the information obtained from CbiB-LacZ and CbiB-PhoA translational fusion analysis. Fusion sites are boxed, and the remaining residues are conserved amino acids among all CbiB orthologs analyzed.
TABLE 4.
Activity of CbiB-PhoA and CbiB-LacZ fusion proteinsa
| Residue no. of CbiB fusion site | Plasmid encoding CbiB-PhoA fusion | Plasmid encoding CbiB-LacZ fusion | No. of molecules ± SD of alkaline phosphatase activity | No. of molecules ± SD of β-galactosidase activity |
|---|---|---|---|---|
| Control | pCBIB4 (no fusion) | pCBIB4 (no fusion) | 2 ± 1 | 0.2 ± 0.1 |
| P23 | None | pCBIB21 | ND | NA |
| F35 | pCBIB22 | pCBIB24 | 8 ± 1 | 0.3 ± 0.1 |
| V56 | pCBIB25 | None | 3 ± 0.3 | ND |
| A76 | None | pCBIB26 | ND | 8 ± 2.4 |
| Q136 | pCBIB28 | pCBIB29 | 1 ± 0.2 | 0.3 ± 0.02 |
| Y191 | pCBIB30 | pCBIB31 | 84 ± 6 | 2 ± 1 |
| G259 | pCBIB32 | None | 2 ± 1 | ND |
| C313 | pCBIB33 | None | 79 ± 6 | ND |
Units are expressed as the number of molecules ± standard deviation (SD) of active alkaline phosphatase or active β-galactosidase per cell, respectively. ND, not determined; NA, no activity. Data obtained from control experiments were considered background noise.
Periplasm- and cytoplasm-exposed residues are critical to CbiB activity.
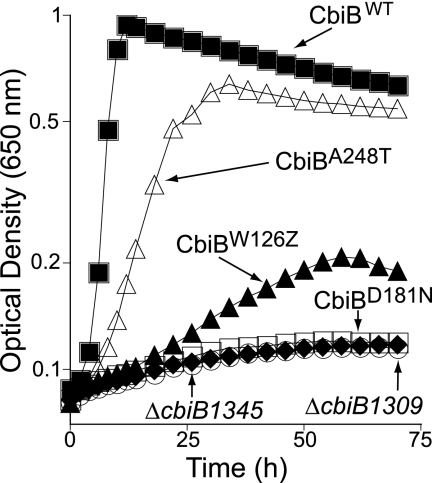
Fifteen cbiB mutant strains were isolated using chemical localized mutagenesis. DNA sequencing of cbiB mutant alleles showed one of the following changes: W126Z (isolated eight times), A248T (isolated six times), or D181N (isolated once). The growth behavior of mutant strains carrying the above-mentioned cbiB alleles was assessed in minimal NCE medium supplemented with glycerol, Cby, and DMB. The growth of strain JE9283 (cbiB1265, which encodes CbiBA248T) displayed a significantly negative, but not dramatic, defect in Cby salvaging compared to a cbiB+ strain (Fig. 3, open triangles versus closed squares).
FIG. 3.
In vivo assessment of CbiB function. Cbl-dependent growth of the chromosomal cbiB mutants of serovar Typhimurium was assessed in minimal medium supplemented with glycerol, Cby, and DMB, demand in Cbl-dependent methionine synthesis. Strains: JE9283 (cbiB1265, which encodes CbiBA248T); JE9268 (cbiB1261, encodes CbiBW126Z); JE9266 (cbiB1260, encodes CbiBD181N); JE9535 (ΔcbiB1345, which encodes CbiBW126Z Δ127-319); JE8185 (ΔcbiB1309, which encodes ΔCbiB).
A cbiB allele encoding a truncated variant of CbiB (CbiBW126Z) where only the first 125 amino acids of the protein were present salvaged Cby, albeit poorly (Fig. 3, closed triangles). We considered this result to reflect read-through by a suppressor tRNA. We explored this possibility by constructing a cbiB deletion strain encoding only the first 125 amino acids of CbiB in addition of the W126Z change (strain JE9535, synthesizes CbiBW126Z Δ127-319). Strain JE9535 failed to salvage Cby, consistent with the explanation that the limited growth of a strain carrying the cbi allele encoding CbiBW126Z (JE9268) was likely due to nonsense codon suppression.
The inability to salvage Cby by a strain that synthesized variant CbiBD181N (JE9266) or a strain lacking CbiB (JE8185, ΔcbiB) was tight, suggesting that residue D181 was critical to CbiB function or stability. In the topological model derived from this work, residue D181 was located in the periplasmic loop between helices IV and V, among several other conserved amino acids (Fig. 2).
We changed seven conserved amino acids (Fig. 4) whose location (according to our model) was periplasmic or cytosolic. The cbiB mutant alleles were constructed in a pT7-7 plasmid carrying the wild-type cbiB+ gene (pCBIB4). After mutagenesis, plasmids were moved into strain JE9515 (cbiB::cat+ ΔcobU ΔycfN/pCOBY38 Methanosarcina mazei cobY+) to assess their effect on CbiB activity. Strain JE9515 lacks all known chromosomally encoded AdoCbi kinase activity and can grow only if endogenous synthesis of AdoCbi-P occurs. Doubling times for strains carrying the cbiB mutant alleles were determined and compared to that of a strain harboring a plasmid carrying the wild-type cbiB allele (Table 5). Our data indicated that chromosomal levels of CbiBD181N protein did not support Cby salvaging (Fig. 3, open versus closed squares). However, when the synthesis of CbiBD181N was directed by a high-copy-number plasmid, slow growth was measured (Table 5).
FIG. 4.
Sequence alignment of CbiB orthologs from the archaeal and bacterial domains. All sequences are available through the National Center for Biotechnology Information. The alignment was obtained with DNAssist software (27). Black boxes indicate conserved amino acids.
TABLE 5.
Growth behavior of strains synthesizing CbiB variantsa
| pCbiB variant | Doubling time (h) |
|---|---|
| pCbiBH24A | 1.6 |
| pCbiBE150A | 23 |
| pCbiBR121A | 2.0 |
| pCbiBT179I | 1.9 |
| pCbiBG195D | 7.1b |
| pCbiBD181N | 23.5 |
| pCbiBL262A | 2.2 |
| pCbiBWT | 1.8 |
Cultures were grown in NCE medium supplemented with glycerol, Mg2+, Cby, and DMB, as indicated in Materials and Methods.
Time period includes a 6-h lag.
We investigated the effect of changes in conserved residues facing the cytosol, where the catalytic site of CbiB is expected to be. Variant proteins CbiBH24A, CbiBR121A, and CbiBL262A did not lose significant catalytic activity compared to the wild-type protein. CbiB variants with mutated residues exposed to the periplasm (i.e., CbiBG195D, CbiBE150A, and CbiBD181N) supported growth but at much lower rates than the CbiB wild-type (CbiBWT) protein (i.e., at 4-, 13-, and 13.5-fold-lower rates, respectively). The CbiBT179I protein, however, was as effective as the CbiBWT enzyme (Table 5).
The increased level of CbiB enzyme allows Cbi salvaging in a strain that lacks AdoCbi kinase activity.
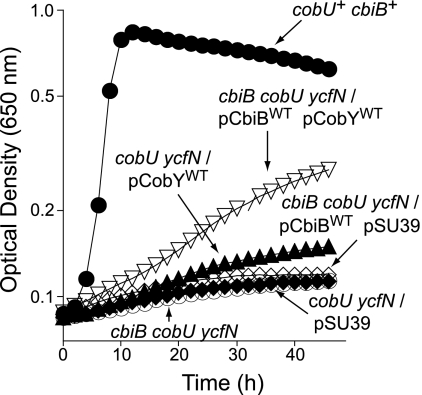
During the course of this work, we noticed that high levels of CbiBWT enzyme allowed strain JE9709 (cbiB cobU ycfN/pCBIB4 cbiB+ pCOBY38 M. mazei cobY+) to salvage Cbi, albeit poorly. This was a striking observation, since strain JE9709 lacks AdoCbi kinase activity because it lacks CobU and the nonspecific Cbi kinase YcfN enzymes (26). This observation strongly suggested that CbiB phosphorylated AdoCbi, providing the substrate for CobY (Fig. 1). Strain JE9709 grew on medium supplemented with Cbi to a final optical density at 650 nm of 0.3, with a 27-h doubling time (Fig. 5, open, inverted triangles), while the negative control strain JE9710 (lacking cobY+) did not grow (Fig. 5, open diamonds), and the positive control strain TR6583 (carrying cbiB+ cobU+) grew to a final optical density of 0.8, with a 1.5-h doubling time (Fig. 5, closed circles). Results of control experiments using strains carrying cbiB mutant alleles were impaired for Cbi salvaging (data not shown). These data were consistent with the idea that the observed growth response of strain JE9709 was due to AdoCbi phosphorylation by CbiB.
FIG. 5.
Cbi salvaging by the cbiB cobU ycfN mutant is partially restored when the expression of CbiB is increased. In these experiments, AdoCbi-P nucleotidyltransferase was provided in trans by a plasmid carrying the CobY enzyme from Methanosarcina mazei strain Gö1. Cbl-dependent growth of serovar Typhimurium strains was assessed in minimal medium supplemented with glycerol, Cbi, and DMB. Genotypes shown in the figure correspond to the strains as follows: JE8311, ΔcobU ΔycfN/pSU39; JE8312, ΔcobU ΔycfN/pCobYWT; JE9515, cbiB ΔcobU ΔycfN; JE9709, cbiB ΔcobU ΔycfN/pCbiBWT/pCobYWT; JE9710, cbiB ΔcobU ΔycfN/pCbiBWT pSU39; TR6583, cbiB+ cobU+.
The observed effect depended on high levels of CbiB. We reached this conclusion after comparing Cbi-dependent growth between strains expressing multiple copies of the cbiB+ gene (strain JE9709) and those expressing chromosomal levels (strain JE8312). As seen in Fig. 5, growth of JE8312 (cobU ycfN/pcobY+) was not significantly different from that of the control strains (JE8311, cobU ycfN/pSU39) or strain JE9710 (cbiB cobU ycfN/pcbiB+/pSU39). These results have important implications for our understanding of how CbiB may function (see Discussion).
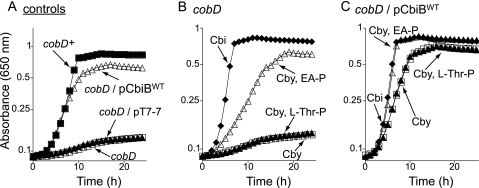
Increased expression of the cbiB+ gene restores the ability of a strain carrying cobD to convert Cby to Cbl.
When a plasmid carrying the cbiB+ gene was introduced into a strain lacking the l-Thr-P decarboxylase (CobD) enzyme (JE2216, cobD1302::Tn10d(cat+)), growth of the resulting strain (JE8836) on medium containing Cby was comparable to that of the cobD+ strain and significantly better than that of the control strains (Fig. 6A). These results suggested that either (i) CbiB could use l-Thr-P as a substrate, (ii) an alternative l-Thr-P decarboxylase existed in serovar Typhimurium, or (iii) CbiB could use a substrate other than l-Thr-P to synthesize a distinct, functionally active cobamide.
FIG. 6.
Cobyric acid salvaging by the cobD mutant is restored by exogenous EA-P but not by l-Thr-P. Cbl-dependent growth of serovar Typhimurium strains was assessed in minimal medium supplemented with glucose, Cby, and DMB. The genotypes described in the figure correspond to the strains as follows: JE2216, cobD; JE6045, cobD/pT7-7; JE8836, cobD/pCbiBWT; TR6583, cobD+. Plasmids were introduced into strain JE2216 (cobD); plasmid pCBIB4 encoded pCbiBWT.
In the second scenario, the level of AP-P generated by the hypothetical alternative l-Thr-P decarboxylase would be low but could be detected if the cell synthesized enough CbiB to allow the synthesis of AdoCbl. The same rationale would apply to the third scenario.
To distinguish between these possibilities, we assessed the growth of the cobD strain on minimal medium containing Cby, DMB, and l-Thr-P or EA-P. We chose EA-P (H2NCH2CH2O4P) because it is structurally very similar to AP-P [H2NCH2(CH3) CH2O4P], and unlike AP-P, EA-P is commercially available. l-Thr-P did not support growth of the cobD strain, but EA-P did (Fig. 6B). In addition, the growth of strain JE8836 [cobD1302::Tn10d(cat+)/pCBIB4 cbiB+] was improved when EA-P was added to the medium but not when l-Thr-P was added (Fig. 6C), suggesting that strain JE8836 incorporated EA-P into the final complete corrinoid but could not do the same with l-Thr-P. Internalization of l-Thr-P by serovar Typhimurium has been demonstrated (42), ruling out the explanation that l-Thr-P is not being transported into the cytosol.
High levels of CbiBWT protein allow a strain unable to make AP-P (cobD) to synthesize norCbl from Cby, DMB, and EA-P.
As shown in Fig. 6, increased levels of cbiB+ expression allowed the cobD strain to salvage Cby when it was grown in the presence of EA-P and DMB. The predicted end product of the pathway would lack the carbon C-176, i.e., the methyl group of AP-P. Such a cobamide is known as norcobalamin (norCbl).
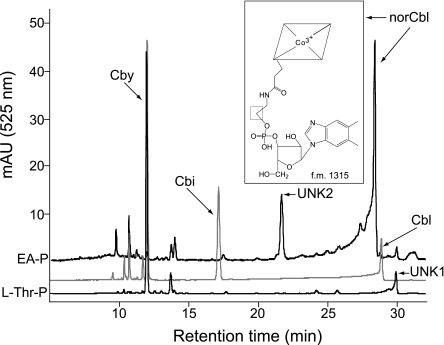
To determine whether EA-P was incorporated into the final product of the pathway without modifications, corrinoids were extracted from strain JE8836 (cobD/pcbiB+) grown in minimal NCE medium supplemented with Cby and DMB in the presence or absence of l-Thr-P or EA-P. Samples were resolved by RP HPLC to separate incomplete and complete corrinoids, and their identity was investigated by bioassay and mass spectrometry.
Cobamide synthesized in the presence of l-Thr-P.
Two corrinoids were purified from the cells grown in the presence of l-Thr-P. One corrinoid (eluted 12 min postinjection; Fig. 7, bottom trace); its identity was established by UV-visible spectroscopy and mass spectrometry as Cby (data not shown) (40). A second corrinoid eluted 29.8 min postinjection (Fig. 7, bottom trace, unknown 1 [UNK1]). Corrinoid UNK1 was identified by bioassay as a complete cobamide because it supported the growth of strain JE8214 (carrying cobS), a known Cbl auxotroph. The rate of growth of strain JE8214 on UNK1 was slower than the one supported by equimolar amounts of Cbl (Fig. 8, open versus closed circles). The UNK1 cobamide was presumed to be one in which l-Thr-P substituted for AP-P; this assignment was not confirmed. Similar growth behavior was observed regardless of the final concentration of UNK1 present in the medium (e.g., 10, 20, or 50 nM) (data not shown).
FIG. 7.
Incorporation of EA-P into a cobamide known as norcobalamin. HPLC was performed with corrinoid extracts from strain JE8836 grown on minimal NCE media supplemented with Cby, DMB, and EA-P. Corrinoid standards, gray; Cby eluted at 12 min, Cbi eluted at 17.2 min, and Cbl eluted at 28.8 min. Extracted corrinoid from l-Thr-P-supplemented cell cultures (bottom black trace) eluted at 12 min (Cby) and 29.8 min (UNK1, cobamide of unknown identity 1). Extracted corrinoids from cell cultures grown with EA-P (upper black trace) eluted at 21.6 (UNK2, cobamide of unknown identity 2) and 28.3 (norCbl) min. The chemical structure of norcobalamin (peak at 28.3 min) is shown. f.m., formula mass.
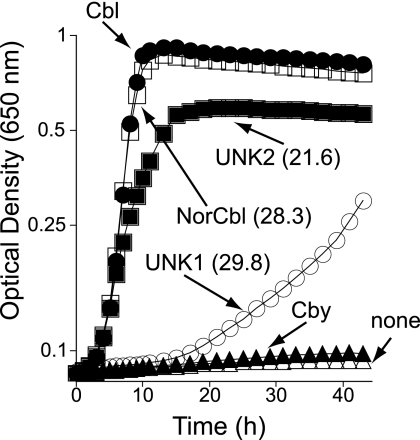
FIG. 8.
Bioassay used to detect cobamides in extracts. Cbl-dependent growth of serovar Typhimurium was assessed in minimal medium supplemented with glycerol, DMB, and HPLC-purified corrinoids. NorCbl and UNK2 are cobamides purified from cells grown on minimal medium supplemented with Cby, DMB, and EA-P. UNK1 identifies a cobamide purified from cells grown in medium supplemented with Cby, DMB, and l-Thr-P. The numbers between parentheses show retention times on RP HPLC analysis. The indicator strain used in these studies was strain JE8214 (cobS1312::cat+), a cobamide auxotroph.
Cobamides synthesized in the presence of EA-P.
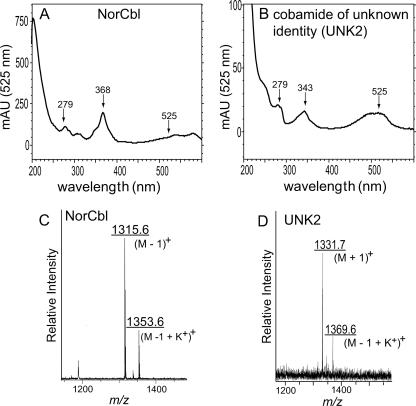
The mixture of corrinoids extracted from cells grown in the presence of EA-P (Fig. 7, upper trace) contained very small amounts of Cby (12 min postinjection; masked in the overlay of spectra shown) and two additional corrinoids that eluted 21.6 and 28.3 min postinjection. The mass spectrum of the corrinoid eluting after 28.3 min (m/z = 1,315.6 atomic mass units [a.m.u.]) was consistent with norCbl, i.e., Cbl without the methyl group at C-176 (f.w. 1,315) (Fig. 9C) (20). The mass spectrum for the corrinoid eluting after 21.6 min (UNK2, m/z = 1331 a.m.u.) could be that of norpseudovitamin B12 (Fig. 9D). However, additional experiments are needed to firmly establish its identity. Interestingly, the absorption spectrum of UNK2 (Fig. 9B) was different from those of authentic CNCbl (and other corrinoids), with a distinct lambda maximum at 343 nm. Results from bioassays employing strain JE8214 (cobS; a Cbl auxotroph) indicated that UNK2 was also a complete cobamide (Fig. 8). Corrinoid UNK2 (putatively norpseudoCbl) was a better cofactor for methionine synthase (MetH) than UNK1.
FIG. 9.
UV visible and mass spectra of norcobalamin and UNK2. Shown are the UV-visible spectrum (A and B) and matrix-assisted laser desorption ionization-time of flight mass spectrum (C and D) of HPLC-purified cobamides. The absorption spectrum of the corrinoid eluting at 28.3 min postinjection (A) is identical to that of authentic cyanocobalamin. The signals with m/z values of 1,315.6 and 1,353.6 a.m.u. (C) were consistent with (M − 1)+, and (M − 1 + K+)+ ions of norcobalamin, respectively. The signals with m/z values of 1,331.7 and 1,369.6 a.m.u. (D) suggest this cobamide of unknown identity could be norpseudovitamin B12 (M + 1)+ and deprotonated norpseudovitamin B12 plus a K+ ion (M − 1 + K+)+. Further tests need to be performed to determine the identity of cobamide UNK2.
DISCUSSION
The last step of the de novo corrin ring biosynthetic pathway occurs at the inner membrane of serovar Typhimurium.
Our refined model of CbiB topology (319 amino acids in length) (Fig. 2) is consistent with the data in hand and with the prediction that CbiB is an integral membrane protein. According to this new model, CbiB has at least five membrane-spanning domains, but the data are not unequivocal as to whether the N terminus is embedded in the membrane. The data do support the fact, however, that the C terminus is exposed to the periplasm. Most conserved residues in CbiB are clustered in the periplasm-exposed loop between helices IV and V of the model, with others located throughout the membrane-spanning helices and the loops facing the cytoplasm (Fig. 2). More-detailed studies are needed to better define the topology of CbiB in the membrane. Nevertheless, the data in hand indicate that the last step of the de novo corrin ring biosynthetic pathway occurs at the membrane.
The association of CbiB with the inner membrane is intriguing but not unique to the de novo corrin ring biosynthetic branch of the pathway. The AdoCbl-5′-P synthase (CobS) enzyme, which catalyzes the penultimate step of the nucleotide loop assembly pathway (42), is also localized to the cell membrane (21).
Bioinformatics analyses of CbiB and CobS orthologs suggest that all cobamide producers sequenced to date place CbiB and CobS in their membranes. Clearly, there is a strong positive selection exerted on cobamide-producing prokaryotes to share this strategy. At present, we can only speculate why. Perhaps this location helps the cell increase the local concentration of cobyric acid available to the CbiB enzyme from the environment, accelerating its conversion to AdoCbl. By having the remaining steps of the pathway also associated with the membrane, the cell would avoid dilution of the substrate into the cytoplasm, making the synthesis of the final product more efficient. The prediction derived from this idea is that the late steps of AdoCbl synthesis and the last step of de novo corrin ring biosynthesis may happen within a multiprotein complex anchored to the membrane. This idea is supported by previous studies of aminopropanol attachment in Pseudomonas denitrificans, where enzymes involved in the late steps of AdoCbl synthesis were partially purified as part of large multiprotein complexes (3). It would make physiological sense if such complexes also interacted with the BtuCD corrinoid transport system, which is also located at the inner membrane. We are currently exploring the merit of these predictions.
Residues important for the function of CbiB are found on both sides of the inner membrane.
The computer-generated model of CbiB predicted that residue D181 was exposed to the periplasm; our data support that assignment. Surprisingly, a D181N change severely impaired CbiB function (Fig. 3). Why is a periplasm-exposed residue such as D181 so important to function? We propose that residue D181 may be important for interactions with other proteins and that only when CbiB interacts with them is the conformation of the active site optimized. The role of residue D181 is clearly very important to CbiB activity, because a mutation at residue T179, just two residues apart from D181, did not affect CbiB function (Table 5). We note that the extreme sensitivity of the bioassay used to assess CbiB function allowed us to identify residues that had very severe negative effects on CbiB activity. Mutations resulting in less severe effects would not be identified because the residual activity of the enzyme would be sufficient to satisfy the Cbl requirement to synthesize methionine.
CbiB may activate AdoCby by phosphorylation.
The reaction catalyzed by CbiB involves the formation of an amide bond between AP-P and AdoCby (Fig. 1). This reaction must require a source of energy to proceed because neither the amino group of AP-P nor the carboxylate of Cby is activated. The enzyme that activates either one of the substrates has not been identified. We propose that CbiB activates AdoCby by converting it into AdoCby-P and that the hydrolysis of the phosphoryl moiety of AdoCby-P drives the reaction that joins AdoCby and AP-P. In vivo data obtained in this study support this idea (Fig. 5). The growth response of the cobU ycfN cbiB/pcobY+/pcbiB+ strain to Cbi in the medium was striking and unexpected (Fig. 5). The simplest explanation for these results is that in such a strain, CbiB phosphorylates AdoCbi, allowing its conversion to AdoCbi-GDP by the archaeal CobY guanylyltransferase enzyme. The merit of this hypothesis needs to be demonstrated in vitro. Attempts to isolate CbiBWT protein or to perform in vitro assays utilizing CbiB-enriched cell extracts have thus far been unsuccessful.
Insights into the CbiB active site.
Our data show that CbiB does not use l-Thr-P as a substrate, but it can use EA-P (Fig. 6B). The difference between l-Thr-P and AP-P (the physiological substrate for CbiB) is the carboxyl moiety of l-Thr-P. Since EA-P is structurally smaller than l-Thr-P, it was not surprising to learn that CbiB would use EA-P as the substrate. However, the tight lack of growth in response to l-Thr-P present in the medium (Fig. 6B) strongly suggests that the active site of CbiB cannot accommodate the carboxylate of l-Thr-P and that CobD (l-Thr-P decarboxylase) is the sole source of AP-P in serovar Typhimurium. Verification of this idea has been hampered by the difficulty in isolating the CbiB protein.
It is interesting that the cobD strain can grow when CbiB protein is present at higher-than-chromosomal levels in the absence of any supplement (Fig. 6A). Clearly the cobD strain synthesizes a complete, functional cobamide (Fig. 7), but its identity remains to be determined.
Acknowledgments
This work was supported by NIH grant GM40313 to J.C.E.-S. C.L.Z. was supported in part by NIH grant F31-GM64009.
We thank P. Renz for the gift of CNCby, Aixadelisse Moreno for assistance in the isolation of cbiB mutant strains of serovar Typhimurium used in this work, and Michele M. Otte for the gift of HPLC-purified CNCbi and AdoCby.
Footnotes
Published ahead of print on 7 September 2007.
REFERENCES
- 1.Atlas, R. 1995. Handbook of media for environmental microbiology, p. 6. CRC Press, Boca Raton, FL.
- 2.Berkowitz, D., J. M. Hushon, H. J. Whitfield, Jr., J. Roth, and B. N. Ames. 1968. Procedure for identifying nonsense mutations. J. Bacteriol. 96:215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanche, F., B. Cameron, J. Crouzet, L. Debussche, D. Thibaut, M. Vuilhorgne, F. J. Leeper, and A. R. Battersby. 1995. Vitamin B12: how the problem of its biosynthesis was solved. Angew. Chem. Int. Ed. Engl. 34:383-411. [Google Scholar]
- 4.Blanche, F., D. Thibaut, M. Couder, and J. C. Muller. 1990. Identification and quantitation of corrinoid precursors of cobalamin from Pseudomonas denitrificans by high-performance liquid chromatography. Anal. Biochem. 189:24-29. [DOI] [PubMed] [Google Scholar]
- 5.Brinsmade, S. R., T. Paldon, and J. C. Escalante-Semerena. 2005. Minimal functions and physiological conditions required for growth of Salmonella enterica on ethanolamine in the absence of the metabolosome. J. Bacteriol. 187:8039-8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, K. L. 2005. Chemistry and enzymology of vitamin B12. Chem. Rev. 105:2075-2149. [DOI] [PubMed] [Google Scholar]
- 7.Brushaber, K. R., G. A. O'Toole, and J. C. Escalante-Semerena. 1998. CobD, a novel enzyme with l-threonine-O-3-phosphate decarboxylase activity, is responsible for the synthesis of (R)-1-amino-2-propanol O-2-phosphate, a proposed new intermediate in cobalamin biosynthesis in Salmonella typhimurium LT2. J. Biol. Chem. 273:2684-2691. [DOI] [PubMed] [Google Scholar]
- 8.Casadaban, M. J., A. Martinez-Arias, S. K. Shapira, and J. Chou. 1983. Beta-galactosidase gene fusions for analyzing gene expression in Escherichia coli and yeast. Methods Enzymol. 100:293-308. [DOI] [PubMed] [Google Scholar]
- 9.Chan, R. K., D. Botstein, T. Watanabe, and Y. Ogata. 1972. Specialized transduction of tetracycline resistance by phage P22 in Salmonella typhimurium. II. Properties of a high transducing lysate. Virology 50:883-898. [DOI] [PubMed] [Google Scholar]
- 10.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeVeaux, L. C., D. S. Clevenson, C. Bradbeer, and R. J. Kadner. 1986. Identification of the BtuCED polypeptides and evidence for their role in vitamin B12 transport in Escherichia coli. J. Bacteriol. 167:920-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drennan, C. L., S. Huang, J. T. Drummond, R. G. Matthews, and M. L. Ludwig. 1994. How a protein binds B12: a 3.0Å X-ray structure of B12-binding domains of methionine synthase. Science 266:1669-1674. [DOI] [PubMed] [Google Scholar]
- 13.Elliott, T., and J. R. Roth. 1988. Characterization of Tn10d-Cam: a transposition-defective Tn10 specifying chloramphenicol resistance. Mol. Gen. Genet. 213:332-338. [DOI] [PubMed] [Google Scholar]
- 14.Escalante-Semerena, J. C., S. J. Suh, and J. R. Roth. 1990. cobA function is required for both de novo cobalamin biosynthesis and assimilation of exogenous corrinoids in Salmonella typhimurium. J. Bacteriol. 172:273-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall, D. A., C. W. Vander Kooi, C. N. Stasik, S. Y. Stevens, E. R. Zuiderweg, and R. G. Matthews. 2001. Mapping the interactions between flavodoxin and its physiological partners flavodoxin reductase and cobalamin-dependent methionine synthase. Proc. Natl. Acad. Sci. USA 98:9521-9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heller, K., B. J. Mann, and R. J. Kadner. 1985. Cloning and expression of the gene for the vitamin B12 receptor protein in the outer membrane of Escherichia coli. J. Bacteriol. 161:896-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman, C. S., and A. Wright. 1985. Fusions of secreted proteins to alkaline phosphatase: an approach for studying protein secretion. Proc. Natl. Acad. Sci. USA 82:5107-5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofmann, K., and W. Stoffel. 1993. TMbase: a database of membrane spanning protein segments. Biol. Chem. 374:166. [Google Scholar]
- 19.Jeter, R. M., B. M. Olivera, and J. R. Roth. 1984. Salmonella typhimurium synthesizes cobalamin (vitamin B12) de novo under anaerobic growth conditions. J. Bacteriol. 159:206-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kräutler, B., W. Fieber, S. Osterman, M. Fasching, K.-H. Ongania, K. Gruber, C. Kratky, C. Mikl, A. Siebert, and G. Diekert. 2003. The cofactor of tetrachloroethene reductive dehalogenase of Dehalospirillum multivorans is Norpseudo-B12, a new type of natural corrinoid. Helv. Chim. Acta 86:3698-3716. [Google Scholar]
- 21.Maggio-Hall, L. A., K. R. Claas, and J. C. Escalante-Semerena. 2004. The last step in coenzyme B(12) synthesis is localized to the cell membrane in bacteria and archaea. Microbiology 150:1385-1395. [DOI] [PubMed] [Google Scholar]
- 22.Manoil, C., and J. Beckwith. 1985. TnphoA: a transposon probe for protein export signals. Proc. Natl. Acad. Sci. USA 82:8129-8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melnick, J., E. Lis, J. H. Park, C. Kinsland, H. Mori, T. Baba, J. Perkins, G. Schyns, O. Vassieva, A. Osterman, and T. P. Begley. 2004. Identification of the two missing bacterial genes involved in thiamine salvage: thiamine pyrophosphokinase and thiamine kinase. J. Bacteriol. 186:3660-3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Toole, G. A., and J. C. Escalante-Semerena. 1995. Purification and characterization of the bifunctional CobU enzyme of Salmonella typhimurium LT2. Evidence for a CobU-GMP intermediate. J. Biol. Chem. 270:23560-23569. [DOI] [PubMed] [Google Scholar]
- 25.O'Toole, G. A., M. R. Rondon, and J. C. Escalante-Semerena. 1993. Analysis of mutants of defective in the synthesis of the nucleotide loop of cobalamin. J. Bacteriol. 175:3317-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Otte, M., J. D. Woodson, and J. Escalante-Semerena. 2007. The thiamine kinase (YcfN) enzyme plays a minor but significant role in cobinamide salvaging in Salmonella enterica. J. Bacteriol. 189:7310-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patterton, H.-G., and S. Graves. 2000. DNAssist: the integrated editing and analysis of molecular biology sequences in Windows. Bioinformatics 16:652-653. [DOI] [PubMed] [Google Scholar]
- 28.Peariso, K., Z. S. Zhou, A. E. Smith, R. G. Matthews, and J. E. Penner-Hahn. 2001. Characterization of the zinc sites in cobalamin-independent and cobalamin-dependent methionine synthase using zinc and selenium X-ray absorption spectroscopy. Biochemistry 40:987-993. [DOI] [PubMed] [Google Scholar]
- 29.Raux, E., A. Lanois, F. Levillayer, M. J. Warren, E. Brody, A. Rambach, and C. Thermes. 1996. Salmonella typhimurium cobalamin (vitamin B12) biosynthetic genes: functional studies in S. typhimurium and Escherichia coli. J. Bacteriol. 178:753-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodionov, D. A., A. G. Vitreschak, A. A. Mironov, and M. S. Gelfand. 2003. Comparative genomics of the vitamin B12 metabolism and regulation in prokaryotes. J. Biol. Chem. 278:41148-41159. [DOI] [PubMed] [Google Scholar]
- 31.Schmieger, H. 1971. A method for detection of phage mutants with altered transduction ability. Mol. Gen. Genet. 110:378-381. [DOI] [PubMed] [Google Scholar]
- 32.Schmieger, H., and H. Bakhaus. 1973. The origin of DNA in transducing particles of P22 mutants with increased transduction frequencies (HT-mutants). Mol. Gen. Genet. 120:181-190. [DOI] [PubMed] [Google Scholar]
- 33.Suh, S., and J. C. Escalante-Semerena. 1995. Purification and initial characterization of the ATP:corrinoid adenosyltransferase encoded by the cobA gene of Salmonella typhimurium. J. Bacteriol. 177:921-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabor, S. 1990. Expression using the T7 RNA polymerase/promoter system, p. 16.2.1.-16.2.11. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. Wiley Interscience, New York, NY. [Google Scholar]
- 35.Taylor, R. T., and H. Weissbach. 1973. N5-methylenetetrahydrofolate-homocysteine methyltransferases, p. 121-165. In P. D. Boyer (ed.), The enzymes, vol. 9. Academic Press, Inc., New York, NY. [Google Scholar]
- 36.Thomas, M. G., and J. C. Escalante-Semerena. 2000. Identification of an alternative nucleoside triphosphate: 5′-deoxyadenosylcobinamide phosphate nucleotidyltransferase in Methanobacterium thermoautotrophicum ΔH. J. Bacteriol. 182:4227-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas, M. G., T. B. Thompson, I. Rayment, and J. C. Escalante-Semerena. 2000. Analysis of the adenosylcobinamide kinase/adenosylcobinamide phosphate guanylyltransferase (CobU) enzyme of Salmonella typhimurium LT2. Identification of residue H46 as the site of guanylylation. J. Biol. Chem. 275:27376-27386. [DOI] [PubMed] [Google Scholar]
- 38.Van Bibber, M., C. Bradbeer, N. Clark, and J. R. Roth. 1999. A new class of cobalamin transport mutants (btuF) provides genetic evidence for a periplasmic binding protein in Salmonella typhimurium. J. Bacteriol. 181:5539-5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warren, M. J., E. Raux, H. L. Schubert, and J. C. Escalante-Semerena. 2002. The biosynthesis of adenosylcobalamin (vitamin B12). Nat. Prod. Rep. 19:390-412. [DOI] [PubMed] [Google Scholar]
- 40.Woodson, J. D., and J. C. Escalante-Semerena. 2004. CbiZ, an amidohydrolase enzyme required for salvaging the coenzyme B12 precursor cobinamide in archaea. Proc. Natl. Acad. Sci. USA 101:3591-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodson, J. D., C. L. Zayas, and J. C. Escalante-Semerena. 2003. A new pathway for salvaging the coenzyme B12 precursor cobinamide in archaea requires cobinamide-phosphate synthase (CbiB) enzyme activity. J. Bacteriol. 185:7193-7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zayas, C. L., and J. C. Escalante-Semerena. 2007. Reassessment of the late steps of coenzyme B12 synthesis in Salmonella enterica: evidence that dephosphorylation of adenosylcobalamin-5′-phosphate by the CobC phosphatase is the last step of the pathway. J. Bacteriol. 189:2210-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]