Abstract
Human T-cell leukemia virus type 1 (HTLV-1) but not HTLV-2 is associated with adult T-cell leukemia, and the distinct pathogenicity of these two closely related viruses is thought to stem from the distinct biological functions of the respective transforming proteins, HTLV-1 Tax1 and HTLV-2 Tax2. In this study, we demonstrate that Tax1 but not Tax2 interacts with NF-κB2/p100 and activates it by inducing the cleavage of p100 into the active transcription factor p52. Using RNA interference methods, we further show that NF-κB2/p100 is required for the transformation induced by Tax1, as determined by the ability to convert a T-cell line (CTLL-2) from interleukin-2 (IL-2)-dependent to -independent growth. While Tax2 shows a reduced transforming activity relative to Tax1, Tax2 fused with a PDZ domain binding motif (PBM) present only in Tax1 shows transforming activity equivalent to that of Tax1 in CTLL-2 cells expressing an inducer of p52 processing. These results reveal that the activation of NF-κB2/p100 plays a crucial role in the Tax1-mediated transformation of T cells and that NF-κB2/p100 activation and PBM function are both responsible for the augmented transforming activity of Tax1 relative to Tax2, thus suggesting that these Tax1-specific functions play crucial roles in HTLV-1 leukemogenesis.
Adult T-cell leukemia (ATL) is an aggressive form of leukemia characterized by the malignant proliferation of CD4 T cells infected with human T-cell leukemia virus type 1 (HTLV-1) (10, 26, 39). HTLV-1 is an oncoretrovirus that immortalizes human CD4 T cells in vitro (11). This immortalization event per se is not, however, sufficient for ATL development since only a fraction of HTLV-1-infected individuals (approximately 5%) suffer ATL after a long latency period (60 years on average) (10, 26). Accumulating evidence suggests that both genetic and epigenetic changes in HTLV-1-infected T cells and the deterioration of the host immune systems are involved in ATL development (10, 26).
HTLV-2 is genetically and biologically similar to HTLV-1. HTLV-2 can also immortalize primary human T cells in vitro, thereby establishing a life-long persistent infection in infected individuals, although HTLV-2 preferentially immortalizes CD8 T cells. In spite of such similarities, HTLV-2 is not associated with the development of ATL or any other malignancies, thus suggesting that HTLV-2 is unable to promote a certain step(s) in leukemogenesis (8).
HTLV-1 and HTLV-2 encode functionally and structurally similar proteins, Tax1 and Tax2, respectively, whose expression plays a central role in immortalizations of infected T cells and their persistent infections (1, 10, 12, 13, 33, 35, 37). Tax1 and Tax2 have multiple functions in T cells, including the activation of cellular genes through transcription factors NF-κB, AP-1, serum response factor, and CREB/ATF, and the inactivation of several tumor suppressor genes, such as p53 (5, 9, 11, 13, 17, 25, 37, 44). Among these, the activation of NF-κB is essential for the Tax-mediated transformation of human T-cell lines by HTLV-1 as well as HTLV-2 (37). For instance, mutant HTLV-1 and HTLV-2 viruses carrying tax1 and tax2 genes, which are null for NF-κB activation, cannot immortalize primary human T cells (33, 34).
There are two NF-κB-signaling pathways that mediate the transcriptional activations of two different NF-κB binding activities, derived from the precursor proteins NF-κB1/p105 and NF-κB2/p100, and these two pathways are called the “canonical” and “noncanonical” NF-κB pathways, respectively (6). While the activation of the canonical pathway results in the degradation of its inhibitor IκB and the translocation of the p50 (NF-κB1)/RelA complex into the nucleus, the activation of the noncanonical pathway results in the processing of the p100 (NF-κB2)/RelB complex into p52 (NF-κB2)/RelB and its translocation to the nucleus. These activated p50/RelA and p52/RelB complexes transcriptionally regulate different sets of genes involved in innate or adaptive immunity, cell proliferation, and cell survival (6). While Tax1 is known to activate both the canonical and noncanonical pathways (41), there is no evidence that Tax2 also activates the noncanonical NF-κB pathway. This study demonstrates that Tax2 cannot activate the noncanonical NF-κB pathway and that the activation of that pathway by Tax1 is crucially important for the transformation of the mouse T-cell line CTLL-2. It will also further be shown that the augmented transforming activity of Tax1 relative to Tax2 in the T-cell line CTLL-2 is caused by the interaction of NF-κB2/p100 activation and the PDZ domain binding motif (PBM), C-terminal (S/T)XV (where S/T is serine or threonine, X is any amino acid, and V is valine), which exists in only Tax1 but not Tax2. This study, therefore, reveals a cooperative role of the noncanonical NF-κB pathway and PBM in Tax1-mediated T-cell transformation and leukemogenesis.
MATERIALS AND METHODS
Cells and cell culture conditions.
CTLL-2 is a mouse cytotoxic T-cell line that grows in an interleukin-2 (IL-2)-dependent manner. CTLL-2/Tax1 is a Tax1-transformed CTLL-2 cell line that grows in an IL-2-independent manner (16). The human T-cell lines used in the present study have been characterized previously (18, 29). Jurkat is an HTLV-1-negative human T-cell line. SLB-1 and ILT-Koy are HTLV-1-positive human T-cell lines. PBL01 and PBL2 are HTLV-2-positive human T-cell lines (31). Jurkat, SLB-1, and CTLL-2/Tax1 were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) (RPMI-10% FBS). CTLL-2 was cultured in RPMI-10% FBS medium supplemented with 55 μM 2-mercaptoethanol and 500 pM recombinant human IL-2. ILT-Koy, PBL01, and PBL2 were cultured in RPMI-20% FBS medium containing 500 pM IL-2. 293T is a human embryonic kidney cell line and was cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS.
Plasmids.
The lentiviral expression vectors CSII-EF-MCS and CSII-CMV-MCS-IRES2-Bsd (where EF is elongation factor, CMV is cytomegalovirus, MCS is multiple cloning site, and IRES is internal ribosome entry site) were kindly provided by H. Miyoshi (RIKEN Tsukuba Institute). CSII-EF-IG is a bicistronic enhanced green fluorescent protein (EGFP) expression vector, constructed by inserting an IRES-EGFP fragment from pMXs-IG (a gift from T. Kitamura) into the NotI and HpaI sites of CSII-EF-MCS. CSII-EF-IB is a bicistronic blasticidin resistance gene expression vector, constructed by inserting an IRES-Bsd fragment from CSII-CMV-MCS-IRES2-Bsd into the EcoRI and XbaI sites of CSII-EF-MCS. The Gateway destination vectors, CS-EF-IG-RfA and CS-EF-IB-RfA, were constructed by inserting the Gateway reading frame cassette A (Invitrogen) into the EcoRI site of CSII-EF-IG and CSII-EF-IB, respectively. Tax expression vectors pHβPr-1-neoTax1, pHβPr-1-neoTax1ΔC, pHβPr-1-neoTax2B, pHβPr-1-neoTax2B+C, pHβPr-1-neoTax221, and pHβPr-1-neoTax2A have been described previously (15). The respective tax genes and an EGFP gene from pEGFP-N3 (Clontech) were amplified by PCR, cloned into pENTR/D-TOPO (Invitrogen), and then transferred to CSII-EF-IG-RfA or CSII-EF-IB-RfA by an LR recombination reaction using LR Clonase (Invitrogen). pMX-kBL7 is a retroviral expression vector for an N-terminal deletion mutant of NF-κB-inducing kinase (ΔN-NIK) as described previously (14). An AccI-PmaCI fragment containing the ΔN-NIK coding sequence from pMX-kBL7 was inserted into the EcoRI site of pMX by blunt-end ligation to generate pMX-kBL7-417. The ΔN-NIK gene fragment, excised from pMX-kBL7-417 using BamHI and SalI, was inserted into the BamHI and XhoI sites of pENTR2B (Invitrogen) and transferred to CSII-EF-IB-RfA using the LR recombination reaction. Human NF-κB2/p100 expression vector pEFneo-p100 was generated by inserting a HindIII-NotI fragment from pBluescript-p100 into the BamHI and NotI sites of pEFneo by blunt-end ligation. Lentiviral vectors expressing short hairpin RNAs (shRNA) against mouse NF-κB2/p100 or control shRNA were purchased from Sigma.
Lentiviruses.
Recombinant lentiviruses were generated by transfecting pCAG-HIVgp, pCMV-VSV-G-RSV-Rev (provided by H. Miyoshi) and the respective lentiviral vectors into 293T cells by using FuGENE 6 (Roche). The resultant lentiviruses were used to infect CTLL-2 or Jurkat cells (4 × 105) in a final volume of 2 ml of RPMI-10% FBS medium containing 8 μg/ml polybrene with 500 pM IL-2 for CTLL-2 or without the addition of IL-2 for Jurkat cells. To establish stably infected cell lines, cells were further cultured in the selection medium containing 14 μg/ml blasticidin or 2 μg/ml puromycin.
Immunoprecipitation and Western blotting.
To prepare total cell extracts, cells were lysed in sodium dodecyl sulfate (SDS) sample buffer (2% SDS, 62.5 mM Tris-HCl, pH 6.8, 10% glycerol, 50 mM dithiothreitol, 0.01% bromophenol blue). For immunoprecipitation assays, 293T cells were transiently transfected with the indicated plasmids (see Fig. 2). The cells were lysed in ice cold lysis buffer (1% Nonidet P-40, 25 mM Tris-HCl, pH 7.2, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 20 μg/ml aprotinin) 48 h after the transfection. Cleared cell lysates were immunoprecipitated with anti-p100 antibody (C-5). Precipitated proteins or total cell extracts were separated by SDS-polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane, and probed with anti-Tax1 antibody (Taxy-7), anti-Tax2 antibody (GP3738), anti-p100 antibody, anti-NIK antibody (H-248), or anti-α-tubulin antibody (DM1A), followed by visualization using an ECL Western blotting detection system (GE Health Science). Anti-Tax2 antibody has been previously described (28). Anti-p100 antibody and anti-NIK antibody were purchased from Santa Cruz Biotechnology. Anti-α-tubulin antibody was purchased from Calbiochem.
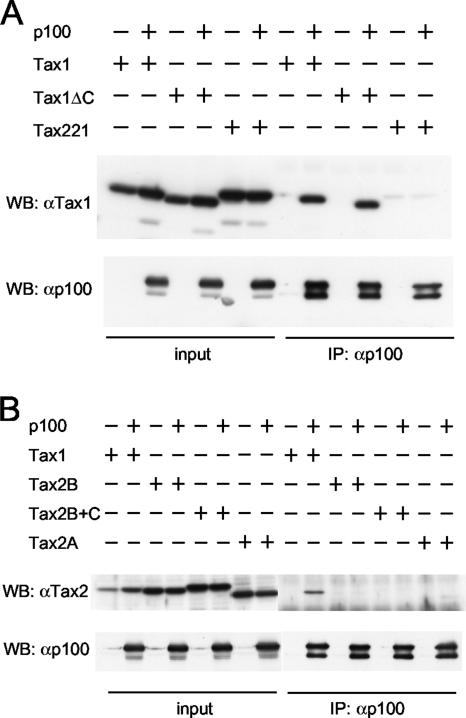
FIG. 2.
Tax1 but not Tax2 interacts with p100 in 293T cells. 293T cells were transfected with the indicated Tax expression plasmids together with a p100 expression plasmid. At 48 h following transfection, the cell lysates were prepared and immunoprecipitated (IP) with anti-p100 antibody. Precipitated proteins were characterized by Western blot analysis with anti-Tax1 (A), anti-Tax2 (B), or anti-p100 antibody (A and B). A 2% aliquot of the lysates removed before immunoprecipitation was also characterized by Western blot (WB) analysis with anti-Tax1 (A), anti-Tax2 (B), or anti-p100 antibody. α, anti.
Transformation assay.
The IL-2-independent transformation assay was done as previously described (16). Briefly, CTLL-2 cells infected with Tax-encoding lentiviruses were cultured in 96-well plates (1 × 104, 3−1 × 104, 3−2 × 104, and 3−3 × 104 cells/well) without IL-2 for 4 weeks. The number of wells containing outgrowing cells was counted using light microscopy.
RESULTS
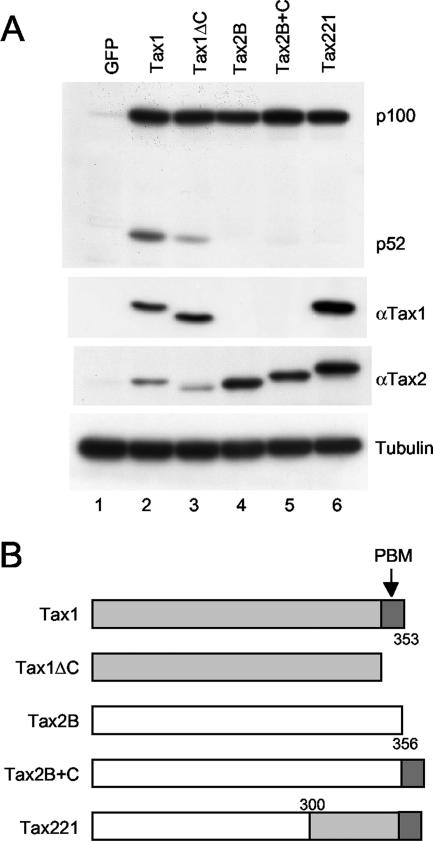
Tax1 but not Tax2 induces processing of NF-κB2/p100.
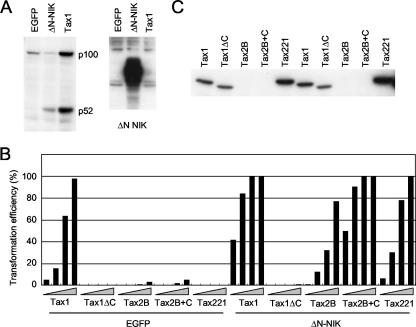
To examine the activity of Tax1 and Tax2 toward the noncanonical NF-κB pathway, a human T-cell line, Jurkat, was infected with lentiviruses encoding Tax1 or Tax2B, and the amount of NF-κB2/p100 and its processed product p52 was determined by a Western blot analysis using anti-p100/p52 antibody (Fig. 1A). Tax1 in Jurkat cells efficiently induced p100 expression and its processing to p52, and these findings are consistent with those of previous reports (Fig. 1A, lane 2) (30, 41). The increased expression of p100 in Tax1-infected cells probably occurs at the transcriptional level, since Tax1 activates transcription of the NF-κB2/p100 gene through the canonical NF-κB pathway (22). Tax2 also induced p100 expression at a level similar to that for Tax1, but processed p52 was undetectable (Fig. 1A, lane 4). A Western blot analysis using anti-Tax1 or anti-Tax2 antibody showed that Tax1 and Tax2 were both expressed in the respective infected cells. The amount of Tax1 and Tax2B in the infected cells could not be compared, since the anti-Tax1 antibody did not recognize Tax2B while the anti-Tax2 antibody recognized Tax1 with a reduced affinity. However, the lentiviral vectors that were used here express a bicistronic transcript encoding Tax1 or Tax2B, together with an EGFP, which enables us to monitor the efficiency of lentivirus infections. Approximately 50 to 60% of the Jurkat cells were EGFP positive with equivalent intensity as determined by fluorescence-activated cell sorting analysis, suggesting that Tax1 and Tax2B are equivalently expressed at least at the transcriptional level (data not shown). Taken together, these results indicate that Tax2 is unable to induce the processing of NF-κB2/p100 in Jurkat cells.
FIG. 1.
The status of NF-κB2/p100 in Tax-transduced Jurkat cells. (A) Jurkat cells were infected with lentiviruses encoding the indicated proteins. Cell lysates were prepared 48 after infection and probed with anti-p100, anti-Tax1, anti-Tax2, or anti-alpha-tubulin antibody. Infection titers of the indicated lentiviruses were normalized by EGFP expression translated from bicistronic transcript encoding Tax genes. Anti-Tax2 antibody recognizes Tax1 protein with lower affinity than Tax2. (B) The structure of Tax1, Tax2B, and their mutants. The boundary amino acids of the chimeras are indicated. α, anti.
Previously, our studies demonstrated that Tax1 transforms the mouse T-cell line CTLL-2 much more efficiently than Tax2, as measured by the conversion from IL-2 dependent to -independent cell growth. The difference was governed by a PBM present only in Tax1 but not in Tax2 (21, 40). To examine whether the Tax1 PBM is also involved in the activation of NF-κB2/p100, the Tax PBM mutants characterized previously were used (15). Tax1ΔC is a Tax1 mutant with a 4-amino-acid deletion corresponding to PBM. Tax2B+C is a Tax2B mutant with an additional 10 amino acids at the Tax1 C terminus containing a PBM. Tax221 is a chimeric gene between Tax2B and Tax1, which also has a PBM at its C terminus (Fig. 1B). Lentiviruses encoding these Tax mutants were transduced into Jurkat cells, and the efficiency of NF-κB2/p100 processing was determined by Western blot analysis. Tax1ΔC also induced p100 expression at a similar level as Tax1, but it processed less p100 than Tax1 (Fig. 1A, lane 3). The reduced activity of Tax1ΔC relative to Tax1 is not explained by the lower level of protein expression, since the infected cells equivalently expressed Tax1ΔC and Tax1 proteins. However, Tax1 PBM alone was not sufficient to induce p100 processing, since cells infected with lentiviruses encoding either Tax2B+C or Tax221, which have the intact PBM sequence, did not show any p100 processing into p52. It should be noted that Tax221 is recognized by both anti-Tax1 and anti-Tax2 antibodies but had no p100 processing activity, and the amount of Tax221 was even greater than that of Tax1. This further confirms that the inability of Tax2B to induce p100 processing was not due to its lower expression relative to Tax1. In addition, our previous study showed that the transcriptional activity of Tax1, Tax2B, and their PBM mutants to the NF-κB and CREB pathways was equivalent in an embryonic kidney cell line (293T) as determined by a luciferase reporter assay, thus further suggesting that they are equivalently expressed in cells (32). Taken together, these results suggest that Tax1 activates both the canonical and noncanonical NF-κB pathways, that Tax2B can activate only the canonical NF-κB pathway, and that Tax1 requires the PBM for efficient p100 processing.
Tax1 but not Tax2 interacts with p100.
A previous report demonstrated that Tax1 physically interacts with NF-κB2/p100, and this interaction is required for Tax1-mediated p100 processing (41). To investigate whether the inability of Tax2 to induce p100 processing is due to its inability to interact with p100, 293T cells were transiently transfected with the Tax1, Tax1ΔC, Tax221, Tax2B, Tax2B+C, or Tax2A expression vectors together with the p100 expression vector. Forty-eight hours after the transfection, the cell lysates were subjected to immunoprecipitation with anti-p100 antibody, and the immunoprecipitates were characterized by Western blot analysis using either anti-Tax1 (Fig. 2A) or anti-Tax2 antibody, which also recognizes Tax1 but with reduced affinity (Fig. 2B). Tax1 and Tax1ΔC were significantly coimmunoprecipitated with p100 (Fig. 1A), indicating that the interaction of Tax1 with p100 is independent of the Tax1 PBM and that the reduced p100 processing activity of Tax1ΔC is not due to its lack of interaction with p100. On the other hand, there was little Tax2B, Tax2B+C, Tax2A, or Tax221 coimmunoprecipitated with p100 (Fig. 1A and B). These results suggest that Tax2 is not able to interact with p100 and that the inability to interact with p100 is a major cause for the defect of Tax2 in NF-κB2/p100 processing.
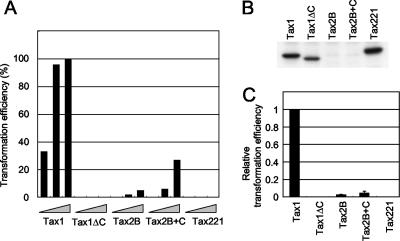
Activation of NF-κB2/p100 by Tax1 correlates with the transforming activity.
An assay system was established to investigate the transforming activity of Tax in an IL-2-dependent T-cell line, based on the lentiviral transduction of tax genes into CTLL-2 cells. Using this system, the transforming activities of Tax1, Tax2B, and their mutants described above were compared (Fig. 3). CTLL-2 cells infected with the appropriate Tax-encoding lentiviruses were seeded in 96-well plates in the absence of IL-2. The level of Tax protein expression was assessed by probing with anti-Tax1 antibody, and the expression levels of Tax1, Tax1ΔC, and Tax221 were almost equivalent (Fig. 3B). Unfortunately, anti-Tax2 antibody was insufficiently reactive to detect the introduced Tax2B and Tax2B+C in CTLL-2 cells, since the lentiviruses infected only about 1 to 2% of cells in this experimental setting as monitored by EGFP expression (data not shown) and since the sensitivity of anti-Tax2 antibody (GP3738) was lower than that of anti-Tax1 antibody (Taxy-7). Instead, the virus titers had been normalized using EGFP staining in Jurkat cells, and the amount of Tax2B was equivalent to that of Tax1 in those cells (Fig. 1A). After 4 weeks of culture, the wells containing outgrowing cells were counted using light microscopy (Fig. 3A and C). As observed in our previous report, Tax1 efficiently transformed CTLL-2 cells from IL-2-dependent growth to IL-2-independent growth whereas the transforming activity of Tax1ΔC was greatly compromised (40). Tax2B also transformed CTLL-2 cells, though with a significantly lower efficiency than Tax1 (21). The addition of the PBM to Tax2B (Tax2B+C and Tax221) had a minimal positive effect on the transforming activity of Tax2B. Since these Tax proteins with low transforming activity (Tax1ΔC, Tax2B, Tax2B+C, and Tax221) showed reduced or no p100 processing (Fig. 1A), these results suggest that the activation of NF-κB2/p100 by Tax1 plays an important role in the transforming activity toward CTLL-2 cells.
FIG. 3.
The transforming activity of Tax1, Tax2B, and their mutants. (A and B) CTLL-2 cells were infected with lentiviruses encoding the indicated Tax genes in the presence of IL-2. At 48 h after infection, the cells were cultured in 96-well plates without IL-2 (3−2 × 104, 3−1 × 104, and 1 × 104 cells/well). After 4 weeks of culture, the wells containing outgrowing cells were counted using light microscopy. The transformation efficiency (percent) was calculated as the ratio of the number of positive wells to the total of 96 wells (A). The cell lysates prepared at 48 h after infection were characterized by Western blot analysis with anti-Tax1 antibody (B). Data are representative of three independent experiments. (C) The relative transformation efficiencies of Tax1ΔC, Tax2B, Tax2B+C, and Tax221 were presented as a ratio of the transformation efficiency of the indicated Tax protein to that of Tax1. The transformation efficiency observed in the plate of 3−1 × 104 cells/well was used for the calculation. Error bars indicate standard deviations from three independent experiments.
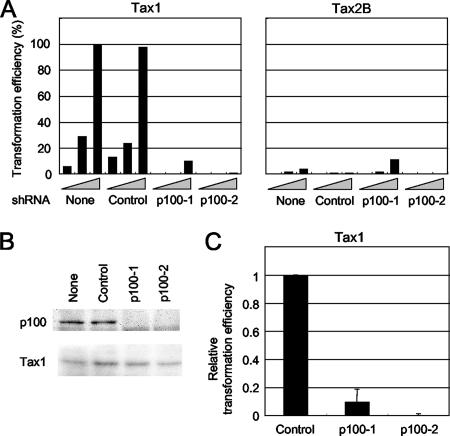
NF-κB2/p100 is required for Tax1-mediated transformation of CTLL-2 cells.
The clear correlation between p100 processing and the transforming activity of Tax suggests that NF-κB2/p100 is involved in Tax1-mediated transformation of CTLL-2 cells. A lentivirus-based RNA interference system was used to knock down NF-κB/p100 expression in CTLL-2 cells. Lentiviruses encoding shRNA against p100 together with a puromycin resistance gene were transduced to CTLL-2 cells, and the cells were cultured in the presence of puromycin for more than 10 days. Western blot analysis showed p100 expression to be almost absent in the cells infected with two distinct p100 shRNA viruses but not in those treated with a control virus (Fig. 4B), and the knock-down of p100 in CTLL-2 cells did not change the cell growth rate in the presence of IL-2 (data not shown). Next, the transformation capacities of these transduced cells were examined by assessing their IL-2-independent growth phenotypes (Fig. 4A and C). Tax1 still transformed the p100 knock-down cells (p100-1 and p100-2), but the transforming activities in the knock-down cells were dramatically reduced relative to those in the control cells. Western blot analysis showed that Tax1 was equivalently expressed in these cells, thus indicating that the distinctive transformation susceptibility of knock-down cells was not due to the expression level of Tax1 (Fig. 4B). Therefore, these results demonstrate that NF-κB2/p100 is crucially involved in the Tax1-induced transformation of CTLL-2 cells. In contrast, at least one knock-down (p100-1) showed an equivalent (or slightly augmented) capability to control cells in Tax2B-mediated transformation, thus suggesting that the inhibitory effect of p100 knock-down in CTLL-2 cells was specific to Tax1-mediated transformation.
FIG. 4.
NF-κB2/p100 knock-down reduces the transforming activity of Tax1. (A and B) CTLL-2 cells were infected with lentiviruses encoding shRNA against mouse NF-κB2/p100 (p100-1 and p100-2) or control shRNA and then were cultured in the presence of puromycin for more than 10 days. The cells were further infected with lentiviruses encoding either Tax1 or Tax2B, and at 48 h after infection, the cells were cultured in a 96-well plate without IL-2 (3−2 × 104, 3−1 × 104, and 1 × 104 cells/well). Parental CTLL-2 cells (labeled None) were also used for the assay. After culturing for 4 weeks, the wells containing outgrowing cells were counted using light microscopy. The transformation efficiency (percent) was calculated as the ratio of the number of positive wells to the total of 96 wells (A). Cell lysates prepared at 48 h after infection were characterized by Western blot analysis with anti-Tax1 antibody (B). Data are representative of three (Tax1) or two (Tax2B) independent experiments. (C) The relative transformation efficiencies of Tax1 in p100-1 and p100-2 cells (3−1 × 104 cells/well) were calculated as the ratio of the transformation efficiencies to the values in control CTLL-2 cells. The error bars indicate the standard deviations from three independent experiments.
Active NIK augments the transforming activity of Tax2 but not Tax1ΔC.
The data presented above suggested that the inability of Tax2 to activate NF-κB2/p100 caused its reduced transforming activity relative to Tax1. If this is true, activation of NF-κB2/p100 in CTLL-2 cells should augment Tax2B-mediated transformation. To pursue this hypothesis, CTLL-2 cells were established that expressed an active form of NIK (ΔN-NIK), a physiological inducer of p100 processing (42), and CTLL-2 cells expressing EGFP were used as a control. Western blot analysis by anti-NIK antibody confirmed the expression of ΔN-NIK as well as an increase of p52 and a decrease of p100 protein in ΔN-NIK cells relative to controls (Fig. 5A). Active NIK could not confer the ability of CTLL-2 cells to grow in the absence of IL-2 (data not shown). These cells were subjected to the transformation assay described above. The transforming activity of Tax2B was prominently augmented by the expression of active NIK in CTLL-2 cells (Fig. 5B). Interestingly, the transforming activities of Tax2B+C and Tax221 were augmented much more prominently than the activity of Tax2B, and the activity of Tax2B+C was equivalent to that of Tax1. These results suggest that the distinct activation of NF-κB2/p100 in CTLL-2 cells is a major factor responsible for the distinct transforming activity of Tax1 and Tax2 and that activation of NF-κB2/p100 and Tax1 PBM function cooperatively to augment the transforming activity of Tax2B. In contrast to the dramatic effect on the transforming activity of Tax2B, active NIK expression had no effect on the transforming activity of Tax1ΔC, thus suggesting that the Tax1 PBM is involved in not only NF-κB2/p100 processing but also other cell proliferation or survival signals, probably mediated through the modulation of the PDZ protein activity. Moreover, the transforming activity of Tax1 was augmented by active NIK, although the augmentation was much less than that of Tax2B, Tax2B+C, and Tax221, thus further supporting the cooperative role of the noncanonical NF-κB pathway and PBM in IL-2-independent growth transformation of CTLL-2 cells by Tax1.
FIG. 5.
An active form of NIK augments the transforming activity of Tax2 in CTLL-2 cells. (A) CTLL-2 cells were infected with lentiviruses encoding ΔN-NIK or control virus (EGFP) and cultured in the presence of blasticidin for more than 14 days. Cell lysates, including those from CTLL-2/Tax1 cells, were then prepared and characterized by Western blot analysis with anti-p100 or anti-NIK antibody. (B and C) The indicated CTLL-2 cells (EGFP and ΔN-NIK) were infected with lentiviruses encoding Tax1, Tax2B, or their mutants, and at 48 h after infection, the cells were cultured in a 96-well plate without IL-2 (3−3 × 104, 3−2 × 104, 3−1 × 104, and 1 × 104 cells/well). After culturing for 4 weeks, the wells containing outgrowing cells were counted using light microscopy. Transformation efficiency (percent) was calculated as the ratio of the number of positive wells to the total of 96 wells (B). The cell lysates prepared at 48 h after infection were characterized by Western blot analysis with anti-Tax1 antibody (C). Data are representative of two independent experiments.
NF-κB2/p100 processing in HTLV-1- and HTLV-2-infected cell lines.
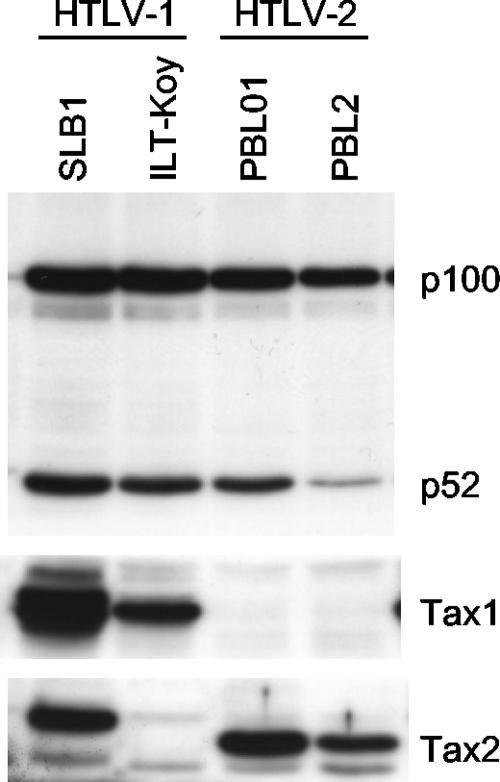
Finally, p100/p52 expression was examined in HTLV-2-infected cell lines (Fig. 6). Unexpectedly, efficient p100 processing was evident in not only the HTLV-1-infected cell lines (SLB-1 and ILT-Koy) but also two HTLV-2-infected cell lines (PBL01 and PBL2). We discuss the possible mechanism and the biological significance of NF-κB2/p100 processing in HTLV-2-infected cell lines below.
FIG. 6.
The status of NF-κB2/p100 in HTLV-1- or HTLV-2-transformed human T-cell lines. Cell lysates were prepared from SLB, ILT-Koy (HTLV-1), PBL01, and PBL2 (HTLV-2) lines and characterized by Western blot analysis with anti-p100, anti-Tax1, or anti-Tax2 antibody.
DISCUSSION
In HTLV-1-infected cells, Tax1 has been shown to activate the noncanonical NF-κB pathway to induce the processing of inactive p100 into active p52, resulting in the transcriptional activation of target genes (41). By using an RNA interference method, we demonstrated that activation of NF-κB2/p100 plays a crucial role in Tax1-induced transformation of the T-cell line CTLL-2. Moreover, we showed that Tax2, a transforming protein of nonleukemogenic HTLV-2, cannot activate the noncanonical NF-κB pathway because of its inability to interact with NF-κB2/p100. Considering the fact that the noncanonical NF-κB pathway is activated by transforming proteins of various oncoviruses, such as Epstein Barr virus LMP1 (3, 7, 24, 36), Kaposi sarcoma herpes virus vFLIP (27), and human papilloma virus (HPV) E6 (19), the present results strongly support the assumption that the activation of the noncanonical NF-κB pathway by Tax1 is crucially involved in T-cell transformation by HTLV-1 as well as leukemogenesis.
The p100 knock-down cells could still be transformed by Tax1, though with a lower efficiency, and these cells showed a minimal expression of p100 as well as p52 (data not shown). These results suggested that the activation of NF-κB2/p100 is not absolutely indispensable for Tax1-induced transformation in CTLL-2 cells, but it significantly augments the transforming activity of Tax1. Tax1 activates both the canonical and noncanonical NF-κB pathways, and a Tax1 mutant inactive to both pathways shows no transforming activity in CTLL-2 cells (18). Taken together, these results suggest that activation of the canonical NF-κB pathway is crucially important (perhaps indispensable) for transformation of CTLL-2 cells by Tax1.
Tax1ΔC showed a reduced p100 processing activity relative to Tax1, but active NIK did not augment the transforming activity of Tax1ΔC at all. These results indicate that the Tax1 PBM has alternative transformation-associated functions distinct from NF-κB2/p100 activation. In contrast to Tax1ΔC, the transforming activity of Tax2B without the PBM was augmented by an active NIK. Previous studies have reported that Tax2 but not Tax1 strongly activates NFAT, and this activity is important for Tax2-mediated transformation of CTLL-2 cells (21, 31). Therefore, NFAT activation may play a role in the augmentation of Tax2B's transforming activity by active NIK.
The present results suggest that the activation of NF-κB2/p100 and PBM are two crucial factors for the augmented transforming activity of Tax1 relative to Tax2 in CTLL-2 cells. First, the transforming activity of Tax1 in p100 knock-down cells was equivalent to that of Tax2B. Second, the transforming activity of Tax2B was prominently augmented in cells expressing an activated form of NIK, an activator of the NF-κB2/p100 pathway, and the activity of Tax2B+C in these cells was greater than that of Tax2B and equivalent to that of Tax1. These results support the hypothesis that two distinct Tax1 functions, activation of the noncanonical NF-κB pathway and modulation of PDZ protein activity, play a cooperative role in the transformation of T cells by HTLV-1 and, thereby, ATL pathogenesis.
It was unexpected that the transforming activity of Tax221 was lower than that of Tax2B in CTLL-2 cells, in spite of the presence of the PBM. This may be due to the conformational changes induced by swapping the C terminus of Tax2B with that of Tax1, thus possibly resulting in reduced NFAT activation. However, the transforming activity of Tax221 was higher than that of Tax2B in the presence of active NIK, thus further confirming the cooperative role of the noncanonical NF-κB pathway and Tax1 PBM function in the transformation of T cells.
Previous studies showed that Tax2B is less active than Tax1 in the transformation of the fibroblast cell line Rat-1, as measured by colony formation in soft agar (15). Again, the PBM was a main determinant for the distinct transforming activity of Tax1 and Tax2B in colony formation in soft agar. However, unlike the results in CTLL-2 cells, the transforming activity of Tax2B+C in Rat-1 cells was equivalent to that of Tax1 without the transduction of an activator of the noncanonical NF-κB pathway. It should be noted that NF-κB2/p100 is already activated in parental Rat-1 cells, since Rat-1 cells express a significant amount of p52, and that amount is greater than the amount of p100 expressed (2). Therefore, the differential status of the NF-κB2/p100 activity in CTLL-2 versus Rat-1 cells can explain the distinct activities of Tax2B+C in these two types of cells.
Bernal-Mizrachi et al. showed that two large granular leukemia cell lines derived from a tax1-transgenic mouse express high levels of the xIAP, cIAP and FLIP antiapoptotic genes, and these expression levels were reduced by treating the cells with NF-κB2/p100 shRNA. Moreover, the knock-down cells simultaneously showed an increased sensitivity to the apoptosis induced by a DNA damaging agent and tumor necrosis factor alpha (4). Therefore, these antiapoptotic genes regulated by the NF-κB2/p100 pathway are potential mediators of Tax1 transformation of CTLL-2 cells.
Unexpectedly, HTLV-2-transformed human T-cell lines express a level of p52 equivalent to that of HTLV-1-transformed cells. These results suggest that a viral or cellular molecule(s) other than Tax2, such as a Tax2-induced cytokine, triggers processing of p100 in HTLV-2-transformed cells, since HTLV-2-transformed T-cell lines produce many types of cytokines. Since NF-κB2/p100 activation by NIK cooperates with Tax2B to transform CTLL-2 cells, activation of the noncanonical NF-κB pathway may also play a relevant role in HTLV-2-mediated T-cell transformation. If that is true, the difference in the modes of NF-κB2/p100 activation by HTLV-1 and HTLV-2 may therefore be associated with a distinct pathogenicity in vivo.
The present results suggest that the Tax1 PBM is involved in the maximum processing of NF-κB2/p100 but not in the interaction with p100. The E6 oncoprotein of HPV also activates NF-κB2/p100 processing to generate p52, and such activation is dependent on the E6 PBM (19). Moreover, this activity correlates with the transforming activity of E6 as well as E6-induced resistance to apoptosis (19). Since Tax1 and E6 interact with similar sets of PDZ domain proteins, including tumor suppressor Dlg1 (15, 20, 23, 32, 38), these proteins may play a role in the regulation of p100 processing. Further analysis is required to clarify the mechanism of how viral oncoproteins regulate the noncanonical NF-κB pathway through the PBM.
Since E6 proteins from low-risk HPVs do not have a PBM, they are unlikely to activate the noncanonical NF-κB pathway, thus suggesting that an alteration of the noncanonical NF-κB pathway and modulation of the activity of PDZ proteins by PBM in high-risk oncoviruses are common factors associated with viral oncogenesis. Therefore, the elucidation of the molecular mechanism of these two cooperative activities in cellular transformation is expected to advance the understanding of viral oncogenesis.
Using infectious recombinant HTLV-1, Xie et al. reported that the Tax1 PBM is dispensable for the transformation of primary human T cells in vitro, but it is indispensable for the establishment and maintenance of persistent HTLV-1 infection in rabbits (43). These observations together with the findings of the present study suggest a possible scenario to explain the role of the PBM and the noncanonical NF-κB pathway in persistent HTLV-1 infection in vivo. Both Tax1-specific functions may play a role in promoting the cell growth of HTLV-1-infected T cells when only small amounts or no IL-2 is present, such as in in vivo conditions, thereby establishing persistent HTLV-1 infection. On the other hand, HTLV-2 may be able to establish a persistent infection in vivo by inducing IL-2 through Tax2-mediated NFAT activation (31). We believe that further examination of this attractive hypothesis will lead to a better understanding of the mechanism controlling both HTLV-1 persistent infection and the ATL pathogenesis.
Acknowledgments
We thank Hiroyuki Miyoshi at RIKEN Tsukuba Institute and Patrick Green for providing us with the CSII-EF-MCS, pCAG-HIVgp, and pCMV-VSV-G-RSV-Rev plasmids and with the HTLV-2-transformed cell lines, respectively. We also thank the Takeda Pharmaceutical Company for providing recombinant human IL-2. We express our gratitude to Chika Yamamoto and Misako Tobimatsu for their excellent technical assistance.
This work was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas and for Scientific Research (C) of Japan, as well as a Grant for Promotion of Niigata University Research Projects.
Footnotes
Published ahead of print on 22 August 2007.
REFERENCES
- 1.Akagi, T., and K. Shimotohno. 1993. Proliferative response of Tax1-transduced primary human T cells to anti-CD3 antibody stimulation by an interleukin-2-independent pathway. J. Virol. 67:1211-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akita, K., S. Kawata, and K. Shimotohno. 2005. p21WAF1 modulates NF-κB signaling and induces anti-apoptotic protein Bcl-2 in Tax-expressing rat fibroblast. Virology 332:249-257. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson, P. G., H. J. Coope, M. Rowe, and S. C. Ley. 2003. Latent membrane protein 1 of Epstein-Barr virus stimulates processing of NF-kappa B2 p100 to p52. J. Biol. Chem. 278:51134-51142. [DOI] [PubMed] [Google Scholar]
- 4.Bernal-Mizrachi, L., C. M. Lovly, and L. Ratner. 2006. The role of NF-κB-1 and NF-κB-2-mediated resistance to apoptosis in lymphomas. Proc. Natl. Acad. Sci. USA 103:9220-9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cross, S. L., M. B. Feinberg, J. B. Wolf, N. J. Holbrook, F. Wong-Staal, and W. J. Leonard. 1987. Regulation of the human interleukin-2 receptor alpha chain promoter: activation of a nonfunctional promoter by the transactivator gene of HTLV-I. Cell 49:47-56. [DOI] [PubMed] [Google Scholar]
- 6.Dejardin, E. 2006. The alternative NF-κB pathway from biochemistry to biology: pitfalls and promises for future drug development. Biochem. Pharmacol. 72:1161-1179. [DOI] [PubMed] [Google Scholar]
- 7.Eliopoulos, A. G., J. H. Caamano, J. Flavell, G. M. Reynolds, P. G. Murray, J. L. Poyet, and L. S. Young. 2003. Epstein-Barr virus-encoded latent infection membrane protein 1 regulates the processing of p100 NF-κB2 to p52 via an IKKγ/NEMO-independent signalling pathway. Oncogene 22:7557-7569. [DOI] [PubMed] [Google Scholar]
- 8.Feuer, G., and P. L. Green. 2005. Comparative biology of human T-cell lymphotropic virus type 1 (HTLV-1) and HTLV-2. Oncogene 24:5996-6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujii, M., H. Tsuchiya, T. Chuhjo, T. Akizawa, and M. Seiki. 1992. Interaction of HTLV-1 Tax1 with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes Dev. 6:2066-2076. [DOI] [PubMed] [Google Scholar]
- 10.Giam, C. Z., and K. T. Jeang. 2007. HTLV-1 Tax and adult T-cell leukemia. Front. Biosci. 12:1496-1507. [DOI] [PubMed] [Google Scholar]
- 11.Grassmann, R., M. Aboud, and K. T. Jeang. 2005. Molecular mechanisms of cellular transformation by HTLV-1 Tax. Oncogene 24:5976-5985. [DOI] [PubMed] [Google Scholar]
- 12.Grassmann, R., S. Berchtold, I. Radant, M. Alt, B. Fleckenstein, J. G. Sodroski, W. A. Haseltine, and U. Ramstedt. 1992. Role of human T-cell leukemia virus type 1 X region proteins in immortalization of primary human lymphocytes in culture. J. Virol. 66:4570-4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall, W. W., and M. Fujii. 2005. Deregulation of cell-signaling pathways in HTLV-1 infection. Oncogene 24:5965-5975. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi, M., T. Matsuda, N. Mori, Y. Yamada, R. Horie, T. Watanabe, M. Takahashi, M. Oie, and M. Fujii. 2005. Elevated expression of CD30 in adult T-cell leukemia cell lines: possible role in constitutive NF-κB activation. Retrovirology 2:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirata, A., M. Higuchi, A. Niinuma, M. Ohashi, M. Fukushi, M. Oie, T. Akiyama, Y. Tanaka, F. Gejyo, and M. Fujii. 2004. PDZ domain-binding motif of human T-cell leukemia virus type 1 Tax oncoprotein augments the transforming activity in a rat fibroblast cell line. Virology 318:327-336. [DOI] [PubMed] [Google Scholar]
- 16.Ishioka, K., M. Higuchi, M. Takahashi, S. Yoshida, M. Oie, Y. Tanaka, S. Takahashi, L. Xie, P. L. Green, and M. Fujii. 2006. Inactivation of tumor suppressor Dlg1 augments transformation of a T-cell line induced by human T-cell leukemia virus type 1 Tax protein. Retrovirology 3:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwai, K., N. Mori, M. Oie, N. Yamamoto, and M. Fujii. 2001. Human T-cell leukemia virus type 1 tax protein activates transcription through AP-1 site by inducing DNA binding activity in T cells. Virology 279:38-46. [DOI] [PubMed] [Google Scholar]
- 18.Iwanaga, Y., T. Tsukahara, T. Ohashi, Y. Tanaka, M. Arai, M. Nakamura, K. Ohtani, Y. Koya, M. Kannagi, N. Yamamoto, and M. Fujii. 1999. Human T-cell leukemia virus type 1 tax protein abrogates interleukin-2 dependence in a mouse T-cell line. J. Virol. 73:1271-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James, M. A., J. H. Lee, and A. J. Klingelhutz. 2006. Human papillomavirus type 16 E6 activates NF-κB, induces cIAP-2 expression, and protects against apoptosis in a PDZ binding motif-dependent manner. J. Virol. 80:5301-5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiyono, T., A. Hiraiwa, M. Fujita, Y. Hayashi, T. Akiyama, and M. Ishibashi. 1997. Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc. Natl. Acad. Sci. USA 94:11612-11616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondo, R., M. Higuchi, M. Takahashi, M. Oie, Y. Tanaka, F. Gejyo, and M. Fujii. 2006. Human T-cell leukemia virus type 2 Tax protein induces interleukin 2-independent growth in a T-cell line. Retrovirology 3:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanoix, J., J. Lacoste, N. Pepin, N. Rice, and J. Hiscott. 1994. Overproduction of NFKB2 (lyt-10) and c-Rel: a mechanism for HTLV-I Tax-mediated trans-activation via the NF-κB signalling pathway. Oncogene 9:841-852. [PubMed] [Google Scholar]
- 23.Lee, S. S., R. S. Weiss, and R. T. Javier. 1997. Binding of human virus oncoproteins to hDlg/SAP97, a mammalian homolog of the Drosophila discs large tumor suppressor protein. Proc. Natl. Acad. Sci. USA 94:6670-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luftig, M., T. Yasui, V. Soni, M. S. Kang, N. Jacobson, E. Cahir-McFarland, B. Seed, and E. Kieff. 2004. Epstein-Barr virus latent infection membrane protein 1 TRAF-binding site induces NIK/IKK α-dependent noncanonical NF-κB activation. Proc. Natl. Acad. Sci. USA 101:141-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maruyama, M., H. Shibuya, H. Harada, M. Hatakeyama, M. Seiki, T. Fujita, J. Inoue, M. Yoshida, and T. Taniguchi. 1987. Evidence for aberrant activation of the interleukin-2 autocrine loop by HTLV-1-encoded p40x and T3/Ti complex triggering. Cell 48:343-350. [DOI] [PubMed] [Google Scholar]
- 26.Matsuoka, M. 2005. Human T-cell leukemia virus type I (HTLV-I) infection and the onset of adult T-cell leukemia (ATL). Retrovirology 2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matta, H., and P. M. Chaudhary. 2004. Activation of alternative NF-kappa B pathway by human herpes virus 8-encoded Fas-associated death domain-like IL-1 beta-converting enzyme inhibitory protein (vFLIP). Proc. Natl. Acad. Sci. USA 101:9399-9404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meertens, L., S. Chevalier, R. Weil, A. Gessain, and R. Mahieux. 2004. A 10-amino acid domain within human T-cell leukemia virus type 1 and type 2 tax protein sequences is responsible for their divergent subcellular distribution. J. Biol. Chem. 279:43307-43320. [DOI] [PubMed] [Google Scholar]
- 29.Mori, N., Y. Nunokawa, Y. Yamada, S. Ikeda, M. Tomonaga, and N. Yamamoto. 1999. Expression of human inducible nitric oxide synthase gene in T-cell lines infected with human T-cell leukemia virus type-I and primary adult T-cell leukemia cells. Blood 94:2862-2870. [PubMed] [Google Scholar]
- 30.Murakami, T., H. Hirai, T. Suzuki, J. Fujisawa, and M. Yoshida. 1995. HTLV-1 Tax enhances NF-kappa B2 expression and binds to the products p52 and p100, but does not suppress the inhibitory function of p100. Virology 206:1066-1074. [DOI] [PubMed] [Google Scholar]
- 31.Niinuma, A., M. Higuchi, M. Takahashi, M. Oie, Y. Tanaka, F. Gejyo, N. Tanaka, K. Sugamura, L. Xie, P. L. Green, and M. Fujii. 2005. Aberrant activation of the interleukin-2 autocrine loop through the nuclear factor of activated T cells by nonleukemogenic human T-cell leukemia virus type 2 but not by leukemogenic type 1 virus. J. Virol. 79:11925-11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohashi, M., M. Sakurai, M. Higuchi, N. Mori, M. Fukushi, M. Oie, R. J. Coffey, K. Yoshiura, Y. Tanaka, M. Uchiyama, M. Hatanaka, and M. Fujii. 2004. Human T-cell leukemia virus type 1 Tax oncoprotein induces and interacts with a multi-PDZ domain protein, MAGI-3. Virology 320:52-62. [DOI] [PubMed] [Google Scholar]
- 33.Robek, M. D., and L. Ratner. 1999. Immortalization of CD4+ and CD8+ T lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J. Virol. 73:4856-4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross, T. M., M. Narayan, Z. Y. Fang, A. C. Minella, and P. L. Green. 2000. Human T-cell leukemia virus type 2 tax mutants that selectively abrogate NFκB or CREB/ATF activation fail to transform primary human T cells. J. Virol. 74:2655-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ross, T. M., S. M. Pettiford, and P. L. Green. 1996. The tax gene of human T-cell leukemia virus type 2 is essential for transformation of human T lymphocytes. J. Virol. 70:5194-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saito, N., G. Courtois, A. Chiba, N. Yamamoto, T. Nitta, N. Hironaka, M. Rowe, N. Yamamoto, and S. Yamaoka. 2003. Two carboxyl-terminal activation regions of Epstein-Barr virus latent membrane protein 1 activate NF-κB through distinct signaling pathways in fibroblast cell lines. J. Biol. Chem. 278:46565-46575. [DOI] [PubMed] [Google Scholar]
- 37.Sun, S. C., and S. Yamaoka. 2005. Activation of NF-κB by HTLV-I and implications for cell transformation. Oncogene 24:5952-5964. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki, T., Y. Ohsugi, M. Uchida-Toita, T. Akiyama, and M. Yoshida. 1999. Tax oncoprotein of HTLV-1 binds to the human homologue of Drosophila discs large tumor suppressor protein, hDLG, and perturbs its function in cell growth control. Oncogene 18:5967-5972. [DOI] [PubMed] [Google Scholar]
- 39.Takatsuki, K. 2005. Discovery of adult T-cell leukemia. Retrovirology 2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsubata, C., M. Higuchi, M. Takahashi, M. Oie, Y. Tanaka, F. Gejyo, and M. Fujii. 2005. PDZ domain-binding motif of human T-cell leukemia virus type 1 Tax oncoprotein is essential for the interleukin 2 independent growth induction of a T-cell line. Retrovirology 2:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao, G., M. E. Cvijic, A. Fong, E. W. Harhaj, M. T. Uhlik, M. Waterfield, and S. C. Sun. 2001. Retroviral oncoprotein Tax induces processing of NF-κB2/p100 in T cells: evidence for the involvement of IKKα. EMBO J. 20:6805-6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao, G., E. W. Harhaj, and S. C. Sun. 2001. NF-κB-inducing kinase regulates the processing of NF-αB2 p100. Mol. Cell 7:401-409. [DOI] [PubMed] [Google Scholar]
- 43.Xie, L., B. Yamamoto, A. Haoudi, O. J. Semmes, and P. L. Green. 2006. PDZ binding motif of HTLV-1 Tax promotes virus-mediated T-cell proliferation in vitro and persistence in vivo. Blood 107:1980-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao, L. J., and C. Z. Giam. 1992. Human T-cell lymphotropic virus type I (HTLV-I) transcriptional activator, Tax, enhances CREB binding to HTLV-I 21-base-pair repeats by protein-protein interaction. Proc. Natl. Acad. Sci. USA 89:7070-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]