Abstract
Mitogen-activated protein kinases (MAPKs) often play important roles in virus infection. To explore intracellular signaling pathways induced by baculovirus infection, we examined the involvement of MAPKs in Bombyx mori nucleopolyhedrovirus (BmNPV) infection of BmN cells. We found that specific inhibitors of extracellular signal-regulated kinase (ERK) kinase and c-Jun NH2-terminal kinase (JNK) significantly reduced occlusion body (OB) formation and budded virus (BV) production. Next, we quantified OB and BV production after applying the inhibitors at different times postinfection (p.i.). The inhibitors significantly reduced OB and BV production to various extents when applied at 12 h p.i., indicating that the reduction of BmNPV infectivity by these inhibitors occurs at the late stage of infection. Also, we observed that these inhibitors markedly repressed or deregulated the expression of delayed early, late, and very late gene products. Western blot analysis using phospho-MAPK-specific antibodies showed that ERK and JNK were activated at the late stage of BmNPV infection. In addition, the magnitude and pattern of MAPK activation were dependent on the multiplicity of infection. To verify the effects of the inhibitors on BmNPV infection, we also attempted to knock down the B. mori genes BmErk and BmJnk, which encode ERK and JNK, respectively. Knockdown of BmErk and BmJnk resulted in the reduced production of OBs and BVs, confirming that BmERK and BmJNK are involved in the BmNPV infection process. Taken together, these results indicate that the activation of MAPK signaling pathways is required for efficient infection by BmNPV.
The Baculoviridae are a diverse family of pathogens that are infectious for arthropods, particularly insects of the order Lepidoptera. Nucleopolyhedroviruses (NPVs), a genus of the Baculoviridae, contain a large circular and double-stranded DNA genome within a rod-shaped virion. NPVs produce two types of virions during the infection cycle to bring about efficient viral replication within infected insect larvae and to spread virus from insect to insect in nature. Occlusion-derived virus transmits virus from insect to insect via oral infection, whereas budded virus (BV) spreads infection to neighboring cells (10, 33).
Viral infection causes the deregulation of various host cellular pathways, some of which reflect cellular responses to infection, while others are the result of viral modification of cellular environments (37, 47, 55). A common strategy that a virus uses to facilitate its infection and replication is to exploit these altered cellular pathways. For example, modulation of mitogen-activated protein kinase (MAPK) pathways is essential for infection and replication of hepatitis B virus, Epstein-Barr virus, and vaccinia virus (6, 13, 59). Deregulated cellular signaling pathways can also contribute to the pathogenesis induced by viral infections (47).
Multicellular organisms have three well-characterized subfamilies of MAPKs that control a vast array of physiological processes (23). These enzymes are regulated by a characteristic phosphorelay system in which a series of three protein kinases phosphorylate and activate one another. The extracellular signal-regulated kinases (ERKs) are widely expressed and are involved in the regulation of meiosis, mitosis, and postmitotic functions in differentiated cells. Many different stimuli including growth factors, cytokines, and virus infection activate the ERK pathways. Inhibitors of these enzymes are being explored as anticancer agents (52). The c-Jun NH2-terminal kinases (JNKs) were isolated and characterized as stress-activated protein kinases on the basis of their activation in response to the inhibition of protein synthesis. JNKs were then discovered to bind and phosphorylate the DNA binding protein c-Jun and increase its transcriptional activity. c-Jun is a component of the AP-1 transcription complex, which is an important regulator of gene expression. JNK inhibitors may be effective in controlling rheumatoid arthritis (16). p38 MAPKs regulate the expression of many cytokines. p38 is activated in immune cells by inflammatory cytokines and has an important role in the activation of the immune response (1).
It is well known that baculovirus infection alters both host protein and host mRNA levels. Infection typically causes a global shutoff of host protein synthesis and gene expression in insect cells beginning at around 12 to 18 h postinfection (p.i.) (hpi) (29, 42, 46). Conversely, particular host genes are induced or remain stably expressed until the late stage of baculovirus infection. Quadt et al. previously observed a dramatic increase in the levels of TATA-binding protein (TBP) during the late phases of Autographa californica multiple NPV (AcMNPV) infection and proposed a role for TBP during late viral transcription (50). By using expressed-sequence-tag analysis of Bombyx mori NPV (BmNPV)-infected BmN cells, Okano et al. showed that the expression of cytochrome c oxidase 1 was stable until 24 hpi (44). Similarly, using a differential display approach, Nobiron et al. found that a heat shock protein 70 cognate of Spodoptera frugiperda Sf9 cells is transiently induced at 6 hpi during AcMNPV infection (42). Nonetheless, the mechanism of viral modulation of host mRNA levels remains largely unknown due to a dearth of information on the signaling cascades with which baculoviruses interact during their infection.
To begin to identify the signaling pathways induced by baculovirus infection, we examined the involvement of host MAPK pathways on BmNPV infection. Using chemical inhibitors and double-stranded RNA (dsRNA), we show here that two B. mori MAPKs, BmERK and BmJNK, are required for efficient infection by BmNPV. This is the first report to explore the signaling pathways of baculovirus-infected host cells.
MATERIALS AND METHODS
Materials.
Inhibitors of ERK kinase (U0126 and PD98059), p38 (SB203580), and JNK (SP600125) were purchased from Calbiochem. Inhibitors were dissolved in dimethyl sulfoxide (DMSO). The final concentration of DMSO in cell culture medium was 0.1% (vol/vol). V-CATH and BmCHI-h polyclonal antibodies were described previously (5). Antibodies against phospho-ERK and phospho-JNK were purchased from Promega. Antibodies against ERK and phospho-p38 were obtained from Cell Signaling Technology. Antibodies against GP64 and actin were obtained from Santa Cruz Biotechnology. Antibodies against BmNPV DNA-binding protein (DBP)(43) and baculovirus repeated open reading frames (BRO) (25) were kindly provided by W. Kang (Riken). Antibodies against AcMNPV IE1 and LEF3 (4, 21) were kindly provided by E. Carstens (Queen's University). The polyhedrin polyclonal antibody (54) was a gift from M. Nagata (University of Tokyo).
Cell lines and viruses.
The BmN-4 (BmN) cell line was cultured at 27°C in TC-100 or IPL-41 medium supplemented with 10% fetal bovine serum (26). BmNPV T3 (14) and BmFGFD, a BmNPV mutant lacking functional vfgf (31), were used in this study. Viruses were propagated in BmN cells, and BV titers were determined by plaque assay (26).
Assays for BV and OB production.
For virus growth curves, BmN cells were infected with BmNPV at a multiplicity of infection (MOI) of 5. After 1 h of incubation, virus-containing culture medium was removed, the cells were washed twice with serum-free TC-100 medium, and fresh serum-free medium with or without chemical inhibitors was added (0 hpi). A small amount of culture medium was harvested at various time points, and BV production was determined by plaque assay. Occlusion bodies (OBs) were counted as described previously (17).
Cell viability.
BmN cells were serum starved for 24 h and infected with BmNPV at an MOI of 5. After 1 h of incubation, virus-containing culture medium was removed, the cells were washed twice with serum-free TC-100 medium, and fresh serum-free medium with or without chemical inhibitors was added (0 hpi). We used the WST-1 assay kit (Roche Applied Science) to assess viable cell numbers as described previously (28).
SDS-PAGE and Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting were performed as described previously (28). Western blot analysis of B. mori MAPKs was carried out using anti-MAPK antibodies. MAPK activation was quantified by densitometry using ImageGauge software (Fujifilm). Polyhedrin expression was examined by SDS-PAGE as described previously (31).
Reverse transcription-PCR.
Total RNA was prepared using TRIzol reagent (Invitrogen) as described previously (29). One microgram total RNA was reverse transcribed, diluted, and used for PCR as described elsewhere previously (29).
Knockdown of BmErk and BmJnk in BmN cells.
dsRNA for BmErk, BmJnk, and the enhanced green fluorescent protein gene (egfp) was generated by using a MEGAscript RNA interference kit (Ambion). Primers used were as follows: BmERKF (5′-TGTTTCTGCCTTCGACAACG-3′), T7-BmERKF (5′-TAATACGACTCACTATAGGGAGATGTTTCTGCCTTCGACAACG-3′), BmERKR (5′-TGATGCAATCAAGATCTTCC-3′), and T7-BmERKR (5′-TAATACGACTCACTATAGGGAGATGATGCAATCAAGATCTTCC-3′) for BmErk; BmJNKF (5′-ACTTATGAAACTTGTCAACC-3′), T7-BmJNKF (5′-TAATACGACTCACTATAGGGAGAACTTATGAAACTTGTCAACC-3′), BmJNKR (5′-CCAGTTGCTCGATGATCTTG-3′), and T7-BmJNKR (5′-TAATACGACTCACTATAGGGAGACCAGTTGCTCGATGATCTTG-3′) for BmJnk; and T7-EGFPF (5′-TAATACGACTCACTATAGGACGTAAACGGCCACAAGTTC-3′) and T7-EGFPR (5′-TAATACGACTCACTATAGGTGCTCAGGTAGTGGTTGTCG-3′) for egfp. BmN cells (5 × 105 cells) were transfected with 5 μg of dsRNA using Cellfectin reagent (Invitrogen). Twenty-four hours after transfection, cells were infected with BmNPV at an MOI of 5. Three days after infection, culture medium was harvested, and OB production was examined. BV production was determined by plaque assay. Knockdown of BmErk and BmJnk was examined at 24 h posttransfection by Western blot analysis using anti-ERK and anti-JNK, respectively.
cDNA cloning of BmJnk.
To determine the sequence of the full-length cDNA of BmJnk, we screened B. mori cDNA libraries (41; T. Shimada et al., unpublished data) and found two clones (ce-1787 and NV021723) showing homology to the product of basket, Drosophila melanogaster JNK. Their nucleotide sequences were determined using the ABI Big Dye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems) and the ABI Prism 3100 DNA sequencer (Applied Biosystems).
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper has been submitted to the DDBJ/EMBL/GenBank Database under accession number AB302934 (BmJnk).
RESULTS
Effects of inhibitors of MAPK pathways on BmNPV infection.
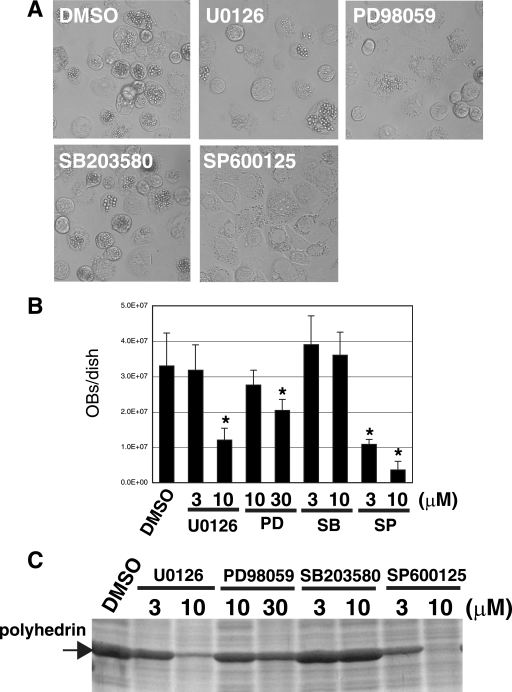
To examine whether MAPK activation is involved in BmNPV infection, we assessed the effects of specific inhibitors on the MAPK pathway components ERK kinase (inhibitors U0126 and PD98059), JNK (inhibitor SP600125), and p38 (inhibitor SB203580). We first measured OB production in BmNPV-infected BmN cells incubated with or without these inhibitors. As shown in Fig. 1A and B, treatment with U0126, PD98059, and SP600125 significantly reduced OB production (64%, 39%, and 88% reduction with 10 μM of U0126, PD98059, and SP600125, respectively), whereas SB203580 had no effect. SP600125 had the strongest effect on OB production. SDS-PAGE analysis indicated that U0126, PD98059, and SP600125 also inhibited polyhedrin protein expression, suggesting that the reduced OB production by U0126, PD98059, and SP600125 was due to the inhibition of polyhedrin synthesis (Fig. 1C).
FIG. 1.
Effects of inhibitors of MAPK pathways on OB production. (A) Light microscopy observations of BmNPV-infected BmN cells at 72 hpi after treatment at 0 hpi with DMSO, U0126 (10 μM), PD98059 (30 μM), SB203580 (10 μM), and SP600125 (10 μM). (B) Quantification of OB production at 72 hpi in BmNPV-infected BmN cells treated at 0 hpi with DMSO, U0126, PD98059 (PD), SB203580 (SB), and SP600125 (SP). *, P < 0.05 versus DMSO-treated control. Data show means ± standard errors (SE) of triplicates, and similar results were obtained in three independent experiments. (C) SDS-PAGE analysis of polyhedrin synthesis at 72 hpi in BmNPV-infected BmN cells treated at 0 hpi with DMSO, U0126, PD98059, SB203580, and SP600125. The gel was stained with Coomassie brilliant blue.
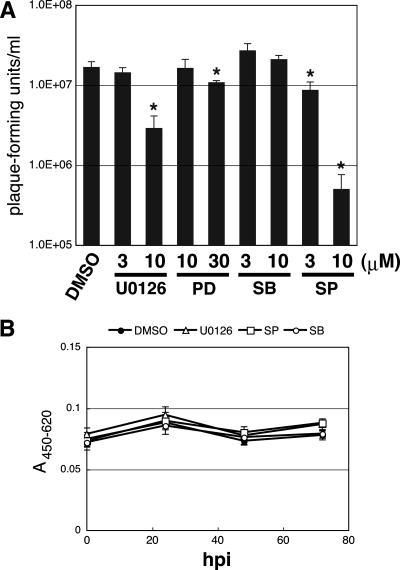
We then assessed the effects of these inhibitors on BV production at 72 hpi. As shown in Fig. 2A, U0126, PD98059, and SP600125, but not SB203580, significantly reduced BV production in BmNPV-infected BmN cells. SP600125 was, again, the strongest suppressor of BV production. Taken together, these results suggest that the activation of MAPK pathways involving ERK and JNK, but not p38, is required for the efficient production of OBs and BVs during BmNPV infection.
FIG. 2.
Effects of inhibitors of MAPK pathways on BV production and cell viability. (A) BV production at 72 hpi in BmNPV-infected BmN cells treated at 0 hpi with DMSO, U0126, PD98059 (PD), SB203580 (SB), and SP600125 (SP) was quantified by plaque assay. *, P < 0.05 versus DMSO-treated control. Data show means ± SE of triplicates, and similar results were obtained in three independent experiments. (B) Cell viability of BmNPV-infected BmN cells treated at 0 hpi with DMSO, U0126 (10 μM), SB203580 (SB) (10 μM), and SP600125 (SP) (10 μM) was assessed by WST-1 assay. Data show means ± SE of 10 replicates, and similar results were obtained in two independent experiments.
A cell viability experiment was conducted to assess whether the observed reductions in OB and BV production by inhibitors were caused by cytotoxicity. BmN cells were infected with BmNPV and treated with 10 μM of inhibitors, and cellular metabolic activity was measured by cleavage of a tetrazolium salt, WST-1 (Fig. 2B). No decrease in cell viability compared to DMSO-treated cells was observed during BmNPV infection, indicating that the effects of MAPK pathway inhibitors on OB and BV production were not associated with cytotoxicity.
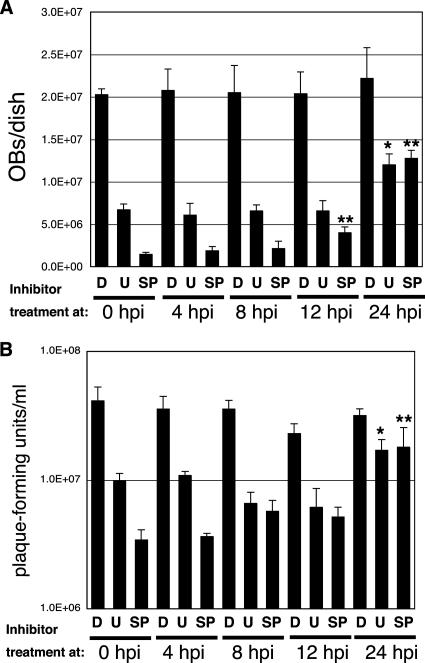
MAPK pathway inhibitors reduce OB and BV production at the late stage of infection.
To more specifically identify the stage of BmNPV infection that the inhibitors targeted, we examined OB or BV production after applying the inhibitors at different time points p.i. As shown in Fig. 3, the inhibitors significantly reduced OB and BV production to different extents at 12 hpi, showing that the reduction of BmNPV infectivity by these inhibitors occurs at the late stage of infection. However, the effects of U0126 and SP600125 on BmNPV infection were slightly different. Whereas U0126 and SP600125 caused significant reductions in OB and BV production, the effect on OB production was reduced for SP600125 when it was applied after 12 hpi and for U0126 and SP600125 when they were applied at 24 hpi. Also, we observed that the effects of U0126 and SP600125 on BV production were significantly reduced when applied at 24 hpi (Fig. 3). These results indicate that U0126 modulates BmNPV infection mainly after 12 hpi, whereas SP600125 affects infection from 12 hpi, suggesting that the activation patterns of ERK and JNK might be different during BmNPV infection in BmN cells.
FIG. 3.
OB and BV production after application of inhibitors at different time points p.i. OB (A) or BV (B) production was assessed at 72 hpi in BmNPV-infected BmN cells after application of DMSO (D), U0126 (U) (10 μM), and SP600125 (SP) (10 μM) at different time points p.i. *, P < 0.05 versus cells treated with U0126 at 0 hpi; **, P < 0.05 versus cells treated with SP600125 at 0 hpi. Data show means ± SE of triplicates, and similar results were obtained in two independent experiments.
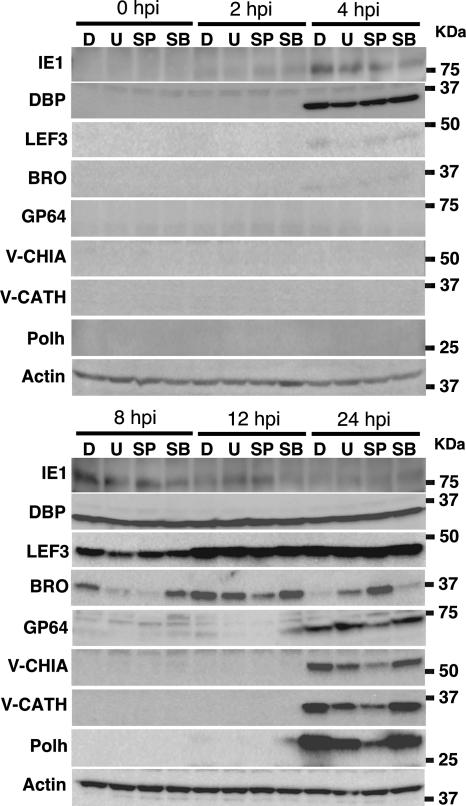
MAPK pathway inhibitors reduce or deregulate expression of viral delayed-early, late, and very late gene products.
It was likely that inhibitors of MAPK pathways reduced BmNPV infectivity by modulating the late stage of infection. We thus examined the effects of these inhibitors on the expression of BmNPV gene products. Western blot analysis showed that the expression of the immediate-early gene product IE1 in cells treated with U0126, SP600125, or SB203580 was comparable to that observed in DMSO-treated cells (Fig. 4), suggesting that these inhibitors had little effect on the expression of immediate-early gene products. Also, we observed that the expression of DBP, which is encoded by one of the BmNPV early gene, was not affected by the inhibitors. However, the expression patterns of two delayed-early gene products, LEF3 and BRO, were different from those observed in DMSO-treated cells: LEF3 expression was significantly reduced in U0126- or SP600125-treated cells at 8 hpi, but its level returned to a similar level at 12 hpi compared to that observed in DMSO-treated cells (Fig. 4). Similarly, the expression pattern of BRO in U0126-treated cells was markedly reduced at 8 hpi, but the level of its expression was comparable to that of DMSO-treated cells at 12 hpi. Interestingly, the pattern of BRO expression in SP600125-treated cells was quite different from that observed in DMSO- or U0126-treated cells: BRO expression in SP600125-treated cells gradually increased and peaked at 24 hpi, although its expression in DMSO- and U0126-treated cells was very low at 24 hpi (Fig. 4). Expression levels of late (GP64, V-CHIA, and V-CATH) and very late (Polh) gene products in cells treated with U0126 or SP600125 were also much lower than those observed in DMSO-treated cells (Fig. 4). This was consistent with the finding that polyhedrin expression in cells treated with U0126 or SP600125 was markedly lower than that in DMSO-treated cells (Fig. 1C). These results suggest that U0126 and SP600125 reduce or deregulate expression of viral delayed-early, late, and very late gene products.
FIG. 4.
Effects of inhibitors of MAPK pathways on expression of BmNPV gene products. BmN cells infected with BmNPV were treated with DMSO (D), U0126 (U) (10 μM), SP600125 (SP) (10 μM), and SB203580 (SB) (10 μM) for 0, 2, 4, 8, 12, and 24 h, lysed, and immunoblotted with antibodies against IE1, DBP, LEF3, BRO, GP64, V-CHIA, V-CATH, Polh, and actin. Similar results were obtained in two independent experiments.
Activation of ERK and JNK by BmNPV in BmN cells.
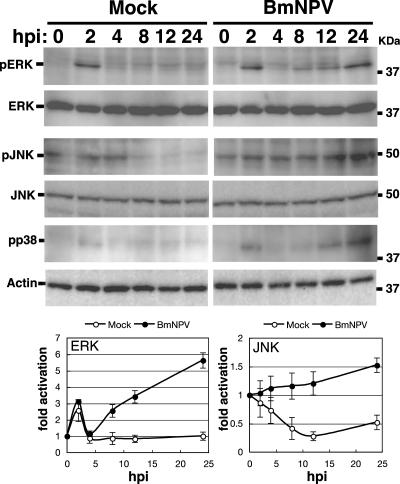
The activation states of ERK and JNK during BmNPV infection were examined by Western blotting using phospho-MAPK-specific antibodies. As shown in Fig. 5, activation of ERK was clearly detected at 2 hpi in both mock- and BmNPV-infected BmN cells, but the activated form almost completely disappeared at 4 hpi, suggesting that this transient early ERK activation is not caused by BmNPV infection but is caused by culture medium containing 10% fetal bovine serum. In BmNPV-infected cells, ERK activation was again detected from 8 hpi, and the level continued to increase at 24 hpi. Total levels of ERK were similar at all times. ERK activation was not seen in mock-infected BmN cells at any time point other than 2 hpi. In BmNPV-infected BmN cells, JNK activation gradually increased until 24 hpi. In contrast, in mock-infected cells, JNK activation levels decreased to 24 hpi. Total levels of JNK were similar at all times in either mock- or BmNPV-infected BmN cells. Collectively, these results clearly show that BmNPV infection causes ERK and JNK activation in BmN cells. In addition, we examined p38 activation during BmNPV infection and found that p38 was also activated by BmNPV infection from 12 hpi to 24 hpi (Fig. 5).
FIG. 5.
Activation of ERK and JNK by BmNPV in BmN cells. BmN cells were mock infected or infected with BmNPV at an MOI of 5. Activation of ERK and JNK at 0, 2, 4, 8, 12, and 24 hpi was assessed by Western blotting using phospho-ERK (pERK)- and phospho-JNK (pJNK)-specific antibodies, respectively. Total levels of ERK and JNK were examined by anti-ERK and anti-JNK antibodies, respectively. Western blots using anti-phospho-p38 (pp38) and anti-actin were also shown. MAPK activation plots show means ± SE of quantitative data from four to six independent experiments.
Dependence of BmNPV-induced ERK and JNK activation on viral MOI.
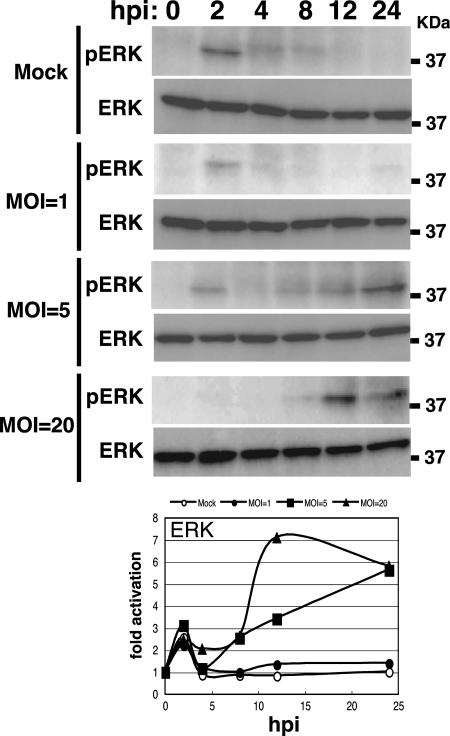
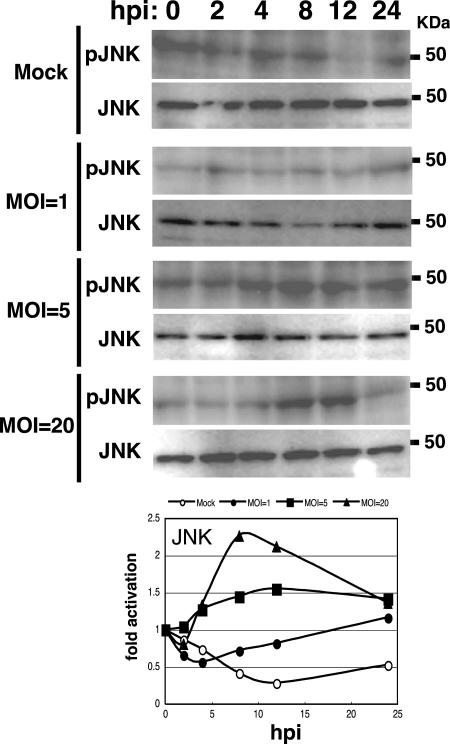
The level of BmNPV required to induce the activation of ERK and JNK in BmN cells was examined. At an MOI of 1, a low level of ERK or JNK activation was observed at 24 hpi as assessed by Western blotting (Fig. 6 and 7). Strong activation of ERK or JNK was seen at an MOI of 5 or 20. The peak of activation was shifted to earlier stages of infection when BmN cells were infected with BmNPV at an MOI of 20 (Fig. 6 and 7). These results suggest that BmNPV-induced ERK and JNK activation depends on viral MOI.
FIG. 6.
Dependence of BmNPV-induced ERK activation on viral MOI. BmN cells were mock infected or infected with BmNPV at MOIs of 1, 5, and 20. Activation of ERK at 0, 2, 4, 8, 12, and 24 hpi was assessed by Western blotting using the phospho-ERK (pERK)-specific antibody. MAPK activation plots show averages of quantitative data from two independent experiments.
FIG. 7.
Dependence of BmNPV-induced JNK activation on viral MOI. BmN cells were mock infected or infected with BmNPV at MOIs of 1, 5, and 20. Activation of JNK at 0, 2, 4, 8, 12, and 24 hpi was assessed by Western blotting using the phospho-JNK (pJNK)-specific antibody. MAPK activation plots show averages of quantitative data from two independent experiments.
Knockdown of BmErk or BmJnk reduces OB and BV production in BmNPV-infected BmN cells.
To verify the effects of ERK kinase and JNK inhibitors on BmNPV infection, we attempted to knock down the B. mori genes BmErk and BmJnk, encoding ERK and JNK, respectively. BmErk was cloned and reported previously by Fujiwara et al. (12), whereas BmJnk has not been identified. Thus, we tried to identify a Jnk homolog of B. mori (BmJnk) from B. mori cDNA libraries. We found two clones (ce-1787 and NV021723) showing homology to Drosophila JNK encoded by basket (GenBank accession number NM_164900) and designated this gene BmJnk. Sequence analysis of these clones identified a 1,188-bp open reading frame that potentially encodes a protein with 396 amino acids. BmJNK has 79.8% and 74.5% amino acid sequence identities to Drosophila Basket and human JNK1, respectively (data not shown).
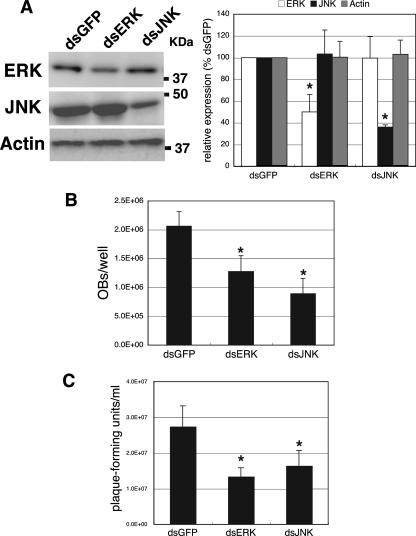
BmN cells were transfected with dsRNA for BmErk or BmJnk 24 h before BmNPV infection. dsRNA for egfp was also transfected into BmN cells as a control. Western blot analysis showed that levels of expression of BmERK and BmJNK were significantly reduced by dsRNA for BmErk and BmJnk, respectively, compared with that in cells transfected with egfp dsRNA (Fig. 8A). In contrast, actin expression was not affected by dsRNA transfection. Next, we examined the effects of dsRNA-mediated knockdown on OB and BV production. As shown in Fig. 8B, the knockdown of BmErk and BmJnk resulted in a reduced production of OBs in BmNPV-infected BmN cells. BV production was also reduced in either BmErk- or BmJnk-knocked-down BmN cells (Fig. 8C), suggesting that BmERK and BmJNK are involved in OB and BV production. Taking these data together with the results obtained by using chemical inhibitors, we conclude that the activation of ERK- and JNK-dependent signaling pathways is required for efficient infection by BmNPV.
FIG. 8.
Effects of dsRNA-mediated knockdown of BmErk and BmJnk on OB and BV production in BmNPV-infected BmN cells. (A) dsRNA-mediated knockdown of BmErk and BmJnk. BmN cells were transfected with dsRNA for BmErk (dsERK), BmJnk (dsJNK), and egfp (dsGFP). Twenty-four hours after transfection, the expression of BmERK, BmJNK, and actin was examined by Western blot analysis using anti-ERK, anti-JNK, and anti-actin antibodies, respectively. (B) Effects of dsRNA-mediated knockdown of BmErk and BmJnk on OB production. Twenty-four hours after transfection, BmN cells were infected with BmNPV at an MOI of 5, and OB production was assessed. (C) Effects of dsRNA-mediated knockdown of BmErk and BmJnk on BV production. Twenty-four hours after transfection, BmN cells were infected with BmNPV at an MOI of 5, and BV production was assessed by plaque assay. *, P < 0.05 versus egfp dsRNA-treated control. Data show means ± SE of triplicates, and similar results were obtained in two independent experiments.
vFGF is not required for ERK and JNK activation during BmNPV infection.
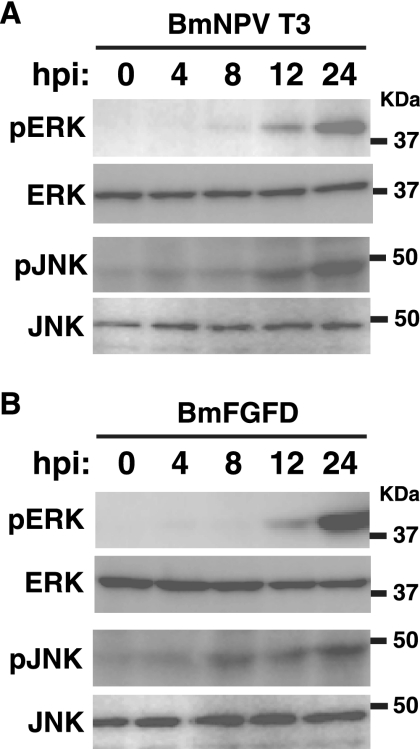
In mammals and Drosophila, fibroblast growth factors (FGFs) have been reported to activate MAPK pathways via cell surface FGF receptors (35, 45, 49). Recently, we found that a BmNPV-encoded FGF, vFGF, is secreted from BmNPV-infected BmN cells and induces cell migration by activating its receptor, BmBtl (27, 30). To examine whether the FGF-BmBtl pathway is required for MAPK activation during BmNPV infection, we assessed the activation of ERK and JNK in BmN cells infected with BmFGFD, a BmNPV mutant lacking functional vfgf (31). Western blot analysis showed that, as observed in BmNPV T3-infected cells (Fig. 9A), BmFGFD infection clearly activated ERK and JNK in BmN cells (Fig. 9B), suggesting that vFGF is not essential for ERK and JNK activation during BmNPV infection.
FIG. 9.
MAPK activation in BmN cells infected with vfgf-deficient BmNPV. BmN cells were infected with BmNPV T3 (A) or BmFDFD (B), a BmNPV mutant lacking functional vfgf, at an MOI of 5. Activation of ERK and JNK at 0, 4, 8, 12, and 24 hpi was assessed by Western blotting using phospho-ERK (pERK)- and phospho-JNK (pJNK)-specific antibodies, respectively. Similar results were obtained in two independent experiments.
DISCUSSION
Infection with a wide variety of viruses can result in the perturbation of host cell signaling pathways including MAPK cascades. Some viruses show a dependence on the ERK signaling cascades for replication, and viral proteins inducing ERK activation have been identified (2, 22, 24, 34, 38, 39). Virus-induced activation of two other MAPKs, JNK and p38, has also been described (9, 18, 20, 36, 40, 48, 53, 58). However, to date, the signaling pathways induced by baculovirus infection are largely unknown. In this study, using chemical inhibitors and dsRNA-mediated gene knockdown, we discovered that two MAPKs, ERK and JNK, are involved in the efficient infection of BmN cells by BmNPV. This is the first direct evidence that baculoviruses modulate the signaling pathways of infected host cells.
We examined the effects of inhibitors of MAPK pathways on BV and OB production and found that U0126, PD98059, and SP600125 significantly reduced the production of BVs and OBs (Fig. 1). Western blot analysis also clearly showed that these inhibitors markedly inhibited the expression of delayed-early, late, and very late gene products of BmNPV (Fig. 4). These results suggest that the activation of ERK and JNK plays important roles in the regulated expression of BmNPV gene products.
Baculovirus pk1 encodes a serine/threonine protein kinase that is expressed during the beginning of the late and throughout the very late phases of AcMNPV infection (51). Fan et al. previously reported that temperature-sensitive mutations in pk1 of AcMNPV completely blocked very late gene expression (11). Also, deletion analysis showed that BmNPV pk1 is essential for virus replication in BmN cells (15; S. Katsuma and T. Shimada, unpublished results), suggesting that the phosphorylation of host and/or viral proteins by PK1 is required for virus replication. We propose a possibility that BmNPV PK1 might be involved in the pathways of Bombyx ERK and/or JNK activation. In this study, we do not clarify the molecules that activate these two MAPKs and/or that directly affect the expression of BmNPV gene products interacting with the MAPK pathways. We are now attempting to identify the host or viral proteins involved in these cascades.
In our previous work, we cloned and characterized an FGF homolog, vFGF, of BmNPV (27). BmNPV vFGF is secreted from BmNPV-infected BmN cells, phosphorylates a cell surface receptor, BmBtl, and induces cell migration (30, 32). Furthermore, we recently reported that the deletion of vfgf from the BmNPV genome causes a delay in BV production and a reduction in the level of polyhedrin expression in BmN cells (31). These results suggest that vFGF can activate or modulate host signaling molecules downstream of the vFGF-Btl cascade, resulting in cell motility and viral late gene expression. In mammals and Drosophila, FGFs have been shown to activate MAPK pathways via cell surface FGF receptors (35, 45, 49). Thus, we speculated that vFGF might activate MAPK pathways in BmNPV-infected BmN cells. However, we observed that BmFGFD infection activated ERK and JNK in BmN cells, convincingly showing that vFGF is not required for MAPK activation during BmNPV infection. BmNPV vFGF must therefore regulate BV and OB production by modulating proteins other than ERK and JNK in BmN cells.
Detvisitsakun et al. previously characterized the properties of AcMNPV vFGF (7) and reported that an AcMNPV mutant lacking functional vfgf displayed no striking phenotype in Sf21 or TN-368 cells in terms of BV production, protein and DNA synthesis, and growth advantage (8). Further experiments have shown that U0126 significantly reduced OB and BV production in AcMNPV-infected Sf9 cells, suggesting that the ERK-mediated cascade might be a common pathway for OB and BV production among NPVs (Katsuma and Shimada, unpublished). These results also suggest that vFGF does not play an important role in ERK activation during infection by these two NPVs.
Baculovirus infection can induce a number of perturbations of host cell properties including alteration of the cellular cytoskeleton (3), arrest of the cell cycle in G2/M phase (19), and global shutoff of host protein translation. Previous studies have shown that mRNA levels of actin, histones, heat shock protein 70 (46), and the translation initiation factors eIF4E (57) and eIF5A (56) were substantially reduced from 12 to 24 h following infection with AcMNPV. In contrast, a dramatic increase in TBP was observed in AcMNPV-infected TN-368 and Sf21 cells (50); those authors also showed that TBP localized to viral DNA replication centers at the late stage of infection, suggesting a role for TBP during late viral transcription. In the present study, we revealed that the inhibitors of ERK kinase and JNK significantly reduced the expression of delayed-early, late, and very late gene products (Fig. 4). This indicates that the activities of ERK and JNK during BmNPV infection are required for the efficient expression of these viral gene products. We further observed that the protein levels of ERK and JNK remained unchanged until 24 hpi in BmNPV-infected BmN cells (Fig. 5). Collectively, these results suggest that host proteins that play important roles in baculovirus infection are selectively increased or escape from the global shutoff, even at the late stage of infection.
In summary, we have shown that the activation of ERK- and JNK-dependent signaling pathways is required for efficient infection by BmNPV. We are currently searching for other molecules that could be activated by BmNPV infection to further delineate the cellular signaling pathways involved in baculovirus infection. By discovering the host signaling cascades that baculoviruses utilize during infection, we may elucidate the mechanism by which baculoviruses can trigger a global shutoff of host protein synthesis and gene expression in host cells.
Acknowledgments
We thank Eric B. Carstens, WonKyung Kang, and Masao Nagata for providing antibodies; Ian Smith for critical reading of the manuscript; Shinpei Kawaoka for preparing dsRNAs; and Naoko Omuro for DNA sequencing. The cDNA clones used in this study were supplied by the National Bioresource Project (Silkworm).
This work was supported by grants from MEXT (grant 17018007 to T.S. and grant 19688004 to S.K.), MAFF-NIAS (Insect Genome Program), and JST (Professional Program for Agricultural Bioinformatics), Japan.
Footnotes
Published ahead of print on 3 October 2007.
REFERENCES
- 1.Ashwell, J. D. 2006. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat. Rev. Immunol. 6:532-540. [DOI] [PubMed] [Google Scholar]
- 2.Cai, Y., Y. Liu, and X. Zhang. 2007. Suppression of coronavirus replication by inhibition of the MEK signaling pathway. J. Virol. 81:446-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charlton, C. A., and L. E. Volkman. 1993. Penetration of Autographa californica nuclear polyhedrosis virus nucleocapsids into IPLB Sf 21 cells induces actin cable formation. Virology 197:245-254. [DOI] [PubMed] [Google Scholar]
- 4.Chen, T., D. Sahri, and E. B. Carstens. 2004. Characterization of the interaction between P143 and LEF-3 from two different baculovirus species: Choristoneura fumiferana nucleopolyhedrovirus LEF-3 can complement Autographa californica nucleopolyhedrovirus LEF-3 in supporting DNA replication. J. Virol. 78:329-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daimon, T., S. Katsuma, and T. Shimada. 2007. Mutational analysis of active site residues of chitinase from Bombyx mori nucleopolyhedrovirus. Virus Res. 124:168-175. [DOI] [PubMed] [Google Scholar]
- 6.de Magalhaes, J. C., A. A. Andrade, P. N. Silva, L. P. Sousa, C. Ropert, P. C. Ferreira, E. G. Kroon, R. T. Gazzinelli, and C. A. Bonjardim. 2001. A mitogenic signal triggered at an early stage of vaccinia virus infection: implication of MEK/ERK and protein kinase A in virus multiplication. J. Biol. Chem. 276:38353-38360. [DOI] [PubMed] [Google Scholar]
- 7.Detvisitsakun, C., M. F. Berretta, C. Lehiy, and A. L. Passarelli. 2005. Stimulation of cell motility by a viral fibroblast growth factor homolog: proposal for a role in viral pathogenesis. Virology 336:308-317. [DOI] [PubMed] [Google Scholar]
- 8.Detvisitsakun, C., E. L. Hutfless, M. F. Berretta, and A. L. Passarelli. 2006. Analysis of a baculovirus lacking a functional viral fibroblast growth factor homolog. Virology 346:258-265. [DOI] [PubMed] [Google Scholar]
- 9.Eliopoulos, A. G., and L. S. Young. 1988. Activation of c-Jun N-terminal kinase (JNK) by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP-1). Oncogene 16:1731-1742. [DOI] [PubMed] [Google Scholar]
- 10.Engelhard, E. K., L. N. Kam-Morgan, J. O. Washburn, and L. E. Volkman. 1994. The insect tracheal system: a conduit for the systemic spread of Autographa californica M nuclear polyhedrosis virus. Proc. Natl. Acad. Sci. USA 91:3224-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan, X., K. Thirunavukkarasu, and R. F. Weaver. 1996. Temperature-sensitive mutations in the protein kinase-1 (pk-1) gene of the Autographa californica nuclear polyhedrosis virus that block very late gene expression. Virology 224:1-9. [DOI] [PubMed] [Google Scholar]
- 12.Fujiwara, Y., C. Shindome, M. Takeda, and K. Shiomi. 2006. The roles of ERK and P38 MAPK signaling cascades on embryonic diapause initiation and termination of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 36:47-53. [DOI] [PubMed] [Google Scholar]
- 13.Gao, X., H. Wang, and T. Sairenji. 2004. Inhibition of Epstein-Barr virus (EBV) reactivation by short interfering RNAs targeting p38 mitogen-activated protein kinase or c-Myc in EBV-positive epithelial cells. J. Virol. 78:11798-11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomi, S., K. Majima, and S. Maeda. 1999. Sequence analysis of the genome of Bombyx mori nucleopolyhedrovirus. J. Gen. Virol. 80:1323-1337. [DOI] [PubMed] [Google Scholar]
- 15.Gomi, S., S. G. Kamita, and S. Maeda. 1999. Deletion analysis of all genes of Bombyx mori nucleopolyhedrovirus (BmNPV). RIKEN Rev. 22:39-41. [Google Scholar]
- 16.Han, Z., D. L. Boyle, K. R. Aupperle, B. Bennett, A. M. Manning, and G. S. Firestein. 1999. Jun N-terminal kinase in rheumatoid arthritis. J. Pharmacol. Exp. Ther. 291:124-130. [PubMed] [Google Scholar]
- 17.Hong, H. K., S. D. Woo, J. Y. Choi, H. K. Lee, M. H. Kim, Y. H. Je, and S. K. Kang. 2000. Characterization of four isolates of Bombyx mori nucleopolyhedrovirus. Arch. Virol. 145:2351-2361. [DOI] [PubMed] [Google Scholar]
- 18.Huttunen, P., T. Hyypia, P. Vihinen, L. Nissinen, and J. Heino. 1998. Echovirus 1 infection induces both the stress- and growth-activated mitogen-activated protein kinase pathways and regulates the transcription of cellular immediate-early genes. Virology 250:85-93. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda, M., and M. Kobayashi. 1999. Cell-cycle perturbation in Sf9 cells infected with Autographa californica nucleopolyhedrovirus. Virology 258:176-188. [DOI] [PubMed] [Google Scholar]
- 20.Iordanov, M. S., J. M. Paranjape, A. Zhou, J. Wong, B. R. G. Williams, E. F. Meurs, R. H. Silverman, and B. E. Magun. 2000. Activation of p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase by double-stranded RNA and encephalomyocarditis virus: involvement of RNase L, protein kinase R and alternative pathways. Mol. Cell. Biol. 20:617-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito, E., D. Sahri, R. Knippers, and E. B. Carstens. 2004. Baculovirus proteins IE-1, LEF-3, and P143 interact with DNA in vivo: a formaldehyde cross-linking study. Virology 329:337-347. [DOI] [PubMed] [Google Scholar]
- 22.Jacque, J. M., A. Mann, H. Enslen, N. Sharuva, B. Brichacek, R. J. Davis, and M. Stevenson. 1998. Modulation of HIV-1 infectivity by MAPK, a virion associated kinase. EMBO J. 17:2607-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson, G. L., and R. Lapadat. 2002. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911-1912. [DOI] [PubMed] [Google Scholar]
- 24.Jung, J. U., and R. C. Desrosiers. 1995. Association of viral oncoprotein, STP-C488, with cellular ras. Mol. Cell. Biol. 15:6506-6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang, W., M. Suzuki, E. Zemskov, K. Okano, and S. Maeda. 1999. Characterization of baculovirus repeated open reading frames (bro) in Bombyx mori nucleopolyhedrovirus. J. Virol. 73:10339-10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katsuma, S., Y. Noguchi, C. L. Zhou, M. Kobayashi, and S. Maeda. 1999. Characterization of the 25K FP gene of the baculovirus Bombyx mori nucleopolyhedrovirus: implications for post-mortem host degradation. J. Gen. Virol. 80:783-791. [DOI] [PubMed] [Google Scholar]
- 27.Katsuma, S., T. Shimada, and M. Kobayashi. 2004. Characterization of the baculovirus Bombyx mori nucleopolyhedrovirus gene homologous to the mammalian FGF gene family. Virus Genes 29:211-217. [DOI] [PubMed] [Google Scholar]
- 28.Katsuma, S., N. Hatae, T. Yano, Y. Ruike, M. Kimura, A. Hirasawa, and G. Tsujimoto. 2005. Free fatty acids inhibit serum deprivation-induced apoptosis through GPR120 in a murine enteroendocrine cell line STC-1. J. Biol. Chem. 280:19507-19515. [DOI] [PubMed] [Google Scholar]
- 29.Katsuma, S., S. Tanaka, N. Omuro, L. Takabuchi, T. Daimon, S. Imanishi, S. Yamashita, M. Iwanaga, K. Mita, S. Maeda, M. Kobayashi, and T. Shimada. 2005. Novel macula-like virus identified in Bombyx mori cultured cells. J. Virol. 79:5577-5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katsuma, S., T. Daimon, K. Mita, and T. Shimada. 2006. Lepidopteran ortholog of Drosophila Breathless is a receptor for the baculovirus fibroblast growth factor. J. Virol. 80:5474-5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katsuma, S., S. Horie, T. Daimon, M. Iwanaga, and T. Shimada. 2006. In vivo and in vitro analyses of a Bombyx mori nucleopolyhedrovirus mutant lacking functional vfgf. Virology 355:62-70. [DOI] [PubMed] [Google Scholar]
- 32.Katsuma, S., T. Daimon, S. Horie, M. Kobayashi, and T. Shimada. 2006. N-linked glycans of Bombyx mori nucleopolyhedrovirus fibroblast growth factor are crucial for its secretion. Biochem. Biophys. Res. Commun. 350:1069-1075. [DOI] [PubMed] [Google Scholar]
- 33.Keddie, B. A., G. W. Aponte, and L. E. Volkman. 1989. The pathway of infection of Autographa californica nuclear polyhedrosis virus in an insect host. Science 243:1728-1730. [DOI] [PubMed] [Google Scholar]
- 34.King, C. S., J. V. Cooper, B. Moss, and D. R. Twardzik. 1986. Vaccinia virus growth factor stimulates tyrosine protein kinase activity of A431 cell epidermal growth factor receptors. Mol. Cell. Biol. 6:332-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kouhara, H., Y. R. Hadari, T. Spivak-Kroizman, J. Schilling, D. Bar-Sagi, I. Lax, and J. Schlessinger. 1997. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 89:693-702. [DOI] [PubMed] [Google Scholar]
- 36.Ludwig, S., C. Ehrhardt, E. R. Neumeier, M. Kracht, U. R. Rapp, and S. Pleschka. 2001. Influenza virus-induced AP-1 dependent gene expression requires activation of the Jun-N-terminal kinase (JNK) signaling pathway. J. Biol. Chem. 276:10990-10998. [PubMed] [Google Scholar]
- 37.Ludwig, S., O. Planz, S. Pleschka, and T. Wolff. 2003. Influenza-virus-induced signaling cascades: targets for antiviral therapy? Trends Mol. Med. 9:46-52. [DOI] [PubMed] [Google Scholar]
- 38.Luo, H., B. Yanagawa, J. Zhang, Z. Luo, M. Zhang, M. Esfandiarei, C. Carthy, J. E. Wilson, D. Yang, and B. M. McManus. 2002. Coxsackievirus B3 replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. J. Virol. 76:3365-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin, P., W. C. Vass, J. T. Schiller, D. R. Lowry, and T. J. Velu. 1989. The bovine papilloma virus E5 transforming protein can stimulate the transforming activity of EGF and CSF-1 receptors. Cell 59:21-32. [DOI] [PubMed] [Google Scholar]
- 40.McLean, T. I., and S. L. Bachenheimer. 1999. Activation of cJun N-terminal kinase by herpes simplex virus type 1 enhances viral replication. J. Virol. 73:8415-8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mita, K., M. Morimyo, K. Okano, Y. Koike, J. Nohata, H. Kawasaki, K. Kadono-Okuda, K. Yamamoto, M. G. Suzuki, T. Shimada, M. R. Goldsmith, and S. Maeda. 2003. The construction of an EST database for Bombyx mori and its application. Proc. Natl. Acad. Sci. USA 100:14121-14126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nobiron, I., D. R. O'Reilly, and J. A. Olszewski. 2003. Autographa californica nucleopolyhedrovirus infection of Spodoptera frugiperda cells: a global analysis of host gene regulation during infection, using a differential display approach. J. Gen. Virol. 84:3029-3039. [DOI] [PubMed] [Google Scholar]
- 43.Okano, K., V. S. Mikhailov, and S. Maeda. 1999. Colocalization of baculovirus IE-1 and two DNA-binding proteins, DBP and LEF-3, to viral replication factories. J. Virol. 73:110-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okano, K., T. Shimada, K. Mita, and S. Maeda. 2001. Comparative expressed-sequence-tag analysis of differential gene expression profiles in BmNPV-infected BmN cells. Virology 282:348-356. [DOI] [PubMed] [Google Scholar]
- 45.O'Neill, E. M., I. Rebay, R. Tjian, and G. M. Rubin. 1994. The activities of two Ets-related transcription factors required for Drosophila eye development are modulated by the Ras/MAPK pathway. Cell 78:137-147. [DOI] [PubMed] [Google Scholar]
- 46.Ooi, B. G., and L. K. Miller. 1988. Regulation of host RNA levels during baculovirus infection. Virology 166:515-523. [DOI] [PubMed] [Google Scholar]
- 47.Panteva, M., H. Korkaya, and S. Jameel. 2003. Hepatitis viruses and the MAPK pathway: is this a survival strategy? Virus Res. 92:131-140. [DOI] [PubMed] [Google Scholar]
- 48.Popik, W., and P. M. Pitha. 1998. Early activation of mitogen-activated protein kinase kinase, extracellular signal-regulated kinase, p38 mitogen-activated protein kinase, and c-Jun N-terminal kinase in response to binding of simian immunodeficiency virus to Jurkat T cells expressing CCR5 receptor. Virology 252:210-217. [DOI] [PubMed] [Google Scholar]
- 49.Powers, C. J., S. W. McLeskey, and A. Wellstein. 2000. Fibroblast growth factors, their receptors and signaling. Endocr. Relat. Cancer 7:165-197. [DOI] [PubMed] [Google Scholar]
- 50.Quadt, I., D. Mainz, R. Mans, A. Kremer, and D. Knebel-Mörsdorf. 2002. Baculovirus infection raises the level of TATA-binding protein that colocalizes with viral DNA replication sites. J. Virol. 76:11123-11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reilly, L. M., and L. A. Guarino. 1994. The pk-1 gene of Autographa californica multinucleocapsid nuclear polyhedrosis virus encodes a protein kinase. J. Gen. Virol. 75:2999-3006. [DOI] [PubMed] [Google Scholar]
- 52.Roberts, P. J., and C. J. Der. 2007. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26:3291-3310. [DOI] [PubMed] [Google Scholar]
- 53.See, B. H., and Y. Shi. 1998. Adenovirus E1B 19,000-molecular-weight protein activates c-Jun N-terminal kinase and c-Jun-mediated transcription. Mol. Cell. Biol. 18:4012-4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shimada, T., I. C. Chan, Y. Noguchi, M. Nagata, and M. Kobayashi. 1994. Structural and functional abnormality of the polyhedrin gene in a polyhedron-deficient mutant of the Bombyx mori nuclear polyhedrosis virus. J. Ser. Sci. Jpn. 63:353-360. [Google Scholar]
- 55.Tyler, K. L., P. Clarke, R. L. DeBiasi, D. Kominsky, and G. J. Poggioli. 2001. Reoviruses and the host cell. Trends Microbiol. 9:560-564. [DOI] [PubMed] [Google Scholar]
- 56.Van Oers, M. M., M. Van Marwijk, M. S. Kwa, J. M. Vlak, and A. A. Thomas. 1999. Cloning and analysis of cDNAs encoding the hypusine-containing protein eIF5A of two lepidopteran insect species. Insect. Mol. Biol. 8:531-538. [DOI] [PubMed] [Google Scholar]
- 57.Van Oers, M. M., L. T. Van der Veken, J. M. Vlak, and A. A. Thomas. 2001. Effect of baculovirus infection on the mRNA and protein levels of the Spodoptera frugiperda eukaryotic initiation factor 4E. Insect Mol. Biol. 10:255-264. [DOI] [PubMed] [Google Scholar]
- 58.Zachos, G., B. Clements, and J. Conner. 1999. Herpes simplex virus type 1 infection stimulates p38/c-Jun N-terminal mitogen-activated protein kinase pathways and activates transcription factor AP-1. J. Biol. Chem. 274:5097-5103. [DOI] [PubMed] [Google Scholar]
- 59.Zheng, Y., J. Li, D. L. Johnson, and J. H. Ou. 2003. Regulation of hepatitis B virus replication by the Ras-mitogen-activated protein kinase signaling pathway. J. Virol. 77:7707-7712. [DOI] [PMC free article] [PubMed] [Google Scholar]