Abstract
Streptococcus pneumoniae (the pneumococcus) produces 1 of 91 capsular polysaccharides (CPS) that define the serotype. The cps loci of 88 pneumococcal serotypes whose CPS is synthesized by the Wzy-dependent pathway were compared with each other and with additional streptococcal polysaccharide biosynthetic loci and were clustered according to the proportion of shared homology groups (HGs), weighted for the sequence similarities between the genes encoding the shared HGs. The cps loci of the 88 pneumococcal serotypes were distributed into eight major clusters and 21 subclusters. All serotypes within the same serogroup fell into the same major cluster, but in six cases, serotypes within the same serogroup were in different subclusters and, conversely, nine subclusters included completely different serotypes. The closely related cps loci within a subcluster were compared to the known CPS structures to relate gene content to structure. The Streptococcus oralis and Streptococcus mitis polysaccharide biosynthetic loci clustered within the pneumococcal cps loci and were in a subcluster that also included the cps locus of pneumococcal serotype 21, whereas the Streptococcus agalactiae cps loci formed a single cluster that was not closely related to any of the pneumococcal cps clusters.
Many important bacterial pathogens produce a polysaccharide capsule that is believed to contribute to virulence by aiding bacterial survival in blood (47). Capsular polysaccharides (CPS) are surface exposed and immunogenic and provide a target for the host immune response. Consequently, there has been selection for mechanisms by which encapsulated bacteria evade the host immune system, and in most encapsulated species, this has been achieved by the generation of antigenic diversity, such that strains of the pathogen may express one of a number of different CPS (40).
In Streptococcus pneumoniae, 90 immunochemically distinct CPS types have been identified and sera that recognize the differences between these capsules provide a serological typing scheme, resolving pneumococci into individual serotypes or into serogroups, which include multiple immunologically related serotypes (16, 17). The serotyping scheme for pneumococci has been updated many times, and the 90 currently recognized serotypes probably represent a high proportion of the total capsule diversity in the species, although a new variant within serogroup 6 (serotype 6C) has recently been reported (33).
The biochemical structures of 54 capsular types are known (22), and the sequences of the CPS biosynthetic (cps) loci of several serotypes have been reported over the last decade (15, 18, 26, 51), but the sequences of the cps loci of all pneumococcal serotypes have only recently become available, allowing relationships among serology, genetics, and structure to be explored (5).
The CPS of 88 serotypes are known to be synthesized by the Wzy-dependent pathway, and the cps loci encoding this pathway in these serotypes are located at the same chromosomal location between dexB and aliA (5, 18, 36, 51); the capsules of the other two serotypes (3 and 37) are synthesized by the synthase pathway and are not considered further in this paper. The Wzy-dependent pathway is also found in several other streptococcal species (25). For example, it is found in CPS biosynthesis of Streptococcus agalactiae (10) and Streptococcus suis (39), in receptor polysaccharide synthesis (RPS) in the viridans group streptococci, Streptococcus gordonii, Streptococcus mitis, and Streptococcus oralis (12, 48-50) and exopolysaccharide synthesis in Streptococcus thermophilus (7, 8).
The evolution of these streptococcal loci is almost certainly very complex, with a long history of gene capture and loss and genetic rearrangements, and it is probably unrealistic to expect to be able to untangle their evolutionary history. Rather than take a phylogenetic approach, which seems inappropriate, we have explored the relatedness of the pneumococcal cps loci (and other streptococcal polysaccharide biosynthetic loci) by cluster analysis, incorporating similarities in both gene content and nucleotide sequence, and used this as a framework to relate differences in the cps loci of closely related serotypes to differences in their CPS structures.
MATERIALS AND METHODS
Polysaccharide biosynthetic sequences.
A total of 116 streptococcal polysaccharide biosynthetic sequences were used in this study; those from strains of each of the 88 pneumococcal serotypes that are known to use the Wzy-dependent pathway (accession numbers CR931632, CR931633, CR931635, CR931637 to CR931708, and CR931710 to CR931722; 5), 17 pneumococcal cps loci sequenced by other groups (see Table S1 in the supplemental material), including the serotype 4 locus extracted from the TIGR4 genome (42), and 11 S. agalactiae or viridans group streptococcal sequences. The latter included sequences of the cps loci of S. agalactiae serotypes Ia and II to VIII (accession numbers AB028896, AY375362, AF163833, AF355776, AF349539, AF337958, AY376403, and AY375363, respectively; 10, 11) and the RPS loci of S. oralis strains 34 (AB181234; 50) and J22 (AB181235; 49) and of S. mitis NCTC12261.
The RPS locus of S. mitis strain NCTC12261 (nucleotides 215361 to 240666) was extracted from the genome sequence available at the J. Craig Venter Institute website (www.tigr.org). This sequence is not completely finished and includes some unresolved nucleotides; therefore, only the region between the wzg (cpsA) and rmlD (rfbD) genes was included. It should be noted that GenBank accession no. AB181235 was initially submitted as the sequence of the RPS biosynthetic locus of strain S. mitis J22, but this strain has subsequently been recharacterized as S. oralis (49). S. pneumoniae serotype 6C (33) could not be included in the cluster analysis, as its cps sequence was unavailable.
Bioinformatic methods and cluster analysis.
TribeMCL (14) was run on the data set of 116 polysaccharide biosynthetic sequences with a TBLASTX cutoff of 1e−50 to assign the gene products to homology groups (HGs). The cps gene products were classified into Pfam families based on hidden Markov model profiles by using the Pfam database (4; http://www.sanger.ac.uk/Software/Pfam/). The pairwise nucleotide sequence similarities among cps genes whose products were assigned to the same HG were calculated by the Needleman-Wunsch algorithm by using the EMBOSS program NEEDLE (32).
Visual representation of the alignments using nucleotide similarities (BLASTN) and similarities of the gene products (TBLASTX) of the polysaccharide biosynthetic loci were performed by ACT version 6 and WebACT, an online version of the Artemis comparison tool (2, 9). The nucleotide differences between similar cps loci were calculated by the EMBOSS program Diffseq (MRC Rosalind Franklin Centre for Genomics Research, Hinxton, United Kingdom) and are presented elsewhere (see Table S1 in the supplemental material).
For clustering of the cps sequences, a modification of the method described by Tekaia et al. (41) was applied. A total of 99 polysaccharide biosynthetic loci that encode capsules synthesized by the Wzy-dependent pathway were included in the cluster analysis, i.e., 88 S. pneumoniae cps loci (5) and 11 other streptococcal sequences. A profile of each cps locus (gene content) was produced according to the presence or absence of each of the 254 HGs. The dexB, aliA, aliB, transposase, and group II intron genes were not included in the profiles. The products of the four cps genes wzg, wzh, wzd, and wze of all pneumococcal cps loci and those of the S. mitis, S. oralis, and S. agalactiae loci each fall into a single HG, and these genes also were not included in the cps profiles used for clustering.
Each polysaccharide biosynthetic locus was compared with all other loci, calculating the similarity scores in sequence-weighted gene content, and a 99-by-99 data table (T) resulting from the pairwise comparisons of the 99 polysaccharide biosynthetic loci was produced, where the similarity score (Tij) for each pair of biosynthetic loci is the sum of the percentage of nucleotide sequence similarities of the HGs shared between loci i and j divided by the total number of HGs present in locus j. It should be noted that Tij is normalized because it is divided by the total number of HGs in locus j (and is 100% for comparisons of each cps locus with itself), and the data table is not symmetrical since Tij is different from Tji if the loci i and j differ in the number of HGs. The data table was used as the input for cluster analysis by the XL-STAT software version 7.5.3 (Addinsoft, New York, NY). A distance (or dissimilarity) matrix was produced by the Pearson distance by transformation of Pearson's coefficient into dissimilarity values in a range of 0 to 1 (rows were clustered according to the columns). The distance matrix was used for cluster analysis by the unweighted-pair group method using arithmetic averages (19).
Biochemical structures and antigenic formulas.
The CPS structures for 52 pneumococcal serotypes that use the Wzy-dependent pathway have been adopted from a report by Kamerling (22), incorporating the revised CPS structures of serotypes 15B, 17F, and 33F (20, 21, 24), and their symbolic representations were previously presented by Bentley et al. (5). The biochemical structures of the S. agalactiae CPS and the cell wall polysaccharides of S. oralis 34 (type 1Gn RPS) and S. oralis J22 (type 2G RPS) were from reports by Cieslewicz et al. (10) and Cisar et al. (12), respectively. The serotypes that react with each of the pneumococcal factor (typing) sera were obtained from data reported by Henrichsen (17).
RESULTS
Assignment of HGs.
The products of the four cps genes (wzg, wzh, wzd, and wze) are relatively conserved in sequence in all 88 pneumococcal serotypes, but there are multiple highly divergent or nonhomologous groups of flippases, polysaccharide polymerases, initial transferases (ITs), and other cps gene products, including a large number of different groups of glycosyltransferases (GTs). Previously, we have classified the gene products of the pneumococcal cps loci into those that share significant homology (HGs) by using the program TribeMCL (5). Genes that encode proteins within the same HG have been given the same name, except for those that encode the polymerases and flippases, where the widely used generic gene names (wzy and wzx, respectively) have been retained (5, 38).
In this study, the gene products encoded within 28 additional streptococcal biosynthetic loci were added to those of the 88 pneumococcal cps loci encoding capsules synthesized by the Wzy-dependent pathway (see Materials and Methods) and TribeMCL was rerun on this combined set to allow these additional proteins to be assigned to pneumococcal HGs or to novel HGs. Table 1 shows the HGs that were common to both pneumococcal and other streptococcal polysaccharide biosynthetic loci. In all cases, the cps loci from different S. pneumoniae strains of the same serotype had identical HG profiles (data not shown) and almost identical sequences (see Table S1 in the supplemental material); therefore, only the pneumococcal sequences of Bentley et al. (5) were used in all further analyses.
TABLE 1.
Products of other streptococcal polysaccharide biosynthetic loci that are within pneumococcal HGs
| Name in S. pneumoniaea | Organism | Name in other species |
|---|---|---|
| WchA | S. agalactiae Ia | CpsIaE |
| S. agalactiae II | CDS | |
| S. agalactiae III | CpsE | |
| S. agalactiae IV | CpsE | |
| S. agalactiae V | CpsE | |
| S. agalactiae VI | CpsE | |
| S. agalactiae VII | CDS | |
| S. agalactiae VIII | CDS | |
| S. mitis NCTC12261 | CpsE | |
| S. oralis 34 | WchA | |
| S. oralis J22 | WchA | |
| Glf | S. mitis NCTC12261 | Glf |
| S. oralis 34 | Glf | |
| S. oralis J22 | Glf | |
| RmlB | S. mitis NCTC12261 | RfbB |
| S. oralis 34 | RmlB | |
| S. oralis J22 | RmlB | |
| RmlD | S. mitis NCTC12261 | RfbD |
| S. oralis 34 | RmlD | |
| S. oralis J22 | RmlD | |
| RmlA | S. mitis NCTC12261 | RfbA |
| S. oralis 34 | RmlA | |
| S. oralis J22 | RmlA | |
| RmlC | S. mitis NCTC12261 | SMT0231 |
| S. oralis 34 | RmlC | |
| S. oralis J22 | RmlC | |
| Wzx-1 | S. mitis NCTC12261 | SMT0226 |
| S. oralis 34 | Wzx | |
| S. oralis J22 | Wzx | |
| WchF | S. agalactiae VIII | CDS |
| S. mitis NCTC12261 | SMT0220 | |
| S. oralis 34 | WchF | |
| S. oralis J22 | WchF | |
| WchJ | S. agalactiae Ia | CpsIaF |
| S. agalactiae II | CDS | |
| S. agalactiae III | CpsF | |
| S. agalactiae IV | CpsF | |
| S. agalactiae V | CpsF | |
| S. agalactiae VI | CpsF | |
| S. agalactiae VII | CDS | |
| WciF | S. oralis 34 | WefD |
| S. oralis J22 | WefG | |
| WcrH | S. mitis NCTC12261 | Eps6N |
| S. oralis 34 | WefE | |
| S. oralis J22 | WefE | |
| WcwA | S. mitis NCTC12261 | SMT0221 |
| S. oralis 34 | WefA | |
| S. oralis J22 | WefA | |
| WcwC | S. mitis NCTC12261 | Eps9H |
| WchV | S. agalactiae VIII | CDS |
| Wzy-19 | S. agalactiae VIII | CDS |
| WcwK | S. mitis NCTC12261 | CpsY |
| S. oralis 34 | WefH | |
| S. oralis J22 | WefF | |
| Wzy-32 | S. oralis 34 | Wzy |
| S. oralis J22 | Wzy | |
| WcwF | S. mitis NCTC12261 | SMT0223 |
Gene products that fall into the same HG as pneumococcal Wzg, Wzh, Wzd, and Wze were present in all of the S. agalactiae and viridans group streptococci listed.
Using the TBLASTX cutoff of 1e−50, the average amino acid sequence similarities of proteins assigned by TribeMCL to the same HG ranged from 42 to 100%, and 83% of the HGs included proteins with average sequence similarities of >70%. Members of the same HG should therefore correspond to proteins (and thus genes) with broadly similar functions.
Predicted function of cps gene products.
A detailed analysis of the predicted functions and specificities of the products of the genes in the pneumococcal cps loci is presented elsewhere (1), and we have used these functional assignments (and gene names) when discussing cps loci that were shown to be similar by cluster analysis. The assignment of the initial sugars of the repeat units and the specificity of the GTs and other transferases are also discussed in detail elsewhere, together with the basis for these assignments (1). In many cases, the comparison of closely related cps loci and their CPS structures provides strength to these assignments of GT specificity (discussed below).
Cluster analysis.
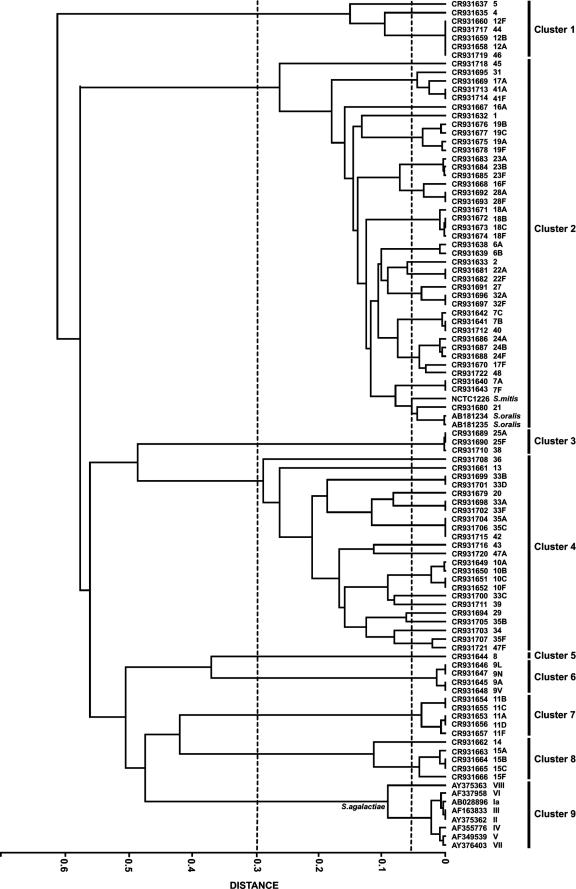
Figure 1 shows the clustering of the cps loci, based on a measurement of the extent of sharing of HGs and the degree of sequence similarity of the genes encoding the shared HGs as described in Materials and Methods. This approach was used as shared HGs could be encoded by genes that are almost identical, or that are divergent, in nucleotide sequence, and adjusting for sequence similarity results in tighter clustering when the genes encoding the shared HGs have very similar sequences than when they have less similar sequences. Clustering was used to identify those cps loci that possess a high level of similarity in sequence-weighted gene content, which forms a useful framework for discussing the relationships among the cps loci of the different pneumococcal serotypes.
FIG. 1.
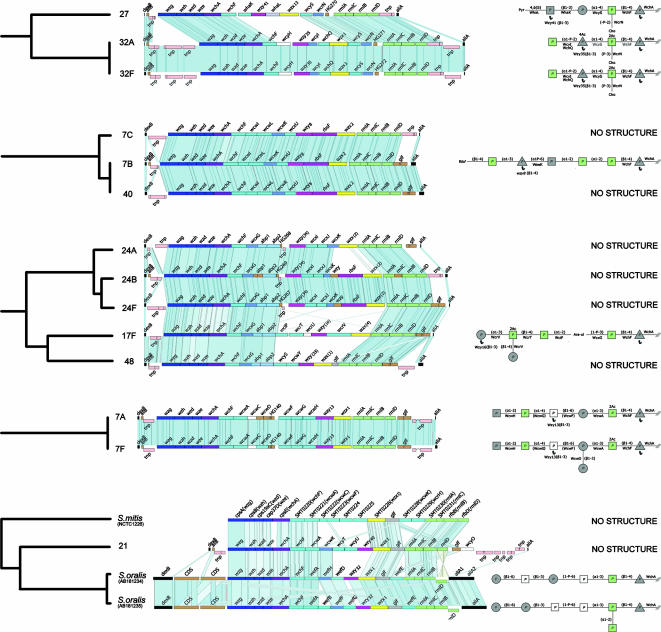
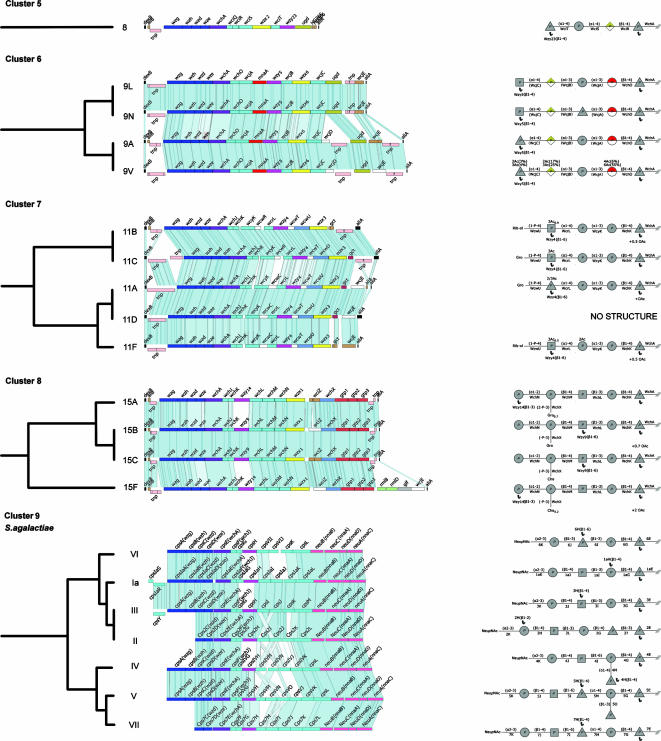
Cluster analysis of streptococcal polysaccharide biosynthetic loci. The relatedness of the loci was determined from the extent of sharing of HGs, adjusted for the percentage DNA sequence similarity between the genes encoding the shared HGs, as described in Materials and Methods. Clusters are defined by the dotted line at a distance of 0.3, and subclusters are defined by the dotted line at 0.05. Serotypes of S. pneumoniae (and S. agalactiae) or species of viridans group streptococci are shown at the end of each branch of the tree.
The cps loci of the 88 pneumococcal serotypes fell into eight clusters at a distance of 0.3 (Fig. 1). The cps loci of pneumococcal serotypes within the same serogroup were invariably in the same cluster. In most cases, each cluster included cps loci of several different serogroups but the cps locus of serotype 8 was sufficiently different from those of the other cps loci to cluster alone, as were the cps loci of the serotypes within both serogroups 9 and 11. A ninth cluster included all of the cps loci of S. agalactiae but no pneumococcal cps loci. In contrast, the S. mitis and two S. oralis RPS loci clustered among pneumococcal cps loci within cluster 2 (Fig. 1).
Subclusters were defined at a distance of 0.05 to identify very closely related cps loci (Fig. 1). All S. pneumoniae serotypes within the same serogroup fell into the same subcluster, except for the serotypes within serogroups 7, 16, 17, 33, 35, and 47, which were split into two or three different subclusters. Cluster analysis identified nine pneumococcal subclusters that included the cps loci of completely different serotypes (Fig. 1), and in three cases these loci were fully syntenic (synteny is defined here as the same genes being present in the same order). Thus, the cps loci of serotypes 44 and 46 were syntenic with those of serogroup 12 (Fig. 2), as shown previously (5), and those of serotypes 35A, 35C, and 42 (see Fig. 4) were syntenic, as were those of serotypes 7B, 7C, and 40 (see Fig. 3). For seven of these nine subclusters, there were factor sera that cross-reacted with the different serotypes within the subclusters (Table 2).
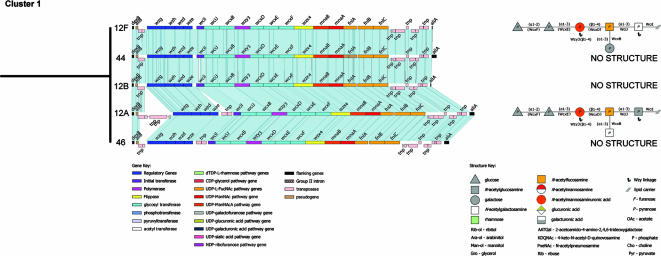
FIG. 2.
Comparisons of those cps loci within cluster 1 assigned to the same subcluster. The relatedness of the cps loci in the subcluster is from Fig. 1. The cps loci and ACT comparisons based on the amino acid sequence similarities of the products of the cps loci are shown, along with the CPS repeat unit structures, where known. CPS structures are represented as in the report by Bentley et al. (5), with the initial sugar that is attached to the lipid carrier (///) on the right. The linkage between repeat units catalyzed by the Wzy polymerase is between the two sugars marked by arrows. The genes that are predicted to catalyze the linkages in the repeat units are shown on the structure. The basis of these gene assignments is discussed in detail by Aanensen et al. (1), and uncertain GT assignments are presented in parentheses. The color keys for the functional classes of genes in the cps loci and for the sugars (and other constituents) in the CPS repeat units are shown at the bottom.
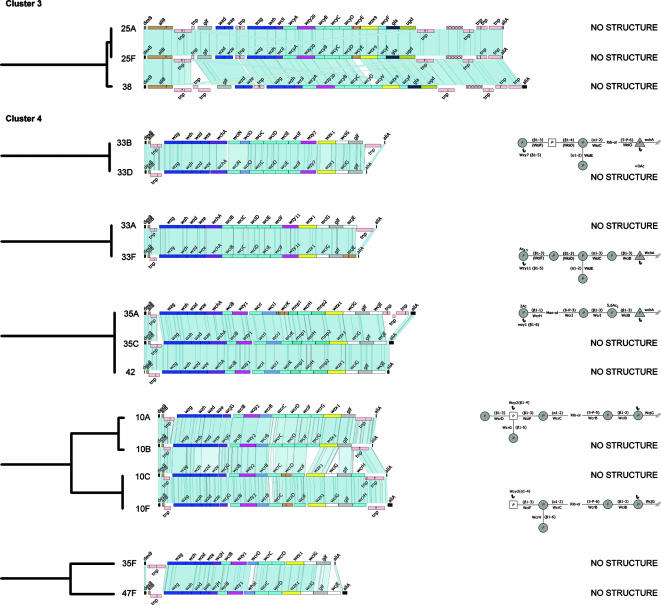
FIG. 4.
Comparisons of those cps loci within clusters 3 and 4 assigned to the same subcluster. Details are as in Fig. 2.
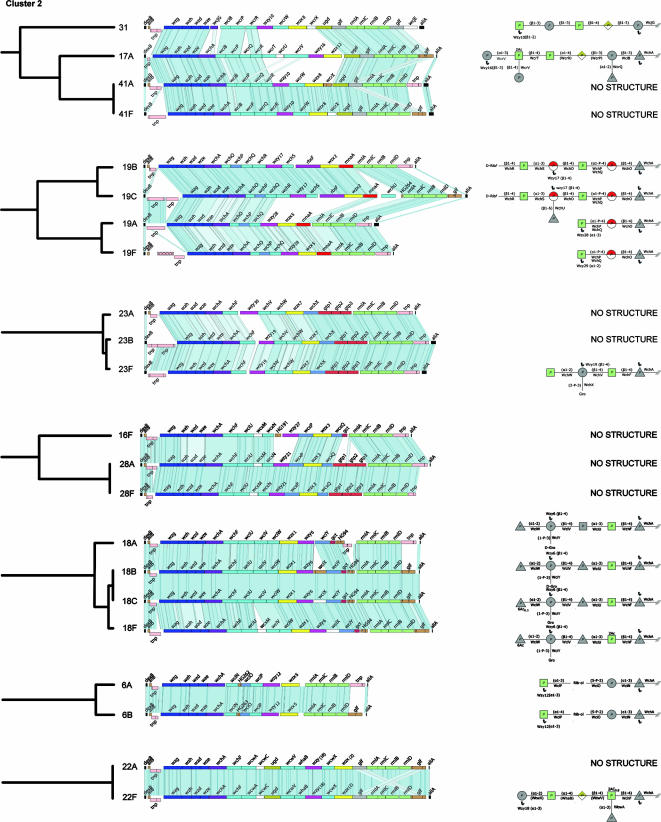
FIG. 3.
Comparisons of those cps loci within cluster 2 assigned to the same subcluster. Details are as in Fig. 2.
TABLE 2.
Serotypes that react with each pneumococcal typing serum
| Seruma | Serotype(s) |
|---|---|
| 1 | 1 |
| 2 | 2 |
| 3 | 3 |
| 4 | 4 |
| 5 | 5 |
| 6a | 6A, 6B, 33D |
| 6b | 6A |
| 6c | 6B |
| 7a | 7F, 7A, 7B, 7C |
| 7b | 7F, 7A |
| 7c | 7A |
| 7d | 7B, 7C |
| 7e | 7B |
| 7f | 7C |
| 7g | 7C, 20, 40 |
| 7h | 7B, 7C, 19B, 19C, 24F, 24B, 40 |
| 8 | 8 |
| 9a | 9A, 9L, 9N, 9V |
| 9b | 9L, 9N |
| 9c | 9A, 9L, 9V |
| 9d | 9A, 9V |
| 9e | 9N, 36 |
| 9f | 9L |
| 9g | 9V |
| 10a | 10F, 10A, 10B, 10C |
| 10b | 10F, 10B, 10C |
| 10c | 10A, 10B, 10C |
| 10d | 10A, 10B, 39 |
| 10e | 10B |
| 10f | 10C |
| 11a | 11F, 11A, 11B, 11C, 11D |
| 11b | 11F, 11B, 11C, 11D |
| 11c | 11A, 11C, 11D |
| 11d | 11A, 11C, 16F |
| 11e | 11F, 11A, 11D |
| 11f | 11B, 11C |
| 11g | 11F, 11B |
| 12a | 12F, 12A, 12B |
| 12b | 12F, 12B, 44 |
| 12c | 12A, 12B, 46 |
| 12d | 12F, 12A, 44 |
| 12e | 12B |
| 13 | 13 |
| 13b | 13, 29 |
| 14 | 14 |
| 15a | 15F, 15A, 15B, 15C, 23A |
| 15b | 15F, 15B |
| 15c | 15F, 15A |
| 15d | 15A, 15B, 15C |
| 15e | 15B, 15C |
| 15f | 15F |
| 15g | 15A |
| 15h | 15B |
| 16a | 16F, 16A |
| 16b | 16F, 28F |
| 16c | 16A |
| 17a | 17F, 17A |
| 17b | 17F |
| 17c | 17A |
| 18a | 18F, 18A, 18B, 18C |
| 18b | 18F, 18A, 18B, 18C, 23F |
| 18c | 18F, 18C |
| 18d | 18A |
| 18e | 18B, 18C |
| 18f | 18F |
| 18g | 18B |
| 19a | 19F, 19A, 19B, 19C |
| 19b | 19F |
| 19c | 19A, 19B, 19C |
| 19d | 19F, 19A |
| 19e | 19B |
| 19f | 19C |
| 20 | 20 |
| 20b | 20, 31, 33A, 35A, 35C, 42 |
| 21 | 21 |
| 22a | 22F, 22A |
| 22b | 22F |
| 22c | 22A |
| 23a | 23F, 23A, 23B |
| 23b | 23F, 23B |
| 23c | 23A |
| 23d | 23B, 28F, 28A |
| 24a | 24F, 24A, 24B |
| 24b | 24F, 24B |
| 24c | 24A |
| 24c | 24F, 24A |
| 24e | 24B |
| 25a | 25F, 25A |
| 25b | 25F, 38 |
| 25c | 25A |
| 27 | 27 |
| 27b | 27, 32F, 32A |
| 28a | 28F, 28A |
| 28b | 28F |
| 28c | 28 |
| 29 | 29 |
| 29b | 29, 35B |
| 31 | 31 |
| 32a | 32F, 32A |
| 32b | 32A |
| 33a | 33F, 33A, 33B, 33C, 33D |
| 33b | 33F, 33A |
| 33c | 33B, 33C, 33D |
| 33d | 33F, 33A, 33B, 33D |
| 33e | 33C |
| 33f | 33B, 33D |
| 34 | 34 |
| 34b | 34, 35F |
| 35a | 35F, 35A, 35B, 35C, 47F |
| 35b | 35F, 47F |
| 35c | 35A, 35B, 35C, 42 |
| 36 | 36 |
| 37 | 37 |
| 38 | 38 |
| 38a | 25A, 38 |
| 39 | 39 |
| 40 | 40 |
| 41a | 41F, 41A |
| 41b | 41F |
| 42 | 42 |
| 42a | 42, 35C |
| 43 | 43 |
| 43b | 43, 47A |
| 44 | 44 |
| 44b | 44, 46 |
| 45 | 45 |
| 46 | 46 |
| 47a | 47F, 47A |
| 48 | 48 |
Type or factor serum.
The similarities between the cps loci of different serotypes were sometimes greater than that between serotypes within the same serogroup (Fig. 1). For example, serotype 40 cps was more similar in sequence-weighted gene content to serotype 7B than 7B was to 7C (similarity scores of 97.5% and 90.3%, respectively) and was much more similar to the latter two serotypes, falling into the same subcluster, than these were to serotypes 7F and 7A (Fig. 1).
The serotypes within each major cluster are considered in the next sections, focusing on those that are sufficiently similar to be placed within the same subcluster (Fig. 1). The cps loci of those serotypes that are not within a subcluster are shown elsewhere (see Fig. S1 in the supplemental material).
Cluster 1.
The cps loci of serogroup 12 and serotypes 44 and 46 form a subcluster and, ignoring transposon-related genes, are syntenic, presumably having diverged recently from a common ancestral cps locus (Fig. 2). The structures of serotypes 12F and 12A are known and differ only in the initial sugar and a side branch. As there is synteny, these differences in repeat unit structure are presumably due to the amino acid sequence variation in the IT WciI and the GT (WcxB) that is predicted to make this side branch (1, 5; see below). The structures of types 12B, 44, and 46 are unknown, but the synteny and immunological cross-reactivity between the serotypes in this subcluster (Table 2) suggest structural similarities with the CPS of serotypes 12F and 12A.
Serotype 4 and 5 cps loci are also members of cluster 1, but they are less closely related to the other members of the cluster (Fig. 1). Compared with serogroup 12 and types 44 and 46, the cps locus of type 4 shares the wciI, wciJ, mnaA, and fnlA-C genes and type 5 shares the wciI, wciJ, and fnlA-C genes, which explains why these serotypes cluster together (Fig. 2; see Fig. S1 in the supplemental material). The type 45 cps locus also shares the wciI, wciJ, wcxB, and fnlA-C genes but their sequences are distantly related to those of cluster 1 (data not shown) and it is placed in cluster 2 (Fig. 1; see Fig. S1 in the supplemental material).
The l-FucpNAc biosynthetic pathway genes (fnlA-C) are present in the cps loci of cluster 1, and l-FucpNAc is known to be present in the CPS of types 4, 5, 12A, and 12F, as well as the constituents of type 46. The type 44 cps locus has fnlC frameshifted; therefore, l-FucpNAc is not expected in its CPS structure. The type 5 CPS contains, in addition to l-FucpNAc, two sugars (PnepNAc and KQDNAc) which are intermediates in the l-FucpNAc biosynthetic pathway (30) and within pneumococci are uniquely present in the type 5 CPS. The products of the fnlA, fnlB, and fnlC genes in type 5 are 76%, 98%, and 99% similar to the other members of the cluster. Therefore, the sequence divergence of FnlA may be implicated in the above difference in the l-FucpNAc pathway which results in these unusual sugars in the CPS of serotype 5 (1).
The ManpNAcA biosynthetic pathway genes mnaA and mnaB are both present in the cps loci of serogroup 12 and serotypes 44 and 46; therefore, ManpNAcA is present in the CPS structures of types 12A and 12F (and is predicted also to be present in types 12B, 44, and 46). In serotype 4, only mnaA is present and accordingly the type 4 CPS contains ManpNAc β-1,4 linked to l-FucpNAc. In the serotype 5 cps locus, neither of these genes is present and consequently the above sugars are absent from the type 5 CPS.
The IT gene wciI is present in the cps loci of cluster 1 and also in the cps loci of types 25A, 25F, 38, and 45, although the sequences of the latter are very divergent from those in cluster 1 (data not shown). WciI appears to have different specificities, as the initial sugar in cluster 1 varies (d-GlcpNAc in serotype 12A, d-GalpNAc in serotypes 4 and 12F, and KQDNAc in serotype 5), although the sequences of WciI within this cluster are very similar (70 to 100% pairwise identity; average, 81.4%). This highlights the difficulties in predicting the enzymatic specificities of transferases from their amino acid sequence similarity.
The GT genes wcxD, wcxE, and wcxF are present only in serogroup 12 and types 44 and 46, and their products are predicted to form the linkages common to these serotypes. Inspection of the two known structures, types 12A and 12F, suggests that these GTs catalyze the formation of the common tetrasaccharide element d-Glcp-(α1-2)-d-Glcp-(α1-3)-d-ManpNAcA-(β1-4)-l-FucpNAc. The GT gene wciJ is present in the cps loci of all serotypes of cluster 1 (and in type 45), and l-FucpNAc is α-1,3 linked to the repeat units but to different initial sugars (see above). Therefore, we have tentatively assigned wciJ as the putative α-1,3-l-FucpNAc transferase gene. As in the case of the IT of cluster 1, the specificities of the linkage made by similar members (average pairwise identity, 83.9%) of the same HG (WciJ) appear to vary. The GT gene wcxB is present in serogroup 12 and types 44 and 46 (and in type 45) and is suggested to encode the GT catalyzing the α-1,3 linkage of d-GalpNAc to α-l-FucpNAc in the CPS of type 12A and of d-Galp to α-l-FucpNAc in type 12F (and type 45).
Cluster 2.
The cps loci of 40 pneumococcal serotypes and the S. oralis and S. mitis polysaccharide biosynthetic loci (the latter two are discussed in a separate section below) are within this large cluster, and all cps loci within the cluster contain the rhamnose biosynthesis genes (rml). All of the available CPS structures of these serotypes contain rhamnose (except for types 1 and 24B, due to the presence of frameshift mutations within the rmlC and rmlD genes, respectively), and there is a perfect correlation between the presence of the linkage l-Rhap-(β1-4)-β-d-Glcp (49) in the repeat units where the cps locus possesses the putative rhamnosyltransferase gene wchF. It is therefore predicted that WchF is the GT that catalyzes the latter linkage. The other cps loci in this cluster have the rml genes present but in association with rhamnosyltransferase genes other than wchF, and where possible we have assigned these transferases (1).
Serotype 1 is the only case where there is no candidate within the cps locus for the IT gene. The serotype 1 repeat unit contains AAT-Galp, which is a component of pneumococcal teichoic acid, and this unusual sugar is also present in Bacteroides fragilis CPS A, the Shigella sonnei form I antigen, and the Plesiomonas shigelloides serotype O17 antigen (1). In these latter species, an IT for AAT-Galp has been identified and there is a homolog in the pneumococcal chromosome (13). It seems likely that this chromosomal IT synthesizes lipid-linked AAT-Galp, which can be used for repeat unit synthesis in serotype 1, as further discussed by Aanensen et al. (1).
The serotypes within serogroups 6, 18, 19, 22, 23, 24, 28, 32, and 41 each fall within the same subcluster, suggesting that they are each descended from a common ancestral cps locus, whereas the cps loci of serogroups 7, 16, and 17 are split into different subclusters (Fig. 1). The cps loci of serotypes within serogroups 6, 22, 28, 32, and 41 are in each case syntenic, and differences in structure should be due to amino acid variation in one or more of the cps gene products (Fig. 3).
Serogroup 6.
CPS of serotypes 6A and 6B differ only in the rhamnose linkage to Rib-ol (α-1,3 in 6A or α-1,4 in 6B), which correlates with a single nucleotide polymorphism in the rhamnosyltransferase gene wciP (27). The structure of the repeat unit of serotype 6C has been reported (33) and is most similar to that of 6A, containing the Rhap(α1-3)d-Rib-ol linkage. Serotype 6C differs from type 6A only in the presence of a Glcp residue as the second sugar, as opposed to Galp, presumably due to amino acid differences in the GT, WciN (1).
Serogroup 7.
The cps loci form two syntenic pairs (7F-7A and 7B-7C) that cluster apart from each other (Fig. 3), and the CPS structures of 7F, 7A, and 7B are known (22). Type 7A lacks only the side branch d-Galp-(β1-2)-α-d-Galp compared with type 7F, and this difference can be attributed to a frameshift mutation in the GT gene wcwD. The CPS of serotype 7B has little in common with those of types 7F and 7A. The CPS structures of types 7A, 7B, and 7F possess the l-Rhap-(β1-4)-β-d-Glcp linkage attributed to the presence of WchF, but l-Rhap is acetylated in types 7A and 7F, presumably due to the presence of the wcwC acetyltransferase gene in their cps loci. Additionally, the d-GlcpNAc-(α1-2)-α-l-Rhap linkage is present as a side branch in types 7A and 7F and in the main chain in type 7B and has been suggested to be the epitope common to all serotypes of serogroup 7 recognized by factor serum 7a (22), but there is no common GT as a candidate for forming the latter linkage. As mentioned previously, types 7B, 7C, and 40 constitute a separate subcluster, reflected in the serology (Table 2), with factor serum 7h cross-reacting with 7B, 7C, and 40, but not 7A or 7F, and 7g reacting with 7C and 40 (the CPS structures of types 7C and 40 are not known).
Serogroup 17.
Serotype 17F and 17A cps loci are in different subclusters (Fig. 1) but share the IT gene wchA, three genes in the central cps region (the putative GT genes wcrT and wcrV and the acetyltransferase gene wcrU), and the polymerase gene wzy-16, although the order and the nucleotide sequences of the latter four shared genes differ substantially. The products of these shared genes should account for the synthesis of the common structural elements d-Galp-(α1-3)-l-Rhap2Ac-(β1-4)-α-l-Rhap in the main chain of the repeat unit, the side branch d-Galp-(β1-4)-l-Rhap2Ac (21), and the polymerase linkage d-Glcp(β1-3)d-Galp, which are common to both serotypes 17F and 17A. WcrV contains two functional GT domains and is suggested to catalyze two transferase reactions (1). Except for these commonalities, serogroup 17 cps loci differ considerably and consequently type 17A clusters with serogroup 41 and type 31, whereas type 17F clusters with serogroup 24 and type 48 (Fig. 3).
Serogroup 18.
Serotype 18F, 18A, 18B, and 18C cps loci form a separate subcluster; types 18B and 18C are syntenic, whereas type 18F has an extra acetyltransferase gene (wcxM) and type 18A lacks the acetyltransferase gene wciX (Fig. 3). As in serogroup 15, the O-acetylation state correlates with the presence of an intact acetyltransferase gene (wciX) in the cps loci of types 18F and 18C and accordingly the last Glcp residue of the repeat unit is acetylated, whereas it has a frameshift mutation in type 18B and is absent in type 18A. Type 18F additionally has the rhamnose residue acetylated due to the presence of the extra acetyltransferase gene wcxM.
Apart from the differences in the acetylation patterns, the CPS of types 18B, 18C, and 18F are identical; type 18A differs only in the third residue of the repeat unit (α-d-GlcpNAc instead of α-d-Glcp). Besides the rhamnosyltransferase gene wchF, serogroup 18 cps loci possess three shared GT genes (wciU, wciV, and wciW). WciV has homology to β-1,4-d-Gal transferases and is likely to catalyze the linkage of β-1,4-d-Galp to α-d-GlcpNAc in type 18A but to α-d-Glcp in types 18B, 18C, and 18F. WciW possesses the same Pfam domain (PF05704) as WchN, which has been suggested to form the d-Galp-(α1-2)-β-d-Galp linkage in serogroup 15 (5). Serogroup 18 CPS structures do not have the latter linkage but do have a d-Glcp-(α1-2)-β-d-Galp linkage. Thus, we propose that WciW functions as the α-1,2 d-Glcp transferase in serogroup 18. WciU would therefore, by a process of elimination, function as the putative d-GlcpNAc-(α1-3)-β-l-Rhap GT in type 18A or the d-Glcp-(α1-3)-β-l-Rhap GT in types 18F, 18B, and 18C. Serogroup 18 CPS contains glycerol-1-phosphate (Gro-1P), and accordingly, the sugar phosphate transferase wciY and the Gro-1P biosynthesis (gct) genes are present in their cps loci (5, 18).
Serogroup 19.
A comparative analysis of serotype 19F, 19A, 19B, and 19C cps loci and the putative biosynthetic pathways has been presented previously (29). The cps loci of all four serotypes are in the same subcluster (Fig. 3), and those of types 19F and 19A are syntenic and their CPS structures differ only in the polymerization linkage between d-Glcp and α-l-Rhap (α-1,2 in type 19F and α-1,3 in type 19A). Thus, it has been proposed that the differences between the products of the wzy genes should account for the different polymerization linkages (29). The products of the wzy-28 genes of types 19F and 19A fall into the same HG, but they display 22% sequence divergence, which supports this view. The products of the wzy and wzx genes of types 19A and 19F fall into different HGs than those of types 19B and 19C.
Serogroup 22.
The cps loci of serotypes 22F and 22A are syntenic (Fig. 3). The chemical differences between the CPS of these serotypes are not known since only the former structure is available.
Serogroup 23.
The cps loci of serotypes 23F, 23A, and 23B are in the same subcluster (Fig. 3) and differ only in their polymerase genes; wzy-19 in types 23F and 23B is replaced by wzy-30 in type 23A, suggesting a different polymerase linkage (only the type 23F CPS structure is known). Type 23F CPS contains only one d-Glcp residue linked with a β-1,4 bond to β-d-Galp. The IT gene wchA is present in the type 23F cps locus, and as this transfers Glcp to the lipid carrier, we are confident that the polymerization linkage is d-Glcp-(β1-4)-β-d-Galp. Type 23F CPS contains glycerol-2-phosphate (Gro-2P), and as the wchX and gtp1 and -3 genes are also present in the cps loci of types 23A and 23B, it is assumed that they also contain Gro-2P (28).
Serogroup 24.
Serotype 24F, 24A, and 24B cps loci form a subcluster with those of types 17F and 48, the latter two serotypes being less related as they differ in gene content (Fig. 3). The cps loci of types 24B and 24F are syntenic and, compared with the type 24A cps locus, have an extra fragment of a polymerase gene and the putative ribofuranose biosynthetic gene (rbsF) that also are present in types 7B, 7C, and 40. In the type 24B cps locus, the abp1 and rmlC genes are frameshifted; therefore, no arabinitol or rhamnose is expected in the CPS. The type 24A cps locus lacks the rbsF gene (and the wzy fragment), and therefore no ribofuranose is expected in the CPS. The sugar phosphate transferase gene wcxG and the arabinitol biosynthetic pathway genes (abp1 and -2) are only found in the cps loci of serogroup 24 and types 17F and 48.
Serogroup 28.
Serotype 28F and 28A and type 16F cps loci constitute a subcluster (Fig. 3), and their CPS structures (none of these are known) show immunological cross-reactions, factor serum 16b reacting with both serotypes 28F and 16F (Table 2). The type 16F cps locus is very similar to those of serotypes 28F and 28A, but it lacks the gtp1 and -3 genes and has an additional gene (gct) and a gene fragment (HG191). In addition, the wzy gene product of type 16F falls into a different HG. In contrast, the type 16A cps locus has relatively little in common with that of serotype 16F and is in a different subcluster (Fig. 1).
Serogroup 32.
Serotype 32F, 32A, and 27 cps loci constitute a subcluster (Fig. 3), and the extensive similarity is reflected in the commonalities of their CPS structures and immunological cross-reaction with factor serum 27b (Table 2). The CPS structures of serogroup 32 differ only in the acetylation pattern (type 32A has an extra acetyl group at the α-d-Glcp residue), presumably due to sequence differences in the acetyltransferase WcyH (5% sequence divergence between types 32A and 32F). The cps locus of type 27 shows extensive similarity at the 5′ and 3′ regions, but the central cps region differs considerably. Following assignment of WchF to the catalysis of the l-Rhap(β1-4)-β-d-Glcp linkage of the repeat units, the GT WcyS is common and presumably catalyzes the d-Galp-(α1-4)-β-l-Rhap linkage in type 27 CPS but the d-Glcp-(α1-4)-β-l-Rhap linkage in serogroup 32 CPS. The putative sugar phosphate transferase WcrN should catalyze the incorporation of choline via a P-2 linkage in type 27 CPS or a P-3 linkage in serogroup 32 CPS (1).
Serogroup 41.
Serotype 41F, 41A, 17A, and 31 cps loci constitute a subcluster (Fig. 3). The cps loci of serogroup 41 differ only in the 3′ end, where type 41F has a second, nonfunctional, copy of glf, whereas type 41A has a defective transposase gene instead. The latter serotype also has a stop codon in the putative acetyltransferase gene wcrX; thus, there should be differences in the acetylation patterns (neither of the CPS structures is known). Serogroup 41 and type 31 cps loci differ in their IT genes (wchA in serogroup 41 and wcjG in serotype 31), and type 31 has a second copy of glf, which appears to be functional, and an extra acetyltransferase gene (wcjE) at the 3′ end, but it lacks the putative GT gene wcrQ.
The type 17A cps locus is syntenic with that of type 41F at the 5′ and 3′ ends. The formation of the similar trisaccharide backbone l-Rhap-(α1-4)-β-d-GlcpA-(β1-3)-β-d-Galf of serotype 17A and l-Rhap-(β1-4)-d-GlcpA-(β1-3)-β-d-Galf of serotype 31 (differing only in the anomeric status of l-Rhap) should be attributed to the presence of the GT genes wcrP and wcrR that are common to both cps loci. In all serotypes where wciB is present in the cps loci, there is a Galf residue β-1,3 linked in the repeat units of the available CPS structures and we have assigned WciB as the GT that makes the β-1,3 linkage of d-Galf to β-d-Glcp in type 17A (1). wciB is also present in serotype 31 and could be assigned to the Galf-(β1-3)-β-l-Rhap linkage; however, we are not confident in the assignment of linkages in serotype 31, in the absence of more recent nuclear magnetic resonance data for the CPS structure (3).
Cluster 3.
Serotypes 25A, 25F, and 38 are assigned to cluster 3, and all fall within the same subcluster (Fig. 1 and 4). Their cps loci are very similar and have the first four cps regulatory genes interrupted by a transposase gene and rearranged in order. The cps loci of types 25A and 25F are syntenic, and type 38 differs only in the presence of the GT WcyV instead of the truncated WcyE in the former types. None of the three structures are available, but the synteny and immunological cross-reactivity (factor sera 25b and 38a cross-react with CPS of types 25F and 38 and types 25A and 38, respectively; Table 2) suggest structural similarities and recent common ancestry.
Cluster 4.
Cluster 4 includes the cps loci of 23 pneumococcal serotypes (Fig. 1 and 4). The presence of certain GT genes (for example, wciB, wcrC, wciF, wcrD, and wciE) and the commonalities in structure at the 3′ end of the cps loci of several of these serotypes appear to account for their inclusion in the same cluster. The cross-reactions of some typing factor sera with several serotypes of cluster 4 also reflect some structural commonalities, for example, 13b with types 13 and 29, 34b with types 34 and 35F, and 35a with types 35A, 35B, 35C, 35F, and 47F (Table 2). The cps loci of types 13 and 36 are not very closely related to any of the others in the cluster. The cps loci of the serotypes within serogroup 10 cluster together, but those of serogroups 33, 35, and 47 do not (Fig. 1). The cps loci of some serotypes in cluster 4 are much more similar to those of a serotype within another serogroup than the latter are to the other members of that serogroup, for example, types 35A, 35C, and 42 or 35F and 47F (Fig. 1).
Serogroup 10.
Serotype 10F, 10A, 10B, and 10C cps loci are in the same subcluster (Fig. 4) and are divided into two syntenic pairs, types 10A and 10B and types 10C and 10F, but there are structures only for types 10A and 10F. The GT genes and the ribitol phosphate transferase gene that direct the synthesis of each linkage in the common backbone of their repeat units, and the different side branches, are discussed by Aanensen et al. (1). The putative acetyltransferase gene wciG is present in type 10C-10F loci but absent from type 10A-10B cps loci; nevertheless, type 10F CPS has not been reported to be acetylated.
Serogroup 33.
There are also two syntenic pairs of cps loci in serogroup 33 (types 33A-33F and 33B-33D), but in this case the pairs are in different subclusters (Fig. 1 and 4), and the available CPS structures of types 33F and 33B are also quite different. The type 33C cps locus differs considerably from both of the above pairs and clusters separately from both of the above syntenic pairs (Fig. 1; see Fig. S1 in the supplemental material). The cps locus of type 33F, compared with the type 33A locus, differs only in the acetyltransferase gene (wcjE), which is frameshifted in type 33F but intact in 33A. The antigenic formulas of types 33F and 33A differ in an extra reaction of type 33A with factor serum 20b, and this serum might recognize a difference in acetylation (Table 2) (18). Type 33D reacts additionally with factor serum 6a compared to type 33B, but the difference in structure is not known as the 33D CPS structure is unavailable.
Serogroup 35.
Serogroup 35, like serogroup 33, is unusual as it includes serotypes that fall into three different subclusters (Fig. 1); type 35A, 35C, and 42 cps loci (only the CPS structure of type 35A is known) are in the same subcluster (Fig. 4), whereas type 35F clusters apart and is very similar to serotype 47F (both CPS structures are unknown), and similarly, type 35B clusters apart and is most similar to type 29 (both structures are known). Although the serotypes within serogroup 35 fall into three subclusters, commonalities in their structures are reflected by the reactivity of factor serum 35a with all four serotypes (Table 2). The differences in the gene complements within serogroup 35 and the close similarity of some of the serotypes to unrelated serotypes are also reflected by serological cross-reactivity patterns (see below).
Except for the presence of defective transposase genes in the cps locus of type 35A, upstream of aliA, types 35A and 35C are syntenic with the type 42 cps locus (Fig. 4), and accordingly factor serum 35c cross-reacts with types 35A, 35C, and 42, 20b cross-reacts with types 35A and 35C, and 42a cross-reacts with types 35C and 42 (Table 2). Type 35A has a frameshift mutation in the GT gene wcrK, and this difference could explain the loss of reactivity with factor serum 42a.
Type 35B cps locus is also similar to 35A and 35C at the 5′ end (and cross-reacts with factor serum 35c) but resembles the type 29 locus in the central region (sharing wcrJ, wcrM, and wcrH) (Fig. 4; see Fig. S1 in the supplemental material). The cross-reaction of types 35B and 29 with factor serum 29b is also reflected by the commonalities in their repeat units; they differ only in the initial sugar (Galp transferred by WcjH and Glcp transferred by WchA, respectively), and the acetylation pattern of type 35B, due to the presence of an extra acetyltransferase gene (wciG).
Type 35F is similar at the 5′ and 3′ ends to the other serotypes within serogroup 35 but clusters with type 47F (Fig. 4), due to similarities in the central cps region, and accordingly, both 35F and 47F cross-react with factor sera 35a and 35b (Table 2); the CPS structures of both types are unknown. Types 35F and 47F differ only in a sugar phosphate transferase gene (type 35F has the wcrO gene replaced by the whaI gene in type 47F) and the presence of an extra acetyltransferase gene (wcjE) in type 47F.
Serogroup 47.
As mentioned above, type 47F is more similar to type 35F than to 47A, which is in a different subcluster (Fig. 1). The central cps region of type 47A is similar to type 43, reflected by the cross-reaction of both serotypes with factor serum 43b; the CPS structures of both types are unknown.
Cluster 5.
Serotype 8 is the only member of this cluster (Fig. 1), and the biochemical functions of the IT WchA and the GT WciS and a biosynthetic pathway have previously been suggested (31, 34, 37). WciS is an α-1,4 galactosyltransferase, WciQ and WciR are homologs of the N-terminal and C-terminal halves, respectively, of the characterized glucuronosyl β-1,4 transferase SpsK of Sphingomonas (35) and are suggested to catalyze the formation of the d-GlcpA-(β1-4)-β-d-Glcp linkage, and by a process of elimination, WciT is suggested to catalyze the d-Glcp-(α1-4)-α-d-Galp linkage.
Cluster 6.
The cps loci of the four serotypes within serogroup 9 fall into the same subcluster (Fig. 5), and they form two syntenic pairs (9A-9V and 9L-9N). A putative model of CPS biosynthesis has been suggested previously (5, 45).
FIG. 5.
Comparisons of those cps loci within clusters 5 to 9 assigned to the same subcluster. Details are as in Fig. 2.
Cluster 7.
The five serotypes within serogroup 11 have very similar cps loci and fall into the same subcluster (Fig. 5). There are two syntenic groups of serogroup 11 cps loci, 11F-11A-11D and 11B-11C, differing only in their acetyltransferase genes, and presumably they have diverged from a recent common ancestral cps locus. All five serotypes possess the acetyltransferase gene wcwT, but types 11F, 11A, and 11D have two extra acetyltransferase genes (wcjE and wcwC) whereas types 11B and 11C have one extra acetyltransferase gene (wcwR). WcwU has been assigned as a glycerol phosphate transferase, and the presence of Gro-1P correlates with an intact gct gene in types 11A and 11C; gct is frameshifted in types 11F and 11B, and Rib-ol is present in the CPS instead of Gro.
The wchJ and wchK genes are present in the serogroup 11 cps loci. These genes are also present in serotype 14 cps, where biochemical studies have shown that WchK is a β-1,4-galactosyltransferase that catalyzes the linkage d-Galp-(β1-4)-β-d-Glcp, whereas WchJ is suggested to act as an enhancer of WchK activity (23). It is therefore likely that the homologous gene products in serogroup 11 are responsible for the d-Galp-(β1-4)-β-d-Glcp linkage found in their CPS. WcrL possesses the same Pfam domain (PF04488) as WciT of type 8, which, as mentioned above, has been suggested to catalyze the d-Glcp-(α1-4)-α-d-Galp linkage (37). The last sugar of the repeat unit is d-Glcp in type 11A but d-GlcpNAc in types 11B, 11C, and 11F (the type 11D structure is unknown), linked in all cases via an α-1,4 bond to α-d-Galp. Thus, we suggest that the last sugar of the repeat unit in serogroup 11 is transferred by WcrL and differences in amino acid sequence probably affect the specificity for the donor sugar. WcyK would therefore, by a process of elimination, catalyze the linkage d-Galp-(α1-3)-β-d-Galp in serogroup 11 CPS.
Cluster 8.
Cluster 8 includes serogroup 15 and serotype 14, and the relatedness of their cps loci has been presented previously (5, 23, 44). The differences in cps profile and the sequences of the shared genes result in the serotype 14 cps locus being in a different subcluster from those of serogroup 15 (Fig. 1 and 5).
Cluster 9.
Cluster 9 includes only the cps loci of the eight S. agalactiae serotypes (Fig. 1), with type VIII being the most divergent member of the cluster (see Fig. S1 in the supplemental material), as has been suggested previously (10). Only a partial sequence was available for the cps locus of S. agalactiae type Ib (46), and therefore it was not included in our study. All of these loci, except that of type VIII, fall into the same subcluster (Fig. 5). These loci are not very similar to any of the pneumococcal cps loci (Fig. 1), although some of the gene products of the cps loci of the two species fall into the same HGs, including wzg, wzh, wzd, wze, the IT gene wchA, and the GT gene wchJ (Table 1). It has been suggested that S. agalactiae type VIII and S. pneumoniae type 23F could have exchanged DNA segments due to the commonalities of their CPS structures and the sequence similarities in the central cps region (10, 28), and this observation was confirmed by our results. Thus, the products of the wchF, wchV, and wzy-19 (HG168) genes present in the type 23F cps locus each fall into the same HG as the corresponding S. agalactiae type VIII gene products and the linkages catalyzed are also thought to be similar.
Relatedness of S. mitis and S. oralis RPS loci to the pneumococcal cps loci.
The wzg, wzh, wzd, and wze genes are present in the S. oralis and S. mitis RPS biosynthetic loci (Fig. 3). They also possess the rhamnose biosynthetic genes (rml), although the direction of transcription of rmlD is reversed compared to that of the pneumococcal cps loci (48) and the rhamnosyltransferase gene wchF. The glf gene is also present in S. mitis and S. oralis, as are the IT gene wchA (the most common IT gene in pneumococci), the wzx-1 (HG7) flippase gene (present in 40 pneumococcal serotypes), and several GT genes that also are found in pneumococcal cps loci (Table 1).
The S. mitis and S. oralis sequences differ somewhat in the central region (Fig. 3), and for example, the Wzy polymerase of S. oralis 34 is in the same HG as that of pneumococcal serotype 36 whereas that of S. mitis is unique. Overall, the RPS loci of S. mitis and S. oralis are most similar to the cps loci of pneumococcal serotypes 7A, 7F, and 21 (Fig. 1 and 3), and they fall in the same subcluster as the pneumococcal serotype 21 locus. The two S. oralis sequences are the most similar to the pneumococcal serotype 21 cps locus (Fig. 3) and have similar HG profiles, although the sequences of the genes encoding the shared HGs differ considerably.
DISCUSSION
Clustering of pneumococcal cps loci on the basis of shared gene content (shared HGs), adjusted for the sequence similarity among the shared genes, provides a useful way of identifying those cps loci that are the most similar. The assignment of serotypes to subclusters by using a cutoff of 0.05 identifies cps loci that are sufficiently similar to predict that they have diversified relatively recently from a common ancestor. In some cases, these serotypes are completely syntenic and differences in CPS structure must be due to the ability of variant forms of the same IT or GTs to catalyze different reactions, but in other serotypes in the same subcluster the cps loci are syntenic except for a single cps gene that is nonhomologous (for example, types 15A and 15B, types 23A and 23B, serogroup 25 and type 38, and types 35F and 47F). The mechanism that gave rise to this phenomenon is not understood, although gene acquisition or replacement by some illegitimate recombination event is the most likely candidate. In effect, the alternative nonhomologous genes constitute a form of polymorphism and strains of these serotypes should interconvert by recombination in the flanking common genes, as has been shown to occur for the interconversion of serotypes 6A and 6B, which differ by a single amino acid polymorphism (27).
Cluster analysis also provides a framework for correlating cps gene content, and gene function, of very similar cps loci with the known CPS structures and the immunochemical similarities inferred from cross-reactivity with factor typing sera. A single difference in gene content between cps loci, that correlates with a single difference in the CPS structure, provides a strong prediction of the specific reaction catalyzed by the products of these alternative genes, and in many cases prediction can be extended to situations where more that one gene differs between very similar cps loci, but in most cases predictions must be made by using comparative methods (1). The uncertainties in predicting the precise functions of HGs (particularly the specificity of the transferases) have been discussed elsewhere (1). However, there is little option but to predict function as extremely few of the gene products involved in pneumococcal CPS biosynthesis have been the subject of any biochemical study. We stress that many of our assignments of specific linkages to gene products are tentative (the basis for these assignments is presented by Aanensen et al. [1]), but we expect most to be correct. We provide them as they form a basis for further biochemical or structural work to firmly establish the linkages catalyzed by individual GTs.
The similarities between the genes of the S. pneumoniae cps loci and the polysaccharide biosynthetic loci of other streptococci have been reported previously (7, 25, 43, 51), and TribeMCL proved a useful tool to identify these homologies (Table 1). Cluster analysis allowed us to recognize the similarities between the S. mitis and S. oralis RPS loci and those of several pneumococcal serotypes, notably serotype 21, suggesting a history of interspecies exchange of polysaccharide biosynthetic loci.
In general, the immunological subdivision of pneumococci into serotypes is supported by the structures of the cps loci. Thus, all serotypes in the same serogroup were in the same cluster and in many cases were in the same subcluster. In those cases where serotypes in the same serogroup are syntenic, it is assumed that selection imposed by the host immune response has led to the accumulation of nonsynonymous substitutions (or in some case frameshift mutations) in one or more of the shared genes, which leads to a difference in their CPS structure, which is recognized by the typing sera. There are, however, several instances in which serotypes in the same serogroup have dissimilar cps loci and, conversely, where the cps loci of completely different serotypes are very similar. In these cases, a classification based on genetic similarity would be quite different from that which has been developed by serology. For example, serotypes 44 and 46 could be reassigned as serotypes within serogroup 12, and in other cases serotypes within the same serogroup could more logically be placed in different serogroups.
However, lumping different serotypes together, or splitting serogroups, would cause confusion and there are no strong arguments for realigning serotypes and serogroups based on genetic similarities or differences between cps loci. Indeed, in the context of pneumococcal conjugate vaccines (6), it is the extent of immunological cross-reactivity and the resulting presence or absence of cross-protection between serotypes that matter, and this is more likely to be captured by the current serotyping scheme than by genetic similarities and differences between cps loci. The serological cross-reactivity between serotypes has been defined in the rabbit, and cross-reactivity between serotypes may be different for antibodies produced in humans, but the genetic similarities between some completely different serotypes and common reactions with some typing sera suggest that there may be situations where antibodies induced in humans by CPS of one serotype might provide some cross-protection against CPS of completely different serotypes.
Conjugate vaccines that include the CPS from a limited number of serotypes (6) are likely to be the only effective way of protecting against pneumococcal disease until vaccines are developed that use antigens which can protect against disease caused by all pneumococcal strains. The availability of the sequences of the cps loci of all 90 serotypes and the analysis of the relatedness of the cps loci of the 88 serotypes that use the Wzy-dependent pathway will provide the basis to develop novel molecular serotyping methods to monitor changes in the serotypes of pneumococci following the introduction of conjugate vaccines. More generally, the clustering approach we used here can be applied to look at the relatedness of other genetic elements that share variable numbers of genes with various levels of sequence similarity.
Supplementary Material
Acknowledgments
This work was funded by the Wellcome Trust. B.G.S. is a Wellcome Trust Principal Research Fellow.
Footnotes
Published ahead of print on 31 August 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Aanensen, D. M., A. Mavroidi, S. D. Bentley, P. R. Reeves, and B. G. Spratt. 2007. Predicted functions and linkage specificities of the products of the Streptococcus pneumoniae capsular biosynthetic loci. J. Bacteriol. 189:7856-7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott, J. C., D. M. Aanensen, K. Rutherford, S. Butcher, and B. G. Spratt. 2005. WebACT—an online companion for the Artemis comparison tool. Bioinformatics 21:3665-3666. [DOI] [PubMed] [Google Scholar]
- 3.Batavyal, L., and N. Roy. 1983. Structure of the capsular polysaccharide of Diplococcus pneumoniae type 31. Carbohydr. Res. 119:300-302. [Google Scholar]
- 4.Bateman, A., L. Coin, R. Durbin, R. D. Finn, V. Hollich, S. Griffiths-Jones, A. Khanna, M. Marshall, S. Moxon, E. L. Sonnhammer, D. J. Studholme, C. Yeats, and S. R. Eddy. 2004. The Pfam protein families database. Nucleic Acids Res. 32:D138-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentley, S. D., D. M. Aanensen, A. Mavroidi, D. Saunders, E. Rabbinowitsch, M. Collins, K. Donohoe, D. Harris, L. Murphy, M. A. Quail, G. Samuel, I. C. Skovsted, M. S. Kaltoft, B. Barrell, P. R. Reeves, J. Parkhill, and B. G. Spratt. 2006. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogaert, D., P. W. Hermans, P. V. Adrian, H. C. Rumke, and R. de Groot. 2004. Pneumococcal vaccines: an update on current strategies. Vaccine 22:2209-2220. [DOI] [PubMed] [Google Scholar]
- 7.Bourgoin, F., A. Pluvinet, B. Gintz, B. Decaris, and G. Guedon. 1999. Are horizontal transfers involved in the evolution of the Streptococcus thermophilus exopolysaccharide synthesis loci? Gene 233:151-161. [DOI] [PubMed] [Google Scholar]
- 8.Broadbent, J. R., D. J. McMahon, D. L. Welker, C. J. Oberg, and S. Moineau. 2003. Biochemistry, genetics, and applications of exopolysaccharide production in Streptococcus thermophilus: a review. J. Dairy Sci. 86:407-423. [DOI] [PubMed] [Google Scholar]
- 9.Carver, T. J., K. M. Rutherford, M. Berriman, M. A. Rajandream, B. G. Barrell, and J. Parkhill. 2005. ACT: the Artemis comparison tool. Bioinformatics 21:3422-3423. [DOI] [PubMed] [Google Scholar]
- 10.Cieslewicz, M. J., D. Chaffin, G. Glusman, D. Kasper, A. Madan, S. Rodrigues, J. Fahey, M. R. Wessels, and C. E. Rubens. 2005. Structural and genetic diversity of group B streptococcus capsular polysaccharides. Infect. Immun. 73:3096-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cieslewicz, M. J., D. L. Kasper, Y. Wang, and M. R. Wessels. 2001. Functional analysis in type Ia group B Streptococcus of a cluster of genes involved in extracellular polysaccharide production by diverse species of streptococci. J. Biol. Chem. 276:139-146. [DOI] [PubMed] [Google Scholar]
- 12.Cisar, J. O., A. L. Sandberg, G. P. Reddy, C. Abeygunawardana, and C. A. Bush. 1997. Structural and antigenic types of cell wall polysaccharides from viridans group streptococci with receptors for oral actinomyces and streptococcal lectins. Infect. Immun. 65:5035-5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coyne, M. J., A. O. Tzianabos, B. C. Mallory, V. J. Carey, D. L. Kasper, and L. E. Comstock. 2001. Polysaccharide biosynthesis locus required for virulence of Bacteroides fragilis. Infect. Immun. 69:4342-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enright, A. J., S. Van Dongen, and C. A. Ouzounis. 2002. An efficient algorithm for large-scale detection of protein families. Nucleic Acids Res. 30:1575-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.García, E., D. Llull, R. Muñoz, M. Mollerach, and R. López. 2000. Current trends in capsular polysaccharide biosynthesis of Streptococcus pneumoniae. Res. Microbiol. 151:429-435. [DOI] [PubMed] [Google Scholar]
- 16.Heidelberger, M. 1983. Precipitating cross-reactions among pneumococcal types. Infect. Immun. 41:1234-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henrichsen, J. 1995. Six newly recognized types of Streptococcus pneumoniae. J. Clin. Microbiol. 33:2759-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang, S. M., L. Wang, and P. R. Reeves. 2001. Molecular characterization of Streptococcus pneumoniae type 4, 6B, 8, and 18C capsular polysaccharide gene clusters. Infect. Immun. 69:1244-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jobson, J. D. 1992. Applied multivariate data analysis. Volume II. Categorical and multivariate methods, p. 483-568. Springer-Verlag, New York, NY.
- 20.Jones, C., and X. Lemercinier. 2005. Full NMR assignment and revised structure for the capsular polysaccharide from Streptococcus pneumoniae type 15B. Carbohydr. Res. 340:403-409. [DOI] [PubMed] [Google Scholar]
- 21.Jones, C., C. Whitley, and X. Lemercinier. 2000. Full assignment of the proton and carbon NMR spectra and revised structure for the capsular polysaccharide from Streptococcus pneumoniae type 17F. Carbohydr. Res. 325:192-201. [DOI] [PubMed] [Google Scholar]
- 22.Kamerling, J. P. 2000. Pneumococcal polysaccharides: a chemical view, p. 81-114. In A. Tomasz (ed.), Streptococcus pneumoniae: molecular biology and mechanisms of disease. Mary Ann Liebert Inc., Larchmont, NY.
- 23.Kolkman, M. A., B. A. van der Zeijst, and P. J. Nuijten. 1997. Functional analysis of glycosyltransferases encoded by the capsular polysaccharide biosynthesis locus of Streptococcus pneumoniae serotype 14. J. Biol. Chem. 272:19502-19508. [DOI] [PubMed] [Google Scholar]
- 24.Lemercinier, X., and C. Jones. 2006. Full assignment of the 1H and 13C spectra and revision of the O-acetylation site of the capsular polysaccharide of Streptococcus pneumoniae type 33F, a component of the current pneumococcal polysaccharide vaccine. Carbohydr. Res. 341:68-74. [DOI] [PubMed] [Google Scholar]
- 25.Llull, D., R. López, and E. García. 2001. Genetic bases and medical relevance of capsular polysaccharide biosynthesis in pathogenic streptococci. Curr. Mol. Med. 1:475-491. [DOI] [PubMed] [Google Scholar]
- 26.López, R., and E. García. 2004. Recent trends on the molecular biology of pneumococcal capsules, lytic enzymes, and bacteriophage. FEMS Microbiol. Rev. 28:553-580. [DOI] [PubMed] [Google Scholar]
- 27.Mavroidi, A., D. Godoy, D. M. Aanensen, D. A. Robinson, S. K. Hollingshead, and B. G. Spratt. 2004. Evolutionary genetics of the capsular locus of serogroup 6 pneumococci. J. Bacteriol. 186:8181-8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morona, J. K., D. C. Miller, T. J. Coffey, C. J. Vindurampulle, B. G. Spratt, R. Morona, and J. C. Paton. 1999. Molecular and genetic characterization of the capsule biosynthesis locus of Streptococcus pneumoniae type 23F. Microbiology 145:781-789. [DOI] [PubMed] [Google Scholar]
- 29.Morona, J. K., R. Morona, and J. C. Paton. 1999. Comparative genetics of capsular polysaccharide biosynthesis in Streptococcus pneumoniae types belonging to serogroup 19. J. Bacteriol. 181:5355-5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulrooney, E. F., K. K. Poon, D. J. McNally, J. R. Brisson, and J. S. Lam. 2005. Biosynthesis of UDP-N-acetyl-l-fucosamine, a precursor to the biosynthesis of lipopolysaccharide in Pseudomonas aeruginosa serotype O11. J. Biol. Chem. 280:19535-19542. [DOI] [PubMed] [Google Scholar]
- 31.Muñoz, R., M. Mollerach, R. López, and E. García. 1999. Characterization of the type 8 capsular gene cluster of Streptococcus pneumoniae. J. Bacteriol. 181:6214-6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Needleman, S. B., and C. D. Wunsch. 1970. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J. Mol. Biol. 48:443-453. [DOI] [PubMed] [Google Scholar]
- 33.Park, I. H., D. G. Pritchard, R. Cartee, A. Brandao, M. C. Brandileone, and M. H. Nahm. 2007. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J. Clin. Microbiol. 45:1225-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelosi, L., M. Boumedienne, N. Saksouk, J Geiselmann, and R. A. Geremia. 2005. The glucosyl-1-phosphate transferase WchA (Cap8E) primes the capsular polysaccharide repeat unit biosynthesis of Streptococcus pneumoniae serotype 8. Biochem. Biophys. Res. Commun. 327:857-865. [DOI] [PubMed] [Google Scholar]
- 35.Pollock, T. J., W. A. van Workum, L. Thorne, M. J. Mikolajczak, M. Yamazaki, J. W. Kijne, and R. W. Armentrout. 1998. Assignment of biochemical functions to glycosyl transferase genes which are essential for biosynthesis of exopolysaccharides in Sphingomonas strain S88 and Rhizobium leguminosarum. J. Bacteriol. 180:586-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reeves, P. R., M. Hobbs, M. A. Valvano, M. Skurnik, C. Whitfield, D. Coplin, N. Kido, J. Klena, D. Maskell, C. R. Raetz, and P. D. Rick. 1996. Bacterial polysaccharide synthesis and gene nomenclature. Trends Microbiol. 4:495-503. [DOI] [PubMed] [Google Scholar]
- 37.Saksouk, N., L. Pelosi, P. Colin-Morel, M. Boumedienne, P. L. Abdian, and R. A. Geremia. 2005. The capsular polysaccharide biosynthesis of Streptococcus pneumoniae serotype 8: functional identification of the glycosyltransferase WciS (Cap8H). Biochem. J. 389:63-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samuel, G., and P. Reeves. 2003. Biosynthesis of O-antigens: genes and pathways involved in nucleotide sugar precursor synthesis and O-antigen assembly. Carbohydr. Res. 338:2503-2519. [DOI] [PubMed] [Google Scholar]
- 39.Smith, H. E., V. Veenbergen, J. van der Velde, M. Damman, H. J. Wisselink, and M. A. Smits. 1999. The cps genes of Streptococcus suis serotypes 1, 2, and 9: development of rapid serotype-specific PCR assays. J. Clin. Microbiol. 37:3146-3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spratt, B. G., W. P. Hanage, and A. B. Bruegemann. 2004. Evolutionary and population biology of Streptococcus pneumoniae, p. 119-135. In E. I. Tuomanen (ed.), The pneumococcus. ASM Press, Washington, D.C.
- 41.Tekaia, F., A. Lazcano, and B. Dujon. 1999. The genomic tree as revealed from whole proteome comparisons. Genome Res. 9:550-557. [PMC free article] [PubMed] [Google Scholar]
- 42.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 43.van Kranenburg, R., H. R. Vos, I. I. van Swam, M. Kleerebezem, and W. M. de Vos. 1999. Functional analysis of glycosyltransferase genes from Lactococcus lactis and other gram-positive cocci: complementation, expression, and diversity. J. Bacteriol. 181:6347-6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Selm, S., L. M. van Cann, M. A. Kolkman, B. A. van der Zeijst, and J. P. van Putten. 2003. Genetic basis for the structural difference between Streptococcus pneumoniae serotype 15B and 15C capsular polysaccharides. Infect. Immun. 71:6192-6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Selm, S., M. A. Kolkman, B. A. van der Zeijst, K. A. Zwaagstra, W. Gaastra, and J. P. van Putten. 2002. Organization and characterization of the capsule biosynthesis locus of Streptococcus pneumoniae serotype 9V. Microbiology 148:1747-1755. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe, M., K. Miyake, K. Yanae, Y. Kataoka, S. Koizumi, T. Endo, A. Ozaki, and S. Iijima. 2002. Molecular characterization of a novel beta1,3-galactosyltransferase for capsular polysaccharide synthesis by Streptococcus agalactiae type Ib. J. Biochem. (Tokyo) 131:183-191. [DOI] [PubMed] [Google Scholar]
- 47.Watson, D. A., D. M. Musher, and J. Verhoef. 1995. Pneumococcal virulence factors and host immune responses to them. Eur. J. Clin. Microbiol. Infect. Dis. 14:479-490. [DOI] [PubMed] [Google Scholar]
- 48.Xu, D. Q., J. Thompson, and J. O. Cisar. 2003. Genetic loci for coaggregation receptor polysaccharide biosynthesis in Streptococcus gordonii 38. J. Bacteriol. 185:5419-5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshida, Y., S. Ganguly, C. A. Bush, and J. O. Cisar. 2005. Carbohydrate engineering of the recognition motifs in streptococcal co-aggregation receptor polysaccharides. Mol. Microbiol. 58:244-256. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida, Y., S. Ganguly, C. A. Bush, and J. O. Cisar. 2006. Molecular basis of l-rhamnose branch formation in streptococcal coaggregation receptor polysaccharides. J. Bacteriol. 188:4125-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yother, J. 2004. Capsules, p. 30-48. In E. I. Tuomanen (ed.), The pneumococcus. ASM Press, Washington, D.C.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.