Abstract
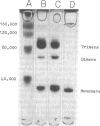
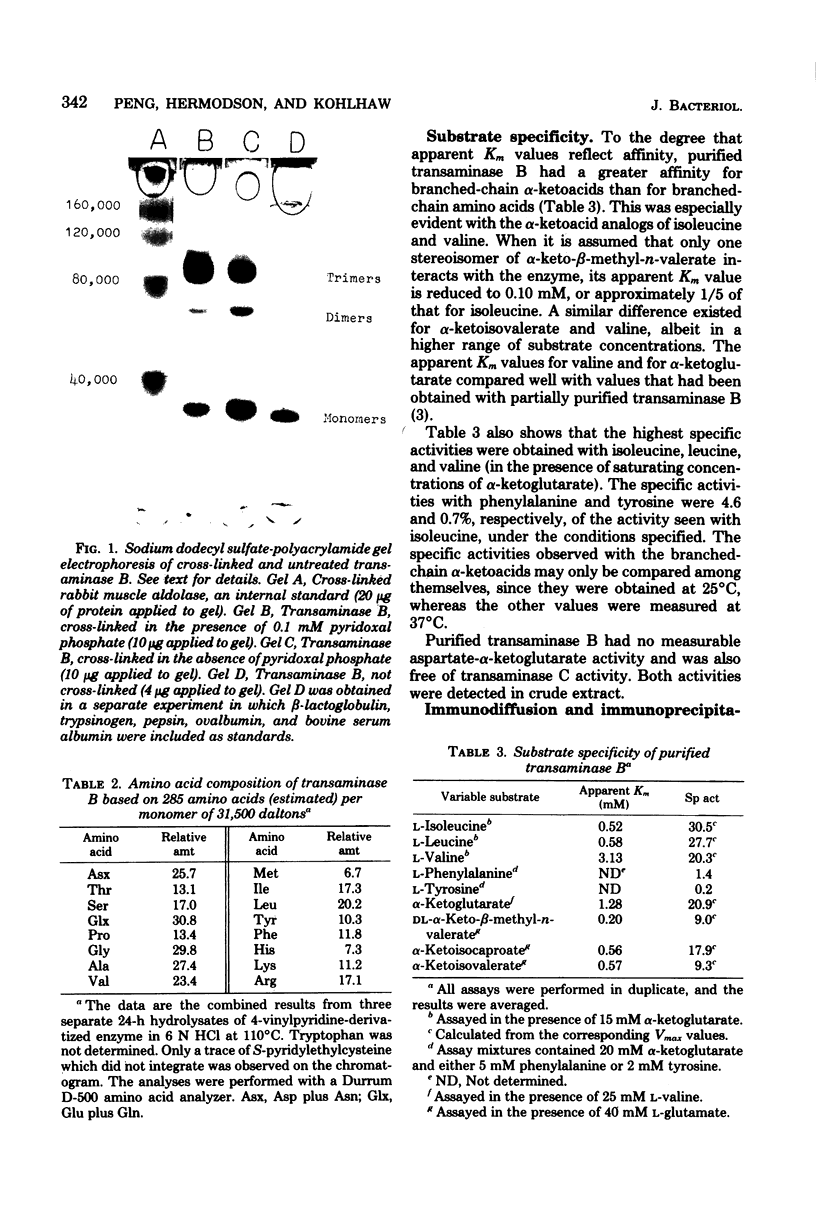
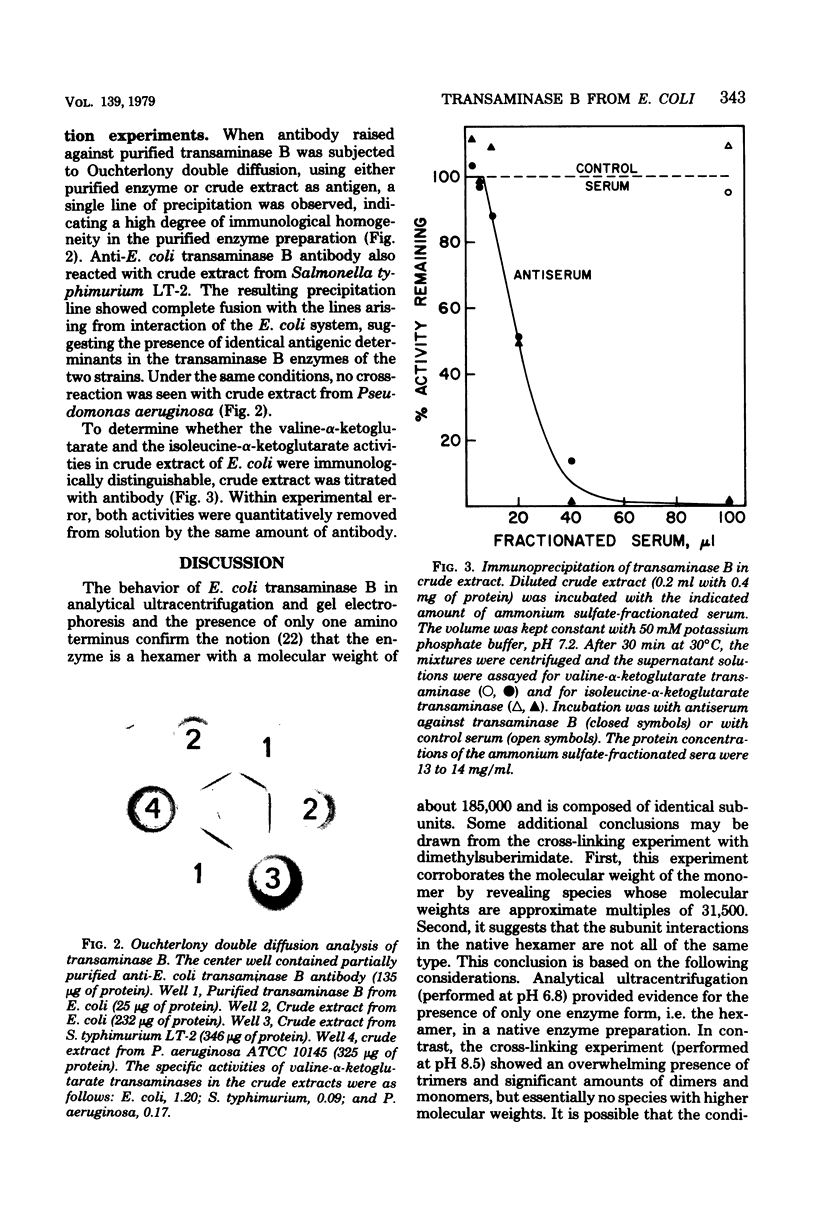
Transaminase B (branched-chain amino acid aminotransferase, EC 2.6.1.42), the ilvE gene product, was purified to apparent homogeneity from an Escherichia coli K-12 strain which carries the ilvE gene both on the host chromosome and on a plasmid. The oligomeric structure of the enzyme, as determined by analytical ultracentrifugation and sodium dodecyl sulfate-polyacrylamide gel electrophoresis, was confirmed to be that of a hexamer with a molecular weight of about 182,000 and apparently identical subunits. Cross-linking with dimethylsuberimidate yielded trimers, dimers, and monomers, but essentially no species of higher molecular weight. These results are consistent with a double-trimer arrangement of the subunits in native enzyme. The amino-terminal sequence was found to be: Gly Thr Lys Lys Ala Asp Tyr Ile (Trp) Phe Asn Gly (Thr) (Met) Val. Purified transaminase B catalyzed transamination between α-ketoglutarate and l-isoleucine, l-leucine, l-valine, and, to a lesser extent, l-phenylalanine and l-tyrosine, the latter reacting very sluggishly. The enzyme was free of aspartate transaminase and of transaminase C. The apparent Km values for the branched-chain α-ketoacids were smaller than those for the corresponding amino acids. The lowest Km was recorded for dl-α-keto-β-methyl-n-valerate, and the highest was recorded for l-valine. The ratio of the valine- and isoleucine-α-ketoglutarate activities did not change significantly during purification, and both activities were quantitatively removed from crude extract by antibody raised against purified transaminase B. These observations argue against the existence of a separate valine-α-ketoglutarate transaminase. Anti-E. coli transaminase B antibody cross-reacted with crude extract from Salmonella typhimurium, but not with extract obtained from Pseudomonas aeruginosa.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baez M., Patin D. W., Calhoun D. H. Deletion mapping of the ilvGOEDAC genes of Escherichia coli K-12. Mol Gen Genet. 1979 Feb 1;169(3):289–297. doi: 10.1007/BF00382275. [DOI] [PubMed] [Google Scholar]
- Chervenka C. H. Long-column meniscus depletion sedimentation equilibrium technique for the analytical ultracentrifuge. Anal Biochem. 1970 Mar;34:24–29. doi: 10.1016/0003-2697(70)90082-5. [DOI] [PubMed] [Google Scholar]
- Chesne S., Montmitonnet A., Pelmont J. Transamination du L-aspartate et de la L-phénylalanine chez Escherichia coli K 12. Biochimie. 1975;57(9):1029–1034. doi: 10.1016/s0300-9084(75)80358-0. [DOI] [PubMed] [Google Scholar]
- Chesne S., Pelmont J. Glutamate-oxaloacétate transaminase d'Escherichia coli. I. Purification et spécificité. Biochimie. 1973;55(3):237–244. doi: 10.1016/s0300-9084(73)80121-x. [DOI] [PubMed] [Google Scholar]
- Cohen B. M., Jones E. W. New Map Location of ilvO in ESCHERICHIA COLI. Genetics. 1976 Jun;83(2):201–225. doi: 10.1093/genetics/83.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. S., Armstrong F. B. Branched-chain amino-acid aminotransferase of Salmonella typhimurium. I. Crystallization and preliminary characterization. Biochim Biophys Acta. 1971 Jan 13;227(1):56–66. doi: 10.1016/0005-2744(71)90167-7. [DOI] [PubMed] [Google Scholar]
- Collier R. H., Kohlhaw G. Nonidentity of the aspartate and the aromatic aminotransferase components of transaminase A in Escherichia coli. J Bacteriol. 1972 Oct;112(1):365–371. doi: 10.1128/jb.112.1.365-371.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan D. E., Wechsler J. A. An assay for transaminase B enzyme activity in Escherichia coli K-12. Anal Biochem. 1973 Jan;51(1):67–79. doi: 10.1016/0003-2697(73)90453-3. [DOI] [PubMed] [Google Scholar]
- Gelfand D. H., Rudo N. Mapping of the aspartate and aromatic amino acid aminotransferase genes tyrB and aspC. J Bacteriol. 1977 Apr;130(1):441–444. doi: 10.1128/jb.130.1.441-444.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand D. H., Steinberg R. A. Escherichia coli mutants deficient in the aspartate and aromatic amino acid aminotransferases. J Bacteriol. 1977 Apr;130(1):429–440. doi: 10.1128/jb.130.1.429-440.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermodson M., Schmer G., Kurachi K. Isolation, crystallization, and primary amino acid sequence of human platelet factor 4. J Biol Chem. 1977 Sep 25;252(18):6276–6279. [PubMed] [Google Scholar]
- Kline E. L., Brown C. S., Coleman W. G., Jr, Umbarger H. E. Regulation of isoleucine-valine biosynthesis in an ilvDAC deletion strain of Escherichia coli K-12. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1144–1151. doi: 10.1016/0006-291x(74)90816-x. [DOI] [PubMed] [Google Scholar]
- Kline E. L., Manross D. N., Jr, Warwick M. L. Multivalent regulation of isoleucine-valine transaminase in an Escherichia coli K-12 ilvA deletion strain. J Bacteriol. 1977 May;130(2):951–953. doi: 10.1128/jb.130.2.951-953.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb E. L., Horton H. R., Armstrong F. B. Molecular weight, subunit structure, and amino acid composition of the branched chain amino acid aminotransferase of Salmonella typhimurium. Biochemistry. 1974 May 7;13(10):2070–2077. doi: 10.1021/bi00707a011. [DOI] [PubMed] [Google Scholar]
- McCorkle G. M., Leathers T. D., Umbarger H. E. Physical organization of the ilvEDAC genes of Escherichia coli strain K-12. Proc Natl Acad Sci U S A. 1978 Jan;75(1):89–93. doi: 10.1073/pnas.75.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGilvray D., Umbarger H. E. Regulation of transaminase C synthesis in Escherichia coli: conditional leucine auxotrophy. J Bacteriol. 1974 Nov;120(2):715–723. doi: 10.1128/jb.120.2.715-723.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier N., Montmitonnet A., Chesne S., Pelmont J. Transaminase B d'Escherichia coli. I. - Purification et premières propriétés. Biochimie. 1976;58(6):663–675. doi: 10.1016/s0300-9084(76)80390-2. [DOI] [PubMed] [Google Scholar]
- Norton J. E., Sokatch J. R. Purification and partial characterization of the branched chain amino acid transaminase of Pseudomonas aeruginosa. Biochim Biophys Acta. 1970 May 13;206(2):261–269. doi: 10.1016/0005-2744(70)90109-9. [DOI] [PubMed] [Google Scholar]
- Powell J. T., Morrison J. F. Role of the Escherichia coli aromatic amino acid aminotransferase in leucine biosynthesis. J Bacteriol. 1978 Oct;136(1):1–4. doi: 10.1128/jb.136.1.1-4.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. T., Morrison J. F. The purification and properties of the aspartate aminotransferase and aromatic-amino-acid aminotransferase from Escherichia coli. Eur J Biochem. 1978 Jun 15;87(2):391–400. doi: 10.1111/j.1432-1033.1978.tb12388.x. [DOI] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. 3. MAP ORDER OF THE STRUCTURAL GENES AND OPERATOR GENES. J Bacteriol. 1965 Mar;89:661–664. doi: 10.1128/jb.89.3.661-664.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUDMAN D., MEISTER A. Transamination in Escherichia coli. J Biol Chem. 1953 Feb;200(2):591–604. [PubMed] [Google Scholar]
- Smith J. M., Smith F. J., Umbarger H. E. Mutations affecting the formation of acetohydroxy acid synthase II in Escherichia coli K-12. Mol Gen Genet. 1979 Feb 1;169(3):299–314. doi: 10.1007/BF00382276. [DOI] [PubMed] [Google Scholar]
- Soper T. S., Doellgast J., Kohlhaw G. B. Mechanism of feedback inhibition by leucine. Purification and properties of a feedback-resistant alpha-isopropylmalate synthase. Arch Biochem Biophys. 1976 Mar;173(1):362–374. doi: 10.1016/0003-9861(76)90271-x. [DOI] [PubMed] [Google Scholar]
- Tracy J. W., Kohlhaw G. B. Evidence for two distinct CoA binding sites on yeast alpha-isopropylmalate synthase. J Biol Chem. 1977 Jun 25;252(12):4085–4091. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. L., Appella E., Pisano J. J. Rapid analysis of amino acid phenylthiohydantoins by high-performance liquid chromatography. Anal Biochem. 1977 Feb;77(2):569–573. doi: 10.1016/0003-2697(77)90276-7. [DOI] [PubMed] [Google Scholar]