Abstract
Lymphocytic choriomeningitis virus (LCMV) is a murine arenavirus whose glycoprotein consists of a transmembrane subunit (GP-2) and a receptor-binding subunit (GP-1). LCMV-neutralizing antibodies (nAbs) are directed against a single site on GP-1 and occur 1 month after the infection of cytotoxic-T-lymphocyte (CTL) deficient mice. In wild-type mice, however, CTLs control early infection, and weak nAb titers emerge very late (after 70 to 150 days) if at all. Production of recombinant GP-1 in native conformation enabled us to study the emergence of GP-1-binding antibodies directed against the neutralizing epitope. By combining binding and neutralization assays, we correlated the development of binding antibodies versus nAbs in wild-type and CTL-deficient mice after infection with different LCMV doses. We found that wild-type mice developed GP-1-specific antibodies already by day 8 after exposure to high but not low doses, demonstrating that naive GP-1-specific B cells were infrequent. Furthermore, the induced antibodies bound to the neutralizing GP-1 epitope but failed to neutralize the virus and therefore were of low affinity. In CTL-deficient mice, where massive viremia quickly levels initial differences in viral load, low and high doses induced low-affinity non-neutralizing GP-1-binding antibodies with kinetics similar to high-dose-infected wild-type mice. Only in CTL-deficient mice, however, the GP-1-specific antibodies developed into nAbs within 1 month. We conclude that LCMV uses a dual strategy to evade nAb responses in wild-type mice. First, LCMV exploits a “hole” in the murine B-cell repertoire, which provides only a small and narrow initial pool of low-affinity GP-1-specific B cells. Second, affinity maturation of the available low-affinity non-neutralizing antibodies is impaired.
Lymphocytic choriomeningitis virus (LCMV) is an enveloped RNA virus, which belongs to the Old World group of the arenavirus family. The virus surface is densely covered with viral fusion glycoprotein spikes, which appear as 9-nm-long projections perpendicular the viral membrane (32). As a typical class I viral fusion protein, the LCMV glycoprotein forms heterodimeric trimers, consisting of the peripheral receptor-binding subunit glycoprotein 1 (GP-1) and of the transmembrane subunit GP-2, which mediates viral membrane fusion (19). An extensive epitope mapping study on purified infectious LCMV strain WE (LCMV-WE) identified several surface epitopes on the native glycoprotein spikes (34): a major conformational epitope (GP-1A/B) and a minor linear epitope (GP-1C) on GP-1 and a linear epitope (GP-2A/B/C) on GP-2. The linear GP-2A/B/C epitope comprises amino acids 370 to 378 and is located within the stem of the glycoprotein spike (20, 39). The GP-1A epitope, which overlaps with the GP-1B epitope, is known to be the only target site for LCMV-WE neutralizing antibodies (nAbs) (8, 34, 40). nAbs binding to the GP-1A site prevent attachment of the virus to the host cell (6), most likely by sterically blocking the receptor-binding site and thus inhibiting attachment to carbohydrates on the cellular receptor α-dystroglycan (12, 26). The neutralizing GP-1A epitope was also mapped by sequencing of LCMV-WE escape mutants evading a strong and focused nAb response in mice (15, 16, 24, 37).
Since LCMV has coevolved with Mus musculus, it has served as an important model system to study persistence, tolerance, and antiviral immune responses in mice. In its natural host, LCMV is noncytopathic and establishes persistent lifelong infections with continuous virus production and with little or no disease (14).
LCMV nAbs are not involved in the clearance of the acute viremia, are completely absent within the first months after the infection, and sometimes develop very late, i.e., 70 to 200 days after infection (3, 9, 23). For example, wild-type (wt) C57BL/6 mice infected with 200 PFU (low dose) LCMV-WE fail to induce measurable nAb titers, whereas infection with 2 × 106 PFU (high dose) induces weak nAb titers normally after day 70 (16, 22, 36).
Acute viremia is controlled by a strong CD8+ cytotoxic T-lymphocyte (CTL) response (17, 25, 30), peaking 1 week after infection (21). Compared to wt mice, where nAb responses are completely absent or delayed, the emergence of nAbs is dramatically accelerated in CTL-deficient mice. CTL-deficient C57BL/6 mice develop strong nAb titers between day 20 and day 30, causing virus clearance from the blood (3, 15, 16). However, nAb-mediated virus clearance is often only transient as LCMV-WE escape mutants emerge, which have acquired single point mutations in the GP-1 subunit of the glycoprotein spike (15, 16).
Taken together, these findings lead to the hypothesis that the accelerated and increased generation of GP-1A-specific nAbs in CTL-deficient mice was due to antigen-driven antibody (Ab) affinity maturation by somatic hypermutation (3, 22). This process is thought to be impaired by CTL-caused immunopathology in wt mice (1, 5, 29, 31, 33), where the destruction of infected follicular dendritic cells, marginal zone macrophages and organized follicles in lymphoid organs leads to immunosuppression (5, 18, 33) and delay of specific Ab responses (1, 29, 31).
The hypothesis presented above would predict that low-affinity, non-neutralizing GP-1A binding Abs are induced within the first days after the infection in wt and in CTL-deficient mice. By expressing recombinant, correctly folded GP-1 and using it in a binding assay, we found that the nAb response is indeed preceded by the emergence of non-neutralizing GP-1A-specific Abs in high-dose-infected wt mice and in CTL-deficient mice. Unexpectedly, we found no GP-1A-specific Abs at all in low-dose-infected wt mice, whereas GP-2-specific Abs were readily detected. Taken together, these results indicate that the low frequency and affinity of GP-1A-specific naive B cells, together with CTL-mediated immunopathology is responsible for the delayed and weak nAb response in wt mice.
MATERIALS AND METHODS
Mice.
All mice were bred and maintained in specific pathogen-free conditions, and experiments were done in accordance with the institutional and Swiss guidelines. Cd8a−/− mice were on a C57BL/6 background. All mice were obtained from the Institut für Labortierkunde Zürich-Irchel, Zürich, Switzerland.
In vivo depletion of CD8+ T cells.
C57BL/6 mice were treated intraperitoneally with rat α-CD8 monoclonal Ab (YTS 169.4) ascites fluids at day −3 and day −1 prior to LVMV-WE infection. This treatment lead to the elimination of > 95% of CD8+ T cells from spleen and lymph nodes (28).
Virus.
The LCMV-WE strain was originally obtained from F. Lehmann-Grube (Heinrich Pette Institut, Hamburg, Germany) and was propagated in L929 mouse fibroblast cells. Mice were infected intravenously (i.v.) either with a low dose (200 PFU) or a high dose (2 × 106 PFU) of LCMV-WE.
Abs.
A hybridoma cell line producing KL 25 was originally obtained from F. Lehmann-Grube (Heinrich Pette Institut, Hamburg, Germany) (8). The KL 25 hybridoma was generated from a BALB/c mouse immunized i.v. with 106 PFU of LCMV-WE and boosted intraperitoneally with purified glycoprotein from 109 PFU LCMV-WE. Fusion was carried out 5 weeks after the initial immunization. Hybridomas producing Wen 1, Wen 3, and Wen 4 were established by this laboratory (2, 36). Wen 1 was generated from a Cd8a−/− C57BL/6 mouse immunized i.v. with 106 PFU LCMV-WE, boosted intraperitoneally with 5 μg of purified virus at day 44 before being sacrificed at day 48. Wen 3 was generated from a Cd8a−/− C57BL/6 mouse immunized i.v. with 106 PFU LCMV-WE, boosted two times i.v. with 106 PFU of LCMV-WE at days 66 and 69 before being sacrificed at day 73. Wen 4 was generated from an α-CD8 monoclonal Ab-treated BALB/c mouse immunized i.v. with 106 PFU of LCMV-WE, boosted two times i.v. with 106 PFU of LCMV-WE at days 66 and 69 before being sacrificed at day 73. Ascites fluids of the Abs WE 18.8, WE 83.4, and WE 11.4 were a generous gift from M. J. Buchmeier (Scripps Research Institute, La Jolla, CA) and have been characterized in a comprehensive epitope-mapping study (10, 34). Detection of the Abs was performed with horseradish peroxidase (HRP)-coupled α-mouse immunoglobulin G (IgG) isotype-specific Abs (Zymed, San Francisco, CA).
Generation of the GP-1-IgG expression plasmid.
A sequence corresponding to the GP-1 sequence (amino acids 1 to 265) was amplified by PCR. In this way, NheI and XbaI restriction sites were introduced. After sequencing, PCR products were cloned into the vector pCMV FC B7H (gift from Cytos Biotechnology AG, Schlieren, Switzerland), replacing the original B7H sequence and thus fusing the GP-1 sequence at its 5′ end to human IgG1 Fc(γ). The assembled GP-1-IgG fusion sequence was excised using NheI and HpaI and was blunt end ligated into the expression plasmid pHCMV-glycoprotein (WE) (AJ318513). In this way the wt LCMV-WE glycoprotein sequence (4), which had been excised with BamHI, was replaced. The expression vector pHCMV contains the human cytomegalovirus immediate-early promoter, the rabbit beta-globin intron B, and the rabbit beta-globin polyadenylation sequence, as well as an ampicillin resistance gene.
Expression of the GP-1-IgG fusion protein.
Human embryonic kidney (HEK) T 293 cells were simultaneously calcium phosphate transfected with the GP-1-IgG expression plasmid and with the plasmid pHygEGFP (DB Biosciences Clontech), carrying a hygromycin resistance gene for selection. Transfected cells were grown adherently for 3 days in nonselective Dulbecco modified Eagle medium (Invitrogen) containing 10% fetal calf serum (FCS), before the addition of 0.3 mg of hygromycin B (Roche)/ml. After 1 week of selection the dead cells were removed, whereas adherent cells were treated with trypsin and transferred to a new flask. Cells were expanded for 2 weeks before being subcloned in 96-well plates. Cell supernatants were screened for secreted GP-1-IgG fusion protein by enzyme linked immunosorbent assay (ELISA). In brief, 96-well plates were coated with goat α-human IgG Fc(γ) Ab (Jackson Immunoresearch) diluted 1:800 in 0.1 M sodium carbonate buffer (pH 9.6). Afterwards plates were blocked with 5% milk in phosphate-buffered saline containing 0.05% Tween (PBS-T). Then, 100 μl of cell supernatant was transferred to the coated plate and incubated for 1 h at room temperature before being washed five times with PBS-T. Bound GP-1-IgG was detected either with HRP-coupled rabbit α-human IgG Fc(γ) Ab (Jackson Immunoresearch) or with KL 25 (5 μg/ml) and HRP-coupled rabbit α-mouse IgG1 Ab (Zymed). Large-scale production was carried out in triple-layer tissue culture flasks. Cells were expanded in Dulbecco modified Eagle medium with 10% FCS until they were confluent. For production cells were washed with PBS and kept in FCS-free InVitrus medium (Cell Culture Technologies GmbH, Gravesano, Switzerland) supplemented with 0.5 mM sodium butyrate (Fluka). GP-1-IgG-containing cell supernatant was collected twice, after 2 and 4 weeks.
Purification and gel analysis of GP-1-IgG.
GP-1-IgG-containing cell supernatant was filtered through 0.2-μm-pore-size filter devices (TPP AG, Trasadingen, Switzerland) and passed over a protein A affinity column (Amersham Biosciences). Bound GP-1-IgG was eluted with 0.1 M glycine buffer (pH 2.7) and was neutralized with 1 M Tris-HCl (pH 7.0). Eluted GP-1-IgG was dialyzed against PBS using 50-kDa-molecular-mass cutoff membranes (Spectrum Laboratories, Inc., Rancho Dominguez, CA), and the final protein concentration was determined by using a BCA assay (Pierce).
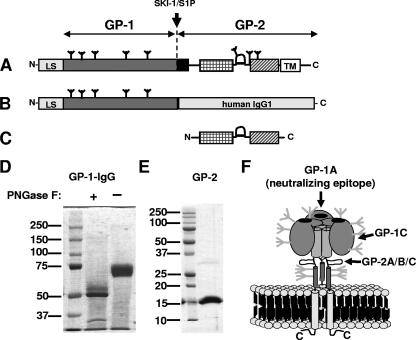
Purified protein was analyzed glycosylated or fully deglycosylated by Coomassie gel analysis. Native GP-1-IgG was completely deglycosylated by digestion with N-glycosidase F (PNGase F) (New England Biolabs) for a total of 96 h at room temperature (shaking at 200 rpm). Initially, 500 U of enzyme was added to 100 μg of purified GP-1-IgG. An additional 500 U was added after 24 h and another 1,000 U was added after 48 h. A total of 5 μg of glycosylated or fully deglycosylated GP-1-IgG was separated by sodium dodecyl sulfate-8% polyacrylamide gel electrophoresis (SDS-8% PAGE). After separation, the gel was fixed in 7% acetic acid-50% methanol and stained with GelCode Blue staining reagent (Pierce).
GP-1-IgG ELISA.
The ELISA was carried out in 96-well polystyrene plates. Plates were coated overnight at 4°C with goat α-human IgG Fc capturing Ab (Jackson Immunoresearch) diluted 1:800 in 0.1 M sodium carbonate buffer (pH 9.6). Afterward, the plates were blocked for 3 h with 5% milk diluted in PBS-T. Thereafter, the plates were incubated with 100 μl per well of GP-1-IgG-containing cell supernatant for 1 h. On a parallel plate serum samples were prediluted 1:8 in PBS-T and a threefold dilution series was performed. A total of 50 μl per well of the diluted serum samples was transferred to the GP-1-IgG-saturated plates, followed by incubation for 1 h. Finally, the plates were incubated for 45 min with HRP-coupled rabbit α-mouse IgG Ab (Zymed) diluted 1:1,000 in PBS-T. HRP was detected by using an ABTS [2,2′azinobis(3-ethylbenzthiazolinesulfonic acid)] color reaction (Roche). All steps were carried out at room temperature. Between each step the plates were washed five times with PBS-T. Titers represent double-above-background values.
Nonspecific binding of serum Abs to the human Fc part of the GP-1-IgG fusion protein was controlled for by two unrelated IgG fusion proteins. Nonspecific binding was never observed (data not shown). As an additional control, we also analyzed sera of C57BL/6 mice infected with vesicular stomatitis virus. Again, no binding to GP-1-IgG was observed (data not shown). Taken together, the controls verified that the GP-1-IgG ELISA exclusively detects GP-1-specific Abs in the sera of LCMV-WE-infected mice.
Expression, purification, and refolding of the GP-2 ectodomain.
Expression, purification, and refolding of the GP-2 ectodomain was described previously (19). In short, a sequence coding for amino acids 312 to 435 of the LCMV-WE glycoprotein with a C316S point mutation was expressed in the cytoplasm of Escherichia coli. The recombinant protein contained in inclusion bodies was separated from the cell lysate by centrifugation, resolved in 8 M urea, and purified by nickel-nitrilotriacetic acid affinity chromatography (Amersham Biosciences). Purified urea denatured protein was refolded by a 1:100 drop-by-drop dilution in ice-cold refolding buffer while stirring. After 48 h of continuous stirring at 4°C, the refolded protein was concentrated by using an Amicon stirred cell (Millipore Corp., Billerica, MA). Precipitated protein was removed by centrifugation, and the supernatant was applied to a Superdex 75 gel filtration column, thereby exchanging the buffer to PBS (pH 7.0).
GP-2 ELISA.
Ninety-six-well polystyrene plates were coated overnight at 4°C with refolded GP-2 at a concentration of 3 μg/ml in PBS (pH 7.0). Afterward, the plates were blocked for 3 h with 5% milk diluted in PBS-T. On a parallel plate serum samples were prediluted 1:8 with PBS-T, and a threefold dilution series was performed. A total of 50 μl per well of the diluted serum samples was transferred to the GP-2-coated plates, followed by incubation for 1 h. Finally, the plates were incubated for 45 min with HRP-coupled rabbit α-mouse IgG Ab (Zymed) diluted 1:1,000 in PBS-T. HRP was detected by using the ABTS color reaction. All steps were carried out at room temperature. Between each step the plates were washed five times with PBS-T. Titers represent double-above-background values.
Epitope mapping on recombinantly expressed GP-1-IgG and refolded GP-2.
To characterize all epitopes present on GP-1-IgG and on the refolded GP-2 ectodomain, ELISAs according to the protocols described above were performed. Instead of serum, monoclonal Abs were used. The Abs KL 25, Wen 1, Wen 3, and Wen 4 were applied as purified Abs, whereas the Abs WE 18.8, WE 83.4, and WE 11.4 were applied as ascites fluids. All Abs were detected either with HRP-coupled rabbit α-mouse IgG Ab (Zymed) or with HRP-coupled rabbit α-mouse IgG isotype-specific Abs (Zymed).
NP ELISA.
Detection of nucleoprotein (NP)-specific serum Abs was carried out in a manner similar to a previously published protocol (27). Instead of coating the plates with a NP containing cell lysate, the NP-specific capturing Ab VL-4 was used: 96-well polystyrene plates were coated with VL-4 at 5 μg/ml diluted in 0.1 M sodium carbonate buffer (pH 9.6). Plates were blocked for 3 h with 5% milk (200 μl per well) in PBS-T and were incubated for 1 h with the cell lysate containing 4 μg of recombinant NP/ml. On a parallel plate serum samples were prediluted 1:8 with PBS-T, and a threefold dilution series was performed. A total of 50 μl per well of the diluted serum samples was transferred to the NP-saturated plate, followed by incubation for 1 h. Finally, the plates were incubated for 45 min with HRP-coupled rabbit α-mouse IgG Ab (Zymed) diluted 1:1,000 in PBS-T. HRP was detected by using the ABTS color reaction. All steps were carried out at room temperature. Between each step the plates were washed five times with PBS-T.
KL 25 competition ELISA.
To show that the GP-1-specific Abs induced in the LCMV-WE-infected C57BL/6 mice were binding to the neutralizing GP-1A epitope on GP-1, we performed a competition ELISA with the nAb KL 25. For this reason, 96-well polystyrene plates were coated overnight at 4°C with a goat α-human IgG Fc Ab (Jackson Immunoresearch) diluted 1:800 in 0.1 M sodium carbonate buffer (pH 9.6). Thereafter, the plates were blocked for 3 h with 5% milk diluted in PBS-T, before being incubated with GP-1-IgG-containing cell supernatant for 1 h. GP-1-IgG-saturated plates were preincubated for 1 h with KL 25 (50 μl per well) at a concentration of 5 μg/ml (or with PBS) before an additional 50 μl of a 1:20 prediluted serum sample per well was added. Competition lasted for 1 h, before bound serum IgG was detected with a mixture of HRP-coupled α-mouse IgG2a, IgG2b, and IgG3 isotype-specific Abs (Zymed). Detection of bound serum IgG1 was excluded, since the competing Ab KL 25 was a mouse IgG1 Ab. HRP was detected by using the ABTS color reaction. All steps were carried out at room temperature. Between each step the plates were washed five times with PBS-T.
To control for specific inhibition by KL 25, we used as negative control mouse myeloma IgG1 (Zymed) at a concentration of 10 μg/ml.
LCMV-WE neutralization assay.
Neutralizing serum titers were determined in a focus-forming assay according to a previously published protocol (2, 3). To increase the sensitivity of the assay, sera were prediluted 1:4 instead of 1:10. In brief, sera were prediluted 1:2 in PBS and again 1:2 in modified Eagle medium supplemented with 5% FCS before a twofold dilution series was performed. Diluted serum samples were UV irradiated and then preincubated for 90 min with 60 PFU of LCMV-WE per well at 37°C. Thereafter, MC57G mouse fibroblast cells were added to each well, and the cells were evenly distributed on a plate shaker. After the cells had adhered (2 to 3 h later), 1% methylcellulose was added, and the cells were put into the incubator for 48 h. Once the cells were confluent, they were fixed with 4% formaldehyde in PBS before being permeabilized with 1% Triton X-100. Foci were visualized by staining for the NP with the Ab VL-4. Titers represent half-maximal inhibition values.
To demonstrate that serum mediated neutralization was completely caused by serum Abs, we performed additional controls (data not shown). Neutralizing serum samples of individual mice were separated by gel filtration chromatography; the total serum IgG was purified and tested in the neutralization assay. The total purified serum IgG always neutralized the virus to the same extent as the original serum sample, thus demonstrating that no other serum factors or proteins contributed to the measured neutralization.
RESULTS AND DISCUSSION
Recombinant GP-1 and GP-2 contain all known epitopes of native glycoprotein spikes.
To measure GP-1- and GP-2-specific Ab responses in LCMV-WE-infected mice, we established two novel ELISAs using the recombinantly expressed GP-1 subunit and the GP-2 ectodomain of the LCMV-WE glycoprotein (Fig. 1A to C). Since folding of the GP-1 subunit depends on N-linked glycosylation (40), the GP-1 subunit was expressed in mammalian cells as GP-1-IgG fusion protein (Fig. 1B and D). Secreted GP-1-IgG was fully glycosylated, as judged by PNGase F digestion (Fig. 1D). Purified GP-1-IgG was captured on ELISA plates and the presence of various epitopes was tested with a panel of well-characterized monoclonal Abs (Table 1 and Fig. 1F). The neutralizing GP-1A epitope was probed with the neutralizing Abs KL 25, Wen 1, Wen 3, and Wen 4; the GP-1C epitope was probed with the Ab WE 18.8, and the GP-2A/B/C epitope was probed with the Abs WE 83.4 and WE 11.4. We found that GP-1-IgG contained the conformational GP-1A and the linear GP-1C epitopes and thus was properly folded (Table 1). Pairwise competition experiments between KL 25, Wen 1, Wen 3, and Wen 4 confirmed that these Abs bound to a single site (data not shown). This finding was consistent with a previous report stating that only a single neutralizing epitope GP-1A exists on GP-1 (34, 40).
FIG. 1.
Recombinant expression of GP-1 and of the GP-2 ectodomain of the LCMV-WE glycoprotein. (A) Schematic representation of the full-length wt LCMV-WE glycoprotein. The LCMV-WE glycoprotein is translated as single precursor protein into the lumen of the endoplasmic reticulum and is subsequently cleaved in the Golgi compartment by the cellular protease SKI1/S1P. Cleavage yields the transmembrane subunit GP-2 and the peripheral subunit GP-1. Several features within the GP-2 sequence are indicated: a potential fusion peptide (in black), a trimeric coiled-coil region, a hypothetical disulfide-bonded loop region, and an α-helical region prior to the transmembrane region (TM). At the N terminus the leader sequence (LS) is shown. N-linked glycans are indicated as trees. (B) Sequence of the GP-1-IgG fusion protein expressed in mammalian cells. (C) Sequence of the GP-2 ectodomain expressed in E. coli. (D) SDS-PAGE gel analysis of reduced GP-1-IgG. The sample was either fully glycosylated (lane 2) or PNGase F deglycosylated (lane 1). (E) SDS-PAGE of the reduced and denatured GP-2 ectodomain purified from inclusion bodies. (F) Model of the LCMV glycoprotein spike (19) showing identified surface epitopes (34): the receptor-binding subunit GP-1 contains the only neutralizing epitope (GP-1A). Neutralizing Abs binding to the GP-1A site prevent attachment of the virus to host cells (6). GP-1A partly overlaps with the non-neutralizing epitope GP-1B (not shown). Besides the GP-1A/B epitope there is an additional minor non-neutralizing linear epitope on GP-1 (GP-1C). The GP-2 subunit contains a single non-neutralizing linear epitope, formed by the overlapping epitopes GP-2A, GP-2B and GP-2C. The GP-2A/B/C epitope was shown to be located in a hypothetical disulfide-bonded loop region comprising amino acids 370 to 378 (39).
TABLE 1.
Epitope mapping of recombinant GP-1-IgG and GP-2
| Epitope | Antibodya | Dilution factor at half-maximal binding value
|
Neutralization titer (IC50)b | |
|---|---|---|---|---|
| GP-1-IgG | GP-2 | |||
| GP-1A | KL 25 | 1:5,555 | >1 | 1:714 |
| Wen 1 | 1:125 | >1 | 1:833 | |
| Wen 3 | 1:666 | >1 | 1:833 | |
| Wen 4 | 1:285 | >1 | 1:313 | |
| GP-1C | WE 18.8 | 1:40,960 | >1 | >1 |
| GP-2A/B/C | WE 83.4 | >1 | 1:163,840 | >1 |
| WE 11.4 | >1 | 1:81,920 | >1 | |
*, KL 25, Wen 1, Wen 3, and Wen 4 were applied as purified antibodies at a starting concentration of 1 mg/ml. WE 18.8, WE 83.4, and WE 11.4 were applied as ascites fluids at unknown starting concentrations.
IC50, 50% inhibitory concentration (as determined in a focus-forming assay).
Other than the glycosylated GP-1 subunit, the GP-2 ectodomain (Fig. 1C) was purified from E. coli inclusion bodies and refolded as described previously (19) (Fig. 1E). Refolded GP-2 trimers were directly bound to ELISA plates and were probed with the Abs mentioned above (Table 1). None of the GP-1-specific Abs but all GP-2-specific Abs detected recombinant GP-2, demonstrating that the linear GP-2A/B/C epitope was accessible.
In summary, the epitope mapping demonstrated that none of the tested epitopes did rely on native glycoprotein trimer assembly. Thus, applying the recombinant subunits in ELISA enabled us to study the emergence of binding Abs against GP-1 and GP-2 and in particular against the neutralizing GP-1A epitope. By combining ELISA and neutralization assays, the development of binding versus neutralizing GP-1A-specific Abs in wt and CTL-deficient mice after infection with different LCMV-WE doses was compared.
C57BL/6 mice infected with a low dose (200 PFU) of LCMV-WE induce a strong Ab response against GP-2 and the NP but not against GP-1.
Previous studies demonstrated that C57BL/6 mice infected with 200 PFU of LCMV-WE failed to develop an nAb response (37). Therefore, we analyzed these mice for GP-1-specific Ab responses. C57BL/6 mice were i.v. infected with 200 PFU of LCMV-WE, and blood was collected during a period of about 100 days. Sera were analyzed by ELISA for GP-1-, GP-2-, and NP-specific Abs (Fig. 2A, B, and C). In addition, the serum was tested for its potential to neutralize LCMV-WE in a focus-forming assay. Since neutralization is known to be solely mediated by GP-1A-specific Abs, the results of the GP-1-specific Ab ELISA and of the neutralization assay are presented in the same diagram (see Fig. 2A).
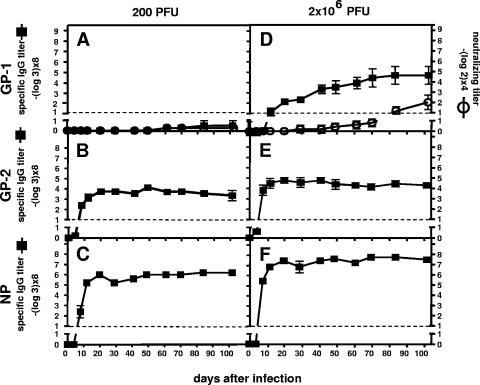
FIG. 2.
C57BL/6 mice infected with a low dose (200 PFU) of LCMV-WE fail to induce GP-1-specific Abs, whereas high-dose (2 × 106 PFU)-infected mice induce a GP-1-specific Ab response, which fails to neutralize the virus. C57BL/6 mice were i.v. infected with 200 (A to C) or with 2 × 106 PFU (D to F) of LCMV-WE, and blood was collected for a period of about 100 days. Sera were analyzed for their LCMV-WE neutralization potential in a focus-forming assay (A and D) and for virus-specific Ab responses. GP-1-specific IgG titers were detected by ELISA using recombinant GP-1-IgG (A and D), GP-2-specific IgG titers were detected by ELISA using refolded recombinant GP-2 (B and E), and NP-specific IgG titers were detected by ELISA using recombinant NP (C and F). Titers represent twofold above background values. The data are expressed as means ± the standard deviation (SD) of sera from five mice per group and are representative of three separate experiments.
As expected, no measurable serum neutralization of LCMV-WE was detected (Fig. 2A). No GP-1-specific Abs were induced, whereas high GP-2- and NP-specific Ab titers were measured (Fig. 2B and C). This finding indicated that GP-1-specific naive B cells were rare compared to GP-2- and NP-specific naive B cells and could not be activated by the low virus dose used.
Since C57BL/6 mice infected with a high dose (2 × 106 PFU) instead of 200 PFU have been reported to develop weak nAb responses after 2 to 3 months (16, 22), we next investigated whether high dose infection induced GP-1-specific Abs.
C57BL/6 mice infected with a high dose (2 × 106 PFU) of LCMV-WE induce a GP-1-specific Ab response, which initially fails to neutralize the virus.
C57BL/6 mice were i.v. infected with 2 × 106 PFU of LCMV-WE, and blood was taken during a period of about 100 days. Sera were tested for GP-1-specific Abs and for neutralizing capacity (Fig. 2D).
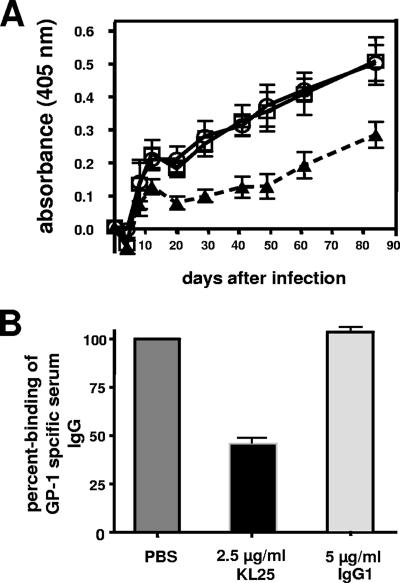
All animals developed a pronounced GP-1-specific Ab response, which was detected as early as day 12. In contrast, no neutralizing activity was detected before day 80. To test whether the induced GP-1-specific Abs were binding to the neutralizing GP-1A epitope, we performed a competition ELISA with the neutralizing Ab KL 25 (Fig. 3). We found that KL 25 caused a ∼55% reduction of the polyclonal serum Abs binding to GP-1, indicating that about half of the GP-1 binding serum Abs were directed to the neutralizing GP-1A epitope. Thus, the failure of the GP-1-specific serum Abs to neutralize the virus was not due to a different epitope specificity but rather to their low affinity.
FIG. 3.
GP-1-specific Abs induced in high-dose (2 × 106 PFU) LCMV-WE-infected C57BL/6 mice are predominantly directed against the neutralizing GP-1A epitope. (A) C57BL/6 mice were i.v. infected with 2 × 106 PFU of LCMV-WE, and blood was collected for a period of about 80 days. GP-1-IgG-coated ELISA plates were preincubated for 1 h with the nAb KL 25 (▴), with nonspecific mouse myeloma IgG1 (○), or with buffer (□). Thereafter, serum samples were added, and the plates were incubated for 1 h. Finally, plates were washed, and bound polyclonal serum Abs were detected with a mixture of HRP-coupled α-mouse IgG2a, IgG2b, and IgG3 isotype-specific Abs. HRP-coupled α-mouse IgG1 isotype-specific Ab was excluded, since the competing Ab KL 25 is a mouse monoclonal IgG1. The data are expressed as means ± the SD of sera from five mice per group and are representative of two separate experiments. (B) Average percent binding of polyclonal serum IgG to GP-1-IgG-coated ELISA plates, which were preincubated with KL 25, mouse myeloma IgG1, or buffer (PBS). The signals of the buffer control were defined as 100% binding. The percent binding values represent the average of all time points shown in panel A.
Since high-dose infection induced GP-1-specific Abs, whereas low-dose infection failed to do so (compare Fig. 2A and D), we sought to determine whether the titers of GP-2- and NP-specific Abs were similarly dependent on the initial virus dose. Indeed, high-dose-infected mice exhibited threefold-higher GP-2- and NP-specific Ab titers compared to low-dose-infected mice (compare Fig. 2E and B and Fig. 2F and C).
Taken together, the results demonstrated that Ab titers against all viral proteins were increased if C57BL/6 mice were infected with 2 × 106 PFU instead of 200 PFU of LCMV-WE. Importantly, GP-1-specific Abs binding to the neutralizing GP-1A epitope, which could only be induced after high-dose infection, were of low affinity and thus unable to neutralize the virus. However, weak nAb titers were detected by day 80, suggesting that the early GP-1A binding Abs slowly developed into nAbs (Fig. 2D).
CTL-deficient mice infected with a low (200 PFU) or high (2 × 106 PFU) dose of LCMV-WE induce high GP-1-specific Ab titers and develop a strong nAb response early after infection.
CD8+-T-cell depleted or Cd8a−/− mice were i.v. infected with either 200 PFU or 2 × 106 PFU of LCMV-WE, and blood was taken during a period of about 100 days. Sera were analyzed for GP-1-specific Abs and for neutralizing capacity (Fig. 4).
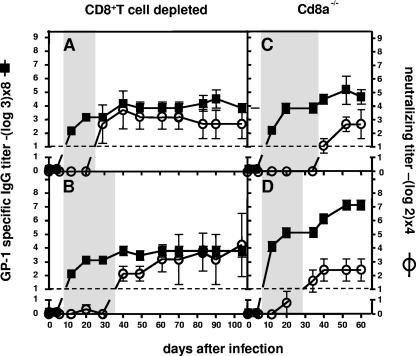
FIG. 4.
CTL-deficient C57BL/6 mice infected with a low or high dose of LCMV-WE alike produce increased GP-1-specific Ab titers and develop early and strong nAb responses. CD8+-T-cell-depleted (A and B) or Cd8a−/− (C and D) C57BL/6 mice were i.v. infected with 200 PFU (A and C) or with 2 × 106 PFU (B and D) of LCMV-WE, and blood was collected over a time period of about 100 days. Sera were analyzed for their neutralization potential and for GP-1-specific Ab titers. GP-1-specific IgG titers were detected by ELISA using recombinant GP-1-IgG. The shaded areas indicate the interval that elapses between the initial detection of GP-1-specific IgG (day 12) and the detection of serum-mediated neutralization (days 30 to 40). The data are expressed as means ± the SD of the sera of five mice per group and are representative of two separate experiments.
Consistent with previous reports, all animals developed strong nAb responses by day 35, irrespective of the dose of infection with low (Fig. 4A and C) or high (Fig. 4B and D) doses of LCMV-WE. In addition, we found that all mice developed similar GP-1-specific Ab responses, which reached maximal titers by day 20 (Fig. 4A to D). GP-1-specific IgG concentrations at day 20 reached up to 0.085 mg/ml and were increased at least threefold compared to high-dose-infected wt mice (compare Fig. 4 and Fig. 2D). Binding of polyclonal GP-1-specific serum Abs was inhibited more than 50% by the nAb KL 25 (data not shown), demonstrating that GP-1-specific serum Abs were predominantly binding to the neutralizing GP-1A epitope.
The results obtained for the CTL-deficient mice showed that initially only non-neutralizing GP-1A binding Abs could be induced similar to high-dose-infected wt mice. The finding that GP-1-specific Ab titers in CTL-deficient mice did not correlate with the injected virus dose can be explained by the fact that a massive viremia quickly levels initial differences in viral load (15, 16, 25, 30). In clear contrast to wt mice, all CTL-deficient mice developed high nAb titers 3 weeks after the initial onset of the non-neutralizing GP-1A-specific IgG response (compare Fig. 4 and Fig. 2D).
In summary, our data suggest that LCMV-WE evades an nAb response in wt C57BL/6 mice by exploiting a limited B-cell repertoire in combination with CTL-mediated immunosuppression: the murine B-cell repertoire appears to have only low frequencies of GP-1A-specific B cells, which are activated only by high virus doses. If activated, these early, germ line-encoded GP-1A-specific Abs initially fail to neutralize the virus due to their low affinity, regardless of their high serum concentration. Consequently, extensive Ab affinity maturation appears to be necessary to eventually generate LCMV nAbs capable of irreversibly blocking the receptor-binding site by binding GP-1A. In wt C57BL/6 mice this latter process seems severely impaired by the massive CTL response, which causes destruction of organized lymphoid follicles (33) and results in immunosuppression of specific Ab responses 6 days and later after infection (1, 29, 31).
LCMV's twofold strategy of avoiding an effective humoral immune response is likely to result from coevolution between the virus and its host, as suggested by many studies (7, 13, 14). The continuous selection pressure by the murine humoral immune response seems to have primarily made an impact on the LCMV glycoprotein spike, since a recombinant vesicular stomatitis virus expressing the LCMV-WE glycoprotein elicits the same delayed and weak Ab response as had wt LCMV-WE (35). This also is supported by the structural similarities shared between the LCMV-WE glycoprotein and other class I glycoprotein spikes, such as influenza virus hemagglutinin or human immunodeficiency virus gp160, which also face a continuous selection pressure by nAbs (11, 38). Among these are the extensive N-linked glycosylation (41) and the presence of highly variable regions within the receptor binding subunit (15, 16, 24, 37). In the case of LCMV-WE, the importance of N-linked glycans in shielding sensitive epitopes has been demonstrated (24, 40); the variable regions identified in the GP-1 subunit most likely represent small surface loops surrounding the conserved carbohydrate binding site. Mutations at these sites would allow abrogation of nAb binding without affecting receptor binding. Future work on the structure of the LCMV glycoprotein spike will be needed to explain how the GP-1 subunit has managed to minimize Ab binding and thereby avoid early neutralization.
Acknowledgments
We thank Michael J. Buchmeier for providing the Abs WE 11.4, WE 18.8, and WE 83.4; Dorothee von Laer for providing the codon optimized LCMV-WE glycoprotein sequence; and Winfried Beyer for the plasmid pHCMV-GP (WE) (AJ318513).
This study was supported by the Swiss National Science Foundation.
Footnotes
Published ahead of print on 15 August 2007.
REFERENCES
- 1.Althage, A., B. Odermatt, D. Moskophidis, T. Kundig, U. Hoffman-Rohrer, H. Hengartner, and R. M. Zinkernagel. 1992. Immunosuppression by lymphocytic choriomeningitis virus infection: competent effector T and B cells but impaired antigen presentation. Eur. J. Immunol. 22:1803-1812. [DOI] [PubMed] [Google Scholar]
- 2.Battegay, M., S. Cooper, A. Althage, J. Banziger, H. Hengartner, and R. M. Zinkernagel. 1991. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J. Virol. Methods 33:191-198. [DOI] [PubMed] [Google Scholar]
- 3.Battegay, M., D. Moskophidis, H. Waldner, M. A. Brundler, W. P. Fung-Leung, T. W. Mak, H. Hengartner, and R. M. Zinkernagel. 1993. Impairment and delay of neutralizing antiviral antibody responses by virus-specific cytotoxic T cells. J. Immunol. 151:5408-5415. [PubMed] [Google Scholar]
- 4.Beyer, W. R., H. Miletic, W. Ostertag, and D. von Laer. 2001. Recombinant expression of lymphocytic choriomeningitis virus strain WE glycoproteins: a single amino acid makes the difference. J. Virol. 75:1061-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow, P., C. F. Evans, and M. B. Oldstone. 1995. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J. Virol. 69:1059-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borrow, P., and M. B. Oldstone. 1992. Characterization of lymphocytic choriomeningitis virus-binding protein(s): a candidate cellular receptor for the virus. J. Virol. 66:7270-7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowen, M. D., C. J. Peters, and S. T. Nichol. 1997. Phylogenetic analysis of the Arenaviridae: patterns of virus evolution and evidence for cospeciation between arenaviruses and their rodent hosts. Mol. Phylogenet. Evol. 8:301-316. [DOI] [PubMed] [Google Scholar]
- 8.Bruns, M., J. Cihak, G. Muller, and F. Lehmann-Grube. 1983. Lymphocytic choriomeningitis virus. VI. Isolation of a glycoprotein mediating neutralization. Virology 130:247-251. [DOI] [PubMed] [Google Scholar]
- 9.Buchmeier, M. J., M. D. Bowen, and C. J. Peters. 2001. Arenaviridae: the viruses and their replication, 4th ed., vol. 2. Lippincott/The Williams & Wilkins Co., Philadelphia, PA.
- 10.Buchmeier, M. J., H. A. Lewicki, O. Tomori, and M. B. Oldstone. 1981. Monoclonal antibodies to lymphocytic choriomeningitis and pichinde viruses: generation, characterization, and cross-reactivity with other arenaviruses. Virology 113:73-85. [DOI] [PubMed] [Google Scholar]
- 11.Burton, D. R., R. L. Stanfield, and I. A. Wilson. 2005. Antibody versus HIV in a clash of evolutionary titans. Proc. Natl. Acad. Sci. USA 102:14943-14948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao, W., M. D. Henry, P. Borrow, H. Yamada, J. H. Elder, E. V. Ravkov, S. T. Nichol, R. W. Compans, K. P. Campbell, and M. B. Oldstone. 1998. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 282:2079-2081. [DOI] [PubMed] [Google Scholar]
- 13.Charrel, R. N., J. J. Lemasson, M. Garbutt, R. Khelifa, P. De Micco, H. Feldmann, and X. de Lamballerie. 2003. New insights into the evolutionary relationships between arenaviruses provided by comparative analysis of small and large segment sequences. Virology 317:191-196. [DOI] [PubMed] [Google Scholar]
- 14.Childs, J. E., and C. J. Peters. 1996. The Arenaviridae: ecology and epidemiology of arenaviruses and their hosts. Plenum Press, Inc., New York, NY.
- 15.Ciurea, A., L. Hunziker, P. Klenerman, H. Hengartner, and R. M. Zinkernagel. 2001. Impairment of CD4+ T-cell responses during chronic virus infection prevents neutralizing antibody responses against virus escape mutants. J. Exp. Med. 193:297-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciurea, A., P. Klenerman, L. Hunziker, E. Horvath, B. M. Senn, A. F. Ochsenbein, H. Hengartner, and R. M. Zinkernagel. 2000. Viral persistence in vivo through selection of neutralizing antibody-escape variants. Proc. Natl. Acad. Sci. USA 97:2749-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole, G. A., N. Nathanson, and R. A. Prendergast. 1972. Requirement for theta-bearing cells in lymphocytic choriomeningitis virus-induced central nervous system disease. Nature 238:335-337. [DOI] [PubMed] [Google Scholar]
- 18.Doyle, M. V., and M. B. Oldstone. 1978. Interactions between viruses and lymphocytes. I. In vivo replication of lymphocytic choriomeningitis virus in mononuclear cells during both chronic and acute viral infections. J. Immunol. 121:1262-1269. [PubMed] [Google Scholar]
- 19.Eschli, B., K. Quirin, A. Wepf, J. Weber, R. Zinkernagel, and H. Hengartner. 2006. Identification of an N-terminal trimeric coiled-coil core within arenavirus glycoprotein 2 permits assignment to class I viral fusion proteins. J. Virol. 80:5897-5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallaher, W. R., C. DiSimone, and M. J. Buchmeier. 2001. The viral transmembrane superfamily: possible divergence of arenavirus and filovirus glycoproteins from a common RNA virus ancestor. BMC Microbiol. 1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallimore, A., A. Glithero, A. Godkin, A. C. Tissot, A. Pluckthun, T. Elliott, H. Hengartner, and R. Zinkernagel. 1998. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 187:1383-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hangartner, L., B. M. Senn, B. Ledermann, U. Kalinke, P. Seiler, E. Bucher, R. M. Zellweger, K. Fink, B. Odermatt, K. Burki, R. M. Zinkernagel, and H. Hengartner. 2003. Antiviral immune responses in gene-targeted mice expressing the immunoglobulin heavy chain of virus-neutralizing antibodies. Proc. Natl. Acad. Sci. USA 100:12883-12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotchin, J., L. Benson, and E. Sikora. 1969. The detection of neutralizing antibody to lymphocytic choriomeningitis virus in mice. J. Immunol. 102:1128-1135. [PubMed] [Google Scholar]
- 24.Hunziker, L., A. Ciurea, M. Recher, H. Hengartner, and R. M. Zinkernagel. 2003. Public versus personal serotypes of a viral quasispecies. Proc. Natl. Acad. Sci. USA 100:6015-6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kagi, D., B. Ledermann, K. Burki, P. Seiler, B. Odermatt, K. J. Olsen, E. R. Podack, R. M. Zinkernagel, and H. Hengartner. 1994. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 369:31-37. [DOI] [PubMed] [Google Scholar]
- 26.Kunz, S., N. Sevilla, D. B. McGavern, K. P. Campbell, and M. B. Oldstone. 2001. Molecular analysis of the interaction of LCMV with its cellular receptor α-dystroglycan. J. Cell Biol. 155:301-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyburz, D., D. E. Speiser, M. Battegay, H. Hengartner, and R. M. Zinkernagel. 1993. Lysis of infected cells in vivo by antiviral cytolytic T cells demonstrated by release of cell internal viral proteins. Eur. J. Immunol. 23:1540-1545. [DOI] [PubMed] [Google Scholar]
- 28.Leist, T. P., S. P. Cobbold, H. Waldmann, M. Aguet, and R. M. Zinkernagel. 1987. Functional analysis of T lymphocyte subsets in antiviral host defense. J. Immunol. 138:2278-2281. [PubMed] [Google Scholar]
- 29.Leist, T. P., E. Ruedi, and R. M. Zinkernagel. 1988. Virus-triggered immune suppression in mice caused by virus-specific cytotoxic T cells. J. Exp. Med. 167:1749-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moskophidis, D., S. P. Cobbold, H. Waldmann, and F. Lehmann-Grube. 1987. Mechanism of recovery from acute virus infection: treatment of lymphocytic choriomeningitis virus-infected mice with monoclonal antibodies reveals that Lyt-2+ T lymphocytes mediate clearance of virus and regulate the antiviral antibody response. J. Virol. 61:1867-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moskophidis, D., H. Pircher, I. Ciernik, B. Odermatt, H. Hengartner, and R. M. Zinkernagel. 1992. Suppression of virus-specific antibody production by CD8+ class I-restricted antiviral cytotoxic T cells in vivo. J. Virol. 66:3661-3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neuman, B. W., B. D. Adair, J. W. Burns, R. A. Milligan, M. J. Buchmeier, and M. Yeager. 2005. Complementarity in the supramolecular design of arenaviruses and retroviruses revealed by electron cryomicroscopy and image analysis. J. Virol. 79:3822-3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Odermatt, B., M. Eppler, T. P. Leist, H. Hengartner, and R. M. Zinkernagel. 1991. Virus-triggered acquired immunodeficiency by cytotoxic T-cell-dependent destruction of antigen-presenting cells and lymph follicle structure. Proc. Natl. Acad. Sci. USA 88:8252-8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parekh, B. S., and M. J. Buchmeier. 1986. Proteins of lymphocytic choriomeningitis virus: antigenic topography of the viral glycoproteins. Virology 153:168-178. [DOI] [PubMed] [Google Scholar]
- 35.Pinschewer, D. D., M. Perez, E. Jeetendra, T. Bachi, E. Horvath, H. Hengartner, M. A. Whitt, J. C. de la Torre, and R. M. Zinkernagel. 2004. Kinetics of protective antibodies are determined by the viral surface antigen. J. Clin. Investig. 114:988-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seiler, P., U. Kalinke, T. Rulicke, E. M. Bucher, C. Bose, R. M. Zinkernagel, and H. Hengartner. 1998. Enhanced virus clearance by early inducible lymphocytic choriomeningitis virus-neutralizing antibodies in immunoglobulin-transgenic mice. J. Virol. 72:2253-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seiler, P., B. M. Senn, M. A. Brundler, R. M. Zinkernagel, H. Hengartner, and U. Kalinke. 1999. In vivo selection of neutralization-resistant virus variants but no evidence of B-cell tolerance in lymphocytic choriomeningitis virus carrier mice expressing a transgenic virus-neutralizing antibody. J. Immunol. 162:4536-4541. [PubMed] [Google Scholar]
- 38.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69:531-569. [DOI] [PubMed] [Google Scholar]
- 39.Weber, E. L., and M. J. Buchmeier. 1988. Fine mapping of a peptide sequence containing an antigenic site conserved among arenaviruses. Virology 164:30-38. [DOI] [PubMed] [Google Scholar]
- 40.Wright, K. E., M. S. Salvato, and M. J. Buchmeier. 1989. Neutralizing epitopes of lymphocytic choriomeningitis virus are conformational and require both glycosylation and disulfide bonds for expression. Virology 171:417-426. [DOI] [PubMed] [Google Scholar]
- 41.Wright, K. E., R. C. Spiro, J. W. Burns, and M. J. Buchmeier. 1990. Post-translational processing of the glycoproteins of lymphocytic choriomeningitis virus. Virology 177:175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]