Abstract
Rabies virus glycoprotein (RVG) is known to be the only factor that mediates rabies infection. The neurotrophin receptor (p75NTR), through its cysteine-rich domain 1, is a specific receptor for RVG and neutralizes virus infectivity, but its role in virus infection has remained obscure. We used adult mouse dorsal root ganglion (DRG) neurons as a model to study the role of p75NTR in RV infection of primary neurons. We show that RV infects around 20% of DRG neurons, of which more than 80% are p75NTR positive, have large diameters, and are capsaicin insensitive. Surprisingly, RV binding and infection are absent in about half of the p75NTR-expressing DRG neurons which have small diameters and are often capsaicin sensitive. This indicates that p75NTR is not sufficient to mediate RV interaction in sensory neurons. The rate and specificity of neural infection are unchanged in RV-infected p75NTRExonIV−/− mice that lack all extracellular receptor domains and in wild-type mice infected with two independent RV mutants that lack p75NTR binding. Accordingly, the mortality rate is unchanged in the absence of RV-p75NTR interaction. We conclude that although p75NTR is a receptor for soluble RVG in transfected cells of heterologous expression systems, an RVG-p75NTR interaction is not necessary for RV infection of primary neurons. This means that other receptors are required to mediate RV infection in vivo and in vitro.
Rabies causes severe progressive encephalitis, myelitis, and paralysis and affects more than 50,000 people worldwide each year (59). After onset of symptoms, the infection progresses relentlessly, and there is only one documented case of a nonvaccinated human patient who survived the disease (56). Rabies virus (RV) belongs to the genus Lyssavirus in the family Rhabdoviridae and is a simple, enveloped, nonsegmented, negative-strand RNA virus that encodes only five proteins (44). The glycoprotein (G) is known to be the only protein component of the viral envelope that mediates viral entry into host cells (44).
RV is highly neurotropic, and after inoculation it is retrogradely transported by peripheral neurons before being passed on to second and higher-order neurons without being taken up by glia. It is this unique property of the virus that makes it a useful transsynaptic neuronal tracer over many synapses (28, 52), which is dependent on RVG (17).
Although several receptors have been suggested to be responsible for RV infection, none has been conclusively identified. In previous studies we adopted an expression cloning strategy using a soluble form of RVG as a ligand. That work identified the neurotrophin receptor p75NTR as a unique interacting receptor (50). We have also shown that the glycoprotein from another lyssavirus genotype (EBL2) is also able to interact with p75NTR (51). p75NTR belongs to the tumor necrosis factor receptor family (11, 14) and is the common receptor for all known mature neurotrophins as well immature proneurotrophins (35). In addition, several other ligands implicated in the pathology of neurodegenerative or infectious diseases bind to p75NTR, including β-amyloid (41) and prion (16) peptides. p75NTR is highly conserved in vertebrates and contains four extracellular cysteine-rich domains (CRDs) and an intracellular type II death domain (11). Whereas neurotrophins bind to the second to fourth CRDs (2, 21), we have previously shown that RVG binding occurs on the first CRD without competition for the neurotrophin binding site (30) Although RVG binds to p75NTR, the role of this receptor for neuronal infection has been questioned, because RV infects and kills mutant mice lacking exon III of the p75NTR gene (25). However, the significance of this finding is unclear, because these mice express the short splice variant of p75NTR that contains CRD1 (53), which we have shown to be sufficient for RVG binding (30). Therefore, we revisited the question of the relative importance of p75NTR for RV infection of primary neurons of the peripheral nervous system. RV infection of the central nervous system in vivo occurs primarily through axonal transport of peripheral neurons, and we used cultured sensory neurons from the dorsal root ganglia (DRG) of adult mice as a model system. RV can infect subpopulations of DRG neurons in vivo (13) or in vitro (6, 7, 38).
The heterogeneity of DRG neurons allowed us to ask how specific the neuronal interactions with RV or RVG are. p75NTR is strongly expressed in a subpopulation of DRG neurons (39, 40). The p75NTR-positive neurons are in themselves diverse and can be further distinguished by cell size (60) and the differential expression of tyrosine kinase (Trk) receptors (40), transient receptor potential (TRP) channels (26), or neuropeptides (32). TRP channels have emerged as main determinants for the functional heterogeneity of DRG neurons, and the capsaicin receptor TRPV1 is the crucial receptor in the pain pathway and conveniently allows in culture the separation of small-diameter nociceptive sensory neurons from large-diameter nonnociceptive neurons (8). Here, we used two lines of mutant mice lacking p75NTR and two RV mutants that do not bind to p75NTR (31) to systematically investigate the importance of RVG-p75NTR interaction in vivo and in vitro for RV binding and infections.
MATERIALS AND METHODS
Animals.
Adult mice of either sex weighing 20 to 30 g at 8 to 12 weeks of age were used. Naïve mice were either BALB/c or C57/B6 and were purchased from Centre d'Elevage et de Recherche, Janvier, Legenest, St.-Isle, France. Mutant mice lacking exon III (p75NTRExonIII−/−) (34) were obtained from Jackson Laboratories (Bar Harbor, ME). The generation of mice lacking all four CRDs (p75NTRExonIV−/−) has been described before (53). All in vivo experiments were approved by the local animal care committee and conducted in accordance with French law.
Cell culture.
DRG from all spinal levels were dissected from wild-type or mutant mice and collected in Dulbecco's modified Eagle medium (DMEM; Sigma). The DRG neurons were then incubated in 0.28 U/ml collagenase IV (Biochrom Seromed) for 90 min followed by 3 mg/ml trypsin (Sigma) for 10 min at 37°C. The DRG were then suspended in DMEM, mechanically dissociated by trituration, and finally centrifuged over a Percoll gradient (Amersham) for 5 min. Cells were plated on poly-d-lysine-coated (200 μg/ml; Sigma) coverslips and maintained at 37°C and 5% CO2 in 800 to 1,000 μl of Ham's F-12 medium (Invitrogen) containing 10% heat-inactivated horse serum (Biochrom Seromed), 0.8% glucose, 100 U penicillin, 100 μg/ml streptomycin (Invitrogen), and 50 ng/ml of recombinant human nerve growth factor (a generous gift from Genentech Inc.).
Cos7 cells were grown in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (FBS; Invitrogen). BSR cells (cloned from baby hamster kidney cells) were grown in DMEM supplemented with 8% FBS.
Virus preparation.
Several strains of RV were used. Stocks of challenge virus standard (CVS) and mutants defective for p75NTR binding derived from the CVS parental strain were prepared for infection as described previously (30). Briefly, viruses were propagated on BSR cells. Cells were infected at a multiplicity of infection of 0.1 to 0.2 PFU/cell. After viral adsorption, minimum essential medium (Invitrogen) containing 2% FBS was added, and the cells were incubated for 3 days at 37°C. Amplification of mutant virus strains was performed in selection pressure conditions to prevent the growth of revertants. The cell supernatant was then collected and clarified by centrifugation at 1,500 × g for 30 min at 4°C, and viral aliquots were stored at −80°C. For concentrated virus, the clarified supernatant was then loaded on a 25% glycerol cushion in TNE buffer (10 mM Tris [pH 7.5], 150 mM NaCl, and 1 mM EDTA) and ultracentrifuged for 1 h at 120,000 × g at 4°C. The virus pellet was then resuspended in a minimal volume, and aliquots were stored at −80°C. Viral titers were determined by plaque assay on BSR cells as described before (30).
Inoculation of mice with RV in vivo.
Concentrated RV (CVS and mutants deficient in p75NTR binding) were inoculated into wild-type, p75NTRExonIII−/−, or p75NTRExonIV−/− mice. The inoculum was injected into the right forelimb muscles of six animals of each genotype. For morbidity studies, the inoculum was 107 PFU, whereas for immunohistochemical studies, it was 108 PFU. A total of 50 μl were given at two to five injection sites. For morbidity studies, animals were monitored daily for clinical symptoms and signs, including weight loss, until death. Six days after infection, three mice of each strain were prepared for immunohistochemistry. After anesthesia with an intraperitoneal injection of pentobarbital (0.2 mg/g body weight), animals were transcardially perfused with 0.1 M phosphate-buffered saline (PBS) followed by 2% paraformaldehyde in PBS. Tissue blocks containing the cervical and thoracic spinal cord and ipsilateral DRG were dissected, cryoprotected in 10% sucrose in PBS, and decalcified (0.1 M EDTA for 8 days). Tissue sections (30 μm thick) were cut on a cryostat, mounted on gelatinized slides, and stained with a fluorescein isothiocyanate (FITC)-conjugated antinucleocapsid antibody (1:100 dilution; a gift from P. Perrin, Institute Pasteur, Paris, France) and visualized under a fluorescent microscope at a ×200 or ×400 magnification.
Infection of DRG cells in culture.
Four to twenty hours after plating of the DRG cells, CVS or mutant virus (diluted in F-12 medium) was added to the coverslip at room temperature for 30 min in a volume of 50 to 200 μl, resulting in a final dose of 2 × 104 to 1 × 106 PFU. Subsequently, cells were maintained in a final volume of 1,000 μl of F-12 medium for 12 to 20 h in a 5% CO2 incubator before further processing.
For virus binding analysis, CVS virus (2 × 106 and 7 × 106 PFU/coverslip) was applied to the DRG cells that had been cultured for 4 to 20 h in a volume of 50 to 200 μl at room temperature for 30 min.
Calcium imaging and vital staining of p75NTR.
DRG neurons were studied as described previously (22). Briefly, coverslips were transferred into extracellular fluid (ECF) (145 mM NaCl, 5 mM KCl, 10 mM HEPES 10, 2 mM CaCl2, 1 mM MgCl2, 10 mM d-glucose) buffered to pH 7.4 and containing 2 μM Fura-2 acetoxymethyl ester (derived from a stock solution of 2 mM Fura dissolved in dimethyl sulfoxide) and 80 μM Pluronic (F-127; both from Molecular Probes) for 30 min. Coverslips were subsequently placed for 10 min in ECF containing a TRITC (tetramethyl rhodamine isothiocyanate)-tagged antibody directed against the extracellular domain of p75NTR (a generous gift from Gipi Schiavo) (15) to allow vital staining of cells and de-esterization of Fura-AM. Afterwards, the coverslips were transferred to a recording chamber, and pairs of images were collected at intervals of 2 s with alternating exposure of 340 and 380 nm each for 50 to 200 ms using a Polychrome IV and an air-cooled Imago charge-coupled device camera (640- by 480-pixel resolution) that were controlled by TILLviSION 4.0 software (all from Till Photonics, Gräfeling, Germany). This software was also used to compute ratios of fluorescent images, which were displayed as pseudocolor images. Coverslips were superfused with ECF, and 1 μM capsaicin solution was applied for 10 s.
Capsaicin-induced cobalt uptake.
Cells were washed twice with an incubation buffer containing (in mM) 58 NaCl, 5 KCl, 2 MgCl2, 0.75 CaCl2, 12 glucose, 137 sucrose, and 10 HEPES at pH 7.3 to 7.4 adjusted with KOH for 2 min (58). They were subsequently incubated in the same buffer containing 1 μM capsaicin (Sigma; diluted from a 20 mM stock solution in 100% ethanol) and containing 5 mM CoCl2 (Sigma) for 5 min followed by two washes with incubation buffer for 2 min. Cobalt was precipitated with a 1% solution of ammonium sulfide (Sigma) in incubation buffer for 3 min. Capsaicin-sensitive neurons showed a brown reaction product.
Immunohistochemistry.
For immunohistochemical staining, cells were fixed in 4% paraformaldehyde in PBS (Merck, Heidelberg, Germany) for 10 min. After rinsing in PBS containing 0.1% Triton X-100 (Sigma) cells were incubated in PBS containing 10% normal donkey serum (Chemicon, Temecula, CA), followed by application of primary antibodies diluted in PBS with 0.1% Triton X-100 and 1% normal donkey serum for 10 h at room temperature. A rabbit polyclonal antibody (Advanced Targeting System) followed by Cy3-conjugated secondary antibody was used to stain for p75NTR. RV was detected either with a mouse monoclonal antiphosphoprotein antibody (43) followed by AMCA (7-amino-4-methylcoumarin-3-acetic acid)-conjugated secondary antibody or a rabbit polyclonal FITC-conjugated antinucleocapsid antibody (13). Secondary antibodies (Jackson Immuno Research, West Grove, PA) were applied at a dilution of 1:200 at room temperature for 2 h. Coverslips containing DRG cells were inverted on a slide over a drop of PBS-glycerol (1:3) containing 2.5% 1,4 diazabicyclo-(2,2,2)-octane (Merck, Duisburg, Germany).
Imaging, data collection, and statistical tests.
Images of fields of cells were randomly collected at a magnification of ×200 on a Zeiss Axiovert microscope connected to a color charge-coupled device camera with a resolution of 640 by 480 pixels and imported to a PC version of NIH Image (Scion Image) to measure cell surface area. At least five fields and at least 150 cells were studied per animal to obtain one data point for computation of percentages. All data are given as means ± standard errors of the means for each group of animals. Statistical analysis was carried out with unpaired t tests by using the StatSoft 6.0 software package (StatSoft, Tulsa, OK).
RESULTS
RV infects a subpopulation of large-diameter sensory neurons in vivo and in vitro.
RV was capable of infecting a subpopulation of DRG neurons in culture (Fig. 1). We studied viral infection by immunohistochemical staining for the viral phosphoprotein, which is part of the viral nucleocapsid. The concentration of the inoculum is too low to be detected by immunohistochemistry, and therefore the appearance of this marker indicates viral replication (45). Infected cells typically displayed a punctate and speckled distribution of immunoreactivity (Fig. 1). Although we used a technique that reduces nonneuronal cells, cultures of the DRG still contained satellite cells and fibroblasts as the major nonneuronal cell types of DRG. The neurotropic specificity of the RV was maintained in culture, and never did we observe infection of nonneuronal cells. The appearance of neurons and the specificity of neuronal infection were similar regardless of whether the cells had been infected in vitro or whether cultures were obtained from animals which had been infected in vivo. In both situations, only a subpopulation of neurons was infected.
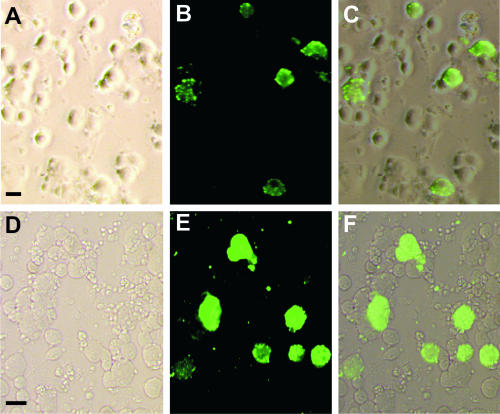
FIG. 1.
DRG neurons from wild-type mice infected by RV. (A to C) Neurons cultured for 16 h and subsequently infected for 20 h; (D to E) DRG neurons infected in vivo 6 days earlier and subsequently cultured for 4 h. Pictures were taken from areas containing many infected neurons to illustrate their appearance rather than to provide a representative picture of the percentage of infected cells. (A) Phase-contrast image; (D) bright-field image. (B and E) Immunoreactivity for RV phosphoprotein; (C and F) overlay of both pictures. Only large cells are infected in vitro or in vivo. Bars, 50 μm.
Six days after inoculation of RV into the forelimb, DRG were dissected from all spinal levels and analyzed for infection to determine whether the properties of infected cells were similar in vivo and in vitro. In concordance with our observation in vitro, infected DRG neurons in vivo had large diameters. Although qualitatively the appearance of the infected cells was similar in both conditions, there was a higher infection rate in culture. We did not quantify the results from in vivo experiments, because in contrast to the in vitro conditions, where all cells were exposed to RV, not all neurons from the ganglia studied project to the peripheral tissue where the inoculum had been deposited in vivo. Therefore, we systematically investigated the factors that influenced the infection rate in vitro.
Time in culture prior to infection influences the infection rate.
The time in culture prior to infection has a significant effect on infection rate (Table 1). Using a standard inoculum of 25,000 PFU, we analyzed the cultures 20 h after infection. This time point was chosen because previous studies in cell lines found robust expression of nucleocapsid L protein (45). When RV was added 4 h after plating of the cultures, an average of 10.7% neurons were found to be infected (Table 1). This number increased significantly (P < 0.05, unpaired t test) and almost doubled to 19.3% when infection was started 16 h after seeding of the DRG cells.
TABLE 1.
Effect of varying culture or inoculation time or inoculum size on the percentage of DRG neurons infected by CVS RV in vitro
| Time (h) in culture
|
Inoculum size (PFU) | % of cells infected | No. of animals | Total no. of cells analyzed | |
|---|---|---|---|---|---|
| Before infection | After infection | ||||
| 4 | 20 | 25,000 | 10.7 ± 0.3 | 3 | 819 |
| 16 | 20 | 25,000 | 19.3 ± 2.0 | 3 | 911 |
| 16 | 20 | 625,000 | 22.3 ± 1.7 | 3 | 608 |
| 16 | 12 | 25,000 | 17.0 ± 0.4 | 4 | 948 |
We also investigated whether the standard dose of the inoculum provided the maximal infection rate and increased the RV concentration 25-fold in neurons cultured for 16 h prior to the 20 h incubation time. When an inoculum of 625,000 PFU was used, the infection rate was 22.3%, and therefore increasing the viral dose does not lead to a significant increase (P > 0.1, t test) of the infection rate in vitro.
In DRG cells cultured for 16 h, we also tested a shorter interval, 12 h, and found that on average 17.0% of the neurons showed evidence of infection, which was not significantly different (P > 0.1, t test) from the 20-h infection protocol.
Thus, in all subsequent experiments, infection was initiated 16 h after plating of the neurons and carried out for a duration of 12 h, using a viral dose of 25,000 PFU to provide an optimized and convenient in vitro model for studying RV infection in culture.
RV binds to a subpopulation of DRG neurons.
There are two possible explanations for why only a subpopulation of neurons becomes infected in culture. One limiting step could be the binding of RV to a select number of cells; the alternative is that binding of the virus is promiscuous but only a small number of cells are supportive of viral entry or replication. In the former case, we would expect a binding pattern similar to that of infected cells, whereas in the latter case a more widespread staining would be observed. To test these two alternatives, we investigated RV binding for 30 min after DRG cells had been cultured for 16 h (Fig. 2D) and compared this with the distribution pattern of infected neurons (Fig. 2B). In our culture system, a typical ring structure indicating specific RV binding was observed in a fifth of the neurons, most of which had a large diameter (Fig. 2D). We never observed binding to nonneuronal cells.
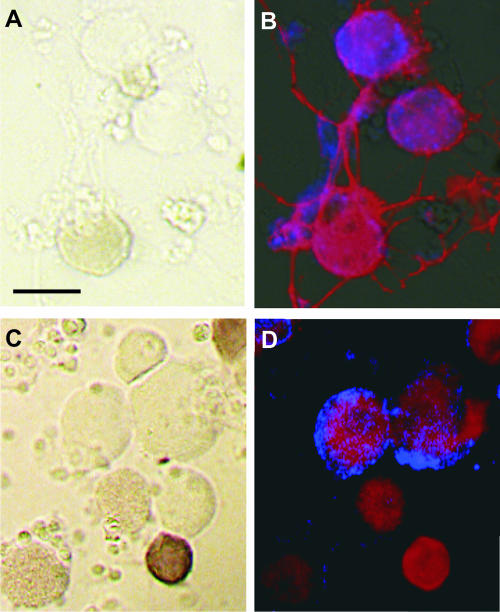
FIG. 2.
DRG neurons cultured for 16 h and subsequently infected with RV (A and B) for 12 h or exposed to RV (C and D), each for 30 min, before further processing. (A and C) Bright-field images; (B and D) fluorescent images. (A and C) Dark brown cells are neurons showing capsaicin-induced cobalt uptake. (B and D) Immunoreactivity for p75NTR (red, extending through the whole soma) and RV phosphoprotein (blue). Bars, 50 μm.
Quantitative analysis of these binding studies showed a rate of positive neurons similar to that in infection studies (Fig. 3). RV produced infection of 17.0% ± 0.4% (four mice, 948 cells) of the cells (Fig. 3A), whereas 16.0% ± 2.1% (three mice, 538 cells) of the neurons showed RV binding (Fig. 3B). These results are in line with the view that virus can bind to only a small population of neurons rather than exhibiting promiscuous binding, indicating that it is the specificity of the binding process that underlies the specificity of the infection.
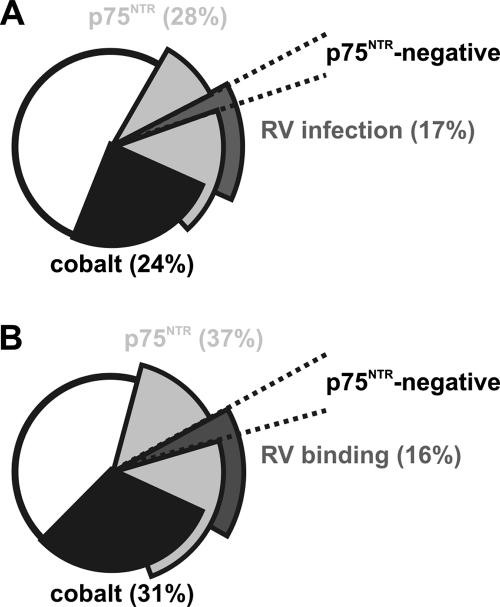
FIG. 3.
Distribution of subpopulations of DRG neurons cultured for 16 h and subsequently infected with RV (25,000 PFU/μl) for 12 h (A) or incubated with RV (5 × 106 PFU/μl) (B) for 30 min. Note the very similar distribution of neuronal subpopulations. There is partial overlap with neurons expressing p75NTR immunoreactivity (the total population is represented by the two light gray segments), no overlap with neurons showing capsaicin-induced cobalt uptake (in black), and very few p75NTR-negative neurons (dashed lines) in the RV binding or RV infection segments (dark gray).
RV infects only a subpopulation of p75NTR-expressing sensory neurons.
DRG neurons are immunohistochemically and functionally heterogeneous. One important distinguishing feature is the expression of neurotrophin receptors. Because p75NTR has been implicated as one of the receptors mediating infection, we asked whether there was a concordance between p75NTR receptor expression of DRG cells and their susceptibility to RV infection and binding. Using immunohistochemistry, we found that a subpopulation of approximately 30% of DRG neurons were immunoreactive for p75NTR, and this population included neurons with small and large diameters. The discrepancy between the prevalence of infected neurons (17%) and p75NTR-expressing cells (30%) shows that there was no complete overlap. This was directly confirmed in double-labeling experiments, which showed that many of the p75NTR-positive neurons were not infected (Fig. 2B and D). The immunohistochemical analysis showed that p75NTR was localized to the cell membranes of both large- and small-diameter neurons (Fig. 4). However, despite the decoration of the cell surface with p75NTR, only the large-diameter sensory neurons became infected; the majority of neurons that did show evidence for RV binding or replication did express p75NTR (Fig. 2B and 3A). Quantitative analysis of staining in double-labeling experiments showed that 82.3% ± 3.4% of the cells that bound RV (three mice, total of 538 neurons, 87 bound RV) were also p75NTR positive (Fig. 3B). Similarly, after 12 h incubation, 80.3% ± 4.4% of the infected neurons (four mice, total of 948 neurons, 169 infected) were p75NTR positive (Fig. 3A). This means that binding of RV and RV infection is largely restricted to p75NTR-positive neurons but that expression of p75NTR alone is not sufficient for binding or infection.
FIG. 4.
Immunostaining of DRG cells for p75NTR (red) shows surface expression in both large- and small-diameter sensory neurons (asterisk). Large- but not small-diameter p75NTR-positive cells become infected, as shown by immunoreactivity for RV phosphoprotein (green, arrows). Bar, 10 μm.
On the other hand, only 37.3% ± 1.5% of the p75NTR-positive neurons (three mice, total of 538 neurons, 203 p75NTR positive) bound RV and only 51.8 ± 6.3% (four mice, total of 948 neurons, 266 p75NTR positive) of p75NTR-positive cells were found to be infected by the virus. This means that only a subpopulation of the p75NTR-expressing neurons bind RV and are susceptible to RV infection.
One possible explanation for the incomplete infection rate of p75NTR-positive neurons is that infection requires additional specific mechanisms or that there is a stochastic infection process. Several lines of evidence converge to indicate that specific mechanisms are involved. First, the exposure time and the inoculum dose are supramaximal (Table 1). Furthermore, cell size distribution and costaining with other markers of DRG neurons strongly endorse a specific mechanism.
Neurons expressing p75NTR are heterogeneous in size and function, and capsaicin sensitivity is a hallmark of nociceptive sensory neurons and restricted to sensory neurons expressing TRPV1 (8, 9). We used ratiometric calcium imaging (8, 22) on cultured cells that were vitally stained using a TRITC-tagged p75NTR antibody directed against the extracellular domain of p75NTR. The staining pattern reveals that both large- and small-diameter DRG cells exhibit surface expression of the receptor. However, among p75NTR-positive neurons, capsaicin sensitivity is found only among small-diameter neurons, not among large-diameter p75NTR-positive neurons (Fig. 5). Capsaicin sensitivity can also be detected by capsaicin-induced cobalt uptake (58). Intriguingly, only 1.8% ± 1.4% (four mice, total of 948 neurons, 266 p75NTR positive) of the p75NTR-positive neurons that were infected after 12 h were cobalt positive (Fig. 2A and B and 3A), indicating that most p75NTR neurons that coexpress TRPV1 are not susceptible to RV infection. While we do not wish to imply that TRPV1 is a receptor preventing RV infection, the data strongly indicate that only the capsaicin-insensitive subpopulation of p75NTR-expressing neurons are susceptible to RV infection. This conclusion is also supported by the analysis of cell size distribution of p75NTR-positive neurons (Fig. 6). This shows that p75NTR-positive neurons are found among the small and large sensory neurons (Fig. 6A). However, infected neurons are found only in the population of cells with large diameters (Fig. 6B and C), whereas cells displaying capsaicin-induced cobalt uptake are concentrated among the small-diameter cells (Fig. 6E). The size spectrum of infected and noninfected cells shows a striking correspondence to these two groups (Fig. 6D and E). This means that p75NTR alone is not sufficient to mediate the specific viral infection and suggests the presence of other factors permitting or preventing RV binding and infection.
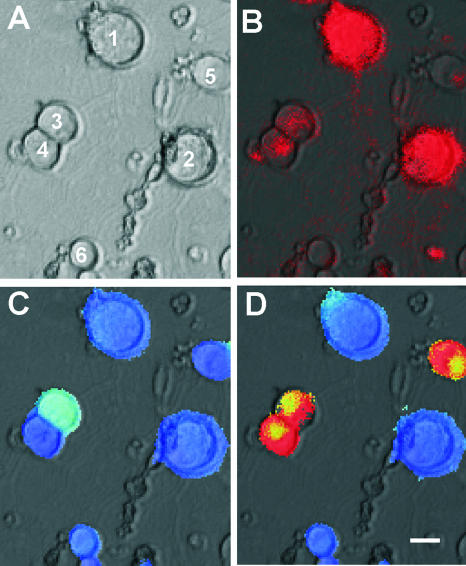
FIG. 5.
Cultured DRG neurons. (A) Bright field; (B) vital staining for p75NTR. Both large (cells 1 and 2) and small (cells 3 and 4) cells are labeled. (C and D) Pseudo-color coding of ratiometric Fura-2 fluorescence at baseline (C) and after 10 s application of 1 μM capsaicin (D). Capsaicin-sensitive cells show a dramatic increase in intracellular calcium (red; cells 3, 4, and 5), whereas there is no change in fluorescence intensity in capsaicin-insensitive neurons. Bar, 10 μm.
FIG. 6.
Cell size histogram of subpopulations of DRG neurons cultured for 16 h and subsequently infected for 12 h, showing the relationship of p75NTR immunoreactivity (A and C to E), RV infection (B and C), and capsaicin-induced cobalt uptake (E). (A) p75NTR-expressing neurons are found among small and large neurons. (B and C) Histograms of infected neurons (B) and infected neurons expressing p75NTR (C) show that only large p75NTR-positive neurons are infected. (D) Noninfected sensory neurons expressing p75NTR are small. (E) Many of these noninfected p75NTR-positive cells show capsaicin-induced cobalt uptake.
RV infects neurons and causes rabies in animals lacking extracellular domains of p75NTR.
To unequivocally test the role of p75NTR in mediating infection, we used two independent strategies. First, we used two strains of knockout mice lacking the extracellular domains of p75NTR, and second, we investigated mutant virus lacking binding to p75NTR (31) to determine whether RV mediates infections through p75NTR.
We confirmed that mice lacking p75NTR exon III (p75NTRExonIII−/−) (33) succumb to RV infection. Wild-type and mutant mice were inoculated in one forelimb and monitored for signs of rabies pathology, which includes weight loss, the appearance of ruffled fur, paralysis, and ultimately death. In agreement with previously published work (25), the clinical course of the disease was similar in both strains, with the first symptoms appearing 4 to 5 days after inoculation and all animals dying within 2 weeks. However, the mutant mice still express the splice variant of p75NTR that retains CRD1 (53). Because CRD1 is sufficient for RVG binding (30), we repeated the experiment in mutant mice lacking exon IV (p75NTRexonIV−/−) and thus all extracellular domains of the receptor (53). Again, these animals showed a clinical course similar to that in previous experiments and died in the same time period as wild-type animals. We did, however, notice that both strains of mutant mice showed an increased ocular and periocular inflammation that was not observed in wild-type mice. These results conclusively show that p75NTR is not an essential factor for the mortality rate.
We state above that less than 20% of the infected DRG neurons in culture (i.e., approximately 3% of all DRG neurons) do not express p75NTR. One possible explanation for the persistent pathogenicity of RV in the p75NTR-null mice is that it could be mediated by this small population of p75NTR-negative DRG cells (Fig. 3). To test this possibility, we repeated the infection experiments in cell cultures using both strains of mutant animals. In p75NTRExonIII−/− (9% and 11%, two mice, 637 neurons) and p75NTRExonIV−/− (15.0 ± 2.7%, four mice, 937 neurons) mice, there was only a slight reduction in the number of infected neurons. As in studies with wild-type animals, less than 3% of the neurons showing capsaicin-induced cobalt uptake were infected. This means that the lack of the extracellular domains of the p75NTR does not lead to a grossly reduced percentage of infected neurons. The somewhat lower numbers of infected cells in the mutant mice (Fig. 7), compared to the 17% seen in wild-type animals, might be explained by the considerable developmental loss of sensory neurons in the p75NTR-deficient animals (3, 34, 53). Cells that were infected in mutant mice lacking p75NTR showed little overlap with cobalt uptake and were large, suggesting that it was the same population of neurons as in wild-type mice. This means that RV infection of sensory neurons still occurs in the absence of p75NTR and in the same population of neurons regardless of their p75NTR expression.
FIG. 7.
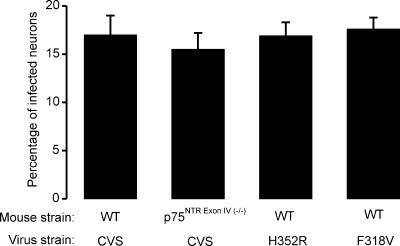
Percentage of neurons cultured for 16 h and subsequently infected for 20 h with the CVS RV strain in wild-type (WT) mice or in mice lacking p75NTRExonIV−/− or with H352R or F318V CVS mutants in WT mice. There is no significant difference in the infection rate.
Mutant RV strains that do not bind to p75NTR infect neurons and cause rabies.
The same conclusion was reached by investigations using mutant RV in wild-type mice. We used two different CVS mutants that harbor a point mutation (histidine 352 replaced by arginine [H352R] or phenylalanine 318 replaced by valine [F318V]) in the glycoprotein and do not bind to p75NTR (31). Wild-type animals were inoculated in the forelimb and monitored for disease progression. As in previous experiments using the CVS parental strain, animals developed the first symptoms within 4 or 5 days and succumbed to the disease within 14 days. The 50% lethal dose for the H352R virus was 3 × 104 PFU, whereas that for the CV parental strain was 1.25 × 104 PFU. Interestingly, wild-type mice infected with mutant virus also displayed ocular and periocular infections.
Studies were also conducted in cell culture and showed that both strains infected the same proportion of neurons as the parental virus (Fig. 7). Thus, mutant virus H352R infected 16.9% ± 1.4% (four mice, 2,235 cells) of DRG neurons, whereas the percentage for the mutant virus F318V was 17.6% ± 1.2% (four mice, 1,656 cells).
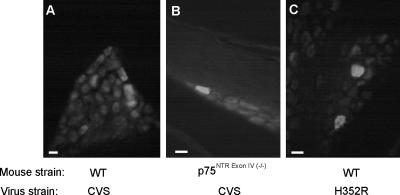
We also investigated whether DRG neurons become infected in vivo by using immunohistochemistry for the nucleocapsid in tissue sections of the DRG (Fig. 8). In wild-type mice inoculated with parental CVS, it is possible to observe positive cells that show immunoreactivity within the cell soma. In accordance with the clinical disease progression in mutant mice exposed to the CVS parental strain, a staining pattern similar to that in wild-type mice inoculated with the mutant virus H352R was observed. The numbers of infected neurons detected in DRG in vivo were similar in all conditions and ranged from 10 to about 50 infected neurons per animal. This indicates that the infection of DRG neurons obtained from p75NTR-deficient mice is not an artifact of tissue culture. In all three conditions, motoneurons in the ventral horn of the spinal cord were also infected.
FIG. 8.
Identification of infected DRG neurons in tissue sections from cervical spinal levels 30 h after inoculation of the forelimb in vivo. Infection was detected by immunohistochemistry using antinucleocapsid antibodies in wild-type mice (A) or in mice lacking p75NTRExonIV (B) using the CVS RV strain or in wild-type mice using the H352R CVS mutant (C). Bars, 50 μm.
DISCUSSION
The crucial receptor mediating RV infection has remained elusive. Many studies have proposed different receptors, including the nicotinic acetylcholine receptor, the neural cell adhesion molecule (NCAM), and the neurotrophin receptor p75NTR. None of them have been unequivocally proven (29). Using an expression cloning strategy, we have previously shown that p75NTR can serve as a receptor for RVG (50). We have also show that a soluble form of p75NTR can bind to RV and neutralizes its infectivity (31), indicating that p75NTR is sufficient for RVG interaction and that no coreceptor is required. In the present study, we found that RV infection is restricted to a subpopulation of large-diameter, capsaicin-insensitive neurons and that the vast majority of infected neurons express p75NTR. Even though RVG is the only molecule of the viral envelope to make contact with host cells and mediate viral entry (44), we now show that the RV, paradoxically, does not require p75NTR for binding or infection, indicating that other receptors are required. Furthermore, small-diameter, capsaicin-sensitive, p75NTR-expressing neurons are resistant to infection and appropriately do not bind RV. This could mean that there are mechanisms that can inhibit the ability of RV to bind to p75NTR.
Why do not all p75NTR expressing neurons bind RV?
Approximately a third of the neurons in DRG express p75NTR (60), and this population can be further subdivided (23, 42). One group consists of nociceptors, which have small cell bodies and express TrkA, neuropeptides, and the capsaicin receptor TRPV1. Another group, with large diameters, does not express TrkA receptors or neuropeptides and presumably functions as mechanoreceptors. It is likely that these different neuronal subtypes also differ in their expression of many other molecules (37).
There are two possibilities that might explain the lack of binding of RV to the population of small-diameter p75NTR-expressing neurons. First, p75NTR might require a cofactor that is not found in these neurons. This is unlikely, because we have shown that a soluble form of p75NTR is able and sufficient to interact with RV and RVG on its own (31, 46) and its affinity constant is 2 nM (46). Second, binding of RV is prevented, and there are several molecules which are tightly associated with p75NTR and could modify its binding affinity (14).
In the adult rodent, p75NTR is not expressed in DRG neurons independently of Trk expression (60). The interaction of TrkA and p75NTR in signaling has been a long and controversial debate. High-affinity cross-linking between TrkA and p75NTR has been shown (24), and ankyrin-rich membrane-spanning proteins have been suggested to play an important role in regulating the interactions between TrkA and p75NTR (10), whereas more recent studies do not support the notion of a direct interaction on the cell surface by cooperation through downstream signaling (55).
One could surmise that the RVG binding domain of p75NTR is no longer accessible or is in the wrong stoichiometric valence in this complex. Consistent with this view, we have demonstrated that RVG trimerization is required for p75NTR interaction (46) and that RVG interacts with the oligomeric but not with the monomeric form of p75NTR (C. Tuffereau, unpublished results). These results suggest the need for a 3-to-3 complex for efficient binding. However, the stoichiometry of liganded and unliganded p75NTR is not well established (62). Interestingly, sympathetic neurons in which p75NTR is always coexpressed with TrkA (18) are also not susceptible to RV infection (13). Furthermore, expression levels in neurons, as judged by amounts of mRNA or 125I-labeled nerve growth factor binding, indicate that TrkA expression is limited and that p75NTR levels are 5- to 10-fold higher (12), making it unlikely that TrkA could prohibit binding of RVG to p75NTR.
Another p75NTR-interacting molecule is the Nogo receptor (54, 57), However, the expression profile of Nogo receptor on sensory neurons includes neurons of all sizes (27), which is in contrast to the clear size difference we have observed for RV infection.
Finally, the ganglioside GT1b forms complexes with p75NTR (61). However, GT1b is expressed in almost all DRG neurons (20), and it is therefore unlikely to specifically inhibit RV binding to p75NTR.
What are the other potential neuronal receptors for RV?
Our results clearly show that RV binding and infection is mediated by molecules that are specifically expressed in a subpopulation of large, capsaicin-insensitive p75NTR-positive neurons (Fig. 6). Our studies also show that the expression of these pivotal receptors increases with the time in culture prior to the infection (10% after 4 h versus up to 20% after 16 h).
Several other receptors have been proposed for RV binding (29). One of the first receptors to be suggested was the nicotinic acetylcholine receptor (36), which is also found on cultured DRG neurons. Nicotinic antagonists partially (25%) block infection of DRG neurons (5), suggesting that this receptor could play a role in RV binding and infection. Most large cells express the α7 subunit; however, this is also found in a third of small DRG neurons (19), which is in contrast to the size profile we have established for infected DRG cells.
Another possible receptor is the NCAM (49). It is found on many cell lines as well as in DRG neurons, and there is a good correlation between RV susceptibility and magnitude of NCAM expression. However, animals lacking NCAM can still be infected with RV binding and die, albeit with a slight delay (49).
Gangliosides, principally GD1b, GQ1b, and GT1b, have also been proposed to play a role in RV binding (48). While some of these gangliosides are prevalently found in subpopulations of DRG neurons, none have the specific expression pattern that is found in infected neurons (20).
We cannot exclude the possibility that other receptors are involved. One important clue is the fact that the susceptibility to RV infection varies between different subtypes of peripheral neurons, and many neurons in the central nervous system that can be infected do not express the above-mentioned receptors. Furthermore, many viruses use different receptors for binding and entry into host cells, as in the case of herpes simplex virus, which can infect many DRG neurons (47).
Since p75NTR is not the essential factor for mediating RV infection, what could its role in rabies pathology be?
Following inoculation of RV in vivo, it became clear that a deficient interaction of the virus with p75NTR alters the clinical manifestations of the disease. This was observed irrespective of whether the parental CVS strain of RV was used to infect p75NTR-deficient animals or whether wild-type mice were infected with mutant RV that was unable to bind to p75NTR. In addition to classical rabies symptoms, such as weight loss, ruffled fur, and paralysis, we consistently observed severe ocular inflammation. This observation suggests that the p75NTR-RV interaction could play a role in the control of the inflammatory response of the host through the stimulation of a specific signaling pathway. Indeed, these ocular symptoms are strikingly similar to the observation made when animal lacking the p55 tumor necrosis factor alpha receptor or athymic nude mice were infected with RV (4). The genus Lyssavirus of the family Rhabdoviridae is currently divided into seven genotypes. Interestingly, only the evolutionarily recently evolved RV and European bat lyssavirus 2 (1) are capable of binding to p75NTR (51), and the reduced virulence might be interpreted as an improved host acceptance.
Thus, RV-p75NTR interaction appears to be important for the clinical manifestations of rabies but not for viral binding to and entry into and infection of host neurons.
Acknowledgments
We thank Marc Labetoulle for help with animal experiments and tissue sections and Pierre Perrin (Institut Pasteur, Paris, France) for the gift of a rabbit FITC-conjugated antinucleocapsid antibody. We also thank Barbara Hempsteadt (Cornell University, New York, NY) for the gift of the human p75NTR plasmid and Gipi Schiavo (Cancer UK, London, United Kingdom) for the gift of TRITC-conjugated p75NTR antibody.
This work was supported by a grant from the European Commission (QLRT-1999-00573) to C.T. and M.K. and a Ph.D. fellowship from the Ministère de l'Education Nationale et de la Recherche to C.L.
Footnotes
Published ahead of print on 10 October 2007.
REFERENCES
- 1.Badrane, H., C. Bahloul, P. Perrin, and N. Tordo. 2001. Evidence of two Lyssavirus phylogroups with distinct pathogenicity and immunogenicity. J. Virol. 75:3268-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin, A. N., and E. M. Shooter. 1995. Zone mapping of the binding domain of the rat low affinity nerve growth factor receptor by the introduction of novel N-glycosylation sites. J. Biol. Chem. 270:4594-4602. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann, I., J. V. Priestley, S. B. McMahon, E. B. Brocker, K. V. Toyka, and M. Koltzenburg. 1997. Analysis of cutaneous sensory neurons in transgenic mice lacking the low affinity neurotrophin receptor p75. Eur. J. Neurosci. 9:18-28. [DOI] [PubMed] [Google Scholar]
- 4.Camelo, S., J. Castellanos, M. Lafage, and M. Lafon. 2001. Rabies virus ocular disease: T-cell-dependent protection is under the control of signaling by the p55 tumor necrosis factor alpha receptor, p55TNFR. J. Virol. 75:3427-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castellanos, J. E., D. R. Castaneda, A. E. Velandia, and H. Hurtado. 1997. Partial inhibition of the in vitro infection of adult mouse dorsal root ganglion neurons by rabies virus using nicotinic antagonists. Neurosci. Lett. 229:198-200. [DOI] [PubMed] [Google Scholar]
- 6.Castellanos, J. E., M. Martinez, O. Acosta, and H. Hurtado. 2000. Nerve growth factor and neurotrophin-3 modulate the rabies infection of adult sensory neurons in primary cultures. Brain Res. 871:120-126. [DOI] [PubMed] [Google Scholar]
- 7.Castellanos, J. E., M. Martinez-Gutierrez, H. Hurtado, R. Kassis, H. Bourhy, O. Acosta, and M. Lafon. 2005. Studying neurotrophin antiviral effect on rabies-infected dorsal root ganglia cultures. J. Neurovirol. 11:403-410. [DOI] [PubMed] [Google Scholar]
- 8.Caterina, M. J., A. Leffler, A. B. Malmberg, W. J. Martin, J. Trafton, K. R. Petersen-Zeitz, M. Koltzenburg, A. I. Basbaum, and D. Julius. 2000. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306-313. [DOI] [PubMed] [Google Scholar]
- 9.Caterina, M. J., M. A. Schumacher, M. Tominaga, T. A. Rosen, J. D. Levine, and D. Julius. 1997. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816-824. [DOI] [PubMed] [Google Scholar]
- 10.Chang, M. S., J. C. Arevalo, and M. V. Chao. 2004. Ternary complex with Trk, p75, and an ankyrin-rich membrane spanning protein. J. Neurosci. Res. 78:186-192. [DOI] [PubMed] [Google Scholar]
- 11.Chao, M. V. 2003. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat. Rev. Neurosci. 4:299-309. [DOI] [PubMed] [Google Scholar]
- 12.Chao, M. V. 1994. The p75 neurotrophin receptor. J. Neurobiol. 25:1373-1385. [DOI] [PubMed] [Google Scholar]
- 13.Coulon, P., C. Derbin, P. Kucera, F. Lafay, C. Prehaud, and A. Flamand. 1989. Invasion of the peripheral nervous systems of adult mice by the CVS strain of rabies virus and its avirulent derivative AvO1. J. Virol. 63:3550-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dechant, G., and Y. A. Barde. 2002. The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system. Nat. Neurosci. 5:1131-1136. [DOI] [PubMed] [Google Scholar]
- 15.Deinhardt, K., S. Salinas, C. Verastegui, R. Watson, D. Worth, S. Hanrahan, C. Bucci, and G. Schiavo. 2006. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron 52:293-305. [DOI] [PubMed] [Google Scholar]
- 16.Della-Bianca, V., F. Rossi, U. Armato, I. Dal Pra, C. Costantini, G. Perini, V. Politi, and V. G. Della. 2001. Neurotrophin p75 receptor is involved in neuronal damage by prion peptide-(106-126). J. Biol. Chem. 276:38929-38933. [DOI] [PubMed] [Google Scholar]
- 17.Etessami, R., K. K. Conzelmann, B. Fadai-Ghotbi, B. Natelson, H. Tsiang, and P. E. Ceccaldi. 2000. Spread and pathogenic characteristics of a G-deficient rabies virus recombinant: an in vitro and in vivo study. J. Gen. Virol. 81:2147-2153. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Suarez, O., F. J. Naves, M. E. Del Valle, I. Esteban, E. Bronzetti, E. Vazquez, and J. A. Vega. 1996. Distribution of p75 and trk-neurotrophin receptor proteins in adult human sympathetic ganglia. Anat. Embryol. (Berlin) 193:577-583. [DOI] [PubMed] [Google Scholar]
- 19.Genzen, J. R., W. Van Cleve, and D. S. McGehee. 2001. Dorsal root ganglion neurons express multiple nicotinic acetylcholine receptor subtypes. J. Neurophysiol. 86:1773-1782. [DOI] [PubMed] [Google Scholar]
- 20.Gong, Y., Y. Tagawa, M. P. Lunn, W. Laroy, M. Heffer-Lauc, C. Y. Li, J. W. Griffin, R. L. Schnaar, and K. A. Sheikh. 2002. Localization of major gangliosides in the PNS: implications for immune neuropathies. Brain 125:2491-2506. [DOI] [PubMed] [Google Scholar]
- 21.He, X. L., and K. C. Garcia. 2004. Structure of nerve growth factor complexed with the shared neurotrophin receptor p75. Science 304:870-875. [DOI] [PubMed] [Google Scholar]
- 22.Hjerling-Leffler, J., M. AlQatari, P. Ernfors, and M. Koltzenburg. 2007. Emergence of functional sensory neuron subtypes as studied by TRP channel expression. J. Neurosci. 27:2435-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang, E. J., and L. F. Reichardt. 2001. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24:677-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber, L. J., and M. V. Chao. 1995. A potential interaction of p75 and trkA NGF receptors revealed by affinity crosslinking and immunoprecipitation. J. Neurosci. Res. 40:557-563. [DOI] [PubMed] [Google Scholar]
- 25.Jackson, A. C., and H. Park. 1999. Experimental rabies virus infection of p75 neurotrophin receptor-deficient mice. Acta Neuropathol. (Berlin) 98:641-644. [DOI] [PubMed] [Google Scholar]
- 26.Jordt, S. E., D. D. McKemy, and D. Julius. 2003. Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr. Opin. Neurobiol. 13:487-492. [DOI] [PubMed] [Google Scholar]
- 27.Josephson, A., A. Trifunovski, H. R. Widmer, J. Widenfalk, L. Olson, and C. Spenger. 2002. Nogo-receptor gene activity: cellular localization and developmental regulation of mRNA in mice and humans. J. Comp. Neurol. 453:292-304. [DOI] [PubMed] [Google Scholar]
- 28.Kelly, R. M., and P. L. Strick. 2000. Rabies as a transneuronal tracer of circuits in the central nervous system. J. Neurosci. Methods 103:63-71. [DOI] [PubMed] [Google Scholar]
- 29.Lafon, M. 2005. Rabies virus receptors. J. Neurovirol. 11:82-87. [DOI] [PubMed] [Google Scholar]
- 30.Langevin, C., H. Jaaro, S. Bressanelli, M. Fainzilber, and C. Tuffereau. 2002. Rabies virus glycoprotein (RVG) is a trimeric ligand for the N-terminal cysteine-rich domain of the mammalian p75 neurotrophin receptor. J. Biol. Chem. 277:37655-37662. [DOI] [PubMed] [Google Scholar]
- 31.Langevin, C., and C. Tuffereau. 2002. Mutations conferring resistance to neutralization by a soluble form of the neurotrophin receptor (p75NTR) map outside of the known antigenic sites of the rabies virus glycoprotein. J. Virol. 76:10756-10765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawson, S. N. 2002. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Aδ- or Aα/β-fibres. Exp. Physiol. 87:239-244. [DOI] [PubMed] [Google Scholar]
- 33.Lee, K. F., A. M. Davies, and R. Jaenisch. 1994. p75-deficient embryonic dorsal root sensory and neonatal sympathetic neurons display a decreased sensitivity to NGF. Development 120:1027-1033. [DOI] [PubMed] [Google Scholar]
- 34.Lee, K. F., E. Li, L. J. Huber, S. C. Landis, A. H. Sharpe, M. V. Chao, and R. Jaenisch. 1992. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell 69:737-749. [DOI] [PubMed] [Google Scholar]
- 35.Lee, R., P. Kermani, K. K. Teng, and B. L. Hempstead. 2001. Regulation of cell survival by secreted proneurotrophins. Science 294:1945-1948. [DOI] [PubMed] [Google Scholar]
- 36.Lentz, T. L., T. G. Burrage, A. L. Smith, J. Crick, and G. H. Tignor. 1982. Is the acetylcholine receptor a rabies virus receptor? Science 215:182-184. [DOI] [PubMed] [Google Scholar]
- 37.Luo, L., R. C. Salunga, H. Guo, A. Bittner, K. C. Joy, J. E. Galindo, H. Xiao, K. E. Rogers, J. S. Wan, M. R. Jackson, and M. G. Erlander. 1999. Gene expression profiles of laser-captured adjacent neuronal subtypes. Nat. Med. 5:117-122. [DOI] [PubMed] [Google Scholar]
- 38.Lycke, E., and H. Tsiang. 1987. Rabies virus infection of cultured rat sensory neurons. J. Virol. 61:2733-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molliver, D. C., D. E. Wright, M. L. Leitner, A. S. Parsadanian, K. Doster, D. Wen, Q. Yan, and W. D. Snider. 1997. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron 19:849-861. [DOI] [PubMed] [Google Scholar]
- 40.Mu, X., I. Silos Santiago, S. L. Carroll, and W. D. Snider. 1993. Neurotrophin receptor genes are expressed in distinct patterns in developing dorsal root ganglia. J. Neurosci. 13:4029-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perini, G., V. Della-Bianca, V. Politi, V. G. Della, I. Dal Pra, F. Rossi, and U. Armato. 2002. Role of p75 neurotrophin receptor in the neurotoxicity by beta-amyloid peptides and synergistic effect of inflammatory cytokines. J. Exp. Med. 195:907-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Priestley, J. V., G. J. Michael, S. Averill, M. Liu, and N. Willmott. 2002. Regulation of nociceptive neurons by nerve growth factor and glial cell line derived neurotrophic factor. Can. J. Physiol. Pharmacol. 80:495-505. [DOI] [PubMed] [Google Scholar]
- 43.Raux, H., F. Iseni, F. Lafay, and D. Blondel. 1997. Mapping of monoclonal antibody epitopes of the rabies virus P protein. J. Gen. Virol. 78:119-124. [DOI] [PubMed] [Google Scholar]
- 44.Rose, J. K., and M. A. Whitt. 2001. Rhabdoviridae: the viruses and their replication, p. 1221-1244. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Field's virology, 4th ed. Lippincott Williams and Wilkins, Philadelphia, PA.
- 45.Saghi, N., and A. Flamand. 1979. Biochemical characterization of temperature-sensitive rabies virus mutants. J. Virol. 31:220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sissoeff, L., M. Mousli, P. England, and C. Tuffereau. 2005. Stable trimerization of recombinant rabies virus glycoprotein ectodomain is required for interaction with the p75NTR receptor. J. Gen. Virol. 86:2543-2552. [DOI] [PubMed] [Google Scholar]
- 47.Spear, P. G. 2004. Herpes simplex virus: receptors and ligands for cell entry. Cell Microbiol. 6:401-410. [DOI] [PubMed] [Google Scholar]
- 48.Superti, F., B. Hauttecoeur, M. J. Morelec, P. Goldoni, B. Bizzini, and H. Tsiang. 1986. Involvement of gangliosides in rabies virus infection. J. Gen. Virol. 67:47-56. [DOI] [PubMed] [Google Scholar]
- 49.Thoulouze, M. I., M. Lafage, M. Schachner, U. Hartmann, H. Cremer, and M. Lafon. 1998. The neural cell adhesion molecule is a receptor for rabies virus. J. Virol. 72:7181-7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tuffereau, C., J. Benejean, D. Blondel, B. Kieffer, and A. Flamand. 1998. Low-affinity nerve-growth factor receptor (P75NTR) can serve as a receptor for rabies virus. EMBO J. 17:7250-7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tuffereau, C., E. Desmezieres, J. Benejean, C. Jallet, A. Flamand, N. Tordo, and P. Perrin. 2001. Interaction of lyssaviruses with the low-affinity nerve-growth factor receptor p75NTR. J. Gen. Virol. 82:2861-2867. [DOI] [PubMed] [Google Scholar]
- 52.Ugolini, G. 1995. Specificity of rabies virus as a transneuronal tracer of motor networks: transfer from hypoglossal motoneurons to connected second-order and higher order central nervous system cell groups. J. Comp. Neurol. 356:457-480. [DOI] [PubMed] [Google Scholar]
- 53.von Schack, D., E. Casademunt, R. Schweigreiter, M. Meyer, M. Bibel, and G. Dechant. 2001. Complete ablation of the neurotrophin receptor p75NTR causes defects both in the nervous and the vascular system. Nat. Neurosci. 4:977-978. [DOI] [PubMed] [Google Scholar]
- 54.Wang, K. C., J. A. Kim, R. Sivasankaran, R. Segal, and Z. He. 2002. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature 420:74-78. [DOI] [PubMed] [Google Scholar]
- 55.Wehrman, T., X. He, B. Raab, A. Dukipatti, H. Blau, and K. C. Garcia. 2007. Structural and mechanistic insights into nerve growth factor interactions with the TrkA and p75 receptors. Neuron 53:25-38. [DOI] [PubMed] [Google Scholar]
- 56.Willoughby, R. E., K. S. Tieves, G. M. Hoffman, N. S. Ghanayem, C. M. Amlie-Lefond, M. J. Schwabe, M. J. Chusid, and C. E. Rupprecht. 2005. Survival after treatment of rabies with induction of coma. N. Engl. J. Med. 352:2508-2514. [DOI] [PubMed] [Google Scholar]
- 57.Wong, S. T., J. R. Henley, K. C. Kanning, K. H. Huang, M. Bothwell, and M. M. Poo. 2002. A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat. Neurosci. 5:1302-1308. [DOI] [PubMed] [Google Scholar]
- 58.Wood, J. N., J. Winter, I. F. James, H. P. Rang, J. Yeats, and S. Bevan. 1988. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J. Neurosci. 8:3208-3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.World Health Organization. 2006. Rabies. http://www.who.int/mediacentre/factsheets/fs099/en/.
- 60.Wright, D. E., and W. D. Snider. 1995. Neurotrophin receptor mRNA expression defines distinct populations of neurons in rat dorsal root ganglia. J. Comp. Neurol. 351:329-338. [DOI] [PubMed] [Google Scholar]
- 61.Yamashita, T., H. Higuchi, and M. Tohyama. 2002. The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J. Cell Biol. 157:565-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zampieri, N., and M. V. Chao. 2004. Structural biology. The p75 NGF receptor exposed. Science 304:833-834. [DOI] [PubMed] [Google Scholar]