Abstract
Recombinant vaccines based on modified vaccinia virus Ankara (MVA) have an excellent record concerning safety and immunogenicity and are currently being evaluated in numerous clinical studies for immunotherapy of infectious diseases and cancer. However, knowledge about the biological properties of target antigens to efficiently induce MVA vaccine-mediated immunity in vivo is sparse. Here, we examined distinct antigen presentation pathways and different antigen formulations contained in MVA vaccines for their capability to induce cytotoxic CD8+ T-cell (CTL) responses. Strikingly, we found that CTL responses against MVA-produced antigens were dominated by cross-priming in vivo, despite the ability of the virus to efficiently infect professional antigen-presenting cells such as dendritic cells. Moreover, stable mature protein was preferred to preprocessed antigen as the substrate for cross-priming. Our data are essential for improved MVA vaccine design, as they demonstrate the need for optimal adjustment of the target antigen properties to the intrinsic requirements of the delivering vector system.
Antigens derived from tumors, viral infections, or intracellular parasites can be recognized by cytotoxic T cells (CTL). The induction of strong CTL immunity directed against those antigens is the aim of vaccination and immunotherapy. While numerous clinical studies currently evaluate viral vectors as recombinant vaccines, very little is known about the antigen presentation pathways that are important to induce efficient T-cell immunity with these vectors. The initiation of adaptive immune responses requires antigen to be presented by professional antigen-presenting cells (pAPC), such as dendritic cells (DC) (19, 27, 50). Viral antigen presented by DC in a major histocompatibility complex (MHC) class I-restricted manner can be derived from intracellular or extracellular sources, since DC can present peptides from proteins synthesized within the infected DC itself (direct presentation) or from acquired antigen produced by other infected donor cells (cross-presentation) (1, 6, 24). Metabolic stability has been discussed as a critical factor for the availability and access of antigen for the two antigen presentation pathways. Stable antigen is thought to be the substrate for efficient cross-priming, whereas the expression of peptides or rapidly degradable protein has been reported to enhance endogenous presentation and thereby direct priming (34, 47, 55, 59). Consequently, for efficient priming of CD8+ T cells (TCD8+), antigens require distinct features to be presented optimally by a particular presentation pathway. Therefore, it might be essential to define for each vector which antigen presentation pathways govern the induction of TCD8+ to enable the selection of efficient antigen formulations.
Here, we investigated this issue for vaccines based on the modified vaccinia virus Ankara (MVA). In principle, MVA bears characteristics that enable direct priming as well as cross-priming. MVA has the ability to infect and to efficiently produce viral and recombinant antigens in both pAPC and non-pAPC (25). Interestingly, MVA induces TCD8+, which recognize so-called late viral antigens that are not synthesized within infected DC due to an early block of the viral life cycle in these pAPC; thus, they appear to be cross-primed (10, 11, 14). We further chose MVA for this study as it is one of the viral vectors being extensively evaluated for vaccination and immunotherapy. Due to its excellent safety record and immunogenicity (22, 60), recombinant MVA vaccines are now widely used as vector vaccines in clinical studies (16, 17, 30, 32, 38). Furthermore, replication-deficient vaccinia viruses (VACV), like MVA, are considered the next-generation smallpox vaccines (for a review, see reference 15).
We observed that DC are indeed infected in vivo upon vaccination with MVA, and they are able to efficiently express recombinant and viral antigens and to present them to TCD8+. The present study, however, showed that the induction of CTL immunity with vaccines based on MVA strongly, if not exclusively, depends on cross-priming. Our data suggest that antigen produced by MVA as long-lived mature protein is the preferred substrate for efficient TCD8+ priming. These findings highlight the importance of adjusting antigen formulations to the applied vector system to achieve the induction of strong CTL immunity.
MATERIALS AND METHODS
Mice and vaccinations.
HLA-A*0201-transgenic HHD mice (A*0201 mice) (36) or C57BL/6 mice were derived from in-house breeding under specific-pathogen-free conditions by following institutional guidelines. Only female mice between 8 and 12 weeks of age were used. Where indicated, mice received 20 ng of synthetic phosphorothioated CpG1668 (TIB-Molbiol, Berlin, Germany) in 100 μl phosphate-buffered saline intravenously (i.v.) the day prior to vaccination for in vivo maturation of DC. Mice were vaccinated with 107 IU MVA. For vaccination with infected cells, 106 cells per mouse were infected at a multiplicity of infection (MOI) of 10, as described for the pulse-chase experiments. Two hours postinfection, cells were washed extensively and were used for vaccination via the intraperitoneal (i.p.) route. Where indicated, infection and incubation were carried out in the presence of the irreversible proteasome inhibitor lactacystin (20 μM). For peptide labeling, DC were incubated with 1 μg/ml synthetic peptide (Biosyntan, Berlin, Germany) and extensively washed. Peptide-coated or infected DC (106 cells) were injected into the tail vein of CpG-treated or control mice. For peptide vaccination, mice were immunized subcutaneously with 0.1 mg synthetic peptide (Biosynthan) and 10 ng of synthetic CpG1668. Mice were sacrificed on day 8 postvaccination, and spleens were harvested and analyzed by intracellular cytokine staining.
Cell lines.
RMA, RMA-HHD, and TAP-deficient RMA-S-HHD mice were kindly provided by F. Lemmonier. DC2.4 cells were kindly provided by K. L. Rock. Tyrosinase-negative A375 human melanoma cells (CRL-1619) and NIH 3T3 mouse fibroblasts (CRL-1658) were purchased from the ATCC. All cells were cultured in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin, and 100 μg/ml streptomycin.
Viruses.
MVA expressing the human tyrosinase gene under control of the VACV natural early/late promoter P7.5 has been described previously (13). To obtain MVA expressing tyrosinase with a stable N-terminal fusion of ubiquitin, we mutated the ubiquitin gene at bp 227 from G to C, resulting in a change at residue 76 from glycine to alanine (ubiquitinA76), which prevents cytosolic cleavage of ubiquitin (42), and constructed the MVA transfer vector pIIIdHR-P7.5-Ub/Tyr containing the ubiquitinA76-tyrosinase fusion construct (see the supplemental material).
The plasmid pBSMelPoly was a kind gift of Andreas Suhrbier. It encodes Tyr369 as part of a polytope that recently has been described (28) and was used to construct the MVA transfer vector pIIIdHR-P7.5-Mini-Tyr.
MVA vector plasmid pIIIΔHR-P7.5-OVA was constructed by inserting the entire ovalbumin gene derived from plasmid pC-OVA (a generous gift of Hermann Wagner, Institute of Immunology, Munich, Germany) into a unique PmeI restriction site of pIIIΔHR-P7.5, placing it under the control of the VACV-specific P7.5 promoter.
A DNA sequence of the ovalbumin-specific TCD8+ epitope SIINFEKL (Ova257-264) was generated by PCR from plasmid pIIIΔHR-P7.5 and was used to construct the MVA transfer vector pIIIΔHR-P7.5-M-SIINFEKL (see the supplemental material).
MVA-Ub/Tyr, MVA-Mini-Tyr, MVA-ovalbumin (MVA-OVA), MVA-SIINFEKL, and MVA-green fluorescent protein (GFP) were generated by homologous recombination as described previously (52) using the respective transfer plasmids mentioned above. DNA genomes of recombinant viruses were analyzed by PCR. MVA strains were propagated and titrated by following standard methodology.
DC isolation, analysis, and injection.
To mature DC in vivo, mice were treated with 20 ng CpG i.v. the day before DC isolation. Spleen suspensions were digested for 30 min at 37°C with collagenase VIII and DNase I (Sigma) and then were treated for 5 min with EDTA. Splenocytes then were incubated with CD11c microbeads (Miltenyi, Bergisch Gladbach, Germany). CD11c+ DC were isolated by magnetic bead isolation according to the manufacturer's recommendations (Miltenyi). Purity was confirmed to be >80% by fluorescence-activated cell sorter (FACS) analysis. DC were stained with antibodies specific for CD11c (HL3), CD80 (16-10A1), CD86 (G/1), CD54 (3E2), I-Ab (M5/114.15.2), and HLA-A2 (BB7.2) (all from Pharmingen). Fluorochrome-conjugated isotype-matched monoclonal antibodies (MAbs) were used as controls. Propidium iodide (Molecular Probes) was added immediately before analysis for live/dead discrimination.
Quantification of antigen-specific TCD8+ responses.
Splenocytes from vaccinated mice were stimulated with either the human tyrosinase peptide 1-9 or 369-377 (58); the VACV-specific peptides derived from H3L(184-192) (14), A6L(6-14), and I1L(211-219) (37); or a control peptide for 5 h. Brefeldin A (Sigma) at 1 mg/ml was added for the last 3 h. Cells were live/dead stained with ethidium monoazide bromide (Molecular Probes) and blocked with anti-CD16/CD32-Fc-Block (Pharmingen). Surface markers were stained with APC-conjugated anti-CD8α and phycoerythrin-conjugated anti-CD62L (Caltag). Intracellular cytokine staining for gamma interferon (IFN-γ) production was performed with fluorescein isothiocyanate-anti-IFN-γ (XMG1.2) using the Cytofix/Cytoperm kit (Pharmingen) according to the manufacturer's recommendations. Data were acquired by FACS analysis on a FACSCanto (Becton Dickinson) and were analyzed with FLOWJO (Tree Star) software.
Western blot analysis.
NIH 3T3 fibroblasts were infected at an MOI of 10. Where indicated, lactacystin (10 μM) and MG-132 (20 μM) (both from Sigma) were added to inhibit proteasomal degradation. Cells were harvested in lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1% Nonidet P-40, 0.02% NaN3, and 100 μg/ml phenylmethylsulfonyl fluoride), dissolved on 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and blotted onto a nitrocellulose membrane (0.45 μM; Bio-Rad, Munich, Germany). Tyrosinase was visualized with the MAb T311 (Novocastra, Newcastle, United Kingdom) and a horseradish peroxidase-labeled anti-mouse Ab (Dianova, Hamburg, Germany) using enhanced chemiluminescence (Roche, Mannheim, Germany) as described previously (13).
Pulse-chase experiments and radioimmunoprecipitation.
For each time point, 2 × 106 RMA cells were infected at a density of 107 cells/ml and an MOI of 10. Cells were incubated on ice for 20 min and then were shifted to 37°C and diluted with fresh medium to a concentration of 106 cells/ml. Where indicated, treatment with 20 μM lactacystin was started during the starvation time and was maintained throughout all subsequent incubations. After 4 h postinfection, cells were washed twice and starved for 20 min with methionine/cysteine-free Dulbecco's modified Eagle's medium containing ultraglutamine and pyruvate, each at 1%. Cells then were adjusted to a density of 107 cells per 100 μl starving medium and transferred into prewarmed Eppendorf tubes. 35S-labeled methionine/cysteine (150 μCi) was added to each 100-μl cell suspension, and cells were immediately incubated for 45 min at 37°C under continuous shaking. To stop the pulse, 800 μl ice-cold RPMI 1640 was added, and the cells were immediately washed. Cells were diluted to 3 × 106cells/ml in RPMI 1640 and 10% FCS, transferred into a T35 culture flask (Nunc), and incubated at 37°C. At the indicated chase times, cells were resuspended and equal-sized aliquots of cells were taken, spun, lysed with Western blotting lysis buffer, and subsequently frozen on dry ice. After the last sample was taken, lysates were freeze-thawed twice and subjected to immunoprecipitation with anti-tyrosinase MAb C-19 and protein G-Sepharose (both from Santa Cruz Biotechnology) in immunoprecipitation buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 0.25% sodium deoxycholate, and 1% Nonidet P-40 with complete protease inhibitors [Roche] and the proteasome inhibitor MG132 at 20 μM). Precipitates were boiled in reduced Laemmli buffer and separated by SDS-8% PAGE before the analysis of radioactivity on fixed and dried gels was visualized with a phosphorimager.
Chromium release assays.
Specific lysis by A*0201-restricted murine CTL reactive to human tyrosinase peptide 1-9 or 369-377 or against the VACV-specific peptide B22R(79-7) (54) was determined in a 6-h standard [51Cr] release assay as described previously (14). Briefly, HLA-A*0201-positive A375 or RMA-HHD cells were infected for 2 h at an MOI of 10, washed, labeled for 1 h at 37°C with 100 μCi Na51CrO4, and then washed four times. Labeled target cells were plated in U-bottomed 96-well plates at 1 × 104 cells/well and incubated with effector cells at various effector-to-target ratios. The specific 51Cr release was determined in supernatants, which were taken at different time points after coincubation for kinetic analysis.
Antigen presentation assays.
RMA-HHD cells or freshly isolated DC were infected for 2 h at an MOI of 10 and then were washed. For ex vivo assays, purified splenic DC were isolated at the indicated times postvaccination. APC were cocultured at different ratios with antigen-specific CTL lines in the presence of 1 mg/ml brefeldin A (Sigma) for 5 h. Staining and analysis for intracellular IFN-γ production were carried out as described above. When DC were used as APC, CD11c and CD3 antibodies were included in the intracellular cytokine staining protocol. For the degranulation assay, fluorescein isothiocyanate-conjugated anti-CD107a/b (Pharmingen) and 1:1,000 monensin (eBiosciences) were added during the stimulation time, and CTL then were stained with surface markers. To detect kb/SIINFEKL complexes, infected DC2.4 cells were stained with the 25-D1.16 antibody (39) and then were labeled with Alexa Fluor 633-conjugated goat anti-mouse immunoglobulin G F(ab′)2 fragments (Molecular Probes).
Statistical analysis.
All statistical analyses were performed using GraphPad Prism4 software. Results are expressed as means ± standard errors of the means. Differences between groups were analyzed for statistical significance using two-tailed Student's t tests.
RESULTS
MVA efficiently infects murine DC and allows for antigen expression, processing, and presentation.
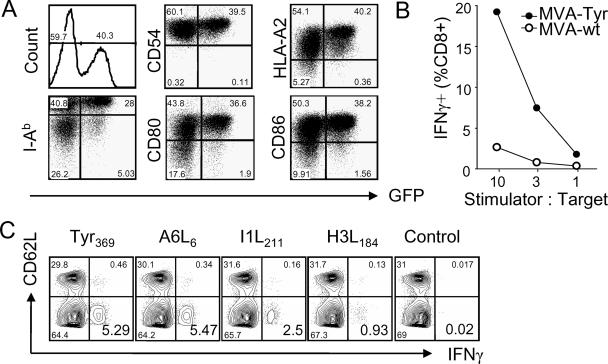
We have shown previously that MVA has the ability to infect human DC and to express viral and recombinant antigens (25). To characterize infection of murine DC, we isolated in-vivo-matured splenic DC (mDC) and infected them with MVA expressing GFP (MVA-GFP). We found that MVA efficiently infected DC, leading to strong GFP expression (Fig. 1A). Infection of DC did not reduce the surface expression of MHC class I or II glycoproteins or that of costimulatory molecules CD80 and CD86. Importantly, DC infected with MVA expressing tyrosinase (MVA-Tyr) were specifically recognized by tyrosinase-specific TCD8+, confirming expression and processing of tyrosinase as well as peptide loading onto MHC class I molecules (Fig. 1B). Experiments using bone-marrow-derived murine DC yielded similar results (data not shown). Our findings closely reflect those for MVA infection of human DC (10, 25).
FIG. 1.
MVA efficiently infects DC and induces TCD8+ specific for recombinant and viral antigens. (A) FACS analysis of MVA-GFP-infected mDC. Surface expression of MHC class I (HLA-A2), MHC class II (I-Ab), costimulatory molecules B7.1 (CD80) and B7.2 (CD86), and ICAM-1 (CD54) was analyzed for infected and noninfected mDC 6 h postinfection. (B) IFN-γ production of Tyr369-specific TCD8+ after stimulation with MVA-Tyr-infected mDC. (C) A*0201 mice were vaccinated i.p. with MVA-Tyr. Eight days later, splenocytes were incubated with the A*0201-restricted tyrosinase peptide Tyr369, the MVA-specific peptides A6L6, I1L211, and H3L184, or a control peptide and were permeabilized, stained with anti-CD8, anti-CD62L, and anti-IFN-γ antibodies, and then analyzed by flow cytometry. Depicted blots were gated on live TCD8+. Results are representative of more than three independent experiments. MVA-wt, wild-type MVA.
MVA expressing tyrosinase primes TCD8+ specific for recombinant and viral antigens.
Several HLA-A*0201-restricted peptides derived from MVA proteins have been identified recently and allowed us to monitor and to compare priming of TCD8+ specific for viral vectors to that for recombinant antigens (14, 33, 37). Vaccination of HLA-A*0201-transgenic mice (A*0201 mice) with MVA-Tyr induced robust TCD8+ responses against tyrosinase and several MVA-specific determinants (Fig. 1C). Tyrosinase-specific TCD8+ were reactive against the Tyr369 internal peptide, which is contained within the mature protein. Tyr369 is generated by proteasomal degradation and is loaded onto MHC class I molecules in a TAP-dependent manner (51, 58). Since the immunogenicity of target antigens expressed by VACV has been correlated with the expression of these antigens in DC (9), we hypothesized that increased proteasomal turnover should enhance peptide generation and MHC class I-restricted presentation and thus improve the induction of TCD8+ by MVA-infected DC (55). We therefore targeted tyrosinase for rapid proteasomal degradation by the expression of a tyrosinase gene stably fused to monomeric ubiquitin (Ub/Tyr) (see the supplemental material).
Ubiquitylation of tyrosinase leads to rapid proteasomal degradation.
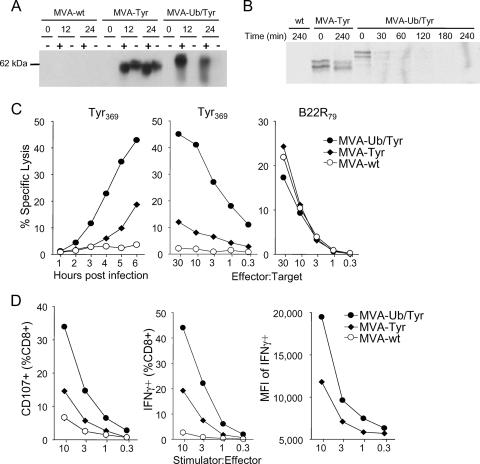
In Western blot analyses (Fig. 2A), ubiquitylated tyrosinase produced by MVA-Ub/Tyr was slightly larger in size than authentic tyrosinase expressed by MVA-Tyr, in line with the fusion to the 8-kDa ubiquitin. Notably, ubiquitylated tyrosinase expressed by MVA-Ub/Tyr was detectable only in the presence of specific proteasome inhibitors. Under these conditions, the total amount of accumulating protein was comparable between viruses. Without proteasome inhibition, the amount of tyrosinase in MVA-Ub/Tyr-infected cells was below the detection limit, indicating that ubiquitylation of tyrosinase resulted in rapid proteasomal degradation. Pulse-chase experiments conducted with a 45-min (Fig. 2B) or 10-min pulse (data not shown) indicated that ubiquitylated tyrosinase was subject to rapid degradation with a half-life of less than 30 min, whereas authentic tyrosinase, which is known to have a half-life of more than 10 h (23), was stable over the entire observation period. These experiments confirmed that comparable amounts of tyrosinase protein were expressed by MVA-Tyr and MVA-Ub/Tyr.
FIG. 2.
Ubiquitylation of tyrosinase leads to rapid proteasomal degradation and enhanced MHC class I/peptide loading. (A) Western blot analysis of NIH cells infected with wild-type MVA (MVA-wt), MVA-Tyr, or MVA-Ub/Tyr in the presence (+) or absence (−) of specific proteasome inhibitors. At 0, 12, or 24 h postinfection, cell lysates were resolved by SDS-PAGE. (B) Pulse-chase labeling of RMA cells infected with wild-type MVA, MVA-Tyr, or MVA-Ub/Tyr. After a brief pulse with 35S-labeled methionine, cells were further incubated. At the indicated time points after pulsing, immunoprecipitation was performed. (C) Cr51 release assay of infected A375 target cells. Tyr369- or VACV-B22R79-specific lysis is shown after the indicated time points postinfection (left graph) or for different effector-to-target ratios (middle and right graphs). (D) FACS analysis of staining of degranulation marker CD107 (left graph) or IFN-γ production (middle and right graphs) of Tyr369-specific TCD8+ after coincubation with in-vitro-infected mDC (MOI = 10) at the indicated stimulator-to-effector ratios. All results are representative of at least three independent experiments. MFI, mean fluorescent intensity.
Ubiquitylation of tyrosinase enhances MHC class I peptide loading of pAPC and non-pAPC.
Next, we tested whether rapid proteasomal degradation of tyrosinase increased peptide generation and loading onto MHC class I molecules. In chromium release assays, expression of ubiquitylated tyrosinase resulted in significantly enhanced Tyr369-specific CTL recognition of infected target cells compared to that of MVA-Tyr-infected cells. MVA-Ub/Tyr-infected A375 cells were lysed at lower effector-to-target ratios and earlier during the viral infection (Fig. 2C). As a control, infected target cells assayed against VACV-specific T cells (B22R79) showed lysis levels that were comparable between the two viruses. These results indicated that rapid degradation of tyrosinase leads to enhanced peptide processing and presentation of peptide/MHC class I complexes. Similar results were obtained with A*0201-transfected mouse target cells (data not shown). Additionally, MVA-Ub/Tyr-infected pAPC consistently had a superior capacity to stimulate Tyr369-specific degranulation and IFN-γ production (Fig. 2D), the latter being greater not only in the number of IFN-γ+ cells but also in the total amount of IFN-γ produced per cell. Therefore, both pAPC and non-pAPC present increased amounts of Tyr369/MHC class I complexes on their surfaces when infected with MVA-Ub/Tyr, resulting in more efficient activation of CTL in vitro.
Rapid degradation of MVA-delivered antigen impairs T-cell priming.
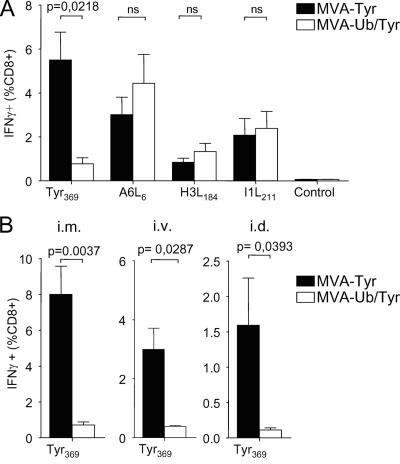
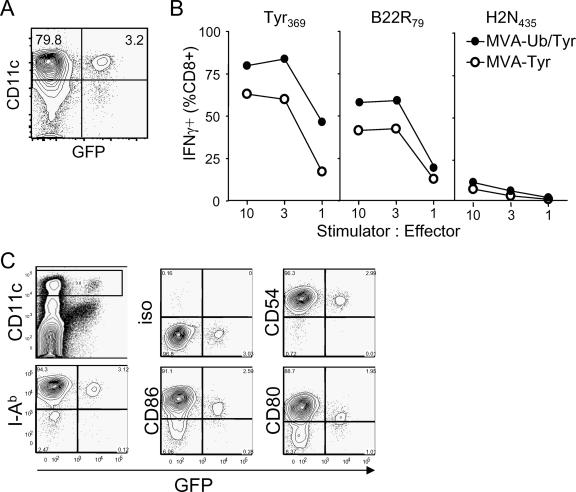
As infection of DC with MVA-Ub/Tyr resulted in enhanced presentation of Tyr369 peptides compared to that with MVA-Tyr-infected DC, we reasoned that vaccination with MVA-Ub/Tyr would enhance TCD8+ priming if direct priming by infected DC dominated the TCD8+ response. Surprisingly, the induction of tyrosinase-specific TCD8+ was dramatically reduced (by more than 80%) in mice that received MVA-Ub/Tyr compared to that of mice that were vaccinated with MVA-Tyr (Fig. 3A). Notably, frequencies of TCD8+ directed against determinants derived from viral proteins were comparable between the two groups, indicating that the differences observed for tyrosinase-specific TCD8+ were due merely to the altered metabolic stability of tyrosinase. Route-specific effects could be ruled out, since similar results were obtained with intramuscular, i.v., and intradermal administration of the vaccine (Fig. 3B). To exclude the possibility that DC were infected in vitro but not in vivo, we vaccinated mice i.v. with MVA-GFP, purified splenic DC 8 h postvaccination, and analyzed them for GFP expression. We found that 3 to 5% of the CD11cbright DC were infected (Fig. 4A). To test whether in-vivo-infected DC were able to stimulate T cells and if enhanced degradation of Ub/Tyr also would increase the presentation of tyrosinase peptides, we vaccinated mice with MVA-Tyr and MVA-Ub/Tyr and purified splenic DC 8 h postvaccination. DC from vaccinated mice were able to stimulate VACV-B22R79- as well as Tyr369-specific T cells (Fig. 4B). DC isolated from groups of mice that received MVA-Ub/Tyr efficiently activated Tyr369-specific T cells, which confirmed the results obtained with in-vitro-infected cells (Fig. 2D). These experiments indicated that DC were indeed infected in vivo by vaccination with MVA and that the expression of ubiquitylated tyrosinase in these DC also enhanced direct presentation and the capacity to stimulate CTL ex vivo. However, this antigen presentation by infected DC did not correlate with the primary T-cell response. As there was no apparent inhibition of direct antigen presentation in vitro and in vivo, we further analyzed infected DC for the expression of costimulatory molecules. We failed to detect any down-regulation of costimulatory molecules after infecting DC in vitro (Fig. 1A); however, in-vivo-infected DC expressed smaller amounts of CD80 and CD86 than uninfected (GFP-negative) DC (Fig. 4C). Based on these observations, we speculated that the primary CTL response induced by MVA vaccination does not depend on antigen presentation by directly infected DC but rather is induced by DC that acquire antigen from other infected cells and cross-present it to naïve T cells.
FIG. 3.
Rapid degradation of antigen impairs TCD8+ priming. (A) Groups of A*0201 mice (n = 4) were vaccinated i.p. with MVA-Tyr or MVA-Ub/Tyr. Tyrosinase- and vector-specific TCD8+ responses on day 8 postvaccination are indicated as the percentage of TCD8+ splenocytes producing IFN-γ in response to the indicated peptides. (B) The same experiment was repeated with mice vaccinated by different routes: intramuscular (i.m.) (left), i.v. (middle), or intradermal (i.d.) (right). Tyr369-specific responses are indicated. No significant differences in the responses to vector-specific peptides were detected (data not shown). Data are representative of three independent experiments. ns, not significant.
FIG. 4.
DC are infected and present MVA-delivered antigen on MHC class I in vivo. (A) Mice were infected i.v. with MVA-GFP. After 8 h, CD11c-sorted splenic DC were analyzed for purity and GFP expression. (B) Mice were infected i.v. with MVA-Tyr or MVA-Ub/Tyr. After 8 h, splenic DC were purified and coincubated with T-cell lines reactive against the indicated peptides. Specific activation of T cells was detected by analyzing them for IFN-γ production. (C) FACS analysis of in-vivo-infected splenic DC 8 h postvaccination. Data are representative of three independent experiments. iso, isotope control.
Cross-presentation of MVA-encoded antigen is sufficient to prime TCD8+.
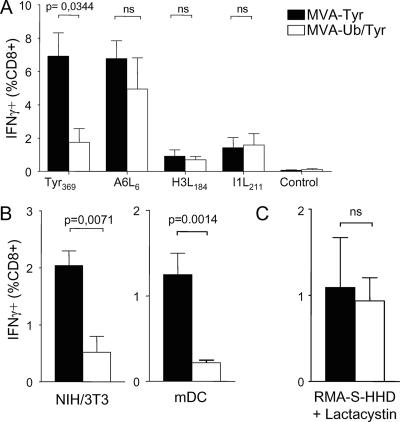
To test this hypothesis, we prevented direct presentation of viral and recombinant antigens. We vaccinated mice with TAP-deficient RMA-S-HHD cells that were infected with recombinant MVA. In these experiments, TCD8+ responses are induced by cross-presentation of antigen synthesized in the infected non-pAPC. We found that vaccination with MVA-infected cells yielded T-cell responses comparable to those induced by the live vaccines with regard to size and the immunodominance hierarchy of TCD8+ specific for viral and recombinant determinants (Fig. 5A). To exclude that residual infectivity was inoculated with these cells, we titrated aliquots of the vaccine preparations. The amount of transferred virus was reduced by more than 2 logs, which proved to be insufficient to prime detectable TCD8+ responses when given as a live vaccine (data not shown). Comparable results were obtained with analogous experiments using HLA-mismatched NIH 3T3 cells (Fig. 5B). Similarly to TAP-deficient or MHC-mismatched non-pAPC, vaccination with syngeneic DC induced a strong T-cell response against Tyr369 only when DC were infected with MVA-Tyr expressing long-lived antigen. When DC where infected with MVA-Ub/Tyr, T-cell priming against Tyr369 was markedly reduced (Fig. 5B), despite the strong direct TCD8+ stimulatory capacity of these DC (Fig. 2D and 4B). These experiments demonstrated that cross-presentation alone is sufficient to elicit a strong T-cell response to MVA vaccines. Importantly, cross-presentation of tyrosinase induced a strong TCD8+ response only when the MVA-infected cells expressed long-lived antigen and not ubiquitylated tyrosinase, indicating a reduced suitability of short-lived antigen for cross-presentation in the context of MVA vaccines. To test whether this was due specifically to the degradation of ubiquitylated tyrosinase, we vaccinated mice with RMA-S-HHD cells that were infected in the presence of the irreversible proteasome inhibitor lactacystin. As a consequence of the inhibited proteasomal degradation, infected cells accumulate similar amounts of tyrosinase whether they are infected with MVA-Tyr or MVA-Ub/Tyr (Fig. 2A) and therefore should be able to cross-prime comparable tyrosinase-specific TCD8+ responses. Indeed, infected RMA-S-HHD cells induced similar amounts of Tyr369-specific IFN-γ-producing TCD8+ when infected with MVA-Tyr or MVA-Ub/Tyr (Fig. 5C). Consistent with the observation that lactacystin also reduces protein synthesis (40; and data not shown) and rapidly induces apoptosis in RMA cells (45; and data not shown), the magnitude of the T-cell response was reduced compared to that of RMA-S cells that were infected with MVA without lactacystin treatment. We deduce from these experiments that efficient cross-priming requires the accumulation of antigens that can serve as proteasomal substrates.
FIG. 5.
Cross-presentation of MVA-encoded antigen is sufficient to prime TCD8+. (A) TAP-deficient RMA-S-HHD cells were infected with MVA-Tyr or MVA-Ub/Tyr for 2 h, washed extensively, and used to vaccinate groups of A*0201 mice (n = 4). The images depict tyrosinase- and vector-specific TCD8+ responses on day 8 postvaccination. (B) The same experiment was repeated with NIH 3T3 cells (left graph) or syngeneic bone-marrow-derived mDC (right graph). (C) The experiment was repeated as described for panel A with infected RMA-S-HHD cells, which were additionally treated with the irreversible proteasome inhibitor lactacystin (20 μM). Tyr369-specific responses are indicated. No significant differences in the responses to vector-specific peptides were detected (data not shown). Data are representative of three independent experiments. ns, not significant.
In vivo maturation of DC abrogates TCD8+ priming with MVA vaccines.
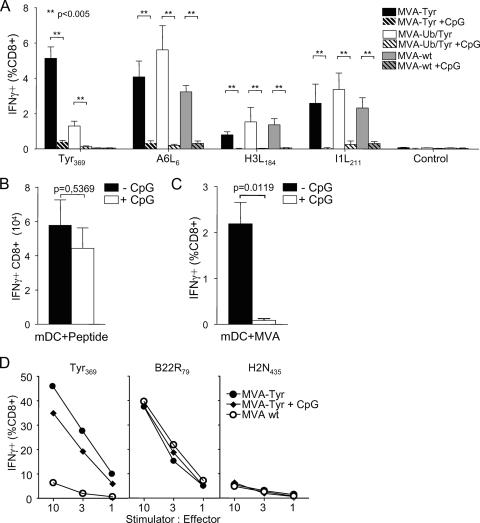
Exclusive cross-priming induced a TCD8+ response that was similar to that using MVA as a live vaccine, which should allow for both antigen-presenting pathways. We next interfered with the cross-presentation pathway and analyzed the MVA-induced T-cell responses. We took advantage of CpG-induced in vivo maturation of DC recently described to down-regulate the uptake of exogenous antigen, an essential step for cross-presentation (57). In animals treated with CpG prior to infection, TCD8+ priming for recombinant and viral antigenic determinants was reduced by more than 90% compared to that of untreated MVA-infected mice (Fig. 6A). As a control, we assessed the capacity of antigen-presenting DC to prime TCD8+ responses in CpG-treated mice. In vivo, peptide-pulsed DC primed comparable amounts of antigen-specific TCD8+ in CpG-pretreated and untreated mice (Fig. 6B). This confirmed that the abrogation of T-cell priming was due specifically to the inhibition of cross-presentation and was not caused by a general impairment of T-cell induction through CpG treatment. Interestingly, vaccination with ex-vivo-infected DC only induced a Tyr369-specific TCD8+ response in untreated mice, suggesting an impaired ability of infected DC to prime TCD8+ if cross-presentation was disabled (Fig. 6C). To exclude the possibility that CpG treatment could alter the capacity of DC to endogenously express, process, or present recombinant and viral antigens in vivo, we isolated splenic DC 6 to 8 h postimmunization and coincubated them with CTL lines reactive against viral and recombinant antigens. Independently, whether mice received CpG treatment 15 h before vaccination or not, splenic DC were fully capable of stimulating antigen-specific IFN-γ production in TCD8+ (Fig. 6D). Although we could detect direct presentation in both groups, this appeared to be insufficient to prime T cells. The interference with cross-presentation, however, abrogated T-cell priming. From these experiments, we conclude that cross-presentation is the dominating pathway for the priming of TCD8+ T cells immunized with MVA.
FIG. 6.
In vivo maturation of DC abrogates TCD8+ priming with MVA vaccines. (A) Groups of A*0201 mice (n = 4) either were CpG treated or were left untreated 1 day prior to vaccination with wild-type MVA (MVA-wt), MVA-Tyr, or MVA-Ub/Tyr. On day 8 postvaccination, tyrosinase- and vector-specific TCD8+ responses were analyzed by intracellular cytokine staining in splenocytes (**, P < 0.005). (B) CpG-pretreated or untreated mice (n = 4) were immunized with Tyr369-peptide-coated in-vivo-matured mDC. Total numbers of Tyr369-specific TCD8+ on day 8 postvaccination are indicated. (C) In-vivo-matured mDC were infected with MVA-Tyr, washed extensively, and used to vaccinate groups of A*0201 mice (n = 4) that either had received CpG treatment the day before or were left untreated. Images show Tyr369-specific TCD8+ responses on day 8 postvaccination. (D) Groups of A*0201 mice (n = 5) were either CpG treated or left untreated 1 day prior to vaccination with wild-type MVA or MVA-Tyr. Seven hours postvaccination, splenic mDC were isolated and coincubated with TCD8+ specific for Tyr369, VACV-B22R79, or irrelevant H2N435. Specific activation of TCD8+ was detected by intracellular staining for IFN-γ production. Results are representative for three independent experiments.
The dominating pathway of antigen presentation dictates antigen requisites.
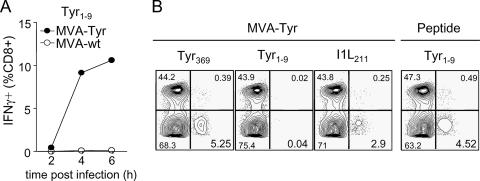
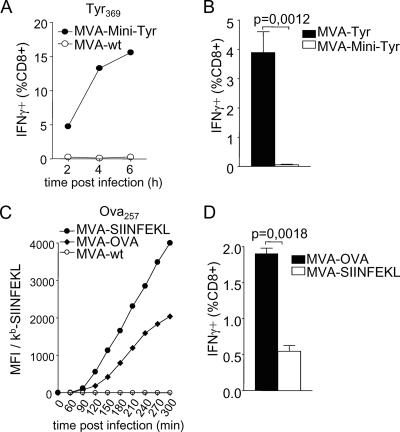
Our findings suggested that the underlying pathway for TCD8+ priming has major consequences for antigen formulations contained in MVA-based vaccines (Fig. 5). To test this prediction, we explored whether mice vaccinated with MVA-Tyr were able to prime TCD8+ responses to a second tyrosinase peptide (Tyr1-9) that, in contrast to Tyr369, is derived from the signal sequence and is presented independently of TAP (58). Peptides located in the signal sequence can be efficiently presented via the classical endogenous route but are not cross-presented (59). Consistently, target cells infected with MVA-Tyr also were recognized by Tyr1-9-specific CTL (Fig. 7A), confirming efficient endogenous processing and presentation of Tyr1-9. Mice vaccinated with MVA-Tyr mounted strong TCD8+ responses to Tyr369 but failed to prime TCD8+ specific for Tyr1-9. Importantly, Tyr1-9-specific TCD8+ could be induced by peptide vaccination (Fig. 7B). We next investigated the efficiency of polytope or minigene formulations, which are commonly used in vector-based vaccines and are currently being evaluated in clinical studies. Again, our data predicted a reduced capacity of minigene constructs to prime TCD8+ compared to that of vaccines expressing the mature protein. We used a recombinant MVA expressing Tyr369 as part of a polytope (MVA-Mini-Tyr). MVA-Mini-Tyr-infected target cells presented the Tyr369 peptide (Fig. 8A), but vaccination with MVA-Mini-Tyr completely failed to prime Tyr369-specific TCD8+ (Fig. 8B). Additionally, we used a well-established model antigen and constructed MVA expressing full-length OVA (MVA-OVA) or the Ova257 peptide SIINFEKL as a minigene (MVA-SIINFEKL) (see the supplemental material). Surface staining of kb/SIINFEKL complexes showed that direct presentation was enhanced when non-pAPC (data not shown) or pAPC were infected with MVA-SIINFEKL compared to direct presentation of cells infected with MVA-OVA (Fig. 8C). For vaccination of C57BL/6 mice with the respective viruses, both vaccines induced a comparable vector-specific response (data not shown), but MVA expressing the long-lived antigen primed about four times more SIINFEKL-specific TCD8+ than MVA encoding the SIINFEKL minigene (Fig. 8D).
FIG. 7.
Cross-priming of TCD8+ dictates antigen requisites. (A) RMA-HHD cells were infected with MVA-Tyr or wild-type MVA (MVA-wt) and coincubated with Tyr1-9-reactive CTL. Specific activation of TCD8+ was assessed by intracellular staining for IFN-γ production. (B) Groups of A*0201 mice (n = 4) were vaccinated with MVA-Tyr or with the Tyr1-9 peptide. Representative plots from intracellular IFN-γ staining on day 8 postvaccination are depicted. Data are representative of three independent experiments.
FIG. 8.
Dominating pathway of antigen presentation can be targeted by the antigen formulation. (A) RMA-HHD cells were infected with MVA expressing the tyrosinase peptide Tyr369 encoded in a minigene (MVA-Mini-Tyr) or wild-type MVA (MVA-wt) and were coincubated with Tyr369-reactive CTL. Specific activation of TCD8+ was assessed by intracellular staining for IFN-γ production. (B) Groups of A*0201 mice (n = 4) were vaccinated with MVA-Mini-Tyr or MVA expressing full-length tyrosinase (MVA-Tyr) and were analyzed on day 8 postvaccination for Tyr369-specific IFN-γ production. (C) DC2.4 cells were infected with wild-type MVA, MVA expressing full-length OVA, or the OVA peptide SIINFEKL as a minigene. At indicated times postinfection, cells were analyzed for the surface expression of kb/SIINFEKL complexes. Data are shown as mean fluorescence intensities (MFI). Note that wild-type MVA-infected cells define background staining. (D) Groups of C57BL/6 mice (n = 4) were vaccinated with MVA-OVA or MVA-SIINFEKL. TCD8+ responses on day 8 postvaccination are indicated. All results are representative of three independent experiments.
Taking the results together, our study demonstrates that the induction of CTL immunity with vaccines based on MVA strongly, if not exclusively, depends on cross-priming and therefore requires vaccines to be designed for the expression of long-lived antigens suitable for cross-presentation.
DISCUSSION
Recently, substantial progress has been made in the characterization of the antigen presentation pathways for MHC class I-restricted determinants. However, for many vectors it is still unknown which pathways contribute to the primary induction of TCD8+ responses. This is of particular interest, because the biological properties of an antigen allowing efficient direct presentation or cross-presentation seem to differ (8, 34, 47, 55, 59). As we have demonstrated, a short half-life of endogenous antigens delivered by a viral vector leads to efficient processing and direct presentation of antigenic determinants. On the other hand, rapid degradation of antigen limits the availability for the cross-presentation pathway, which efficiently exploits mature protein. We have shown that the infection with the viral vector MVA comprises central features that in principle would allow for direct TCD8+ priming. MVA-infected DC exhibited strong antigen expression and presentation in vitro and in vivo. A role for direct priming in MVA immunity therefore seemed to be likely. Strikingly, however, we found that TCD8+ priming is dominated by cross-presentation when MVA-based vaccines are used. This notion is supported by several lines of evidence. (i) Recombinant MVA expressing rapidly degradable protein enhanced direct presentation in vitro and in vivo but failed to prime strong CTL immunity. (ii) Cross-priming experiments using infected TAP-deficient or MHC class I-mismatched non-pAPC donor cells evoked similar TCD8+ responses regarding CTL frequencies and the immunodominance hierarchy when MVA was given as a live vaccine. (iii) Inhibition of cross-presentation by in vivo maturation of DC almost completely abrogated priming of MVA-specific TCD8+. Importantly, systemic maturation of DC did not inhibit their capacity for endogenous expression, processing, or presentation of antigens or their ability to directly prime TCD8+ in vivo. (iv) In all the experiments performed, we never found a correlation between the ensuing TCD8+ response and the generation of antigenic peptides or direct presentation of these determinants by infected cells. The priming of TCD8+ instead required the expression of antigenic determinants as a substrate suitable for cross-presentation. Accordingly, we observed no TCD8+ priming against an epitope derived from a signal peptide (59). TCD8+ responses strongly correlated with the steady-state level of long-lived antigen, which we found to be the substrate for efficient cross-presentation when delivered by MVA. Importantly, our findings are not dependent on the route of vaccination and apply to different model antigens tested in different mouse strains.
Taking our results together, we conclude that the functionally relevant pathway to induce TCD8+ responses with MVA vaccines in vivo is cross-priming. Although we cannot exclude a minor role for direct priming, our data strongly argue that this potential contribution to T-cell priming is not relevant for vaccine design with this vector. Residual TCD8+ responses observed upon vaccination with MVA encoding rapidly degradable antigen or minigene constructs also could be explained by cross-presentation of peptides or polypeptides, which has been found to be quite inefficient (34, 47, 59). Therefore, our findings possibly indicate a functionally exclusive role of cross-presentation for the induction of primary TCD8+ responses with MVA vaccines. In the past, this has been postulated only for those viruses that do not infect DC (49, 53) or that considerably interfere with DC antigen presentation (61). To our knowledge, the present study is the first to provide evidence that cross-priming can dominate the induction of CTL to a virus that efficiently infects DC and allows strong antigen presentation in these pAPC.
Our observations strongly support the hypothesis that the transfer of substrates for the proteasome (34, 47, 59) rather than postproteasomal products (7, 8, 46) enables efficient TCD8+ cross-priming. In accordance with studies conducted with replication-competent VACV (34), we found that the expression of rapidly degradable proteins abrogated T-cell priming when infected cells were used as cross-priming vaccines. We additionally observed that T cells were efficiently cross-primed against viral antigens. However, our experiments revealed considerable differences between immunizations with the attenuated MVA strain and immunizations with replication-competent VACV. Although cross-presentation of VACV-derived antigens has been observed (5, 26, 46, 48), several studies demonstrated a functional role of direct priming for the response to VACV infection (5, 35, 48). Consistently, the delivery of destabilized antigens or minigenes by immunization with VACV had no disadvantage or even enhanced T-cell priming (34, 55). When contained in MVA vaccines, by contrast, these antigen formulations failed to induce strong CTL responses, and in this regard they resembled data obtained with Semliki Forest virus, a vector that is unable to infect DC, and therefore TCD8+ are thought to be induced via cross-priming (20).
Beyond replication resulting in sustained antigen expression (versus abortive infection with a single round of antigen expression), there are other explanations that could account for the dominating role of cross-presentation in MVA responses in contrast to that seen with VACV infection. During its host range adaptation, MVA lost multiple gene products, including at least two viral proteins with proposed antiapoptotic functions (3, 4, 12). Accordingly, a recent study showed that DC undergo apoptosis earlier with MVA infection than with VACV infection and that MVA infection leads to an accelerated shutdown of host cell protein synthesis in DC (10). We found that MVA-infected DC are unable to mature even when treated with cytokines (25). In addition, VACV, including MVA, have been reported to impair the capacity of DC to migrate and to adequately respond to chemokines (21). We speculate that the inability of MVA-infected DC to prime TCD8+ could result from an altered functional plasticity and possibly the incapacity to form immunological synapses. Interestingly, VACV infection has been found to affect cytoskeleton arrangement and cell contractility (43, 44, 56). However, we believe that the accelerated induction of apoptosis combined with the severe host cell protein synthesis shutdown could be sufficient to prevent several steps required for T-cell priming. Recent data from our laboratory indicate an in vivo half-life of MVA-infected DC of ∼9 h (data not shown). As stable interactions between T cells and DC start to form after 8 h during priming (31), the rapid induction of apoptosis in MVA-infected DC per se could explain the observed inefficient direct priming.
Extending the above observations, our data suggest that MVA-infected DC used in transfer vaccination protocols (11) could function mainly as carriers for antigen to be cross-presented by endogenous pAPC (2, 41). Similar to vaccinations with the live vaccine, primary T-cell responses elicited by MVA-infected DC did not correlate with direct presentation of antigenic peptides but depended on the availability of mature protein within these pAPC. Since DC restrict VACV gene expression to the early viral life cycle (25), the amount of antigen available for cross-presentation may even be limited. Further evaluation is needed to determine whether other cell types that allow for extended target gene expression and that might be easier to obtain from patients could improve such vaccination protocols.
The insights gained here open the door for a variety of potential targets to improve vaccine efficacy by improving cross-presentation. In this respect, localizing antigen to a certain subcellular compartment, the coexpression of cytokines or molecules that enhance cross-presentation, or the use of adjuvants should be studied. Moreover, our data underscore the requirement for a more detailed understanding of how candidate vaccines for immunotherapy elicit TCD8+ responses. As we demonstrate, it is essential to adjust the properties of target antigens to the delivering vector and the underlying pathway of antigen presentation. In the case of vectors that work via direct presentation, such as lentiviral vectors (18), targeting proteins for rapid degradation should enhance CTL immunity. On the other hand, our immunization experiments confirmed that the preferred substrate for cross-priming in vivo is stable mature protein. Therefore, the synthesis of large amounts of long-lived antigen needs to be optimized for vectors that rely on cross-priming, such as MVA. Since MVA-based vectors can easily accommodate large inserts, the expression of full-length antigens provides a strategy to optimally target the dominant antigen presentation pathway and to overcome the restriction to defined MHC haplotypes or immunodominant epitopes targeted by minigene constructs. Furthermore, large vaccine inserts might also minimize microbial escape from CTL immunity by eliciting T-cell responses to multiple epitopes.
The vector-specific antigen requirements that we found here might help to explain the somewhat disappointing results of a recent series of clinical studies in which antigen-specific T-cell responses were not detectable ex vivo after primary vaccination with MVA-human immunodeficiency virus type 1 vaccines (16, 38). Similarly, only the protein portion, and not a multiepitope string, simultaneously produced by an MVA malaria vaccine proved to be immunogenic in another clinical trial (29), indicating that our findings also may apply to humans.
The present study suggests that the primary induction of CTL immunity with MVA vaccines above all depends on cross-presentation, which actually dictates the antigen formulation required to design potent vectors. As several clinical protocols already apply MVA as vector vaccines, it should be feasible to rapidly investigate these issues with humans and potentially enhance vaccine efficacy against infectious diseases and cancer.
Supplementary Material
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 456-B7 to I.D.) and the EU (LSHP-CT-2006-037536 to G.S.).
We thank R. Baier for expert technical assistance and Hermann Wagner for critical reading of the manuscript.
Footnotes
Published ahead of print on 15 August 2007.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86-89. [DOI] [PubMed] [Google Scholar]
- 2.Allan, R. S., J. Waithman, S. Bedoui, C. M. Jones, J. A. Villadangos, Y. Zhan, A. M. Lew, K. Shortman, W. R. Heath, and F. R. Carbone. 2006. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25:153-162. [DOI] [PubMed] [Google Scholar]
- 3.Antoine, G., F. Scheiflinger, F. Dorner, and F. G. Falkner. 1998. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology 244:365-396. [DOI] [PubMed] [Google Scholar]
- 4.Aoyagi, M., D. Zhai, C. Jin, A. E. Aleshin, B. Stec, J. C. Reed, and R. C. Liddington. 2007. Vaccinia virus N1L protein resembles a B cell lymphoma-2 (Bcl-2) family protein. Protein Sci. 16:118-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basta, S., W. Chen, J. R. Bennink, and J. W. Yewdell. 2002. Inhibitory effects of cytomegalovirus proteins US2 and US11 point to contributions from direct priming and cross-priming in induction of vaccinia virus-specific CD8(+) T cells. J. Immunol. 168:5403-5408. [DOI] [PubMed] [Google Scholar]
- 6.Bevan, M. J. 1976. Minor H antigens introduced on H-2 different stimulating cells cross-react at the cytotoxic T cell level during in vivo priming. J. Immunol. 117:2233-2238. [PubMed] [Google Scholar]
- 7.Binder, R. J., and P. K. Srivastava. 2005. Peptides chaperoned by heat-shock proteins are a necessary and sufficient source of antigen in the cross-priming of CD8+ T cells. Nat. Immunol. 6:593-599. [DOI] [PubMed] [Google Scholar]
- 8.Blachère, N. E., R. B. Darnell, and M. L. Albert. 2005. Apoptotic cells deliver processed antigen to dendritic cells for cross-presentation. PLoS Biol. 3:e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bronte, V., M. W. Carroll, T. J. Goletz, M. Wang, W. W. Overwijk, F. Marincola, S. A. Rosenberg, B. Moss, and N. P. Restifo. 1997. Antigen expression by dendritic cells correlates with the therapeutic effectiveness of a model recombinant poxvirus tumor vaccine. Proc. Natl. Acad. Sci. USA 94:3183-3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chahroudi, A., D. A. Garber, P. Reeves, L. Liu, D. Kalman, and M. B. Feinberg. 2006. Differences and similarities in viral life cycle progression and host cell physiology after infection of human dendritic cells with modified vaccinia virus Ankara and vaccinia virus. J. Virol. 80:8469-8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Nicola, M., C. Carlo-Stella, R. Mortarini, P. Baldassari, A. Guidetti, G. F. Gallino, M. Del Vecchio, F. Ravagnani, M. Magni, P. Chaplin, N. Cascinelli, G. Parmiani, A. M. Gianni, and A. Anichini. 2004. Boosting T cell-mediated immunity to tyrosinase by vaccinia virus-transduced, CD34(+)-derived dendritic cell vaccination: a phase I trial in metastatic melanoma. Clin. Cancer Res. 10:5381-5390. [DOI] [PubMed] [Google Scholar]
- 12.Dobbelstein, M., and T. Shenk. 1996. Protection against apoptosis by the vaccinia virus SPI-2 (B13R) gene product. J. Virol. 70:6479-6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drexler, I., E. Antunes, M. Schmitz, T. Wolfel, C. Huber, V. Erfle, P. Rieber, M. Theobald, and G. Sutter. 1999. Modified vaccinia virus Ankara for delivery of human tyrosinase as melanoma-associated antigen: induction of tyrosinase- and melanoma-specific human leukocyte antigen A*0201-restricted cytotoxic T cells in vitro and in vivo. Cancer Res. 59:4955-4963. [PubMed] [Google Scholar]
- 14.Drexler, I., C. Staib, W. Kastenmuller, S. Stevanovic, B. Schmidt, F. A. Lemonnier, H. G. Rammensee, D. H. Busch, H. Bernhard, V. Erfle, and G. Sutter. 2003. Identification of vaccinia virus epitope-specific HLA-A*0201-restricted T cells and comparative analysis of smallpox vaccines. Proc. Natl. Acad. Sci. USA 100:217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drexler, I., C. Staib, and G. Sutter. 2004. Modified vaccinia virus Ankara as antigen delivery system: how can we best use its potential? Curr. Opin. Biotechnol. 15:506-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goonetilleke, N., S. Moore, L. Dally, N. Winstone, I. Cebere, A. Mahmoud, S. Pinheiro, G. Gillespie, D. Brown, V. Loach, J. Roberts, A. Guimaraes-Walker, P. Hayes, K. Loughran, C. Smith, J. De Bont, C. Verlinde, D. Vooijs, C. Schmidt, M. Boaz, J. Gilmour, P. Fast, L. Dorrell, T. Hanke, and A. J. McMichael. 2006. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J. Virol. 80:4717-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrop, R., N. Connolly, I. Redchenko, J. Valle, M. Saunders, M. G. Ryan, K. A. Myers, N. Drury, S. M. Kingsman, R. E. Hawkins, and M. W. Carroll. 2006. Vaccination of colorectal cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) induces immune responses which correlate with disease control: a phase I/II trial. Clin. Cancer Res. 12:3416-3424. [DOI] [PubMed] [Google Scholar]
- 18.He, Y., J. Zhang, C. Donahue, and L. D. Falo, Jr. 2006. Skin-derived dendritic cells induce potent CD8(+) T cell immunity in recombinant lentivector-mediated genetic immunization. Immunity 24:643-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang, A. Y., P. Golumbek, M. Ahmadzadeh, E. Jaffee, D. Pardoll, and H. Levitsky. 1994. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science 264:961-965. [DOI] [PubMed] [Google Scholar]
- 20.Huckriede, A., L. Bungener, M. Holtrop, J. de Vries, B. L. Waarts, T. Daemen, and J. Wilschut. 2004. Induction of cytotoxic T lymphocyte activity by immunization with recombinant Semliki Forest virus: indications for cross-priming. Vaccine 22:1104-1113. [DOI] [PubMed] [Google Scholar]
- 21.Humrich, J. Y., P. Thumann, S. Greiner, J. H. Humrich, M. Averbeck, C. Schwank, E. Kampgen, G. Schuler, and L. Jenne. 2007. Vaccinia virus impairs directional migration and chemokine receptor switch of human dendritic cells. Eur. J. Immunol. 37:954-965. [DOI] [PubMed] [Google Scholar]
- 22.Imoukhuede, E. B., T. Berthoud, P. Milligan, K. Bojang, J. Ismaili, S. Keating, D. Nwakanma, S. Keita, F. Njie, M. Sowe, S. Todryk, S. M. Laidlaw, M. A. Skinner, T. Lang, S. Gilbert, B. M. Greenwood, and A. V. Hill. 2006. Safety and immunogenicity of the malaria candidate vaccines FP9 CS and MVA CS in adult Gambian men. Vaccine 24:6526-6533. [DOI] [PubMed] [Google Scholar]
- 23.Jiménez, M., K. Kameyama, W. L. Maloy, Y. Tomita, and V. J. Hearing. 1988. Mammalian tyrosinase: biosynthesis, processing, and modulation by melanocyte-stimulating hormone. Proc. Natl. Acad. Sci. USA 85:3830-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung, S., D. Unutmaz, P. Wong, G. Sano, K. De los Santos, T. Sparwasser, S. Wu, S. Vuthoori, K. Ko, F. Zavala, E. G. Pamer, D. R. Littman, and R. A. Lang. 2002. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity 17:211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kastenmuller, W., I. Drexler, H. Ludwig, V. Erfle, C. Peschel, H. Bernhard, and G. Sutter. 2006. Infection of human dendritic cells with recombinant vaccinia virus MVA reveals general persistence of viral early transcription but distinct maturation-dependent cytopathogenicity. Virology 350:276-288. [DOI] [PubMed] [Google Scholar]
- 26.Larsson, M., J. F. Fonteneau, S. Somersan, C. Sanders, K. Bickham, E. K. Thomas, K. Mahnke, and N. Bhardwaj. 2001. Efficiency of cross presentation of vaccinia virus-derived antigens by human dendritic cells. Eur. J. Immunol. 31:3432-3442. [DOI] [PubMed] [Google Scholar]
- 27.Lenz, L. L., E. A. Butz, and M. J. Bevan. 2000. Requirements for bone marrow-derived antigen-presenting cells in priming cytotoxic T cell responses to intracellular pathogens. J. Exp. Med. 192:1135-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mateo, L., J. Gardner, Q. Chen, C. Schmidt, M. Down, S. L. Elliott, S. J. Pye, H. Firat, F. A. Lemonnier, J. Cebon, and A. Suhrbier. 1999. An HLA-A2 polyepitope vaccine for melanoma immunotherapy. J. Immunol. 163:4058-4063. [PubMed] [Google Scholar]
- 29.McConkey, S. J., W. H. Reece, V. S. Moorthy, D. Webster, S. Dunachie, G. Butcher, J. M. Vuola, T. J. Blanchard, P. Gothard, K. Watkins, C. M. Hannan, S. Everaere, K. Brown, K. E. Kester, J. Cummings, J. Williams, D. G. Heppner, A. Pathan, K. Flanagan, N. Arulanantham, M. T. Roberts, M. Roy, G. L. Smith, J. Schneider, T. Peto, R. E. Sinden, S. C. Gilbert, and A. V. Hill. 2003. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat. Med. 9:729-735. [DOI] [PubMed] [Google Scholar]
- 30.McShane, H., A. A. Pathan, C. R. Sander, S. M. Keating, S. C. Gilbert, K. Huygen, H. A. Fletcher, and A. V. Hill. 2004. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat. Med. 10:1240-1244. [DOI] [PubMed] [Google Scholar]
- 31.Mempel, T. R., S. E. Henrickson, and U. H. Von Andrian. 2004. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427:154-159. [DOI] [PubMed] [Google Scholar]
- 32.Meyer, R. G., C. M. Britten, U. Siepmann, B. Petzold, T. A. Sagban, H. A. Lehr, B. Weigle, M. Schmitz, L. Mateo, B. Schmidt, H. Bernhard, T. Jakob, R. Hein, G. Schuler, B. Schuler-Thurner, S. N. Wagner, I. Drexler, G. Sutter, N. Arndtz, P. Chaplin, J. Metz, A. Enk, C. Huber, and T. Wolfel. 2005. A phase I vaccination study with tyrosinase in patients with stage II melanoma using recombinant modified vaccinia virus Ankara (MVA-hTyr). Cancer Immunol. Immunother. 54:453-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moutaftsi, M., B. Peters, V. Pasquetto, D. C. Tscharke, J. Sidney, H. H. Bui, H. Grey, and A. Sette. 2006. A consensus epitope prediction approach identifies the breadth of murine TCD8+-cell responses to vaccinia virus. Nat. Biotechnol. 24:817-819. [DOI] [PubMed] [Google Scholar]
- 34.Norbury, C. C., S. Basta, K. B. Donohue, D. C. Tscharke, M. F. Princiotta, P. Berglund, J. Gibbs, J. R. Bennink, and J. W. Yewdell. 2004. CD8+ T cell cross-priming via transfer of proteasome substrates. Science 304:1318-1321. [DOI] [PubMed] [Google Scholar]
- 35.Norbury, C. C., D. Malide, J. S. Gibbs, J. R. Bennink, and J. W. Yewdell. 2002. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nat. Immunol. 3:265-271. [DOI] [PubMed] [Google Scholar]
- 36.Pascolo, S., N. Bervas, J. M. Ure, A. G. Smith, F. A. Lemonnier, and B. Perarnau. 1997. HLA-A2.1-restricted education and cytolytic activity of CD8(+) T lymphocytes from β2 microglobulin (β2m) HLA-A2.1 monochain transgenic H-2Db β2m double knockout mice. J. Exp. Med. 185:2043-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasquetto, V., H. H. Bui, R. Giannino, C. Banh, F. Mirza, J. Sidney, C. Oseroff, D. C. Tscharke, K. Irvine, J. R. Bennink, B. Peters, S. Southwood, V. Cerundolo, H. Grey, J. W. Yewdell, and A. Sette. 2005. HLA-A*0201, HLA-A*1101, and HLA-B*0702 transgenic mice recognize numerous poxvirus determinants from a wide variety of viral gene products. J. Immunol. 175:5504-5515. [DOI] [PubMed] [Google Scholar]
- 38.Peters, B. S., W. Jaoko, E. Vardas, G. Panayotakopoulos, P. Fast, C. Schmidt, J. Gilmour, M. Bogoshi, G. Omosa-Manyonyi, L. Dally, L. Klavinskis, B. Farah, T. Tarragona, P. A. Bart, A. Robinson, C. Pieterse, W. Stevens, R. Thomas, B. Barin, A. J. McMichael, J. A. McIntyre, G. Pantaleo, T. Hanke, and J. Bwayo. 2007. Studies of a prophylactic HIV-1 vaccine candidate based on modified vaccinia virus Ankara (MVA) with and without DNA priming: effects of dosage and route on safety and immunogenicity. Vaccine 25:2120-2127. [DOI] [PubMed] [Google Scholar]
- 39.Porgador, A., J. W. Yewdell, Y. Deng, J. R. Bennink, and R. N. Germain. 1997. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity 6:715-726. [DOI] [PubMed] [Google Scholar]
- 40.Qian, S. B., J. R. Bennink, and J. W. Yewdell. 2005. Quantitating defective ribosome products. Methods Mol. Biol. 301:271-281. [DOI] [PubMed] [Google Scholar]
- 41.Racanelli, V., S. E. Behrens, J. Aliberti, and B. Rehermann. 2004. Dendritic cells transfected with cytopathic self-replicating RNA induce crosspriming of CD8+ T cells and antiviral immunity. Immunity 20:47-58. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez, F., J. Zhang, and J. L. Whitton. 1997. DNA immunization: ubiquitination of a viral protein enhances cytotoxic T-lymphocyte induction and antiviral protection but abrogates antibody induction. J. Virol. 71:8497-8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanderson, C. M., M. Way, and G. L. Smith. 1998. Virus-induced cell motility. J. Virol. 72:1235-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schepis, A., B. Schramm, C. A. de Haan, and J. K. Locker. 2006. Vaccinia virus-induced microtubule-dependent cellular rearrangements. Traffic 7:308-323. [DOI] [PubMed] [Google Scholar]
- 45.Schwarz, K., R. de Giuli, G. Schmidtke, S. Kostka, M. van den Broek, K. B. Kim, C. M. Crews, R. Kraft, and M. Groettrup. 2000. The selective proteasome inhibitors lactacystin and epoxomicin can be used to either up- or down-regulate antigen presentation at nontoxic doses. J. Immunol. 164:6147-6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serna, A., M. C. Ramirez, A. Soukhanova, and L. J. Sigal. 2003. Cutting edge: efficient MHC class I cross-presentation during early vaccinia infection requires the transfer of proteasomal intermediates between antigen donor and presenting cells. J. Immunol. 171:5668-5672. [DOI] [PubMed] [Google Scholar]
- 47.Shen, L., and K. L. Rock. 2004. Cellular protein is the source of cross-priming antigen in vivo. Proc. Natl. Acad. Sci. USA 101:3035-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen, X., S. B. Wong, C. B. Buck, J. Zhang, and R. F. Siliciano. 2002. Direct priming and cross-priming contribute differentially to the induction of CD8+ CTL following exposure to vaccinia virus via different routes. J. Immunol. 169:4222-4229. [DOI] [PubMed] [Google Scholar]
- 49.Sigal, L. J., S. Crotty, R. Andino, and K. L. Rock. 1999. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature 398:77-80. [DOI] [PubMed] [Google Scholar]
- 50.Sigal, L. J., and K. L. Rock. 2000. Bone marrow-derived antigen-presenting cells are required for the generation of cytotoxic T lymphocyte responses to viruses and use transporter associated with antigen presentation (TAP)-dependent and -independent pathways of antigen presentation. J. Exp. Med. 192:1143-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skipper, J. C., R. C. Hendrickson, P. H. Gulden, V. Brichard, A. Van Pel, Y. Chen, J. Shabanowitz, T. Wolfel, C. L. Slingluff, Jr., T. Boon, D. F. Hunt, and V. H. Engelhard. 1996. An HLA-A2-restricted tyrosinase antigen on melanoma cells results from posttranslational modification and suggests a novel pathway for processing of membrane proteins. J. Exp. Med. 183:527-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Staib, C., I. Drexler, M. Ohlmann, S. Wintersperger, V. Erfle, and G. Sutter. 2000. Transient host range selection for genetic engineering of modified vaccinia virus Ankara. BioTechniques 28:1137-1148. [DOI] [PubMed] [Google Scholar]
- 53.Subklewe, M., C. Paludan, M. L. Tsang, K. Mahnke, R. M. Steinman, and C. Munz. 2001. Dendritic cells cross-present latency gene products from Epstein-Barr virus-transformed B cells and expand tumor-reactive CD8(+) killer T cells. J. Exp. Med. 193:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Terajima, M., J. Cruz, G. Raines, E. D. Kilpatrick, J. S. Kennedy, A. L. Rothman, and F. A. Ennis. 2003. Quantitation of CD8+ T cell responses to newly identified HLA-A*0201-restricted T cell epitopes conserved among vaccinia and variola (smallpox) viruses. J. Exp. Med. 197:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tobery, T. W., and R. F. Siliciano. 1997. Targeting of HIV-1 antigens for rapid intracellular degradation enhances cytotoxic T lymphocyte (CTL) recognition and the induction of de novo CTL responses in vivo after immunization. J. Exp. Med. 185:909-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valderrama, F., J. V. Cordeiro, S. Schleich, F. Frischknecht, and M. Way. 2006. Vaccinia virus-induced cell motility requires F11L-mediated inhibition of RhoA signaling. Science 311:377-381. [DOI] [PubMed] [Google Scholar]
- 57.Wilson, N. S., G. M. Behrens, R. J. Lundie, C. M. Smith, J. Waithman, L. Young, S. P. Forehan, A. Mount, R. J. Steptoe, K. D. Shortman, T. F. de Koning-Ward, G. T. Belz, F. R. Carbone, B. S. Crabb, W. R. Heath, and J. A. Villadangos. 2006. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat. Immunol. 7:165-172. [DOI] [PubMed] [Google Scholar]
- 58.Wölfel, C., I. Drexler, A. Van Pel, T. Thres, N. Leister, W. Herr, G. Sutter, C. Huber, and T. Wolfel. 2000. Transporter (TAP)- and proteasome-independent presentation of a melanoma-associated tyrosinase epitope. Int. J. Cancer 88:432-438. [PubMed] [Google Scholar]
- 59.Wolkers, M. C., N. Brouwenstijn, A. H. Bakker, M. Toebes, and T. N. Schumacher. 2004. Antigen bias in T cell cross-priming. Science 304:1314-1317. [DOI] [PubMed] [Google Scholar]
- 60.Wyatt, L. S., P. L. Earl, L. A. Eller, and B. Moss. 2004. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc. Natl. Acad. Sci. USA 101:4590-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yewdell, J. W., and A. B. Hill. 2002. Viral interference with antigen presentation. Nat. Immunol. 3:1019-1025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.