Abstract
The rotavirus NSP5 protein directs the formation of viroplasm-like structures (VLS) and is required for viroplasm formation within infected cells. In this report, we have defined signals within the C-terminal 21 amino acids of NSP5 that are required for VLS formation and that direct the insolubility and hyperphosphorylation of NSP5. Deleting C-terminal residues of NSP5 dramatically increased the solubility of N-terminally tagged NSP5 and prevented NSP5 hyperphosphorylation. Computer modeling and analysis of the NSP5 C terminus revealed the presence of an amphipathic α-helix spanning 21 C-terminal residues that is conserved among rotaviruses. Proline-scanning mutagenesis of the predicted helix revealed that single-amino-acid substitutions abolish NSP5 insolubility and hyperphosphorylation. Helix-disrupting NSP5 mutations also abolished localization of green fluorescent protein (GFP)-NSP5 fusions into VLS and directly correlate VLS formation with NSP5 insolubility. All mutations introduced into the hydrophobic face of the predicted NSP5 α-helix disrupted VLS formation, NSP5 insolubility, and the accumulation of hyperphosphorylated NSP5 isoforms. Some NSP5 mutants were highly soluble but still were hyperphosphorylated, indicating that NSP5 insolubility was not required for hyperphosphorylation. Expression of GFP containing the last 68 residues of NSP5 at its C terminus resulted in the formation of punctate VLS within cells. Interestingly, GFP-NSP5-C68 was diffusely dispersed in the cytoplasm when calcium was depleted from the medium, and after calcium resupplementation GFP-NSP5-C68 rapidly accumulated into punctate VLS. A potential calcium switch, formed by two tandem pseudo-EF-hand motifs (DxDxD), is present just upstream of the predicted α-helix. Mutagenesis of either DxDxD motif abolished the regulatory effect of calcium on VLS formation and resulted in the constitutive assembly of GFP-NSP5-C68 into punctate VLS. These results reveal specific residues within the NSP5 C-terminal domain that direct NSP5 hyperphosphorylation, insolubility, and VLS formation in addition to defining residues that constitute a calcium-dependent trigger of VLS formation. These studies identify functional determinants within the C terminus of NSP5 that regulate VLS formation and provide a target for inhibiting NSP5-directed VLS functions during rotavirus replication.
Rotavirus infection is the single most important cause of severe dehydrating diarrhea in infants and young children, causing approximately 600,000 deaths every year (20). Rotaviruses are icosahedral viruses with a segmented double-stranded RNA genome that infect villous-tip epithelial cells of the proximal small intestine (31). Rotavirus replication is fully cytoplasmic and occurs within highly specialized regions called viroplasms (32, 33). Viroplasms are punctate perinuclear structures that are sites of rotavirus protein assembly, RNA packaging, and replication (13, 14, 25, 26, 30, 46). Viroplasm formation is a characteristic of several members of the Reoviridae family, including rotaviruses, reoviruses, orbiviruses, and phytoreoviruses (8, 19, 32, 33, 39). The mechanism(s) of viroplasm formation is an area of intense research, and each member of the Reoviridae family contains a primary protein that directs viroplasm formation (7, 28, 39, 43, 47).
Recent studies have revealed a critical role for the rotavirus nonstructural protein NSP5 in the formation of viroplasms and rotavirus replication (11, 25, 26, 28, 37, 46). Inhibiting NSP5 expression using RNA interference results in defective viroplasm formation, reduced viral RNA synthesis, and decreased rotavirus titers (11, 26). NSP5 is encoded by gene segment 11 and has a predicted molecular mass of 21 kDa (2, 5, 6, 48). NSP5 is reportedly O-glycosylated, and basal 26- and 28-kDa isoforms (21) are phosphorylated to 32- to 35-kDa hyperphosphorylated isoforms (2, 6). Hyperphosphorylation of NSP5 occurs in the absence of coexpressed NSP2 or N-terminal epitope tags when cellular phosphatases are inhibited (5, 6, 10, 28, 34, 37). This indicates that NSP5 itself is constitutively phosphorylated by cellular kinases and dephosphorylated by cellular phosphatases. The rotavirus NSP2 protein interacts with the N terminus of NSP5, permitting the hyperphosphorylation of NSP5, NSP5 insolubility, and its localization into punctate viroplasm-like structures (VLS) (1, 3, 16-18, 25, 28, 42). Similarly, the addition of N-terminal epitope tags to NSP5 appears to mimic the effect of NSP2 binding by directing NSP5 hyperphosphorylation, insolubility, and VLS formation in the absence of NSP2 coexpression (10, 28, 37).
Several important NSP5 functions also are reportedly directed by the NSP5 C-terminal domain (1, 3, 10, 15, 17, 18, 28, 37, 42). The NSP5 C terminus directs dimer formation (15, 28, 42), NSP5 binding to the NSP6 protein (42), and the increased insolubility of NSP5 (10, 37). Recently, we and others have demonstrated that fusing the C-terminal 68 residues of NSP5 to the green fluorescent protein (GFP) C terminus is sufficient to direct the formation of VLS within cells (28, 37). The ability of N-terminally tagged NSP5 proteins to intrinsically form viroplasms and undergo hyperphosphorylation permits the study of functional determinants of viroplasm formation that are embedded within NSP5.
In this study, we have further examined functional elements within the NSP5 C terminus that direct VLS formation and protein insolubility. Our results demonstrate that residues within a predicted helical domain at the C terminus of NSP5 are critical for VLS formation. The insolubility of expressed N-tagged NSP5 is directly correlated with VLS formation by chimeric GFP-NSP5 proteins. Our studies further demonstrate that two upstream DxDxD motifs upstream and adjacent to the NSP5 C-terminal helix form a calcium switch that regulates NSP5 assembly into VLS. Mutagenesis of either DxDxD motif results in the constitutive formation of VLS by GFP-NSP5-C68 and abolishes the ability of calcium to regulate VLS formation. These studies provide a basic understanding of the mechanism of NSP5-directed VLS formation and define specific residues within the NSP5 C terminus that direct and regulate VLS formation.
MATERIALS AND METHODS
Cell culture, virus, and reagents.
African green monkey kidney-derived COS-7 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (Sigma) containing 10% fetal bovine serum (Sigma) and antibiotic-antimycotic solution (final concentration of 100 U penicillin per ml, 100 μg streptomycin per ml, and 250 ng amphotericin B per ml; Sigma). Calyculin A was purchased from Calbiochem and was used at the indicated concentration. Antibodies used for Western blotting were mouse monoclonal anti-N-HisG antibody (Invitrogen), anti-β-actin (clone AC-15; Sigma), and anti-mouse horseradish peroxidase conjugate (Amersham). NP-40 and sodium dodecyl sulfate (SDS) were purchased from Sigma.
Cloning and mutagenesis.
The rhesus rotavirus (RRV) NSP5 open reading frame was cloned into pcDNA3.1 in frame and downstream of an N-terminal 6× His-Gly tag as previously described (37). C-terminal truncations of NSP5 were obtained by inserting stop codons into reverse primers and performing PCR with a common forward primer (37). Site-directed mutagenesis was used to introduce single-amino-acid mutations in the NSP5 C terminus using the QuickChange site-directed mutagenesis kit (Stratagene) per the manufacturer's protocol. All clones were verified by automated fluorescent sequencing using Big Dye terminator chemistry (Perkin Elmer).
Transfection and protein expression.
Transfections were carried out using 50% confluent COS-7 cells grown in 6-well plates 18 to 24 h after being seeded using FuGene6 (Roche) as directed by the company. Cells were lysed 48 h posttransfection in NP-40 lysis buffer (40 mM Tris-HCl, pH 8, 150 mM NaCl, 2 mM EDTA, 10% glycerol, 1% NP-40, 1× protease inhibitor cocktail; Sigma) on ice for 20 min, followed by centrifugation at 18,000 × g for 30 min to separate soluble and pellet fractions. Following collection of the soluble fraction, insoluble pellet fractions were resuspended in an equal volume of NP-40 lysis buffer, and each fraction was mixed with an equal volume of 2× Laemmli sample buffer (4% SDS, 20% glycerol, 100 mM Tris-HCl, pH 6.8, 0.002% bromophenol blue, 10% 2-mercaptoethanol) to solubilize NSP5 prior to analysis by SDS-15% polyacrylamide gel electrophoresis. Proteins were transferred to nitrocellulose membranes and were immunoblotted using the indicated antibodies as described earlier (37). The amount of NSP5 present in equivalent volumes of soluble and pellet fractions was quantified using ImageJ software. As an internal control, blots were reprobed for levels of β-actin in soluble and pellet fractions. The ratio of soluble NSP5 to pellet NSP5 (designated the S/P ratio) was obtained after normalization to actin levels.
Calyculin A and calcium switch.
Cells were transfected with 1.5 μg of plasmid DNA, and 48 h later medium was replaced with fresh medium containing 200 nM calyculin A for 30 min before lysis and immunoblot analysis as described above. For the calcium switch experiments, cells were transfected as described above, and 18 to 24 h later they were subjected to calcium starvation for 24 h by replacing normal DMEM with low-calcium medium (DMEM containing 2 mM EGTA) as previously described (22, 23). Cells were visualized using an Olympus X51 inverted fluorescence microscope and were photographed using an Olympus DP-71 charge-coupled device camera. A calcium switch was induced by replacing low-calcium medium with DMEM containing 10 mM Ca2+ (DMEM supplemented with CaCl2), and cellular fluorescence was monitored in real time by time-lapse photography for the times indicated. Images were analyzed using Adobe Photoshop mask and overlay functions. Fluorescence microscopy of GFP chimeras was similarly recorded at 48 h posttransfection.
RESULTS
Insolubility and hyperphosphorylation are dependent on the N-NSP5 C terminus.
We have previously shown that N-terminally tagged NSP5 (N-NSP5) is present in detergent-insoluble fractions and is hyperphosphorylated in the absence of other rotavirus proteins (10, 37). In order to define functional determinants of NSP5 insolubility and hyperphosphorylation, we generated a C-terminal truncation of N-terminally tagged NSP5 lacking 30 C-terminal residues (NSP5-ΔC30). Identical amounts of wild-type or truncated NSP5 expression plasmids were transfected into COS-7 cells. Cells were lysed in buffer containing 1% NP-40, and soluble and insoluble cellular fractions were separated by centrifugation at 18,000 × g for 30 min. Western blot analysis of equivalent amounts of pellets and supernatants indicated that wild-type NSP5 is present predominantly in the insoluble fraction (Fig. 1A). In contrast, C-terminal truncation of NSP5 resulted in a protein that was present primarily in soluble cellular fractions (Fig. 1A). Thus, deletion of 30 residues from the NSP5 C terminus converts primarily insoluble NSP5 into a soluble protein.
FIG. 1.
Truncation of the C-terminal 30 residues from NSP5 abolishes its insolubility and hyperphosphorylation. (A) N-NSP5 lacking the C-terminal 30 residues (ΔC-30) was expressed in COS-7 cells, and soluble and insoluble fractions were collected 48 h posttransfection. Protein expression in each fraction was analyzed by Western blotting with anti-HisG antibody. Unlike full-length N-NSP5 (N-NSP5 wt) (lanes 1 and 3), the ΔC-30 mutant was detected only in the soluble fraction (lanes 2 and 4). (B) Cells expressing either full-length N-NSP5 or the ΔC-30 mutant were analyzed as described above after treatment with the phosphatase inhibitor calyculin A (+) or with no treatment (−). The brackets denote the basal (b) and hyperphosphorylated (hp) isoforms of NSP5. Numbers refer to the migration of protein markers, and the asterisk indicates a nonspecific cellular protein.
It has been reported that NSP5 is constitutively dephosphorylated by cellular phosphatases, since calyculin A inhibition of phosphatase activity enhances the appearance of hyperphosphorylated isoforms (5, 6, 34, 37). We observed that NSP5-ΔC30 lacks slower-migrating isoforms representing NSP5 hyperphosphorylation (Fig. 1A), and this could result from either the lack of protein phosphorylation or enhanced dephosphorylation. To address this, we expressed NSP5-ΔC30 in the presence of the phosphatase inhibitor calyculin A. In contrast to accumulation with the wild type, Fig. 1B shows that there is no significant accumulation of basal NSP5-ΔC30 within slower-migrating hyperphosphorylated isoforms in the presence of calyculin A, and as a result the NSP5-ΔC30 isoform observed does not result from phosphatase-directed dephosphorylation. Taken together, these findings suggest that the C-terminal 30 residues of NSP5 are critical for both its insolubility and hyperphosphorylation.
The NSP5 C terminus contains a predicted α-helix.
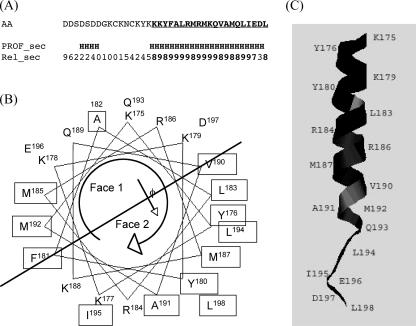
Secondary-structure analysis of the C-terminal 30 residues of NSP5 reveals the presence of a right-handed α-helix between residues 178 and 198 (Fig. 2A). Using a helical wheel program (Pepwheel; available online at http://bioweb.pasteur.fr/docs/EMBOSS/pepwheel.html), 10 out of 13 hydrophobic residues are present on a single face of the predicted C-terminal α-helix. Thus, the helical domain within the NSP5 C terminus is amphipathic and can be divided into two halves, with hydrophilic and hydrophobic faces (faces 1 and 2 in Fig. 2B). The asymmetrical distribution of hydrophobic residues also is apparent by using a fold comparison program (Phyre version 1), which similarly predicted an α-helical three-dimensional structure of the NSP5 C terminus (Fig. 2C). Alignment of the C-terminal NSP5 residues from different rotavirus strains (n = 43) revealed a high level of amino acid conservation within the predicted helix (94% similarity), further suggesting the functional importance of the C-terminal α-helix of NSP5.
FIG. 2.
Rotavirus NSP5 protein contains a conserved predicted amphipathic α-helix within the carboxyl 30 amino acids. (A) Prediction of the secondary structure present in the carboxyl 37 amino acids of the RRV NSP5 protein (AA) using the profile network Heidelberg (PROF_sec) method. Numbers indicate the reliability index values (REL_sec) of the prediction (0 = low; 9 = high); H denotes the predicted helical structure. (B) Helical wheel plot of NSP5 residues (178 to 198) predicted to form an α-helical structure. Hydrophobic residues are boxed, and the line divides the helix into a hydrophilic face (face 1) and hydrophobic face (face 2) along the overall hydrophobic moment of the helix (indicated by a solid arrow). Numbers refer to amino acid positions in the RRV NSP5 protein, and the open arrow indicates the residue arrangement along the helix viewed from the top. (C) Predicted structure of the NSP5 C terminus using a fold recognition algorithm. Numbers refer to amino acid positions in the RRV NSP5 protein.
Proline-scanning mutagenesis of the predicted C-terminal NSP5 α-helix.
In order to address the function of the predicted α-helix within the NSP5 C terminus, proline was systematically introduced at 12 positions in both faces of the predicted NSP5 α-helix, and the presence of NSP5 mutants in soluble and insoluble fractions was determined. Helix mutants of His6-NSP5 were expressed in COS-7 cells, and after separation of soluble and insoluble fractions, equivalent amounts of each fraction were analyzed for NSP5 and β-actin by Western blotting (Fig. 3). S/P ratios were calculated by quantitating protein expression using NIH Image. Wild-type His6-NSP5 was predominantly insoluble, with an S/P ratio of 0.3. An alanine-to-methionine mutation at position 191 of NSP5 (A191M) had little effect on NSP5 insolubility and resulted in an S/P ratio of 0.6. In contrast, introducing proline residues at positions 183, 190, 191, 192, 193, 194, and 195 resulted in an ∼5- to 33-fold increase in the S/P ratio of the NSP5 mutants, with the bulk of the expressed NSP5 protein present in the soluble fraction (Fig. 3). Proline substitutions at residues 179, 182, 185, 189, and 196 either did not alter NSP5 solubility compared to that of the wild type or increased the S/P ratio to less than threefold over that of the wild type. Interestingly, all proline substitutions within the hydrophobic face of the predicted α-helix dramatically enhanced NSP5 solubility. Mutation of two upstream, highly conserved cysteine residues (C166A and C169A) had no effect on NSP5 solubility (data not shown). The S/P ratio of the internal control, β-actin, consistently ranged from 0.6 to 0.9 for all samples. Normalizing S/P ratios of NSP5 to actin ratios further supports the enhanced solubility of mutants with proline residue substitutions within the hydrophobic face (face 2) of the NSP5 C-terminal α-helix (Fig. 3B). These findings suggest that the hydrophobic face of the helix is critical for NSP5 insolubility.
FIG. 3.
Role of C-terminal residues in NSP5 insolubility. (A) Thirteen NSP5 C-terminal mutants were expressed in COS-7 cells, and equivalent amounts of pellet and soluble fractions were analyzed by Western blotting using anti-His antibody as detailed in Materials and Methods. Blots were reprobed for β-actin as an internal control for comparable fractionation and equal loading. For wild-type N-NSP5 (wt) or each of the mutants tested, the S/P ratio was derived (indicated below each protein) as described in Materials and Methods. An asterisk indicates a nonspecific cellular band to demonstrate comparable protein separation for the panels shown. (B) Soluble fraction-pellet fraction distribution of N-NSP5 and proline mutants. The bars represent densitometric values obtained after scanning the blots shown in panel A. NSP5 S/P ratios were normalized to the S/P ratio of actin, internal to each sample and represented as fold increases over the level for wild-type NSP5 protein.
Proline substitutions within the C-terminal α-helix alter NSP5 hyperphosphorylation.
NSP5 synthesized during rotavirus infection or as a result of expressing N- or C-terminally tagged NSP5 are both phosphorylated by cellular kinases and are dephosphorylated by cellular phosphatases, and the sum of these two opposing processes regulates the appearance of hyperphosphorylated NSP5 isoforms (5, 6, 34, 37). We have previously demonstrated that basal forms of soluble NSP5 are hyperphosphorylated following phosphatase inhibition by a potent phosphatase inhibitor, calyculin A (37). In order to determine the role of the C-terminal α-helical domain on NSP5 hyperphosphorylation, we evaluated hyperphosphorylated isoforms of His6-NSP5 helix mutants in the presence and absence of calyculin A. COS-7 cells were transfected with equal amounts of plasmids expressing N-NSP5 or its mutants, and cells were treated with calyculin A. Cell lysates were prepared in 2% SDS, which solubilizes even highly insoluble NSP5 isoforms and permits the collective analysis of total cellular NSP5 (37). NSP5 present in equivalent amounts of cell lysates from cells treated with calyculin A or left untreated was analyzed by Western blotting (Fig. 4). Calyculin A treatment results in an increase in the amount of NSP5 present in hyperphosphorylated isoforms following expression of wild-type NSP5 protein or K179P, A182P, M185P, Q189P, and E196P mutant NSP5 proteins (Fig. 4A). A more dramatic effect of calyculin A-directed phosphatase inhibition was observed in NSP5 mutants with prolines substituted at positions 183, 190, 191, 192, 193, 194, and 195. In these mutants, there is little hyperphosphorylation of the protein in the absence of the phosphatase inhibitor; however, following calyculin A inhibition of cellular phosphatases, hyperphosphorylated isoforms are apparent but their levels are dramatically reduced compared to those of wild-type NSP5 (Fig. 4), and most of the protein was in basal forms. Proline introduction at L194 and I195 resulted in a minimal accumulation of hyperphosphorylated NSP5 isoforms (less than 5% of total NSP5). These results indicate that disrupting the predicted α-helical domain in the N-NSP5 C terminus profoundly affects protein hyperphosphorylation.
FIG. 4.
Proline substitution of residues in the predicted NSP5 α-helical domain affects the accumulation of hyperphosphorylated isoforms. Proline mutants or wild-type N-NSP5 (N-NSP5 wt) were expressed in COS-7 cells, and cells were exposed to 200 nM of calyculin A (lanes indicated by a plus sign) for 30 min before lysis in buffer containing 2% SDS in order to recover all hyperphosphorylated NSP5 isoforms (37). Basal (b) and hyperphosphorylated (hp) isoforms are indicated by brackets. The asterisk indicates a nonspecific cellular band present in total cell lysates that serves as an internal loading control.
Mutations in the α-helix that increase NSP5 solubility inhibit the formation of VLS.
Recently, the C-terminal 68 residues of NSP5 were shown to form VLS when expressed in cells lacking other rotavirus proteins (28, 37) and to significantly increase the insolubility of GFP (37). These findings suggested that this region is the minimal determinant of VLS formation and correlated VLS formation with the insolubility of NSP5. In this study, we tested the relationship between NSP5 insolubility and VLS formation by using proline mutations that either disrupt N-NSP5 insolubility (A191P, M192P, and L194P) or that lack any significant effect on N-NSP5 insolubility (A182P, M185P, and Q189P). Cells were transfected with equal amounts of plasmids expressing GFP-C68 wild-type or mutant proteins, and GFP was visualized 48 h later by fluorescence microscopy (Fig. 5A). Wild-type GFP-NSP5 chimeras formed punctate VLS within cells, as did chimeras with proline substitutions at positions 182, 185, and 189, and each of these NSP5 mutants is present predominantly in insoluble fractions, with S/P ratios of <0.8 (Fig. 3). In contrast, GFP-NSP5-C68 mutants containing A191P, M192P, and L194P substitutions were devoid of VLS within cells. Consistent with this finding, mutations at these positions within full-length N-NSP5 resulted in predominantly soluble proteins with S/P ratios ≥5-fold higher than those for wild-type NSP5 (Fig. 3B). A control mutation in which A191 was mutated to another hydrophobic residue, methionine, also displayed normal punctate VLS (Fig. 5B) and S/P ratios similar to those of the wild type (Fig. 3). These results support correlations between the insolubility of N-NSP5 and its localization into cellular VLS.
FIG. 5.
Effect of mutations in the α-helix of NSP5 on VLS formation by GFP-C68-NSP5. Cells were transfected with plasmids expressing GFP-NSP5 chimeras, and protein localization was observed 48 h later. (A) Shown are the GFP-NSP5-C68 wild-type protein (left panel), A191M mutant (middle panel), and A191P mutant (right panel). (B) GFP-NSP5-C68 mutants A191P, M192P, and L194P abolish VLS assembly (lower row), whereas A182P, M185P, and Q189P mutant proteins (upper row) do not.
VLS formation by GFP-NSP5-C68 constructs is regulated by a calcium switch.
During our analysis, we noted that DxDxD sequences, similar to those found in calcium binding proteins (35), are present just upstream of the C-terminal α-helix (residues 153 to 170). In order to examine whether NSP5-directed VLS formation is calcium regulated, we monitored the localization of GFP-NSP5-C68 following calcium addition by fluorescence microscopy. We expressed GFP-NSP5-C68 and then starved the cells of calcium for 24 h. Under these conditions, GFP-NSP5-C68 displayed a largely diffuse localization with a small number of punctate regions within individual cells (Fig. 6A). Exposure of cells to medium supplemented with calcium resulted in the rapid redistribution of GFP-NSP5-C68 within 2 to 12 min after calcium addition, and virtually all the detectable protein was found in punctate VLS 12 min after the calcium switch (Fig. 6A). A time course of GFP-NSP5-C68 fluorescence is shown in Fig. 6B and clearly demonstrates the appearance of VLS over time within the same cells. A small number of punctate structures, observed in the absence of calcium, may be the result of the inability of calcium removal to completely disassemble preformed VLS. Although indirect effects arising from the calcium switch cannot be ruled out at this stage, these findings suggest that VLS formation directed by the NSP5 C terminus is likely regulated by calcium.
FIG. 6.
VLS formation is regulated by a calcium switch. (A) The GFP-NSP5-C68 protein was expressed in cells, and 24 h later cells were starved of calcium (−Ca2+; left panel) as described in Materials and Methods (22, 23). GFP-C68-NSP5 subsequently was monitored 12 min after calcium resupplementation by fluorescence microscopy (+Ca2+; right panel). (B) Time-lapse images of rapid VLS formation by GFP-NSP5-C68 following a calcium switch. The left panel shows a field of cells that were starved of calcium as described above. The inset follows GFP-NSP5-C68 fluorescence within a group of cells at 1, 5, and 10 min after exposure to high-calcium medium by time-lapse photography.
Calcium-dependent VLS formation is affected by changes in DxDxD motifs and the C-terminal α-helix.
Two potential calcium-coordinating domains upstream of the C-terminal α-helix could be responsible for the observed calcium switch in NSP5-directed VLS formation (12, 24, 35, 45, 49-51). Here, we examined whether mutations in the potential calcium binding motifs and α-helical domains alter VLS formation and the calcium switch. Cells were transfected with the GFP-NSP5-C68 constructs with mutation A191P, M192P, or L194P, and a calcium switch was introduced 48 h posttransfection and 24 h after calcium starvation (Fig. 7). The diffuse localization of GFP-NSP5-C68 mutants A191P, M192P, and L194P was not altered by a calcium switch and is consistent with the inability of these NSP5 mutants to form VLS (Fig. 5). In contrast, in the absence of calcium the GFP-NSP5-C68 mutants A182P, M185P, Q189P, and A191M were primarily diffusely dispersed in the cytoplasm but responded to calcium addition by displaying a VLS pattern similar to that of wild-type GFP-NSP5-C68.
FIG. 7.
Effect of helix disruption on the calcium-switch-mediated VLS formation by GFP-NSP5-C68. (A) Wild-type GFP-NSP5-C68 and mutants A182P, M185P, M192P, and L194P were subjected to a calcium switch as described in the legend to Fig. 6. (B) GFP-NSP5-C68 A191 was mutated to either Met or Pro, and the ability of mutants to respond to a calcium switch was visualized by fluorescence microscopy.
We further mutated all aspartic acid residues, within each of the two potential calcium-coordinating DxDxD motifs, to alanines singly in the GFP-NSP5-C68 protein (residues 153 to 157, DXM1; residues 163 to 170, DXM2) (Fig. 8A) and expressed the mutants in COS-7 cells (Fig. 8B). Unlike wild-type GFP-NSP5-C68, we observed that, following calcium starvation, neither of the DX mutants displayed a diffuse localization (Fig. 8). In contrast, we observed intense punctate VLS in both GFP-NSP5-C68 DXM1 and DXM2, even in the presence of EGTA for 24 h (Fig. 8A). After switching to a high-calcium medium, there was no increase in DXM1 or DXM2 VLS formation. These findings indicate that the absence of either DxDxD motif results in the constitutive formation of VLS in the presence or absence of calcium. This suggests that the presence of two tandem DxDxD motifs upstream of the NSP5 C-terminal α-helical domain is required for the regulation, but not formation, of VLS.
FIG. 8.
Identification of functional DxDxD motifs in the NSP5 C terminus. (A) The C terminus of NSP5 contains two tandem DxDxD pseudo-EF-hand calcium motifs (in boldface) upstream of the predicted α-helix. The predicted NSP5 C-terminal α-helix is underlined. Alanine mutations introduced to disrupt DxDxD motifs individually are shown (DXM1 and DXM2), with mutations in boldface. (B) Expression of DXM1 and DXM2 mutant GFP-NSP5-C68 constructs following calcium starvation (upper row) or following exposure to high-calcium medium (lower row).
DISCUSSION
Rotavirus replication and assembly occurs within punctate cytoplasmic structures called viroplasms (13, 14, 25, 26, 30, 46). Several studies indicate that VLS are formed by the coexpression of two rotavirus nonstructural proteins, NSP5 and NSP2 (16-18, 30). More recently, it was reported that VLS formation results from the expression of only NSP5 containing an N-terminal epitope tag and that VLS formation maps to the C-terminal 68 residues of NSP5 (28, 37). NSP5 is essential for viroplasm formation, since small interfering RNAs, which block NSP5 expression, inhibit viroplasm formation within rotavirus-infected cells (11, 26). The nature of viroplasms, as nonmembranous structures within infected cells or following NSP5 expression, suggests that viroplasms result from the assembly of insoluble cytoplasmic complexes. Recent studies demonstrate that untagged NSP5 is insoluble when complexed with NSP2 and suggest an association of NSP5-NSP2 complexes with highly insoluble cytoskeletal proteins (3, 9). VLS formation following expression of only N-tagged NSP5 is coincident with the presence of NSP5 within SDS (0.1%)-insoluble fractions, and both insolubility and VLS formation map to the C-terminal 68 residues of NSP5 (37). Studies presented here define elements within the NSP5 C-terminal 68 residues that direct and regulate NSP5 insolubility and VLS assembly.
During infection or following NSP5 expression, NSP5 also is hyperphosphorylated, although a functional role for NSP5 phosphorylation has yet to be defined (1, 2, 5, 6, 13, 15, 17, 28, 34, 37, 42, 48). Soluble NSP5 is reportedly not hyperphosphorylated and is present primarily in basal 26- and 28-kDa isoforms (1, 2, 15-18). However, the addition of phosphatase inhibitors results in the hyperphosphorylation of soluble NSP5, indicating that soluble NSP5 is phosphorylated and concomitantly dephosphorylated by a cellular phosphatase (5, 6, 28, 34, 37). In contrast, insoluble NSP5 is present primarily in hyperphosphorylated isoforms, suggesting either that the insoluble NSP5 is a better substrate for phosphorylation or that it is less susceptible to cellular phosphatase regulation than soluble NSP5 (10, 37). Similar to the requirement for NSP5 insolubility, our findings demonstrate that the C terminus of NSP5 is required for NSP5 hyperphosphorylation. Deletion of the last 30 residues from N-NSP5 resulted in a loss of NSP5 insolubility and hyperphosphorylation, and this is consistent with C-terminal residue requirements for the hyperphosphorylation of untagged NSP5 (1, 15, 17, 42). Thus, NSP5 insolubility, VLS formation, and hyperphosphorylation all depend on the presence of an intact NSP5 C terminus.
The NSP5 C terminus is highly conserved among group A rotaviruses, and in analyzing this domain we found that the NSP5 of all rotaviruses examined contained a predicted right-handed α-helix spanning 21 residues. The predicted helix is amphipathic in nature, with most hydrophobic residues occupying a single helical face (Fig. 2). To address whether the predicted α-helix is a functional entity within the NSP5 C terminus, we used a systematic proline-scanning mutagenesis approach to alter both hydrophobic and hydrophilic faces, and we determined whether these changes altered NSP5 insolubility. We found that proline substitutions at 7 out of 12 positions in the helical region blocked NSP5 insolubility. Proline substitutions that did not alter NSP5 insolubility (changing residues 179, 182, 185, 189, and 196) were invariably found to occur within the hydrophilic face of the predicted α-helix (Fig. 3). Consistent with these findings, studies on naturally occurring amphipathic helical domains have revealed that prolines are tolerated and often occur within the hydrophilic helical face of α-helical domains, but they almost never occur within hydrophobic helical faces (29). Reported explanations for this indicate that residues on the hydrophilic face of the helix form compensatory hydrogen bonds with solvent molecules and that hydrophobic face residues are not capable of these interactions (29, 36). Although studies on the physical structure of the NSP5 C terminus are needed, it is not easy to obtain structural data on insoluble domains. Findings presented here demonstrate the functional presence of an amphipathic α-helix at the NSP5 C terminus.
We previously reported that NSP5 insolubility and hyperphosphorylation were discrete properties, since the appearance of hyperphosphorylated NSP5 isoforms is regulated by cellular phosphatases (37). As a result, the hyperphosphorylation of C-terminal NSP5 proline mutants was evaluated in the presence or absence of the phosphatase inhibitor calyculin A. Our data indicate that proline substitution for hydrophobic face residues prevents the efficient accumulation of NSP5 into hyperphosphorylated isoforms. In contrast, proline substitutions were mostly tolerated within the hydrophilic face (at positions K179, A182, M185, Q189, and E196) of the C-terminal α-helix, since these mutations still resulted in the substantial accumulation of NSP5 into higher isoforms following phosphatase inhibition. Interestingly, we noted that NSP5 mutants resulting in predominantly soluble NSP5 (L183P, V190P, A191P, and M192P) still were hyperphosphorylated following phosphatase inhibition. These results substantiate previous findings that NSP5 insolubility is not required for hyperphosphorylation (37) and also demonstrate that disruption of the C-terminal α-helix reduces the level of NSP5 phosphorylation.
The C-terminal 68 residues of NSP5 direct GFP to both insoluble cellular fractions (37) and VLS (28, 37), correlating NSP5's insolubility with VLS formation. Since mutations in the NSP5 C terminus altered NSP5 insolubility, we analyzed the effect of these α-helical domain mutations on VLS formation within GFP-NSP5-C68 fusion proteins. Mutations in the NSP5 C-terminal α-helix, which result in soluble NSP5, completely abrogated VLS formation by GFP-NSP5-C68 (A191P, M192P, and L194P) (Fig. 5). In contrast, the neutral substitution of methionine for alanine at residue 191 did not alter VLS formation by GFP-NSP5-C68 (Fig. 5A), indicating that insertion of a proline residue rather than sequence specificity altered VLS formation. Although an α-helix at the NSP5 C terminus needs to be demonstrated biophysically, these findings functionally demonstrate that VLS formation is dependent on residues within the NSP5 C-terminal α-helical domain.
Analysis of the NSP5 protein revealed the presence of pseudo-EF-hand calcium binding motifs, tandem DxDxD sequences (35, 52), adjacent and just upstream of the C-terminal α-helical domain (Fig. 8A). EF-hand motifs direct calcium binding by cellular proteins, often occur upstream of predicted α-helices, and are associated with regulating large conformational changes in helical domain function in a cooperative manner (12, 24, 35, 38, 44, 45, 49, 51, 52). Interestingly, we found that starving cells of calcium for 24 h resulted in GFP-NSP5-C68 that was diffusely distributed in the cytoplasm. However, following calcium addition, GFP-NSP5-C68 rapidly formed VLS (Fig. 6), suggesting that a calcium switch regulates NSP5-directed VLS formation. Disrupting either of the two DxDxD sequences in GFP-NSP5-C68 by mutagenesis resulted in NSP5 that was constitutively present in punctate VLS and no longer responsive to calcium starvation or addition. Thus, VLS formation was not dependent on the presence of two pseudo-EF-hand motifs, but the regulation of VLS formation by a calcium switch required both DxDxD motifs upstream of the NSP5 α-helical domain.
Interestingly, the rotavirus NSP4 protein recently was reported to form vesicular structures in cells following a calcium switch, and NSP4 reportedly is associated with viroplasmic NSP5 (4). Since NSP4 expression reportedly mobilizes intracellular calcium (4, 40, 41), NSP4 may serve as a viroplasm assembly trigger that directs NSP5 to form viroplasms during viral infection. Additionally, reovirus and bluetongue virus members of the Reoviridae family contain a similar motif adjacent to a helical domain at the C termini of μNS and NS2 proteins, respectively, and μNS has been suggested to have a zinc-coordinating function (7, 27). This suggests the potential for conserved divalent cation regulatory functions of viroplasm forming proteins within the Reoviridae, although the coordinate regulation of VLS formation during viral infection requires further study.
NSP5 is required for VLS formation during rotavirus infection, and as a result, NSP5 plays a critical role in rotavirus replication and assembly (30). In this report, we have defined two domains within the C-terminal 68 residues of NSP5 that regulate VLS formation. One domain defines a calcium-sensitive switch that regulates VLS formation, while the hydrophobic face of a second C-terminal α-helical domain appears to be essential for NSP5 insolubility and VLS formation. These findings suggest the need for studies on the role of calcium in VLS formation and suggest that the NSP5 C-terminal domain may be a target for the design and analysis of inhibitors of VLS formation that impact rotavirus assembly and replication.
Acknowledgments
We thank Karen Endriss and Varvara Kirrilov for technical assistance and Timothy Pepini for critical comments.
This work was supported by National Institutes of Health grants R01AI47873, PO1AI055621, and U54AI57158 (Northeast Biodefense Center-Lipkin) and by a Veterans Affairs merit award.
Footnotes
Published ahead of print on 15 August 2007.
REFERENCES
- 1.Afrikanova, I., E. Fabbretti, M. C. Miozzo, and O. R. Burrone. 1998. Rotavirus NSP5 phosphorylation is up-regulated by interaction with NSP2. J. Gen. Virol. 79:2679-2686. [DOI] [PubMed] [Google Scholar]
- 2.Afrikanova, I., M. C. Miozzo, S. Giambiagi, and O. Burrone. 1996. Phosphorylation generates different forms of rotavirus NSP5. J. Gen. Virol. 77:2059-2065. [DOI] [PubMed] [Google Scholar]
- 3.Arnoldi, F., M. Campagna, C. Eichwald, U. Desselberger, and O. R. Burrone. 2007. Interaction of rotavirus polymerase VP1 with nonstructural protein NSP5 is stronger than that with NSP2. J. Virol. 81:2128-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkova, Z., S. E. Crawford, G. Trugnan, T. Yoshimori, A. P. Morris, and M. K. Estes. 2006. Rotavirus NSP4 induces a novel vesicular compartment regulated by calcium and associated with viroplasms. J. Virol. 80:6061-6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackhall, J., A. Fuentes, K. Hansen, and G. Magnusson. 1997. Serine protein kinase activity associated with rotavirus phosphoprotein NSP5. J. Virol. 71:138-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackhall, J., M. Munoz, A. Fuentes, and G. Magnusson. 1998. Analysis of rotavirus nonstructural protein NSP5 phosphorylation. J. Virol. 72:6398-6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broering, T. J., M. M. Arnold, C. L. Miller, J. A. Hurt, P. L. Joyce, and M. L. Nibert. 2005. Carboxyl-proximal regions of reovirus nonstructural protein μNS necessary and sufficient for forming factory-like inclusions. J. Virol. 79:6194-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brookes, S. M., A. D. Hyatt, and B. T. Eaton. 1993. Characterization of virus inclusion bodies in bluetongue virus-infected cells. J. Gen. Virol. 74:525-530. [DOI] [PubMed] [Google Scholar]
- 9.Cabral-Romero, C., and L. Padilla-Noriega. 2006. Association of rotavirus viroplasms with microtubules through NSP2 and NSP5. Mem. Inst. Oswaldo Cruz. 101:603-611. [DOI] [PubMed] [Google Scholar]
- 10.Campagna, M., and O. R. Burrone. 2006. Fusion of tags induces spurious phosphorylation of rotavirus NSP5. J. Virol. 80:8283-8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campagna, M., C. Eichwald, F. Vascotto, and O. R. Burrone. 2005. RNA interference of rotavirus segment 11 mRNA reveals the essential role of NSP5 in the virus replicative cycle. J. Gen. Virol. 86:1481-1487. [DOI] [PubMed] [Google Scholar]
- 12.Chen, B., M. U. Mayer, L. M. Markillie, D. L. Stenoien, and T. C. Squier. 2005. Dynamic motion of helix A in the amino-terminal domain of calmodulin is stabilized upon calcium activation. Biochemistry 44:905-914. [DOI] [PubMed] [Google Scholar]
- 13.Chnaiderman, J., M. Barro, and E. Spencer. 2002. NSP5 phosphorylation regulates the fate of viral mRNA in rotavirus infected cells. Arch. Virol. 147:1899-1911. [DOI] [PubMed] [Google Scholar]
- 14.Chnaiderman, J., J. Diaz, G. Magnusson, F. Liprandi, and E. Spencer. 1998. Characterization of a rotavirus rearranged gene 11 by gene reassortment. Arch. Virol. 143:1711-1722. [DOI] [PubMed] [Google Scholar]
- 15.Eichwald, C., G. Jacob, B. Muszynski, J. E. Allende, and O. R. Burrone. 2004. Uncoupling substrate and activation functions of rotavirus NSP5: phosphorylation of Ser-67 by casein kinase 1 is essential for hyperphosphorylation. Proc. Natl. Acad. Sci. USA 101:16304-16309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eichwald, C., J. F. Rodriguez, and O. R. Burrone. 2004. Characterization of rotavirus NSP2/NSP5 interactions and the dynamics of viroplasm formation. J. Gen. Virol. 85:625-634. [DOI] [PubMed] [Google Scholar]
- 17.Eichwald, C., F. Vascotto, E. Fabbretti, and O. R. Burrone. 2002. Rotavirus NSP5: mapping phosphorylation sites and kinase activation and viroplasm localization domains. J. Virol. 76:3461-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fabbretti, E., I. Afrikanova, F. Vascotto, and O. R. Burrone. 1999. Two non-structural rotavirus proteins, NSP2 and NSP5, form viroplasm-like structures in vivo. J. Gen. Virol. 80:333-339. [DOI] [PubMed] [Google Scholar]
- 19.Fukushi, T., E. Shikata, and I. Kimura. 1962. Some morphological characters of rice dwarf virus. Virology 18:192-205. [DOI] [PubMed] [Google Scholar]
- 20.Glass, R. I., U. D. Parashar, J. S. Bresee, R. Turcios, T. K. Fischer, M. A. Widdowson, B. Jiang, and J. R. Gentsch. 2006. Rotavirus vaccines: current prospects and future challenges. Lancet 368:323-332. [DOI] [PubMed] [Google Scholar]
- 21.González, S. A., and O. R. Burrone. 1991. Rotavirus NS26 is modified by addition of single O-linked residues of N-acetylglucosamine. Virology 182:8-16. [DOI] [PubMed] [Google Scholar]
- 22.Gumbiner, B., and K. Simons. 1986. A functional assay for proteins involved in establishing an epithelial occluding barrier: identification of a uvomorulin-like polypeptide. J. Cell Biol. 102:457-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirose, T., Y. Izumi, Y. Nagashima, Y. Tamai-Nagai, H. Kurihara, T. Sakai, Y. Suzuki, T. Yamanaka, A. Suzuki, K. Mizuno, and S. Ohno. 2002. Involvement of ASIP/PAR-3 in the promotion of epithelial tight junction formation. J. Cell Sci. 115:2485-2495. [DOI] [PubMed] [Google Scholar]
- 24.Huq, N. L., K. J. Cross, and E. C. Reynolds. 2003. Nascent helix in the multiphosphorylated peptide alphaS2-casein(2-20). J. Pept. Sci. 9:386-392. [DOI] [PubMed] [Google Scholar]
- 25.Jiang, X., H. Jayaram, M. Kumar, S. J. Ludtke, M. K. Estes, and B. V. Prasad. 2006. Cryoelectron microscopy structures of rotavirus NSP2-NSP5 and NSP2-RNA complexes: implications for genome replication. J. Virol. 80:10829-10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.López, T., M. Rojas, C. Ayala-Breton, S. Lopez, and C. F. Arias. 2005. Reduced expression of the rotavirus NSP5 gene has a pleiotropic effect on virus replication. J. Gen. Virol. 86:1609-1617. [DOI] [PubMed] [Google Scholar]
- 27.Modrof, J., K. Lymperopoulos, and P. Roy. 2005. Phosphorylation of bluetongue virus nonstructural protein 2 is essential for formation of viral inclusion bodies. J. Virol. 79:10023-10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohan, K. V., J. Muller, I. Som, and C. D. Atreya. 2003. The N- and C-terminal regions of rotavirus NSP5 are the critical determinants for the formation of viroplasm-like structures independent of NSP2. J. Virol. 77:12184-12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orzáez, M., J. Salgado, A. Gimenez-Giner, E. Perez-Paya, and I. Mingarro. 2004. Influence of proline residues in transmembrane helix packing. J. Mol. Biol. 335:631-640. [DOI] [PubMed] [Google Scholar]
- 30.Patton, J. T., L. S. Silvestri, M. A. Tortorici, R. Vasquez-Del Carpio, and Z. F. Taraporewala. 2006. Rotavirus genome replication and morphogenesis: role of the viroplasm. Curr. Top. Microbiol. Immunol. 309:169-187. [DOI] [PubMed] [Google Scholar]
- 31.Pesavento, J. B., S. E. Crawford, M. K. Estes, and B. V. Prasad. 2006. Rotavirus proteins: structure and assembly. Curr. Top. Microbiol. Immunol. 309:189-219. [DOI] [PubMed] [Google Scholar]
- 32.Petrie, B. L., D. Y. Graham, H. Hanssen, and M. K. Estes. 1982. Localization of rotavirus antigens in infected cells by ultrastructural immunocytochemistry. J. Gen. Virol. 63:457-467. [DOI] [PubMed] [Google Scholar]
- 33.Petrie, B. L., H. B. Greenberg, D. Y. Graham, and M. K. Estes. 1984. Ultrastructural localization of rotavirus antigens using colloidal gold. Virus Res. 1:133-152. [DOI] [PubMed] [Google Scholar]
- 34.Poncet, D., P. Lindenbaum, R. L'Haridon, and J. Cohen. 1997. In vivo and in vitro phosphorylation of rotavirus NSP5 correlates with its localization in viroplasms. J. Virol. 71:34-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rigden, D. J., and M. Y. Galperin. 2004. The DxDxDG motif for calcium binding: multiple structural contexts and implications for evolution. J. Mol. Biol. 343:971-984. [DOI] [PubMed] [Google Scholar]
- 36.Ryan, P., and C. O. Edwards. 1995. Systematic introduction of proline in a eukaryotic signal sequence suggests asymmetry within the hydrophobic core. J. Biol. Chem. 270:27876-27879. [DOI] [PubMed] [Google Scholar]
- 37.Sen, A., D. Agresti, and E. R. Mackow. 2006. Hyperphosphorylation of the rotavirus NSP5 protein is independent of serine 67, NSP2, or the intrinsic insolubility of NSP5 and is regulated by cellular phosphatases. J. Virol. 80:1807-1816. [title corrected per Erratum, 80:3692.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka, T., N. Miwa, S. Kawamura, H. Sohma, K. Nitta, and N. Matsushima. 1999. Molecular modeling of single polypeptide chain of calcium-binding protein p26olf from dimeric S100B(ββ). Protein Eng. 12:395-405. [DOI] [PubMed] [Google Scholar]
- 39.Thomas, C. P., T. F. Booth, and P. Roy. 1990. Synthesis of bluetongue virus-encoded phosphoprotein and formation of inclusion bodies by recombinant baculovirus in insect cells: it binds the single-stranded RNA species. J. Gen. Virol. 71:2073-2083. [DOI] [PubMed] [Google Scholar]
- 40.Tian, P., M. K. Estes, Y. Hu, J. M. Ball, C. Q. Zeng, and W. P. Schilling. 1995. The rotavirus nonstructural glycoprotein NSP4 mobilizes Ca2+ from the endoplasmic reticulum. J. Virol. 69:5763-5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian, P., Y. Hu, W. P. Schilling, D. A. Lindsay, J. Eiden, and M. K. Estes. 1994. The nonstructural glycoprotein of rotavirus affects intracellular calcium levels. J. Virol. 68:251-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torres-Vega, M. A., R. A. Gonzalez, M. Duarte, D. Poncet, S. Lopez, and C. F. Arias. 2000. The C-terminal domain of rotavirus NSP5 is essential for its multimerization, hyperphosphorylation and interaction with NSP6. J. Gen. Virol. 81:821-830. [DOI] [PubMed] [Google Scholar]
- 43.Touris-Otero, F., J. Martinez-Costas, V. N. Vakharia, and J. Benavente. 2004. Avian reovirus nonstructural protein μNS forms viroplasm-like inclusions and recruits protein σNS to these structures. Virology 319:94-106. [DOI] [PubMed] [Google Scholar]
- 44.Tsuji, T., and E. T. Kaiser. 1991. Design and synthesis of the pseudo-EF hand in calbindin D9K: effect of amino acid substitutions in the alpha-helical regions. Proteins 9:12-22. [DOI] [PubMed] [Google Scholar]
- 45.Ueki, S., M. Nakamura, T. Komori, and T. Arata. 2005. Site-directed spin labeling electron paramagnetic resonance study of the calcium-induced structural transition in the N-domain of human cardiac troponin C complexed with troponin I. Biochemistry 44:411-416. [DOI] [PubMed] [Google Scholar]
- 46.Vascotto, F., M. Campagna, M. Visintin, A. Cattaneo, and O. R. Burrone. 2004. Effects of intrabodies specific for rotavirus NSP5 during the virus replicative cycle. J. Gen. Virol. 85:3285-3290. [DOI] [PubMed] [Google Scholar]
- 47.Wei, T., T. Shimizu, K. Hagiwara, A. Kikuchi, Y. Moriyasu, N. Suzuki, H. Chen, and T. Omura. 2006. Pns12 protein of Rice dwarf virus is essential for formation of viroplasms and nucleation of viral-assembly complexes. J. Gen. Virol. 87:429-438. [DOI] [PubMed] [Google Scholar]
- 48.Welch, S. K., S. E. Crawford, and M. K. Estes. 1989. Rotavirus SA11 genome segment 11 protein is a nonstructural phosphoprotein. J. Virol. 63:3974-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yap, K. L., J. B. Ames, M. B. Swindells, and M. Ikura. 1999. Diversity of conformational states and changes within the EF-hand protein superfamily. Proteins 37:499-507. [DOI] [PubMed] [Google Scholar]
- 50.Zetina, C. R. 2001. A conserved helix-unfolding motif in the naturally unfolded proteins. Proteins 44:479-483. [DOI] [PubMed] [Google Scholar]
- 51.Zhang, M., and T. Yuan. 1998. Molecular mechanisms of calmodulin's functional versatility. Biochem. Cell Biol. 76:313-323. [DOI] [PubMed] [Google Scholar]
- 52.Zhou, Y., W. Yang, M. Kirberger, H. W. Lee, G. Ayalasomayajula, and J. J. Yang. 2006. Prediction of EF-hand calcium-binding proteins and analysis of bacterial EF-hand proteins. Proteins 65:643-655. [DOI] [PubMed] [Google Scholar]