Abstract
Cytotoxic T cells (CTL) play a central role in the control of viral infections. Their antiviral activity can be mediated by at least two cytotoxic pathways, namely, the granule exocytosis pathway, involving perforin and granzymes, and the Fas-FasL pathway. However, the viral factor(s) that influences the selection of one or the other pathway for pathogen control is elusive. Here we investigate the role of viral replication levels in the induction and activation of CTL, including their effector potential, during acute Friend murine leukemia virus (F-MuLV) infection. F-MuLV inoculation results in a low-level infection of adult C57BL/6 mice that is enhanced about 500-fold upon coinfection with the spleen focus-forming virus (SFFV). Both the low- and high-level F-MuLV infections generated CD8+ effector T cells that were essential for the control of viral replication. However, the low-level infection induced CD8+ T cells expressing solely FasL but not the cytotoxic molecules granzymes A and B, whereas the high-level infection resulted in induction of CD8+ effector T cells secreting molecules of the granule exocytosis pathway. By using knockout mouse strains deficient in one or the other cytotoxic pathway, we found that low-level viral replication was controlled by CTL that expressed FasL but control of high-level viral replication required perforin and granzymes. Additional studies, in which F-MuLV replication was enhanced experimentally in the absence of SFFV coinfection, supported the notion that only the replication level of F-MuLV was the critical factor that determined the differential expression of cytotoxic molecules by CD8+ T cells and the pathway of CTL cytotoxicity.
CD8+ cytotoxic T cells (CTL) are the key players in the immune response against intracellular pathogens including viruses. CTL can eliminate target cells by two independent cytolytic pathways, the granule exocytosis pathway and the Fas pathway (37). In the first pathway the membrane-disrupting protein perforin and the two principal serine proteases, termed granzymes A and B, are secreted by the CTL and act together to induce apoptosis in the target cell (31). The second pathway involves the binding of the cell death receptor Fas on the target cell to its cognate ligand FasL on the killer cell membrane. This engagement results in caspase-dependent apoptosis of the target (23, 40). Previous studies demonstrated that both cytotoxic pathways are of relevance, though differentially, for the control of individual viral infections. For example, studies with knockout mice provide evidence for the granule exocytosis pathway as the key mechanism in recovery from lymphocytic choriomeningitis virus (3, 21, 41), ectromelia virus (mouse pox virus) (27, 33), and Friend retrovirus infection (44). Other studies showed that certain viruses are readily controlled by the Fas pathway (3, 8, 32). However, the viral factor(s) responsible for the differential induction of CTL-associated cytotoxic molecules during infection is largely unknown.
We have used the Friend virus (FV) model to determine whether the level of viral replication is a key factor for CTL in the induction and usage of different cytotoxic pathways for antiviral defense. FV is a complex comprised of two retroviruses, the replication-competent but nonpathogenic helper virus, known as Friend murine leukemia virus (F-MuLV), and the replication-defective but pathogenic spleen focus-forming virus (SFFV) (20). Infection of adult mice with the FV complex induces various degrees of acute viremia and splenomegaly depending on the genetic background of the mouse strain (7, 17). Susceptible strains progress to lethal erythroleukemia after FV infection (28), whereas resistant mice, such as the C57BL/6 (B6) mice used in the current experiments, recover from acute splenomegaly and do not develop leukemia. Previous studies showed that both virus-specific humoral and cellular immune responses, in particular CD8+ CTL, are essential for the recovery of resistant mice from primary FV infection (17, 19).
The F-MuLV helper virus expresses all the relevant T-cell epitopes that are necessary for recovery from disease as well as for vaccine protection against FV (12, 13). Thus, the current study focuses on the CTL response to F-MuLV during acute infection and compares the mechanisms of specific CTL-mediated virus control during low- and high-level F-MuLV infection. Infection of adult mice with the F-MuLV helper virus alone results in poor viral replication without any clinical symptoms. However, replication of F-MuLV can be greatly enhanced by coinfection with the replication-defective SFFV. SFFV encodes an envelope protein that binds to the erythropoietin receptor, leading to activation and polyclonal proliferation of erythroid precursor cells, which are the most important target cells for F-MuLV (20).
In the current paper we show that in B6 mice F-MuLV replication is about 500 times lower in the absence than in the presence (coinjected) of SFFV. However, both infection protocols induced virus-specific CTL responses that were critical for controlling viral replication. Interestingly, protective CTL generated during low-level infection with F-MuLV expressed only FasL, whereas CTL induced during high-level infection expressed granzymes in addition, which in conjunction with perforin were required for efficient virus control. The results demonstrate that in FV infection the level of viral replication has a strong impact on the mode of the antiviral CTL activity.
MATERIALS AND METHODS
Mice.
Inbred C57BL/6 (B6) and gld (35) mice as well as the knockout mouse strain perforin×granzymeA×B (perf×A×B−/−) (39) were maintained under pathogen-free conditions. The knockout mice were originated from B6 embryonic stem cells. Fas-deficient mice (Fas−/−) were generated in S. Nagata's lab (1) and were kindly provided by M. Klein (Zürich, Switzerland). In all experiments 3- to 6-month-old mice were used.
Viral infection.
In all viral infection experiments, mice were injected intravenously with 0.5 ml phosphate-buffered saline containing 10,000 focus-forming units (FFU) of F-MuLV. The titer of SFFV in the F-MuLV-plus-SFFV stock was 10,000 spleen FFU. Both virus stocks were free of lactate dehydrogenase-elevating virus.
Infectious-center assays.
Titrations of single-cell suspensions from infected mouse spleens were plated onto susceptible Mus dunni cells (22), cocultivated for 3 days, fixed with ethanol, stained with F-MuLV envelope-specific monoclonal antibody 720 (34), and developed with peroxidase-conjugated goat anti-mouse antibodies and aminoethylcarbazol to detect foci.
Lymphocyte depletion.
CD4+ and CD8+ T-cell and NK cell depletions were performed essentially as described previously (18, 26, 29). Briefly, mice were inoculated intraperitoneally with 0.5 ml of supernatant fluid obtained from hybridoma cell culture for the CD4-, CD8-, or NK1.1-specific monoclonal antibodies 191.1, 169.4, and PK136, respectively. Mice were inoculated every other day four times starting on the day of FV infection. The treatment depleted 92% of CD4+ cells, 90% of CD8+ cells, and 86% of NK1.1+ cells in the spleens of the respective mice (measured at 10 days postinfection).
Tetramer staining.
For the detection of activated virus-specific CD8+ T cells, 5 × 105 nucleated spleen cells were stained with anti-CD8- and anti-CD43-labeled (Pharmingen, Heidelberg, Germany) and phycoerythrin-labeled major histocompatibility complex class I H-2Db tetramers specific for the immunodominant GagL CTL epitope gPr80gag85-93 (6) for 15 min at room temperature. The tetramers contained modified versions of the Gag epitope in which all three cysteine residues were replaced with aminobutyric acid (38). After washing, cells were analyzed by flow cytometry. Dead cells were excluded by 7-aminoglycoside adenylyltransferase (Becton Dickinson, Heidelberg, Germany) staining.
Intracellular granzyme staining and flow cytometry.
Cell surface staining was performed using Becton Dickinson (Heidelberg, Germany) reagents. T-cell antibodies were anti-CD43, directed against the activation-associated glycosylated isoform of CD43 (1B11), anti-CD44 (Pgp-1), anti-CD8 (53-6.7), anti-CD127 (A7R34), and anti-CD107a (1D4B). Ter119 (Ly-76) was used as a marker for erythroblasts. Dead cells (7-aminoglycoside adenylyltransferase or propidium iodide positive) were excluded from all cell surface analyses. Intracellular granzyme A (polyclonal rabbit anti-mouse granzyme A immunoglobulin G [IgG], protein A purified) and granzyme B (monoclonal anti-human granzyme B antibody, allophycocyanin conjugated, clone GB12; Caltag Laboratories, Hamburg, Germany) staining was performed using the Cytofix/Cytoperm intracellular staining kit (Becton Dickinson). After being labeled for cell surface markers the spleen cells were washed and intracellular staining for granzyme B was performed according to the manufacturer's protocol. Granzyme A was reacted with a polyclonal rabbit anti-mouse granzyme A IgG antiserum (45) and stained with a fluorescein isothiocyanate-conjugated goat anti-rabbit IgG secondary antibody (Dianova, Hamburg, Germany). Analysis was always done on gated CD8+ T cells. To test for the resolution of the granzyme staining assay, we engineered a 10-fold dilution of spleen cells from F-MuLV-plus-SFFV-infected mice with splenocytes from naïve mice. In these control samples only 1.5 to 3% of the CD8+ cells were CD43+ in comparison to 15 to 25% of the CD8+ cells from infected animals. However, in the diluted and undiluted samples 20 to 40% of the activated CD8+ T cells were positive for granzyme A or B, indicating the high resolution of the assay. Data were acquired on a FACSCalibur flow cytometer (Becton Dickinson) from 10,000 to 30,000 lymphocyte-gated events per sample. Analyses were done using BD Cellquest Pro software (version 4.0.1; Becton Dickinson).
FasL staining.
Spleen cells were isolated after gradient centrifugation with 70% Percoll (Sigma, Taufkirchen, Germany) and stimulated for 1 h with anti-mouse CD3 and CD28 (Becton Dickinson, Heidelberg, Germany) in RPMI medium with 10% fetal calf serum, 10−5 M 2-mercaptoethanol, and 10 μmol/liter metalloproteinase inhibitor TAPI-1 (Calbiochem, Bad Soden, Germany). The expression of FasL was measured by flow cytometry after staining with anti-mouse FasL (CD178.1) antibody (Becton Dickinson, Heidelberg, Germany).
Quantitative PCR.
Total RNA was extracted from 1 × 105 CD8+ T cells by using the RNeasy Micro kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. mRNA was transcribed by incubating total RNA with an oligo(dT)12-18 primer (500 ng; Pharmacia, Freiburg, Germany) and RevertAid H Minus Moloney murine leukemia virus reverse transcriptase (Fermentas, St. Leon-Rot, Germany) as advised by the manufacturer. The resulting cDNA was used as a template for 18S rRNA (housekeeping gene) amplification in the LightCycler system (Roche Diagnostics, Mannheim, Germany) by using FastStart DNA Master SYBR green I (Roche, Basel, Switzerland). Plasmid vectors expressing FasL or 18S rRNA were used for quantification of mRNA molecules.
The primers were as follows: FasL, 5′ TAG ACAGCA GTG CCA CTT CAT 3′ and 5′ AAC TCA CGG AGT TCT GCC AGT 3′, and 18S rRNA, 5′ GCC CGA GCC GCC TGG ATA C 3′ and 5′ CCG GCG GGT CAT GGG AAT AAC 3′.
RESULTS
Both low- and high-level F-MuLV replications were controlled by CD8+ T cells.
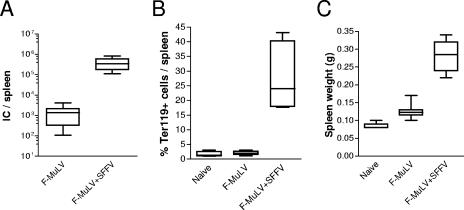
To investigate the mechanism of CTL-mediated immune control of low- and high-level retroviral infection, B6 mice were inoculated with F-MuLV in the absence or presence of SFFV. At 10 days postinfection levels of F-MuLV replication were determined in splenocytes using an infectious-center assay. Inoculation of mice with F-MuLV alone resulted in a low-level infection (Fig. 1A). In contrast, coinjection of the same dose of F-MuLV together with SFFV led to an almost-500-fold increase in F-MuLV loads. This enhanced viral replication was due to an SFFV envelope-mediated activation and proliferation of Ter119-positive erythroblasts (Fig. 1B), which created an expanded target cell pool for F-MuLV infection. The proliferation of the erythroid precursor cells resulted in a mild and temporary splenomegaly (Fig. 1C). However, both infection protocols did not lead to any other symptoms of acute or chronic disease (data not shown).
FIG. 1.
Levels of infection in F-MuLV versus F-MuLV-plus-SFFV-challenged mice. C57BL/6 mice were infected with 10,000 FFU F-MuLV, and the outcome of infection was determined 10 days later. In one group the animals were coinjected with SFFV to enhance F-MuLV replication. (A) F-MuLV infection levels in the spleen (infectious centers [IC]) were determined at 10 days postinfection. (B and C) Expansion of the Ter119+ erythroid precursor cell population (B) and spleen weights (C) are shown to demonstrate proliferation of the F-MuLV target cells. Experiments were repeated three times with comparable results.
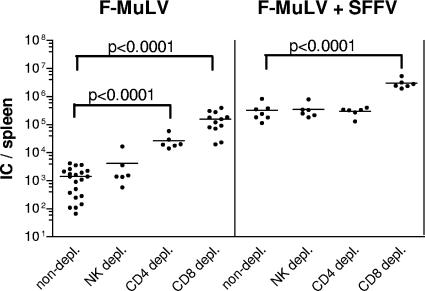
Next we investigated the involvement of the three different cytotoxic lymphocyte subsets, reported before to be critical in cytolysis of virus-infected target cells (37), i.e., CD4+ T cells, CD8+ T cells, and NK cells, in the control of low- versus high-level F-MuLV replication. Thus, B6 mice were depleted of CD4+, CD8+, or NK1.1+ cells by antibody treatment starting at the time of infection. On day 10 postinfection numbers of F-MuLV-infected cells in the spleen were significantly higher in mice depleted of CD8+ cells during both F-MuLV and F-MuLV-plus-SFFV infection than in untreated controls (Fig. 2). In contrast, following depletion of CD4+ cells, only splenocytes from F-MuLV- but not from F-MuLV-plus-SFFV-challenged mice showed an increased viral replication, which was, however, lower than that of CD8+ T-cell-depleted mice. Depletion of NK cells had no effect on the viral load by either of the two infection protocols. These data suggest that CD8+ CTL are the most critical effectors for the control of both low- and high-level F-MuLV infection. Consequently, we investigated the expression of cytotoxic molecules by CD8+ T cells and determined their role in the immune control of retroviral infections with different replication kinetics.
FIG. 2.
Depletion of T cells or NK cells during acute infection of mice with F-MuLV. C57BL/6 mice were infected with F-MuLV or F-MuLV plus SFFV and depleted for their CD8+, CD4+, or NK1.1+ cells by injecting monoclonal antibodies. Ten days postinfection the mice were sacrificed and analyzed for viral loads in the spleen. The results for depleted, infected animals were compared with those from untreated FV-infected mice. Each dot represents the results from a single mouse. Statistically significant differences between the groups are given in the figures. Differences between the group of infected wild-type mice and the groups of depleted mice were compared by using the unpaired t test. Statistically significant differences between the groups are given in the figure. Experiments were repeated two times with comparable results. IC, infectious centers.
Low-level retroviral infection was controlled by FasL-mediated CTL activity, whereas the granule exocytosis pathway was required for the control of a high-level infection.
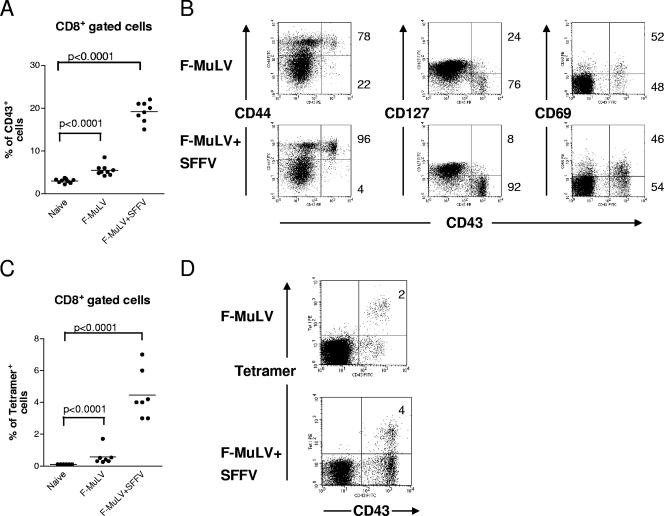
Since CD8+ T cells were critical for the control of F-MuLV infection, the activation and specificity of these cells were determined. Flow cytometry analysis at 10 days postinfection demonstrated that CD8+ T cells were activated, as measured by the expression of the activation-associated glycoform of CD43 (15), after infection of mice with both F-MuLV (mean of 5.5%) and F-MuLV plus SFFV (mean of 19%) (Fig. 3A). The increased frequency of CD8+ CD43+ T cells in F-MuLV-plus-SFFV- compared to F-MuLV-infected mice correlated with the level of viral replication following the two infection protocols (Fig. 1A). During both infections the majority of the CD43+ T cells also expressed the early activation marker CD69 but only a few cells expressed CD127 (the interleukin-7 [IL-7] receptor), indicating that these cells had an effector phenotype (Fig. 3B). In addition, the CD43+ cells coexpressed CD44, showing that they had encountered antigen. A subpopulation of the CD43+ effector T cells recognized the H-2Db-restricted F-MuLV glycosylated gag epitope (Fig. 3C), demonstrating their viral specificity. Whereas the numbers of tetramer+ CD8+ T cells were higher during high-level (mean of 4.4%) than during low-level (mean of 0.6%) F-MuLV infection (Fig. 3A and C), no significant differences were found regarding the cell surface phenotype of the CTL populations after the different infections.
FIG. 3.
CD8+ T-cell activation in mice with low- versus high-level F-MuLV replication. C57BL/6 mice were infected with F-MuLV or F-MuLV plus SFFV, and their CD8+ T cells were analyzed by flow cytometry. (A) Gated CD8+ T cells from infected mice were stained for the activation-associated glycoform of CD43 to detect effector T cells at 10 days post-F-MuLV infection. (B) CD43+ T cells were also positive for CD69 and CD44 and mostly negative for CD127, showing their effector phenotype. Percentages of positive (upper right quadrant) and negative (lower right quadrant) cells are given right next to the dot blots. (C) The percentages of virus-specific CD8+ T cells reactive with DbgagL class I tetramers are shown. (D) The representative costaining experiment indicates that all tetramer+ CD8+ T cells expressed the CD43 activation marker. Percentages of tetramer+ T cells of whole CD8+ cells are given in the upper right quadrant. Each dot represents an individual mouse. The mean percentage for each group is indicated by a line. Differences between the two groups were analyzed by using the unpaired t test. Statistically significant differences between the groups are given in the figures. Experiments were repeated three times with comparable results.
Thus, we could compare the expression of cytotoxic molecules in CTL during a low-level infection with that during high-level infection with the same virus. To this end, the expression of three key effector proteins of cytolytic CD8+ T cells, granzymes A and B and Fas ligand, was measured in CD8+ T cells.
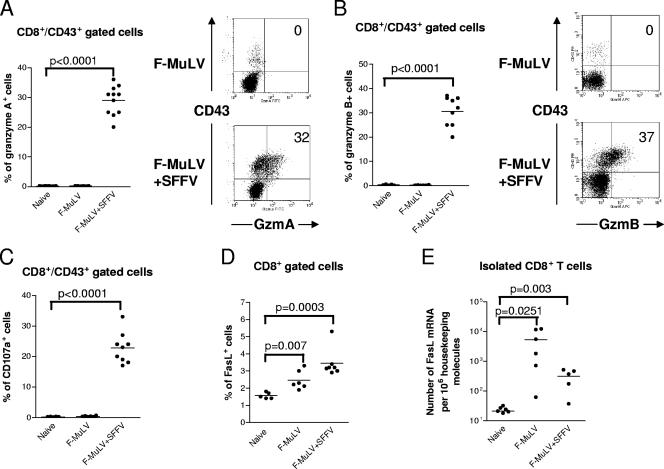
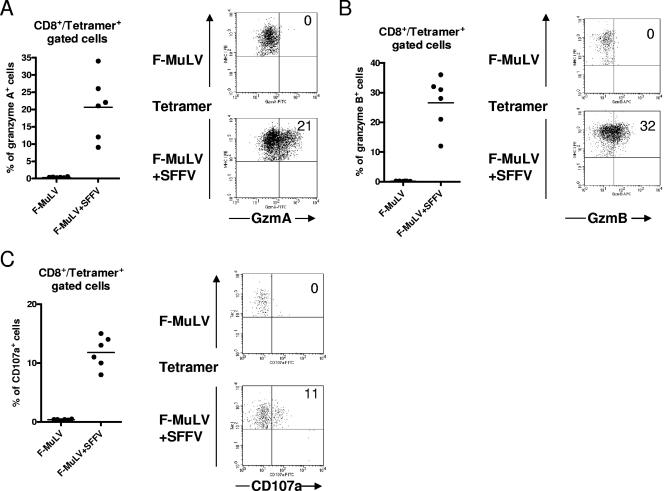
Figure 4A and B show that a mean of 29% and 31% of effector (CD43+) CD8+ T cells from F-MuLV-plus-SFFV-infected mice expressed granzymes A and B, respectively, whereas granzymes were not detectable in effector CD8+ T cells from F-MuLV-infected mice. The granule exocytosis pathway was further analyzed by examining cell surface expression of CD107a (LAMP-1) on CD8+ T cells. CD107a is located at the luminal side of the membranes of cytotoxic vesicles and becomes exposed on the cell surface as a result of exocytosis (4, 36). Thus, cell surface expression of CD107a indicates recent cytotoxic granule degranulation and has been used as a surrogate marker for granzyme/perforin-meditated killing by CTL in the FV model (45). A mean of 22% of the CD43+ CD8+ T cells from mice with high-level F-MuLV replication expressed CD107a on their surface, whereas no evidence of degranulation was found in cells from mice with low-level viral replication. Similar findings were obtained when tetramer+ CD8+ T cells were analyzed. Figure 5 shows that tetramer+ T cells did not produce granzymes or express CD107a after F-MuLV infection. In contrast, after F-MuLV-plus-SFFV infection around 20% of the virus-specific CD8+ T cells expressed granzymes and more than 10% showed evidence for recent degranulation. These results indicate that only the virus-specific CTL generated during high-level F-MuLV replication degranulate in response to antigenic stimulation. When enriched CD8+ T-cell populations were tested for the expression of FasL, cells from both F-MuLV- and F-MuLV-plus-SFFV-infected mice were found to express this molecule and the expression was only slightly higher on CD8+ T cells from mice with high-level F-MuLV replication (Fig. 4D). By quantifying FasL transcripts, even more mRNA molecules were observed in CD8+ T cells from F-MuLV-infected than from F-MuLV-plus-SFFV-infected mice, but the differences were not statistically significant (Fig. 4E). The results suggest that the CTL-mediated control of low-level F-MuLV infection was mainly elicited by the FasL-Fas pathway, whereas the exocytosis pathway was required for the control of high-level viral replication.
FIG. 4.
Production of granzymes and FasL and detection of degranulation in effector CD8+ T cells from mice with low- and high-level F-MuLV replication. Flow cytometry was used to detect intracellular granzymes, degranulation marker CD107a, or Fas ligand in activated effector CD8+ T cells (CD43+) at 10 days post-F-MuLV infection. In one group the animals were coinjected with SFFV to enhance F-MuLV replication. (A) Percentages of effector CD8+ T cells expressing granzyme A. Accumulated results are shown on the left with representative flow data on the right. The percentages of CD43+ cells that were positive for granzyme A are given in the upper right quadrants. (B) Percentages of effector CD8+ T cells expressing granzyme B. Accumulated results are shown on the left with representative flow data on the right. The percentages of CD43+ cells that were positive for granzyme B are given in the upper right quadrants. (C) Percentages of effector CD8+ T cells expressing the degranulation marker CD107a. (D) Percentages of CD8+ T cells expressing FasL after restimulation with anti-CD3 and anti-CD28 antibodies in vitro. (E) mRNA levels for FasL in CD8+ T cells isolated from F-MuLV-infected mice. The 18S rRNA was used as an internal standard. Each dot represents an individual mouse. The mean percentage for each group is indicated by a line. Differences between the two groups were analyzed by using the unpaired t test. Statistically significant differences between the groups are given in the figures. All experiments except those for panel E were repeated three times with comparable results.
FIG. 5.
Production of granzymes and FasL and detection of degranulation in virus-specific (tetramer+) CD8+ T cells from mice with low- and high-level F-MuLV replication. Flow cytometry was used to detect intracellular granzymes or degranulation marker CD107a in activated virus-specific CD8+ T cells (tetramer+) at 10 days post-F-MuLV infection. In one group the animals were coinjected with SFFV to enhance F-MuLV replication. (A) Percentages of virus-specific CD8+ T cells expressing granzyme A. Accumulated results are shown on the left with representative flow data on the right. The percentages of tetramer+ cells that were positive for granzyme A are given in the upper right quadrants. (B) Percentages of virus-specific CD8+ T cells expressing granzyme B. Accumulated results are shown on the left with representative flow data on the right. The percentages of tetramer+ cells that were positive for granzyme B are given in the upper right quadrants. (C) Percentages of virus-specific CD8+ T cells expressing the degranulation marker CD107a. Accumulated results are shown on the left with representative flow data on the right. The percentages of tetramer+ cells that were positive for CD107a are given in the upper right quadrants. Each dot represents an individual mouse. The mean percentage for each group is indicated by a line. Differences between the two groups were analyzed by using the unpaired t test. Statistically significant differences between the groups are given in the figures. All experiments were repeated three times with comparable results.
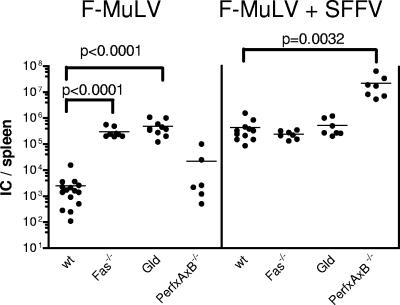
To support this hypothesis, the course of low- and high-level F-MuLV infection was monitored in mutant mouse strains with defects in either the exocytosis or the Fas-FasL pathway. At 10 days postinfection F-MuLV loads in the spleen were measured as a surrogate marker for CTL-mediated control of viral replication (Fig. 6). As shown in Fig. 6, in the case of a F-MuLV infection, viral replication was significantly increased in comparison to wild-type mice in Fas and gld (FasL−/−) mice but not in mice lacking perforin and granzymes A and B. In contrast, during high-level F-MuLV infection viral replication was significantly increased compared to that in wild-type mice in animals with defects in the exocytosis pathway but in neither of the Fas/FasL-knockout mice, supporting previous findings (44). These results indicate that different F-MuLV replication kinetics were selectively controlled by different pathways of the cytotoxic CD8+ T-cell response.
FIG. 6.
Infection with F-MuLV versus F-MuLV plus SFFV of mice deficient for perforin, granzyme A, and granzyme B or the Fas-FasL pathway. C57BL/6 wild-type mice and knockout mice that lack the cytotoxic molecules perforin, granzyme A, and granzyme B or Fas or FasL (gld mice) were infected with F-MuLV. In half of the groups, animals were coinjected with SFFV to enhance F-MuLV replication. Ten days postinfection the mice were sacrificed and analyzed for viral loads. The results for the knockout mice indicated were compared with those from wild-type animals. Viral loads were measured in the spleens of the infected mice. Each dot represents the results from a single mouse. The mean value for each group is indicated by a bar. Differences between the group of infected wild-type mice and the groups of knockout mice were compared by using the unpaired t test. Statistically significant differences between the groups are given in the figure. Experiments were repeated three times with comparable results. IC, infectious centers.
The pathway of CTL-mediated virus control was dictated by the level of F-MuLV replication and not by SFFV coinfection.
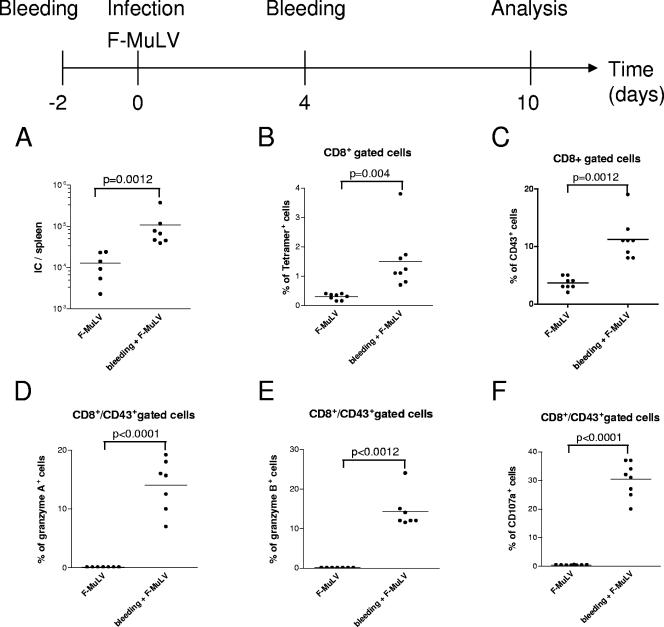
It is generally accepted that a major consequence of a simultaneous challenge of mice with F-MuLV and SFFV is the amplification of F-MuLV replication in erythroblasts. However, we could not completely rule out that the SFFV infection might have had some direct effects on CD8+ T-cell responses. To verify that only the level of F-MuLV replication but not the SFFV infection itself was responsible for the different induction of cytotoxic effector molecules, we increased F-MuLV replication by means other than SFFV coinfection. Bleeding of mice induces anemia and subsequent proliferation of Ter119+ erythroid precursor cells to replenish the deprived hematopoietic compartment, thus creating an expanded pool of target cells for the virus. Using the protocol depicted in Fig. 7, F-MuLV replication was significantly enhanced by bleeding compared to that in untreated mice infected with F-MuLV (Fig. 7A). The enhanced F-MuLV replication resulted in a significant expansion of the populations of virus-specific (tetramer+) as well as activated (CD43+) CD8+ T cells (Fig. 7B and C), with a mean of 14% to 30% expressing granzyme A or B or the degranulation marker CD107a (Fig. 7D and E). As seen before, CD43+ CD8+ T cells from untreated mice infected with F-MuLV were negative for all three markers. The data indicate that the level of F-MuLV replication rather than SFFV infection itself determined the different expression of cytotoxic molecules in CTL.
FIG. 7.
Detection of T-cell responses in mice after enhancement of F-MuLV replication due to an experimental induction of anemia. The bleeding of mice pre- and postinfection with F-MuLV results in anemia and subsequent activation and proliferation of Ter119+ target cell for the virus. (A) Spleen viral loads in mice with and without experimentally induced anemia were compared at 10 days postinfection. IC, infectious centers. (B and C) Flow cytometry was used to detect virus-specific (tetramer+) (B) and activated (CD43+) (C) CD8+ T cells in both groups. (D to F) In addition, intracellular granzyme A (D), granzyme B (E), or degranulation marker CD107a (F) in activated effector CD8+ T cells was measured. Accumulated results are shown as percentages of the CD43+ CD8+ T-cell population. Each dot represents an individual mouse. The mean percentage for each group is indicated by a line. Differences between the two groups were analyzed by using the unpaired t test. Statistically significant differences between the groups are given in the figures. Experiments were repeated three times with comparable results.
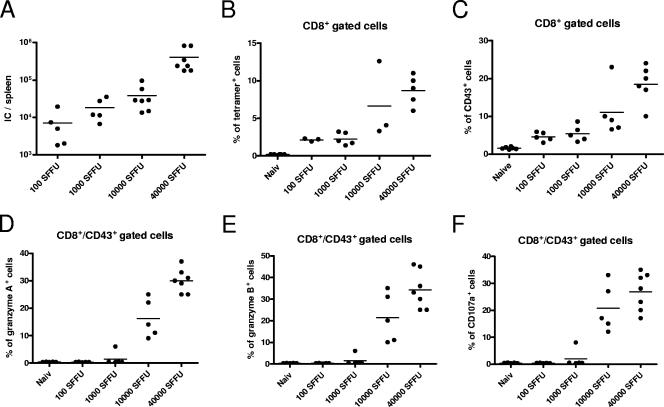
Similar findings were made when the inoculation dose of F-MuLV plus SFFV was reduced in titer. Figure 8A clearly shows that low doses of F-MuLV plus SFFV (100 to 1,000 spleen FFU) resulted in a low-level F-MuLV infection of spleen cells. This was associated with a weak but detectable activation of virus-specific CD8+ T cells (Fig. 8B and C). However, these activated (CD43+) CD8+ T cells did not express granzyme A or B or the degranulation marker CD107a. In contrast, the inoculation of mice with 10,000 spleen FFU or more of the two viruses resulted in high F-MuLV loads in the spleen (Fig. 8A) and induced strong activation of virus-specific CD8+ T cells (Fig. 8B and C). Furthermore, a mean of 15 to 30% of the activated (CD43+) as well as tetramer+ (data not shown) T cells expressed granzyme A or B or CD107a (Fig. 8D to F). These results indicate that for the induction and degranulation of granzymes, a certain threshold of viral replication is mandatory.
FIG. 8.
Detection of T-cell responses after infection of mice with different doses of F-MuLV plus SFFV. Mice were infected with four different doses of F-MuLV plus SFFV to investigate the effect of the inoculation dose on the type of T-cell response. (A) Spleen viral loads were compared between the different groups (doses of 100 to 40,000 spleen FFU) at 10 days postinfection. IC, infectious centers. (B and C) Flow cytometry was used to detect virus-specific (tetramer+) (B) and activated (CD43+) (C) CD8+ T cells in all groups. (D to F) In addition, intracellular granzyme A (D), granzyme B (E), or degranulation marker CD107a (F) in activated effector CD8+ T cells was measured. Accumulated results are shown as percentages of the CD43+ CD8+ T-cell population. Each dot represents an individual mouse. Experiments were repeated two times with comparable results.
DISCUSSION
Cell-mediated cytotoxicity delivered by CTL comes from either the exocytosis pathway or the FasL-Fas pathway. Although both pathways have been shown to be of importance for the control of viral infections, it was unknown which viral factors determine the differential expression of cytotoxic molecules in CTL (37). Here we show that the level of viral replication is a critical factor in this process and that the same virus can be controlled by one or the other pathway. We have previously shown that high-level replication of F-MuLV is controlled by CTL that secrete the cytotoxic molecules granzyme A, granzyme B, and perforin, all of which mediated nonredundant antiviral functions (44). The current data indicate that a threshold of viral replication is mandatory to induce the expression of these molecules in virus-specific effector CD8+ T cells. CD8+ T cells were also activated by F-MuLV replication below this threshold, and they developed antiviral activity, but they expressed only FasL and used this pathway for the control of viral spread. The data suggest that the different pathways of CTL-mediated cytotoxicity described for several viruses (27) might reflect their different replication capacities in vivo. As shown here, in many viral infections, initial viral replication level might be influenced by the inoculation dose. As a consequence the viral dose might determine the expression of cytotoxic molecules in CTL during an acute viral infection. In a large number of natural viral infections the inoculation dose is rather low. The current data imply that in such infections acute viral replication might be controlled by FasL-expressing CTL rather then CD8+ T cells expressing granzymes and perforin. In several chronic infections T cells that control viral replication via the Fas-FasL pathway also seem to play an important role. During such chronic infections, e.g., those with hepatitis C virus, human immunodeficiency virus, or FV, viral loads are usually low but at the same time CD8+ T cells often develop an impairment in the exocytosis pathway (2, 24, 25, 42, 45). In the FV model we could show that Fas-FasL-mediated cytotoxicity becomes important to control the low-level viral replication during chronic infection (44). However, in this model CD4+ T cells rather than CD8+ T cells were critical for this control (18). Interestingly, the depletion experiments in the current study showed that CD4+ T cells had an antiviral activity during F-MuLV but not F-MuLV-plus-SFFV infection. It is likely that these cells also mediated their antiviral activity by Fas-FasL interaction, which was effective during low-level but not high-level infection. Thus, it might be a common feature of the immune system to control low-level viral infections by T cells using the Fas-FasL pathway rather than the exocytosis pathway. However, the biological advantages of this strategy remain to be determined. It is, for example, possible that FasL-expressing CTL might be easier to induce than CTL expressing molecules of the exocytosis pathway or that such cells have a lower potential to induce immunopathology. For the latter scenario it would make biological sense to activate only the less-powerful weapons against a weak enemy to avoid self-damage during the battle.
An important question to answer is how the activation of the different cytotoxic pathways is regulated and what the signals in this regulation are. One likely answer to this question is that the dose of antigen available for CTL stimulation is one critical factor. The current data and data from others (5, 14) show that CTL frequencies and the level of CTL activation were strongly influenced by the level of viral replication. Viral replication is the key factor that determines the dose of antigen available for the induction and activation of T cells. In fact, Wherry et al. (43) reported that the magnitude of a CTL response generated in vivo after vaccinia virus infection of mice is proportional to the CTL epitope density. Our current data strongly suggest that the antigen dose also determines the cytotoxic pathway that CTL use for immune control of virus-infected cells. Beside the antigen dose the cytokine milieu induced by a viral infection might also play a role in antiviral CTL activity. Most viral infections induce inflammatory responses that are characterized by the production of different cytokines. Some of these cytokines have been shown to play an important role in CD8+ T-cell activation and function. Along theses lines, it has been reported that CD8+ T cells that respond to antigen and costimulation in the absence of a third signal, such as IL-12 (9), alpha interferon (IFN-α) (11), or IFN-γ (16), fail to develop cytolytic functions. In these studies, the expression of granzyme B was strongly dependent upon IL-12 as the third signal in T-cell activation (10). For the experiments described here, it is therefore possible that the low- and high-level F-MuLV infections might differ in their abilities to induce inflammation and cytokine production, such as that of IL-12 or IFN-α. This could then result in differences in CTL activation and function. However, we infected IL-12- and type I IFN receptor-knockout mice with F-MuLV plus SFFV and found that the expression of granzymes in CTL of these mice was comparable to that of wild-type mice (data not shown). Thus, either these signals did not play a role in the differential expression of cytotoxic molecules in CTL in vivo or the system is redundant in that other cytokines could compensate for the lack of IL-12 or the type I IFN signaling.
The current results have some relevance for monitoring immune responses in human infectious diseases. The evidence for a protective role of T cells in human viral infections remains mainly indirect, and in most studies, the expression of perforin or IFN-γ is used as a surrogate marker for CD8+ T-cell functions. Low antigen loads during acute or chronic viral infections are often associated with low expression of cytotoxic molecules of the exocytosis pathway or antiviral cytokines by CD8+ T cells (30). However, according to our data this does not always mean that such T cells must be functionally inactive. In contrast, they might be very important for the control of the low-level infection but use other pathways to mediate their antiviral activity.
Acknowledgments
The work was supported in part by a grant to U.D. from the Deutsche Forschungsgemeinschaft (Di 714/8-1).
Footnotes
Published ahead of print on 29 August 2007.
REFERENCES
- 1.Adachi, M., S. Suematsu, T. Kondo, J. Ogasawara, T. Tanaka, N. Yoshida, and S. Nagata. 1995. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nat. Genet. 11:294-300. [DOI] [PubMed] [Google Scholar]
- 2.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balkow, S., A. Kersten, T. T. Tran, T. Stehle, P. Grosse, C. Museteanu, O. Utermohlen, H. Pircher, F. von Weizsacker, R. Wallich, A. Mullbacher, and M. M. Simon. 2001. Concerted action of the FasL/Fas and perforin/granzyme A and B pathways is mandatory for the development of early viral hepatitis but not for recovery from viral infection. J. Virol. 75:8781-8791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betts, M. R., J. M. Brenchley, D. A. Price, S. C. De Rosa, D. C. Douek, M. Roederer, and R. A. Koup. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 281:65-78. [DOI] [PubMed] [Google Scholar]
- 5.Bocharov, G., B. Ludewig, A. Bertoletti, P. Klenerman, T. Junt, P. Krebs, T. Luzyanina, C. Fraser, and R. M. Anderson. 2004. Underwhelming the immune response: effect of slow virus growth on CD8+-T-lymphocyte responses. J. Virol. 78:2247-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, W., H. Qin, B. Chesebro, and M. A. Cheever. 1996. Identification of a gag-encoded cytotoxic T-lymphocyte epitope from FBL-3 leukemia shared by Friend, Moloney, and Rauscher murine leukemia virus-induced tumors. J. Virol. 70:7773-7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chesebro, B., M. Miyazawa, and W. J. Britt. 1990. Host genetic control of spontaneous and induced immunity to Friend murine retrovirus infection. Annu. Rev. Immunol. 8:477-499. [DOI] [PubMed] [Google Scholar]
- 8.Corazza, N., S. Muller, T. Brunner, D. Kagi, and C. Mueller. 2000. Differential contribution of Fas- and perforin-mediated mechanisms to the cell-mediated cytotoxic activity of naive and in vivo-primed intestinal intraepithelial lymphocytes. J. Immunol. 164:398-403. [DOI] [PubMed] [Google Scholar]
- 9.Curtsinger, J. M., C. M. Johnson, and M. F. Mescher. 2003. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J. Immunol. 171:5165-5171. [DOI] [PubMed] [Google Scholar]
- 10.Curtsinger, J. M., D. C. Lins, C. M. Johnson, and M. F. Mescher. 2005. Signal 3 tolerant CD8 T cells degranulate in response to antigen but lack granzyme B to mediate cytolysis. J. Immunol. 175:4392-4399. [DOI] [PubMed] [Google Scholar]
- 11.Curtsinger, J. M., J. O. Valenzuela, P. Agarwal, D. Lins, and M. F. Mescher. 2005. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J. Immunol. 174:4465-4469. [DOI] [PubMed] [Google Scholar]
- 12.Dittmer, U., D. M. Brooks, and K. J. Hasenkrug. 1998. Characterization of a live-attenuated retroviral vaccine demonstrates protection via immune mechanisms. J. Virol. 72:6554-6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dittmer, U., D. M. Brooks, and K. J. Hasenkrug. 1999. Requirement for multiple lymphocyte subsets in protection by a live attenuated vaccine against retroviral infection. Nat. Med. 5:189-193. [DOI] [PubMed] [Google Scholar]
- 14.Doherty, P. C., and J. P. Christensen. 2000. Accessing complexity: the dynamics of virus-specific T cell responses. Annu. Rev. Immunol. 18:561-592. [DOI] [PubMed] [Google Scholar]
- 15.Ellies, L. G., A. T. Jones, M. J. Williams, and H. J. Ziltener. 1994. Differential regulation of CD43 glycoforms on CD4+ and CD8+ T lymphocytes in graft-versus-host disease. Glycobiology 4:885-893. [DOI] [PubMed] [Google Scholar]
- 16.Haring, J. S., V. P. Badovinac, and J. T. Harty. 2006. Inflaming the CD8+ T cell response. Immunity 25:19-29. [DOI] [PubMed] [Google Scholar]
- 17.Hasenkrug, K. J. 1999. Lymphocyte deficiencies increase susceptibility to Friend virus-induced erythroleukemia in Fv-2 genetically resistant mice. J. Virol. 73:6468-6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasenkrug, K. J., D. M. Brooks, and U. Dittmer. 1998. Critical role for CD4+ T cells in controlling retrovirus replication and spread in persistently infected mice. J. Virol. 72:6559-6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasenkrug, K. J., and U. Dittmer. 2000. The role of CD4 and CD8 T cells in recovery and protection from retroviral infection: lessons from the Friend virus model. Virology 272:244-249. [DOI] [PubMed] [Google Scholar]
- 20.Kabat, D. 1989. Molecular biology of Friend viral erythroleukemia. Curr. Top. Microbiol. Immunol. 148:1-42. [DOI] [PubMed] [Google Scholar]
- 21.Kagi, D., B. Ledermann, K. Burki, P. Seiler, B. Odermatt, K. J. Olsen, E. R. Podack, R. M. Zinkernagel, and H. Hengartner. 1994. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 369:31-37. [DOI] [PubMed] [Google Scholar]
- 22.Lander, M. R., and S. K. Chattopadhyay. 1984. A Mus dunni cell line that lacks sequences closely related to endogenous murine leukemia viruses and can be infected by ecotropic, amphotropic, xenotropic, and mink cell focus-forming viruses. J. Virol. 52:695-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lieberman, J. 2003. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat. Rev. Immunol. 3:361-370. [DOI] [PubMed] [Google Scholar]
- 24.McMichael, A. J., and S. L. Rowland-Jones. 2001. Cellular immune responses to HIV. Nature 410:980-987. [DOI] [PubMed] [Google Scholar]
- 25.Migueles, S. A., A. C. Laborico, W. L. Shupert, M. S. Sabbaghian, R. Rabin, C. W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, and M. Connors. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061-1068. [DOI] [PubMed] [Google Scholar]
- 26.Mullbacher, A., R. T. Hla, C. Museteanu, and M. M. Simon. 1999. Perforin is essential for control of ectromelia virus but not related poxviruses in mice. J. Virol. 73:1665-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mullbacher, A., M. Regner, Y. Wang, E. Lee, M. Lobigs, and M. Simon. 2004. Can. we really learn from model pathogens? Trends Immunol. 25:524-528. [DOI] [PubMed] [Google Scholar]
- 28.Munroe, D. G., J. W. Peacock, and S. Benchimol. 1990. Inactivation of the cellular p53 gene is a common feature of Friend virus-induced erythroleukemia: relationship of inactivation to dominant transforming alleles. Mol. Cell. Biol. 10:3307-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olbrich, A. R., S. Schimmer, K. Heeg, K. Schepers, T. N. Schumacher, and U. Dittmer. 2002. Effective postexposure treatment of retrovirus-induced disease with immunostimulatory DNA containing CpG motifs. J. Virol. 76:11397-11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pantaleo, G., and A. Harari. 2006. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat. Rev. Immunol. 6:417-423. [DOI] [PubMed] [Google Scholar]
- 31.Pardo, J., A. Bosque, R. Brehm, R. Wallich, J. Naval, A. Mullbacher, A. Anel, and M. M. Simon. 2004. Apoptotic pathways are selectively activated by granzyme A and/or granzyme B in CTL-mediated target cell lysis. J. Cell Biol. 167:457-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parra, B., M. T. Lin, S. A. Stohlman, C. C. Bergmann, R. Atkinson, and D. R. Hinton. 2000. Contributions of Fas-Fas ligand interactions to the pathogenesis of mouse hepatitis virus in the central nervous system. J. Virol. 74:2447-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramshaw, I. A., A. J. Ramsay, G. Karupiah, M. S. Rolph, S. Mahalingam, and J. C. Ruby. 1997. Cytokines and immunity to viral infections. Immunol. Rev. 159:119-135. [DOI] [PubMed] [Google Scholar]
- 34.Robertson, M. N., M. Miyazawa, S. Mori, B. Caughey, L. H. Evans, S. F. Hayes, and B. Chesebro. 1991. Production of monoclonal antibodies reactive with a denatured form of the Friend murine leukemia virus gp70 envelope protein: use in a focal infectivity assay, immunohistochemical studies, electron microscopy and western blotting. J. Virol. Methods 34:255-271. [DOI] [PubMed] [Google Scholar]
- 35.Roths, J. B., E. D. Murphy, and E. M. Eicher. 1984. A new mutation, gld, that produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J. Exp. Med. 159:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubio, V., T. B. Stuge, N. Singh, M. R. Betts, J. S. Weber, M. Roederer, and P. P. Lee. 2003. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat. Med. 9:1377-1382. [DOI] [PubMed] [Google Scholar]
- 37.Russell, J. H., and T. J. Ley. 2002. Lymphocyte-mediated cytotoxicity. Annu. Rev. Immunol. 20:323-370. [DOI] [PubMed] [Google Scholar]
- 38.Schepers, K., M. Toebes, G. Sotthewes, F. A. Vyth-Dreese, T. A. Dellemijn, C. J. Melief, F. Ossendorp, and T. N. Schumacher. 2002. Differential kinetics of antigen-specific CD4+ and CD8+ T cell responses in the regression of retrovirus-induced sarcomas. J. Immunol. 169:3191-3199. [DOI] [PubMed] [Google Scholar]
- 39.Simon, H. G., U. Fruth, M. D. Kramer, and M. M. Simon. 1987. A secretable serine proteinase with highly restricted specificity from cytolytic T lymphocytes inactivates retrovirus-associated reverse transcriptase. FEBS Lett. 223:352-360. [DOI] [PubMed] [Google Scholar]
- 40.Trapani, J. A., and M. J. Smyth. 2002. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 2:735-747. [DOI] [PubMed] [Google Scholar]
- 41.Walsh, C. M., M. Matloubian, C. C. Liu, R. Ueda, C. G. Kurahara, J. L. Christensen, M. T. Huang, J. D. Young, R. Ahmed, and W. R. Clark. 1994. Immune function in mice lacking the perforin gene. Proc. Natl. Acad. Sci. USA 91:10854-10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wherry, E. J., J. N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wherry, E. J., K. A. Puorro, A. Porgador, and L. C. Eisenlohr. 1999. The induction of virus-specific CTL as a function of increasing epitope expression: responses rise steadily until excessively high levels of epitope are attained. J. Immunol. 163:3735-3745. [PubMed] [Google Scholar]
- 44.Zelinskyy, G., S. Balkow, S. Schimmer, K. Schepers, M. M. Simon, and U. Dittmer. 2004. Independent roles of perforin, granzymes, and Fas in the control of Friend retrovirus infection. Virology 330:365-374. [DOI] [PubMed] [Google Scholar]
- 45.Zelinskyy, G., S. J. Robertson, S. Schimmer, R. J. Messer, K. J. Hasenkrug, and U. Dittmer. 2005. CD8+ T-cell dysfunction due to cytolytic granule deficiency in persistent Friend retrovirus infection. J. Virol. 79:10619-10626. [DOI] [PMC free article] [PubMed] [Google Scholar]