Abstract
Since the first discovery of Torque teno virus (TTV) in 1997, many researchers focused on its epidemiology and transcriptional regulation, but the function of TTV-encoded proteins remained unknown. The function of the TTV open reading frame (ORF) in the nuclear factor κB (NF-κB) pathway has not yet been established. In this study, we found for the first time that the TTV ORF2 protein could suppress NF-κB activity in a dose-dependent manner in the canonical NF-κB pathway. By Western blot analysis, we proved that the TTV ORF2 protein did not alter the level of NF-κB expression but prevented the p50 and p65 subunits from entering the nucleus due to the inhibition of IκBα protein degradation. Further immunoprecipitation assays showed that the TTV ORF2 protein could physically interact with IKKβ as well as IKKα, but not IKKγ. Luciferase assays and Western blot experiments showed that the TTV ORF2 protein could also suppress NF-κB activity in the noncanonical NF-κB pathway and block the activation and translocation of p52. Finally, we found that the TTV ORF2 protein inhibited the transcription of NF-κB-mediated downstream genes (interleukin 6 [IL-6], IL-8, and COX-2) through down-regulation of NF-κB. Together, these data indicate that the TTV ORF2 protein suppresses the canonical and noncanonical NF-κB pathways, suggesting that the TTV ORF2 protein may be involved in regulating the innate and adaptive immunity of organisms, contributing to TTV pathogenesis, and even be related to some diseases.
Nuclear factor κB (NF-κB) plays a central role in the regulation of diverse biological processes, including immune responses, development, cell growth, and survival (1, 19, 25, 55). The mammalian NF-κB family contains five members: NF-κB1 (p105 and p50), NF-κB2 (p100 and p52), RelA (p65), RelB, and c-Rel. They share a highly conserved N-terminal Rel homology domain responsible for DNA binding, homo- or heterodimerization, and nuclear translocation (20). In mammals, there exist two major NF-κB pathways: the canonical NF-κB pathway and the noncanonical NF-κB pathway. The canonical NF-κB pathway is triggered by proinflammatory cytokines and dependent on the inhibitor of κB (IκB) kinase (IKK), which is composed of two catalysis subunits (IKKα and IKKβ) and a regulatory subunit, IKKγ or NEMO (NF-κB essential modulator) (15, 31, 48). Among these subunits, IKKβ is known as a major kinase and IKKγ is required for the full activation of IKKβ upon proinflammatory stimulation (27, 49, 50). Normally, NF-κB resides in the cytoplasm, forming complexes with IκB (5). When stimulated with proinflammatory stimuli, such as tumor necrosis factor alpha (TNF-α) or lipopolysaccharide (LPS), the IKK complex phosphorylates specific serines within the IκB proteins, triggering their ubiquitination by a ubiquitin ligase. The IκB protein is then degraded by the 26S proteasome, thus allowing the release, modification, and translocation of the NF-κB dimer (p65 and p50 subunits) into the nucleus to regulate gene transcription (25). In the noncanonical NF-κB pathway, the NF-κB dimer (RelB and p52 subunits) is activated by members of the TNF-α family, such as lymphotoxin-β, CD40L, and BAFF (B-cell-activating factor of the TNF family) (12, 13, 14, 34, 53). Unlike the canonical NF-κB pathway, this activation does not require IKKβ phosphorylation and degradation but depends on the activation of IKKα by NF-κB-inducing kinase (NIK). NIK recruits IKKα to p100, phosphorylates the N terminus of p100, and leads to the generation of p52. Then the p52/RelB complex translocates into the nucleus and regulates the transcription of target genes (8, 53). Through the two pathways described above, NF-κB controls the expression of genes, including those for the proinflammatory cytokines (e.g., interleukin 1 [IL-1], IL-2, IL-6, TNF-α, BAFF, and Blys, etc.), chemokines (e.g., IL-8, MIP-1α, MCP1, RANTES, eotaxin, B-lymphocyte chemoattractant, and secondary lymphoid tissue chemokine, etc.), adhesion molecules (e.g., intercellular adhesion molecule 1, vascular cell adhesion molecule 1, and E-selectin), inducible enzymes (e.g., COX-2 and inducible nitric oxide synthase), growth factors, some of the acute-phase proteins, and immune receptors, all of which play critical roles in controlling most inflammatory processes (2, 36).
In 1997, the Japanese researchers Nishizawa et al. identified Torque teno virus (TTV) by representational-difference display analysis with the serum of a Japanese patient with acute posttransfusion hepatitis of unknown etiology (37). The virus was first named after the initials of the patient and then was renamed Torque teno virus or transfusion-transmitted virus. TTV is a small, unenveloped, single-stranded, and circular DNA virus with a genome of approximately 3,800 nucleotides and was originally classified as belonging to the Circoviridae family (33, 35, 58). Until now, at least 39 genotypes of TTV have been identified, and they can be classified into five distantly related groups (44). Molecular epidemiological studies reveal a wide spectrum of TTVs displaying over 30% nucleotide diversity (39). Like chicken anemia virus (CAV), which is the other member of the Circoviridae family, TTV has two main open reading frames (ORFs) (ORF1 and ORF2), which can be deduced directly from the nucleotide sequence. By analogy with VP1 of CAV, ORF1 might encode a structural protein corresponding to the capsid of the virus. Moreover, the presence of conserved motifs related to the Rep protein suggests that replication of TTV might follow a rolling circle mechanism (38, 59). ORF2 might encode nonstructural protein that possesses the activity of a novel dual-specificity protein phosphatase, possibly involved in viral replication or another function of regulation. A conserved motif, WX7HX3CX1CX5H, present in VP2 of CAV is also found in the TTV group (7, 40, 45). Later analysis of the TTV transcription pattern in COS-1 and bone marrow cells has revealed the existence of at least three species of spliced mRNA molecules of 2.9 to 3, 1.2, and 1.0 kb in length, with common 5′ and 3′ termini, suggesting that more ORFs emerged through mRNA splicing, even though their functions are not clear (24, 42, 46).
Since the first isolation of TTV, most studies have shown that it might be relevant to liver disorders and liver damage and have a possible association with fulminant hepatitis, cryptogenic liver disease, non-A-G hepatitis, posttransfusion hepatitis, liver cirrhosis, and hepatocellular carcinoma (11, 22, 37, 41, 57, 65). At the same time, epidemiological associations of TTV with B-cell lymphoma, Hodgkin's disease, aplastic anemia, idiopathic pulmonary fibrosis, acute respiratory disease, and autoimmune rheumatic disorders have also been described (6, 16, 29, 30, 32, 52). Although TTV is potentially related to many diseases, its actual function is still questioned. Due to its global presence in healthy populations and the lack of morphological or molecular abnormalities of TTV-infected cells, there exist many conflicting opinions about its association with several diseases, and whether TTV is the major or minor cause of any human diseases is still under investigation (17, 23).
Until recently, most researchers have focused on the epidemiology of TTV, and its potential function was rarely mentioned. In this study, we found that the TTV ORF2 protein (SANBAN isolate, which belongs to a novel group 3 TTV genotype) suppressed NF-κB activity and subsequent products. It is probable that TTV is involved in regulating the innate and adaptive immunity of an organism by altering the expression of key inflammatory molecules.
MATERIALS AND METHODS
Plasmid construction and polyantibody production.
The cDNA of TTV ORF2 was amplified by PCR from pCR-SBFL (provided by Tetsuro Suzuki, National Institute of Infectious Diseases, Japan) by using the forward primer 5′-A GTC GGA TCC ATG GGC AAG GCT CTT AGG GTC-3′ and reverse primer 5′-G GCT GAA TTC TTA CTG TGC GTC GTC TTC GAT-3′ or the forward primer 5′-A TAC GCT AGC ATG GGC AAG GCT CTT AGG GTC-3′ and reverse primer 5′-G GCT GAA TTC TTA CTG TGC GTC GTC TTC GAT-3′) and then cloned into the prokaryotic expression vector pET-His (Invitrogen) or eukaryotic expression vector pcDNA3.1(−) (Invitrogen) by using standard cloning methods, which resulted in pET-His-TTV2 and pcDNA3.1(−)-TTV2, respectively. The recombinant plasmids were analyzed by restriction digestion and PCR, and their sequences were confirmed by sequencing. The plasmid pET-His-TTV2 was transformed into the Escherichia coli strain BL21(DE3) to produce the TTV ORF2 protein. Anti-TTV ORF2 polyclonal serum was obtained by immunizing rabbits with purified ORF2 protein (64). The plasmid expression vector for pcDNA3.1(+)-NIK and dominant negative NIK [pcDNA3.1(+)-dnNIK], which has the kinase-dead mutation K429A/K430A, were kindly provided by Brian M. J. Foxwell and Alison Davis (Kennedy Institute of Rheumatology Division, Imperial College School of Medicine, London, United Kingdom). The plasmids pGL3 COX-2-p-Luc and its mutant pGL3 COX-2-p-Luc (ΔNF-κB), pGL3 IL-6-p-Luc and its mutant pGL3 IL-6-p-Luc (ΔNF-κB), and pGL3 IL-8-p-Luc and its mutant pGL3 IL-8-p-Luc (ΔNF-κB) were gifted by Ying Zhu (State Key Laboratory of Virology, College of Life Science, Wuhan University). The plasmids pcDNA3.1(−)-Flag-IKKα and pCMV-Flag-IKKβ were gifted by Rohit Mittal (Neurobiology Division, MRC Laboratory of Molecular Biology, Cambridge, United Kingdom).
Cell lines and cell culture.
HepG2, HeLa, HEK293, and RAW264.7 cells were obtained from the China Center for Type Culture Collection (Wuhan, China). HepG2, HeLa, and HEK293 cells were cultured in Dulbecco's modified Eagle's medium (Gibco), while RAW264.7 cells were cultured in RPMI 1640 (Gibco). All these cell lines were maintained at 37°C with 5% CO2 and supplemented with 10% heat-inactivated fetal bovine serum (HyClone), 100 U of penicillin per ml, and 100 μg of streptomycin per ml.
Plasmid transfection; TNF-α, LPS, and/or MG-132 treatment; and luciferase assay.
DNA transfection was performed using Lipofectamine 2000 (Gibco) according to the manufacturer's instructions. Each transfection was normalized with appropriate vector plasmids. At 48 h posttransfection, the cells were harvested for the subsequent experiments. For the TNF-α or LPS treatment, the posttransfected cells were maintained without serum overnight and then treated with TNF-α (10 ng/ml; Sigma), LPS (1 μg/ml; Sigma), and/or MG-132 (20 μmol/liter; Alexis). For the luciferase assay, the cells were cotransfected with the plasmids indicated in the figures, luciferase reporter vectors, and the Renilla luciferase reporter tk-Renilla-Luc (Invitrogen), which was an internal control for transfection efficiency. Luciferase activity was measured by the dual-luciferase assay system (Promega) according to the protocol recommended by the manufacturer. The luciferase assay was done in triplicate, and the results are shown as means ± standard errors (SE).
Preparation of cytoplasmic and nuclear fractions.
At 48 h posttransfection, the treated or untreated cells were washed three times with ice-cold phosphate-buffered saline and added to hypotonic buffer (10 mM HEPES [pH 7.9], 5 mM KCl, 1.5 mM MgCl2, 1 mM NaF, and 1 mM Na3VO4) supplemented with 1 mM dithiothreitol, phenylmethylsulfonyl fluoride (1 mM), aprotinin (2 μg/ml), leupeptin (2 μg/ml), and soybean trypsin inhibitor (37.5 μg/ml). After lysis for 15 min on ice, the cytoplasmic fraction was prepared by centrifugation at 3,000 rpm for 5 min, and it was cleared by centrifugation at 12,000 rpm for 15 min before further analysis. To prepare the nuclear fraction, the cell pellet generated after initial centrifugation was washed three times with the hypotonic buffer and suspended in high-salt buffer (20 mM HEPES [pH 7.9], 1.5 mM MgCl2, 0.2 mM EDTA [pH 8.0], 420 mM NaCl, 25% [vol/vol] glycerol, 50 mM β-glycerophosphate, 1 mM NaF, and 1 mM Na3VO4) supplemented with 1 mM dithiothreitol, phenylmethylsulfonyl fluoride (1 mM), aprotinin (2 μg/ml), leupeptin (2 μg/ml), and soybean trypsin inhibitor (37.5 μg/ml). The suspended cell pellet was incubated for 30 min on ice with occasional vortexing, and the nuclear fraction was collected after centrifugation at 12,000 rpm for 10 min.
Western blot assay and immunoprecipitation.
At 48 h posttransfection, total cell lysate was washed with cold phosphate-buffered saline and dissolved in lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EDTA, 0.1 mM phenylmethylsulfonyl fluoride, 50 mM NaF, 1 mM sodium orthovanadate, and 0.06 mg/ml aprotinin) on ice for 30 min. After centrifugation at 12,000 rpm for 15 min, the supernatants were separated and the protein concentration was determined. Proteins were then separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred to nitrocellulose membranes with a mini Trans-blot cell (Bio-Rad). The specific immunoreactive proteins were detected by enhanced chemiluminescence (Pierce) and exposed to X-ray film (Eastman Kodak). For the immunoprecipitation assay, total cell lysate was prepared with immunoprecipitation assay cell lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 2 mM EDTA, 1% sodium orthovanadate, 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS) supplemented with phenylmethylsulfonyl fluoride (1 mM), aprotinin (2 μg/ml), leupeptin (2 μg/ml), and soybean trypsin inhibitor (37.5 μg/ml). Then, 1 to 2 μg of appropriate antibodies were added to precleared cell lysate and incubated overnight at 4°C. Immune complexes were captured with 30 μl of protein A-Sepharose (Santa Cruz Biotechnology) for 30 min at 4°C and washed five times with immunoprecipitation assay buffer. Antibodies used in our study were as follows: anti-COX-2, anti-β-actin, anti-GAPDH (anti-glyceraldehyde-3-phosphate dehydrogenase; Santa Cruz Biotechnology), and anti-TTV ORF2 (prepared in this study) polyclonal antibodies and anti-p65, anti-p50, anti-IκBα, anti-p100/p52, anti-Rel-B (Santa Cruz Biotechnology), anti-Flag (Sigma), anti-IKKα, anti-IKKβ, anti-IKKγ, and anti-phosphoserine IκBα (Cell Signaling Technology) monoclonal antibodies.
Reverse transcription (RT)-PCR.
At 48 h posttransfection, total RNA was extracted by using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. The cDNA was reverse transcribed from 1.0 μg total RNA with random hexamer primers using an avian myeloblastosis virus-reverse transcriptase kit (TaKaRa) as recommended by the supplier. Subsequently, the cDNA preparations were used as the templates for PCR to amplify the GAPDH, COX-2, IL-6, or IL-8 gene by using the forward primer 5′-ATC ACT GCC ACC CAG AAG AC-3′ and reverse primer 5′-AGG ATG AGC TGC CCT ATG ATG-3′ for GAPDH, the forward primer 5′-AGA GGC TAG TGC CTC AGA GAG AA-3′ and reverse primer 5′-TCG CAT ACA CAA CCC AAA TTC CC-3′ for COX-2, the forward primer 5′-CAA AAG TCC TGA TCC AGT TCC-3′ and reverse primer 5′-ACA TAA TTT CTG TGC CCA GTG-3′ for IL-6, and the forward primer 5′-ATG ACT TCC AAG CTG GCC GTG GCT-3′ and reverse primer 5′-TCT CAG CCC TCT TCA AAA ACT TCT-3′ for IL-8. The PCR conditions were as follows: denaturation at 94°C, annealing at 56°C, and extension at 72°C, for 25 cycles using rTaq (TaKaRa).
Measurement of IL-6 and IL-8 in the supernatant of cultured cells.
At 48 h posttransfection, RAW264.7 cells were stimulated with LPS. IL-6 and IL-8 in the culture supernatant of RAW264.7 cells were measured by using enzyme-linked immunosorbent assay (ELISA) kits (Bender MedSystems) according to the manufacturer's instructions.
Statistical analysis.
The results of our study are expressed as the means ± SE of results of experiments performed in triplicate. Statistical analysis was performed using the Statistical Package Social Sciences (SPSS) program, version 11.5, with one-way analysis of variance, and significant differences among groups were determined by least significant difference analysis. The accepted level of statistical significance was a P of <0.05.
RESULTS
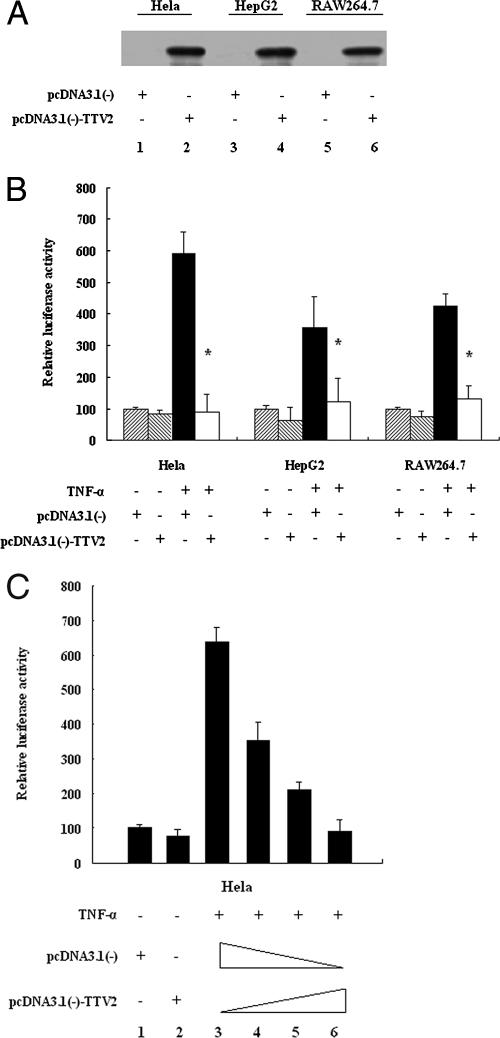
TTV ORF2 protein suppresses NF-κB activity in a dose-dependent manner in HeLa, HepG2, and RAW264.7 cells.
The expression of TTV ORF2 protein in the pcDNA3.1(−)-TTV2-transfected cell lines was confirmed by Western blotting, as shown in Fig. 1A. The effect of TTV ORF2 protein on NF-κB activity in these cell lines was investigated by a luciferase assay. The results in Fig. 1B showed that the TTV ORF2 protein significantly suppressed the activity of NF-κB elicited by TNF-α stimulation, compared with its activity in control HeLa, HepG2, and RAW264.7 cells. With the increase in the concentration of pcDNA3.1(−)-TTV2 (0, 200, 400, and 600 ng), NF-κB activity was suppressed in a dose-dependent manner (lanes 3 to 6) in HeLa cells (Fig. 1C). Similar results were also obtained for the two other cell lines (data not shown).
FIG. 1.
The TTV ORF2 protein suppresses NF-κB activation by TNF-α in a dose-dependent manner in HeLa, HepG2, and RAW264.7 cells. (A) A Western blot analysis detected the expression of the TTV ORF2 protein in HeLa (lanes 1 and 2), HepG2 (lanes 3 and 4), and RAW264.7 (lanes 5 and 6) cells. Cells were transfected with 0.8 μg of pcDNA3.1(−) as a control (lanes 1, 3, and 5) or with 0.8 μg of plasmid pcDNA3.1(−)-TTV2, expressing the TTV ORF2 protein (lanes 2, 4, and 6). At 48 h posttransfection, cell extracts were prepared and expression was determined using rabbit anti-TTV ORF2 antibody. (B) HeLa, HepG2, and RAW264.7 cells were cotransfected with 0.1 μg of pNF-κB-Luc and 10 ng of tk-Renilla-Luc along with 0.6 μg of pcDNA3.1(−)-TTV2 or 0.6 μg of pcDNA3.1(−) as a negative control. At 48 h posttransfection, cells were treated with TNF-α (10 ng/ml) or left untreated for 1 h as indicated and then harvested for luciferase assay. (C) HeLa cells were cotransfected with 0.1 μg of pNF-κB-Luc and 10 ng of tk-Renilla-Luc along with different amounts of pcDNA3.1(−)-TTV2 (lane 3, 0 ng; lane 4, 200 ng; lane 5, 400 ng; lane 6, 600 ng). The total amount of plasmid was adjusted with the empty vector pcDNA3.1(−). At 48 h posttransfection, cells were treated with TNF-α (10 ng/ml) or left untreated for 1 h as indicated and then harvested for the luciferase assay. Luciferase activities correspond to an average of results from at least three independent experiments, and data are shown as means ± SE (*, P < 0.05).
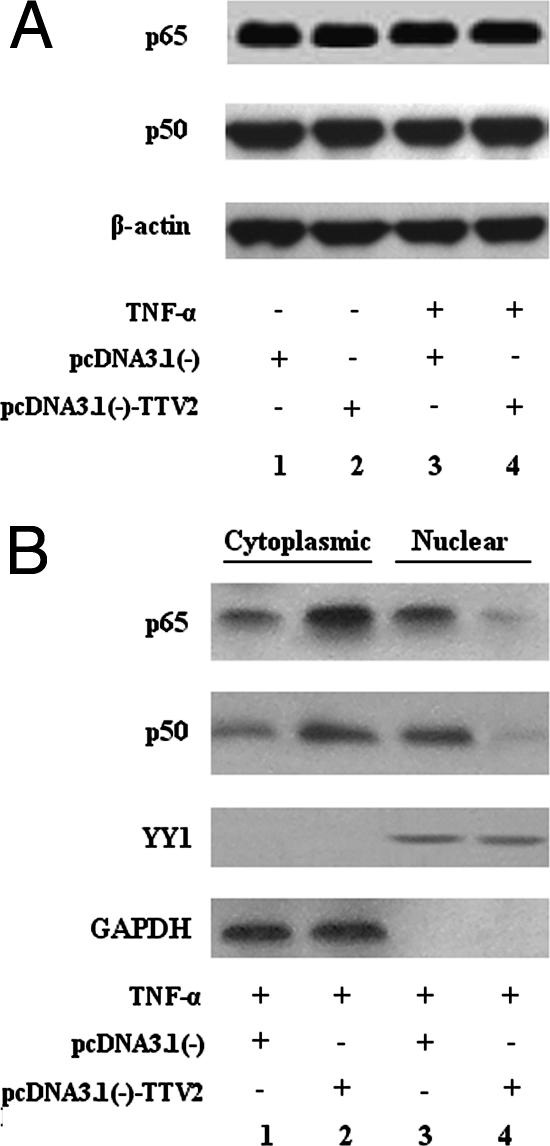
TTV ORF2 protein does not alter the expression of NF-κB but affects its nuclear translocation.
To investigate the molecular mechanism of NF-κB suppression in TTV ORF2-expressing cells, the total quantity of NF-κB in HeLa cells was examined by Western blot analysis using antibodies specific for the subunits of NF-κB. As shown in Fig. 2A, the levels of the NF-κB subunits p50 and p65 remained the same as in the control, no matter when the cells were stimulated or if they were not stimulated (compare lane 1 with lane 2 and lane 3 with lane 4), suggesting that the TTV ORF2 protein did not alter the expression of NF-κB. To determine the localization of NF-κB, cytoplasmic and nuclear fractions were prepared and detected by Western blot analysis using the above-named antibodies. The nucleus-specific antibody anti-YY1 was used as a control to exclude the possibility of cross contamination. Meanwhile, we used a GAPDH control to ensure the equal loadings of the cytoplasmic fractions as well as of YY1 in the nuclear fractions. As shown in Fig. 2B, TTV ORF2 protein inhibited the translocation of p50 and p65 to the nucleus, thereby increasing the amounts of cytoplasmic p50 and p65, which clearly demonstrated that TTV ORF2 protein prevented p50 and p65 from entering the nucleus and, hence, down-regulated the activity of NF-κB.
FIG. 2.
TTV ORF2 protein does not alter the expression of NF-κB but affects its nuclear translocation in HeLa cells. (A) HeLa cells were transfected with the empty vector pcDNA3.1(−) as a control (lanes 1 and 3) or with pcDNA3.1(−)-TTV2 (lanes 2 and 4). At 48 h posttransfection and after stimulation with TNF-α (10 ng/ml) for 1 h (lanes 3 and 4), cell extracts were prepared and detected by Western blot analysis with anti-p65, anti-p50, and anti-β-actin antibodies. (B) HeLa cells were transfected with 0.8 μg of either the empty vector pcDNA3.1(−) or pcDNA3.1(−)-TTV2. At 48 h posttransfection and after stimulation with TNF-α (10 ng/ml) for 1 h, cytoplasmic and nuclear fractions were prepared as described in Materials and Methods. Both cytoplasmic proteins and nuclear proteins were analyzed by SDS-PAGE and Western blotting with anti-p65 and anti-p50 antibodies to reveal the localization of NF-κB subunits. Nucleus-specific anti-YY1 antibody and anti-GAPDH antibody were used as controls. Results are representative of three different experiments.
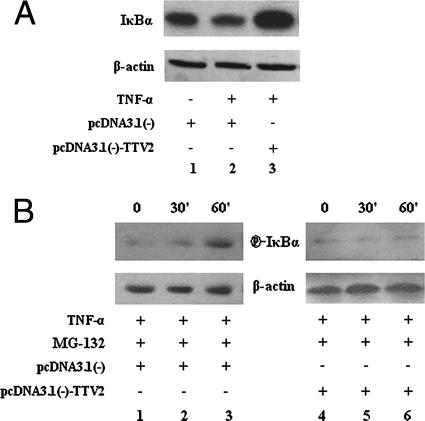
TTV ORF2 protein inhibits IκBα protein degradation.
To investigate how TTV ORF2 protein suppresses NF-κB activity, IκBα was measured by Western blot analysis. When we compared lane 3 with lanes 1 and 2 in Fig. 3A, it was obvious that TTV ORF2 protein could substantially increase the quantity of IκBα protein when the pcDNA3.1(−)-TTV2 plasmid was transfected (Fig. 3A). The activation of NF-κB depends on the phosphorylation and polyubiquitination of IκBα, but phosphorylated IκBα is an unstable intermediate due to its rapid proteasome-mediated degradation, so we preincubated the cells with the proteasome inhibitor MG-132 to allow the accumulation of phosphorylated IκBα. As shown in Fig. 3B, the level of phosphorylated IκBα decreased in pcDNA3.1(−)-TTV2-transfected cells (compare lane 1 with lane 4, lane 2 with lane 5, and lane 3 with lane 6). Taking these results together, it was reasonable to conclude that TTV ORF2 protein inhibited IκBα protein degradation.
FIG. 3.
TTV ORF2 protein inhibits IκBα protein degradation. (A) HeLa cells were transfected with either the empty vector pcDNA3.1(−) or pcDNA3.1(−)-TTV2. At 48 h posttransfection and after stimulation with TNF-α (10 ng/ml) for 1 h or no stimulation as indicated, cell extracts were prepared and IκBα expression was determined by Western blotting using anti-IκBα antibody. β-Actin was used as a control. (B) HeLa cells were transfected with either the empty vector pcDNA3.1(−) or pcDNA3.1(−)-TTV2. At 48 h posttransfection and after being treated with TNF-α (10 ng/ml) and the proteasome inhibitor MG-132 (20 μM) for the indicated times, cell extracts were prepared and phosphoserine IκBα levels were determined by Western blotting using anti-phosphoserine IκBα (P-IκBα) antibody. β-Actin was used as a control. Results are representative of three different experiments.
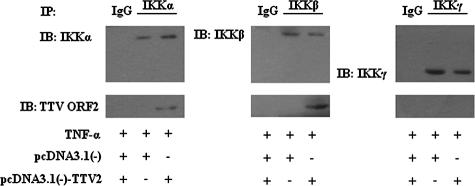
TTV ORF2 protein can physically interact with IKKα and IKKβ but not IKKγ.
To understand how the TTV ORF2 protein affects IκBα protein, we examined the physical interaction between the IKK complex and TTV ORF2 protein by an immunoprecipitation assay. As shown in Fig. 4, TTV ORF2 protein coprecipitated with IKKα as well as IKKβ but not IKKγ in HeLa cells, suggesting that TTV ORF2 protein can physically interact with IKKα and IKKβ. An immunoprecipitation assay of overexpressed IKKα or IKKβ [using the plasmids pcDNA3.1(−)-Flag-IKKα and pCMV-Flag-IKKβ] and TTV ORF2 protein was performed, and we obtained similar results (data not shown).
FIG. 4.
TTV ORF2 protein interacts with IKKα and IKKβ. HeLa cells were transfected with either the empty vector pcDNA3.1(−) or pcDNA3.1(−)-TTV2. At 48 h posttransfection and after being treated with TNF-α (10 ng/ml) for 4 h, cell lysate was prepared for immunoprecipitation with monoclonal anti-IKKα, anti-IKKβ, or anti-IKKγ antibodies, with immunoglobulin G (IgG) taken as a control. Immune complex captured by protein A-Sepharose was separated by SDS-PAGE and analyzed by Western blotting with anti-TTV ORF2 antibody. One-tenth of the total cell lysates used for immunoprecipitation was loaded as a positive control for IKKα, IKKβ, and IKKγ. Results are representative of three different experiments. IP, immunoprecipitation; IB, immunoblotting.
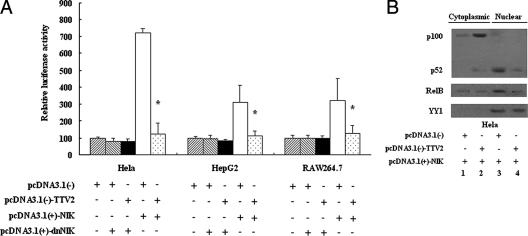
TTV ORF2 protein also suppresses NF-κB activity in the noncanonical NF-κB pathway.
As we all know, IKKα does not respond to the phosphorylation of IκBα in the canonical NF-κB pathway, while it does affect the processing of p100 into p52 in the noncanonical NF-κB pathway, so we assumed that TTV ORF2 protein might be involved in the noncanonical NF-κB pathway. To examine whether TTV ORF2 protein could affect the activity of NF-κB in the noncanonical NF-κB pathway or not, we performed an NF-κB luciferase assay. As shown in Fig. 5A, TTV ORF2 protein suppressed NF-κB activity elicited by NIK in the noncanonical NF-κB pathway, as expected. When it was transfected with dominant negative NIK [pcDNA3.1(+)-dnNIK], there was no difference between them. By detecting the localization and amount of p100/p52 (Fig. 5B), we got the following prospective result: TTV ORF2 protein could inhibit the activity of NF-κB in the noncanonical NF-κB pathway, and this suppression might be correlative with the interaction between TTV ORF2 protein and IKKα.
FIG. 5.
The TTV ORF2 protein suppresses NF-κB activity in the noncanonical NF-κB pathway. (A) HeLa, HepG2, and RAW264.7 cells were cotransfected with 0.1 μg of pcDNA3.1(+)-NIK or pcDNA3.1(+)-dnNIK, 0.1 μg of pNF-κB-Luc, 10 ng of tk-Renilla-Luc along with 0.6 μg of pcDNA3.1(−)-TTV2, or 0.6 μg of pcDNA3.1(−) as indicated below the graph. At 48 h posttransfection, cells were harvested for luciferase assay. Luciferase activities correspond to averages of results from at least three independent experiments, and data are means ± SE (*, P < 0.05). (B) HeLa cells were transfected with 0.1 μg of pcDNA3.1(+)-NIK and 0.6 μg of either the empty vector pcDNA3.1(−) or pcDNA3.1(−)-TTV2 as indicated below the graph. At 48 h posttransfection, cytoplasmic and nuclear fractions were prepared as described in Materials and Methods. Both cytoplasmic proteins and nuclear proteins were analyzed by Western blotting with anti-p100/p52 and anti-Rel-B antibodies to reveal the localization of NF-κB subunits. Nucleus-specific anti-YY1 antibody was used as a control. Results are representative of three different experiments.
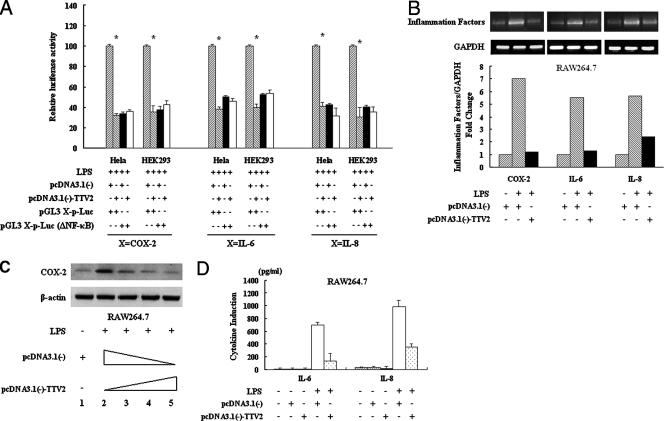
TTV ORF2 protein inhibits the transcription of NF-κB-mediated downstream genes through the down-regulation of NF-κB.
IL-6, IL-8, and COX-2 are all NF-κB-mediated downstream genes, and NF-κB can bind directly to their promoters as a transcript factor, regulating their expression. Now that we knew that TTV ORF2 protein can suppress NF-κB activity in vivo, we wished to detect whether TTV ORF2 protein can affect these genes. From the results of a luciferase assay, it was verified that TTV ORF2 protein does affect the activity of the IL-6, IL-8, and COX-2 promoters through NF-κB sites in different cells (Fig. 6A). Then, we detected the expression of the IL-6, IL-8, and COX-2 genes at the mRNA and protein levels. As shown in Fig. 6B, RT-PCR analysis of the extracted mRNA revealed that LPS caused a remarkable increase in IL-6, IL-8, and COX-2 mRNA compared with the level in the control group, while the cells transfected with pcDNA3.1(−)-TTV2, with the same stimuli, blocked this induction in a dose-dependent manner in the RAW264.7 cells. At the bottom of Fig. 6B, the quantification of IL-6, IL-8, and COX-2 mRNA expression as measured by gel analysis software, normalized by calculating the ratio of mRNA to GAPDH, is shown. In the HeLa cells, we repeated the experiments under the same conditions and obtained similar results (data not shown). At the protein level, Western blot analysis of cell lysate using COX-2 antibody showed that the level of COX-2 expression decreased in TTV ORF2 protein-expressing cells treated with LPS compared to the level in the control in the RAW264.7 cells (Fig. 6C). The levels of IL-6 and IL-8 were measured by ELISA, and Fig. 6D shows that the expression of both IL-6 and IL-8 decreased in pcDNA3.1(−)-TTV2-transfected RAW264.7 cells, even though their ranges of reduction were not the same.
FIG. 6.
TTV ORF2 protein inhibits the expression of NF-κB-mediated downstream genes through its effect on NF-κB. (A) HeLa and HEK293 cells were cotransfected with 0.1 μg of pGL3 X-p-Luc or pGL3 X-p-Luc (ΔNF-κB) (where X is COX-2, IL-6, or IL-8) and 10 ng of tk-Renilla-Luc along with 0.6 μg of pcDNA3.1(−)-TTV2 or 0.6 μg of pcDNA3.1(−) as indicated below the graph. At 48 h posttransfection, cells were treated with LPS (1 μg/ml) for 6 h and then harvested for luciferase activity. Luciferase activities correspond to averages from at least three independent experiments, and data shown are means ± SE (*, P < 0.05). (B) RAW264.7 cells were transfected with 0.6 μg of pcDNA3.1(−)-TTV2 or 0.6 μg of pcDNA3.1(−). At 48 h posttransfection, cells were stimulated with LPS (1 μg/ml) or left unstimulated for 6 h as indicated. The RT-PCR products of COX-2, IL-6, or IL-8 mRNA were detected by agarose gel electrophoresis. GAPDH was used as a control. IL-6, IL-8, and COX-2 mRNAs were measured by gel analysis software and normalized by calculating the ratio of mRNA to GAPDH. (C) RAW264.7 cells were transfected with 0.6 μg of the empty vector pcDNA3.1(−) without stimulation as a control or with different amounts of pcDNA3.1(−)-TTV2 (lane 2, 0 ng; lane 3, 200 ng; lane 4, 400 ng; lane 5, 600 ng) and treated with LPS (1 μg/ml) for 6 h. Cell extracts were prepared, and the protein amounts were determined using anti-COX-2 antibody. (D) RAW264.7 cells were transfected with 0.6 μg of pcDNA3.1(−)-TTV2 or 0.6 μg of pcDNA3.1(−). At 48 h posttransfection, cells were stimulated with LPS (1 μg/ml) or left unstimulated for 6 h as indicated below the graph. IL-6 and IL-8 in the culture supernatant of RAW264.7 cells were measured using the ELISA method. Results are representative of three different experiments.
DISCUSSION
As known to all, NF-κB activation is a protective response of the host to viral pathogens, and mice deficient in different members of the NF-κB family are more susceptible to viral infection, suggesting that NF-κB plays a crucial role in the process of viral infection (21, 54, 60). How to regulate and evade the monitoring of the host immune system must be an all-important step for the virus itself. Some viruses have evolved strategies to interfere with NF-κB activation in order to evade the immune response. For example, vaccinia virus encodes a viral protein, A52R, that is a homolog of the scaffolding protein MyD88, abrogating IL-1-mediated signaling, which is important for resistance to vaccinia virus (10). Another example is the human immunodeficiency virus (HIV) accessory protein Vpu. It was shown to inhibit NF-κB activation by interfering with the β-transducin repeat-containing protein-mediated degradation of IκBα and to promote the apoptosis of infected cells by suppressing the NF-κB-dependent expression of antiapoptotic factors (3, 9). The African swine fever virus A238L protein, a homolog of IκB, contains ankyrin repeats that bind to NF-κB dimers, blocking NF-κB's nuclear translocation (47). Since it was first discovered in 1997, TTV has been considered a chronic and long-standing virus, but its pathogenic nature is still a mystery. In our study, we proved that the TTV ORF2 protein of the SANBAN isolate, which belongs to the group 3 TTV genotype, can interact with IKKα and IKKβ and then inhibit IκBα degradation and suppress the activation of NF-κB in the canonical and noncanonical NF-κB pathways, implying that TTV may share with other viruses a mechanism to infect a host, proliferate, and survive.
Although the signals inducing NF-κB in cancer cells are still not well understood, overactivation of NF-κB and the subsequent production of cytokines, chemokines, inflammatory enzymes, growth factors, and antiapoptotic proteins have been found to be involved in cancer progression and chemoresistance. In our study, the inflammation factors IL-6, IL-8, and COX-2, which have been proved to be involved in the formation and metastasis of a wide variety of cancers, including bladder cancer, colorectal cancer, renal cell carcinoma, gastric cardia adenocarcinoma, lung carcinoma, prostate cancer, and so on (4, 26, 43, 51, 56, 62), are down-regulated in the presence of the TTV ORF2 protein, implying that TTV ORF2 protein may disable the mechanism that allows up-regulation of the inflammatory response by means of down-regulating these factors. This finding might explain why TTV can replicate to a high DNA copy level without leading to much inflammation, and it might be a survival mechanism that the virus has developed over time to avoid being eliminated by immune responses. Furthermore, this also might partially account for the fact that TTV infection cannot lead to cancer directly.
Recently, Gergely et al. reported that patients with systemic lupus erythematosus (SLE) produced autoantibodies to HRES-1/p28, a 28-kDa nuclear autoantigen encoded by a human endogenous retrovirus. BLAST searching for cross-reactive viral epitopes to HRES-1/p28 yielded two viral epitopes similar to that of the TTV ORF2 protein. These epitopes may be related to the pathogenicity of TTV in SLE (18). As early as 1999, Wong et al. reported that, compared with that in normal cells, the activation of NF-κB activity was significantly decreased in SLE patients (63). So we can speculate that the high prevalence of TTV DNA in lupus patients might be caused by the inhibitory effect of the TTV ORF2 protein on NF-κB activation.
The viral load of TTV may be dependent on the immunological status of the host. In 2001, Zhong et al. reported that 93.2% of cancer patients were positive for TTV, as determined by a cutoff value of more than 250 TTV copies per 105 peripheral blood mononuclear cells (66). In HIV+ patients receiving highly active antiretroviral therapy, the TTV load decreased after the highly active antiretroviral therapy probably because of immunological improvement even in the absence of CD4 improvement (28). In 2007, Thom and Petrik reported that TTV viral loads of an AIDS patient group were significantly higher than those in the bone marrow of both HIV-positive and -negative groups, which shows a link between the deterioration of the immune system and increased viral loads in studied tissues (61). Considering the significance of NF-κB in the innate and adaptive immunity of organisms, more studies are needed to define the relationship between the NF-κB and TTV loads and to determine whether the TTV ORF2 protein plays an important role in this course.
In conclusion, we for the first time demonstrated that the TTV ORF2 protein could suppress the canonical and noncanonical NF-κB pathways via interacting with IKKβ and IKKα, thereby decreasing IL-6, IL-8, and COX-2 expression. This inhibitory effect suggests that the TTV ORF2 protein may be involved in regulating the innate and adaptive immune responses of organisms, contribute to TTV pathogenesis, and even be related to some diseases.
Acknowledgments
This work was supported by grant 99J129 from the National Nature Science Foundation of Hubei Province.
Footnotes
Published ahead of print on 8 August 2007.
REFERENCES
- 1.Aggarwal, B. B. 2004. Nuclear factor-kappaB: the enemy within. Cancer Cell 6:203-208. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal, B. B., S. Shishodia, S. K. Sandur, M. K. Pandey, and G. Sethi. 2006. Inflammation and cancer: how hot is the link? Biochem. Pharmacol. 72:1605-1621. [DOI] [PubMed] [Google Scholar]
- 3.Akari, H., S. Bour, S. Kao, A. Adachi, and K. Strebel. 2001. The human immunodeficiency virus type 1 accessory protein Vpu induces apoptosis by suppressing the nuclear factor kappaB-dependent expression of antiapoptotic factors. J. Exp. Med. 194:1299-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angelo, L. S., M. Talpaz, and R. Kurzrock. 2002. Autocrine interleukin-6 production in renal cell carcinoma: evidence for the involvement of p53. Cancer Res. 62:932-940. [PubMed] [Google Scholar]
- 5.Baeuerle, P. A., and D. Baltimore. 1988. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science 242:540-546. [DOI] [PubMed] [Google Scholar]
- 6.Bando, M., S. Ohno, K. Oshikawa, M. Takahashi, H. Okamoto, and Y. Sugiyama. 2001. Infection of TT virus in patients with idiopathic pulmonary fibrosis. Respir. Med. 95:935-942. [DOI] [PubMed] [Google Scholar]
- 7.Biagini, P., H. Attoui, P. Gallian, M. Touinssi, J. F. Cantaloube, P. de Micco, and X. de Lamballerie. 2000. Complete sequences of two highly divergent European isolates of TT virus. Biochem. Biophys. Res. Commun. 271:837-841. [DOI] [PubMed] [Google Scholar]
- 8.Bonizzi, G., and M. Karin. 2004. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25:280-288. [DOI] [PubMed] [Google Scholar]
- 9.Bour, S., C. Perrin, H. Akari, and K. Strebel. 2001. The human immunodeficiency virus type 1 Vpu protein inhibits NF-kappa B activation by interfering with beta TrCP-mediated degradation of Ikappa B. J. Biol. Chem. 276:15920-15928. [DOI] [PubMed] [Google Scholar]
- 10.Bowie, A., E. Kiss-Toth, J. A. Symons, G. L. Smith, S. K. Dower, and L. A. O'Neill. 2000. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 97:10162-10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camci, C., C. Guney, A. Balkan, N. Buyukberber, S. Buyukberber, A. Kadayifci, and A. Kubar. 2002. The prevalence of TT virus in cancer patients. New Microbiol. 25:463-468. [PubMed] [Google Scholar]
- 12.Claudio, E., K. Brown, S. Park, H. Wang, and U. Siebenlist. 2002. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nat. Immunol. 3:958-965. [DOI] [PubMed] [Google Scholar]
- 13.Coope, H. J., P. G. Atkinson, B. Huhse, M. Belich, J. Janzen, M. J. Holman, G. G. Klaus, L. H. Johnston, and S. C. Ley. 2002. CD40 regulates the processing of NF-kappaB2 p100 to p52. EMBO J. 21:5375-5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dejardin, E., N. M. Droin, M. Delhase, E. Haas, Y. Cao, C. Makris, Z. W. Li, M. Karin, C. F. Ware, and D. R. Green. 2002. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity 17:525-535. [DOI] [PubMed] [Google Scholar]
- 15.DiDonato, J. A., M. Hayakawa, D. M. Rothwarf, E. Zandi, and M. Karin. 1997. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature 388:548-554. [DOI] [PubMed] [Google Scholar]
- 16.Garbuglia, A. R., T. Iezzi, M. R. Capobianchi, P. Pignoloni, A. Pulsoni, J. Sourdis, E. Pescarmona, D. Vitolo, and F. Mandelli. 2003. Detection of TT virus in lymph node biopsies of B-cell lymphoma and Hodgkin's disease, and its association with EBV infection. Int. J. Immunopathol. Pharmacol. 16:109-118. [DOI] [PubMed] [Google Scholar]
- 17.Gergely, P., Jr., A. Perl, and G. Poor. 2006. Possible pathogenic nature of the recently discovered TT virus: does it play a role in autoimmune rheumatic diseases? Autoimmun. Rev. 6:5-9. [DOI] [PubMed] [Google Scholar]
- 18.Gergely, P., Jr., R. Pullmann, C. Stancato, L. Otvos, Jr., A. Koncz, A. Blazsek, G. Poor, K. E. Brown, P. E. Phillips, and A. Perl. 2005. Increased prevalence of transfusion-transmitted virus and cross-reactivity with immunodominant epitopes of the HRES-1/p28 endogenous retroviral autoantigen in patients with systemic lupus erythematosus. Clin. Immunol. 116:124-134. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-kappaB puzzle. Cell 109(Suppl.):S81-S96. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 21.Harling-McNabb, L., G. Deliyannis, D. C. Jackson, S. Gerondakis, G. Grigoriadis, and L. E. Brown. 1999. Mice lacking the transcription factor subunit Rel can clear an influenza infection and have functional anti-viral cytotoxic T cells but do not develop an optimal antibody response. Int. Immunol. 11:1431-1439. [DOI] [PubMed] [Google Scholar]
- 22.Hu, Z. J., Z. W. Lang, Y. S. Zhou, H. P. Yan, D. Z. Huang, W. R. Chen, and Z. X. Luo. 2002. Clinicopathological study on TTV infection in hepatitis of unknown etiology. World J. Gastroenterol. 8:288-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irshad, M., Y. K. Joshi, Y. Sharma, and I. Dhar. 2006. Transfusion transmitted virus: a review on its molecular characteristics and role in medicine. World J. Gastroenterol. 12:5122-5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamahora, T., S. Hino, and H. Miyata. 2000. Three spliced mRNAs of TT virus transcribed from a plasmid containing the entire genome in COS1 cells. J. Virol. 74:9980-9986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 26.Landi, S., V. Moreno, L. Gioia-Patricola, E. Guino, M. Navarro, J. de Oca, G. Capella, and F. Canzian. 2003. Association of common polymorphisms in inflammatory genes interleukin (IL)6, IL8, tumor necrosis factor alpha, NFKB1, and peroxisome proliferator-activated receptor gamma with colorectal cancer. Cancer Res. 63:3560-3566. [PubMed] [Google Scholar]
- 27.Li, Z. W., W. Chu, Y. Hu, M. Delhase, T. Deerinck, M. Ellisman, R. Johnson, and M. Karin. 1999. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J. Exp. Med. 189:1839-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madsen, C. D., J. Eugen-Olsen, O. Kirk, J. Parner, J. Kaae Christensen, M. S. Brasholt, J. Ole Nielsen, and K. Krogsgaard. 2002. TTV viral load as a marker for immune reconstitution after initiation of HAART in HIV-infected patients. HIV Clin. Trials 3:287-295. [DOI] [PubMed] [Google Scholar]
- 29.Maggi, F., C. Fornai, A. Morrica, F. Casula, M. L. Vatteroni, S. Marchi, P. Ciccorossi, L. Riente, M. Pistello, and M. Bendinelli. 1999. High prevalence of TT virus viremia in Italian patients, regardless of age, clinical diagnosis, and previous interferon treatment. J. Infect. Dis. 180:838-842. [DOI] [PubMed] [Google Scholar]
- 30.Maggi, F., M. Pifferi, C. Fornai, E. Andreoli, E. Tempestini, M. Vatteroni, S. Presciuttini, S. Marchi, A. Pietrobelli, A. Boner, M. Pistello, and M. Bendinelli. 2003. TT virus in the nasal secretions of children with acute respiratory diseases: relations to viremia and disease severity. J. Virol. 77:2418-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mercurio, F., H. Zhu, B. W. Murray, A. Shevchenko, B. L. Bennett, J. Li, D. B. Young, M. Barbosa, M. Mann, A. Manning, and A. Rao. 1997. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science 278:860-866. [DOI] [PubMed] [Google Scholar]
- 32.Miyamoto, M., H. Takahashi, I. Sakata, and Y. Adachi. 2000. Hepatitis-associated aplastic anemia and transfusion-transmitted virus infection. Intern. Med. 39:1068-1070. [DOI] [PubMed] [Google Scholar]
- 33.Miyata, H., H. Tsunoda, A. Kazi, A. Yamada, M. A. Khan, J. Murakami, T. Kamahora, K. Shiraki, and S. Hino. 1999. Identification of a novel GC-rich 113-nucleotide region to complete the circular, single-stranded DNA genome of TT virus, the first human circovirus. J. Virol. 73:3582-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller, J. R., and U. Siebenlist. 2003. Lymphotoxin beta receptor induces sequential activation of distinct NF-kappa B factors via separate signaling pathways. J. Biol. Chem. 278:12006-12012. [DOI] [PubMed] [Google Scholar]
- 35.Mushahwar, I. K., J. C. Erker, A. S. Muerhoff, T. P. Leary, J. N. Simons, L. G. Birkenmeyer, M. L. Chalmers, T. J. Pilot-Matias, and S. M. Dexai. 1999. Molecular and biophysical characterization of TT virus: evidence for a new virus family infecting humans. Proc. Natl. Acad. Sci. USA 96:3177-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nam, N. H. 2006. Naturally occurring NF-kappaB inhibitors. Mini Rev. Med. Chem. 6:945-951. [DOI] [PubMed] [Google Scholar]
- 37.Nishizawa, T., H. Okamoto, K. Konishi, H. Yoshizawa, Y. Miyakawa, and M. Mayumi. 1997. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem. Biophys. Res. Commun. 241:92-97. [DOI] [PubMed] [Google Scholar]
- 38.Okamoto, H., T. Nishizawa, N. Kato, M. Ukita, H. Ikeda, H. Lizuka, Y. Miyakawa, and M. Mayumi. 1998. Molecular cloning and characterisation of a novel DNA virus (TTV) associated with posttransfusion hepatitis of unknown etiology. Hepatol. Res. 10:1-16. [Google Scholar]
- 39.Okamoto, H., T. Nishizawa, A. Tawara, Y. Peng, M. Takahashi, J. Kishimoto, T. Tanaka, Y. Miyakawa, and M. Mayumi. 2000. Species-specific TT viruses in humans and nonhuman primates and their phylogenetic relatedness. Virology 277:368-378. [DOI] [PubMed] [Google Scholar]
- 40.Okamoto, H., T. Nishizawa, A. Tawara, M. Takahashi, J. Kishimoto, T. Sai, and Y. Sugai. 2000. TT virus mRNAs detected in the bone marrow cells from an infected individual. Biochem. Biophys. Res. Commun. 279:700-707. [DOI] [PubMed] [Google Scholar]
- 41.Okamoto, H., T. Nishizawa, and M. Ukita. 1999. A novel unenveloped DNA virus (TT virus) associated with acute and chronic non-A to G hepatitis. Intervirology 42:196-204. [DOI] [PubMed] [Google Scholar]
- 42.Okamoto, H., M. Takahashi, T. Nishizawa, A. Tawara, Y. Sugai, T. Sai, T. Tanaka, and F. Tsuda. 2000. Replicative forms of TT virus DNA in bone marrow cells. Biochem. Biophys. Res. Commun. 270:657-662. [DOI] [PubMed] [Google Scholar]
- 43.Okamoto, M., H. Kawamata, K. Kawai, and R. Oyasu. 1995. Enhancement of transformation in vitro of a nontumorigenic rat urothelial cell line by interleukin 6. Cancer Res. 55:4581-4585. [PubMed] [Google Scholar]
- 44.Peng, Y. H., T. Nishizawa, M. Takahashi, T. Ishikawa, A. Yoshikawa, and H. Okamoto. 2002. Analysis of the entire genomes of thirteen TT virus variants classifiable into the fourth and fifth genetic groups, isolated from viremic infants. Arch. Virol. 147:21-41. [DOI] [PubMed] [Google Scholar]
- 45.Peters, M. A., D. C. Jackson, B. S. Crabb, and G. F. Browning. 2002. Chicken anemia virus VP2 is a novel dual specificity protein phosphatase. J. Biol. Chem. 277:39566-39573. [DOI] [PubMed] [Google Scholar]
- 46.Qiu, J., L. Kakkola, F. Cheng, C. Ye, M. Soderlund-Venermo, K. Hedman, and D. J. Pintel. 2005. Human circovirus TT virus genotype 6 expresses six proteins following transfection of a full-length clone. J. Virol. 79:6505-6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Revilla, Y., M. Callejo, J. M. Rodriguez, E. Culebras, M. L. Nogal, M. L. Salas, E. Vinuela, and M. Fresno. 1998. Inhibition of nuclear factor kappaB activation by a virus-encoded IkappaB-like protein. J. Biol. Chem. 273:5405-5411. [DOI] [PubMed] [Google Scholar]
- 48.Rothwarf, D. M., E. Zandi, G. Natoli, and M. Karin. 1998. IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature 395:297-300. [DOI] [PubMed] [Google Scholar]
- 49.Rudolph, D., W. C. Yeh, A. Wakeham, B. Rudolph, D. Nallainathan, J. Potter, A. J. Elia, and T. W. Mak. 2000. Severe liver degeneration and lack of NF-kappaB activation in NEMO/IKKgamma-deficient mice. Genes Dev. 14:854-862. [PMC free article] [PubMed] [Google Scholar]
- 50.Sadikot, R. T., W. Han, M. B. Everhart, O. Zoia, R. S. Peebles, E. D. Jansen, F. E. Yull, J. W. Christman, and T. S. Blackwell. 2003. Selective I kappa B kinase expression in airway epithelium generates neutrophilic lung inflammation. J. Immunol. 170:1091-1098. [DOI] [PubMed] [Google Scholar]
- 51.Savage, S. A., C. C. Abnet, S. D. Mark, Y. L. Qiao, Z. W. Dong, S. M. Dawsey, P. R. Taylor, and S. J. Chanock. 2004. Variants of the IL8 and IL8RB genes and risk for gastric cardia adenocarcinoma and esophageal squamous cell carcinoma. Cancer Epidemiol. Biomarkers Prev. 13:2251-2257. [PubMed] [Google Scholar]
- 52.Seemayer, C. A., S. Viazov, M. Neidhart, P. Bruhlmann, B. A. Michel, R. E. Gay, M. Roggendorf, and S. Gay. 2001. Prevalence of TTV DNA and GBV-C RNA in patients with systemic sclerosis, rheumatoid arthritis, and osteoarthritis does not differ from that in healthy blood donors. Ann. Rheum. Dis. 60:806-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Senftleben, U., Y. Cao, G. Xiao, F. R. Greten, G. Krahn, G. Bonizzi, Y. Chen, Y. Hu, A. Fong, S. C. Sun, and M. Karin. 2001. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 293:1495-1499. [DOI] [PubMed] [Google Scholar]
- 54.Sha, W. C., H. C. Liou, E. I. Tuomanen, and D. Baltimore. 1995. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell 80:321-330. [DOI] [PubMed] [Google Scholar]
- 55.Silverman, N., and T. Maniatis. 2001. NF-kappaB signaling pathways in mammalian and insect innate immunity. Genes Dev. 15:2321-2342. [DOI] [PubMed] [Google Scholar]
- 56.Subbarayan, V., A. L. Sabichi, N. Llansa, S. M. Lippman, and D. G. Menter. 2001. Differential expression of cyclooxygenase-2 and its regulation by tumor necrosis factor-alpha in normal and malignant prostate cells. Cancer Res. 61:2720-2726. [PubMed] [Google Scholar]
- 57.Tagger, A., F. Donato, M. L. Ribero, G. Binelli, U. Gelatti, G. Portera, A. Albertini, M. Fasola, R. Chiesa, G. Nardi, et al. 1999. A case-control study on a novel DNA virus (TT virus) infection and hepatocellular carcinoma. Hepatology 30:294-299. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi, K., Y. Iwasa, M. Hijikata, and S. Mishiro. 2000. Identification of a new human DNA virus (TTV-like mini virus, TLMV) intermediately related to TT virus and chicken anemia virus. Arch. Virol. 145:979-993. [DOI] [PubMed] [Google Scholar]
- 59.Takahashi, K., Y. Ohta, and S. Mishiro. 1998. Partial 2.4-kb sequences of TT virus (TTV) genome from eight Japanese isolates: diagnostic and phylogenetic implications. Hepatol. Res. 12:111-120. [Google Scholar]
- 60.Tato, C. M., and C. A. Hunter. 2002. Host-pathogen interactions: subversion and utilization of the NF-kappa B pathway during infection. Infect. Immun. 70:3311-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thom, K., and J. Petrik. 2007. Progression towards AIDS leads to increased Torque teno virus and Torque teno minivirus titers in tissues of HIV infected individuals. J. Med. Virol. 79:1-7. [DOI] [PubMed] [Google Scholar]
- 62.Wolff, H., K. Saukkonen, S. Anttila, A. Karjalainen, H. Vainio, and A. Ristimaki. 1998. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res. 58:4997-5001. [PubMed] [Google Scholar]
- 63.Wong, H. K., G. M. Kammer, G. Dennis, and G. C. Tsokos. 1999. Abnormal NF-kappa B activity in T lymphocytes from patients with systemic lupus erythematosus is associated with decreased p65-RelA protein expression. J. Immunol. 163:1682-1689. [PubMed] [Google Scholar]
- 64.Ye, L., K. A. Timani, L. Ye, L. Kong, X. Yang, Q. Liao, and J. Wu. 2005. Two cis-acting elements in negative RNA strand of hepatitis C virus involved in synthesis of positive RNA strand in vitro. Acta Virol. 49:83-90. [PubMed] [Google Scholar]
- 65.Yoshida, H., N. Kato, Y. Shiratori, K. H. Lan, S. K. Ono-Nita, Z. Feng, S. Shiina, and M. Omata. 2000. Poor association of TT virus viremia with hepatocellular carcinoma. Liver 20:247-252. [DOI] [PubMed] [Google Scholar]
- 66.Zhong, S., W. Yeo, M. W. Tang, X. R. Lin, F. Mo, W. M. Ho, P. Hui, and P. J. Johnson. 2001. Gross elevation of TT virus genome load in the peripheral blood mononuclear cells of cancer patients. Ann. N. Y. Acad. Sci. 945:84-92. [DOI] [PubMed] [Google Scholar]