Abstract
We have previously shown that treatment of human cytomegalovirus-infected cells with the cyclin-dependent kinase (cdk) inhibitor roscovitine has significant effects on several stages of the virus life cycle depending on the time of addition (V. Sanchez, A. K. McElroy, J. Yen, S. Tamrakar, C. L. Clark, R. A. Schwartz, and D. H. Spector, J. Virol. 78:11219-11232, 2004; V. Sanchez and D. Spector, J. Virol. 80:5886-5896, 2006). In this report, we add to these findings by demonstrating alterations in the phosphorylation and localization of pp65 (UL83) in cells treated with roscovitine. We observed that inhibition of cdk activity causes the retention of pp65 within the nucleus at late times postinfection. At the same time, we observed a change in the phosphorylation pattern of the protein. Interestingly, mutation of potential cdk phosphorylation sites did not affect the ability of pp65 to localize to the nucleus or to relocalize to the cytoplasm late in infection. However, we found that the cytoplasmic accumulation of pp65 late in infection was sensitive to the Crm1 inhibitor leptomycin B.
Human cytomegalovirus (HCMV), the largest member of the herpesvirus family, is a ubiquitous pathogen that remains the leading viral cause of birth defects (13). Like that of other herpesviruses, HCMV gene expression is temporally regulated (10). The immediate-early (IE) class of genes is expressed shortly after infection, and their expression requires no de novo protein synthesis. The IE genes encode proteins important for the modulation of the apoptotic response to infection (for a review, see reference 4) and for the expression of the early (E) genes, which are necessary for viral DNA replication. The early proteins have also been implicated in the altered expression of key cell cycle proteins during infection (8, 19). Viral DNA synthesis is a prerequisite for the synthesis of the late RNAs, which encode structural components of the virion and proteins that function in virion maturation (10).
Numerous studies have examined the assembly pathways for the herpesviruses (for a review, see reference 9). The current model describes the encapsidation of the viral DNA in the nucleus, followed by egress of subviral particles through the nuclear envelope. This process is thought to occur through an initial envelopment at the inner nuclear membrane and a subsequent de-envelopment step at the outer nuclear membrane. The immature virions are then transported to the final site of envelopment in the cytoplasm. The acquisition of the tegument proteins likely occurs in both the nucleus and the cytoplasm, since the steady-state localization of some HCMV tegument proteins is restricted during infection (1, 7, 22, 26). The distribution of other tegument proteins is temporally regulated (5, 11, 18, 27). The best-studied example of this temporal regulation of virion proteins is the biphasic localization of the abundant tegument protein pp65. As part of the incoming virion, pp65 is targeted to the nucleus immediately after infection (24). Expression of UL83 is an early-late event, and the newly synthesized pp65 is observed in the nucleus until some time after 48 h postinfection (p.i.). Thereafter, pp65 accumulates in the cytoplasm, and the nucleus becomes devoid of the protein (18). The pp65 nuclear localization signals (NLS) have been mapped and are contained in the carboxy-terminal one-third of the protein (6, 24); however, the underlying cause of the relocalization of pp65 to the cytoplasm late in infection has not yet been elucidated.
A recent report has described the aggregation of pp65 in cells infected with an HCMV viral kinase UL97 mutant (15). The aggregation of pp65 in large nuclear and cytoplasmic structures was also detected upon treatment of cells with maribavir, a UL97 inhibitor. Interestingly, redistribution of pp65 to the cytoplasm late in infection was still observed in the absence of UL97 activity. A role for UL97 in the regulation of pp65 self-aggregation was supported by experiments in which transient coexpression of UL97 and an enhanced green fluorescent protein (EGFP)-pp65 fusion protein resulted a diffuse pattern of pp65 expression in the nucleus, as opposed to a punctate nuclear pattern in the absence of UL97. Based on these and other data, the authors concluded that pp65 was a likely substrate for UL97 kinase activity. However, the pp65 sequence contains consensus phosphorylation sites for numerous cellular kinases, including the cyclin-dependent kinases (cdk). In fact, a putative phosphorylation site overlaps the bipartite NLS at the carboxy terminus of pp65 (24). Thus, it is possible that phosphorylation of this site could modulate recognition of the NLS by the nuclear import machinery late in infection, resulting in cytoplasmic accumulation of pp65.
Our lab has previously reported the inhibition of HCMV infection by treatment of cells with the cdk inhibitor roscovitine (20, 21). We observed that the level of inhibition was dependent on the time at which the drug was first added to the infected cultures. If roscovitine was added from the onset of infection, the processing of IE transcripts was altered, expression of specific E proteins and viral DNA replication were inhibited, and the titer was reduced 2 to 3 log units (20). The effects on viral gene expression and DNA replication were not as significant if addition of the drug was delayed until 24 h p.i. (21). In this case, we observed a lag in the expression of several viral proteins, a two- to threefold reduction in replication, and a 1- to 2-log-unit drop in titers. The steady-state levels of pp65 were not significantly affected. In contrast, the expression of the IE2-86 protein was markedly reduced. We also noted that ppUL69 accumulated in a hyperphosphorylated form. The reduced titer produced upon drug treatment was attributed to a reduction in the production of extracellular virus particles, based on a reduced amount of viral DNA in the supernatant. Our data thus suggested that cdk activity was required for the maturation and/or release of infectious virus.
We have extended these preliminary studies on the effect of cdk inhibition on late events in HCMV infection. The transition of pp65 from the nucleus to the cytoplasm was delayed in roscovitine-treated cells. These changes in localization may be related to alterations in the phosphorylation pattern of pp65 in the presence of the drug; however, the results of our mutational analyses of cdk phosphorylation sites in the protein suggest that these sites do not regulate pp65 localization. In addition, we report that in infected cells the export of pp65 from the nucleus is dependent on Crm1, and our data suggest that pp65 is a nucleocytoplasmic shuttling protein. Furthermore, our results indicate that late in infection, the equilibrium between nuclear import and export changes, resulting in accumulation of pp65 in the cytoplasm.
MATERIALS AND METHODS
Cell culture and virus.
Human foreskin fibroblasts (HFF) were maintained in minimal essential medium-Earle's medium supplemented with 10% fetal bovine serum, penicillin, streptomycin, amphotericin, and glutamine, as described previously (19). Cells were synchronized in G0 by allowing them to grow to confluence and to arrest for 3 additional days before trypsinization and replating at a lower density. Cells were infected at a multiplicity of infection (MOI) of 5 with the Towne strain of HCMV at the time of release from confluence. Mock-infected cells were incubated with an equal volume of conditioned medium. Cells were plated at a density of 8 × 105 per 10-cm-diameter dish containing glass coverslips and were maintained in 8 ml of complete medium. At 24 h p.i., roscovitine or dimethyl sulfoxide (DMSO) was added as indicated. At 24 h p.i., roscovitine (EMD Biosciences or Sigma) was added to a final concentration of 20 μM. Media and chemicals were replenished every 24 h thereafter.
In separate experiments, the localization phenotypes of wild-type or mutant forms of pp65 were determined by infection of cells transfected with plasmids encoding the constructs of interest. Mutations at threonine 66 (T66A) or threonine 555 (T555A) were made in plasmid pEGFP-pp65 (Clontech Laboratories) using the QuikChange kit from Stratagene according to the manufacturer's instructions. The sequences for the mutagenesis primers are as follows: 5′-GTACACGCCCGACTCGGCGCCATGCCACCG-3′ and its complement for the T66A mutation; 5′-GCTTTTTGGGCGCCGAGGCGATGCATGG-3′ and its complement for the T555A mutation. The generation of the desired mutations was confirmed by sequencing. The EGFP-coding sequences were removed from pp65 by subcloning the pp65-coding sequence into pcDNA3. HFF were electroporated with 10 μg of plasmid DNA and plated onto glass coverslips. For initial experiments, cells transfected with pEGFP-pp65, pEGFP-pp65 T66A, or pEGFP-pp65 T55A were infected 1 day postelectroporation at an MOI of approximately 5 with the Towne strain of HCMV. Cultures were fixed in 2% formaldehyde in phosphate-buffered saline (PBS) 72 h p.i. For later experiments, transfected cells were infected with HCMV Towne at an MOI of approximately 3 at 6 h posttransfection. Cultures were fixed as described above at 72 to 96 h p.i. Similar experiments were performed using pcDNA3-pp65, pcDNA3-pp65 T66A, or pcDNA3-pp65 T555A for transfection, followed by infection with the HCMV Ad169 pp65-deletion mutant RV (25), a kind gift from Bodo Plachter, at an MOI of approximately 3. Cells were fixed 96 h p.i. and stained with monoclonal antibody 65-8.
Immunofluorescence.
Confluence-synchronized cells were seeded on glass coverslips, infected, and treated with roscovitine at 24 h p.i., as described above. To test the reversibility of cdk inhibition by roscovitine, the drug was removed at 48 h p.i., and cells were fed with complete medium without the drug. In some experiments, 20 μM roscovitine, 1.8 μM leptomycin B (LMB) (EMD Biosciences), and/or 100 μg/ml cycloheximide was added starting at 72 h p.i. At 72, 73, 75, and 96 h p.i., coverslips were fixed in 2% paraformaldehyde in PBS for 15 min at room temperature. Cells were stained with monoclonal antibodies to pp65 (28-19 and 65-8) and Hoechst stain as previously described (17). For quantification of nuclear staining, cells were stained with monoclonal antibody 28-19.
Phosphopeptide mapping of pp65.
G0-synchronized HFF were either infected at the time of release from confluence with HCMV Towne at an MOI of 5 or mock infected. At 24 h p.i., roscovitine or DMSO was added. Media and treatments were replenished at 48 h p.i. At 66 h p.i., cells were washed and starved in phosphate-free minimal essential medium (Caisson Laboratories) supplemented with antibiotics and dialyzed fetal bovine serum, and containing roscovitine or DMSO, for 1 to 2 h. The medium on each plate was then replaced with 5 ml of fresh, warm supplemented phosphate-free medium that contained 5 mCi of 32Pi (MP Biomedicals). After labeling for 1 to 2 h, cells were washed with ice-cold Tris-buffered saline and harvested by scraping in NETN buffer (20 mM Tris [pH 8.0], 100 mM NaCl, 1 mm EDTA, 0.5% NP-40) containing protease and phosphatase inhibitors (1 mM sodium metabisulfite, 10 mM sodium pyrophosphate, 50 mM sodium fluoride, 1 mM sodium orthovanadate, 4 mM phenylmethylsulfonyl fluoride, 50 μM leupeptin, 100 μM pepstatin A, 17.5 μM β-glycerophosphate, and 2.5 μM MG132). For pp65 immunoprecipitation, the cleared lysate from each plate was split into two 1.8-ml tubes, and 800 μl of monoclonal anti-pp65 (clone 28-19) was added to each tube. Immunoprecipitation was carried out at 4°C for 4 h. Protein G plus agarose (Santa Cruz) was first washed extensively with PBS, then blocked with an unlabeled HFF NETN lysate (600 μl agarose slurry added to a NETN lysate from approximately 3 million cells incubated for 1 h at 4°C), washed extensively, and finally added to the labeled lysates for the last hour. Immunoprecipitates were washed with NETN buffer containing 0.1% sodium dodecyl sulfate (SDS), boiled in reducing sample buffer, and run out on an 8% SDS-polyacrylamide gel. Extraction of labeled protein, tryptic digestion, and phosphopeptide separation were carried out essentially as described by Schlaepfer and Hunter (23).
RESULTS
Roscovitine alters the localization of pp65 within infected cells.
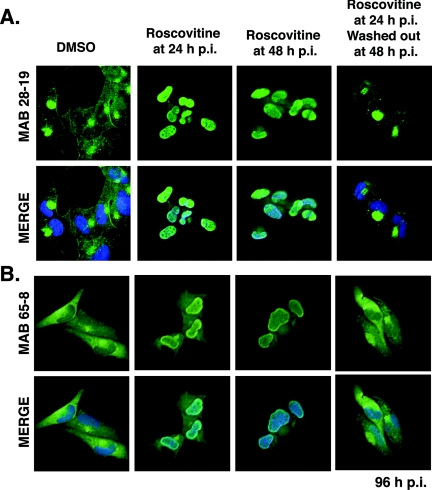
Several virion proteins are phosphorylated, but the importance of this modification for their incorporation into particles has not been extensively studied. As a first step in determining whether virion proteins were substrates for cdk activity, we determined if there were changes in their localization upon treatment of infected cells with roscovitine. The most significant difference was the retention of pp65 within the nuclei of drug-treated infected cells. During a normal infection, input pp65 from the inoculum is rapidly transported to the nucleus (24). The newly synthesized pp65 continues to localize to the nucleus during early times and viral DNA replication. After 48 h p.i., pp65 moves into the cytoplasm and is barely detectable within the nucleus (18). The transport of pp65 out of the nucleus marks a shift to the late phase of infection. In cells treated with roscovitine beginning at 24 h p.i., the accumulation of pp65 in the cytoplasm is markedly inhibited at 96 h p.i. (Fig. 1A and B). Results using two different anti-pp65 antibodies are shown. For Fig. 1A, cells were stained with monoclonal antibody 28-19. As previously reported (18), pp65 was localized in the cytoplasm and accumulated in the juxtanuclear assembly compartment in control cells. In contrast, pp65 was predominantly localized in the nuclei of cells treated with roscovitine from 24 h p.i. This trend was observed for several experiments, and the percentage of cells showing nuclear localization of pp65 in the presence of the drug was high but variable. Two experiments were quantified using monoclonal antibody 28-19, and an average of 85% of cells treated with roscovitine exhibited nuclear pp65.
FIG. 1.
Treatment of HCMV-infected fibroblasts with roscovitine results in nuclear retention of pp65 at late times during infection. Cells were treated with 20 μM roscovitine from 24 or 48 h p.i. and were fixed at 96 h p.i. For experiments testing the reversibility of the drug, roscovitine was added at 24 h p.i. and removed at 48 h p.i. as described in Materials and Methods. Cells were stained with monoclonal antibody (MAB) 28-19 (A) or 65-8 (B) against pp65. The nuclei were counterstained with Hoechst stain (blue), and the anti-pp65 antibody was detected with a fluorescein isothiocyanate-conjugated secondary antibody (green). Original magnification, ×400.
Nuclear accumulation was also observed if addition of roscovitine was delayed until 48 h p.i. (Fig. 1A). In this case, pp65 was detected in the nuclei of approximately 95% of cells in two experiments. If roscovitine was washed out of the cultures at 48 h p.i., pp65 was localized in both the nucleus and the cytoplasm, but its accumulation at the assembly compartment lagged behind that observed in cells that were not treated with the drug (Fig. 1A). Approximately 27% of cells in two experiments contained some pp65 in the nucleus after the drug was removed. These experiments demonstrated the reversibility of cdk inhibition.
In Fig. 1B, we show the distribution of pp65 detected with monoclonal antibody 65-8. In control cells, we observed pp65 in the cytoplasm in a diffuse pattern, with some accumulation at the juxtanuclear assembly compartment. If roscovitine was added at 24 h p.i., pp65 was detected in both the nucleus and the cytoplasm. In addition to the diffuse nuclear staining, pp65 also appeared to accumulate along the periphery of the nucleus. As with monoclonal antibody 28-19, we observed nuclear accumulation of the protein in cells treated with the inhibitor from 48 h p.i.
cdk inhibition with roscovitine alters the phosphorylation pattern of pp65.
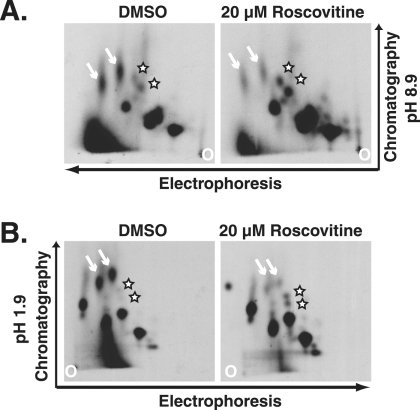
Previous work demonstrated that pp65 was phosphorylated (12, 16). Work from our laboratory showed that cdk inhibition with roscovitine did not cause a significant change in the steady-state levels of pp65 (21), but these experiments did not address the phosphorylation state of the protein. In order to determine if pp65 was a substrate for the cdk in infected cells, we generated phosphopeptide maps of pp65 immunoprecipitated from cells treated with DMSO or roscovitine beginning at 24 h p.i. Cells were labeled with 32Pi at 66 h p.i., at which time pp65 is accumulating in the cytoplasm in control cells. The protein was immunoprecipitated from the radiolabeled cells and gel purified. The isolated protein was then digested with trypsin, and the resulting peptides were resolved in two dimensions. Approximately 10 spots were detected, suggesting that pp65 is heavily phosphorylated (Fig. 2A and B). As expected, the phosphorylation pattern in Fig. 2B was similar to that reported by Roby and Gibson (16). We observed notable differences in the map generated for pp65 derived from cells treated with roscovitine, but we did not observe the complete absence of a particular peptide. Instead, the intensities of two peptides were reduced, and those of two other peptides increased, in cells treated with roscovitine, suggesting that the phosphorylation state of pp65 was altered by treatment with the drug.
FIG. 2.
The phosphorylation pattern of pp65 changes upon treatment of cells with roscovitine from 24 h p.i. Infected cells were labeled with 32Pi beginning at 66 h p.i. in the presence or absence of the drug. The pp65 from labeled lysates was immunoprecipitated with monoclonal antibody 28-19. The protein was isolated from SDS-polyacrylamide gels and subjected to digestion and separation as described in Materials and Methods. (A) Peptides separated at a high pH; (B) peptides separated at a low pH. Arrows indicate peptides whose intensities decreased, and stars indicate peptides whose intensities increased, in cells treated with roscovitine.
Mutation of cdk sites does not alter the localization of pp65.
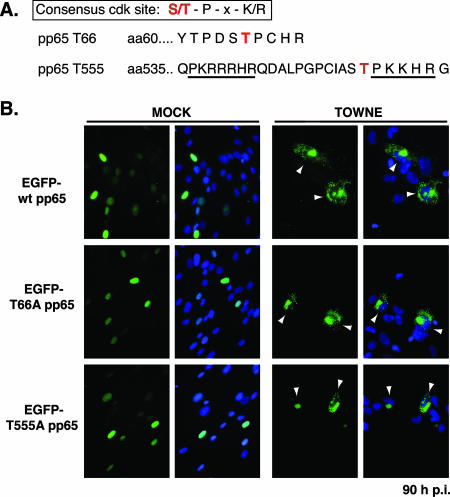
Analysis of the pp65 protein using the NetPhos program (2) revealed a number of potential phosphorylation sites for cellular kinases within the sequence, including two potential cdk sites at Thr66 and Thr555 (Fig. 3). The latter site falls within the previously described bipartite NLS (24); thus, it seemed possible that nuclear retention of pp65 in the presence of roscovitine could be mediated by inhibition of phosphorylation at this site. To test this possibility, we generated mutations at Thr555 and Thr66 in the pp65 sequence. The amino acids were changed to nonphosphorylatable alanine residues. Plasmids encoding wild-type or mutant pp65 proteins fused to EGFP were transfected into human fibroblasts, which were then seeded onto coverslips. Cells were subsequently infected with Towne at a high MOI and were fixed 72 to 96 h p.i. The localization of the EGFP fusion proteins was observed by fluorescence microscopy. As shown in Fig. 3, neither the mutation of Thr66 to alanine nor that of Thr555 affected the ability of pp65 to target to the nucleus in cells transiently expressing the protein. More than 99% of cells exhibited nuclear localization of the pp65 constructs. When cells were infected with HCMV, the wild-type and mutant fusion proteins displayed cytoplasmic localization at late times (Fig. 3), although less than 50% of transfected cells showed this phenotype. Expression of pp65 from the plasmids was driven from the CMV major IE promoter, and upon infection, the high levels of expression led to the formation of intranuclear aggregates of the protein. Only a small percentage of infected cells, approximately 20 to 30%, exhibited cytoplasmic localization of pp65 fusion proteins at this time point.
FIG. 3.
Mutation of putative cdk sites in EGFP-pp65 does not alter relocalization to the cytoplasm late in infection. (A) Sequences of putative cdk sites in pp65. aa, amino acid. (B) Human fibroblasts were transfected with plasmids encoding wild-type (wt) or mutant (T66A or T555A) pp65 proteins fused to EGFP. Cells were either infected with HCMV Towne at a high MOI or mock infected. Cells were fixed at 90 h p.i. Arrowheads indicate cells displaying cytoplasmic pp65 localization. Original magnification, ×400.
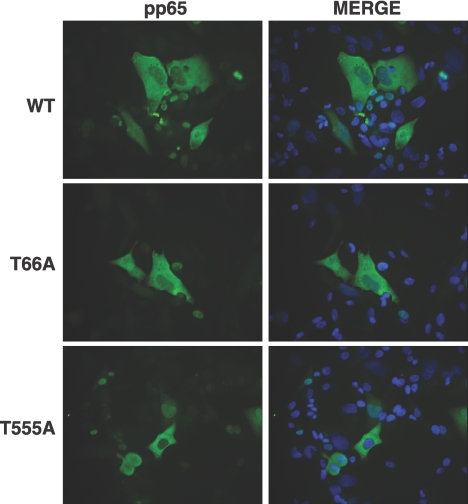
Because EGFP forms a dimer, it was possible that complex formation was affecting the localization of wild-type or mutant pp65 in the assays described above. Moreover, it was also possible that the wild-type pp65 expressed from the virus used for superinfection formed complexes with the mutant pp65 proteins and altered their trafficking. To test these possibilities, experiments similar to those described above were performed with pp65 constructs lacking EGFP. In this case, the pp65-negative Ad169 RV (25) was used for superinfection of cells transfected with plasmids encoding wild-type or mutant pp65 proteins. At 96 h p.i., cells were fixed, and coverslips were stained with monoclonal antibody 65-8 to visualize the localization of pp65 (Fig. 4). The results obtained were similar to those for the experiments using EGFP fusions. The mutation of the consensus cdk phosphorylation site at Thr66 or Thr555 to alanine did not inhibit the ability of pp65 to localize to the cytoplasm late in infection. Thus, phosphorylation at these sites does not appear to play a role in the localization of pp65.
FIG. 4.
Mutation of putative cdk sites in pp65 does not alter relocalization to the cytoplasm in cells infected with the pp65-null virus RV. Human fibroblasts were transfected with plasmids encoding wild-type (WT) or mutant (T66A or T555A) pp65 proteins. Cells were either infected with RV at a high MOI or mock infected. Cells were fixed at 96 h p.i. and stained with monoclonal antibody 65-8 directed against pp65. The nuclei were counterstained with Hoechst stain (blue), and the anti-pp65 antibody was detected with a fluorescein isothiocyanate-conjugated secondary antibody. Original magnification, ×400.
Accumulation of pp65 in the cytoplasm late in infection is dependent on the Crm1 exporter.
The biphasic distribution of pp65 (first nuclear, then cytoplasmic) suggested that there might be an alteration in the relative rates of nuclear import and export of the protein at early and late phases of infection. Given the change in the phosphorylation pattern of pp65 caused by cdk inhibition, it seemed possible that this could alter the protein's localization if the binding of targeting signals by transport receptors, such as importins and exportins, was affected. The interactions between localization signals and the receptors can be modulated by different mechanisms (for a review, see reference 14). First, the signal can be obscured from the receptor by intramolecular mechanisms such as protein folding, disulfide bonds, or phosphorylation. Second, the interaction can be blocked by the binding of other molecules, such as RNA or other proteins, to the cargo. Finally, modification of the protein by phosphorylation can simply change the affinity of the receptor for the signal. Since pp65 accumulated in the nuclei of cells treated with a cdk inhibitor, it seemed unlikely that the altered phosphorylation was affecting nuclear import. In fact, the data were consistent with changes in the ability of the protein to be exported. We therefore asked if pp65 was a target for Crm1, the best-studied nuclear export receptor.
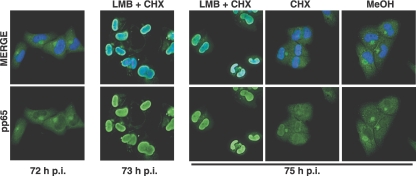
In order to determine whether there might be a change in the relative rates of nucleocytoplasmic transport at late times in infection, we treated infected cells with LMB at 72 h p.i., a time at which the majority of pp65 was localized in the cytoplasm. LMB inhibits the Crm1-dependent export of proteins from the nucleus; thus, if the cytoplasmic localization of pp65 resulted from higher rates of Crm1-dependent export relative to import, treatment with LMB would result in the accumulation of pp65 in the nucleus. Incubations with LMB were carried out with or without cycloheximide. After 1 and 3 h of treatment with the export inhibitor, cells were fixed and stained for pp65. Treatment of cells with LMB for 1 or 3 h resulted in accumulation of pp65 in the nuclei of >95% of cells (Fig. 5). The observation that pp65 accumulated in the nuclei of cells treated with LMB and cycloheximide for merely 1 h had strong implications. First, the data indicated that pp65 was not static in the cell. Furthermore, the results suggested that pp65 was capable of shuttling between the nucleus and the cytoplasm. Interestingly, treatment of cells with cycloheximide alone resulted in a low level of nuclear accumulation of pp65, but it was significantly lower than that observed when LMB was added.
FIG. 5.
The cytoplasmic accumulation of pp65 late in infection is reversed by LMB. HCMV-infected cells were treated with LMB or methanol (MeOH) in the presence of cycloheximide (CHX) beginning at 72 h p.i. Cells were fixed at 73 or 75 h p.i. and stained with monoclonal antibody 65-8. The nuclei were counterstained with Hoechst stain (blue), and the anti-pp65 antibody was detected with a fluorescein isothiocyanate-conjugated secondary antibody. Original magnification, ×400.
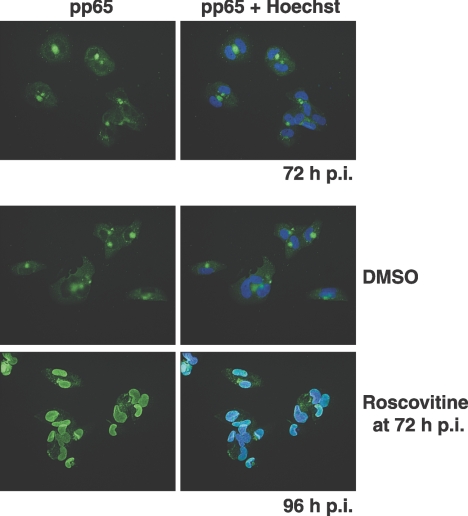
To determine if inhibition of cdk activity for short periods affected pp65 localization like the disruption of Crm1-mediated export, roscovitine was added at 72 h p.i. for only a 3-h period. The effect of the cdk inhibitor on pp65 localization was not as uniform as that of LMB. A mixed pattern of localization was observed, and the percentage of cells showing nuclear pp65 was low and variable between experiments (data not shown). If, however, the cells were incubated with roscovitine for 24 h (between 72 and 96 h p.i.), the effect on pp65 localization was more striking; the protein was relocalized to the nucleus in approximately 86% of the cells in two experiments quantified with monoclonal antibody 28-19 (Fig. 6). These data suggested that roscovitine did not have an immediate effect on pp65 nuclear export like that observed with direct inhibition of the Crm1 exporter but that prolonged incubation with the cdk inhibitor did have a pronounced effect on export.
FIG. 6.
Late addition of roscovitine to infected cells causes the nuclear accumulation of pp65. Roscovitine (20 μM) was added at 72 h p.i., a time when pp65 is predominantly localized in the cytoplasm. Cells were fixed at 96 h p.i., and coverslips were stained with monoclonal antibody 28-19. The nuclei were counterstained with Hoechst stain (blue), and the anti-pp65 antibody was detected with a fluorescein isothiocyanate-conjugated secondary antibody. Original magnification, ×400.
DISCUSSION
Our previous studies using the cdk inhibitor roscovitine have shown that cdk activity is required at multiple stages during HCMV infection. At the early phase of infection, cdk function is required for proper processing of viral transcripts, for expression of early gene products, and for establishing an environment suitable for viral DNA replication (20). At later times, cdk activity appears to be required for virion maturation and the release of infectious virus (3, 21). In this paper we have extended these studies and demonstrate that cdk activity is also important for the posttranslational modification and localization of the abundant virion tegument protein pp65.
Treatment of infected cells with the cdk inhibitor roscovitine led to accumulation of pp65 in the nucleus, even at late phases of infection. In contrast, pp65 in control cells was distributed in the cytoplasm late in infection. Phosphopeptide mapping of pp65 immunoprecipitated from infected cells treated with the drug revealed that the phosphorylation pattern of the protein was also changed when cdk activity was inhibited. Taken together, these observations suggested that pp65 was a substrate for cdk in infected cells and that cdk-mediated phosphorylation controlled pp65 localization. However, mutational analyses of potential cdk sites within the protein sequence did not alter the ability of pp65 to be relocalized to the cytoplasm late in infection, indicating that the two phenomena might not be related. It is possible that both T66 and T555 must be mutated within the same molecule in order to affect protein localization in our assay. Alternatively, the effects on pp65 phosphorylation could be indirect; that is, cdk could activate another kinase or inhibit a phosphatase that acts on pp65. Determination of the identity of pp65 phosphopeptides will allow us to define pathways by which roscovitine leads to altered pp65 localization. It is possible that inhibition of cdk activity could affect the function of other proteins that regulate the trafficking of pp65.
The accumulation of pp65 in the nuclei of infected cells treated with roscovitine prompted us to examine the mechanisms underlying the biphasic localization of this protein during infection. Our data indicate that the nuclear targeting of pp65 is not affected by mutation of a potential phosphorylation site within the bipartite localization signal, because the T555A mutant was localized to the nucleus in transfected cells. In addition, we found that a functional Crm1 exporter is required for relocalization of pp65 to the cytoplasm late in infection. Our strongest evidence that pp65 is a nucleocytoplasmic shuttling protein came from experiments where LMB and cycloheximide were added to infected cell cultures at 72 h p.i., when pp65 was cytoplasmic. After a 1-h incubation, pp65 had accumulated in the nuclei of the majority of cells, suggesting that pp65 was dynamic and retained the ability to be targeted to the nucleus late in infection. Thus, it does not appear that pp65 is retained in the cytoplasm in a static state late in infection. Short incubations with the cdk inhibitor starting at 72 h p.i. did not have the same impact on pp65 localization, but nuclear accumulation of pp65 was observed after 24 h. The delayed effect of roscovitine on pp65 localization compared to that of LMB may reflect the reversibility of the cdk inhibitor as opposed to LMB.
Our preliminary data using glutathione S-transferase-Crm1 pulldown assays indicate that the interaction between pp65 and Crm1 is not direct, but further studies are necessary in the context of infection to characterize the nature of the interactions. Our data do not exclude the possibility that roscovitine inhibits the nuclear export system. However, based on our observations that pp65 appears to shuttle within infected cells, we have developed a model to explain the distribution of pp65 during infection. At early phases of infection, the rate of pp65 nuclear import is greater than the rate of export, and thus, the protein is localized to the nucleus. After 48 h p.i., we believe that there is a shift in the steady-state equilibrium that results in cytoplasmic accumulation of pp65; that is, the rate of export becomes greater than the rate of import. Although the observed changes in the phosphorylation pattern of pp65 in the presence of roscovitine support our model, phosphopeptide mapping of pp65 at early and late phases of infection would confirm our hypothesis that phosphorylation of pp65 mediates this equilibrium shift. Therefore, additional experiments are necessary to decipher the complex trafficking pathways of pp65 during infection.
In summary, we have described the nuclear accumulation of pp65 within infected cells treated with the cdk inhibitor roscovitine. We show that cdk activity is required even at very late stages of infection for the proper posttranslational modification and localization of virion proteins. These studies provide the basis for future experiments designed to elucidate how the virus utilizes multiple pathways to promote nuclear egress and cytoplasmic assembly of the virion.
Acknowledgments
We thank Jill Meisenhelder in Tony Hunter's lab at the Salk Institute for Biological Studies for help with phosphopeptide mapping of pp65 and William Britt at the University of Alabama at Birmingham for antibodies to pp65. We also thank Lance Hall with Leeds Instruments for allowing use of the Olympus IX81 microscope and Julian Leibowitz for use of the electroporator.
This work was funded by NIH grants CA073490 and CA034729 to D.H.S. and CA102094 to V.S.
Footnotes
Published ahead of print on 22 August 2007.
REFERENCES
- 1.Battista, M., G. Bergamini, M. Boccuni, F. Campanini, A. Ripalti, and M. Landini. 1999. Expression and characterization of a novel structural protein of human cytomegalovirus, pUL25. J. Virol. 73:3800-3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blom, N., S. Gammeltoft, and S. Brunak. 1999. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 294:1351-1362. [DOI] [PubMed] [Google Scholar]
- 3.Bresnahan, W. A., I. Boldogh, P. Chi, E. A. Thompson, and T. Albrecht. 1997. Inhibition of cellular Cdk2 activity blocks human cytomegalovirus replication. Virology 231:239-247. [DOI] [PubMed] [Google Scholar]
- 4.Cinatl, J., Jr., J.-U. Vogel, R. Kotchetknov, and H. W. Doerr. 2004. Oncomodulatory signals by regulatory proteins encoded by human cytomegalovirus: a novel role for viral infection in tumor progression. FEMS Microbiol. Rev. 28:59-77. [DOI] [PubMed] [Google Scholar]
- 5.Dal Monte, P., S. Pignatelli, N. Zini, N. Maraldi, E. Perret, M. Prevost, and M. Landini. 2002. Analysis of intracellular and intraviral localization of the human cytomegalovirus UL53 protein. J. Gen. Virol. 83:1005-1012. [DOI] [PubMed] [Google Scholar]
- 6.Gallina, A., E. Percivalle, L. Simoncini, M. Revello, G. Gerna, and G. Milanesi. 1996. Human cytomegalovirus pp65 lower matrix phosphoprotein harbours two transplantable nuclear localization signals. J. Gen. Virol. 77:1151-1157. [DOI] [PubMed] [Google Scholar]
- 7.Jones, T., and S.-W. Lee. 2004. An acidic cluster of human cytomegalovirus UL99 tegument protein is required for trafficking and function. J. Virol. 78:1488-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McElroy, A. K., R. S. Dwarakanath, and D. H. Spector. 2000. Dysregulation of cyclin E gene expression in human cytomegalovirus-infected cells requires viral early gene expression and is associated with changes in the Rb-related protein p130. J. Virol. 74:4192-4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mettenleiter, T., B. Klupp, and H. Granzow. 2006. Herpesvirus assembly: a tale of two membranes. Curr. Opin. Microbiol. 9:423-429. [DOI] [PubMed] [Google Scholar]
- 10.Mocarski, E. S., Jr., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2673. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 11.Munger, J., D. Yu, and T. Shenk. 2006. UL26-deficient human cytomegalovirus produces virions with hypophosphorylated pp28 tegument protein that is unstable within newly infected cells. J. Virol. 80:3541-3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pande, H., T. Lee, M. Churchill, and J. Zaia. 1990. Structural analysis of a 64-kDa major structural protein of human cytomegalovirus (Towne): identification of a phosphorylation site and comparison to pp65 of HCMV (AD169). Virology 178:6-14. [DOI] [PubMed] [Google Scholar]
- 13.Pass, R. P. 2001. Cytomegalovirus, p. 2675-2705. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 14.Poon, I., and D. Jans. 2005. Regulation of nuclear transport: central role in development and transformation? Traffic 6:173-176. [DOI] [PubMed] [Google Scholar]
- 15.Prichard, M., W. Britt, S. Daily, C. Harline, and E. Kern. 2005. Human cytomegalovirus UL97 kinase is required for the normal intranuclear distribution of pp65 and virion morphogenesis. J. Virol. 79:15494-15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roby, C., and W. Gibson. 1986. Characterization of phosphoproteins and protein kinase activity of virions, noninfectious enveloped particles, and dense bodies of human cytomegalovirus. J. Virol. 59:714-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez, V., C. L. Clark, J. Y. Yen, R. Dwarakanath, and D. H. Spector. 2002. Viable human cytomegalovirus recombinant virus with an internal deletion of the IE2 86 gene affects late stages of viral replication. J. Virol. 76:2973-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez, V., K. D. Greis, E. Sztul, and W. J. Britt. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74:975-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez, V., A. K. McElroy, and D. H. Spector. 2003. Mechanisms governing maintenance of cdk1/cyclin B1 kinase activity in cells infected with human cytomegalovirus. J. Virol. 77:13214-13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez, V., A. K. McElroy, J. Yen, S. Tamrakar, C. L. Clark, R. A. Schwartz, and D. H. Spector. 2004. Cyclin-dependent kinase activity is required at early times for accurate processing and accumulation of the human cytomegalovirus UL122-123 and UL37 immediate-early transcripts and at later times for virus production. J. Virol. 78:11219-11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez, V., and D. Spector. 2006. Cyclin-dependent kinase activity is required for efficient expression and posttranslational modification of human cytomegalovirus proteins and for production of extracellular particles. J. Virol. 80:5886-5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez, V., E. Sztul, and W. J. Britt. 2000. Human cytomegalovirus pp28 (UL99) localizes to a cytoplasmic compartment which overlaps the endoplasmic reticulum-Golgi-intermediate compartment. J. Virol. 74:3842-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlaepfer, D., and T. Hunter. 1996. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol. Cell. Biol. 16:5623-5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmolke, S., P. Drescher, G. Jahn, and B. Plachter. 1995. Nuclear targeting of the tegument protein pp65 (UL83) of human cytomegalovirus: an unusual bipartite nuclear localization signal functions with other portions of the protein to mediate its efficient nuclear transport. J. Virol. 69:1071-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmolke, S., H. F. Kern, P. Drescher, G. Jahn, and B. Plachter. 1995. The dominant phosphoprotein pp65 (UL83) of human cytomegalovirus is dispensable for growth in cell culture. J. Virol. 69:5959-5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo, J.-Y., and W. Britt. 2006. Sequence requirements for localization of human cytomegalovirus tegument protein pp28 to virus assembly compartment and for assembly of infectious virus. J. Virol. 80:5611-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trgovcich, J., C. Cebulla, P. Zimmerman, and D. Sedmak. 2006. Human cytomegalovirus protein pp71 disrupts major histocompatibility complex class I cell surface expression. J. Virol. 80:951-963. [DOI] [PMC free article] [PubMed] [Google Scholar]