Abstract
West Nile virus (WNV) has spread throughout the United States and Canada and now annually causes a clinical spectrum of human disease ranging from a self-limiting acute febrile illness to acute flaccid paralysis and lethal encephalitis. No therapy or vaccine is currently approved for use in humans. Using high-throughput screening assays that included a luciferase expressing WNV subgenomic replicon and an NS1 capture enzyme-linked immunosorbent assay, we evaluated a chemical library of over 80,000 compounds for their capacity to inhibit WNV replication. We identified 10 compounds with strong inhibitory activity against genetically diverse WNV and Kunjin virus isolates. Many of the inhibitory compounds belonged to a chemical family of secondary sulfonamides and have not been described previously to inhibit WNV or other related or unrelated viruses. Several of these compounds inhibited WNV infection in the submicromolar range, had selectivity indices of greater than 10, and inhibited replication of other flaviviruses, including dengue and yellow fever viruses. One of the most promising compounds, AP30451, specifically blocked translation of a yellow fever virus replicon but not a Sindbis virus replicon or an internal ribosome entry site containing mRNA. Overall, these compounds comprise a novel class of promising inhibitors for therapy against WNV and other flavivirus infections in humans.
West Nile virus (WNV) is a single-stranded positive polarity RNA Flavivirus that cycles enzootically between Culex species of mosquitoes and birds but also infects and causes disease in humans, horses, and other vertebrate species. It is related to other viruses that cause human disease including dengue virus (DENV), yellow fever virus (YFV), and the Japanese, St. Louis, and tick-borne encephalitis viruses. Historically, WNV caused sporadic outbreaks of a mild febrile illness in regions of Africa, the Middle East, Asia, and Australia. However, in the last decade, the ecology and epidemiology of infection changed. New outbreaks in parts of Eastern Europe and North America were associated with higher rates of severe neurological disease (37, 41, 73). WNV has spread to all 48 continental United States, as well as to Canada, Mexico, Caribbean, and more recently South America (62). The more severe symptoms of WNV infection occur in the elderly and immunocompromised, although serious illness has been observed across all age ranges. The fatality-to-case ratio of recent WNV outbreaks is 4 to 14%, and it can rise to 10 to 19% in hospitalized cases. Annual outbreaks occur in the United States (34), with ∼24,000 human cases diagnosed between 1999 and 2006 (http://www.cdc.gov/ncidod/dvbid/westnile/surv&control.htm#maps), and an estimated 730,000 undiagnosed infections in 2003 alone (12). No vaccine or specific therapy for WNV is currently approved for humans.
Recent animal studies suggest that administration of anti-WNV antibodies may be therapeutic. Three groups have demonstrated that immune human gamma globulin partially protects mice against WNV-induced mortality even when therapy was delayed up to 5 days after infection (6, 27, 46). Small numbers of human patients have received immune gamma globulin therapy against WNV infection, and case reports (36, 84) have documented clinical improvement in humans with neurological WNV infection. Human monoclonal antibodies (MAbs), humanized MAbs, and antibody fragments against the WNV envelope protein have been recently developed (33, 72, 87). These reagents have high neutralizing activity in vitro and provide equivalent or superior protection in vivo in mice and hamsters compared to gamma globulin (33, 65, 72, 87). One possible limitation of MAb therapy, however, is the rapid emergence of escape mutants that could compromise inhibitory activity (53).
Several well-characterized antiviral agents have been tested for inhibitory activity against WNV. Pretreatment of cells in vitro with alpha interferon (IFN-α) potently inhibits flavivirus infection, including WNV infection (2, 15, 21, 22, 28), and mice that lack IFN-α/β receptors are highly susceptible to lethal WNV infection (48, 79). However, the inhibitory effect of IFN is markedly attenuated once viral replication has begun (22, 55) because flavivirus nonstructural proteins block IFN signaling (7, 44, 55-57, 68, 69). Pretreatment of rodents with IFN-α inhibited St. Louis encephalitis virus infection and decreased WNV viral load and mortality (11, 64), and treatment with IFN-α after infection reduced complications in human St. Louis encephalitis virus cases. In an uncontrolled study, a small number of human cases of WNV encephalitis were successfully treated with IFN-α (47, 76, 83). Consequently, a randomized, nonblinded clinical trial of IFN α2b has been initiated for WNV infection (http://nyhq.org/posting/rahal.html).
The cellular enzyme IMP dehydrogenase (IMPDH) has been a target of antiviral development. IMPDH catalyzes an essential step in the de novo biosynthesis of guanine nucleotides. Ribavirin (1-β-d-ribofuranosyl-1H-1,2,4-triazole-3-carboximide) is as a guanosine analogue that competitively inhibits IMPDH resulting in depletion of intracellular GTP pools (52). Although the mechanism of its broad-spectrum antiviral activity is uncertain, low pools of GTP may interfere with the guanylylation step of RNA capping and inhibit viral RNA polymerases (42). Alternatively, phosphorylated ribavirin may incorporate directly into the nascent RNA strand, leading to loss of viral genome integrity by virtue of an error catastrophe effect (16, 18). Although ribavirin has inhibitory activity against WNV infection in vitro (2, 18, 45), animal experiments have not been promising. Treatment of WNV-infected hamsters with ribavirin resulted in increased mortality (64). Moreover, in a WNV outbreak in 2000, an elevated 41% mortality rate was observed among 37 patients that received ribavirin therapy (13). Mycophenolic acid (MPA) is a non-nucleoside, noncompetitive inhibitor of IMPDH that has broad-spectrum antiviral activity in vitro, including against WNV (24, 66). Unfortunately, MPA had poor efficacy in mice against WNV (B. Geiss and M. Diamond, unpublished results), likely because of its significant immunosuppressive effects. VX-497, a phenyloxazole derivative that is structurally unrelated to ribavirin and MPA, is a potent, reversible, noncompetitive inhibitor of IMPDH (61). Although it is has potent antiviral activity against several DNA and RNA viruses, it was relatively inactive against DENV and YFV and has not been tested against WNV.
Novel small molecule inhibitors have been identified that inhibit WNV translation and replication (9, 32, 35, 75). Gu et al. used a cell-based subgenomic replicon screen to identify pyrozolopyrimidine compound with anti-WNV activity, although the selectivity index (SI) was only 8 (35). Puig-Basagoiti et al. (75) identified a triaryl pyrazoline compound that inhibited flavivirus RNA replication. This compound inhibited viral replication of an epidemic strain of WNV in Vero cells with an effective concentration that inhibits replication 50% (EC50) of 15 μM and had broad antiviral activity against related (e.g., DENV or YFV) or unrelated (mouse hepatitis and vesicular stomatitis viruses) RNA viruses.
We describe here a screening strategy for the identification of small molecule inhibitors with antiviral activity against WNV and other flaviviruses. We identify several compounds with submicromolar activity and minimal cell toxicity. Based on our screening platform, these inhibitors likely target steps after entry and before assembly. One compound, AP30451, specifically inhibited translation of flavivirus mRNA and blocked WNV replication in several cell types, including primary neuron cultures.
MATERIALS AND METHODS
Cells and viruses.
Baby hamster kidney cells (BHK-21-15), monkey Vero cells, human Huh-7.5 hepatoma cells (8), and human A549 lung carcinoma cells were cultured in Dulbecco modified Eagle medium with 10% fetal bovine serum as previously described (20). The lineage I New York isolate (3000.0259, 2000, passage 2) was described previously (25, 26), and the Kunjin virus (KUNV) isolate (CH 16532) was a generous gift from J. Anderson (New Haven, CT). The DENV-2 strain (16681) is a prototype dengue hemorrhagic fever isolate from Thailand and has been described previously (77).
WNV lineage I replicon.
A plasmid containing the cDNA of a lineage I WNV subgenomic replicon that expresses Renilla luciferase was generated from an infectious cDNA clone of the New York 1999 strain (provided by R. Kinney, Centers for Disease Control, Fort Collins, CO). Briefly, pWN.CA26-hRUPac was engineered by deleting WNV nucleotides 181 to 2379 and fusing the first 31 amino acids of the capsid protein to the Renilla luciferase gene, the coding sequences of the ubiquitin autocleavage peptide, and the puromycin-resistance conferring gene (pac). The DNA template was prepared by linearizing pWN.CA26-hRUPac with XbaI restriction endonuclease followed by phenol-chloroform extraction and ethanol precipitation. Replicon RNA was generated by using an Amplicap T7 DNA-dependent RNA polymerase High Yield Message Maker kit (Epicenter Technologies, Madison, WI). RNA transcripts were electroporated into BHK-21 cells, and stable clones of replicon-expressing cells (BHK-WNV-Rep) were isolated after selection with 5 μg of puromycin (Sigma-Aldrich, St. Louis, MO)/ml.
Inhibitor screening with the WNV replicon.
A library of more than 80,000 small molecules was obtained from commercial suppliers. Compounds were maintained at −80°C in dimethyl sulfoxide (DMSO) at a stock concentration of 10 mM. Primary screens were performed at a single concentration (25 μM) of compound in 1% DMSO in 96-well plates containing 2.5 × 105 BHK-WNV-Rep cells. At 24 h after treatment, cells were lysed, and a luciferase assay was performed. Compounds that showed greater than 80% inhibition were tested in quadruplicate with an extended dose range (0.75 to 75 μM), and an EC50 was determined. In parallel, these compounds were also tested for cell toxicity using an ATP metabolic assay (Celltiter-Glo; Promega, Madison, WI) to obtain the concentration that is cytotoxic for 50% of cells (CC50). The SI (CC50/EC50) was determined and used to prioritize lead compounds.
Inhibitor screening by NS1 ELISA.
Lead compounds from the replicon screen were tested in a secondary screen for their inhibitory activity on KUNV infection of Vero cells. KUNV is a lineage I strain of WNV that can be manipulated at biosafety level 2 (BSL-2), which facilitated high-throughput screening. Since NS1 is a secreted nonstructural glycoprotein that accumulates in supernatants of flavivirus-infected cells, we developed a highly quantitative capture enzyme-linked immunosorbent assay (ELISA) using previously described anti-NS1 MAbs (3-NS1 and 17-NS1) (14). Vero cells were incubated with different doses of lead compounds and infected with KUNV at a multiplicity of infection (MOI) of 0.01, and supernatants were harvested 48 h after infection. Microtiter plates were coated with 17-NS1 (10 μg/ml) overnight at 4°C and blocked for 1 h at 37°C in 150 mM NaCl-25 mM Tris-HCl (pH 7.5)-1% bovine serum albumin-3% horse serum-0.05% NP-40-0.025% NaN3. Subsequently, supernatants from KUNV-infected Vero cells were treated with NP-40 (final concentration of 0.1% to inactivate virus) and added to wells for 1 h at room temperature. Plates were rinsed four times with wash buffer (150 mM NaCl, 25 mM Tris-HCl [pH 7.5], 1% bovine serum albumin, 0.025% NP-40, 0.025% NaN3) and incubated sequentially for 1 h with biotinylated 3-NS1 (2 μg/ml in wash buffer), streptavidin-horseradish peroxidase (2 μg/ml in wash buffer), and tetramethylbenzidine substrate. After stopping the reaction with 1 N H2SO4, the optical density was measured at 450 nm on a BMG plate reader (BMG Labtech, Durham, NC).
Inhibitor screening with virulent New York strain of WNV.
Compounds that were inhibitory in both the replicon and KUNV NS1 assays were tested for their ability to block infection of WNV 3000.0259, a virulent strain of WNV isolated in New York in 2000 and previously characterized in our laboratory (23). Initially, Vero cells were infected with WNV at an MOI of 0.01 at 37°C in the presence of increasing doses of inhibitor in a 0.5% DMSO solution. One day later, cells were harvested, fixed, permeabilized, stained for intracellular E protein with an anti-WNV MAb (Alexa 647-conjugated E16 [72]), and processed by flow cytometry. Similar studies were performed in BHK-21 cells with the 16681 strain of DENV-2, except that an MOI of 0.01 was used, and cells were harvested 72 h after infection and stained with an anti-DENV-2 MAb (3H5-1) (31).
Neuron cultures and infection studies.
Cortical neurons were prepared from embryonic day 15 (E15) wild-type C57BL/6 mouse embryos as described previously (49). Cells were seeded at a density of 5 × 105 cells/well in poly-d-lysine/laminin-coated 24-well plates, cultured in Dulbecco modified Eagle medium containing 5% heat-inactivated horse serum and 5% heat-inactivated fetal bovine serum, and maintained in NeuroBASAL medium supplemented with B27 (Invitrogen) and l-glutamine. For infection experiments, cortical neurons cultured for 3 to 4 days were infected at an MOI of 0.1 for 1 h at 37°C, followed by serial washing with phosphate-buffered saline (PBS) to remove free virus and the addition of compounds AP30451, AP18417, and AP34456 (at 50 or 16 μM) or of DMSO as a vehicle control. The concentration of DMSO used (0.5%) in these studies did not cause significant toxicity in neurons compared to the medium control. For virus production experiments, supernatants were harvested at 24 h postinfection, and virus titers were determined by plaque assay on BHK-21 cells.
For immunostaining experiments, cortical neurons were infected with WNV at an MOI of 0.1 and treated with compounds. WNV-infected and control uninfected neurons were fixed at 48 h postinfection with 4% paraformaldehyde in PBS at 4°C for 15 min. Cells were permeabilized in PBS with 0.2% Triton X-100, blocked (5% normal goat serum and 0.2% Triton X-100), and stained with rat anti-WNV immune serum and a mouse antibody against the neuronal marker NeuN (Chemicon International, Temecula, CA) or a control mouse immunoglobulin G (IgG). Cells were then incubated with Alexa-488-conjugated anti-mouse IgG (Invitrogen) and Cy3-conjugated anti-rat IgG (Jackson Laboratories, West Grove, PA) and the nucleic acid stain TO-PRO-3 (Invitrogen). Neurons were visualized by using a Zeiss 510 Meta LSM confocal microscope.
Neuronal survival studies were performed as previously described (80). Cortical neurons were treated with 50 or 16 μM concentrations of the compounds AP30451, AP18417, and AP34456 or with DMSO as a vehicle control. Neuron survival was assessed 24 h after treatment by using the CellTiter-Blue cell viability assay according to the manufacturer's instructions (Promega). The data were normalized to DMSO-treated neurons harvested at the same time point.
Transient reporter gene assays.
Transient expression of reporter genes was used to determine the mechanism of compound-mediated inhibition. A YFV replicon (YF-hRupac) was engineered by deleting nucleotides 27 to 755 of the 17D strain of YFV and fusing the first 26 amino acids of the capsid protein to the Renilla luciferase gene, the coding sequences of the ubiquitin autocleavage peptide, and the puromycin resistance gene (pac). The Sindbis virus replicon was described previously and contains the luciferase gene under control of the subgenomic promoter (1). The encephalomyocarditis (EMCV) internal ribosome entry site (IRES)-dependent lacZ mRNA was generated from the pTM1 plasmid by inserting the lacZ open reading frame encoding the β-galactosidase protein into the multiple cloning site to create pTM1lacZ (67). The m7G-capped firefly luciferase mRNA was generated from the luciferase T7 control DNA plasmid purchased from Promega.
DNA template for all reporter constructs was prepared as described above for the WNV replicon, with the following differences. Replicon mRNA was generated using the mMessage mMachine high-yield capped RNA transcription kit (Ambion, Austin, TX). RNA transcripts (2 to 4 μg) were electroporated into 5 × 106 BHK-21 cells. Subsequently, 2 × 104 cells were seeded into 96-well plates, immediately treated with compounds or controls in 1% DMSO, and assayed for reporter gene activity at 2, 4, and 6 h for early translation and at 24, 30, and 48 h for late, replication-dependent translation. The Renilla luciferase, firefly luciferase, and β-galactosidase activities were detected by using commercial assay systems from Promega and Applied Biosystems (Foster City, CA). Similarly seeded and treated 96-well plates were used to measure cell viability in parallel using the CellTiter Glo luminescent assay kit (Promega), which determines the relative cell metabolic activity by measuring the amount of ATP using a luciferase-based reporter gene system. The data from eight wells per treatment were averaged and calculated as transient reporter relative light units (RLU) per ATP-dependent luciferase relative light units to normalize for cell number. Experiments were repeated several times on independent days.
Statistical analysis.
An unpaired Student t test was used to determine statistically significant differences. All data were analyzed by using Prism software (GraphPad Prism4, San Diego, CA).
RESULTS
Stable cell line that constitutively replicates a WNV subgenomic replicon.
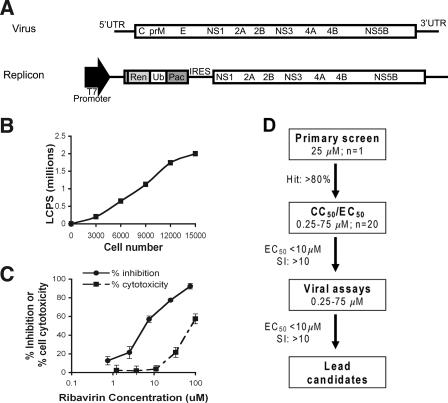
A plasmid (pWN.CA26-hRUPac.N) was constructed that contained a subgenomic WNV replicon with a Renilla luciferase gene and a puromycin acetylase selectable marker (Fig. 1A). After full-length 7-methyl-guanosine (m7G)-capped RNA transcripts in vitro were generated by using a T7 DNA-dependent RNA polymerase, BHK cells were transfected by electroporation and a stable cell line that constitutively propagated a WNV replicon was isolated after selection with puromycin. Replication was verified by reintroduction of total RNA extracted from the stable cell line into naive BHK cells and observing a replication-dependent increase in Renilla expression (data not shown). One high-expression clonal cell line, BHK/WN-CA26-hRUPacN3-2, was chosen for further studies. Luciferase expression from this cell line was linear over a wide range of cell numbers, with >100 RLU per cell (Fig. 1B). Luciferase expression from these cells was also tested after treatment with ribavirin, an established in vitro inhibitor of flavivirus replication (Fig. 1C). The EC50 and CC50 for ribavirin were 6.85 and 87 μM, respectively, in good agreement with previous studies (24).
FIG. 1.
Functional activity of the WNV subgenomic replicon. (A) Scheme of the WNV replicon. Beginning from the cDNA of the 1999 New York strain infectious clone of WNV, most of capsid (C), and all of the premembrane (prM) and envelope (E) genes were deleted, and a Renilla luciferase (Ren) reporter gene and puromycin acetylase gene (Pac) were inserted as a fusion protein linked by a ubiquitin protease cleavage site (Ub) to allow for the expression of individual selectable marker and reporter proteins. The cDNA contains a T7 RNA polymerase promoter to allow for in vitro transcription of replicon RNA. The nonstructural proteins (NS1 to NS5) are translated via the EMCV IRES. (B) Linearity of luciferase activity in BHK cells expressing the WNV replicon. (C) Luciferase expression from BHK-WNV replicon cells after treatment with ribavirin. The EC50 and CC50 for ribavirin were 6.85 and 87 μM, respectively. The results are representative of several independent experiments performed in quadruplicate. (D) Flow diagram of the several stages in the drug screening process.
Primary antiviral screen using the WNV replicon.
We tested the performance of the WNV replicon cells in a primary screening format in 96-well plates. The assay was performed with 104 cells per well and luciferase was measured 24 h after compound addition. The assay had an average signal of 259,168 ± 55,625 RLU with a signal-to-noise ratio of 1,103. Ribavirin was used to monitor screening reliability, and the WN-CA26hRUPac replicon assay was tested for quality control and statistical performance by calculating the percent coefficient of variation (% CV), and the “screening window coefficient” or Z factor (90). The Z factor is defined as the ratio of the separation of two values to the signal range of the assay and is a representation of the dynamic range and variability of a particular assay. A trial screen of 70 plates was performed, and the results were analyzed. All % CVs were <14%, and the Z value was >0.9, which indicated excellent performance (90).
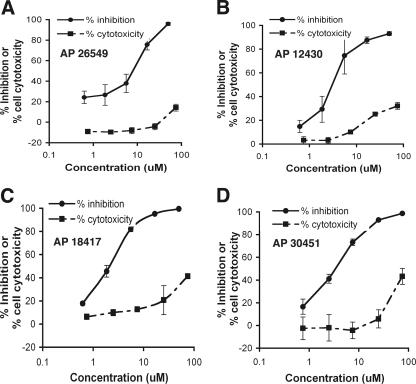
A flow diagram of the primary screening program is shown (Fig. 1D). More than 80,000 compounds were tested at a single dose of 25 μM. A total of 1,355 of these compounds showed a greater than 80% reduction in luciferase signal. These compounds were subsequently tested in complete dose-response analyses for inhibitory (EC50) and cytotoxicity (CC50) with four replicates of each concentration (0.25 to 75 μM). Notably, 242 of 1,355 compounds had EC50 values of ≤10 μM and a CC50/EC50 ratio (i.e., SI) of >10. These compounds were designated replicon hits and were subsequently tested in secondary virological assays. The EC50 and CC50 curves of four example compounds with inhibitory activity against WNV replicon propagation are shown (Fig. 2).
FIG. 2.
Activity and selectivity of four compounds in the WNV replicon assay. The antiviral activity and cytotoxic effect of compounds were assessed at the indicated concentrations in BHK cells by measuring replication-dependent luciferase expression and ATP quantification assay, respectively. Activity in both assays is expressed as a percentage of no-drug controls. Depicted are the EC50 and CC50 curves of four chemical inhibitors from our libraries. (A) AP26549; (B) AP12430; (C) AP18417; (D) AP30451. The results are representative of several independent experiments performed in quadruplicate.
Secondary virological screens.
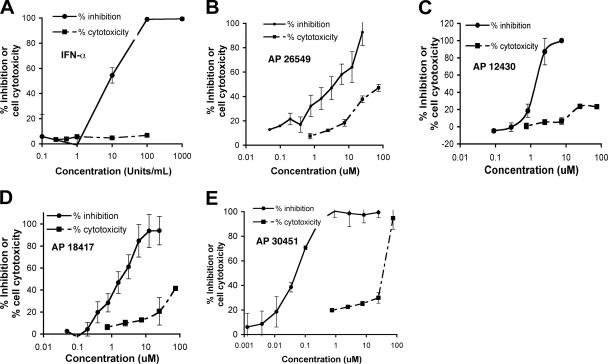
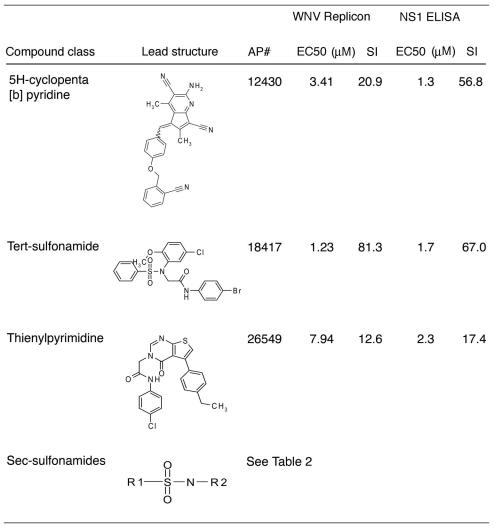
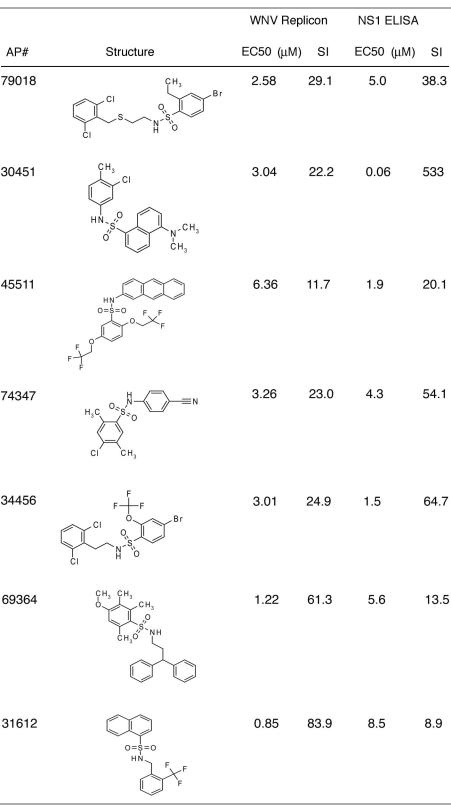
Compounds with favorable inhibitory profiles in the replicon screen were evaluated in virus-based assays. As a secondary screen, compounds were tested for their ability to inhibit KUNV, a lineage I Australian WNV strain that is closely related (97.6% amino acid sequence identity) to North American WNV isolates and yet can be used under BSL-2 conditions. For high-throughput screening, we measured the level of NS1, a secreted nonstructural viral glycoprotein, in the supernatant of KUNV-infected Vero cells as a marker of infection by using an NS1 capture ELISA. This assay was developed with our previously characterized anti-NS1 MAbs (14) and is based on published experiments (54, 59, 89). For validation purposes, we demonstrated the effect of a known inhibitor, IFN-α, on production of KUNV NS1 at 48 h after infection (Fig. 3A). Five concentrations (0.5 to 75 μM) of 242 compounds were evaluated by using the NS1 ELISA; 40 of these compounds showed 50% inhibition in the low micromolar range. These compounds were then tested for a complete dose-response (EC50 and CC50) by using this assay. Ten compounds exhibited an EC50 of <10 μM and an SI of >10: four representative inhibition profiles are shown (Fig. 3B to E). These compounds fell into four compound classes (Table 1). One of them, AP30451, exhibited an excellent EC50 of 60 nM and an SI of 533. Since seven different compounds with potent inhibitory activity in two different assays on distinct cell types were secondary sulfonamides (Table 2), this class of compounds became the focus of further testing.
FIG. 3.
NS1 capture ELISA for assessing antiviral activity against KUNV infection. (A) Effect of IFN-α on NS1 production from KUNV virus-infected cells 48 h after infection (MOI = 0.01). (B to E) Antiviral activity of different compounds from the chemical libraries. Solid curves show the dose response of WNV replication as measured by decrease in detection of secreted NS1 in supernatants of Vero cells by ELISA. Dashed curves show the cytotoxic effect of compounds in Vero cells at the indicated concentrations using an ATP quantification assay. The results are representative of several independent experiments performed in quadruplicate.
TABLE 1.
Chemical classes and virological data of WNV lead compoundsa
The lead compound classes, structures, EC50, and SI (CC50/EC50) derived from replicon and viral assays are shown.
TABLE 2.
Secondary sulfonamide compounds and their virological dataa
The secondary sulfonamide structures, EC50, and SI (CC50/EC50) derived from replicon and viral assays are listed.
Tertiary screens with a virulent North American WNV isolate.
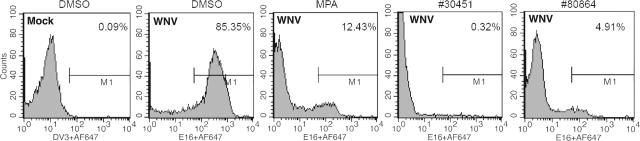
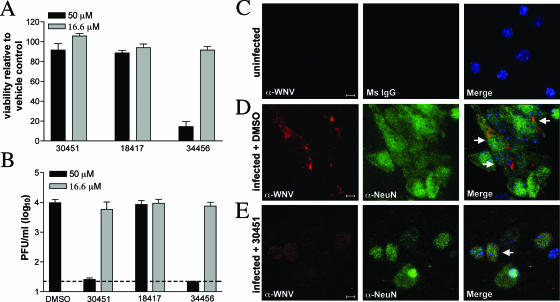
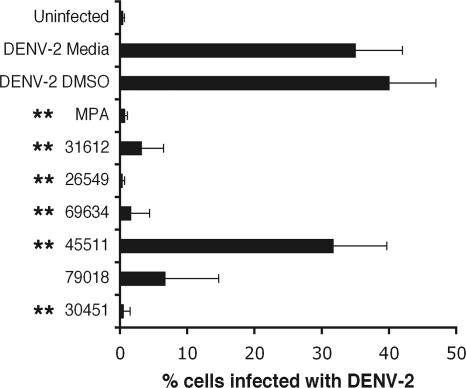
To confirm the inhibitory activity of our lead compounds, we tested their ability to inhibit infection in Vero cells with a virulent North American WNV strain that was isolated from New York in 2000 (25, 26). Compounds were added to Vero cells at the time of infection, and 24 h later inhibition was evaluated by a previously described flow cytometry assay (20) for decreases in WNV envelope (E) protein expression. Notably, several compounds significantly reduced WNV infection in Vero cells (Table 3 and Fig. 4). Some of the most potent inhibitors in the Vero cell assay were also tested for their ability to reduce WNV production in primary mouse cortical neurons. Neurons are the primary target of WNV infection in vivo in the central nervous system of humans and other vertebrate animals (17). One compound (AP30451) showed a potent antiviral effect in neurons at a dose that did not cause significant cell toxicity (Fig. 5). In contrast, other compounds that inhibited infection in Vero cells either had no inhibitory activity (AP18417) or had toxic effects (AP34456) in neurons.
TABLE 3.
Inhibition of WNV infection in Vero cellsa
| Compound | % Positive cells (mean ± SD)
|
||
|---|---|---|---|
| 50 μM | 16.6 μM | 5.6 μM | |
| AP30451 | 1 ± 2* | 40 ± 8* | 80 ± 7 |
| AP79018 | 20 ± 4* | 83 ± 3 | 87 ± 5 |
| AP74347 | 74 ± 26 | 78 ± 20 | 88 ± 4 |
| AP45511 | 60 ± 6* | 84 ± 9 | 84 ± 7 |
| AP12430 | 17 ± 9* | 49 ± 17* | 62 ± 20 |
| AP69634 | 5 ± 2* | 20 ± 5* | 83 ± 6 |
| AP18417 | 5 ± 3* | 6 ± 3* | 61 ± 29 |
| AP26549 | 19 ± 5* | 61 ± 8* | 86 ± 4 |
| AP34456 | 0.5 ± 0.3* | 8 ± 5* | 72 ± 9 |
| AP31612 | 39 ± 41 | 83 ± 10 | 85 ± 5 |
Vero cells were infected with the New York strain of WNV for 1 h at 37°C. Subsequently, cells were washed, and inhibitors were added at the indicated concentrations. One day later, cells were harvested, permeabilized, stained with anti-WNV E MAb, and processed by flow cytometry. The data are an average of three independent experiments performed in duplicate. The percent positive cells for the controls were as follows: WNV-medium, 89 ± 7; WNV-0.5% DMSO, 88 ± 7; and MPA (10 μg/ml), 24 ± 12*. An asterisk indicates a statistically significant difference (P < 0.05) compared to the 0.5% DMSO vehicle control.
FIG. 4.
Effect of candidate inhibitors on infection by a virulent North American strain of WNV. Vero cells were infected with WNV New York 2000 at an MOI of 0.01. One hour later, DMSO vehicle, the indicated inhibitors, or MPA was added. One day later, the cells were harvested and stained with anti-WNV E MAbs and processed by flow cytometry. Histograms are shown, and the M1 gate indicates the percentage of WNV positive cells for each compound. The results are representative of at least three independent experiments performed in duplicate.
FIG. 5.
Compound AP30451 inhibits WNV replication in neurons. Primary cortical neurons were generated from wild-type mice and treated with the indicated compounds or the vehicle control DMSO. (A) Neurons were treated with the compounds AP30451, AP18417, or AP34456 or with DMSO, and survival was evaluated 24 h after addition by a fluorescence-based viability assay. (B) Neurons were infected at an MOI of 0.1 and treated with compound AP30451, AP18417, or AP34456 or with DMSO 1 h after infection. Virus production was determined 24 h after infection by plaque assay. (C to E) Uninfected and WNV-infected neurons were stained for WNV antigen (α-WNV, red) and costained for the neuronal marker NeuN (α-NeuN, green) or a mouse IgG control antibody (Ms IgG) and the nuclear stain TO-PRO-3 (blue). Representative images are shown of uninfected neurons (C), infected neurons treated with DMSO (D), and infected neurons treated with 50 μM AP30451 (E). Cells that show staining for WNV antigen and NeuN are denoted with white arrows. The nuclear stain filter was only included in the merged image.
Effect of inhibitors on other Flaviviridae family members.
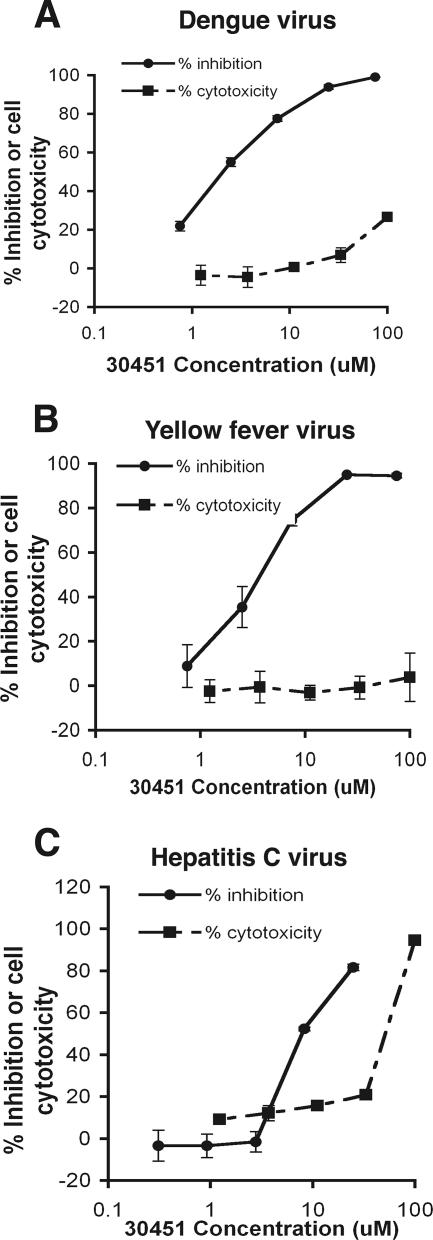
To examine whether candidate lead compounds inhibited other viruses in the Flaviviridae family, we performed analogous replication assays with previously described DENV-2 (88), YFV (10), and hepatitis C virus (HCV) (8, 58) replicons that contain luciferase reporter genes. Notably, compound AP30451 strongly inhibited DENV and YFV, members of the Flavivirus genus (Fig. 6A and B), with EC50 values in the low micromolar range and SI values of >10. In contrast, little, if any, specific inhibition was observed with HCV, a member of the Hepacivirus genus (Fig. 6C). Of note, AP30451 appeared to have slightly greater toxicity in the human Huh 7.5 hepatoma cell lines used in the HCV studies; this was not, however, consistently observed with other human lines, including HeLa and 293T cells (data not shown). Because the compound AP30451 was active against DENV-2, we subsequently tested additional inhibitors of WNV infection against DENV-2 by flow cytometric analysis of intracellular viral antigen in BHK-21 cells at 72 h after infection. Notably, several of the compounds tested that inhibited WNV infection in cells also strongly inhibited DENV-2 infection, including compound AP30451 (Fig. 7). For AP30451, significant inhibition was observed regardless of whether the compound was added immediately before or after infection (data not shown).
FIG. 6.
Anti-flavivirus activity of compound AP30451. BHK (A and B) or Huh-7.5 (C) cells expressing dengue (A), yellow fever (B), or hepatitis C (C) subgenomic replicons were treated with increasing concentrations of lead compound AP30451. Antiviral activity of AP30451 was assessed by measuring replication-dependent luciferase expression. Cytotoxic effect of compounds was measured at the indicated concentrations by using an ATP quantification assay. The activity in both assays is expressed as a percentage of no-drug controls. The results are representative of several independent experiments performed in quadruplicate.
FIG. 7.
Inhibition of DENV-2 infection with lead compounds. BHK cells were incubated for 30 min with inhibitors prior to infection with DENV-2 (MOI = 0.01). Three days later, cells were harvested, permeabilized, stained with anti-DENV-2 MAb, and processed by flow cytometry. The data are an average of three independent experiments performed in triplicate, and error bars indicate standard deviations. The asterisks indicate statistically significant differences (P < 0.05) compared to the 0.5% DMSO vehicle control.
Mechanism of action.
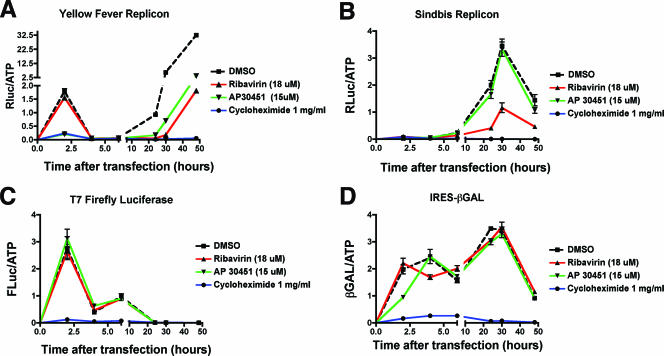
We next assessed the mechanism of action of AP30451, our most consistently potent anti-WNV compound in all screening assays. Since this antiviral compound was initially identified in the replicon screening assay, we speculated that AP30451 should inhibit translation or replication and is unlikely to affect viral entry, encapsidation, secretion, or maturation. To further dissect whether AP30451 inhibited flavivirus translation or replication, we examined its effect on luciferase activity at early time points after transfection of infectious replicon RNA. For these studies, we used the YFV replicon for two reasons. (i) AP30451 inhibited YFV replicon propagation (see Fig. 6B). (ii) The luciferase signal at early time points representing translation of input RNA was significantly higher than that from WNV or DENV-2 replicons and thus easier to interpret potential drug-mediated inhibition (data not shown). In vitro-transcribed m7G-capped RNA from the YFV replicon cDNA was transfected into BHK cells, and the translation of luciferase was measured in the presence of DMSO diluent, AP30451, ribavirin, or the translation inhibitor cycloheximide (Fig. 8A). As expected, cycloheximide virtually abolished replicon translation at early and late times after RNA transfection. Ribavirin, a guanosine analogue and inhibitor of RNA-dependent RNA polymerases (42), had no effect on early translation but did significantly inhibit later translation, likely due to its effect on replication. In contrast, AP30451 blocked both the early and later phases of translation to <10% of the DMSO control (P ≤ 0.001). To confirm that the inhibitory effect was specific for flaviviruses, we evaluated the ability of AP30451 to inhibit translation of other viral and nonviral m7G-capped and uncapped mRNA. Whereas cycloheximide uniformly blocked mRNA translation, AP30451 did not reduce translation of m7G-capped firefly luciferase, or Sindbis virus replicon mRNA, or uncapped mRNA containing an EMCV IRES (Fig. 8B to D). For the Sindbis virus replicon, the expression of the luciferase gene is under control of the 26S subgenomic promoter and thus is only translated after an initial round of replication of the genomic RNA (82). Overall, AP30451 appeared to inhibit the translation of flavivirus RNA specifically.
FIG. 8.
Effect of WNV inhibitors on viral translation and replication. YFV replicon (A), Sindbis virus replicon (B), firefly luciferase (C), and IRES-β-Gal (D) mRNAs were generated in vitro after T7 DNA-dependent RNA transcription and transfected by electroporation into BHK-21 cells. Immediately after transfection, diluent (1% DMSO), ribavirin (18 μM), AP30451 (15 μM), or cycloheximide (1 mg/ml) was added. Parallel sets of cells were harvested at the indicated times, and the reporter activity or ATP levels were measured. The data are expressed as reporter RLU per ATP-dependent RLU to normalize for cell number. One experiment of four performed in octuplicate is shown, and error bars indicate standard deviations. Note the break in the x and y axes to facilitate visualization of both early and later phases of translation.
DISCUSSION
Currently, there is no approved specific therapy for WNV or other flaviviruses. We performed here a primary screen of 80,000 small molecule compounds from a commercial library with a cell-based replicon assay that measured WNV replication as a function of luciferase expression. This primary screen was reproducible, had a robust signal-to-noise ratio, and was amenable to high-throughput applications. Indeed, analogous replicon-based primary screens to identify inhibitors of flavivirus replication have been performed by other groups (35, 74, 75). One limitation of this assay is that candidate inhibitors block translation or replication and not viral entry, encapsidation, secretion, or maturation. Our strategy involved identification of compounds that reduced luciferase activity in lysates of cells, followed by a more thorough dose-response analysis with multiple data points to rule out cytotoxicity and define the SI. Compounds with favorable inhibitory profiles in the replicon assay were then subjected to three lower-throughput assays using infectious virus. These included the use of a capture ELISA that detected the relative amounts of secreted NS1 in the supernatants of infected cells and viral infection assays with Vero cells, BHK cells, and primary cortical neurons, the latter a known target of WNV infection in vivo (43, 80, 81, 85). Based on these screens, we identified several compounds whose antiviral profile is sufficiently attractive to justify further drug development efforts, including structure activity relationships, lead optimization, pharmacokinetic studies, toxicokinetic studies, and animal efficacy studies.
The candidate lead compounds from our screens were classified into four diverse chemical classes. One class, the secondary sulfonamides, which are chemically tractable compounds, encompassed the majority of the candidates. However, this could be a reflection of the particular makeup of our library. Indeed, parallel small molecule inhibitor screens by others that used an analogous replicon assay but different chemical libraries identified parazolotrahydrothophenes (35), pyrozolopyrimidines (35), pyrazolines (32, 75), xanthanes, acridines, and quinolines (32). One secondary sulfonamide compound, AP30451, was particularly promising and had antiviral activity not only in continuous cell lines but also in primary neurons. Compound AP30451 inhibited WNV at low micromolar concentrations in multiple assays, which indicates that it is as potent as any published anti-flavivirus small molecule inhibitor. In general, it is expected that a viable therapeutic antiviral drug should be active at concentrations in the submicromolar range, depending on its pharmacokinetic and pharmacodynamic properties. Ongoing studies have shown that secondary sulfonamides are amenable to structure-activity relationship lead optimization, and efforts to improve potency and drug-like properties are under way.
In addition to compounds that have been identified by replicon screening, existing small molecule drugs have been proposed as antiviral agents for WNV. Some of these were evaluated in our screens as controls. Ribavirin has inhibitory activity against WNV infection in cell culture (2, 18, 45); this was confirmed in our replicon-based screen. However, in vivo studies with ribavirin and WNV or other flaviviruses have not been promising, since no clinical benefit (50, 60) or increased mortality (13, 64) was observed. MPA, a non-nucleoside inhibitor of IMPDH, inhibits WNV infection in cells by preventing viral RNA replication (24, 66, 86). We also observed significant inhibition with this compound in vitro. However, this compound also has significant immunosuppressive properties in vivo; increased mortality after WNV infection was observed in mice treated with MPA (B. Geiss and M. Diamond, unpublished results). Taken together, the preclinical data suggests that inhibitors of guanosine biosynthesis, such as ribavirin and MPA, may not be promising therapeutic candidates against WNV infection in vivo.
Since WNV is a neurotropic virus and most symptomatic infections are associated with neuroinvasive disease and infection of neurons (17, 38, 78), it is essential that a therapeutic agent efficiently control WNV infection in neurons. Indeed, our study is the first to demonstrate that a small molecule inhibitor of WNV replication can directly control infection in neurons. Nonetheless, for a small molecule to have antiviral activity against neuronal infection in vivo, it must efficiently cross the blood-brain barrier. Studies that evaluate and modify lipophilicity and biodistribution of AP30451, its congeners, or other candidate secondary sulfonamides into different tissue compartments are planned.
Compound AP30451 had broad-spectrum anti-flavivirus activity since it also inhibited DENV infection and YFV replicon propagation. RNA transfection and reporter gene studies suggest that AP30451 blocks the initial phase of translation of infectious viral RNA that occurs prior to viral replication. This was not entirely surprising since that this compound was identified using a replicon screening assay and thus would not be expected to inhibit viral entry, encapsidation, secretion, or maturation. Inhibition of the early phase of translation by AP30451 distinguishes it from recent studies with the pyrazoline class of inhibitors, which apparently block RNA synthesis (32). Nonetheless, it remains possible that AP30451 could have independent inhibitory effects on RNA replication. Interestingly, the inhibition of translation appeared specific for flavivirus replicon mRNA, since no significant reduction of translation was observed by AP30451 with an m7G-capped mRNA, an m7G-capped Sindbis virus replicon mRNA, or an IRES-regulated transcript. Thus, AP30451 does not appear to be a general inhibitor of cellular or viral mRNA translation, which, in part, may explain its favorable selectivity profile. Accordingly, AP30451 reduced flavivirus replicon propagation but did not specifically inhibit replication of HCV, a distantly related Flaviviridae family member that uses a unique IRES-dependent translation mechanism (29). AP30451 is also distinguished from broad-spectrum antiviral compounds such as 2-amino-8-(β-d-ribofuranosyl) imidazo [1,2-a]-s-triazine-4-one (ZX-2401), which inhibits infection by several types of RNA viruses, including Flaviviridae family members (71). At present, it remains unclear how AP30451 specifically inhibits translation of m7G-capped flavivirus but not other capped or uncapped cellular or viral mRNA. Studies are under way to define whether AP30451 affects viral RNA stability or the initiation or elongation phases of translation.
Combination drug therapy against rapidly evolving pathogens has become a mainstay of the treatment of infections by a number of viruses, including HCV and human immunodeficiency virus (5, 39), to enhance the potency and decrease emergence of resistant variants. For WNV, novel antiviral strategies with biologic agents have been developed and include type I IFN (2, 15, 47, 76, 83), immune gamma globulin (6, 27), human or humanized neutralizing MAbs (33, 72, 87), peptide inhibitors (3, 40), and oligonucleotide-based platforms, including small interfering RNA (4, 19, 30, 51, 63). Combination therapy in vitro with type I IFN and ZX-2401, a broad-spectrum nucleoside with anti-flavivirus activity, resulted in moderate synergy of inhibition (71). Synergy studies will be important to perform with compounds like AP30451 and existing candidate biological therapeutics against WNV. Indeed, since members of our group have recently developed an inhibitory humanized MAb that blocks virus entry at a postattachment step with significant therapeutic activity in vivo (65, 70, 72), we plan to test our lead compounds, in combination with these agents in animals models of WNV infection.
Acknowledgments
We acknowledge the contribution of members of the Diamond laboratory and Apath to this study. We also thank Richard Kinney (Centers for Disease Control and Prevention, Fort Collins, CO) for the WNV and DENV infectious cDNA plasmids.
This study was supported by the NIH (U01/AI538870 [M.S.D. and P.D.O.]).
Footnotes
Published ahead of print on 22 August 2007.
REFERENCES
- 1.Agapov, E. V., I. Frolov, B. D. Lindenbach, B. M. Pragai, S. Schlesinger, and C. M. Rice. 1998. Noncytopathic Sindbis virus RNA vectors for heterologous gene expression. Proc. Natl. Acad. Sci. USA 95:12989-12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, J. F., and J. J. Rahal. 2002. Efficacy of interferon alpha-2b and ribavirin against West Nile virus in vitro. Emerg. Infect. Dis. 8:107-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai, F., T. Town, D. Pradhan, J. Cox, Ashish, M. Ledizet, J. F. Anderson, R. A. Flavell, J. K. Krueger, R. A. Koski, and E. Fikrig. 2007. Antiviral peptides targeting the West Nile virus envelope protein. J. Virol. 81:2047-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai, F., T. Wang, U. Pal, F. Bao, L. H. Gould, and E. Fikrig. 2005. Use of RNA interference to prevent lethal murine West Nile virus infection. J. Infect. Dis. 191:1148-1154. [DOI] [PubMed] [Google Scholar]
- 5.Barreiro, P., T. Garcia-Benayas, A. Rendon, S. Rodriguez-Novoa, and V. Soriano. 2004. Combinations of nucleoside/nucleotide analogues for HIV therapy. AIDS Rev. 6:234-243. [PubMed] [Google Scholar]
- 6.Ben-Nathan, D., S. Lustig, G. Tam, S. Robinzon, S. Segal, and B. Rager-Zisman. 2003. Prophylactic and therapeutic efficacy of human intravenous immunoglobulin in treating West Nile virus infection in mice. J. Infect. Dis. 188:5-12. [DOI] [PubMed] [Google Scholar]
- 7.Best, S. M., K. L. Morris, J. G. Shannon, S. J. Robertson, D. N. Mitzel, G. S. Park, E. Boer, J. B. Wolfinbarger, and M. E. Bloom. 2005. Inhibition of interferon-stimulated JAK-STAT signaling by a tick-borne flavivirus and identification of NS5 as an interferon antagonist. J. Virol. 79:12828-12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blight, K. J., A. A. Kolykhalov, and C. M. Rice. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972-1974. [DOI] [PubMed] [Google Scholar]
- 9.Borowski, P., M. Lang, A. Haag, H. Schmitz, J. Choe, H. M. Chen, and R. S. Hosmane. 2002. Characterization of imidazo[4,5-d]pyridazine nucleosides as modulators of unwinding reaction mediated by West Nile virus nucleoside triphosphatase/helicase: evidence for activity on the level of substrate and/or enzyme. Antimicrob. Agents Chemother. 46:1231-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bredenbeek, P. J., E. A. Kooi, B. Lindenbach, N. Huijkman, C. M. Rice, and W. J. Spaan. 2003. A stable full-length yellow fever virus cDNA clone and the role of conserved RNA elements in flavivirus replication. J. Gen. Virol. 84:1261-1268. [DOI] [PubMed] [Google Scholar]
- 11.Brooks, T. J., and R. J. Phillpotts. 1999. Interferon-alpha protects mice against lethal infection with St. Louis encephalitis virus delivered by the aerosol and subcutaneous routes. Antivir. Res. 41:57-64. [DOI] [PubMed] [Google Scholar]
- 12.Busch, M. P., S. Caglioti, E. F. Robertson, J. D. McAuley, L. H. Tobler, H. Kamel, J. M. Linnen, V. Shyamala, P. Tomasulo, and S. H. Kleinman. 2005. Screening the blood supply for West Nile virus RNA by nucleic acid amplification testing. N. Engl. J. Med. 353:460-467. [DOI] [PubMed] [Google Scholar]
- 13.Chowers, M. Y., R. Lang, F. Nassar, D. Ben-David, M. Giladi, E. Rubinshtein, A. Itzhaki, J. Mishal, Y. Siegman-Igra, R. Kitzes, N. Pick, Z. Landau, D. Wolf, H. Bin, E. Mendelson, S. D. Pitlik, and M. Weinberger. 2001. Clinical characteristics of the West Nile fever outbreak, Israel, 2000. Emerg. Infect. Dis. 7:675-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung, K. M., G. E. Nybakken, B. S. Thompson, M. J. Engle, A. Marri, D. H. Fremont, and M. S. Diamond. 2006. Antibodies against West Nile virus nonstructural (NS)-1 protein prevent lethal infection through Fc gamma receptor-dependent and independent mechanisms. J. Virol. 80:1340-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crance, J. M., N. Scaramozzino, A. Jouan, and D. Garin. 2003. Interferon, ribavirin, 6-azauridine and glycyrrhizin: antiviral compounds active against pathogenic flaviviruses. Antivir. Res. 58:73-79. [DOI] [PubMed] [Google Scholar]
- 16.Crotty, S., D. Maag, J. J. Arnold, W. Zhong, J. Y. Lau, Z. Hong, R. Andino, and C. E. Cameron. 2000. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat. Med. 6:1375-1379. [DOI] [PubMed] [Google Scholar]
- 17.Davis, L. E., R. DeBiasi, D. E. Goade, K. Y. Haaland, J. A. Harrington, J. B. Harnar, S. A. Pergam, M. K. King, B. K. DeMasters, and K. L. Tyler. 2006. West Nile virus neuroinvasive disease. Ann. Neurol. 60:286-300. [DOI] [PubMed] [Google Scholar]
- 18.Day, C. W., D. F. Smee, J. G. Julander, V. F. Yamshchikov, R. W. Sidwell, and J. D. Morrey. 2005. Error-prone replication of West Nile virus caused by ribavirin. Antivir. Res. 67:38-45. [DOI] [PubMed] [Google Scholar]
- 19.Deas, T. S., I. Binduga-Gajewska, M. Tilgner, P. Ren, D. A. Stein, H. M. Moulton, P. L. Iversen, E. B. Kauffman, L. D. Kramer, and P. Y. Shi. 2005. Inhibition of flavivirus infections by antisense oligomers specifically suppressing viral translation and RNA replication. J. Virol. 79:4599-4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diamond, M. S., D. Edgil, T. G. Roberts, B. Lu, and E. Harris. 2000. Infection of human cells by dengue virus is modulated by different cell types and viral strains. J. Virol. 74:7814-7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diamond, M. S., and E. Harris. 2001. Interferon inhibits dengue virus infection by preventing translation of viral RNA through a PKR-independent mechanism. Virology 289:297-311. [DOI] [PubMed] [Google Scholar]
- 22.Diamond, M. S., T. Roberts, D. Edgil, B. Lu, J. Ernst, and E. Harris. 2000. Modulation of dengue virus infection in human cells by alpha, beta, and gamma interferons. J. Virol. 74:4957-4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diamond, M. S., B. Shrestha, A. Marri, D. Mahan, and M. Engle. 2003. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J. Virol. 77:2578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diamond, M. S., M. Zachariah, and E. Harris. 2002. Mycophenolic Acid inhibits dengue virus infection by preventing replication of viral RNA. Virology 304:211-221. [DOI] [PubMed] [Google Scholar]
- 25.Ebel, G. D., J. Carricaburu, D. Young, K. A. Bernard, and L. D. Kramer. 2004. Genetic and phenotypic variation of West Nile virus in New York, 2002-2003. Am. J. Trop. Med. Hyg. 71:493-500. [PubMed] [Google Scholar]
- 26.Ebel, G. D., A. P. Dupuis III, K. Ngo, D. Nicholas, E. Kauffman, S. A. Jones, D. Young, J. Maffei, P. Y. Shi, K. Bernard, and L. Kramer. 2001. Partial genetic characterization of West Nile Virus strains, New York State, 2000. Emerg. Infect. Dis. 7:650-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engle, M., and M. S. Diamond. 2003. Antibody prophylaxis and therapy against West Nile Virus infection in wild type and immunodeficient mice. J. Virol. 77:12941-12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fredericksen, B. L., M. Smith, M. G. Katze, P. Y. Shi, and M. Gale. 2004. The host response to West Nile virus infection limits spread through the activation of the interferon regulatory factor 3 pathway. J. Virol. 78:7737-7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallego, J., and G. Varani. 2002. The hepatitis C virus internal ribosome-entry site: a new target for antiviral research. Biochem. Soc. Trans. 30:140-145. [DOI] [PubMed] [Google Scholar]
- 30.Geiss, B. J., T. C. Pierson, and M. S. Diamond. 2005. Actively replicating West Nile virus is resistant to cytoplasmic delivery of siRNA. Virology J. 2: 53:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gentry, M. K., E. A. Henchal, J. M. McCown, W. E. Brandt, and J. M. Dalrymple. 1982. Identification of distinct antigenic determinants on dengue-2 virus using monoclonal antibodies. Am. J. Trop. Med. Hyg. 31:548-555. [DOI] [PubMed] [Google Scholar]
- 32.Goodell, J. R., F. Puig-Basagoiti, B. M. Forshey, P. Y. Shi, and D. M. Ferguson. 2006. Identification of compounds with anti-West Nile virus activity. J. Med. Chem. 49:2127-2137. [DOI] [PubMed] [Google Scholar]
- 33.Gould, L. H., J. Sui, H. Foellmer, T. Oliphant, T. Wang, M. Ledizet, A. Murakami, K. Noonan, C. Lambeth, K. Kar, J. F. Anderson, A. M. de Silva, M. S. Diamond, R. A. Koski, W. A. Marasco, and E. Fikrig. 2005. Protective and therapeutic capacity of human single chain Fv-Fc fusion proteins against West Nile virus. J. Virol. 79:14606-14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granwehr, B. P., K. M. Lillibridge, S. Higgs, P. W. Mason, J. F. Aronson, G. A. Campbell, and A. D. Barrett. 2004. West Nile virus: where are we now? Lancet Infect. Dis. 4:547-556. [DOI] [PubMed] [Google Scholar]
- 35.Gu, B., S. Ouzunov, L. Wang, P. Mason, N. Bourne, A. Cuconati, and T. M. Block. 2006. Discovery of small molecule inhibitors of West Nile virus using a high-throughput sub-genomic replicon screen. Antivir. Res. 70:39-50. [DOI] [PubMed] [Google Scholar]
- 36.Hamdan, A., P. Green, E. Mendelson, M. R. Kramer, S. Pitlik, and M. Weinberger. 2002. Possible benefit of intravenous immunoglobulin therapy in a lung transplant recipient with West Nile virus encephalitis. Transpl. Infect. Dis. 4:160-162. [DOI] [PubMed] [Google Scholar]
- 37.Hayes, E. B., N. Komar, R. S. Nasci, S. P. Montgomery, D. R. O'Leary, and G. L. Campbell. 2005. Epidemiology and transmission dynamics of West Nile virus disease. Emerg. Infect. Dis. 11:1167-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayes, E. B., J. J. Sejvar, S. R. Zaki, R. S. Lanciotti, A. V. Bode, and G. L. Campbell. 2005. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg. Infect. Dis. 11:1174-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoofnagle, J. H., and L. B. Seeff. 2006. Peginterferon and ribavirin for chronic hepatitis C. N. Engl. J. Med. 355:2444-2451. [DOI] [PubMed] [Google Scholar]
- 40.Hrobowski, Y. M., R. F. Garry, and S. F. Michael. 2005. Peptide inhibitors of dengue virus and West Nile virus infectivity. Virol. J. 2:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hubalek, Z., and J. Halouzka. 1999. West Nile fever: a reemerging mosquito-borne viral disease in Europe. Emerg. Infect. Dis. 5:643-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huggins, J. W. 1989. Prospects for treatment of viral hemorrhagic fevers with ribavirin, a broad-spectrum antiviral drug. Rev. Infect. Dis. 11(Suppl. 4):S750-S761. [DOI] [PubMed] [Google Scholar]
- 43.Hunsperger, E. A., and J. T. Roehrig. 2006. Temporal analyses of the neuropathogenesis of a West Nile virus infection in mice. J. Neurovirol. 12:129-139. [DOI] [PubMed] [Google Scholar]
- 44.Jones, M., A. Davidson, L. Hibbert, P. Gruenwald, J. Schlaak, S. Ball, G. R. Foster, and M. Jacobs. 2005. Dengue virus inhibits alpha interferon signaling by reducing STAT2 expression. J. Virol. 79:5414-5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jordan, I., T. Briese, N. Fischer, J. Y. Lau, and W. I. Lipkin. 2000. Ribavirin inhibits West Nile virus replication and cytopathic effect in neural cells. J. Infect. Dis. 182:1214-1217. [DOI] [PubMed] [Google Scholar]
- 46.Julander, J. G., Q. A. Winger, A. L. Olsen, C. W. Day, R. W. Sidwell, and J. D. Morrey. 2005. Treatment of West Nile virus-infected mice with reactive immunoglobulin reduces fetal titers and increases dam survival. Antivir. Res. 65:79-85. [DOI] [PubMed] [Google Scholar]
- 47.Kalil, A. C., M. P. Devetten, S. Singh, B. Lesiak, D. P. Poage, K. Bargenquast, P. Fayad, and A. G. Freifeld. 2005. Use of interferon-alpha in patients with West Nile encephalitis: report of 2 cases. Clin. Infect. Dis. 40:764-766. [DOI] [PubMed] [Google Scholar]
- 48.Keller, B. C., B. L. Fredericksen, M. A. Samuel, R. E. Mock, P. W. Mason, M. S. Diamond, and M. Gale, Jr. 2006. Resistance to alpha/beta interferon is a determinant of West Nile virus replication fitness and virulence. J. Virol. 80:9424-9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klein, R. S., E. Lin, B. Zhang, A. D. Luster, J. Tollett, M. A. Samuel, M. Engle, and M. S. Diamond. 2005. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J. Virol. 79:11457-11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koff, W. C., R. D. Pratt, J. L. Elm, Jr., C. N. Venkateshan, and S. B. Halstead. 1983. Treatment of intracranial dengue virus infections in mice with a lipophilic derivative of ribavirin. Antimicrob. Agents Chemother. 24:134-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar, P., S. K. Lee, P. Shankar, and N. Manjunath. 2006. A single siRNA suppresses fatal encephalitis induced by two different flaviviruses. PLoS Med. 3:e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leyssen, P., J. Balzarini, E. De Clercq, and J. Neyts. 2005. The predominant mechanism by which ribavirin exerts its antiviral activity in vitro against flaviviruses and paramyxoviruses is mediated by inhibition of IMP dehydrogenase. J. Virol. 79:1943-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li, L., A. D. Barrett, and D. W. Beasley. 2005. Differential expression of domain III neutralizing epitopes on the envelope proteins of West Nile virus strains. Virology 335:99-105. [DOI] [PubMed] [Google Scholar]
- 54.Libraty, D. H., P. R. Young, D. Pickering, T. P. Endy, S. Kalayanarooj, S. Green, D. W. Vaughn, A. Nisalak, F. A. Ennis, and A. L. Rothman. 2002. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J. Infect. Dis. 186:1165-1168. [DOI] [PubMed] [Google Scholar]
- 55.Lin, R. J., C. L. Liao, E. Lin, and Y. L. Lin. 2004. Blocking of the alpha interferon-induced Jak-Stat signaling pathway by Japanese encephalitis virus. J. Virol. 78:9285-9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu, W. J., H. B. Chen, X. J. Wang, H. Huang, and A. A. Khromykh. 2004. Analysis of adaptive mutations in Kunjin virus replicon RNA reveals a novel role for the flavivirus nonstructural protein NS2A in inhibition of beta interferon promoter-driven transcription. J. Virol. 78:12225-12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu, W. J., X. J. Wang, V. V. Mokhonov, P. Y. Shi, R. Randall, and A. A. Khromykh. 2005. Inhibition of interferon signaling by the New York 99 strain and Kunjin subtype of West Nile virus involves blockage of STAT1 and STAT2 activation by nonstructural proteins. J. Virol. 79:1934-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lohmann, V., F. Korner, J. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110-113. [DOI] [PubMed] [Google Scholar]
- 59.Macdonald, J., J. Tonry, R. A. Hall, B. Williams, G. Palacios, M. S. Ashok, O. Jabado, D. Clark, R. B. Tesh, T. Briese, and W. I. Lipkin. 2005. NS1 protein secretion during the acute phase of West Nile virus infection. J. Virol. 79:13924-13933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malinoski, F. J., S. E. Hasty, M. A. Ussery, and J. M. Dalrymple. 1990. Prophylactic ribavirin treatment of dengue type 1 infection in rhesus monkeys. Antivir. Res. 13:139-149. [DOI] [PubMed] [Google Scholar]
- 61.Markland, W., T. J. McQuaid, J. Jain, and A. D. Kwong. 2000. Broad-spectrum antiviral activity of the IMP dehydrogenase inhibitor VX-497: a comparison with ribavirin and demonstration of antiviral additivity with alpha interferon. Antimicrob. Agents Chemother. 44:859-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mattar, S., E. Edwards, J. Laguado, M. Gonzalez, J. Alvarez, and N. Komar. 2005. West Nile virus antibodies in Colombian horses. Emerg. Infect. Dis. 11:1497-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCown, M., M. S. Diamond, and A. Pekosz. 2003. The utility of siRNA transcripts produced by RNA polymerase I in down regulating viral gene expression and replication of negative- and positive-strand RNA viruses. Virology 313:514-524. [DOI] [PubMed] [Google Scholar]
- 64.Morrey, J. D., C. W. Day, J. G. Julander, L. M. Blatt, D. F. Smee, and R. W. Sidwell. 2004. Effect of interferon-alpha and interferon-inducers on West Nile virus in mouse and hamster animal models. Antivir. Chem. Chemother. 15:101-109. [DOI] [PubMed] [Google Scholar]
- 65.Morrey, J. D., V. Siddharthan, A. L. Olsen, G. Y. Roper, H. Wang, T. J. Baldwin, S. Koenig, S. Johnson, J. L. Nordstrom, and M. S. Diamond. 2006. Humanized monoclonal antibody against West Nile virus E protein administered after neuronal infection protects against lethal encephalitis in hamsters. J. Infect. Dis. 194:1300-1308. [DOI] [PubMed] [Google Scholar]
- 66.Morrey, J. D., D. F. Smee, R. W. Sidwell, and C. Tseng. 2002. Identification of active antiviral compounds against a New York isolate of West Nile virus. Antivir. Res. 55:107-116. [DOI] [PubMed] [Google Scholar]
- 67.Moss, B., O. Elroy-Stein, T. Mizukami, W. A. Alexander, and T. R. Fuerst. 1990. New mammalian expression vectors. Nature 348:91-92. [DOI] [PubMed] [Google Scholar]
- 68.Munoz-Jordan, J. L., M. Laurent-Rolle, J. Ashour, L. Martinez-Sobrido, M. Ashok, W. I. Lipkin, and A. Garcia-Sastre. 2005. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J. Virol. 79:8004-8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Munoz-Jordan, J. L., G. G. Sanchez-Burgos, M. Laurent-Rolle, and A. Garcia-Sastre. 2003. Inhibition of interferon signaling by dengue virus. Proc. Natl. Acad. Sci. USA 100:14333-14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nybakken, G., T. Oliphant, S. Johnson, S. Burke, M. S. Diamond, and D. H. Fremont. 2005. Structural basis for neutralization of a therapeutic antibody against West Nile virus. Nature 437:764-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ojwang, J. O., S. Ali, D. F. Smee, J. D. Morrey, C. D. Shimasaki, and R. W. Sidwell. 2005. Broad-spectrum inhibitor of viruses in the Flaviviridae family. Antivir. Res. 68:49-55. [DOI] [PubMed] [Google Scholar]
- 72.Oliphant, T., M. Engle, G. Nybakken, C. Doane, S. Johnson, L. Huang, S. Gorlatov, E. Mehlhop, A. Marri, K. M. Chung, G. D. Ebel, L. D. Kramer, D. H. Fremont, and M. S. Diamond. 2005. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat. Med. 11:522-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petersen, L. R., A. A. Marfin, and D. J. Gubler. 2003. West Nile virus. JAMA 290:524-528. [DOI] [PubMed] [Google Scholar]
- 74.Puig-Basagoiti, F., T. S. Deas, P. Ren, M. Tilgner, D. M. Ferguson, and P. Y. Shi. 2005. High-throughput assays using a luciferase-expressing replicon, virus-like particles, and full-length virus for West Nile virus drug discovery. Antimicrob. Agents Chemother. 49:4980-4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Puig-Basagoiti, F., M. Tilgner, B. M. Forshey, S. M. Philpott, N. G. Espina, D. E. Wentworth, S. J. Goebel, P. S. Masters, B. Falgout, P. Ren, D. M. Ferguson, and P. Y. Shi. 2006. Triaryl pyrazoline compound inhibits flavivirus RNA replication. Antimicrob. Agents Chemother. 50:1320-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rahal, J. J., J. Anderson, C. Rosenberg, T. Reagan, and L. L. Thompson. 2004. Effect of interferon-alpha2b therapy on St. Louis viral meningoencephalitis: clinical and laboratory results of a pilot study. J. Infect. Dis. 190:1084-1087. [DOI] [PubMed] [Google Scholar]
- 77.Russell, P. K., S. Udomsakdi, and S. B. Halstead. 1967. Antibody response in dengue and dengue hemorrhagic fever. Jpn. J. Med. Sci. Biol. 20:103-108. [PubMed] [Google Scholar]
- 78.Samuel, M. A., and M. S. Diamond. 2006. Pathogenesis of West Nile virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J. Virol. 80:9349-9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Samuel, M. A., and M. S. Diamond. 2005. Type I IFN protects against lethal West Nile Virus infection by restricting cellular tropism and enhancing neuronal survival. J. Virol. 79:13350-13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Samuel, M. A., J. D. Morrey, and M. S. Diamond. 2007. Caspase-3-dependent cell death of neurons contributes to the pathogenesis of West Nile virus encephalitis. J. Virol. 81:2614-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Samuel, M. A., K. Whitby, B. C. Keller, A. Marri, W. Barchet, B. R. G. Williams, R. H. Silverman, M. Gale, and M. S. Diamond. 2006. PKR and RNase L contribute to protection against lethal West Nile virus infection by controlling early viral spread in the periphery and replication in neurons. J. Virol. 80:7009-7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sanz, M. A., A. Castello, and L. Carrasco. 2007. Viral translation is coupled to transcription in Sindbis virus-infected cells. J. Virol. 81:7061-7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sayao, A. L., O. Suchowersky, A. Al-Khathaami, B. Klassen, N. R. Katz, R. Sevick, P. Tilley, J. Fox, and D. Patry. 2004. Calgary experience with West Nile virus neurological syndrome during the late summer of 2003. Can. J. Neurol. Sci. 31:194-203. [DOI] [PubMed] [Google Scholar]
- 84.Shimoni, Z., M. J. Niven, S. Pitlick, and S. Bulvik. 2001. Treatment of West Nile virus encephalitis with intravenous immunoglobulin. Emerg. Infect. Dis. 7:759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shrestha, B., D. I. Gottlieb, and M. S. Diamond. 2003. Infection and injury of neurons by West Nile encephalitis virus. J. Virol. 77:13203-13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takhampunya, R., S. Ubol, H. S. Houng, C. E. Cameron, and R. Padmanabhan. 2006. Inhibition of dengue virus replication by mycophenolic acid and ribavirin. J. Gen. Virol. 87:1947-1952. [DOI] [PubMed] [Google Scholar]
- 87.Throsby, M., C. Geuijen, J. Goudsmit, A. Q. Bakker, J. Korimbocus, R. A. Kramer, M. Clijsters-van der Horst, M. de Jong, M. Jongeneelen, S. Thijsse, R. Smit, T. J. Visser, N. Bijl, W. E. Marissen, M. Loeb, D. J. Kelvin, W. Preiser, J. ter Meulen, and J. de Kruif. 2006. Isolation and characterization of human monoclonal antibodies from individuals infected with West Nile virus. J. Virol. 80:6982-6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Whitby, K., T. C. Pierson, B. Geiss, K. Lane, M. Engle, Y. Zhou, R. W. Doms, and M. S. Diamond. 2005. Castanospermine, a potent inhibitor of dengue virus infection in vitro and in vivo. J. Virol. 79:8698-8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Young, P. R., P. A. Hilditch, C. Bletchly, and W. Halloran. 2000. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J. Clin. Microbiol. 38:1053-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang, J. H., T. D. Chung, and K. R. Oldenburg. 1999. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen 4:67-73. [DOI] [PubMed] [Google Scholar]