Abstract
We generated a monoclonal antibody, RG-1, that binds to highly conserved L2 residues 17 to 36 and neutralizes human papillomavirus 16 (HPV16) and HPV18. Passive immunotherapy with RG-1 was protective in mice. Antiserum to the HPV16 L2 peptide comprising residues 17 to 36 (peptide 17-36) neutralized pseudoviruses HPV5, HPV6, HPV16, HPV 18, HPV31, HPV 45, HPV 52, HPV 58, bovine papillomavirus 1, and HPV11 native virions. Depletion of HPV16 L2 peptide 17-36-reactive antibodies from cross-neutralizing rabbit and human L2-specific sera abolished cross-neutralization and drastically reduced neutralization of the cognate type. This cross-neutralization of diverse HPVs associated with cervical cancer, genital warts, and epidermodysplasia verruciformis suggests the possibility of a broadly protective, peptide-based vaccine.
Minor capsid antigen L2 is a possible alternative to highly multivalent L1 virus-like-particle (VLP) vaccines to obtain broad protection against oncogenic human papillomaviruses (HPVs) (16). Vaccination with L2 as a full-length protein or as polypeptides protects animals against homologous-type viral challenges at both cutaneous and mucosal sites (2-4, 6, 12). Protection is not mediated by cellular immunity, suggesting the importance of neutralizing antibodies (5, 7). L2 is subdominant in the context of L1/L2 VLPs (19), but antibodies elicited by recombinant L2 immunogens are able to neutralize a remarkably broad range of HPV genotypes (15). This suggests that neutralizing epitopes of L2 may be conserved across HPV types due to some critical viral function (13). Furthermore, it raises the possibility that a single L2 protein- or peptide-based vaccine might provide comprehensive protection against the HPV types causing genital cancer and genital warts and possibly even those associated with cutaneous warts and epidermodysplasia verruciformis (EV).
Identification of neutralizing epitopes within HPV16 L2.
The rational design of a broadly protective L2-based preventive vaccine requires knowledge of the relevant neutralizing epitopes. To identify the neutralizing epitopes in L2, we vaccinated BALB/c mice with full-length six-His-tagged HPV16 L2 protein and produced hybridomas by using standard procedures (18). Of the 100 supernatants reactive with L2 protein, only 45 reacted with HPV16 L1/L2 pseudovirions, and only one (RG-1) neutralized HPV16 pseudovirus and was cloned. The RG-1 supernatant exhibited a neutralizing titer of 1,280 and also reacted with HPV16 L1/L2 pseudivirions by an enzyme-linked immunosorbent assay (ELISA). RG-1 and another four monoclonal antibodies (MAbs) that showed the highest ELISA reactivities with HPV16 pseudovirions were all the immunoglobulin G1(κ) [IgG1(κ)] isotype and reacted with HPV16 L2 protein by Western blotting (Table 1).
TABLE 1.
Capsid surface reactivity and neutralizing activity of HPV16 L2 MAbsa
| Hybridoma | OD405
|
HPV16 L2 reactivity on Western blot | HPV 16 neutralization titer | Isotype | Epitope residues (aa) | |
|---|---|---|---|---|---|---|
| L2 protein ELISA | HPV16 pseudovirus ELISA | |||||
| RG-1 | 1.3 | 0.9 | Yes | 1,280 | IgG1(κ) | 17-36 |
| 10 | 1.3 | 0.6 | Yes | <50 | IgG1(κ) | 89-100 |
| B1 | 1.2 | 0.5 | Yes | <50 | IgG1(κ) | 89-100 |
| C9 | 1.14 | 0.7 | Yes | <50 | IgG1(κ) | 73-84 |
| 11 | 1.25 | 0.7 | Yes | <50 | IgG1(κ) | 33-52 |
Undiluted hybridoma supernatants from five clones were tested by L2 protein ELISA and by L1/L2 pseudovirus ELISA (results given as optical densities at 405 nm [OD405] after background subtraction), for L2 reactivity by Western blotting (shown as yes/no), for antibody isotype, and for their ability to neutralize HPV16 pseudovirus. The epitopes recognized by each monoclonal antibody were defined by peptide ELISA using 56 20-mer peptides derived from HPV16 L2, each overlapping by 12 amino acids (aa). Where adjacent peptides reacted, the overlapping sequence is given.
Each MAb was screened for reactivity with 56 20-mer peptides of HPV16 L2 that overlapped each other by 12 amino acids (Table 1). The neutralizing MAb RG-1 reacted with a peptide comprising residues 17 to 36 of HPV 16 L2 (peptide 17-36) (Fig. 1A) but not the overlapping peptides 9-28 and 25-44. Two of the four other nonneutralizing, capsid-reactive MAbs recognized HPV16 L2 residues 89 to 100; one recognized residues 73 to 84 and another recognized residues 33 to 52. RG-1 ascites exhibited a titer of 1,024,000 in an HPV16 L1/L2 VLP ELISA and neutralized both HPV16 and HPV18 pseudovirions with titers of 204,800 and 25,600, respectively, but failed to neutralize HPV5, HPV6, HPV45, HPV52 or HPV58 or bovine papillomavirus 1 (BPV1) pseudovirions at a titer of 40. Sequence comparison suggests that RG-1 recognizes lysine at residue 20, which is conserved in HPV16 and HPV18 but different among other types that were not neutralized (R or Q).
FIG. 1.
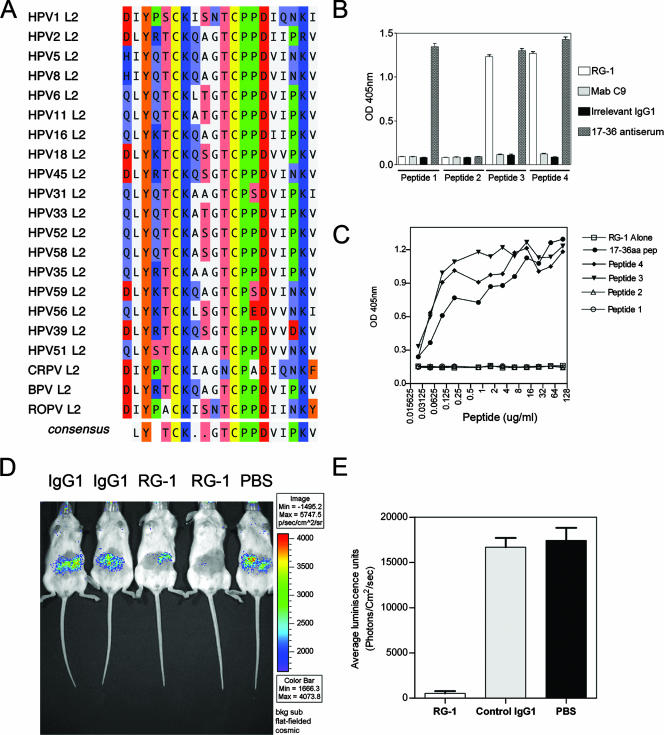
The RG-1 neutralizing MAb recognizes the evolutionarily conserved L2 17-36 motif and provides passive immunity. (A) CLUSTAL W homology comparison of residues 17 to 36 of HPV16 L2 peptide and L2 sequences from different papillomavirus types. The HPV16 L2 sequence comprising amino acids 17 to 36 is highly conserved among different types and exhibits 78% identity with the L2 sequences from HPV2 (skin type), HPV5 (EV related), and HPV45; 80% identity with HPV6 and HPV11 L2 (benign types); and 84% identity with HPV18 (high-risk type). In contrast, L2 as a whole exhibits only ∼25% conservation among these types. This sequence was conserved even in BPV1, which is evolutionarily distant from high-risk HPV. (B) Peptide ELISA using monoclonal antibody and polyclonal antiserum to HPV16 L2 peptide 17-36. Wells were coated with synthetic peptide 1 (ASATQLYKVVKQAGTCPPD), comprising HPV16 L2 residues 13 to 31 in which residues 21 and 22 are both changed to valine (in bold); 2 (TASADPCPKQLYKCQATGT), comprising HPV16 L2 residues 13 to 31 in which the amino acid order is scrambled; 3 (ASATQLYKTCKQAGTCPPD), comprising wild-type HPV16 L2 residues 13 to 31; or 4 (ASATQAAKTCKQAGTCPPD) comprising HPV16 L2 residues 13 to 31 in which residues 18 and 19 are both replaced with alanine (in bold). Plates were probed with RG-1, MAb C9, an irrelevant isotype-matched MAb, or rabbit antiserum to HPV16 L2 peptide 17-36 as indicated. (C) Blockade of RG-1 neutralization of HPV16 pseudovirus by titrations of synthetic peptide comprising wild-type HPV16 L2 residues 17 to 36 and the peptides described above for panel B. (D) Passive transfer of RG-1 (n = 5), but not an irrelevant isotype-matched control MAb (n = 6) or phosphate-buffered saline (PBS) (n = 5), protects mice from cutaneous challenge with HPV16 pseudovirus (see http://home.ccr.cancer.gov/lco/ for plasmid maps and production methods). A patch on the belly of each anesthetized BALB/c mouse was shaved with an electric razor without traumatizing the epithelium. MAb was injected (100 μg intraperitoneally) 5 h prior to challenge by application to the shaved skin of 3 × 109 HPV16 pseudovirions (100 ng) in 10 μl of 0.6% carboxymethylcellulose (Sigma C5013) containing L1 and L2 (or L1 alone for background determination) and carrying an encapsidated luciferase reporter construct. Three days later, the mice were anesthetized and injected with luciferin (100 μl at 7 mg/ml), and their images were acquired for 10 min with a Xenogen IVIS 200. Equally sized areas encompassing the site of inoculation were analyzed using Living Image 2.20 software, and background was determined by challenge with noninfectious HPV pseudovirions lacking L2. A representative image is shown in panel D, and the bioluminescence data for each group are plotted in panel E. OD, optical density.
RG-1 may neutralize by blocking some critical interaction between the highly conserved 17-36 region of L2 (Fig. 1A) and a cellular factor (1, 21, 22). Interestingly, HPV16 L2 residues 13 to 31 bind with a Kd of ∼1 nM to a cell surface receptor, and mutation of L2 residues 18 and 19 or 21 and 22 disrupted both L2 binding to the cell surface and viral infection (21). RG-1 bound to both wild-type HPV16 L2 peptides 13-31 and 17-36 and the 18A-19A mutant, but neither the 21V-22V mutant nor the scrambled-sequence peptides were recognized (Fig. 1B). Similarly, wild-type HPV16 L2 peptides 13-31 and 17-36 and the 18A-19A mutant but neither the 21V-22V mutant nor the scrambled-sequence peptides blocked the neutralization of HPV16 pseudovirions by RG-1 (Fig. 1C), suggesting that its epitope overlaps the surface-binding motif of HPV16 L2 (21).
Passive immunization with RG-1 protects mice against HPV16 pseudovirus challenge.
It is unclear whether L2-specific neutralizing antibodies are sufficient to mediate protection. HPV16 pseudovirus containing the cottontail rabbit papillomavirus (CRPV) genome infects and induces cutaneous warts in domestic rabbits (14), and HPV16 pseudovirus also infects mouse C127 cells (17). Therefore, we tested the ability of HPV16 pseudovirus carrying the luciferase reporter gene to infect cutaneous epithelium in mice (Fig. 1D). Vaccination of mice with HPV16 L1 VLPs, but not HPV45 L1 VLPs, reduced infection to background levels (as determined using noninfectious pseudovirus lacking L2 as a control [17]), demonstrating type-restricted protection (not shown). To test whether passive immunotherapy with RG-1 confers protection, 100 μg of RG-1, an isotype-matched irrelevant MAb, or phosphate-buffered saline was administered intraperitoneally to naïve mice 5 h prior to HPV16 pseudovirus challenge. Administration of RG-1, but not the isotype-matched control antibody, protected the mice from cutaneous HPV16 pseudovirus challenge (P < 0.001, analysis of variance) (Fig. 1E). The mice receiving RG-1 had a serum HPV16 neutralizing titer of 6,400 at the time of challenge.
Pseudovirus and native HPV11 virus-based neutralization with the HPV16 L2 peptide 17-36 antiserum.
Since our aim was to identify a broadly neutralizing epitope and the HPV16 L2 peptide 17-36 was well conserved among different HPVs (Fig. 1A), we immunized a rabbit with HPV16 L2 peptide 17-36 coupled to keyhole limpet hemocyanin. The rabbit antiserum was analyzed by a six-His HPV16 L2 protein ELISA as well as an HPV16 L1/L2 pseudovirion ELISA. The final bleed sample had ELISA titers of 128,000 against L2 protein and 16,000 to L1/L2 pseudovirus (not shown), whereas the preimmunization serum exhibited background reactivities in both assays (not shown). The HPV16 L2 peptide 17-36 antiserum bound to HPV16 L2 peptides17-36 and 13-31 and both mutant peptides but not the scrambled-sequence peptide (Fig. 1B). The HPV16 L2 peptide 17-36 antiserum, but not the preimmunization serum, broadly neutralized the following: HPV16 pseudovirions (titer, 3,200) and pseudovirions from all of the other five oncogenic types tested (that together account for ∼85% of cervical cancer), the benign mucosal type HPV6 (titer, 200), the cutaneous EV type HPV5 (titer, 200), and the evolutionarily divergent BPV1 (titer, 800), but not CRPV (titer, <50), indicating specificity (Table 2). To extend our observation of cross-neutralization to an additional HPV type and to eliminate potential artifacts in the pseudovirus system, the peptide antiserum was tested at 1:50 for neutralization activity against native HPV11 virus derived from a human xenograft (15). E1∧E4 early spliced transcripts were observed in cells exposed to HPV11 treated with a 1:50 dilution of preimmunization serum, demonstrating infection, whereas L2 peptide 17-36 antiserum completely neutralized infection at this concentration (not shown). Thus, L2 peptide 17-36 antiserum neutralized very divergent cutaneous and mucosal HPV types associated with EV, condylomata accuminata, and genital cancers.
TABLE 2.
Neutralizing titers of HPV16 L2 peptide 17-36 antiserum for pseudovirions from different papillomaviruses
| Papillomavirus | Disease (relative contribution)a | Neutralization titer |
|---|---|---|
| HPV5 | EV | 200 |
| HPV6 | Condyloma accuminata | 200 |
| HPV16 | Cervical cancer (53) | 3,200 |
| HPV18 | Cervical cancer (17.2) | 1,600 |
| HPV31 | Cervical cancer (2.9) | 50 |
| HPV45 | Cervical cancer (6.7) | 800 |
| HPV52 | Cervical cancer (2.3) | 400 |
| HPV58 | Cervical cancer (2.2) | 800 |
| BPV1 | Bovine fibropapilloma | 800 |
| CRPV | Rabbit papillomas | <50 |
The nature of disease and/or relative contribution to cervical cancer of each HPV type (indicated as a percentage of total cases) is indicated.
Depletion of region 17-36-specific antibodies eliminates cross-neutralization.
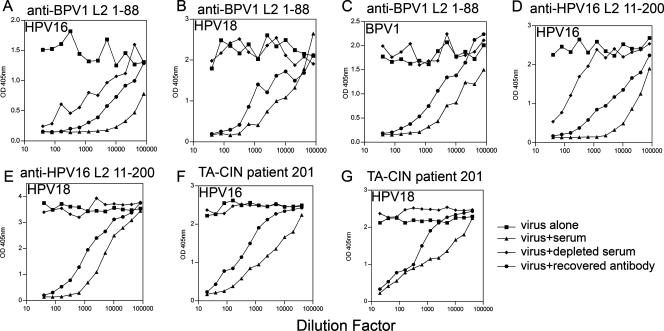
To study the contribution of the 17-36 epitope relative to others in both homologous-type neutralization and cross-neutralization of heterologous papillomavirus types, antibodies specific to this region of L2 were depleted from L2 immune sera using an L2 peptide 17-36-bound column. Depletion of HPV16 L2 peptide 17-36-reactive antibodies from a broadly cross-neutralizing BPV1 L2 peptide 1-88 antiserum (15) reduced the homologous BPV1 neutralization titers from 40,960 to 320 (Fig. 2A). We recovered 25% of the BPV1 neutralizing activity (after factoring for the dilution that occurs upon elution of the peptide column). Notably, depletion of HPV16 L2 peptide 17-36-reactive antibodies from BPV1 L2 peptide 1-88 antiserum eliminated detectable cross-neutralization of HPV16 and HPV18. Again, cross-neutralizing activity was recovered from the fraction eluted from the peptide 17-36 column (Fig. 2B and C).
FIG. 2.
Depletion of anti-HPV16 L2 peptide 17-36 antibodies from L2 immune serum abolishes cross-neutralization. Rabbit antisera to BPV1 L2 peptide 1-88 (A to C) or HPV16 L2 peptide 11-200 (D and E) and serum of an HPV16-positive AGIN patient (no. 201) who had been vaccinated with HPV16 L2E7E6 L2 (F and G) were depleted of HPV16 L2 peptide 17-36-specific antibodies by use of a peptide column. Antibodies bound to the column were recovered by elution at low pH and brought back to neutral pH. The sera, both before and after depletion, as well as the recovered antibodies were tested for neutralizing titers for BPV1, HPV16, or HPV18 pseudovirions, as indicated. The serum dilutions for the antibody recovered from the column are not corrected for the dilution that occurs during their elution and return to neutral pH. Neutralization of BPV1 pseudovirions by BPV1 L2 peptide 1-88 antiserum (A), HPV16 pseudovirions by BPV1 L2 peptide 1-88 antiserum (B), HPV18 pseudovirions by BPV1 L2 peptide 1-88 antiserum (C), HPV16 pseudovirions by HPV16 L2 peptide 11-200 antiserum (D), HPV18 pseudovirions by HPV16 L2 peptide 11-200 antiserum (E), HPV16 pseudovirions by immune serum from a patient vaccinated with HPV16 L2E7E6 (F), and HPV18 pseudovirions by immune serum from a patient vaccinated with HPV16 L2E7E6 (G) is shown. OD, optical density.
Chandrachud et al. demonstrated that vaccination with BPV4 L2 peptide 11-200, but not a C-terminal polypeptide, protects cattle from experimental BPV4 challenge (3). To address the relative contributions of L2 peptide 17-36 and other neutralizing epitopes, we performed similar depletion experiments with serum from a rabbit vaccinated with HPV16 L2 peptide 11-200. The HPV16 neutralizing titer of antiserum to HPV16 L2 peptide 11-200 drops from 40,960 to 80 upon depletion of HPV16 L2 peptide 17-36-reactive antibodies. Again, after correcting for the dilution that occurs upon elution of the peptide column, we recovered 25% of the homologous-type HPV16 neutralizing activity. The failure to completely eliminate neutralizing activity against the homologous virus is consistent with previous descriptions of type-restricted neutralizing epitopes outside of the L2 17-36 region (10, 11). However, depletion of the HPV16 L2 peptide 11-200 antiserum with an HPV16 L2 peptide 17-36 column removed detectable cross-neutralizing activity against HPV18 (2,560 to <20), suggesting that the neutralizing epitopes outside this 17-36 region are predominantly type specific. After correcting for the dilution that occurs upon elution from the peptide column, 25% of cognate as well as cross-neutralizing activity was recovered. Finally, we wished to determine whether L2 peptide 17-36 represents a neutralizing B-cell epitope in humans. We utilized sera from a group of 24 HPV16-positive patients with high-grade anogenital intraepithelial neoplasias (AGIN) vaccinated three times at monthly intervals with 500 μg of HPV16 L2E7E6 fusion protein (TA-CIN) without adjuvant and bled 1 month later at week 12 (20). Vaccination induced L2-specific antibodies in five patients (Table 3). Only one patient, code number 201, had no detectable HPV16-neutralizing antibodies at week 0, whereas the serum after vaccination exhibited an HPV16-neutralizing titer of 1,600 (Table 3). These neutralizing antibodies correlated with the reactivity to HPV16 L2 determined by ELISA (Table 3), and neither preimmunization nor postimmunization sera from patient 201 exhibited antibodies to HPV16 L1 above background levels (20). The week 12 serum from patient 201 also neutralized HPV18 with a titer of 100, whereas the week 0 serum did not (Table 3). Depletion of this serum with an HPV16 L2 peptide 17-36 column removed detectable neutralizing activity against HPV16 (Fig. 2F) and HPV18 (Fig. 2G), and 40% of this neutralizing activity was recovered upon elution of the column. This suggests that although HPV16 L2 peptide 108-120 can induce cross-neutralizing antibodies in mice and humans (8, 9), this peptide may not contain optimal cross-neutralizing epitopes (10). Since the patient was vaccinated with a full-length HPV16 L2 fusion protein, this finding supports the importance of L2 peptide 17-36 as a neutralizing epitope in this patient. The sera from five of five AGIN patients vaccinated with HPV16 L2E7E6 that reacted to full-length HPV16 L2 also reacted to HPV16 L2 peptide 17-36 (Table 3), suggesting that L2 peptide 17-36 is a common human B-cell epitope.
TABLE 3.
In vitro HPV neutralization titers of sera from AGIN patients who were vaccinated with TA-CIN and mounted an HPV16 L2-specific antibody responsea
| Patient | Titer determined by:
|
Titer resulting in:
|
||||||
|---|---|---|---|---|---|---|---|---|
| HPV16 L2 full-length ELISA
|
HPV16 L2 peptide 17-36 ELISA
|
HPV16 neutralization
|
HPV18 neutralization
|
|||||
| Wk 0 | Wk 12 | Wk 0 | Wk 12 | Wk 0 | Wk 12 | Wk 0 | Wk 12 | |
| 201 | <50 | 1,600 | <50 | 1,600 | <50 | 1,600 | <50 | 100 |
| 303 | <50 | 400 | <50 | 400 | 200 | 1,600 | <50 | 50 |
| 307 | <50 | 100 | <50 | 100 | 400 | 1,600 | <50 | 400 |
| 309 | <50 | 200 | <50 | 200 | 3,200 | 3,200 | <50 | <50 |
| 311 | <50 | 1,600 | <50 | 1,600 | 6,400 | 12,800 | 200 | 200 |
Sera were obtained from HPV16-positive AGIN patients either before or 1 month after three monthly vaccinations with 500 μg of HPV16 L2E7E6 fusion protein (TA-CIN) without adjuvant. The sera were tested for ELISA reactivity with either full-length HPV16 L2 protein or HPV16 L2 peptide 17-36 and for their ability to neutralize either HPV16 or HPV18 in vitro. Note that in vitro neutralization titers at week 0 represent L1-specific neutralizing antibody and reflect preexisting HPV infection.
Acknowledgments
The studies with animals and humans described herein were approved by the Johns Hopkins University Animal Use and Welfare Committee and the Johns Hopkins School of Medicine Institutional Review Board, respectively.
This research was supported by grants to R.B.S.R. and N.D.C. from the PHS (National Cancer Institute, SPORE in Cervical Cancer, P50 CA098252) and to both J.N.R. and C.B.B. by the National Institutes of Health intramural research program.
We thank Liz Rollinson (Xenova Pharmaceuticals plc) for TA-CIN trial samples, Chien-fu Hung and Daejin Kim (Johns Hopkins University) for help with imaging, John Schiller and Doug Lowy (NCI, NIH) for reagents, Martin Müller (DKFZ, Germany) for codon-modified HPV16 L1 and L2, Tadahito Kanda (National Institute of Infectious Diseases, Japan) for codon-modified HPV52 and HPV58 L1 and L2, and Lou Laimins (Northwestern University, Chicago) for codon-modified HPV31 L1 and L2.
R.B.S.R. is a paid consultant of Knobbe, Martens, Olson and Bear LLC.
Footnotes
Published ahead of print on 10 October 2007.
REFERENCES
- 1.Bossis, I., R. B. Roden, R. Gambhira, R. Yang, M. Tagaya, P. M. Howley, and P. I. Meneses. 2005. Interaction of tSNARE syntaxin 18 with the papillomavirus minor capsid protein mediates infection. J. Virol. 79:6723-6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campo, M. S. 1997. Vaccination against papillomavirus in cattle. Clin. Dermatol. 15:275-283. [DOI] [PubMed] [Google Scholar]
- 3.Chandrachud, L. M., G. J. Grindlay, G. M. McGarvie, B. W. O'Neil, E. R. Wagner, W. F. Jarrett, and M. S. Campo. 1995. Vaccination of cattle with the N-terminus of L2 is necessary and sufficient for preventing infection by bovine papillomavirus-4. Virology 211:204-208. [DOI] [PubMed] [Google Scholar]
- 4.Christensen, N. D., J. W. Kreider, N. C. Kan, and S. L. DiAngelo. 1991. The open reading frame L2 of cottontail rabbit papillomavirus contains antibody-inducing neutralizing epitopes. Virology 181:572-579. [DOI] [PubMed] [Google Scholar]
- 5.Embers, M. E., L. R. Budgeon, T. D. Culp, C. A. Reed, M. D. Pickel, and N. D. Christensen. 2004. Differential antibody responses to a distinct region of human papillomavirus minor capsid proteins. Vaccine 22:670-680. [DOI] [PubMed] [Google Scholar]
- 6.Embers, M. E., L. R. Budgeon, M. Pickel, and N. D. Christensen. 2002. Protective immunity to rabbit oral and cutaneous papillomaviruses by immunization with short peptides of L2, the minor capsid protein. J. Virol. 76:9798-9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaukroger, J. M., L. M. Chandrachud, B. W. O'Neil, G. J. Grindlay, G. Knowles, and M. S. Campo. 1996. Vaccination of cattle with bovine papillomavirus type 4 L2 elicits the production of virus-neutralizing antibodies. J. Gen. Virol. 77:1577-1583. [DOI] [PubMed] [Google Scholar]
- 8.Kawana, K., Y. Kawana, H. Yoshikawa, Y. Taketani, K. Yoshiike, and T. Kanda. 2001. Nasal immunization of mice with peptide having a cross-neutralization epitope on minor capsid protein L2 of human papillomavirus type 16 elicit systemic and mucosal antibodies. Vaccine 19:1496-1502. [DOI] [PubMed] [Google Scholar]
- 9.Kawana, K., T. Yasugi, T. Kanda, N. Kino, K. Oda, S. Okada, Y. Kawana, T. Nei, T. Takada, S. Toyoshima, A. Tsuchiya, K. Kondo, H. Yoshikawa, O. Tsutsumi, and Y. Taketani. 2003. Safety and immunogenicity of a peptide containing the cross-neutralization epitope of HPV16 L2 administered nasally in healthy volunteers. Vaccine 21:4256-4260. [DOI] [PubMed] [Google Scholar]
- 10.Kawana, K., H. Yoshikawa, Y. Taketani, K. Yoshiike, and T. Kanda. 1999. Common neutralization epitope in minor capsid protein L2 of human papillomavirus types 16 and 6. J. Virol. 73:6188-6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kondo, K., Y. Ishii, H. Ochi, T. Matsumoto, H. Yoshikawa, and T. Kanda. 2007. Neutralization of HPV16, 18, 31, and 58 pseudovirions with antisera induced by immunizing rabbits with synthetic peptides representing segments of the HPV16 minor capsid protein L2 surface region. Virology 358:266-272. [DOI] [PubMed] [Google Scholar]
- 12.Lin, Y. L., L. A. Borenstein, R. Selvakumar, R. Ahmed, and F. O. Wettstein. 1992. Effective vaccination against papilloma development by immunization with L1 or L2 structural protein of cottontail rabbit papillomavirus. Virology 187:612-619. [DOI] [PubMed] [Google Scholar]
- 13.Longworth, M. S., and L. A. Laimins. 2004. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol. Mol. Biol. Rev. 68:362-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mejia, A. F., T. D. Culp, N. M. Cladel, K. K. Balogh, L. R. Budgeon, C. B. Buck, and N. D. Christensen. 2006. Preclinical model to test human papillomavirus virus (HPV) capsid vaccines in vivo using infectious HPV/cottontail rabbit papillomavirus chimeric papillomavirus particles. J. Virol. 80:12393-12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pastrana, D. V., R. Gambhira, C. B. Buck, Y. Y. Pang, C. D. Thompson, T. D. Culp, N. D. Christensen, D. R. Lowy, J. T. Schiller, and R. B. Roden. 2005. Cross-neutralization of cutaneous and mucosal Papillomavirus types with anti-sera to the amino terminus of L2. Virology 337:365-372. [DOI] [PubMed] [Google Scholar]
- 16.Roden, R., and T. C. Wu. 2006. How will HPV vaccines affect cervical cancer? Nat. Rev. Cancer 6:753-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roden, R. B., H. L. Greenstone, R. Kirnbauer, F. P. Booy, J. Jessie, D. R. Lowy, and J. T. Schiller. 1996. In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. J. Virol. 70:5875-5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roden, R. B., E. M. Weissinger, D. W. Henderson, F. Booy, R. Kirnbauer, J. F. Mushinski, D. R. Lowy, and J. T. Schiller. 1994. Neutralization of bovine papillomavirus by antibodies to L1 and L2 capsid proteins. J. Virol. 68:7570-7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roden, R. B., W. H. t. Yutzy, R. Fallon, S. Inglis, D. R. Lowy, and J. T. Schiller. 2000. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology 270:254-257. [DOI] [PubMed] [Google Scholar]
- 20.Smyth, L. J., M. I. Van Poelgeest, E. J. Davidson, K. M. Kwappenberg, D. Burt, P. Sehr, M. Pawlita, S. Man, J. K. Hickling, A. N. Fiander, A. Tristram, H. C. Kitchener, R. Offringa, P. L. Stern, and S. H. Van Der Burg. 2004. Immunological responses in women with human papillomavirus type 16 (HPV-16)-associated anogenital intraepithelial neoplasia induced by heterologous prime-boost HPV-16 oncogene vaccination. Clin. Cancer Res. 10:2954-2961. [DOI] [PubMed] [Google Scholar]
- 21.Yang, R., P. M. Day, W. H. t. Yutzy, K. Y. Lin, C. F. Hung, and R. B. Roden. 2003. Cell surface-binding motifs of L2 that facilitate papillomavirus infection. J. Virol. 77:3531-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang, R., W. H. Yutzy IV, R. P. Viscidi, and R. B. Roden. 2003. Interaction of L2 with beta-actin directs intracellular transport of papillomavirus and infection. J. Biol. Chem. 278:12546-12553. [DOI] [PubMed] [Google Scholar]