Abstract
Human immunodeficiency virus (HIV)-specific CD8 T-cell responses targeting products encoded within the Gag open reading frame have frequently been associated with better viral control and disease outcome during the chronic phase of HIV infection. To further clarify this relationship, we have studied the dynamics of Gag-specific CD8 T-cell responses in relation to plasma viral load and time since infection in 33 chronically infected subjects over a 9-month period. High baseline viral loads were associated with a net loss of breadth (P < 0.001) and a decrease in the total magnitude of the Gag-specific T-cell response in general (P = 0.03). Most importantly, the baseline viral load predicted the subsequent change in the breadth of Gag recognition over time (P < 0.0001, r2 = 0.41). Compared to maintained responses, lost responses were low in magnitude (P < 0.0001) and subdominant in the hierarchy of Gag-specific responses. The present study indicates that chronic exposure of the human immune system to high levels of HIV viremia is a determinant of virus-specific CD8 T-cell loss.
The design of potentially efficacious human immunodeficiency virus (HIV) vaccines relies heavily on the identification of correlates of protection of the adaptive immune response. There is a wealth of evidence that an effective HIV-specific CD8 T-cell response is likely to represent one such correlate. The decline of acute-phase viremia is temporally associated with the appearance of the HIV-specific CD8 T-cell response (7, 21); particular HLA class I alleles are associated with specific mutations in the HIV proteome that may revert to the wild-type sequence upon transmission to a new human host (4, 22); a high proportion of long-term nonprogressors express the HLA class I allele B57 (2, 16), and specifically for these individuals it has been shown that the response restricted by this class I allele can contribute to efficient control of HIV replication (22). In addition to these human studies, CD8 T-cell depletion in the simian immunodeficiency virus-rhesus macaque model results in increased plasma viremia and accelerated loss of CD4 T cells during the chronic phase of infection (33).
During and shortly after the acute phase of infection, the HIV-specific CD8 T-cell response broadens (1, 3, 9) but is thought to remain relatively stabile during the chronic phase. Responses targeting products encoded within the Gag and Nef genes are among the most immunodominant (1, 6, 12, 24, 26). The Gag-specific CD8 T-cell response may play a special role in controlling viral replication, and it was first reported in 1995 that Gag-specific CD8 T cells may contribute to slowing down progression to AIDS (32). In support of this finding, an inverse correlation between the Gag-specific CD8 T-cell response and the HIV load was demonstrated in several cohorts and for different HIV subtypes (8, 12, 15, 16, 21, 26, 29, 33). Recent reports have suggested that the breadth of the Gag-specific T-cell response may play an important role in controlling the viral load during chronic infection (13, 19). No such relationship has been observed for Nef- or Env-specific CD8 T-cell responses. Indeed, some studies have found that Nef or Env responses are positively correlated with a high viral load (6, 19). Together, these results have led to controversy over which gene products should be included in HIV vaccine formulations.
To further clarify the relationship between the HIV-specific CD8 T-cell response and disease progression, we have analyzed the dynamics of the Gag-specific T-cell responses in relation to the plasma viral load and time since infection in 33 antiretroviral-untreated, chronically infected subjects. Longitudinal follow-up of those individuals allowed us to prospectively study changes in the frequency and magnitude of Gag-specific T-cell responses in correlation with clinical parameters (viral load and CD4 cell count) over a period of 9 months. The present study adds important information to previous reports about the relationship between the Gag-specific T-cell response and the plasma viral load.
MATERIALS AND METHODS
Study subjects.
The 33 individuals in this study are part of a larger, well characterized high-risk HIV cohort of female bar workers enrolled in a prospective study of HIV type 1 (HIV-1) superinfection (HISIS) in the Mbeya region of southwestern Tanzania (13, 14, 17, 30, 31). Between September and December 2000, 600 women were recruited after giving informed consent and each participant provided blood samples at enrolment and every 3 months thereafter for up to 4 years. During the study, all participants received health care that included treatment of all acute infectious diseases, screening for and treatment of sexually transmitted diseases, and since 2003, cotrimoxazole prophylaxis for opportunistic infections for women with CD4 T-cell counts below 200/μl. Since 2005, antiretroviral treatment has been available for all participants with AIDS-defining symptoms or CD4 cell counts below 200/μl. During the course of this study, all individuals were antiretroviral naive. HIV-1 status was determined with two diagnostic HIV enzyme-linked immunoassays (Enzygnost Anti HIV1/2 Plus [Dade Behring, Liederbach, Germany] and Determine HIV 1/2 [Abbott, Wiesbaden, Germany]). Discordant results were resolved with a Western blot assay (Genelabs Diagnostics, Geneva, Switzerland). Plasma HIV-1 RNA levels were measured with the Amplicor HIV-1 Monitor assay (Roche Diagnostics, Indianapolis, IN). In April 2003, 56 of the HIV-1-positive participants were enrolled in the HISIS cytotoxic T-lymphocyte substudy. In 33 of these, Gag-specific T-cell responses were determined at follow-up 12 (baseline) and follow-up 15 (9 months). This study was reviewed and approved by the ethics committees of all partners, in compliance with national guidelines and institutional policies.
Synthetic peptides and peptide pools.
Three sets of Gag peptides were used throughout this study, and they consisted of 15-mer peptides overlapping by 11 amino acids of primary isolates 90CF402 (subtype A-Gag, AAB38823), DU422 (subtype C-Gag, CAD62240), and 98UG57143 (subtype D-Gag, AF484514). The three Gag peptide sets were closely related to HIV-1 isolates from the Mbeya region (data not shown), and the use of all three peptide sets afforded the most sensitive detection of Gag-specific T-cell responses within this cohort (13). Peptides were synthesized by 9-fluorenylmethoxy carbonyl chemistry and standard solid-phase techniques with free amino termini. All peptides were >80% pure as determined by high-performance liquid chromatography, mass spectrophotometry, amino acid analysis, and N-terminal sequencing. Peptides were synthesized at the Henry M. Jackson Foundation (Rockville, MD) by Anaspec Incorporated (San Jose, CA) and the NMI (Tuebingen, Germany). Each of the three subtype-specific peptide sets was then pooled into 11 peptide pools, each containing 11 consecutive peptides. Peptide pools corresponded to HXB2 amino acid numbering as follows: pool 1, amino acids (aa) 1 to 55; pool 2, aa 45 to 99; pool 3, aa 89 to 146; pool 4, aa 136 to 186; pool 5, aa 180 to 234; pool 6, aa 224 to 278; pool 7, aa 268 to 322; pool 8, aa 312 to 366; pool 9, aa 360 to 411; pool 10, 397 to 455; pool 11, aa 445 to 500.
ELISPOT assays, determination of cutoffs, and quality controls.
During follow-ups 12 and 15 of the HISIS study, freshly isolated peripheral blood mononuclear cells (PBMC; viability, >95%) were screened for HIV-specific T-cell responses by stimulation with the overlapping peptide pools described above. Gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assays were performed with Nova-Red substrate (Vector, Burlingame, CA) as previously described (25). Gag peptide pool responses with at least 70 spot-forming cells (SFC)/106 PBMC and at least three times the negative control were scored as positive.
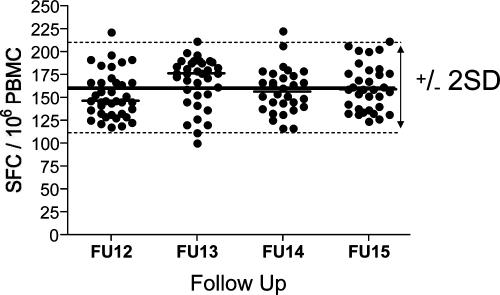
Accurate analysis of the dynamics of T-cell responses is dependent on the precise and sensitive quantification of the frequency of antigen-specific T cells over the entire study period. The IFN-γ ELISPOT assay, in combination with a thorough quality control, was used to meet this goal. To avoid potential variation of sample quality at any time point, all ELISPOT assays were performed with fresh PBMC isolated within 6 h of the blood draw. A response was counted positive when at least one of the three subtype-specific peptide pools covering the same antigenic region was recognized. The background of the assay was negligible (<20 SFC/106 PBMC) over the entire study period. In order to minimize cell-counting errors, the same person at all time points performed the cell counting. The variability of the ELISPOT assay was measured over the 9-month period with four batches of quality control PBMC recovered from the frozen state and restimulated with a peptide pool containing optimal peptides from cytomegalovirus, Epstein-Barr virus, and influenza virus (CEF peptide pool [8]) at >40 different time points and on one to five plates per time point. Figure 1 shows the number of spot-forming cells per well of the quality control sample after restimulation with the CEF peptide pool. During the entire study, the mean of all tests was 160 SFC/well and the standard deviation was 25.9. With this estimate of variability, we defined a range over which we would consider a measurement unchanged. Under a normal distribution, approximately 95% of the samples will fall within 2 standard deviations (SD) of the mean; we used this fact and the estimated SD of the mean of ±16% for the quality control samples to define a range of “unchanged” as within 32% of the original response. Responses that changed over the 9 months by more than 32% were considered to have decreased or increased, whereas responses with changes of less than this magnitude were considered to have stayed the same. Gag peptide pool responses that fell below the threshold of 70 SFC/106 PBMC were counted as lost; newly emerging responses were counted as “new.”
FIG. 1.
Variability of the IFN-γ ELISPOT assay over the study period and determination of cutoffs. Shown are the numbers of spot-forming cells per well after stimulation of quality control (QC) PBMC with the CEF peptide pool from more than 40 time points. Quality control PBMC were recovered from the frozen state and rested overnight before use. For determination of cutoffs, a total of 136 quality controls during the 9-month interval were used.
Statistical analysis.
Data analyses were carried out with GraphPad Prism software. Comparisons of two groups were performed with the Mann-Whitney test. Comparisons of the magnitudes of responses at two different time points were performed with the Wilcoxon signed-rank test. The linear relationship between viral loads or CD4 cell counts and CD8 T-cell responses was determined by linear regression analysis. The tests used for statistical analysis are mentioned in the figure legends.
RESULTS
The Gag-specific T-cell responses decrease in magnitude and breadth during the chronic phase of HIV infection.
We have studied the dynamics of antigen-specific T-cell responses in relation to the time since infection and parameters of disease progression in 33 chronically HIV-infected women over a 9-month period. Table 1 summarizes the clinical characteristics of the study subjects and the net changes in Gag-specific pool responses. At baseline, the time since primary infection ranged from 9 months to more than 3 years (n = 20, subjects already infected at enrolment). The median viral load of all subjects was 77,300 viral RNA (vRNA) copies/ml at baseline and increased to 139,000 vRNA copies/ml (range, <400 to >750,000) 9 months later. The median CD4 cell count was 395 cells/μl (range, 81 to 1,050) at baseline and slightly dropped to 349 cells/μl at 9 months (range, 18 to 957).
TABLE 1.
Clinical characteristics of the subjects in this study
| Subject | HIV infection during HISIS | Time infected (mo) at baseline | pVLa at baseline | pVLa at 9 mo | Highest pVLa,b during study period | CD4 cell countc at baseline | CD4 cell countc at 9 mo | Breadth of Gag pool recognition at baseline | Net change in Gag pool recognition |
|---|---|---|---|---|---|---|---|---|---|
| H398 | Yes | 33 | 261 | <400 | 1,990 | 826 | 783 | 3 | 3 |
| H278 | Yes | 18 | 1,350 | 6,300 | 6,300 | 1050 | 957 | 2 | 1 |
| H561 | Yes | 9 | 4,810 | 22,900 | 43,400 | 524 | 318 | 3 | 0 |
| H604 | Yes | 24 | 14,200 | <400 | 14,300 | 919 | 829 | 3 | 1 |
| H054 | Yes | 24 | 22,200 | 36,700 | 84,000 | 499 | 321 | 2 | 0 |
| H558 | Yes | 18 | 31,400 | 180,000 | 180,000 | 478 | 376 | 3 | 0 |
| H477 | Yes | 27 | 38,100 | 67,000 | 74,300 | 594 | 690 | 2 | 1 |
| H189 | Yes | 9 | 46,300 | 43,200 | 185,000 | 424 | 429 | 5 | −2 |
| H304 | Yes | 30 | 69,200 | 241,000 | 728,000 | 350 | 584 | 1 | 1 |
| H346 | Yes | 24 | 119,000 | 546,000 | 546,000 | 251 | 183 | 3 | 0 |
| H123 | Yes | 33 | 174,000 | 202,000 | 533,000 | 395 | 472 | 4 | −3 |
| H390 | Yes | 33 | 284,000 | 609,000 | 609,000 | 231 | 307 | 4 | 0 |
| H574 | Yes | 9 | 477,000 | 319,000 | >750,000 | 312 | 193 | 2 | −2 |
| H064 | No | >36 | 6,460 | 7,070 | 9,220 | 340 | 494 | 7 | 0 |
| H041 | No | >36 | 6,470 | 13,600 | 107,000 | 488 | 487 | 8 | −2 |
| H456 | No | >36 | 11,700 | 113,000 | 113,000 | 946 | 618 | 3 | 1 |
| H070 | No | >36 | 22,500 | 57,100 | 57,100 | 376 | 391 | 4 | 0 |
| H326 | No | >36 | 28,100 | 38,700 | 163,000 | 855 | 933 | 2 | −1 |
| H349 | No | >36 | 30,600 | 8,700 | 30,600 | 417 | 300 | 5 | 0 |
| H575 | No | >36 | 39,600 | 449,000 | 602,000 | 81 | 68 | 1 | 0 |
| H290 | No | >36 | 85,400 | 6,190 | 85,800 | 391 | 436 | 2 | 0 |
| H048 | No | >36 | 98,300 | 112,000 | 706,000 | 312 | 218 | 3 | 0 |
| H195 | No | >36 | 108,000 | 164,000 | 279,000 | 397 | 18 | 2 | −1 |
| H402 | No | >36 | 112,000 | 750,000 | >750,000 | 209 | 112 | 3 | −2 |
| H325 | No | >36 | 128,000 | 174,000 | 355,000 | 422 | 189 | 3 | −1 |
| H572 | No | >36 | 134,000 | 193,000 | 193,000 | 120 | 72 | 4 | −1 |
| H314 | No | >36 | 182,000 | 187,000 | 257,000 | 565 | 730 | 5 | −1 |
| H446 | No | >36 | 258,000 | 750,000 | >750,000 | 143 | 130 | 4 | −1 |
| H513 | No | >36 | 260,000 | 115,000 | >750,000 | 339 | 282 | 4 | −1 |
| H134 | No | >36 | 425,000 | 426,000 | 426,000 | 468 | 457 | 5 | −1 |
| H149 | No | >36 | 746,000 | 750,000 | >750,000 | 242 | NDd | 3 | −2 |
| H457 | No | >36 | >750,000 | >750,000 | >750,000 | 348 | 237 | 4 | −1 |
| H075 | No | >36 | ND | 568,000 | 568,000 | 185 | 227 | 3 | −1 |
Number of vRNA copies per milliliter of plasma.
Highest plasma viral load measured during the study period.
CD4 cell count per microliter.
ND, not done.
A total of 124 Gag-specific pool responses were analyzed over a 9-month interval (Fig. 2), and wells with no detectable responses were excluded. Over the 9 months, 22.6% of the responses (n = 28) were lost and 24.2% of the responses (n = 30) were of decreasing magnitude, whereas 25.8% of the Gag-specific responses (n = 32) stayed within ±32% of the magnitude measured at the baseline. Increasing responses accounted for 17.7% (n = 22) and newly emerging responses made up only 9.7% (n = 12). Within the larger HISIS cytotoxic T-lymphocyte cohort, 89% of the responses that were detected with the IFN-γ ELISPOT assay were previously shown to be mediated by CD8+ T cells (14). Together, these results indicate that there is a marked decline in the magnitude and breadth of the Gag-specific CD8+ T-cell response during the chronic phase of HIV infection.
FIG. 2.
Decreasing frequencies of Gag-specific T-cell responses during the chronic phase of HIV infection. Gag peptide pool responses were detected at baseline and 9 months later with fresh PBMC and pools of consecutive overlapping 15-mer peptides in an IFN-γ ELISPOT assay. The proportion of Gag pool responses that either remained the same, increased in magnitude, were newly detected, were lost, or decreased in magnitude after a 9-month interval is shown. The estimated SD of the mean of ±16% for the quality control samples was used to define a range of “unchanged” as within 32% of the original response. Responses that changed over the 9 months by more than 32% were considered as decreased or increased.
Loss of Gag-specific responses is associated with high baseline viral loads.
We next determined which parameters contributed to the loss of HIV-specific T-cell responses. First, we subdivided the 33 subjects into two groups—those who had been HIV infected before the start of the HISIS study (group 1, n = 20) for at least 3 years at baseline and those who had been infected for less than 3.0 years (group 2, n = 13). As shown in Figure 3 the loss of pool-specific T-cell responses was biased toward the subjects infected for more than 3 years (P = 0.06, Mann-Whitney test). Thirteen of 20 subjects within group 1 had a net loss in the breadth of detectable Gag recognition after the 9-month period, 6 subjects responded with the same breadth of recognition, and only 1 subject had an increase in the breadth of Gag recognition. The biggest drops in magnitude for single pool responses were observed in four subjects with viral loads continuously above 200,000 (data not shown).
FIG. 3.
The time since primary infection is associated with a decreasing breadth of Gag-specific T-cell recognition. Shown on the y axis is the frequency of subjects who recognized either a greater or a lesser number of Gag peptide pools 9 months after baseline. The white bars represent subjects who were infected during the 3 years of the ongoing study (n = 13). Subjects who were infected for more than 3 years (n = 20) are represented by the dark gray bars.
In contrast, of 13 subjects infected for less than 3 years, only 3 lost breadth of Gag recognition, whereas the remainder of the subjects responded with either increased breadth (n = 5) or the same breadth (n = 5) of detectable Gag responses. Together, these results suggest that the time since primary HIV infection may be an important parameter associated with decreasing breadth of recognition of antigenic regions within Gag.
In 3 of the 13 subjects, the breadth of Gag recognition decreased although they had been infected for less than 3 years. Two of these had been infected for only 9 and 12 months at the baseline. Hence, other parameters may contribute to declining HIV-specific T-cell responses during the chronic phase of infection. A trait common to all three subjects was a high steady-state plasma viremia, reaching 180,000 to >750,000 vRNA copies/ml during the 9 months of this study, suggesting that besides the time since primary infection, high viral loads (and therefore antigenic loads) might be associated with the loss of Gag-specific T-cell responses. Therefore, we next compared the dynamics of breadth of recognition and changes in absolute magnitude of the Gag-specific response from subjects who at baseline had a viral load below (n = 18) or above (n = 14) 100,000 vRNA copies/ml. Indeed, the dynamics of Gag-specific T-cell responses differed between the two groups (P < 0.001, Fig. 4A). Twelve of 14 subjects with a high viral load at baseline lost at least one Gag pool response. Both subjects with high viral loads but no decline in the Gag-specific breadth of T-cell recognition were infected for less than 2 years. In stark contrast, the breadth of the Gag-specific response only declined in 3 of 18 subjects, increased in 6 subjects, and stayed the same in 9 subjects with comparatively low baseline viral loads. A similar trend between the two groups was observed for changes in the absolute magnitudes of the Gag responses detected at baseline and 9 months (Fig. 4B); those of 11 of 18 subjects with low viral loads increased, whereas those of the remaining 7 subjects decreased (P = 0.96, Wilcoxon signed-rank test). In contrast, the responses of only 2 of 14 subjects with high viral loads increased, whereas those of 12 of these subjects decreased in absolute magnitude (P = 0.03, Wilcoxon signed-rank test).
FIG. 4.
Loss of Gag-specific responses is associated with high baseline viral loads. Shown is the net change in Gag pool response breadth (x axis) (A) or the absolute changes in the magnitudes of Gag responses in subjects with a high (>105 vRNA copies/ml; n = 14, gray diamonds) or a low (<105 vRNA copies/ml; n = 18, black circles) baseline viral load (B); the median change is indicated. Gag-specific T-cell responses were detected after restimulation with Gag peptide pools with an IFN-γ ELISPOT assay and fresh PBMC as described in Materials and Methods. Responses that fell below the threshold of 70 SFC/106 PBMC were counted as lost, and responses that newly emerged were counted as new. One subject was excluded because of a missing baseline viral load value. Statistical analysis was done with the Mann-Whitney test (A) or the Wilcoxon signed-rank test (B).
Together, these results suggest that high plasma viral loads may contribute to a decrease in the breadth and magnitude of the Gag-specific T-cell response.
The baseline plasma viral load predicts subsequent changes in the breadth of Gag-specific recognition.
To clarify the relationship between HIV disease progression and the HIV-specific T-cell response, we compared disease progression markers with the subsequent dynamics of HIV-specific T cells for each study subject. Figure 5 shows linear regression analyses of the plasma viral load (panel A) and CD4 cell count (panel B) at baseline versus the change in the number of Gag peptide pools recognized over the next 9 months. The baseline viral load was inversely correlated and the baseline CD4 cell count was positively correlated with the subsequent change in the breadth of Gag-specific recognition (P < 0.0001, r2 = 0.41, and P = 0.0032, r2 = 0.24, respectively). Interestingly, in one HLA B5301-positive individual with a viral load of 182,000 vRNA copies/ml, the immunodominant pool response targeting two peptides within the highly conserved cyclophilin binding region within p24 (one of them a B5301 epitope-containing peptide) increased twofold, from 820 to 1,720 SFC/106 PBMC, whereas one subdominant response targeting pool 7 (160 SFC/106 PBMC at baseline, data not shown) was lost. In this subject, the Gag-specific T-cell response is narrowing despite an increase in magnitude; this subject's viral load remained unchanged. In conclusion, these results demonstrate that classical disease progression markers, the plasma viral load and CD4 cell count, can partly predict subsequent changes in the Gag-specific T-cell population.
FIG. 5.
Inverse linear correlation between HIV disease progression markers at baseline and the subsequent loss of Gag-specific breadth in T-cell recognition. Shown are the net change in the breadth of Gag-specific peptide pool responses over a 9-month interval (x axis) and the baseline viral load (A) or the baseline CD4 cell count (B) for each subject (n = 33). For one subject, the baseline viral load was not determined. GraphPad Prism software was used for the linear regression analysis.
The loss of responses predominantly affects subdominant responses of low magnitude.
A total of 17 subjects lost Gag-specific responses. As shown in Fig. 6A, all lost responses were mostly weak at baseline, with a median magnitude of 110 SFC/106 PBMC (range, 70 to 550 SFC/106 PBMC). Maintained responses varied but were mostly of higher magnitude, with a median of 520 SFC/106 PBMC (range, 70 to 5,030 SFC/106 PBMC). Not surprisingly, the difference in magnitude between lost and maintained responses was highly significant (P < 0.0001). In order to study the immunodominance hierarchy of lost versus maintained responses, we next compared the magnitudes of Gag responses within subjects that had lost at least one response (n = 17, Fig. 6B). Two of 17 subjects lost all Gag-specific responses. In all of the remaining 15 subjects, the lost responses were defined as subdominant responses because they maintained responses of higher baseline magnitude. However, in 8 of these 15 subjects (7 with viral loads above 100,000 vRNA copies/ml) the loss of subdominant responses was accompanied by a decreasing magnitude of the most immunodominant Gag response (data not shown). Hence, the loss of only weak to medium responses is likely to be explained by the short time period studied.
FIG. 6.
Lost responses are mostly weak and subdominant. Shown is a comparison of the magnitudes of responses that were either lost (n = 28) or maintained (n = 84) after a 9-month interval (A). The immunodominance hierarchy of lost responses within a subject is shown in panel B. The y axis shows the percentage of subjects who lost responses (n = 17) that were defined as subdominant, if the same subjects had a response with a greater magnitude. Two subjects lost all responses. The Mann-Whitney test was used for statistical analysis.
DISCUSSION
In the present study, we show that both the time since primary infection and the viral load may influence the dynamics of the HIV-specific T-cell response. Subjects who had been infected for more than 3 years at baseline were more likely to lose Gag-specific T-cell responses than were subjects infected for shorter time periods. Most significantly, a high baseline plasma viral load had an even more detrimental effect and was strongly associated with a subsequent loss of Gag-specific T cells, leading not only to decreasing frequencies of Gag-specific T cells but also to a reduced breadth of recognition.
The dynamics of the early HIV-specific CD8 T-cell response are often characterized by a broadening in specificity from the acute phase to the early chronic phase of infection (3, 9, 18, 34). The appearance of subdominant epitope responses may partly compensate for the loss of recognition of immunodominant epitopes after escape of the virus from CD8 T-cell recognition (11, 18). Our results suggest that during later stages of HIV infection CD8 T-cell recognition of Gag narrows. Immune escape of the virus from T-cell recognition by point mutation of targeted epitopes may have contributed to the decline seen in these patients. However, escape from T-cell recognition is thought to be most pronounced during the acute and early phases of infection and may subside over the course of simian immunodeficiency virus and HIV infections (5, 23, 27, 29). Most importantly, high plasma loads and low CD4 cell counts at baseline predicted the loss of subdominant Gag responses. This observation indicates that T-cell exhaustion due to extended exposure of the immune system to high antigenic loads may be an important factor in the depletion of HIV-specific responses during later stages of this chronic disease (36). Previous observations that HIV-specific CD8 T cells usually exhibit reduced proliferative capacities and are prone to apoptosis further support this hypothesis (10, 28).
During chronic infection, a broad Gag-specific T-cell response is associated with particular HLA class I alleles, low plasma viral loads, and high CD4 T-cell counts (14, 19). Additionally, a low median steady-state plasma viremia was found for extended time periods after the acute phase in subjects that targeted two specific regions of Gag (aa 1 to 75 and 248 to 500) during later phases of chronic infection (14). Together, these observations led to the hypothesis that the breadth of Gag-specific recognition by CD8 T cells is important for viral control during chronic HIV infection. The results presented here add additional information to these previous findings, and it is possible that a broad Gag response could also be secondary in individuals with low viral loads and slower disease progression. Indeed, the observation that Gag-specific responses of high magnitude can be initially mounted but wane during the course of infection has been reported in HIV progressors with high steady-state viremia (20, 35). Alternatively, both aforementioned hypotheses, in conjunction, may provide a supplementary model, a positive-feedback mechanism in which a high viral load contributes to the exhaustion of the HIV-specific CD8 T-cell response and therefore to diminished antiviral immunity. This, in turn, may lead to further increases in viral load and ever-accelerated disease progression during late stages of chronic HIV infection.
In conclusion, the chronic exposure of the human immune system to high levels of HIV viremia is a determinant of virus-specific CD8 T-cell loss. The breadth of the Gag-specific CD8 T-cell response is therefore at least partially reflective of an effective and healthy virus-specific immune response and may, at the same time, also contribute to the control of viral replication. Although technically challenging, it is therefore of great importance to further dissect the mechanisms of the human immune system that contribute to the establishment and maintenance of steady-state viremia during the acute and chronic phases.
Acknowledgments
This work was supported by the European Commission, DG XII, INCO-DC (grant ICA-CT-2002-10048), and by a cooperative agreement between the Henry M. Jackson Foundation for the Advancement of Military Medicine and the U.S. Department of Defense.
The views and opinions expressed herein do not necessarily reflect those of the U.S. Army or the Department of Defense.
We thank all of the HISIS study participants and the excellent staff at the Mbeya Medical Research Programme that conducted the HISIS study, especially Vera Kleinfeldt, Frowin Nichombe, Weston Assisya, and Clemence Konkamkula. Furthermore, we thank Francine McCutchan (USMHRP, Rockville, MD), Rick Koup, Costas Petrovas, and Joe Casazza (Vaccine Research Center, NIH) for many helpful suggestions during the writing of the manuscript.
Footnotes
Published ahead of print on 26 September 2007.
REFERENCES
- 1.Addo, M. M., X. G. Yu, A. Rathod, D. Cohen, R. L. Eldridge, D. Strick, M. N. Johnston, C. Corcoran, A. G. Wurcel, C. A. Fitzpatrick, M. E. Feeney, W. R. Rodriguez, N. Basgoz, R. Draenert, D. R. Stone, C. Brander, P. J. Goulder, E. S. Rosenberg, M. Altfeld, and B. D. Walker. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 77:2081-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altfeld, M., M. M. Addo, E. S. Rosenberg, F. M. Hecht, P. K. Lee, M. Vogel, X. G. Yu, R. Draenert, M. N. Johnston, D. Strick, T. M. Allen, M. E. Feeney, J. O. Kahn, R. P. Sekaly, J. A. Levy, J. K. Rockstroh, P. J. Goulder, and B. D. Walker. 2003. Influence of HLA-B57 on clinical presentation and viral control during acute HIV-1 infection. AIDS 17:2581-2591. [DOI] [PubMed] [Google Scholar]
- 3.Altfeld, M., E. S. Rosenberg, R. Shankarappa, J. S. Mukherjee, F. M. Hecht, R. L. Eldridge, M. M. Addo, S. H. Poon, M. N. Phillips, G. K. Robbins, P. E. Sax, S. Boswell, J. O. Kahn, C. Brander, P. J. Goulder, J. A. Levy, J. I. Mullins, and B. D. Walker. 2001. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J. Exp. Med. 193:169-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey, J. R., T. M. Williams, R. F. Siliciano, and J. N. Blankson. 2006. Maintenance of viral suppression in HIV-1-infected HLA-B*57+ elite suppressors despite CTL escape mutations. J. Exp. Med. 203:1357-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernardin, F., D. Kong, L. Peddada, L. A. Baxter-Lowe, and E. Delwart. 2005. Human immunodeficiency virus mutations during the first month of infection are preferentially found in known cytotoxic T-lymphocyte epitopes. J. Virol. 79:11523-11528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Currier, J. R., E. G. Kuta, E. Turk, L. B. Earhart, L. Loomis-Price, S. Janetzki, G. Ferrari, D. L. Birx, and J. H. Cox. 2002. A panel of MHC class I restricted viral peptides for use as a quality control for vaccine trial ELISPOT assays. J. Immunol. Methods 260:157-172. [DOI] [PubMed] [Google Scholar]
- 9.Dalod, M., M. Dupuis, J. C. Deschemin, C. Goujard, C. Deveau, L. Meyer, N. Ngo, C. Rouzioux, J. G. Guillet, J. F. Delfraissy, M. Sinet, and A. Venet. 1999. Weak anti-HIV CD8+ T-cell effector activity in HIV primary infection. J. Clin. Investig. 104:1431-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350-354. [DOI] [PubMed] [Google Scholar]
- 11.Feeney, M. E., Y. Tang, K. A. Roosevelt, A. J. Leslie, K. McIntosh, N. Karthas, B. D. Walker, and P. J. Goulder. 2004. Immune escape precedes breakthrough human immunodeficiency virus type 1 viremia and broadening of the cytotoxic T-lymphocyte response in an HLA-B27-positive long-term-nonprogressing child. J. Virol. 78:8927-8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frahm, N., B. T. Korber, C. M. Adams, J. J. Szinger, R. Draenert, M. M. Addo, M. E. Feeney, K. Yusim, K. Sango, N. V. Brown, D. SenGupta, A. Piechocka-Trocha, T. Simonis, F. M. Marincola, A. G. Wurcel, D. R. Stone, C. J. Russell, P. Adolf, D. Cohen, T. Roach, A. StJohn, A. Khatri, K. Davis, J. Mullins, P. J. Goulder, B. D. Walker, and C. Brander. 2004. Consistent cytotoxic-T-lymphocyte targeting of immunodominant regions in human immunodeficiency virus across multiple ethnicities. J. Virol. 78:2187-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geldmacher, C., J. R. Currier, M. Gerhardt, A. Haule, L. Maboko, D. Birx, C. Gray, A. Meyerhans, J. Cox, and M. Hoelscher. 2007. In a mixed subtype epidemic, the HIV-1 Gag-specific T-cell response is biased towards the infecting subtype. AIDS 21:135-143. [DOI] [PubMed] [Google Scholar]
- 14.Geldmacher, C., J. R. Currier, E. Herrmann, A. Haule, E. Kuta, F. McCutchan, L. Njovu, S. Geis, O. Hoffmann, L. Maboko, C. Williamson, D. Birx, A. Meyerhans, J. Cox, and M. Hoelscher. 2007. CD8 T-cell recognition of multiple epitopes within specific Gag regions is associated with maintenance of a low steady-state viremia in human immunodeficiency virus type 1-seropositive patients. J. Virol. 81:2440-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goulder, P. J., M. A. Altfeld, E. S. Rosenberg, T. Nguyen, Y. Tang, R. L. Eldridge, M. M. Addo, S. He, J. S. Mukherjee, M. N. Phillips, M. Bunce, S. A. Kalams, R. P. Sekaly, B. D. Walker, and C. Brander. 2001. Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection. J. Exp. Med. 193:181-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendel, H., S. Caillat-Zucman, H. Lebuanec, M. Carrington, S. O'Brien, J. M. Andrieu, F. Schachter, D. Zagury, J. Rappaport, C. Winkler, G. W. Nelson, and J. F. Zagury. 1999. New class I and II HLA alleles strongly associated with opposite patterns of progression to AIDS. J. Immunol. 162:6942-6946. [PubMed] [Google Scholar]
- 17.Herbinger, K. H., M. Gerhardt, S. Piyasirisilp, D. Mloka, M. A. Arroyo, O. Hoffmann, L. Maboko, D. L. Birx, D. Mmbando, F. E. McCutchan, and M. Hoelscher. 2006. Frequency of HIV type 1 dual infection and HIV diversity: analysis of low- and high-risk populations in Mbeya region, Tanzania. AIDS Res. Hum. Retrovir. 22:599-606. [DOI] [PubMed] [Google Scholar]
- 18.Karlsson, A. C., A. K. Iverson, J. M. Chapman, T. de Oliviera, G. Spotts, A. J. McMichael, M. P. Davenport, F. M. Hecht, and D. F. Nixon. 2007. Sequential broadening of CTL responses in early HIV-1 infection is associated with viral escape. PLoS One 2:e225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiepiela, P., K. Ngumbela, C. Thobakgale, D. Ramduth, I. Honeyborne, E. Moodley, S. Reddy, C. de Pierres, Z. Mncube, N. Mkhwanazi, K. Bishop, M. van der Stok, K. Nair, N. Khan, H. Crawford, R. Payne, A. Leslie, J. Prado, A. Prendergast, J. Frater, N. McCarthy, C. Brander, G. H. Learn, D. Nickle, C. Rousseau, H. Coovadia, J. I. Mullins, D. Heckerman, B. D. Walker, and P. Goulder. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13:46-53. [DOI] [PubMed] [Google Scholar]
- 20.Klein, M. R., C. A. van Baalen, A. M. Holwerda, S. R. Kerkhof Garde, R. J. Bende, I. P. Keet, J. K. Eeftinck-Schattenkerk, A. D. Osterhaus, H. Schuitemaker, and F. Miedema. 1995. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J. Exp. Med. 181:1365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leslie, A. J., K. J. Pfafferott, P. Chetty, R. Draenert, M. M. Addo, M. Feeney, Y. Tang, E. C. Holmes, T. Allen, J. G. Prado, M. Altfeld, C. Brander, C. Dixon, D. Ramduth, P. Jeena, S. A. Thomas, A. St John, T. A. Roach, B. Kupfer, G. Luzzi, A. Edwards, G. Taylor, H. Lyall, G. Tudor-Williams, V. Novelli, J. Martinez-Picado, P. Kiepiela, B. D. Walker, and P. J. Goulder. 2004. HIV evolution: CTL escape mutation and reversion after transmission. Nat. Med. 10:282-289. [DOI] [PubMed] [Google Scholar]
- 23.Li, H., B. Li, H. Song, L. Breydo, I. V. Baskakov, and L. X. Wang. 2005. Chemoenzymatic synthesis of HIV-1 V3 glycopeptides carrying two N-glycans and effects of glycosylation on the peptide domain. J. Org. Chem. 70:9990-9996. [DOI] [PubMed] [Google Scholar]
- 24.Masemola, A., T. Mashishi, G. Khoury, P. Mohube, P. Mokgotho, E. Vardas, M. Colvin, L. Zijenah, D. Katzenstein, R. Musonda, S. Allen, N. Kumwenda, T. Taha, G. Gray, J. McIntyre, S. A. Karim, H. W. Sheppard, C. M. Gray, and H. S. Team. 2004. Hierarchical targeting of subtype C human immunodeficiency virus type 1 proteins by CD8+ T cells: correlation with viral load. J. Virol. 78:3233-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mashishi, T., and C. M. Gray. 2002. The ELISPOT assay: an easily transferable method for measuring cellular responses and identifying T cell epitopes. Clin. Chem. Lab. Med. 40:903-910. [DOI] [PubMed] [Google Scholar]
- 26.Novitsky, V., H. Cao, N. Rybak, P. Gilbert, M. F. McLane, S. Gaolekwe, T. Peter, I. Thior, T. Ndung'u, R. Marlink, T. H. Lee, and M. Essex. 2002. Magnitude and frequency of cytotoxic T-lymphocyte responses: identification of immunodominant regions of human immunodeficiency virus type 1 subtype C. J. Virol. 76:10155-10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Connor, D. H., T. M. Allen, T. U. Vogel, P. Jing, I. P. DeSouza, E. Dodds, E. J. Dunphy, C. Melsaether, B. Mothe, H. Yamamoto, H. Horton, N. Wilson, A. L. Hughes, and D. I. Watkins. 2002. Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection. Nat. Med. 8:493-499. [DOI] [PubMed] [Google Scholar]
- 28.Petrovas, C., J. P. Casazza, J. M. Brenchley, D. A. Price, E. Gostick, W. C. Adams, M. L. Precopio, T. Schacker, M. Roederer, D. C. Douek, and R. A. Koup. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 203:2281-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price, D. A., P. J. Goulder, P. Klenerman, A. K. Sewell, P. J. Easterbrook, M. Troop, C. R. Bangham, and R. E. Phillips. 1997. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc. Natl. Acad. Sci. USA 94:1890-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riedner, G., O. Hoffmann, M. Rusizoka, D. Mmbando, L. Maboko, H. Grosskurth, J. Todd, R. Hayes, and M. Hoelscher. 2006. Decline in sexually transmitted infection prevalence and HIV incidence in female barworkers attending prevention and care services in Mbeya region, Tanzania. AIDS 20:609-615. [DOI] [PubMed] [Google Scholar]
- 31.Riedner, G., M. Rusizoka, O. Hoffmann, F. Nichombe, E. Lyamuya, D. Mmbando, L. Maboko, P. Hay, J. Todd, R. Hayes, M. Hoelscher, and H. Grosskurth. 2003. Baseline survey of sexually transmitted infections in a cohort of female bar workers in Mbeya region, Tanzania. Sex. Transm. Infect. 79:382-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivière, Y., M. B. McChesney, F. Porrot, F. Tanneau-Salvadori, P. Sansonetti, O. Lopez, G. Pialoux, V. Feuillie, M. Mollereau, and S. Chamaret. 1995. Gag-specific cytotoxic responses to HIV type 1 are associated with a decreased risk of progression to AIDS-related complex or AIDS. AIDS Res. Hum. Retrovir. 11:903-907. [DOI] [PubMed] [Google Scholar]
- 33.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 34.Streeck, H., M. Lichterfeld, G. Alter, A. Meier, N. Teigen, B. Yassine-Diab, H. K. Sidhu, S. Little, A. Kelleher, J.-P. Routy, E. S. Rosenberg, R.-P. Sekaly, B. D. Walker, and M. Altfeld. 2007. Recognition of a defined region within p24 Gag by CD8+ T cells during primary HIV-1 infection in individuals expressing protective HLA class I alleles. J. Virol. 81:7725-7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Streeck, H., B. Schweighardt, H. Jessen, R. L. Allgaier, T. Wrin, E. W. Stawiski, A. B. Jessen, T. M. Allen, B. D. Walker, and M. Altfeld. 2007. Loss of HIV-1-specific T-cell responses associated with very rapid HIV-1 disease progression. AIDS 21:889-891. [DOI] [PubMed] [Google Scholar]
- 36.Wodarz, D., P. Klenerman, and M. A. Nowak. 1998. Dynamics of cytotoxic T-lymphocyte exhaustion. Proc. Biol. Sci. Royal Soc. 265:191-203. [DOI] [PMC free article] [PubMed] [Google Scholar]