Abstract
Several environmental stresses have been demonstrated to increase polysaccharide intercellular adhesin (PIA) synthesis and biofilm formation by the human pathogens Staphylococcus aureus and Staphylococcus epidermidis. In this study we characterized an adaptive response of S. aureus SA113 to nitrite-induced stress and show that it involves concomitant impairment of PIA synthesis and biofilm formation. Transcriptional analysis provided evidence that nitrite, either as the endogenous product of respiratory nitrate reduction or after external addition, causes repression of the icaADBC gene cluster, mediated likely by IcaR. Comparative microarray analysis revealed a global change in gene expression during growth in the presence of 5 mM sodium nitrite and indicated a response to oxidative and nitrosative stress. Many nitrite-induced genes are involved in DNA repair, detoxification of reactive oxygen and nitrogen species, and iron homeostasis. Moreover, preformed biofilms could be eradicated by the addition of nitrite, likely the result of the formation of toxic acidified nitrite derivatives. Nitrite-mediated inhibition of S. aureus biofilm formation was abrogated by the addition of nitric oxide (NO) scavengers, suggesting that NO is directly or indirectly involved. Nitrite also repressed biofilm formation of S. epidermidis RP62A.
Staphylococcus aureus and Staphylococcus epidermidis are the pathogens of nosocomial sepsis most frequently isolated, and especially those patients with indwelling medical devices are at risk for chronic staphylococcal foreign body-associated infections (39, 51, 52, 69), which are mediated by the organisms' ability to form biofilms on metal or polymeric surfaces (22, 73). Biofilm-embedded bacteria are more resistant to antimicrobial agents than their planktonic counterparts and often cause chronic infections and sepsis, particularly in immunocompromised patients (14, 36, 42, 60, 65). Staphylococcal biofilm formation is a multifactorial process. Primary attachment can be mediated by various cell surface-associated factors such as the major autolysin (5, 25), the teichoic acids (23), or the polysaccharide intercellular adhesin (PIA) (24), the product of the icaADBC gene cluster (26). The accumulation of cells into a multilayered community requires the synthesis of PIA, which consists of polymeric N-acetylglucosamine (40) and is also referred to as PNAG. Furthermore, PIA-independent mechanisms of intercellular adhesion and biofilm formation have been reported and are of overall importance (17, 58). PIA expression and biofilm formation are induced by a variety of environmental stresses, like low oxygen (16), high osmolarity (3% NaCl) (53), the presence of ethanol (34), subinhibitory concentrations of tetracycline and the streptogramin quinupristin-dalfopristin (54), and during the course of a device-related infection (20).
Nitrate (NO3−) and nitrite (NO2−) can be used as terminal electron acceptors under anaerobic conditions. In Staphylococcus carnosus, the membrane-bound respiratory nitrate reductase, NarGHJI, generates nitrite, which under strict anaerobic conditions can be further reduced to ammonium by a cytoplasmic NADH-dependent nitrite reductase encoded by the nirBD genes (44, 45, 49). In contrast to nitrate reduction, NirBD-mediated nitrite dissimilation does not generate a proton motive force and is not a respiratory pathway. It serves rather to detoxify the nitrite that accumulates in nitrate-respiring cells and as an electron sink to regenerate NAD+.
Here we report an interplay between respiratory nitrate reduction and biofilm formation in S. aureus SA113 and S. epidermidis RP62A and show that the presence of nitrite, the product of nitrate respiration, causes a stress response, which concomitantly involves impairment of PIA-mediated biofilm formation. We also provide data suggesting that the acidified nitrite derivative nitric oxide (NO), widely used as a defense or signaling molecule in biological systems, is directly or indirectly involved in the inhibition of S. aureus biofilm formation.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Staphylococcus aureus strain SA113 (ATCC 35556) (30) and Staphylococcus epidermidis strain RP62A (ATCC 35984) (11) were used in this study. All strains were cultured at 37°C in tryptic soy broth (TSB; Sigma) supplemented with 0.5% glucose (TSBg). Conditions under which oxygen tension was decreased were created by growing the cells in closed Eppendorf tubes (2 ml) with moderate shaking (100 rpm). For growth under static biofilm conditions, bacteria were cultured in flat-bottom 96-well polystyrene microtiter plates (200 μl per well; Greiner) or in 6-well polystyrene cell culture plates (6 ml per well; Corning), when higher cell yields were required (e.g., for RNA and PIA extraction). Where applicable, sodium nitrate (NaNO3) or sodium nitrite (NaNO2) was added to final concentrations of 1 to 10 mM. Nitrite concentrations were determined colorimetrically by the Griess reaction as described previously (46, 62).
Construction of the S. aureus narG deletion mutant.
The upstream flanking region (1.1 kbp) of narG was amplified from S. aureus SA113 chromosomal DNA by PCR using DeepVent DNA polymerase (New England Biolabs). The forward primer (5′-TAATGGATCCCGTATGCAAAACACATTCAGC-3′) introduced a BamHI site, and the reverse primer (5′-ATAATGGTACCCCTACGTATAAAAATACGATGTG-3′) introduced a KpnI/Acc65I site (restriction sites are underlined). The region starting at 2.5 kbp downstream of the narG start codon (1.0 kbp) was amplified using the forward primer 5′-ATAATTCTAGAGATGTTGTGACAACTCCACTTAG-3′ and the reverse primer 5′-ATAATAAGCTTTCTGACCCAGGCGTTTGAATATG-3′, introducing the XbaI and HindIII sites, respectively. The erythromycin resistance gene ermB of Tn551 was removed from plasmid pEC3 by Acc65I/XbaI digestion and ligated together with the two PCR-generated fragments into BamHI/HindIII-digested temperature-sensitive shuttle vector pBT2, yielding pBT2ΔnarG. Cloning was performed in Escherichia coli XL1-Blue (stratagene). The plasmid pBT2ΔnarG was introduced into S. aureus SA113 by electroporation (2). Insertional inactivation of narG by homologous recombination was achieved as described previously (8). The insertional disruption of narG was confirmed by PCR and DNA sequence analysis.
Microtiter plate adherence assay.
For quantification of biofilms, bacteria were grown overnight in TSBg medium. Cultures were then diluted to an optical density at 578 nm of 0.07 (approximately 8.3 × 106 CFU ml−1) in fresh TSBg medium, and 200 μl of the cell suspension was used per well to inoculate sterile, flat-bottom 96-well polystyrene microtiter plates (Greiner). After incubation for 24 h at 37°C without shaking, the plate wells were washed twice with phosphate-buffered saline (pH 7.2) and dried in an inverted position, and adherent cells were stained with 0.1% safranin (Serva). The absorbance of stained biofilms was measured at 450 nm, using a microtiter plate reader (SpectraMax 340; Molecular Devices). CFU were determined by serial dilution of homogenized cell suspensions removed from the plate wells by using a modified drop plate method (27). Disintegration of biofilm cell clusters into single cells was achieved by mild sonication on ice using a Branson Sonifier 250 equipped with a microtip (power setting 1, 5 to 10 cycles of 10 bursts at 50% duty cycle with 30-s pauses). Statistical analyses were performed by using Student's unpaired t test. Where applicable, the NO donor S-nitroso-N-acetylpenicillamine (SNAP) (Cayman Chemical), freshly dissolved in dimethyl sulfoxide, and the NO scavengers 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (carboxy-PTIO potassium salt) (Sigma) and bovine hemoglobin (Sigma), dissolved in TSBg, were used at the indicated concentrations.
RNA isolation.
S. aureus SA113 was cultured under static biofilm conditions (6-well cell culture plate) in TSBg in the absence or presence of 5 mM sodium nitrite for 6 h at 37°C. Cells were collected from biofilms and treated with lysostaphin (Dr. Petry genmedics) for 15 min. Lysostaphin-treated cells were mechanically disrupted by vortexing three times for 30 s with glass-beads. RNA was isolated with an RNeasy mini-kit (QIAGEN), followed by treatment with DNase I (Ambion) at 37°C for 30 min, according to the manufacturers' instructions. RNA integrity was confirmed by agarose gel electrophoresis.
Semiquantitative and real-time cDNA-PCR.
The relative expression levels of icaA were determined by semiquantitative cDNA-PCR. As an internal standard, the relative expression levels of the gyrB gene were used. RNA was isolated as described above. DNase I-treated RNA (3 μg) was subjected to reverse transcription with 100 U of Moloney murine leukemia virus reverse transcriptase (Peqlab) in a 30-μl reaction volume for 1 h. Random hexamers (Invitrogen) were used to prime the reaction. For semiquantitative cDNA-PCR, an equal amount (5 μl) of each reaction mixture was used as a template for PCR amplification (25 cycles). Primer sets for gyrB (5′-GACCAGGTATGTATATAGGATC-3′ and 5′-GATGAACCAACACCATGTAAAC-3′) and icaA (5′-CCTGTATTTATGTCTATTTACTGG-3′ and 5′-CTTCTCGTATTTGAGTGCAAG-3′) were used. After agarose gel electrophoresis and ethidium bromide staining, band intensities were calculated by densitometric analysis (ImageMaster 2.0 software; Pharmacia Biotech). For real-time cDNA-PCR quantification of icaR expression, two biological replicates with three technical replicates each were assayed. One microliter of the cDNA reaction mixture and a LightCycler fast-start DNA master SYBR green I kit (Roche) were used in a LightCycler system (Roche), according to the manufacturer's instructions. Primer sets for gyrB (see above) and icaR (5′-TTGAAGGATAAGATTATTGATAACGC-3′ and 5′-TCTTCCACTGCTCCAAATTTTTGCGAAAAG-3′) were used. To monitor specificity, the PCR products were analyzed by melting curves and agarose gel electrophoresis. The values were normalized with respect to gyrB expression, and the data were expressed as the ratio of cycle threshold. The absence of genomic DNA was verified by PCR using DNase I-treated RNA samples as templates with gyrB primers (minus reverse transcription [RT] control). RNA templates for semiquantitative and real-time cDNA-PCR as well as for microarray analyses were from independent experiments.
cDNA microarray analysis.
For DNA microarray analysis, sciTRACER S. aureus N315 full genome microarrays (Scienion) containing PCR products corresponding to 2,334 genes derived from the genome sequence of S. aureus N315 were used. Cells were grown under static biofilm conditions (6-well cell culture plates) in TSBg medium in the absence or presence of 5 mM sodium nitrite for 6 h at 37°C. Bacteria from two samples of each growth condition were collected and pooled. RNA was isolated as described above. Total DNase I-treated RNA (1.5 μg) was used for microarray hybridization, and data analysis was done as described previously (55). The data represent the medians of four parallel microarray analyses, and the significant threshold was set at a level of greater than twofold change in expression, with a P value cutoff of 0.05 (one-sample t test with a Benjamini and Hochberg multiple testing correction).
Semiquantitative PIA detection.
S. aureus SA113 and its PIA-deficient isogenic ica deletion mutant (15), which was included as a negative control, were grown under static biofilm conditions (6-well cell culture plate) in TSBg medium in the absence or presence of 5 mM sodium nitrite for 24 h at 37°C. PIA cell surface extracts were prepared by resuspending the cells in 3 ml 0.5 M EDTA (pH 8.0) per gram wet weight, followed by 5 min incubation at 100°C. After centrifugation, 40 μl of the supernatant was incubated with 10 μl of proteinase K (20 mg/ml; Boehringer) for 2 h at 37°C. Ten microliters was then transferred to a nitrocellulose membrane, using a 96-well dot blot vacuum manifold (Gibco). The dried membrane was blocked with 3% bovine serum albumin and incubated with 3.2 μg/ml wheat germ agglutinin coupled to horseradish peroxidase (WGA-HRP conjugate; Lectinotest Laboratory) for 1 h. HRP activity was visualized via chromogenic detection. As a control, PIA was also quantified using rabbit antiserum raised against S. epidermidis PIA as described previously (16), with detection by HRP-linked antibody (Amersham).
Preparation of crude protein extracts and SDS-PAGE analysis.
Lysostaphin-treated S. aureus cells were mechanically disrupted by glass beads. After centrifugation, protein concentrations were determined by the method of Bradford, with bovine serum albumin as the standard (7). The extracts were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis according to the method of Laemmli (38), followed by staining with Coomassie brilliant blue G 250, and used for enzyme assays.
Enzyme assays.
Fructose-1,6-bisphosphate aldolase activity was measured using a coupled enzyme assay in standard buffer containing α-glycerophosphate dehydrogenase and triosephosphate isomerase as described by the manufacturer (Sigma). The decrease of NADH was monitored spectrophotometrically at 340 nm, and 1 μmol of NADH oxidized was set as equivalent to 0.5 μmol of fructose-1,6-bisphosphate cleaved. Catalase activity was assayed spectrophotometrically at 240 nm, as described previously (4), using 50 mM potassium phosphate buffer (pH 7.4) and 20 mM H2O2. Specific activities were calculated using the molar extinction coefficients of 6,220 M−1 cm−1 for NADH and 43.6 M−1 cm−1 for hydrogen peroxide.
Protein identification by MALDI-TOF MS.
The respective protein bands were excised from Coomassie blue-stained SDS-PAGE gels, and protein identification was performed by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) spectrometry. After tryptic digestions performed as described previously (61), MALDI-TOF analysis was performed on a Bruker Reflex III mass spectrometer (Bruker Daltonik) equipped with a N2 337-nm laser and gridless pulsed ion extraction. Sequence verifications of some fragments were performed by nanoelectrospray tandem mass spectrometry (MS) on a quadrupole time of flight I mass spectrometer (Micromass) equipped with a nanoflow electrospray ionization source. Gold-coated glass capillary nanoflow needles were obtained from Proxeon (Type Medium NanoES spray capillaries). Database searches (NCBI nonredundant [NCBInr] protein database) were done using MASCOT software (Matrix Science) (50).
Agar disc diffusion assay.
To test the pH dependency of NO2− toxicity, approximately 8.3 × 105 CFU was seeded onto TSB agar plates buffered with 100 mM sodium phosphate buffer at the indicated pH. If applicable, sodium nitrate was added to the agar plates at a final concentration of 5 mM. A filter disc containing 20 μl of an aqueous 2 M NaNO2 solution was placed on top of the agar. The plates were incubated for 48 h under oxygen limitation in an anaerobic jar (Merck Anaerocult A) at 37°C and monitored for inhibition zones around the discs.
RESULTS
The presence of nitrate and nitrite leads to reduced cell aggregation and loss of biofilm formation.
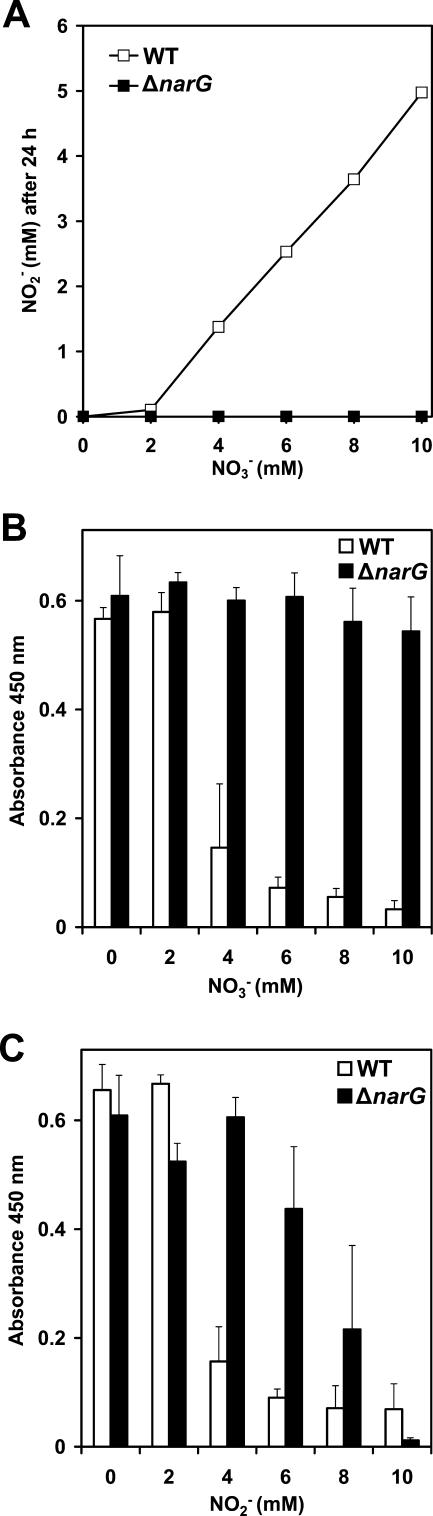
During cultivation studies under conditions with reduced oxygen tension, we observed a severe decrease in cellular aggregation in S. aureus SA113 and S. epidermidis RP62A in TSBg medium in the presence of 5 mM sodium nitrate (NaNO3) or sodium nitrite (NaNO2) (not shown). Thus, we investigated whether the presence of nitrate or nitrite had an influence on biofilm formation, using microtiter plate adherence assays. S. aureus SA113 was no longer able to form a biofilm on the polystyrene surface of microtiter plate wells if the initial concentration of nitrate or nitrite exceeded a concentration of 2 mM (Fig. 1, upper panel). The occurrence of nitrate and nitrite reduction during growth indicated anaerobic microenvironments, resulting in a switch from O2 to NO3− or NO2− as the electron acceptor. After 24 h, the residual nitrite concentrations in the culture supernatants increased accordingly to the initial concentrations of nitrate and nitrite. The cells reduced 1 mM nitrate to nitrite, which was completely reduced further, likely to ammonia, whereas an initial 2 mM concentration and higher concentrations of nitrate led to an accumulation of nitrite in the growth medium. Likewise, 2 mM nitrite was fully reduced, while higher concentrations resulted in remaining nitrite after 24 h of growth under static biofilm conditions. We obtained similar results with S. epidermidis RP62A (Fig. 1, lower panel).
FIG. 1.
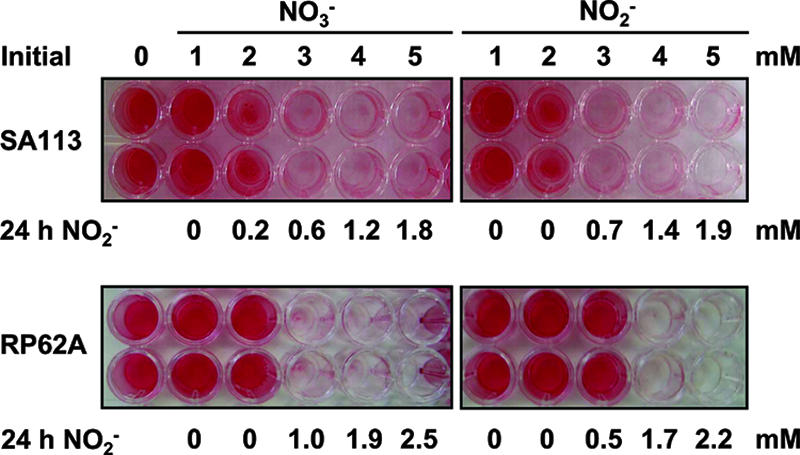
Biofilm formation of S. aureus and S. epidermidis is impaired by sodium nitrate and sodium nitrite. Biofilm formation of S. aureus SA113 (upper panel) and S. epidermidis RP62A (lower panel) was assayed using the microtiter plate adherence assay. The cells were grown in the absence or initial presence of 1 to 5 mM sodium nitrate (NO3−) or sodium nitrite (NO2−). After 24 h, the residual nitrite concentrations in the culture supernatants were determined by colorimetric detection (24 h NO2−). Adherent cells were stained with safranin.
Respiratory nitrate reduction and accumulation of nitrite are required to inhibit biofilm formation.
Since both the presence of nitrate and the presence of nitrite led to impaired biofilm formation, we investigated whether the presence of nitrate per se is sufficient or whether the reduction of nitrate to nitrite is required for the observed phenotype. Therefore, most of the gene encoding the catalytic subunit of respiratory nitrate reductase, narG, was replaced in S. aureus SA113 by an erythromycin resistance cassette (ermB). The deletion mutant (referred to as the ΔnarG strain) was no longer able to reduce nitrate to nitrite under conditions of reduced oxygen tension (not shown) or biofilm conditions (Fig. 2A), indicating that no alternative nitrate-reducing pathway is functional in S. aureus SA113. Moreover, the ΔnarG strain did not exhibit nitrate-responsive inhibition of biofilm formation even in the presence of 10 mM nitrate (Fig. 2B). Multicellular aggregation during growth under oxygen limitation in TSBg medium also remained unaffected by the presence of nitrate (not shown). The accumulation of nitrite in the growth medium as the result of respiratory nitrate reduction correlated with a decrease of biofilm formation in the wild type. Both the wild type and the ΔnarG mutant displayed inhibition of biofilm formation in response to nitrite (Fig. 2C). The rate of nitrite reduction in the nitrate reductase mutant was remarkably higher than in its parental wild type, possibly indicating a regulatory link between dissimilatory nitrate and nitrite reductions in S. aureus SA113. The mutant was able to completely reduce 6 mM nitrite within 24 h and to form a strong biofilm, whereas in the culture supernatant of the wild type, about 3 mM nitrite was remaining, linked with a biofilm-negative phenotype.
FIG. 2.
Respiratory nitrate reduction resulting in the accumulation of nitrite is required to inhibit biofilm formation. (A) S. aureus wild type (WT) and its isogenic narG deletion (ΔnarG) mutant were grown under biofilm conditions in microtiter plate wells in the absence or presence of 2 to 10 mM sodium nitrate (NO3−). After 24 h, the residual nitrite concentrations in the culture supernatants were determined (NO2− after 24 h). Biofilm formation of the S. aureus WT and the ΔnarG mutant in response to nitrate (NO3−) (B) or nitrite (NO2−) (C) was quantified by spectrophotometric measurement of the absorbance of adherent cells after safranin staining at 450 nm. Data represent the means ± standard errors of the means (n = 8) of one representative experiment.
Respiratory nitrate reduction and accumulation of nitrite lead to decreased transcription of icaA and impair PIA production.
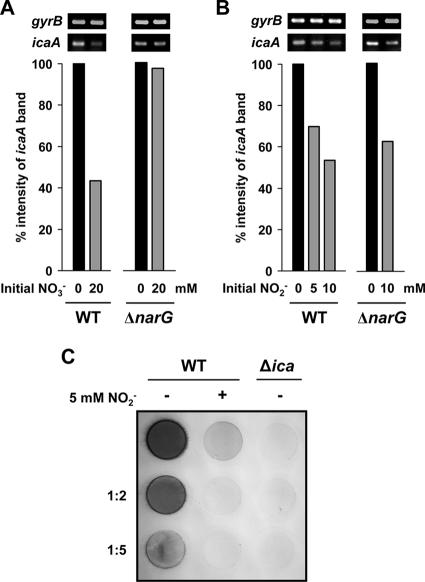
Bacterial accumulation during biofilm maturation requires the PIA, which is synthesized by enzymes encoded by the ica operon (icaABDC) (15, 26). Using semiquantitative RT-PCR, we determined whether the observed nitrate-induced and nitrite-induced impairment of biofilm formation can be attributed to a decrease of icaA transcription. As shown in Fig. 3A, transcription of the icaA gene in the wild-type isolate was reduced after growth under biofilm conditions for 6 h in the presence of 20 mM nitrate. The initially added nitrate had almost completely been reduced to nitrite, which had accumulated in the medium at a concentration of 18.8 mM. A reduction of icaA transcription by the presence of nitrate was observed only in the wild type and not in the nitrate reductase ΔnarG mutant, which had no detectable amounts of nitrite in its culture supernatant. Both the wild type and the mutant showed transcriptional down-regulation of icaA after growth in the presence of nitrite, which appeared to occur in a concentration-dependent manner (Fig. 3B). Moreover, quantification of surface-located PIA demonstrated a severe nitrite-induced decrease in PIA production in the wild-type S. aureus SA113 after growth for 24 h under biofilm conditions (Fig. 3C).
FIG. 3.
Effect of nitrate and nitrite on icaA transcription and PIA synthesis. S. aureus wild type (WT) and its isogenic narG deletion (ΔnarG) mutant were grown under biofilm conditions in 6-well cell culture plate wells in the absence or presence of 20 mM sodium nitrate (NO3−) (A) or 5 and 10 mM sodium nitrite (NO2−) (B). Cells were harvested after 6 h, and total RNA was isolated. The relative abundance levels of icaA mRNAs normalized to gyrB signals were assessed by semiquantitative RT-PCR and densitometric analysis. The levels of icaA expression in the presence of nitrate or nitrite were compared with normalized levels obtained from their matched controls set to 100% (bars). (C) PIA was extracted from cells grown for 24 h under biofilm conditions in 6-well cell culture plates in the absence (−) or presence (+) of 5 mM sodium nitrite (NO2−). PIA was detected by dot blot analysis using wheat germ agglutinin coupled to horseradish peroxidase. The PIA-deficient S. aureus SA113 ica (Δica) mutant was used as a negative control. PIA quantification using rabbit antiserum raised against S. epidermidis PIA yielded comparable results (not shown).
Nitrite-induced cell killing accounts only partially for the impairment of biofilm formation.
While the reduction of adherent cells was significant (P < 0.001; n = 3; not shown), CFU analyses revealed no significant differences in viable cell numbers when S. aureus SA113 biofilms were grown in the presence of 5 mM nitrite for 24 h (P = 0.59; n = 3; not shown). Viable cell counts were merely reduced by 15%. This suggests an adaptive response resulting in a planktonic state of growth rather than in adherence. The pH of the culture supernatant after 24 h remained higher in the presence of nitrite (pH 5.0 to 5.1) than in the absence of nitrite (pH 4.6 to 4.7). Remarkably, the addition of 5 mM nitrite to 24-h-old biofilms of S. aureus SA113 and S epidermidis RP62A, followed by incubation for another 24 h, led to an almost complete elimination of biofilms. However, the addition of 5 mM nitrite after 24 h was linked to an efficient cell killing (>99% for S. aureus, not shown; not tested with S. epidermidis).
Transcriptional microarray analysis of NO2−-exposed S. aureus SA113 cells.
Since CFU analyses suggested an adaptive response of S. aureus SA113 to the presence of NO2− with concomitant impairment of biofilm formation, we compared global levels of transcript abundance from cells grown for 6 h under static biofilm conditions in the absence or presence of 5 mM sodium nitrite by microarray analysis. A total of 638 genes were differentially expressed at least twofold, indicating a global change in gene expression triggered by nitrite. Two hundred ninety genes were up-regulated, and 348 genes were down-regulated (see Tables S1 and S2, respectively, in the supplemental material). Consistent with our previous data, the icaA gene was down-regulated by a factor of 2.2, but the P value (P = 0.09) was higher than the cutoff. Interestingly, icaR, coding for a repressor of the ica operon (12), was up-regulated by a factor of 2.2 (P = 0.02), suggesting a nitrite-responsive IcaR-mediated repression of the ica operon. Real-time PCR analysis confirmed a mean 4.9-fold induction of icaR transcription (not shown). A group of up-regulated genes encoded well-characterized components of the bacterial SOS response (19), like RecA (2.6-fold), UvrA (2.2-fold), UvrB (3.0-fold), and UvrC (3.0-fold), the latter three of which are involved in DNA repair. Also, genes involved in the oxidative stress response displayed enhanced expression upon exposure to nitrite, namely a superoxide dismutase (sodA, 3.1-fold), a flavin mononucleotide-dependent NADPH oxidase (nfrA, 7.7-fold) (66), and a catalase (katA, 5.1-fold), which is subject to iron-dependent coregulation by Fur (3.5-fold up-regulated) and PerR (29). In S. aureus, the PerR regulon is induced by iron (28). We found, apart from katA, several other PerR regulon members up-regulated in response to nitrite, e.g., the genes of the alkylhydroperoxide reductase AhpC (8.5-fold) (13) and AhpF (7.7-fold), the thioredoxin reductase TrxB (3.3-fold), the ferritin-family protein FtnA (12.1-fold) (43), and the ferritin-like Dps homologue MrgA (5.4-fold). Consistently, the group of down-regulated transcripts comprised genes of iron-regulated surface determinants IsdC, IsdE, IsdF, and IsdG (2.4- to 3.7-fold), which transport hemoproteins for use as an iron source (63, 68), and SirA (2.3-fold) as a member of the iron import system SirABC (18). The structural gene of the flavohemoprotein Hmp, responsible for nitric oxide detoxification (56), was induced by a factor of 6.5. The operons of molybdenum cofactor and molybdopterin biosynthesis, moaABCDE and moeAB, respectively, were induced 2.0- to 3.7-fold. The genes of clumping factors A and B, mediating the specific binding of S. aureus to fibrinogen (41, 47), exhibited down-regulation (clfA, 2.7-fold; clfB, 2.2-fold). The fmtA gene, which is involved in alterations of the cell wall structure (37), was repressed 3.5-fold. Also, genes of S. aureus central metabolism were affected by the presence of nitrite. The glycolysis operon regulator gene gapR (11.8-fold up-regulated) (64) and almost all genes involved in glycolysis were induced 2.6- to 3.5-fold (e.g., gap, pgk, tpi, pgm, eno, fdaB, and pgi), whereas fermentation-related genes (adh1, pflAB, and ldh) and the genes of the arginine deiminase pathway (arcABCD, 7.4- to 8.3-fold) were repressed for the most part. Only ddh, encoding a NAD+-dependent d-lactate dehydrogenase (6), was up-regulated threefold. The genes of the tricarboxylic acid cycle remained mainly unaffected, only the gene of fumarate hydratase (citG) displayed a 2.6-fold down-regulation. Quite a number of functionally uncharacterized genes encoding hypothetical proteins showed strong differential expression, indicating a potential role in adaptation to nitrite stress. Among the genes induced were SA2359 (54-fold), SA2360 (26-fold), SA0082 (29-fold), SA0083 (29-fold), and the putative oxidoreductase gene SA0084 (14-fold). The group of repressed genes consisted of SA0739 (10-fold), the glycosyl transferase gene homologue SA2350 (8-fold), the putative phosphosugar-binding transcriptional regulator SA0187, and the putative amino acid/cation transporter SA0180 (both 8-fold).
In summary, these data indicate that the response of S. aureus SA113 to nitrite comprises a stress response to reactive oxygen and nitrogen species as well as to increased levels of free iron. Recent studies characterized the response of S. aureus to hydrogen peroxide-driven oxidative stress (10) and to NO-induced nitrosative stress (56) and the SOS response, induced by mitomycin C challenge (1). Hence, our data for the S. aureus SA113 response to nitrite are consistent with the aforementioned classified stress responses. Strikingly, the icaADBC genes have recently been reported to be repressed upon exposure to 10 mM hydrogen peroxide (10), and a mutation of the fmtA gene was associated with impaired PIA production and biofilm formation (70).
Phenotypic validation of microarray data by mapping physiological states of the cells.
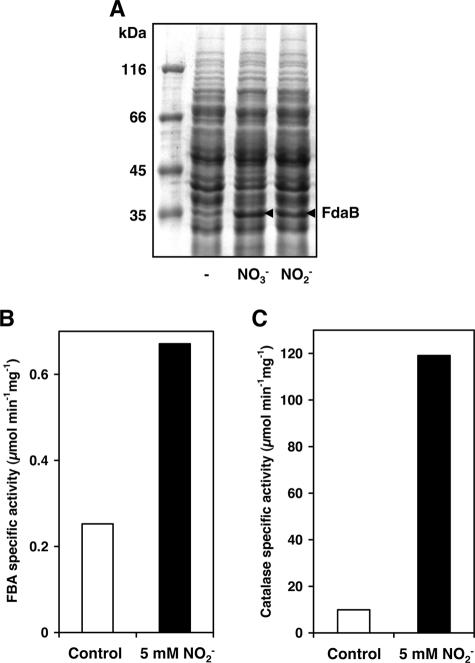
Cells were grown in 6-well cell culture plates under biofilm conditions for 6 h. Crude protein extracts analyzed by SDS-PAGE showed a nitrate- or nitrite-responsive induction of an approximately 35-kDa protein (Fig. 4A), which was identified by MS analysis as the class I fructose-bisphosphate aldolase FdaB (SA2399, 33.04 kDa). FdaB expression was not induced by nitrate in the nitrate reductase-deficient ΔnarG mutant (not shown). An enzymatic assay demonstrated an almost threefold induction of fructose-bisphosphate aldolase-specific activity in the presence of 5 mM nitrite (Fig. 4B). In accordance with the observed enzymatic activity, our microarray data showed a 3.5-fold induction of fdaB transcription upon nitrite exposure. Moreover, we tested cell extracts for catalase activity, since the microarray data indicated an up-regulation of katA transcription by a factor of 5.1. In fact, we could observe a 12-fold increase of catalase activity in response to the presence of nitrite (Fig. 4C).
FIG. 4.
Phenotypic validation of microarray data by mapping physiological states of the cells. (A) Cells were grown in 6-well cell culture plates under biofilm conditions for 6 h. SDS-PAGE analysis of crude protein extracts followed by Coomassie blue staining showed induction of an approximately 35-kDa protein in response to 20 mM nitrate (NO3−) or 10 mM nitrite (NO2−) (arrowheads), which was identified by MS analysis as the class I fructose-bisphosphate aldolase FdaB. Crude protein extracts from cells grown under biofilm conditions for 6 h in the absence (Control) or presence of 5 mM nitrite (NO2−) were assayed for fructose-bisphosphate aldolase (FBA)- and catalase-specific activities (panels B and C, respectively). Data represent the means of two independent experiments.
The acidified nitrite derivative NO is involved in the inhibition of biofilm formation.
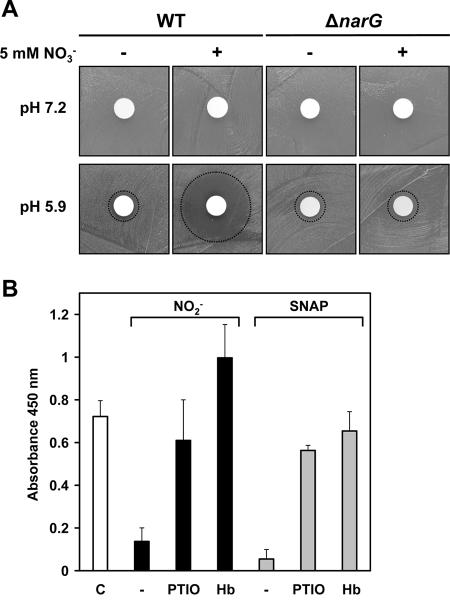
As shown in Fig. 5A, nitrite toxicity toward S. aureus required acidification. Using an agar disc diffusion assay, S. aureus growth was inhibited by high nitrite concentrations only at a lower pH (5.9), while no inhibition was observed at pH 7.2. When 5 mM sodium nitrate was included in the agar plates, the wild type formed a bigger inhibition zone around the nitrite-soaked filter disc than the nitrate reductase ΔnarG mutant, presumably because the concentration of acidified nitrite derivatives around the filter disc was higher due to additional nitrite endogenously generated by nitrate respiration.
FIG. 5.
Nitrite toxicity toward S. aureus requires acidification. (A) S. aureus wild type (WT) and its isogenic narG deletion (ΔnarG) mutant were seeded onto TSB agar plates buffered at the indicated pH values. Five millimolar sodium nitrate (NO3−) was included (+) or omitted (−) from the plates. After the placement of a filter disc soaked with 20 μl of a 2 M NaNO2 solution, the plates were incubated under conditions of oxygen limitation for 48 h and monitored for growth inhibition zones (indicated by dotted circles). (B) Nitrite- and SNAP-mediated inhibition of biofilm formation are abrogated in the presence of NO scavengers. Biofilm formation of S. aureus SA113 in the absence (C, control) or presence of 5 mM nitrite (NO2−) or the NO donor SNAP (0.1 mM) was quantified after 24 h by spectrophotometric measurement of the absorbance of adherent cells after safranin staining at 450 nm. The NO scavengers carboxy-PTIO (PTIO; 2.5 mM) and hemoglobin (Hb; 0.5 mM) were initially added as indicated or omitted (−). Data are means ± standard error of the means of two to four experiments performed at least in triplicate.
Acidification of the growth medium during biofilm maturation to a pH of approximately 5 after 24 h led us to hypothesize that in the presence of nitrite, the formation of nitrous acid (HNO2; pKa = 3.3) and derivatives thereof may account for the inhibition of biofilm formation. HNO2 is unstable and, upon disproportionation, slowly generates NO and NO3− (71). We therefore determined whether NO had an influence on S. aureus biofilm formation. As shown in Fig. 5B, addition of the NO donor SNAP to the growth medium (0.1 mM) led to impairment of biofilm formation, whereas viable cell counts were reduced only by approximately 20% (n = 3; not shown). Moreover, both SNAP- and nitrite-mediated inhibition of biofilm formation were abrogated in the presence of the NO scavenger carboxy-PTIO or hemoglobin, suggesting that nitrite-derived NO is directly or indirectly involved in the inhibition of biofilm formation. The addition of hemoglobin in fact had a stimulatory effect on biofilm formation, irrespectively of the presence of nitrite.
DISCUSSION
Several studies have demonstrated that PIA synthesis and biofilm formation by S. aureus and S. epidermidis are significantly increased by a number of environmental stresses. In this study, we characterized the response of S. aureus to nitrite-induced stress and show that it involves impairment of PIA synthesis and biofilm formation. We provide evidence that nitrite-derived NO plays a role in the inhibition of biofilm formation and that biofilm-embedded staphylococci can be efficiently killed by nitrite in an acidic environment. Moreover, biofilm formation of S. epidermidis RP62A was also abrogated in the presence of nitrite.
We initially observed severely reduced multicellular aggregation when S. aureus SA113 and S. epidermidis RP62A were grown planktonically under oxygen limitation conditions in TSBg medium in the presence of the alternative electron acceptors nitrate (NO3−) and nitrite (NO2−). We could demonstrate that this decrease in cell aggregation is associated with a reduced synthesis of PIA and an impairment of biofilm formation in a static biofilm model. Oxygen depletion was reported to induce PIA production (16). The occurrence of nitrate respiration and nitrite reduction in our biofilm model indicated oxygen limitation. Thus, nitrate respiration and nitrite dissimilation may contribute at least in part to a reversion of anaerobically induced PIA synthesis. Also, other anaerobic effects which emerge during biofilm growth may be weakened. Our microarray data demonstrated that fermentation-related genes as well as several genes that have been found to be up-regulated in the course of S. aureus biofilm maturation (e.g., the cap operon, clfA, clfB, and the staphyloxanthin synthesis gene cluster) (55) were down-regulated in the presence of nitrite. Glycolysis was remarkably increased, likely because nitrite reduction is coupled to a regeneration of NAD+. Commensurate excess pyruvate is presumably shuttled to the tricarboxylic acid cycle as opposed to the fermentative pathways, since the pH remained higher when nitrate or nitrite was present, indicating a reduced accumulation of acidic fermentation products.
We showed that nitrite, either as the endogenous product of nitrate reduction or after external addition, has a major impact on the observed impairment of biofilm formation. Our data provide evidence for a nitrite-driven induction of icaR transcription, resulting in a repression of the icaADBC gene cluster. The icaADBC genes also have been found to be repressed upon exposure to hydrogen peroxide, although IcaR was not found to play a role (10). While multifactorial regulation of icaR expression has been reported (34, 35, 48, 67), the mechanism underlying nitrite-mediated enhancement of icaR transcription remains to be elucidated. Moreover, nitrite-driven repression of fmtA, disruption of which is associated with impaired PIA production (70), may in addition account for impaired biofilm formation in our study by decreasing PIA levels. FmtA was described as a membrane protein, and the fmtA mutant showed a reduced cross-linking and partially reduced amidation of glutamate residues in the peptidoglycan (37).
In this study, we focused on polysaccharide-mediated biofilm formation. However, biofilm formation is multifactorial and can also be mediated by proteins. We found the gene of SasG (SA2285), a homologue of the S. epidermidis accumulation-associated protein Aap (57), was down-regulated 1.9-fold (not shown). Aap mediates intercellular adhesion, leading to biofilm accumulation in a completely PIA-negative background in S. epidermidis (58). Also, the gene coding for the S. aureus major autolysin (Atl) was down-regulated 1.7-fold. Atl is a homologue of S. epidermidis AtlE, which was reported to be involved in mediating the primary attachment to a polymer surface (25). Therefore, one could speculate that nitrite may also interfere with primary adhesion, the initial stage of biofilm formation, and the resulting biofilm-negative phenotype in S. aureus SA113 may in addition be attributed to factors other than PIA.
Nitrite seems to be a mixed blessing for the cells. On the one hand, it can have a growth-promoting role (44); on the other hand, high concentrations of nitrite and nitrite-derived compounds can be toxic. Under acidic conditions, nitrite can form nitrous acid (HNO2), which has strong bactericidal activity (9). We showed that the acidified nitrite derivative NO is directly or indirectly involved in the inhibition of biofilm formation. NO plays a key role in a variety of biological processes, e.g., as an important effector of host innate immunity, where it acts as an antimicrobial compound and signaling molecule. It is premature to conclude that NO is responsible for the inhibition of staphylococcal biofilm formation, since downstream reactive nitrogen species may also be involved. Although NO exposure can reduce staphylococcal viability (32), S. aureus has been described as relatively resistant to growth inhibition by NO and to exhibit efficient NO scavenging mechanisms, where the flavohemoprotein Hmp was shown to play a crucial role in counteracting NO toxicity (56). Nitrite can also react with nitrosable substrates (amines, amides, and amino acids) to produce N-nitroso compounds, and peroxidase oxidation of nitrite results in toxic products. We were able to demonstrate that the S. aureus response to sublethal concentrations of nitrite (5 mM) involves adaptation to oxidative and nitrosative stress, which apart from hmp, also resulted in enhanced expression of the catalase and superoxide dismutase genes katA and sodA, respectively. Superoxide, usually the by-product of cellular oxygen respiration, leads to the release of free iron from iron-sulfur proteins and thus increases the levels of intracellular free iron (33). Free iron can undergo Fenton chemistry and generate hydroxyl radicals which indiscriminately damage all cellular components (72). Hence, iron metabolism is linked to oxidative stress defenses. Our data indicate an attenuation of iron uptake upon nitrite exposure and an enhanced expression of iron scavengers like transferrin, which likely serves to prevent further iron-induced oxidative damage. Iron has been demonstrated to repress biofilm formation of S. aureus Newman, although in a PIA-independent manner (31), and changes in iron status have been reported to affect S. aureus central metabolism (21). Whether and how the presence of nitrite in fact leads to increased levels of free iron, which may via PerR and Fur regulation trigger planktonic growth, remains unclear.
While only modest differences in viable cell numbers could be observed after biofilm maturation for 24 h in the presence of 5 mM nitrite, the addition of 5 mM nitrite to 24-h-old biofilms resulted in efficient killing and detachment of adherent cells. This could be explained by the addition of nitrite to an acidified environment, resulting in an immediate generation of nitrous acid, NO, and toxic derivatives at concentrations which are lethal for nonadapted cells. In contrast, when nitrite is added from the beginning, adaptation to oxidative and nitrosative stress in the course of biofilm maturation can counteract toxicity and therefore confer significant protection against nitrite-induced damage.
Biofilm-associated bacteria were found to be more resistant to antimicrobial agents than their planktonic counterparts (36, 42, 60, 65). Thus, it is of great clinical interest to investigate the physiology of sessile communities and to find mechanisms involved in the inhibition or eradication of biofilms. Killing of mucoid Pseudomonas aeruginosa by acidified nitrite derivatives under cystic fibrosis airway conditions has recently been reported (71), and NO has been identified as an elicitor of active biofilm dispersal (3). S. aureus also frequently infects the lungs of cystic fibrosis patients (59), and therapeutic strategies based on nitrite inhalation to eradicate P. aeruginosa (71) may also be suitable for clearing S. aureus lung infections. It should also be considered whether persistent biofilm-associated infections by pathogenic staphylococcal species may be treated with nitrite or NO as an adjuvant agent.
Supplementary Material
Acknowledgments
We thank Christiane Wolz (Institute of Medical Microbiology and Hygiene, University of Tübingen) for access to a LightCycler system and Stefan Stevanovic (Institute for Cell Biology, University of Tübingen) for MS analyses. We are indebted to Rosmarie Gaupp and Melanie Kull for advice and discussion and to Ralph Bertram for critical reading of the manuscript. We thank Detlinde Futter-Bryniok and Vittoria Bisanzio for technical assistance.
This work was supported by the DFG (GO371/6-1 and TR-SFB 34).
Supplemental material for this article may be found at http://jb.asm.org/.
Footnotes
Published ahead of print on 24 August 2007.
REFERENCES
- 1.Anderson, K. L., C. Roberts, T. Disz, V. Vonstein, K. Hwang, R. Overbeek, P. D. Olson, S. J. Projan, and P. M. Dunman. 2006. Characterization of the Staphylococcus aureus heat shock, cold shock, stringent, and SOS responses and their effects on log-phase mRNA turnover. J. Bacteriol. 188:6739-6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Augustin, J., and F. Götz. 1990. Transformation of Staphylococcus epidermidis and other staphylococcal species with plasmid DNA by electroporation. FEMS Microbiol. Lett. 54:203-207. [DOI] [PubMed] [Google Scholar]
- 3.Barraud, N., D. J. Hassett, S. H. Hwang, S. A. Rice, S. Kjelleberg, and J. S. Webb. 2006. Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J. Bacteriol. 188:7344-7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beers, R. F., Jr., and I. W. Sizer. 1952. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195:133-140. [PubMed] [Google Scholar]
- 5.Biswas, R., L. Voggu, U. K. Simon, P. Hentschel, G. Thumm, and F. Götz. 2006. Activity of the major staphylococcal autolysin Atl. FEMS Microbiol. Lett. 259:260-268. [DOI] [PubMed] [Google Scholar]
- 6.Boyle-Vavra, S., B. L. de Jonge, C. C. Ebert, and R. S. Daum. 1997. Cloning of the Staphylococcus aureus ddh gene encoding NAD+-dependent d-lactate dehydrogenase and insertional inactivation in a glycopeptide-resistant isolate. J. Bacteriol. 179:6756-6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 8.Brückner, R. 1997. Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol. Lett. 151:1-8. [DOI] [PubMed] [Google Scholar]
- 9.Cammack, R., C. L. Joannou, X. Y. Cui, C. Torres Martinez, S. R. Maraj, and M. N. Hughes. 1999. Nitrite and nitrosyl compounds in food preservation. Biochim. Biophys. Acta 1411:475-488. [DOI] [PubMed] [Google Scholar]
- 10.Chang, W., D. A. Small, F. Toghrol, and W. E. Bentley. 2006. Global transcriptome analysis of Staphylococcus aureus response to hydrogen peroxide. J. Bacteriol. 188:1648-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen, G. D., A. L. Bisno, J. T. Parisi, B. McLaughlin, M. G. Hester, and R. W. Luther. 1982. Nosocomial septicemia due to multiply antibiotic-resistant Staphylococcus epidermidis. Ann. Intern. Med. 96:1-10. [DOI] [PubMed] [Google Scholar]
- 12.Conlon, K. M., H. Humphreys, and J. P. O'Gara. 2002. icaR encodes a transcriptional repressor involved in environmental regulation of ica operon expression and biofilm formation in Staphylococcus epidermidis. J. Bacteriol. 184:4400-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosgrove, K., G. Coutts, I. M. Jonsson, A. Tarkowski, J. F. Kokai-Kun, J. J. Mond, and S. J. Foster. 2007. Catalase (KatA) and alkyl hydroperoxide reductase (AhpC) have compensatory roles in peroxide stress resistance and are required for survival, persistence, and nasal colonization in Staphylococcus aureus. J. Bacteriol. 189:1025-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 15.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Götz. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cramton, S. E., M. Ulrich, F. Götz, and G. Döring. 2001. Anaerobic conditions induce expression of polysaccharide intercellular adhesin in Staphylococcus aureus and Staphylococcus epidermidis. Infect. Immun. 69:4079-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cucarella, C., C. Solano, J. Valle, B. Amorena, I. Lasa, and J. R. Penades. 2001. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 183:2888-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dale, S. E., M. T. Sebulsky, and D. E. Heinrichs. 2004. Involvement of SirABC in iron-siderophore import in Staphylococcus aureus. J. Bacteriol. 186:8356-8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisen, J. A., and P. C. Hanawalt. 1999. A phylogenomic study of DNA repair genes, proteins, and processes. Mutat. Res. 435:171-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flückiger, U., M. Ulrich, A. Steinhuber, G. Döring, D. Mack, R. Landmann, C. Goerke, and C. Wolz. 2005. Biofilm formation, icaADBC transcription, and polysaccharide intercellular adhesin synthesis by staphylococci in a device-related infection model. Infect. Immun. 73:1811-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman, D. B., D. L. Stauff, G. Pishchany, C. W. Whitwell, V. J. Torres, and E. P. Skaar. 2006. Staphylococcus aureus redirects central metabolism to increase iron availability. PLoS Pathog. 2:e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Götz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 23.Gross, M., S. E. Cramton, F. Götz, and A. Peschel. 2001. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect. Immun. 69:3423-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heilmann, C., C. Gerke, F. Perdreau-Remington, and F. Götz. 1996. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect. Immun. 64:277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heilmann, C., M. Hussain, G. Peters, and F. Götz. 1997. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 24:1013-1024. [DOI] [PubMed] [Google Scholar]
- 26.Heilmann, C., O. Schweitzer, C. Gerke, N. Vanittanakom, D. Mack, and F. Götz. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20:1083-1091. [DOI] [PubMed] [Google Scholar]
- 27.Herigstad, B., M. Hamilton, and J. Heersink. 2001. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 44:121-129. [DOI] [PubMed] [Google Scholar]
- 28.Horsburgh, M. J., M. O. Clements, H. Crossley, E. Ingham, and S. J. Foster. 2001. PerR controls oxidative stress resistance and iron storage proteins and is required for virulence in Staphylococcus aureus. Infect. Immun. 69:3744-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horsburgh, M. J., E. Ingham, and S. J. Foster. 2001. In Staphylococcus aureus, Fur is an interactive regulator with PerR, contributes to virulence, and is necessary for oxidative stress resistance through positive regulation of catalase and iron homeostasis. J. Bacteriol. 183:468-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iordanescu, S., and M. Surdeanu. 1976. Two restriction and modification systems in Staphylococcus aureus NCTC8325. J. Gen. Microbiol. 96:277-281. [DOI] [PubMed] [Google Scholar]
- 31.Johnson, M., A. Cockayne, P. H. Williams, and J. A. Morrissey. 2005. Iron-responsive regulation of biofilm formation in Staphylococcus aureus involves fur-dependent and fur-independent mechanisms. J. Bacteriol. 187:8211-8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan, S. S., J. R. Lancaster, Jr., R. E. Basford, and R. L. Simmons. 1996. Effect of nitric oxide on staphylococcal killing and interactive effect with superoxide. Infect. Immun. 64:69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keyer, K., and J. A. Imlay. 1996. Superoxide accelerates DNA damage by elevating free-iron levels. Proc. Natl. Acad. Sci. USA 93:13635-13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knobloch, J. K., K. Bartscht, A. Sabottke, H. Rohde, H. H. Feucht, and D. Mack. 2001. Biofilm formation by Staphylococcus epidermidis depends on functional RsbU, an activator of the sigB operon: differential activation mechanisms due to ethanol and salt stress. J. Bacteriol. 183:2624-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knobloch, J. K., S. Jäger, M. A. Horstkotte, H. Rohde, and D. Mack. 2004. RsbU-dependent regulation of Staphylococcus epidermidis biofilm formation is mediated via the alternative sigma factor σB by repression of the negative regulator gene icaR. Infect. Immun. 72:3838-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knobloch, J. K., H. Von Osten, M. A. Horstkotte, H. Rohde, and D. Mack. 2002. Minimal attachment killing (MAK): a versatile method for susceptibility testing of attached biofilm-positive and -negative Staphylococcus epidermidis. Med. Microbiol. Immunol. (Berlin) 191:107-114. [DOI] [PubMed] [Google Scholar]
- 37.Komatsuzawa, H., K. Ohta, H. Labischinski, M. Sugai, and H. Suginaka. 1999. Characterization of fmtA, a gene that modulates the expression of methicillin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 43:2121-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 39.Ludwicka, A., R. Locci, B. Jansen, G. Peters, and G. Pulverer. 1983. Microbial colonization of prosthetic devices. V. Attachment of coagulase-negative staphylococci and “slime”-production on chemically pure synthetic polymers. Zentralbl. Bakteriol. Mikrobiol. Hyg. B 177:527-532. [PubMed] [Google Scholar]
- 40.Mack, D., W. Fischer, A. Krokotsch, K. Leopold, R. Hartmann, H. Egge, and R. Laufs. 1996. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol. 178:175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDevitt, D., P. Francois, P. Vaudaux, and T. J. Foster. 1995. Identification of the ligand-binding domain of the surface-located fibrinogen receptor (clumping factor) of Staphylococcus aureus. Mol. Microbiol. 16:895-907. [DOI] [PubMed] [Google Scholar]
- 42.Melchior, M. B., J. Fink-Gremmels, and W. Gaastra. 2006. Comparative assessment of the antimicrobial susceptibility of Staphylococcus aureus isolates from bovine mastitis in biofilm versus planktonic culture. J. Vet. Med. B Infect. Dis. Vet. Public Health 53:326-332. [DOI] [PubMed] [Google Scholar]
- 43.Morrissey, J. A., A. Cockayne, K. Brummell, and P. Williams. 2004. The staphylococcal ferritins are differentially regulated in response to iron and manganese and via PerR and Fur. Infect. Immun. 72:972-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neubauer, H., and F. Götz. 1996. Physiology and interaction of nitrate and nitrite reduction in Staphylococcus carnosus. J. Bacteriol. 178:2005-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neubauer, H., I. Pantel, and F. Götz. 1999. Molecular characterization of the nitrite-reducing system of Staphylococcus carnosus. J. Bacteriol. 181:1481-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicholas, D. J. D., and A. Nason. 1957. Determination of nitrate and nitrite. Methods Enzymol. 3:981-984. [Google Scholar]
- 47.Ni Eidhin, D., S. Perkins, P. Francois, P. Vaudaux, M. Hook, and T. J. Foster. 1998. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol. Microbiol. 30:245-257. [DOI] [PubMed] [Google Scholar]
- 48.Pamp, S. J., D. Frees, S. Engelmann, M. Hecker, and H. Ingmer. 2006. Spx is a global effector impacting stress tolerance and biofilm formation in Staphylococcus aureus. J. Bacteriol. 188:4861-4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pantel, I., P. E. Lindgren, H. Neubauer, and F. Götz. 1998. Identification and characterization of the Staphylococcus carnosus nitrate reductase operon. Mol. Gen. Genet. 259:105-114. [DOI] [PubMed] [Google Scholar]
- 50.Perkins, D. N., D. J. Pappin, D. M. Creasy, and J. S. Cottrell. 1999. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20:3551-3567. [DOI] [PubMed] [Google Scholar]
- 51.Peters, G. 1984. Pathogenesis of staphylococcal infections of implanted plastics and intravascular catheters. Infection 12:235-239. [DOI] [PubMed] [Google Scholar]
- 52.Peters, G., R. Locci, and G. Pulverer. 1981. Microbial colonization of prosthetic devices. II. Scanning electron microscopy of naturally infected intravenous catheters. Zentralbl. Bakteriol. Mikrobiol. Hyg. B 173:293-299. [PubMed] [Google Scholar]
- 53.Rachid, S., K. Ohlsen, U. Wallner, J. Hacker, M. Hecker, and W. Ziebuhr. 2000. Alternative transcription factor σB is involved in regulation of biofilm expression in a Staphylococcus aureus mucosal isolate. J. Bacteriol. 182:6824-6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rachid, S., K. Ohlsen, W. Witte, J. Hacker, and W. Ziebuhr. 2000. Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesin expression in biofilm-forming Staphylococcus epidermidis. Antimicrob. Agents Chemother. 44:3357-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Resch, A., R. Rosenstein, C. Nerz, and F. Götz. 2005. Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Appl. Environ. Microbiol. 71:2663-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richardson, A. R., P. M. Dunman, and F. C. Fang. 2006. The nitrosative stress response of Staphylococcus aureus is required for resistance to innate immunity. Mol. Microbiol. 61:927-939. [DOI] [PubMed] [Google Scholar]
- 57.Roche, F. M., M. Meehan, and T. J. Foster. 2003. The Staphylococcus aureus surface protein SasG and its homologues promote bacterial adherence to human desquamated nasal epithelial cells. Microbiology 149:2759-2767. [DOI] [PubMed] [Google Scholar]
- 58.Rohde, H., C. Burdelski, K. Bartscht, M. Hussain, F. Buck, M. A. Horstkotte, J. K. Knobloch, C. Heilmann, M. Herrmann, and D. Mack. 2005. Induction of Staphylococcus epidermidis biofilm formation via proteolytic processing of the accumulation-associated protein by staphylococcal and host proteases. Mol. Microbiol. 55:1883-1895. [DOI] [PubMed] [Google Scholar]
- 59.Sadowska, B., A. Bonar, C. von Eiff, R. A. Proctor, M. Chmiela, W. Rudnicka, and B. Rozalska. 2002. Characteristics of Staphylococcus aureus, isolated from airways of cystic fibrosis patients, and their small colony variants. FEMS Immunol. Med. Microbiol. 32:191-197. [DOI] [PubMed] [Google Scholar]
- 60.Saginur, R., M. St Denis, W. Ferris, S. D. Aaron, F. Chan, C. Lee, and K. Ramotar. 2006. Multiple combination bactericidal testing of staphylococcal biofilms from implant-associated infections. Antimicrob. Agents Chemother. 50:55-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shevchenko, A., M. Wilm, O. Vorm, and M. Mann. 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68:850-858. [DOI] [PubMed] [Google Scholar]
- 62.Showe, M. K., and J. A. DeMoss. 1968. Localization and regulation of synthesis of nitrate reductase in Escherichia coli. J. Bacteriol. 95:1305-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skaar, E. P., and O. Schneewind. 2004. Iron-regulated surface determinants (Isd) of Staphylococcus aureus: stealing iron from heme. Microbes Infect. 6:390-397. [DOI] [PubMed] [Google Scholar]
- 64.Sprusansky, O., B. Rezuchova, D. Homerova, and J. Kormanec. 2001. Expression of the gap gene encoding glyceraldehyde-3-phosphate dehydrogenase of Streptomyces aureofaciens requires GapR, a member of the AraC/XylS family of transcriptional activators. Microbiology 147:1291-1301. [DOI] [PubMed] [Google Scholar]
- 65.Stewart, P. S. 2002. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 292:107-113. [DOI] [PubMed] [Google Scholar]
- 66.Streker, K., C. Freiberg, H. Labischinski, J. Hacker, and K. Ohlsen. 2005. Staphylococcus aureus NfrA (SA0367) is a flavin mononucleotide-dependent NADPH oxidase involved in oxidative stress response. J. Bacteriol. 187:2249-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tormo, M. A., M. Marti, J. Valle, A. C. Manna, A. L. Cheung, I. Lasa, and J. R. Penades. 2005. SarA is an essential positive regulator of Staphylococcus epidermidis biofilm development. J. Bacteriol. 187:2348-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Torres, V. J., G. Pishchany, M. Humayun, O. Schneewind, and E. P. Skaar. 2006. Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. J. Bacteriol. 188:8421-8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsukayama, D. T., R. Estrada, and R. B. Gustilo. 1996. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J. Bone Joint Surg. Am. 78:512-523. [DOI] [PubMed] [Google Scholar]
- 70.Tu Quoc, P. H., P. Genevaux, M. Pajunen, H. Savilahti, C. Georgopoulos, J. Schrenzel, and W. L. Kelley. 2007. Isolation and characterization of biofilm formation-defective mutants of Staphylococcus aureus. Infect. Immun. 75:1079-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoon, S. S., R. Coakley, G. W. Lau, S. V. Lymar, B. Gaston, A. C. Karabulut, R. F. Hennigan, S. H. Hwang, G. Buettner, M. J. Schurr, J. E. Mortensen, J. L. Burns, D. Speert, R. C. Boucher, and D. J. Hassett. 2006. Anaerobic killing of mucoid Pseudomonas aeruginosa by acidified nitrite derivatives under cystic fibrosis airway conditions. J. Clin. Investig. 116:436-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng, M., B. Doan, T. D. Schneider, and G. Storz. 1999. OxyR and SoxRS regulation of fur. J. Bacteriol. 181:4639-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ziebuhr, W., C. Heilmann, F. Götz, P. Meyer, K. Wilms, E. Straube, and J. Hacker. 1997. Detection of the intercellular adhesion gene cluster (ica) and phase variation in Staphylococcus epidermidis blood culture strains and mucosal isolates. Infect. Immun. 65:890-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.