Abstract
Escherichia coli SlyD, which is involved in the biosynthesis of the metal cluster in the [NiFe]-hydrogenase enzymes, exhibits several activities including that of a peptidyl-prolyl isomerase (PPIase). Mutations that result in deficient PPIase activity do not produce corresponding decreases in the other activities of SlyD in vitro or in hydrogenase production levels in vivo.
Escherichia coli synthesizes at least three [NiFe]-hydrogenase enzymes that catalyze the production or consumption of hydrogen gas and occupy a central place in cellular energy metabolism (4, 17). Multiple accessory proteins are required for the assembly of the bimetallic catalytic cluster of these enzymes, including the proteins encoded by the hyp genes and slyD (3, 7, 12). SlyD, which contributes to the insertion of nickel into the hydrogenase precursor proteins and cellular nickel accumulation (18), consists of a peptidyl-prolyl isomerase (PPIase) domain as well as a molecular chaperone domain and a C-terminal tail rich in metal-binding residues (10, 14, 15). A combination of in vitro and in vivo experiments demonstrated that both the metal-binding domain and the chaperone activity of SlyD are essential components of its function in hydrogenase production (13), but the role of SlyD's PPIase activity in the hydrogenase maturation pathway was unknown.
The crystal structure of Methanococcus thermolithotrophicus FKBP, a SlyD homolog that also exhibits both PPIase and chaperone activities (8), reveals a hydrophobic cluster in the substrate-binding pocket of its PPIase domain (16). To investigate the role of the PPIase activity of SlyD, one or two amino acids in the corresponding hydrophobic cluster of SlyD were mutated and the two mutant proteins, SlyD(I42S) and SlyD(I42S, F132Y), were expressed in a BL21(DE3)ΔslyD strain and purified as reported for wild-type SlyD (18). To measure the PPIase activity, we employed a protease-free assay (11) that monitors the change in absorption at 330 nm due to the cis-trans isomerization of a tetrapeptide anilide (Suc-Ala-Phe-Pro-Phe-4-nitroanilide). The PPIase activities of SlyD(I42S) and SlyD(I42S F132Y) were 43% and l.6% that of wild-type SlyD, respectively (Fig. 1), demonstrating that these mutations disrupt PPIase activity.
FIG. 1.
PPIase activities of SlyD variants. Upon addition of 1 μM wild-type (WT) SlyD or a SlyD mutant [SlyD(I42S) or SlyD(I42S F132Y)] to 71.3 μM substrate (succinyl-Ala-Phe-Pro-Phe-4-nitroanilide), the decrease in absorbance at 330 nm was monitored for 80 s in 35 mM HEPES, pH 7.6, at 10°C (13). Kinetic traces were fit to a single exponential decay equation, and the value observed in the absence of protein was subtracted, followed by normalization to the enzyme concentration. Data are averages of the values from at least three independent measurements that were normalized to the value for wild-type SlyD (3 × 104 M−1 s−1). Error bars, ±1 standard deviation.
Several additional experiments were performed to determine if the SlyD mutations have any other consequences for the properties of the protein. SlyD forms a complex with the hydrogenase accessory protein HypB (18), and this interaction is critical for SlyD's function during hydrogenase metallocenter assembly (13). A qualitative chemical cross-linking assay (18) performed with each mutant incubated with HypB revealed robust cross-links of similar intensities that migrate at the molecular weight of the heterodimer on a denaturing polyacrylamide gel (data not shown). SlyD also exhibits pronounced chaperone properties and suppresses the aggregation of citrate synthase (CS) (13, 15). The reactivation of CS is significantly enhanced in the presence of a 20-fold excess of SlyD, increasing to 68% ± 2% (13) from the ≈30% activity observed in the absence of a chaperone (5), and the addition of the same amount of SlyD(I42S) or SlyD(I42S, F132Y) results in only slightly lower yields of 61% ± 1% or 63% ± 3%, respectively (data not shown). Similarly, the mutants are competent at suppressing the aggregation of chemically denatured CS (Fig. 2). These experiments demonstrate that the loss of SlyD's PPIase activity has very little impact on its complex formation with HypB or its chaperone activities. The latter observation is consistent with the finding that several PPIase-deficient mutants of M. thermolithotrophicus FKBP17 still exhibit chaperone activity (8).
FIG. 2.
SlyD, SlyD(I42S), and SlyD(I42S F132Y) suppress the aggregation of CS. Chemically denatured, reduced CS was diluted to 0.2 μM with buffer (25 mM HEPES [pH 7.5], 200 mM NaCl) in the presence or absence of SlyD, SlyD(I42S), or SlyD(I42S F132Y) at a SlyD/CS ratio of 20:1, and aggregation was observed by monitoring light scattering (13).
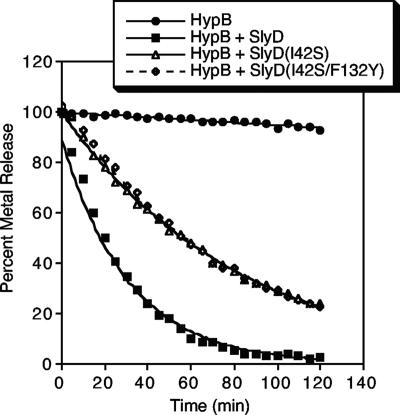
One possible function for SlyD in the hydrogenase maturation pathway is the activation of metal release from the high-affinity nickel-binding site of HypB (equilibrium dissociation constant [KD] = 1 × 10−13 M) (13). In an in vitro metal release assay that detects complex formation between nickel and the colorimetric indicator 4-(2-pyridylazo)resorcinol (PAR), nickel is slowly liberated from the high-affinity site of HypB (half-life [t1/2], ≈20 h), but a 10:1 ratio of SlyD stimulates release of the metal (t1/2, ≈20 min) (13). This activity is only slightly impaired in the PPIase mutants SlyD(I42S) and SlyD(I42S, F132Y) (t1/2, ≈55 min for both variants) (Fig. 3).
FIG. 3.
SlyD mutants stimulate nickel release from HypB to PAR. Purified HypB (5 μM) was incubated with 100 μM PAR with or without 50 μM SlyD, SlyD(I42S), or SlyD(I42S F132Y), and metal release was monitored by measuring the absorbance at 500 nm of the metal-PAR2 complex (13). The data were converted to the percentage of metal bound by determining total metal content and then fit to an exponential decay equation.
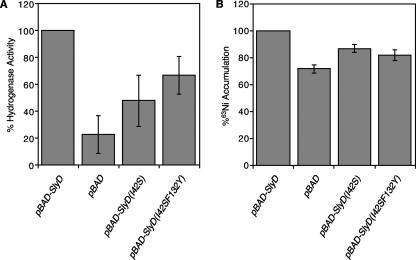
The observation that the main detectable effect of the SlyD mutations is disruption of PPIase activity allows for an assessment of the importance of this activity for the activation of hydrogenase. To this end, arabinose-inducible pBAD plasmids expressing SlyD, SlyD(I42S), or SlyD(I42S, F132Y) were prepared and transformed into ΔslyD cells (18). Protein production at a level similar to that of the wild type (within 5%) (data not shown) was confirmed by Western blot analysis. Solution assays were performed with cell extracts to monitor the reduction of benzyl viologen in the presence of hydrogen gas (2, 18), a measurement of overall hydrogenase activity that does not differentiate between the different isoenzymes. The ΔslyD cells transformed with the empty pBAD vector produced 23% of the activity of cells expressing pBAD-SlyD (18); the same level of activity was observed for the ΔslyD strain in the absence of any plasmid (13). In contrast, cell extracts containing the SlyD(I42S, F132Y) protein, which has less than 1% of the PPIase activity of wild-type SlyD in vitro, produced 67% ± 14% hydrogenase activity (Fig. 4A). This level of activity is significantly higher than that of the pBAD cells (at the 99.9% confidence level by Student's t test), whereas SlyD(I42S) produced 48% ± 19% activity, indicating that the loss of PPIase activity does not directly correlate with impairment in overall hydrogenase production. Similarly, examination of the individual activities of hydrogenases 1 and 2 by using an in-gel assay (18) revealed higher activities for both isoenzymes in extracts of cells expressing the SlyD mutants compared to the ΔslyD strain (data not shown), although in this case the differences between the two PPIase mutants were not significant.
FIG. 4.
In vivo activities of SlyD variants. (A) ΔslyD(DY330) cells transformed with pBAD, pBAD-SlyD, pBAD-SlyD(I42S), or pBAD-SlyD(I42S, F132Y) were grown anaerobically for 6 h in TGYEP medium supplemented with 1 μM sodium selenite, 1 μM sodium molybdate, 0.8% glycerol, and 15 mM sodium fumarate (18). Protein production from the pBAD plasmids was induced with 100 μM arabinose. Western blot analysis of the cell extracts probed with an anti-SlyD polyclonal antibody confirmed that the production of SlyD, SlyD(I42S), and SlyD(I42S, F132Y) from the pBAD vector reached levels comparable to that of SlyD production by the wild-type control (data not shown). Cell extracts were prepared and tested for hydrogenase activity by using benzyl viologen as a chromophoric electron acceptor in an anaerobic solution assay (2, 18). The rates of benzyl viologen reduction were first normalized for total protein concentration and then normalized to the value for the pBAD-SlyD-expressing cells of a given experiment (average value, 0.5 U/mg total protein). (B) Cellular nickel accumulation in SlyD variants. ΔslyD(DY330) cells transformed with pBAD, pBAD-SlyD, pBAD-SlyD(I42S), or pBAD-SlyD(I42S, F132Y) were grown anaerobically in the presence of 0.25 μM 63Ni (Perkin-Elmer, Toronto, Ontario, Canada) under the same growth conditions described for panel A. Cell extracts were prepared, and nickel accumulation was measured by scintillation counting (18). Values were normalized to the value for pBAD-SlyD-expressing cells. Data in both panels are averages from at least four separate experiments. Error bars, ±1 standard deviation.
The level of nickel uptake by E. coli cells grown under anaerobic conditions is also deficient for ΔslyD cells but can be fully restored upon transformation with pBAD-SlyD (18). The nickel uptake level measured in extracts from cells expressing SlyD(I42S) or SlyD(I42S, F132Y) was moderately deficient, at 87% ± 3% and 82% ± 4% that of SlyD, respectively (Fig. 4B), compared with 72% ± 3% in pBAD cells. Binding of nickel to SlyD and its variants was confirmed by using equilibrium dialysis followed by metal analysis (1), revealing that SlyD, SlyD(I42S), and SlyD(I42S, F132Y) can bind 3.8 ± 0.3, 4.5 ± 0.3, and 3.2 ± 0.2 equivalents of nickel in the presence of 1 mM Tris-(2-carboxyethyl)phosphine (TCEP), respectively.
The data presented in this report demonstrate that the loss of the PPIase activity of SlyD is not the main factor that produces the hydrogenase deficiency of ΔslyD cells and does not greatly affect the formation of a HypB complex or the release of metal from HypB. These observations correspond to those of other PPIases for which the PPIase activity itself is not a critical component of the physiological function of proteins (6, 9). The fact that the SlyD mutations result in a measurable decrease in hydrogenase activity suggests that either they produce subtle changes not detected in the in vitro experiments performed in this study or they affect some other function of the protein that has not yet been characterized. In contrast, it is clear that complex formation with HypB, which correlates with chaperone activity, and the metal-binding domain are important for the function of SlyD in hydrogenase biosynthesis (13). It is possible that this HypB-SlyD complex acts as a supply of nickel for the activation pathway of hydrogenase under anaerobic conditions (J. W. Zhang and D. B. Zamble, unpublished data). This hypothesis is consistent with the observation that the PPIase activity of SlyD is not critical for the activation of hydrogenase in E. coli, given that the PPIase activity of SlyD is inhibited by the binding of Ni(II) ions to the C-terminal metal-binding domain (10). Determining the exact role of SlyD in the maturation pathway of hydrogenase will require further investigation.
Acknowledgments
This work was supported in part by funding from the Canadian Institutes of Health Research and the Petroleum Research Fund (ACS). D.B.Z. is funded by a Canada Research Chair.
Footnotes
Published ahead of print on 24 August 2007.
REFERENCES
- 1.Atanassova, A., R. Lam, and D. B. Zamble. 2004. A high-performance liquid chromatography method for determining transition metal content of proteins. Anal. Biochem. 335:103-111. [DOI] [PubMed] [Google Scholar]
- 2.Ballantine, S. P., and D. H. Boxer. 1985. Nickel-containing hydrogenase isoenzymes from anaerobically grown Escherichia coli K-12. J. Bacteriol. 163:454-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Böck, A., P. W. King, M. Blokesch, and M. C. Posewitz. 2006. Maturation of hydrogenases. Adv. Microb. Physiol. 51(Suppl.):1-71. [DOI] [PubMed] [Google Scholar]
- 4.Böck, A., and G. Sawers. 1996. Fermentation, p. 262-282. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, DC.
- 5.Buchner, J., H. Grallert, and U. Jakob. 1998. Analysis of chaperone function using citrate synthase as nonnative substrate protein. Methods Enzymol. 290:323-338. [DOI] [PubMed] [Google Scholar]
- 6.Fischer, G., and T. Aumüller. 2003. Regulation of peptide bond cis/trans isomerization by enzyme catalysis and its implication in physiological processes. Rev. Physiol. Biochem. Pharmacol. 148:105-150. [DOI] [PubMed] [Google Scholar]
- 7.Forzi, L., and G. Sawers. 2007. Maturation of [NiFe]-hydrogenases in Escherichia coli. BioMetals 20:565-578. [DOI] [PubMed] [Google Scholar]
- 8.Furutani, M., A. Ideno, T. Iida, and T. Maruyama. 2000. FK506 binding protein from a thermophilic archaeon, Methanococcus thermolithotrophicus, has chaperone-like activity in vitro. Biochemistry 39:453-462. [DOI] [PubMed] [Google Scholar]
- 9.Göthel, S. F., and M. A. Marahiel. 1999. Peptidyl-prolyl cis-trans isomerases, a superfamily of ubiquitous folding catalysts. Cell. Mol. Life Sci. 55:423-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hottenrott, S., T. Schumann, A. Plückthun, G. Fischer, and J.-U. Rahfeld. 1997. Escherichia coli SlyD is a metal ion-regulated peptidyl-prolyl cis/trans-isomerase. J. Biol. Chem. 272:15697-15701. [DOI] [PubMed] [Google Scholar]
- 11.Janowski, B., S. Wöllner, M. Schutkowski, and G. Fischer. 1997. A protease-free assay for peptidyl prolyl cis/trans isomerases using standard peptide substrates. Anal. Biochem. 252:299-307. [DOI] [PubMed] [Google Scholar]
- 12.Leach, M. R., and D. B. Zamble. 2007. Metallocenter assembly of the hydrogenase enzymes. Curr. Opin. Chem. Biol. 11:159-165. [DOI] [PubMed] [Google Scholar]
- 13.Leach, M. R., J. W. Zhang, and D. B. Zamble. 2007. The role of complex formation between the Escherichia coli hydrogenase accessory factors HypB and SlyD. J. Biol. Chem. 282:16177-16186. [DOI] [PubMed] [Google Scholar]
- 14.Roof, W. D., and R. Young. 1995. φX174 lysis requires slyD, a host gene which is related to the FKBP family of peptidyl-prolyl cis-trans isomerases. FEMS Microbiol. Rev. 17:213-218. [DOI] [PubMed] [Google Scholar]
- 15.Scholz, C., B. Eckert, F. Hagn, P. Schaarschmidt, J. Balbach, and F. X. Schmid. 2006. SlyD proteins from different species exhibit high prolyl isomerase and chaperone activities. Biochemistry 45:20-33. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki, R., K. Nagata, F. Yumoto, M. Kawakami, N. Nemoto, M. Furutani, K. Adachi, T. Maruyama, and M. Tanokura. 2003. Three-dimensional solution structure of an archaeal FKBP with a dual function of peptidyl prolyl cis-trans isomerase and chaperone-like activities. J. Mol. Biol. 328:1149-1160. [DOI] [PubMed] [Google Scholar]
- 17.Vignais, P. M., B. Billoud, and J. Meyer. 2001. Classification and phylogeny of hydrogenases. FEMS Microbiol. Rev. 25:455-501. [DOI] [PubMed] [Google Scholar]
- 18.Zhang, J. W., G. Butland, J. F. Greenblatt, A. Emili, and D. B. Zamble. 2005. A role for SlyD in the Escherichia coli hydrogenase biosynthetic pathway. J. Biol. Chem. 280:4360-4366. [DOI] [PubMed] [Google Scholar]