Abstract
α/β-type small, acid-soluble spore proteins (SASP) are essential for the resistance of DNA in spores of Bacillus species to damage. An α/β-type SASP, Ssp2, from Clostridium perfringens was expressed at significant levels in B. subtilis spores lacking one or both major α/β-type SASP (α− and α− β− strains, respectively). Ssp2 restored some of the resistance of α− β− spores to UV and nitrous acid and of α− spores to dry heat. Ssp2 also restored much of the resistance of α− spores to nitrous acid and restored full resistance of α− spores to UV and moist heat. These results further indicate the interchangeability of α/β-type SASP in DNA protection in spores.
Spores of Bacillus species are much more resistant to a variety of environmental stress factors, including heat, radiation, desiccation, and toxic chemicals, than are their corresponding growing cells (6, 13). Many factors are responsible for spore resistance properties, including the spore coats, the relatively impermeable spore inner membrane, and the spore core's low water content and high levels of dipicolinic acid and associated divalent cations (13). However, the major factor involved in protection of spore DNA against damage is a group of novel proteins, termed the α/β-type small, acid-soluble spore proteins (SASP) (13, 14). These proteins saturate spore DNA and alter both its physical structure and its chemical and photochemical reactivity. The latter changes protect DNA from heat and many genotoxic chemicals and cause a change in spore DNA photochemistry that is the major reason for spore resistance to UV light.
The α/β-type SASP are encoded by a multigene family of three to seven members in Bacillus species, and the sequences of these proteins are well conserved both within and across species (14). While the many α/β-type SASP in any one species are present in spores at quite different levels, studies in Bacillus spores and in vitro have shown that all α/β-type SASP, whether normally expressed at high or low levels, have similar effects on DNA if present at high enough levels (4, 17).
Spores of Clostridium species also contain α/β-type SASP, and these proteins appear at approximately the same time in sporulation as they do in B. subtilis (11). While the sequences of the C. perfringens α/β-type SASP differ somewhat from those of Bacillus species, many features of the sequences of these proteins are conserved in both species (14). Indeed, several purified clostridial α/β-type SASP have effects on DNA's UV photochemistry in vitro similar to those of purified α/β-type SASP from Bacillus species (2, 7). In addition, recent work has indicated that α/β-type SASP are very important in the resistance of C. perfringens spores to UV radiation and moist heat, since C. perfringens spores with reduced levels of α/β-type SASP have reduced resistance to these two agents (10, 11). However, α/β-type SASP may not be important in the resistance of C. perfringens spores to dry heat (10), in contrast to results with B. subtilis spores (13, 14). Dry heat kills B. subtilis spores in large part by DNA damage, and B. subtilis α/β-type SASP protect DNA from damage both in vitro and in spores (7, 12, 13). Consequently, the lack of an effect of decreased α/β-type SASP levels on the dry-heat resistance of C. perfringens spores suggested that the C. perfringens proteins may have different effects on DNA properties than do the comparable proteins from spores of Bacillus species. To more closely assess the functions of C. perfringens α/β-type SASP, we expressed a C. perfringens α/β-type SASP at high levels in spores of B. subtilis strains PS356 and PS260 (termed α− β− and α−, respectively) (3). In the latter two strains, either both sspA and sspB, which encode ∼85% of B. subtilis α/β-type SASP (SASP-α and -β), or only sspA, encoding SASP-α, which comprises ∼55% of wild-type spores’ α/β-type SASP, had been inactivated (3). The resistance properties of the spores of these strains were then compared to those of wild-type, α− β−, and α− B. subtilis spores, as well as α− and α− β− B. subtilis spores expressing high levels of SspC (strain PS1450 [17]), a B. subtilis α/β-type SASP normally present at only low levels. Wild-type and α− β− spores used for comparative purposes were from strains PS533 (wild-type) and PS578 (α− β−), which each also carry plasmid pUB110, encoding resistance to kanamycin (Km) (10 μg/ml) (12).
The C. perfringens gene used for this work was ssp2 from C. perfringens strain 13. The amino acid sequence of the encoded protein is identical to that of Ssp2 from C. perfringens SM101, but the gene in strain 13 has a likely strong transcription terminator just downstream of the translation stop codon (5, 15). We chose the ssp2 gene for this work because it is likely expressed at the highest level of all ssp genes in C. perfringens (10, 11). The ssp2 gene was PCR amplified from C. perfringens strain 13 DNA using the upstream primer 5′-CCCCGGGGAATAACTAAGGAGGAATGAA-3′ and the downstream primer 5′-CCCCGGGGAATATAAAATGAGTTTAATGGG-3′, each containing extra 5′ residues, including a SmaI cleavage site (underlined). The resultant ∼435-bp PCR fragment was cloned in plasmid pCR-TOPO (Invitrogen, Carlsbad, CA) in Escherichia coli, giving plasmid pPS4046. The insert in this plasmid was sequenced to confirm that the PCR product had the DNA sequence of C. perfringens strain 13 DNA. The insert was removed from plasmid pPS4046 by digestion with SmaI (sites within original PCR primers) and cloned in the HpaI site of plasmid pPS4047 in E. coli DH5α. Plasmid pPS4047 was derived from plasmid pPS1393 (17) by digestion with HpaI, removal of a 130-bp fragment that contains a region of the B. subtilis sspB gene beginning just after its promoter and ending midway in its coding sequence, and ligation and transformation of the remaining plasmid backbone into E. coli DH5α. A plasmid termed pPS4048, carrying the C. perfringens ssp2 gene in the HpaI site of plasmid pPS4047, was identified by restriction enzyme digestion, and the orientation of the ssp2 coding sequence just downstream of the strong forespore-specific promoter of the B. subtilis sspB gene was confirmed by digestion with PvuII. Plasmid pPS4048 was cut with BamHI to remove the pUC19 sequence in this hybrid plasmid derived from pPS1393 (17), ligated, and transformed into B. subtilis strain PS356 (α− β−) (3) with selection for Kmr, giving strain PS4049 (α− β− Ssp2). Plasmids from strains PS1450 (α− β− SspC) and PS4049 were used to transform strain PS260 (α− and resistant to 3 μg/ml chloramphenicol) (3) to Kmr, giving strains PS4051 (α− Ssp2) and PS4052 (α− SspC), respectively, in which the ssp2 or sspC gene is present on a plasmid and just downstream of the sspB promoter.
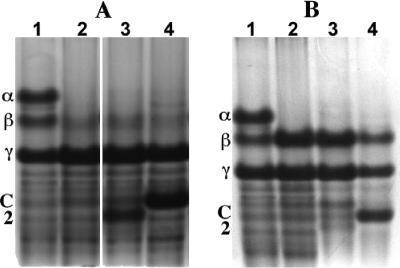
Spores of B. subtilis strains with and without various SASP sporulated with similar efficiencies at 37°C on 2× Schaeffer's glucose medium agar plates, as determined by phase-contrast microscopy (data not shown), and spores were harvested, purified, and stored as described elsewhere (8, 9). All spore preparations used in this work were free (≥98%) of growing or sporulating cells or germinated spores, as determined by phase-contrast microscopy. Spores (∼6 mg, dry weight) were dry ruptured, SASP were extracted with 1 ml and then 0.5 ml ice-cold 0.1 mM HCl, and both supernatant fluids were pooled, dialyzed in 3,500-molecular-weight-cutoff dialysis tubing against 1% acetic acid, and lyophilized as described elsewhere (8). The dry residue was dissolved in 30 μl 8 M urea, 15 μl acid gel diluent was added, aliquots from ∼1.3 mg (dry weight) of spores were subjected to polyacrylamide gel electrophoresis at low pH, and the gel was stained with Coomassie blue as described elsewhere (8). Spores of strain PS4049 (α− β− Ssp2) did not contain SASP-α and -β as expected but did contain an additional SASP migrating at the position expected for Ssp2 (Fig. 1A, compare lanes 1, 2, and 3; also data not shown). Extract from 0.7 mg of PS4049 spores was also subjected to polyacrylamide gel electrophoresis at low pH, the proteins on the gel were transferred to polyvinylidene difluoride paper (Immobilon-P; Millipore Corporation, Billerica, MA), the paper was lightly stained with Coomassie blue, and the putative Ssp2 band was cut out and subjected to automated protein sequence analysis. The first eight amino acids of the major protein in this band were Ser-Gln-His-Leu-Val-Pro-Glu-Ala (data not shown), as expected for Ssp2 with its amino-terminal methionine removed posttranslationally as is the rule in α/β-type SASP (14; also, see below). While PS4049 spores contained Ssp2, the level of this protein appeared to be slightly lower than that of either SASP-α plus SASP-β in wild-type spores or SspC in PS1450 (α− β− SspC) spores (Fig. 1A, compare lanes 1, 3, and 4), although the relative staining efficiencies of Ssp2, SASP-α, SASP-β, and SspC on polyacrylamide gels are not known.
FIG. 1.
Acid-gel electrophoresis of SASP extracts from spores of various strains. Spores were prepared, purified, and extracted; the extracts were processed; aliquots were subjected to acid-gel electrophoresis; and the gels were stained, as described in the text. The extracts run in the various lanes were from spores of the following strains: (A) lane 1, PS533 (wild-type); lane 2, PS578 (α− β−); lane 3, PS4049 (α− β− Ssp2); and lane 4, PS1450 (α− β− SspC); (B) lane 1, PS533 (wild type); lane 2, PS260 (α−); lane 3, PS4051 (α− Ssp2); and lane 4, PS4052 (α− SspC). α, β, C, and 2, migration positions of SASP-α, SASP-β, SspC, and Ssp2, respectively; γ, migration position of the single γ-type SASP in B. subtilis spores. This last protein is very different from α/β-type SASP and plays no role in spore resistance properties (6, 13). The lanes in panel A are from the same gel, but several intervening lanes have been removed for clarity, and a white space was left to indicate where lanes were removed; the lanes in panel B are also from the same gel and were run adjacent to each other.
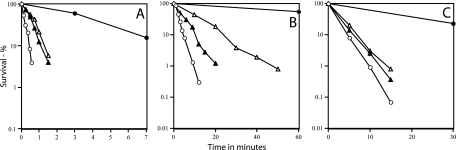
Analysis of the resistance of PS4049 (α− β− Ssp2) spores to various agents determined on plates containing the appropriate antibiotics as described previously (4, 10, 16) indicated that Ssp2 restored significant UV and nitrous acid resistance to α− β− spores, although not as much as did SspC (Fig. 2A and B). However, only a minimal amount of moist-heat resistance was restored to α− β− spores by expression of SspC or Ssp2 (Fig. 2C), and neither protein restored any dry-heat resistance to α− β− spores (data not shown). All of these resistance results were obtained in two separate experiments with two sets of spore preparations.
FIG. 2.
Resistance of wild-type spores and α− β− spores of various strains to UV radiation (A), nitrous acid (B), and moist heat (C). Spores of various strains were prepared and purified and their survival after various treatments was measured as described in the text. The variability in survival values in experiments was ±20%. Symbols: •, PS533 (wild type); ○, PS578 (α− β−); ▵, PS1450 (α− β− SspC); and ▴, PS4049 (α− β− Ssp2).
The relatively low levels of Ssp2 in B. subtilis α− β− spores might well be the reason for the minimal restoration of spore resistance properties by this protein. To further examine the ability of Ssp2 to provide resistance to B. subtilis spores, we expressed either this protein or SspC in spores of a B. subtilis strain lacking only SASP-α, the major B. subtilis α/β-type SASP (3). Previous work has shown that loss of SASP-α alone results in loss of much of the wild-type spore's resistance to UV and moist heat, although not as much as is lost in α− β− spores (4). More importantly, the UV and moist-heat resistance of α− spores is more readily restored to wild-type levels by normally minor α/β-type SASP than is the resistance to these agents of α− β− spores (4). As expected, spores of all α− strains lacked SASP-α but retained SASP-β and -γ (Fig. 1B, compare lanes 1 and 2). The level of SspC in the α− spores relative to that of SASP-γ was also similar to that in spores with an α− β− background (Fig. 1A and B, compare lanes 4). While Ssp2 was expressed in α− spores, the level of this protein relative to SASP-γ was significantly lower than in α− β− spores (Fig. 1A and B, compare lanes 3). The reason for the difference in levels of Ssp2 in α− and α− β− spores is not completely clear, but it may be due to repression of Ssp2 synthesis by SASP-β in α− spores. As seen previously (3, 4), levels of SASP-β increased significantly in α− spores compared to levels in wild-type spores (Fig. 1B, compare lanes 1 and 2). Possibly Ssp2 expression is more suppressed by increased levels of SASP-β than is expression of SspC.
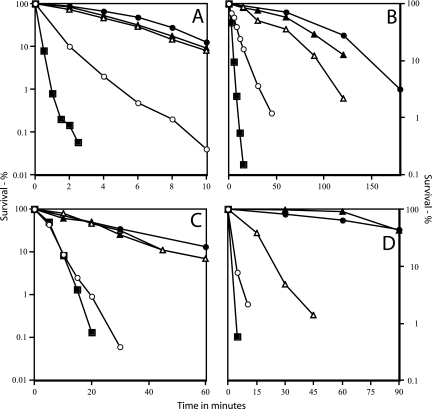
As expected, the UV, nitrous acid, and moist- and dry-heat resistance of α− spores was much lower than that of wild-type spores, although higher than that of α− β− spores (Fig. 3). More importantly, the resistance of the α− spores to UV and moist heat was almost completely restored by expression of Ssp2 or SspC, while nitrous acid resistance was largely restored (Fig. 3A to C). Dry-heat resistance of α− spores was also restored by expression of SspC, although Ssp2 had much less effect (Fig. 3D).
FIG. 3.
Resistance of wild-type spores and α− spores of various strains to UV radiation (A), nitrous acid (B), moist heat (C), and dry heat (D). Spores were prepared and purified and their survival after various treatments was measured as described in the text. The variability in survival values in these experiments was ±15%. Symbols: •, PS533 (wild type); ○, PS260 (α−); ▴, PS4052 (α− SspC); ▵, PS4051 (α− Ssp2); and ▪, PS578 (α− β−).
The major conclusion from this work is that α/β-type SASP from species widely separated in evolutionary time are largely interchangeable in their ability to provide DNA protection in spores. This has been shown previously in vivo for α/β-type SASP from Bacillus species (4, 13, 17), but B. subtilis and C. perfringens diverged ≥1.2 billion years ago (18). While C. perfringens Ssp2 was not as effective in restoring resistance to α− or α− β− B. subtilis spores as was B. subtilis SspC, this may be because of the apparently lower level of Ssp2 accumulated in these spores. However, these proteins may have different staining intensities on gels, so we do not know the precise amounts of these two proteins accumulated in α− and α− β− spores.
The full restoration of UV resistance to α− spores by Ssp2 is consistent with the similar effects of α/β-type SASP from Bacillus and Clostridium species on the UV photochemistry of DNA in vitro (2, 7) and with the markedly decreased UV resistance of C. perfringens spores with an ∼3-fold decrease in levels of total α/β-type SASP (10). C. perfringens spores with low levels of α/β-type SASP also exhibit significantly lower moist-heat resistance (10), consistent with the ability of Ssp2 to restore moist-heat resistance to α− spores. Previous work has shown that C. perfringens spores with lower levels of α/β-type SASP exhibit no decrease in dry heat resistance (10), and the resistance property that Ssp2 was least able to restore to α− B. subtilis spores was dry-heat resistance. However, we do not know if the latter result is because levels of Ssp2 in B. subtilis spores were too low to provide full dry-heat resistance and higher levels would have been more effective. Alternatively, perhaps there is a structural difference between the B. subtilis and C. perfringens α/β-type SASP such that the latter proteins are significantly less able to protect DNA from dry-heat damage than are the B. subtilis proteins.
The general interchangeability of diverse α/β-type SASP in almost all spore resistance properties is also consistent with the high amino acid sequence conservation between α/β-type SASP from widely divergent sporeformers, in particular between two very highly conserved regions in these proteins (Fig. 4; note that the amino-terminal sequence of Ssp2 determined for the protein expressed in B. subtilis spores is identical to that predicted from the gene's sequence) (14). Indeed, the major differences in the amino acid sequences of Ssp2 and an α/β-type SASP, whose structure when bound to DNA is being determined (1), are in the N- and C-terminal areas and in the number of residues between the two most highly conserved regions, with three more in Ssp2 (2, 14) (Fig. 4). Interestingly, while the length of this linking region is constant at 3 amino acids in known α/β-type SASP from Bacillus species, the length of this region varies from 2 to 15 residues in the α/β-type SASP from the anaerobic line of sporeformers (14). Presumably this variability, as well as individual amino acid sequence changes in Ssp2 and other α/β-type SASP, modifies the structure of the α/β-type SASP-DNA complex. Consequently, the specific properties of DNA in the complexes with different α/β-type SASP variants in vitro and in spores will be of great interest in future studies of these novel proteins.
FIG. 4.
Comparison of the amino acid sequence of Ssp2 (C) with that of a B. subtilis-derived α/β-type SASP, SspCΔ11-D13K-C3 (B), whose DNA-bound structure is being determined (1). The N-terminal methionine is not shown, since it is removed posttranslationally from α/β-type SASP (13). In line C, the shading highlights the eight amino acids determined by sequence analysis of this protein expressed in B. subtilis spores, and the boldface residues are conserved in ≥95% of all known B. subtilis α/β-type SASP (14). In line B, the shading highlights the two most highly conserved regions in these proteins from Bacillus species, and the boldface residues are identical in ≥95% of all known α/β-type SASP from Bacillus species (14). The asterisks indicate residues that are identical in both proteins.
Acknowledgments
This work was supported by grants from the National Institutes of Health (GM19698) and the Army Research Office to P. Setlow and from the U.S. Department of Agriculture (2002-35201-12643) to M. R. Sarker.
Footnotes
Published ahead of print on 31 August 2007.
REFERENCES
- 1.Bumbaca, D., J. Kosman, P. Setlow, R. K. Henderson, and M. J. Jedrzejas. 2007. Crystallization and preliminary X-ray analysis of the complex between a Bacillus subtilis α/β-type small, acid-soluble spore protein and DNA. Acta Crystallogr. F 63: 501-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fairhead, H., and P. Setlow. 1992. Binding of DNA to α/β-type small, acid-soluble proteins from spores of Bacillus or Clostridium species prevents formation of cytosine dimers, cytosine-thymine dimers, and bipyrimidine photoadducts upon ultraviolet irradiation. J. Bacteriol. 174: 2874-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mason, J. M., and P. Setlow. 1986. Evidence for an essential role for small, acid-soluble spore proteins in the resistance of Bacillus subtilis spores to ultraviolet light. J. Bacteriol. 167: 174-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mason, J. M., and P. Setlow. 1987. Different small, acid-soluble proteins of the α/β type have interchangeable roles in the heat and ultraviolet irradiation resistance of Bacillus subtilis spores. J. Bacteriol. 169: 3633-3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myers, G. S., D. A. Rasko, J. K. Cheung, J. Ravel, R. Seshadri, R. T. DeBoy, Q. Ren, J. Varga, M. M. Awad, L. M. Brinkac, S. C. Daugherty, D. H. Haft, R. J. Dodson, R. Madupu, W. C. Nelson, M. J. Rosovitz, S. A. Sullivan, H. Khouri, G. I. Dimitrov, K. L. Watkins, S. Mulligan, J. Benton, D. Radune, D. J. Fisher, H. S. Atkins, T. Hiscox, B. H. Jost, S. J. Billington, J. G. Songer, B. A. McClane, R. W. Titball, J. I. Rood, S. B. Melville, and I. T. Paulsen. 2006. Skewed genomic variability in strains of the toxigenic bacterial pathogen, Clostridium perfringens. Genome Res. 16: 1031-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholson, W. L., N. Munakata, G. Horneck, H. J. Melosh, and P. Setlow. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64: 548-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholson, W. L., B. Setlow, and P. Setlow. 1991. Ultraviolet irradiation of DNA complexed with α/β-type small, acid-soluble proteins from spores of Bacillus or Clostridium species makes spore photoproduct but not thymine dimers. Proc. Natl. Acad. Sci. USA 88: 8288-8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 281-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom.
- 9.Paidhungat, M., B. Setlow, A. Driks, and P. Setlow. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182: 5505-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raju, D., P. Setlow, and M. R. Sarker. 2007. Antisense RNA-mediated decreased synthesis of small, acid-soluble spore proteins leads to decreased resistance of Clostridium perfringens spores to moist heat and UV radiation. Appl. Environ. Microbiol. 73: 2048-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raju, D., M. Waters, P. Setlow, and M. R. Sarker. 2006. Investigating the role of small, acid-soluble spore proteins (SASPs) in the resistance of Clostridium perfringens spores to heat. BMC Microbiol. 6: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Setlow, B., and P. Setlow. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 178: 3486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Setlow, P. 2006. Spores of Bacillus subtilis: their resistance to radiation, heat and chemicals. J. Appl. Microbiol. 101: 514-525. [DOI] [PubMed] [Google Scholar]
- 14.Setlow, P. 2007. I will survive: DNA protection in bacterial spores. Trends Microbiol. 15: 172-180. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu, T., K. Ohtani, H. Hirakawa, K. Ohshima, A. Yamashita, T. Shiba, N. Ogasawara, M. Hattori, S. Kuhara, and H. Hayashi. 2002. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc. Natl. Acad. Sci. USA 99: 996-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tennen, R., B. Setlow, K. L. Davis, C. A. Loshon, and P. Setlow. 2000. Mechanisms of killing of spores of Bacillus subtilis by iodine, glutaraldehyde and nitrous acid. J. Appl. Microbiol. 89: 330-338. [DOI] [PubMed] [Google Scholar]
- 17.Tovar-Rojo, F., and P. Setlow. 1991. Analysis of the effects of mutant small, acid-soluble spore proteins from Bacillus subtilis on DNA in vivo and in vitro. J. Bacteriol. 173: 4827-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson, A. C., H. Ochman, and E. M. Prager. 1987. Molecular time scale for evolution. Trends Genet. 3: 241-247. [Google Scholar]