Abstract
The biosynthesis and posttranslational processing of human metapneumovirus attachment G glycoprotein were investigated. After pulse-labeling, the G protein accumulated as three species with molecular weights of 45,000, 50,000, and 53,000 (45K, 50K, and 53K, respectively). N-Glycosidase digestion indicated that these forms represent the unglycosylated precursor and N-glycosylated intermediate products, respectively. After an appropriate chase, these three naive forms were further processed to a mature 97K form. The presence of O-linked sugars in mature G protein was confirmed by O-glycanase digestion and lectin-binding assay using Arachis hypogaea (peanut agglutinin), an O-glycan-specific lectin. In addition, in the O-glycosylation-deficient cell line (CHO ldlD cell), the G protein could not be processed to the mature form unless the exogenous Gal and GalNAc were supplemented, which provided added evidence supporting the O-linked glycosylation of G protein. The maturation of G was completely blocked by monensin but was partially sensitive to brefeldin A (BFA), suggesting the O-linked glycosylation of G initiated in the trans-Golgi compartment and terminated in the trans-Golgi network. Enzymatic deglycosylation analysis confirmed that the BFA-G was a partial mature form containing N-linked oligosaccharides and various amounts of O-linked carbohydrate side chains. The expression of G protein at the cell surface could be detected by indirect immunofluorescence staining assay. Furthermore, cell surface immunoprecipitation displayed an efficient intracellular transport of G protein.
Human metapneumovirus (HMPV) is an etiologic agent that causes a substantial proportion of lower respiratory tract infections in infants and young children and is second only to human respiratory syncytial virus (HRSV) as a cause of bronchiolitis in early childhood (see reference 32 and references therein). It has been tentatively assigned to the Paramyxoviridae family, subfamily Pneumovirinae, and the genus Metapneumovirus (65, 66). Although belonging to a different genus, HMPV shares many features with HRSV (Pneumovirus genus) with respect to the associated diseases (10, 21, 47, 68), susceptible populations (11, 42, 48, 49, 51, 70), and similar genome organization (5, 29, 65).
The HMPV genome encodes three transmembrane proteins including the fusion (F), the attachment (G), and the small hydrophobic (SH) proteins (5, 65). Similar to HRSV, the G protein of HMPV is the most divergent structural protein among isolates (1, 5, 50). The deduced amino acid sequence of the G protein contains a single hydrophobic region that is located near the N terminus (amino acids 30 to 53) and is thought to serve as both an uncleaved signal peptide and a membrane anchor (1, 5, 65). The C-terminal three-fourths of the molecule is thought to be extracellular. Such a type II membrane orientation is also shared by HRSV G protein (15, 45, 71). Sequence analysis also shows that HMPV G protein has a high content of serine and threonine residues (30 to 34%), which are potential acceptor sites for O-linked sugars (1, 5, 50). The G protein also contains a high level of proline residues (7 to 8.5%) (5) and three to six potential N-linked glycosylation sites (1). The excessive potential O-glycosylation sites, together with the high level of proline residues, suggests a heavily glycosylated mucin-like structure for the G protein. This structural feature is shared by the HRSV G protein as well (Fig. 1) and makes both of them distinct from the hemagglutinin-neuraminidase (HN) or hemagglutinin (H) glycoproteins of other paramyxoviruses (31, 45, 56, 71).
FIG. 1.
Primary structures of the G surface glycoproteins of HMPV strain CAN99-80 and HRSV strain A2. CT, cytoplasmic tail (hatched box); TM, transmembrane anchor (black box); ED, extracellular domain (open box); His, eight histidine residues attached to the C terminus of HMPV G protein (dotted box).Upward-facing arrows identify the potential acceptor sites for N-linked carbohydrate. For each G protein, the 26 potential acceptor sites for O-linked sugars predicted to be the most likely to be used are indicated as downward-facing bars.
The function of HMPV G is as yet unclear. Although it has been proposed to play a role as an attachment protein, the observation that a recombinant HMPV deficient in G protein is able to replicate both in vitro and in vivo implies that G protein might not be essential for HMPV replication (3, 7). For HRSV, both G and F protein appear to be protective antigens. Immunization of BALB/c mice with recombinant vaccinia viruses that each express an individual HRSV protein shows that proteins F and G are the only proteins that induce HRSV-neutralizing antibodies and long-lived resistance to challenge HRSV replication (18). It seems likely that the HMPV G might also be capable of stimulating a protective immunity. However, in a recent report it was shown that the G protein of HMPV is only weakly immunogenic in hamsters and might not be a protective antigen (60). Whether or not a protective immunity can be elicited by HMPV G protein requires further study. Particularly, it requires confirmation in a nonhuman primate that more closely models the natural human host (13).
Thus far, numerous reports have demonstrated that the propagation of HMPV in vitro was very difficult (6, 8, 27, 67). Its fastidious replication in cell culture, dependence on trypsin, and restricted numbers of permissive cell lines have posed challenges to research (4, 8). To date, there are no data available yet regarding the biosynthesis of the HMPV G protein. Although a highly glycosylated protein with a mucinous origin has been proposed for G (1, 5, 50), there is no experimental evidence to confirm these predictions. Because of the potential functional importance in the protection against HMPV infections, knowledge of the structure of this glycoprotein is essential. Using a transient-expression method, we analyzed here the expression, glycosylation, intracellular transport, and cell surface expression of G protein. This is the first report that provides proof authenticating previous predictions concerning the structural features of G protein that were made solely on the basis of sequence analysis.
MATERIALS AND METHODS
Cells.
LLC-MK2 cells (CCL7; American Type Culture Collection, Manassas, VA) were maintained as a monolayer culture in Opti-MEM (Invitrogen, Burlington, Ontario, Canada). CHO-K1 (CCL61; American Type Culture Collection) and CHO ldlD cells were grown in Ham F-12 nutrient mixture (Invitrogen). Both media were supplemented with 5% (vol/vol) heat-inactivated fetal bovine serum. The CHO ldlD cells were kindly provided by Monty Krieger (Massachusetts Institute of Technology, Cambridge, MA). All cells were maintained at 37°C in an atmosphere of 5% CO2.
Antibodies.
A rabbit antiserum to peptide sequence corresponding to amino acid residues 148 to 161 of HMPV G protein was generated by Alpha Diagnostic (San Antonio, TX). Anti-His antibody and fluorescein isothiocyanate (FITC)-conjugated anti-mouse antibody were purchased from QIAGEN (Mississauga, Ontario, Canada) and Pierce (Nepean, Ontario, Canada), respectively.
Plasmid construction.
The coding sequence for the G gene of HMPV isolate CAN99-80 (GenBank accession no. AY574247) was amplified by PCR and extended at the C terminus by eight histidine residues using pBacPAK8-CAN99-80G as a template (1). The primer sequences are available upon request. The amplicon was cloned into pTriEX1 vector (Novagen, Madison, WI) and designated pTriEX1-GHis. The sequence of this construct was substantiated by DNA sequencing.
Transfection of cells.
LLC-MK2, CHO-K1, or ldlD cells were seeded on six-well plates at a density of 2.5 × 105/well and grown at 37°C for 24 h. Prior to transfection, 1 μg of DNA, 3 μl of Gene Juice (Novagen), and 100 μl of Opti-MEM/well were mixed thoroughly, followed by incubation for 15 min at room temperature. Subsequently, the transfection mixture was added to the cell monolayer. Transfected cells were maintained at 37°C in an atmosphere of 5% CO2 for 24 h.
Metabolic labeling and immunoprecipitation.
Radioisotopic labeling of transfected cells and immunoprecipitation were performed as described previously (40). Briefly, at 24 h posttransfection, cells were placed in methionine-free minimal essential medium (MP Biomedicals, Solon, OH) for 45 min, after which they were pulse-labeled for various periods (as specified in each figure legend) with 300 μCi of [35S]methionine (MP Biomedicals)/ml. For pulse-chase experiments, the labeling medium was removed after an appropriate pulse and replaced with complete minimal essential medium containing 5% fetal bovine serum. At various intervals, cells were lysed with 1% Nonidet P-40 in 150 mM NaCl-50 mM Tris-HCl-1 mM phenylmethylsulfonyl fluoride, incubated on ice for 15 min, and centrifuged for 5 min. The cell extracts were incubated for 2 h at 4°C with anti-His antibody, after which 10% protein A-Sepharose (Amersham, Baie d'Urfe, Quebec, Canada) was added for 1 h. For cell surface immunoprecipitation, after proper pulse and chase, the cells were first incubated with antibody for 2 h at 4°C and then subjected to extensive washing, followed by cell lysis and incubation with protein A-Sepharose as described above. The precipitated immune complex was washed three times with radioimmunoprecipitation assay buffer consisting of 50 mM Tris-HCl, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate (SDS), 1% sodium deoxycholate, 150 mM NaCl, and 1 mM EDTA. Samples were solubilized by boiling for 5 min in SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer containing 5% β-mercaptoethanol and 2% SDS. Immunoprecipitated G protein was electrophoresed on an SDS-10% polyacrylamide gel, followed by autoradiography.
Glycosidase digestion.
Overnight digestion at 37°C with various glycosidases (PROzyme, San Leandro, CA) was performed as follows. Immunoprecipitated proteins were eluted from protein A-Sepharose beads by boiling them for 5 min in commercial denaturing buffer (PROzyme). Enzymes and appropriate buffer were added exactly according to the protocol provided by the manufacturer.
Lectin-binding assay.
A lectin-binding study was carried out as described previously (16). Briefly, cell lysates were mixed for 1 h at 4°C with a one-tenth volume of 4% agarose beads conjugated to Arachis hypogaea lectin (Sigma, Oakville, Ontario, Canada), which binds to galactose (1-3)-N-acetylgalactosamine (Gal-GalNAc), characteristic sugar components of mucin-type O-linked glycoproteins (12, 38). After a washing step, glycoproteins bound to lectin were eluted in radioimmunoprecipitation assay buffer by heating at 95°C for 5 min. Subsequently, the eluted proteins were subjected to immunoprecipitation.
Metabolic inhibitors.
Monensin and brefeldin A (BFA) were purchased from Sigma. Both drugs were added 45 min prior to labeling, and the drugs were maintained during labeling and chase incubation (16, 17). The working concentrations of monensin and BFA were 20 μM and 5 μg/ml, respectively (16, 17).
Immunoblotting.
Cell lysates prepared from pTriEX1-GHis- or pTriEX1-transfected CHO-K1 or CHO ldlD cells were resolved by SDS-10% PAGE and transferred onto polyvinylidene difluoride membrane sheets (Bio-Rad, Mississauga, Ontario, Canada). Anti-His antibody diluted 1:1,000 was used to probe the G protein. After incubation with horseradish peroxidase-conjugated anti-mouse antibody, the immunoblots were visualized by using enhanced chemiluminescent substrate (Pierce, Ottawa, Ontario, Canada).
Immunofluorescence microscopy.
LLC-MK2 cells were grown to 80% confluence on a 24-well plate and transfected with pTriEX-GHis as described above. At 48 h posttransfection, cells were fixed with 4% paraformaldehyde for 10 min. The fixed cells were incubated with anti-His antibody for 1 h, followed by incubation with FITC-conjugated anti-mouse antibody for 1 h. For intracellular staining, before primary antibody incubation, the cells were first permeabilized with 0.1% Triton X-100 for 15 min. Fluorescence microscopy was done with a Zeiss Axiovert microscope.
RESULTS
Expression of G protein.
The replication of HMPV in vitro was extremely inefficient (6, 8, 28, 67). The time required for viral propagation varied from 10 to 14 days (27, 66). Consistent with this slow replication feature, in a preliminary time course immunoblotting assay the optimum expression of G in the virus-infected cells was not observed until 8 days postinfection (Fig. 2A and B). As shown in Fig. 2A, under reducing conditions, a rabbit antiserum raised against the peptide sequence of G148-161 effectively recognized three forms of G protein, with approximate molecular weights of 40,000, 45,000, and 48,000 (40K, 45K, and 48K, respectively; lanes 3 and 4). In contrast, under nonreducing conditions, an additional form between 80 and 90K could also be detected (Fig. 2B, lane 4). Our result was in accordance with previous reports, in which the molecular weight of the virion-associated mature G protein has been reported to be about 80,000 to 100,000, regardless of the reducing or nonreducing conditions (7, 8, 60). By analogy to RSV G, the 80 to 100K form might represent a fully processed mature product, whereas the smaller forms could be the premature species in the maturation of G glycoprotein (16). Surprisingly, when the peptide antiserum was used to react with the viral G protein in an immunoprecipitation assay, it was unable to recognize the mature form of G (data not shown). This observation might be associated with possible conformational changes between the native protein in the immunoprecipitation assay and the denatured protein in the immunoblotting test. In any case, the slow replication character of HMPV and the conformational dependence of the peptide antiserum deeply hampered our in-depth characterization of the biosynthesis of protein G. Therefore, to express a recombinant G protein containing a readily detectable epitope and out of the context of viral infection appeared to be a reasonable alternative choice.
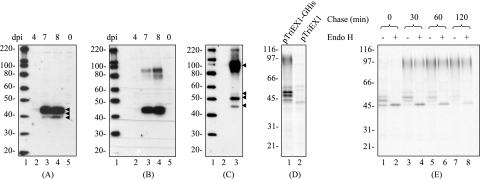
FIG. 2.
Expression of G protein. (A and B) LLC-MK2 cells were infected with (lanes 2 to 4) or without (lane 5) HMPV isolate CAN99-80. At 4, 7, and 8 days postinfection (dpi; lanes 2, 3, and 4), the cell lysates were prepared under reducing (A) and nonreducing (B) conditions and analyzed by immunoblotting assay with rabbit antiserum raised against peptide sequence of G148-161. Lane 1 shows a protein marker (Invitrogen), for which the sizes are shown in kilodaltons (indicated on the left). (C) pTriEX1 (lane 2)- or pTriEX1-GHis (lane 3)-transfected LLC-MK2 cells were analyzed by immunoblotting assay using the peptide antiserum under nonreducing conditions. Lane 1 shows a protein marker (Invitrogen), for which the sizes are shown in kilodaltons (indicated on the left). (D) pTriEX1-GHis (lane 1)- or pTriEX1 (lane 2)-transfected LLC-MK2 cells were pulse-labeled with [35S]methionine at 300 μCi/ml for 2 h. Labeled proteins were immunoprecipitated with anti-His antibody. Recovered proteins were resolved by gel electrophoresis, followed by autoradiography. Molecular size standards are indicated on the left. (E) pTriEX1-GHis-transfected cells were pulse-labeled with [35S]methionine at 300 μCi/ml for 20 min and chased for various times as indicated on the top. Cell lysates were immunoprecipitated with the anti-His antibody. Recovered proteins were treated with (+) or without (−) Endo H and resolved by gel electrophoresis, followed by autoradiography. Molecular size standards are indicated on the left.
To do this, LLC-MK2 cells were transfected with pTriEX1-GHis, which encodes a G protein containing eight histidine residues at the C terminus (Fig. 1). The synthesized G protein was first analyzed by immunoblotting assay using the peptide antiserum under nonreducing conditions (Fig. 2C, lane 3). The recombinant G protein accumulated as three discrete bands and one broad band (45, 50, 53, and 100K). Subsequently, the expression of the recombinant G protein was also analyzed by radioimmunoprecipitation assay using anti-His antibody, which confirmed the production of four major forms of G protein (45K, 50K, 53K, and 97K) (Fig. 2D, lane 1). There was one extra band that migrated slightly slower than 53K, whose signal strength was relatively weak. This band may represent a nonspecific cellular protein because it was also detectable in empty vector-transfected cells (Fig. 2D, lane 2). Obviously, the electrophoresis patterns of the recombinant and viral G protein were similar, except that the recombinant G proteins migrated slightly slower than their counterparts produced in the virus-infected cells. Such a minor variation was likely due to the presence of the His tags on the recombinant proteins that marginally retarded their electrophoresis mobility. It is evident that the recombinant G protein was comparable to the authentic viral protein. In view of its expeditious expression and the great efficacy of the anti-His antibody independent of protein conformation, the pTriEX1-GHis construct was preferentially used in the following experiments.
To examine the kinetic of G protein synthesis, a pulse-chase analysis was performed in conjunction with endo-β-N-acetylglucosaminidase H (Endo H) digestion. After 20 min of pulse-labeling, intracellular G proteins were first detected as three bands: 45, 50, and 53K (Fig. 2E, lane 1). With time of chase, these bands gradually disappeared coincidently with the appearance of the 97K form (lanes 3, 5, and 7). After 2 h of chase, only a trace amount of the 53K form remained visible, and the 97K form became the predominant species (lane 7), suggesting a precursor-product relationship. The molecular sizes of the 50K and 53K forms could be reduced by Endo H digestion to 45K (lanes 2, 4, 6, and 8), indicating the presence of high-mannose type N-linked sugars. In contrast, the mature form of 97K was Endo H resistant, suggesting the intracellular transport of mature G from the endoplasmic reticulum (ER) to the Golgi apparatus (20, 34). The electrophoresis mobility of the 45K precursor was not influenced by Endo H treatment, suggesting that it was deficient in N-linked sugars. The deduced molecular size for the G-protein backbone was only 26K, which was considerably smaller than 45K form. However, because the 45K form was also resistant to O-glycanase digestion (see Fig. 3), it is unlikely that the difference is caused by O glycosylation. Furthermore, when G protein was produced in the rabbit reticulocyte lysates, a cell-free system lacking the activity of posttranslational modifications, the molecular weight of the in vitro-synthesized G was also about 45,000 (data not shown). Therefore, the 45K species was indeed a product representing G-protein backbone and was not a result of O-linked glycosylation or other unidentified modifications. The reason for the slower mobility of this form than that predicted was unclear. We suggested that it might be caused by aberrant migration under electrophoresis (72).
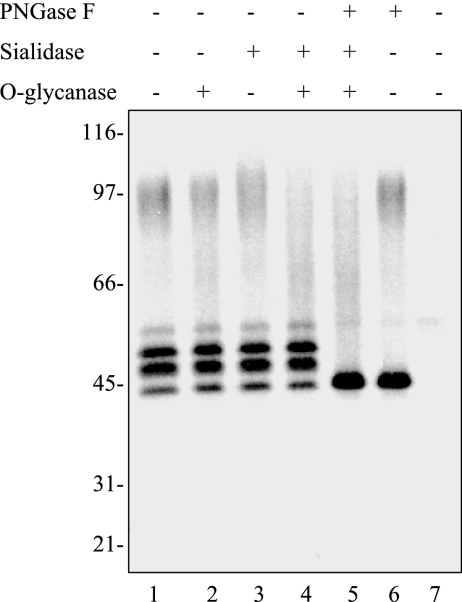
FIG. 3.
Enzymatic deglycosylation of G protein. pTriEX1-GHis (lanes 1 to 6)- and pTriEX1 (lane 7)-transfected cells were pulse-labeled with [35S]methionine for 1 h, followed by immunoprecipitation. The recovered proteins were treated with (+) or without (−) various glycosidases as follows: lanes 1 and 7, mock digested; lane 2, O-glycanase; lane 3, sialidase; lane 4, O-glycanase plus sialidase; lane 5, O-glycanase plus sialidase and PNGase F; lane 6, PNGase F. Digested proteins were resolved by electrophoresis, followed by autoradiography. Molecular size standards are indicated on the left.
Deglycosylation analysis of protein G.
To further characterize the glycosylation of G protein, labeled proteins were subjected to various enzymatic deglycosylations. Treatment of O-glycanase had no effects on the electrophoresis mobilities of the three immature forms, a finding that was in agreement with the conclusion that these premature species were either N glycosylated or unglycosylated (Fig. 3, lane 2). The mature form of the G protein was also resistant to O-glycanase digestion (lane 2). However, this resistance might be due to the presence of sialic acid on the G molecule, which blocked the action of O-glycanase.
Indeed, like HRSV G protein (16), sialidase treatment converted the mature G protein into a somewhat more diffuse form with a slight increase in the apparent molecular weight (lane 3). It has been observed that the electrophoresis migration of a glycoprotein can be either accelerated or retarded after sialidase digestion (16, 19, 59). As reported previously, these alterations were signs of an interplay between effects on shape versus charge (16). The elimination of sialic acid would enable proteins to navigate the separation matrix faster; however, the removal of its negative charge would reduce the negative charge of the protein and thereby impede the migration of protein on the gels (16). In contrast to the mature G protein, three immature forms were not affected by sialidase digestion, indicating that they were not sialylated. This was consistent with the concept that sialic acid is found at the termini of completed N-linked and O-linked side chains and is not known to occupy the internal positions of sugar chains or to be a temporary component of growing chains (16).
Double digestion with sialidase and O-glycanase decreased the amount of the detectable mature G protein and generated a variety of smaller species that migrated dispersedly (lane 4). The absence of complete digestion of mature G was likely due to incomplete enzymatic removal of O-linked oligosaccharides (39). Triple digestion with sialidase, O-glycanase, and PNGase F further converted the heterogeneous mixture, as well as the N-glycosylated 53K and 50K forms into the unglycosylated 45K species with enhanced signal strength (lane 5). Single digestion with PNGase F converted the 53K and 50K forms to 45K (lane 6). The Mr reduction of mature G protein after PNGase F digestion was marginal, a finding which reflected that the relative mass contribution of N-linked sugars to the mature G protein is much less than that of the extensive O-linked oligosaccharides.
Lectin-binding assay of G.
To confirm the presence of O-linked oligosaccharides on G protein, radioisotope-labeled G protein was examined for its ability to bind with A. hypogaea (peanut agglutinin), a lectin specific for the Ser/Thr-linked mucin-type sugar chain (16, 46, 57). A. hypogaea binds Gal-GalNAc (16, 22) and has been used for the purification of O-linked glycoproteins. As shown in Fig. 4, only the mature form of G protein bound to A. hypogaea (lane 2), indicating the presence of Gal-GalNAc, a core structure for O-linked sugars. In contrast, the N-glycosylated intermediates and unglycosylated precursor did not bind to A. hypogaea, a finding that was consistent with the expected specificity.
FIG. 4.
Lectin-binding assay. pTriEX1-GHis (lanes 1 and 2)- or pTriEX1 (lane 3)-transfected cells were pulse-labeled with [35S]methionine at 300 μCi/ml for 2 h, and then cell lysates were prepared. In lane 1, the lysates were directly subjected to immunoprecipitation with anti-His antibody, whereas in lanes 2 and 3 the lysates were incubated with 4% agarose beads conjugated with A. hypogaea lectin for 1 h. Subsequently, recovered proteins were eluted from the lectin beads and subjected to immunoprecipitation. Samples were analyzed by electrophoresis, followed by autoradiography. Molecular size standards are indicated on the left.
Expression of protein G in CHO-K1 and CHO ldlD cells.
To strengthen our conclusion that the G protein is O glycosylated, we also compared the expression of protein G in CHO-K1 and CHO ldlD cell lines by using an immunoblotting assay. The hallmark of the ldlD cell that makes it distinct from the wild-type CHO-K1 cell is that it is deficient in the enzyme activity of UDP-galactose/UDP-N-acetylgalactosamine 4-epimerase (33, 38). Unlike the wild-type CHO cell the ldlD cells, under normal culture conditions in which glucose is the sole external source of monosaccharide, cannot convert the UDP-glucose and UDP-N-acetylglucosamine to UDP-Gal and UDP-GalNAc, which are required to add galactose and N-acetylgalactosamine to N-linked and O-linked oligosaccharides on glycoproteins (33, 38). Galactose is required for the completion of both N- and O-linked chains, whereas N-acetylgalactosamine is only added to O-linked glycans and is always the first sugar linked to serine or threonine residue. Therefore, in ldlD cells O-linked glycosylation is deficient and N-linked glycosylation is incomplete (33, 38, 72). On the other hand, however, all of the structural and functional defects of the ldlD mutants can be reversed by providing the cells with alternative sources of the aforementioned two sugars, namely, galactose and N-acetylgalactosamine, which are the normal products of the epimerase enzyme (33, 38, 72).
When pTriEX1-GHis was expressed in wild-type CHO cells, the detected G protein could be divided into two size groups (Fig. 5, lane 2). The fast-migrating group ranging from 48K to 55K represented the premature forms, including unglycosylated precursor and N-glycosylated intermediates. The slowly migrating group consisted of a broad 97K band representing the fully processed mature form (lane 2). An additional band running between the mature and the premature forms might represent a nonspecific cellular protein because it was also detectable in empty vector-transfected cells (lanes 7 and 8). In contrast, the synthesis of the mature form of G protein was abolished in the O-glycosylation-deficient ldlD cells (lane 3), indicating that the mature G protein was indeed O glycosylated. The generation of the N-glycosylated premature forms in wild-type CHO-K1 and ldlD cells could be further inhibited by tunicamycin treatment, resulting in the production of unglycosylated G protein (data not shown). Subsequently, the synthesis of G protein in ldlD cells under various conditions with respect to sugar addition was further investigated. When galactose was added (lane 4), the Mr of N-glycosylated forms was slightly increased, a result probably due to the addition of galactose to the sugar chains. When N-acetylgalactosamine was added, conditions where incomplete O-linked and N-linked glycosylation were permitted, a heterodisperse population between 48 and 70K was generated (lane 5). Notably, when both galactose and N-acetylgalactosamine were added to restore the complete O-linked and N-linked glycosylation, the mature form of 97K protein emerged as expected (lane 6).
FIG. 5.
Expression of G in CHO-K1 and CHO ldlD cells. pTriEX1-GHis (lanes 2 to 6)- or pTriEX1 (lanes 7 and 8)-transfected CHO-K1 (lanes 2 and 7) or CHO ldlD cells (lanes 3 to 6 and lane 8) were grown in Ham F-12 medium supplemented with (+) or without (−) exogenous galactose (25 mM) and N-acetylgalactosamine (400 μM) as indicated at the top. Cell lysates were prepared and analyzed by immunoblotting. Lane 1 shows a protein marker (Invitrogen) for which the sizes are shown in kilodaltons (indicated on the left).
Altered O glycosylation of protein G in the presence of monensin and BFA.
In general, O-linked glycosylation is mainly a posttranslational and postfolding event that does not occur until the protein reaches the Golgi complex and has already passed the ER quality control (2, 30, 69). Monensin, a polyether antibiotic, blocks transport between the medial and trans-Golgi compartments (24, 61) and can thereby indirectly inhibit the addition or elongation or both of O-linked sugars within the trans-Golgi compartment (16, 45, 54). It has been used to inhibit O-linked glycosylation of HRSV G and rubella virus E2 proteins, respectively (16, 45, 54). BFA, the fungal metabolite, blocks protein traffic from the ER to the Golgi complex (43, 44, 53). In addition, it induces a rapid and reversible disassembly of Golgi complexes (23, 63, 64) and concomitantly induces a relocation of the cis-, medial-, and at least some of the trans-Golgi markers and enzyme activities to the ER (16, 19, 41), whereas enzymes are not redistributed from the more distal trans-Golgi network (TGN) (14, 16, 25). Therefore, BFA interrupts posttranslational processing occurring in the TGN.
To further characterize and dissect the intracellular compartments for O-linked glycosylation of G protein, transfected cells were treated with monensin or BFA. After a 1-h pulse-labeling and a 1-h chase, in the absence of any drugs, the majority of newly synthesized G protein had been converted to the mature form (Fig. 6A, lane 1). Upon monensin treatment, the maturation of the 97K form was dramatically compromised, and the G protein accumulated as N-glycosylated intermediate products (lane 2). It was apparent that the generation of N-glycosylated intermediates took place in a cellular compartment before the trans-Golgi complex, a finding in agreement with the observation that they were Endo H sensitive. The data also showed that O glycosylation of the G protein does not occur in the ER or in cis or medial compartments of the Golgi complex (16). In contrast, in the presence of BFA all of the intermediate forms were efficiently processed into a heterogeneous species migrating between 55 and 80K (lane 3), implying that O glycosylation occurred in the presence of BFA but was incomplete (14). This indicated that O glycosylation of the G protein begins in the trans compartment of the Golgi complex.
FIG. 6.
Effect of monensin and BFA treatment on O-linked glycosylation of G protein. (A) pTriEX1-GHis (lanes 1 to 3)- and pTriEX1 (lane 4)-transfected cells were pulse-labeled with [35S]methionine for 1 h, followed by a 1-h chase and immunoprecipitation. The pulse-labeling and chase were performed with (+) or without (−) inhibitors (monensin or BFA) of exocytosis as indicated at the top. Samples were analyzed on SDS-10% PAGE, followed by autoradiography. Molecular size standards are indicated on the right. (B) Cells were transfected with pTriEX1-GHis (lane 1 to 6) or mock transfected with empty vector pTriEX1 (lane 7). Proteins were synthesized in the presence of BFA and labeled with [35S]methionine for 1 h, followed by a 1-h chase. The immunoprecipitated G proteins were treated with (+) or without (−) various glycosidases as follows: lanes 1 and 7, mock digested; lane 2, O-glycanase; lane 3, sialidase; lane 4, sialidase plus O-glycanase; lane 5, sialidase and O-glycanase plus PNGase F; lane 6, PNGase F. Enzyme-digested samples were resolved by electrophoresis, followed by autoradiography. Molecular size standards are indicated on the left.
Enzymatic deglycosylation of BFA-G.
To further depict the glycosylation of G protein synthesized in the presence of BFA, the BFA-G was subjected to various enzymatic digestions as indicated in Fig. 3. The electrophoresis mobility of BFA-G was not influenced by single digestion with O-glycanase (Fig. 6B, lane 1 versus lane 2), suggesting the potential existence of sialic acid may hinder the cleavage of O-linked sugars. This assumption appeared to be correct since the apparent molecular weight of BFA-G could be substantially reduced by sialidase digestion (lane 3). Unlike the mature G protein, after desialylation the migration of BFA-G became faster. It is likely that the BFA-G may contain a greater content of sialic acid than the mature G protein. The Mr of BFA-G could be further reduced by double digestion with sialidase and O-glycanase (lane 4), indicating the presence of O-linked sugars, which were readily accessed by O-glycanase with the facilitation of sialidase. Triple digestion with sialidase, O-glycanase, and PNGase F gave rise to a band with a smear tail. The leading edge of the band comigrated with the unglycosylated precursor (lane 5). Single digestion with PNGase F converted the BFA-G protein into two major forms: a discrete band corresponding to the unglycosylated precursor and a heterogeneous species containing O-linked sugars and sialic acids (lane 6). Taken together, the results suggested that the BFA-G was a partial mature form of protein G containing both N-linked and O-linked glycosylation.
Cell surface expression of protein G.
To determine the cell surface expression of protein G, pTriEX1-GHis-transfected LLC-MK2 cells were analyzed by indirect immunofluorescence assay with the anti-His antibody. The results showed abundant expression of the G protein both in the cytoplasm (Fig. 7Aa) and at the surface (Fig. 7Ac) of transfected cells. The fluorescent staining observed with the anti-His antibody was specific since no reactivity was detected in the cytoplasm or on the surface of cells transfected with empty vector (Fig. 7Ab and d). The result indicated that G protein was capable of traveling through the secretory pathway and was incorporated on the plasma membrane.
FIG. 7.
Surface expression of G protein. (A) pTriEX1-GHis (a and c)- or pTriEX1 (b and d)-transfected LLC-MK2 cells were fixed with 4% paraformaldehyde and treated with 0.1% Triton X-100 for internal (a and b) or not treated for surface (c and d) immunofluorescence staining assay using anti-His and FITC-conjugated anti-mouse antibodies. (B) pTriEX1-GHis (lanes 1 to 4)- or empty vector (lane 5)-transfected cells were pulse-labeled for 10 min and chased for various times as indicated at the top. Cells were subsequently reacted with anti-His antibody, followed by extensive washing and cell lysis. The antibody-antigen complex was precipitated by using protein A-Sepharose. Eluted proteins were analyzed by electrophoresis, followed by autoradiography. Molecular size standards are indicated on the left.
To determine the time course expression of protein G on plasma membrane, cell surface immunoprecipitation was performed after 10 min of labeling and an appropriate chase. The result showed that the G protein appeared at the cell surface exclusively in its fully processed mature form (Fig. 7B). The transit time required for G protein to reach the cell surface was about 20 min, which was comparable to that of RSV G protein (17). The glycosylation pattern of G protein present on plasma membrane was the same as that of the mature form located intracellularly (data not shown).
DISCUSSION
The aim of the experiments presented here was to characterize the biosynthesis, glycosylation, intracellular transport, and cell surface expression of HMPV G glycoprotein. Such a study has been greatly facilitated by the application of a recombinant G protein containing a His tag at the C terminus. In our experiments, four species of G protein could be detected: 45K, 50K, 53K, and 97K. Pulse-chase analysis and in vitro translation experiments (data not shown) indicated that the 45K form was an unglycosylated protein backbone, the 50K and 53K forms were premature naive forms during posttranslational processing, and the 97K species was the fully processed mature form. The size of the predicted backbone of protein G is considerably smaller than that estimated from SDS-PAGE. This result is consistent with a recent report in which the in vitro-translated protein G had a mass of 45 kilodaltons and was much bigger than that predicted (58). A similar discrepancy has also been reported for HRSV G protein (72). It has been proposed that a high proline content may account for the anomalous mobility of proteins on gels and that all mass determinations by gel electrophoresis of proteins with a high content of proline or carbohydrates are just approximations (72).
The 50K and 53K forms were solely modified by N-linked glycosylation since their sizes could be reduced to 45K by PNGase F but not by O-glycanase digestion. The apparent molecular weight of the mature G protein was about 97,000, which was consistent with former reports (7, 8, 60) and strongly suggested that this form also contains significant amount of O-linked sugars. This notion was supported by three lines of evidence. First, the mature 97K G protein bound to A. hypogaea, an O-glycan-specific lectin (16, 22, 46, 57). Second, the mature G protein could be converted to a heterodisperse species after enzymatic digestion of sialidase and O-glycanase. Complete conversion of the mature G protein to solely N-glycosylated species was not efficiently produced by the treatment described above. A possible explanation could be that blocked oligosaccharide cleavage sites or protein conformational constraints inhibited the complete access of O-glycanase to the O-linked oligosaccharides (39). Finally, the mature form of G protein was only detectable in wild-type CHO-K1 cells but not in CHO ldlD cells, a well-characterized cell line that is deficient in O-linked glycosylation (33, 38). Remarkably, the complete glycosylation of G protein in ldlD cells was rescued by the exogenous addition of Gal and GalNAc to the culture medium. These observations showed that the difference in the electrophoresis mobilities between the immature and mature forms of G protein was due to variable glycosylation and ruled out the possibility that the 97K form was a dimer of the immature forms.
The CHO ldlD cell line has provided a powerful tool for the study of the structure and function of O-linked glycoprotein. Historically, it has been used to characterize a variety of viral and nonviral glycoproteins (9, 12, 33, 35-38, 72). The absence of mature G protein in ldlD cells consolidated our data derived from LLC-MK2 cells and unequivocally demonstrated that HMPV G protein is O glycosylated. It appeared that more than half of the molecular weight of the mature G protein is contributed by O-linked sugars, which was a reminiscent of HRSV G (26, 56, 71). However, it is also likely that, as suggested for the HRSV G, the apparent high molecular weight of the mature HMPV G protein represents an artifact of reduced SDS binding due to the O-linked sugars, and thus the real molecular weight might be much lower than that estimated by SDS-PAGE (16).
The location of the subcellular compartment where O-linked glycosylation is initiated is still controversial and may be depend on the type of UDP-GalNAC and GalNAc transferase (GalNAc-T) (69). The location may range from subregions of the ER, a proximal Golgi compartment, to an intermediate ER-Golgi compartment (ERGIC), and beyond the ERGIC to more distal compartment(s) of the exocytic pathway (52, 55, 62). The maturation of G protein was severely hampered in the presence of monensin. In contrast, in the presence of BFA, although G protein was physically blocked in the ER, it could be partially O glycosylated. As mentioned above, BFA allows the redistribution of enzymes before the TGN into the ER and processing of the immobilized proteins efficiently (16, 19, 41). Therefore, the partial sensitivity of O glycosylation to BFA and the complete sensitivity to monensin indicated that both initiation and elongation of O-linked sugar chains begin in the trans-Golgi compartment, but the O glycosylation is not complete until the G protein reaches the TGN.
BFA-G was sensitive to the sialidase, indicating that sialylation took place before the TGN. In some studies, sialytransferase was not redistributed by BFA (14, 19). On the other hand, sialylation of BFA-arrested protein was reported by others (16, 64). As reported previously, this could be linked to the cell type-specific variability in the distribution of sialytransferase in the trans-Golgi cisternae or TGN (16). It turned out that the BFA-G contained more sialic acids than did the mature G protein. Such a phenomenon was also observed for HRSV G, for which the sialylation had a greater effect on BFA-G's electrophoresis mobility than on that of the mature G protein (16). It has been suggested that this difference might be caused by the redistribution of sialytransferase to the ER, which adds sialic acids excessively on the immobilized protein (16). After sialidase and O-glycanase digestion, the molecular weight shift of BFA-G was more apparent than that of the mature form. This might be related to the different conformational status between the partial and complete mature G protein. In the former status, the O-linked sugars might be well exposed and readily accessible for the O-glycanase.
Using the anti-His antibody, we could detect the cell surface expression of G protein. Considering that the His tag was attached at the extreme C-terminal end of the G protein, these data confirmed that the C terminus of the G protein was exposed on the exterior of the transfected cells, in agreement with a type II membrane orientation that has been proposed for the G protein (45). Cell surface immunoprecipitation demonstrated that after 20 min of chase the mature form of the G protein appeared at the cell membrane, indicating an efficient intracellular transport of G protein. This observation was quite similar to that of RSV G protein and provided additional proof supporting an earlier conclusion that the type II membrane orientation is not obligatorily associated with a reduced transit rate (17).
These results indicate that the biosynthesis and intracellular processing of the HMPV G protein are very similar to those of the HRSV G protein. Both of them were initially synthesized as N-glycosylated intermediate forms that were subsequently processed to mature form, which contained extensive O-linked carbohydrates. The O glycosylation of both G proteins occurred in the Golgi apparatus and terminated in the TGN. All of them had a type II membrane orientation and could be exported to the cell surface efficiently. It is reasonable to speculate that the similar structure features of HMPV G and HRSV G may define comparable functions for both proteins, which in turn may determine equivalent roles in viral pathogenesis. However, such a notion remains to be further clarified.
Acknowledgments
We are grateful to M. Krieger of the Massachusetts Institute of Technology (Cambridge, MA) for permission to use the CHO ldlD cell line.
Footnotes
Published ahead of print on 3 October 2007.
REFERENCES
- 1.Bastien, N., L. Liu, D. Ward, T. Taylor, and Y. Li. 2004. Genetic variability of the G glycoprotein gene of human metapneumovirus. J. Clin. Microbiol. 42:3532-3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benham, A. M., and I. Braakman. 2000. Glycoprotein folding in the endoplasmic reticulum. Crit. Rev. Biochem. Mol. Biol. 35:433-473. [DOI] [PubMed] [Google Scholar]
- 3.Biacchesi, S., Q. N. Pham, M. H. Skiadopoulos, B. R. Murphy, P. L. Collins, and U. J. Buchholz. 2005. Infection of nonhuman primates with recombinant human metapneumovirus lacking the SH, G, or M2-2 protein categorizes each as a nonessential accessory protein and identifies vaccine candidates. J. Virol. 79:12608-12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biacchesi, S., Q. N. Pham, M. H. Skiadopoulos, B. R. Murphy, P. L. Collins, and U. J. Buchholz. 2006. Modification of the trypsin-dependent cleavage activation site of the human metapneumovirus fusion protein to be trypsin independent does not increase replication or spread in rodents or nonhuman primates. J. Virol. 80:5798-5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biacchesi, S., M. H. Skiadopoulos, G. Boivin, C. T. Hanson, B. R. Murphy, P. L. Collins, and U. J. Buchholz. 2003. Genetic diversity between human metapneumovirus subgroups. Virology 315:1-9. [DOI] [PubMed] [Google Scholar]
- 6.Biacchesi, S., M. H. Skiadopoulos, K. C. Tran, B. R. Murphy, P. L. Collins, and U. J. Buchholz. 2004. Recovery of human metapneumovirus from cDNA: optimization of growth in vitro and expression of additional genes. Virology 321:247-259. [DOI] [PubMed] [Google Scholar]
- 7.Biacchesi, S., M. H. Skiadopoulos, L. Yang, E. W. Lamirande, K. C. Tran, B. R. Murphy, P. L. Collins, and U. J. Buchholz. 2004. Recombinant human metapneumovirus lacking the small hydrophobic SH and/or attachment G glycoprotein: deletion of G yields a promising vaccine candidate. J. Virol. 78:12877-12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biacchesi, S., M. H. Skiadopoulos, L. Yang, B. R. Murphy, P. L. Collins, and U. J. Buchholz. 2005. Rapid human metapneumovirus microneutralization assay based on green fluorescent protein expression. J. Virol. Methods 128:192-197. [DOI] [PubMed] [Google Scholar]
- 9.Biller, M., K. Mardberg, H. Hassan, H. Clausen, A. Bolmstedt, T. Bergstrom, and S. Olofsson. 2000. Early steps in O-linked glycosylation and clustered O-linked glycans of herpes simplex virus type 1 glycoprotein C: effects on glycoprotein properties. Glycobiology 10:1259-1269. [DOI] [PubMed] [Google Scholar]
- 10.Boivin, G., Y. Abed, G. Pelletier, L. Ruel, D. Moisan, S. Cote, T. C. Peret, D. D. Erdman, and L. J. Anderson. 2002. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J. Infect. Dis. 186:1330-1334. [DOI] [PubMed] [Google Scholar]
- 11.Boivin, G., G. De Serres, S. Cote, R. Gilca, Y. Abed, L. Rochette, M. G. Bergeron, and P. Dery. 2003. Human metapneumovirus infections in hospitalized children. Emerg. Infect. Dis. 9:634-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruneau, N., A. Nganga, E. A. Fisher, and D. Lombardo. 1997. O-glycosylation of C-terminal tandem-repeated sequences regulates the secretion of rat pancreatic bile salt-dependent lipase. J. Biol. Chem. 272:27353-27361. [DOI] [PubMed] [Google Scholar]
- 13.Buchholz, U. J., K. Nagashima, B. R. Murphy, and P. L. Collins. 2006. Live vaccines for human metapneumovirus designed by reverse genetics. Expert Rev. Vaccines 5:695-706. [DOI] [PubMed] [Google Scholar]
- 14.Chege, N. W., and S. R. Pfeffer. 1990. Compartmentation of the Golgi complex: brefeldin-A distinguishes trans-Golgi cisternae from the trans-Golgi network. J. Cell Biol. 111:893-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins, P. L. 1990. O glycosylation of glycoprotein G of human respiratory syncytial virus is specified within the divergent ectodomain. J. Virol. 64:4007-4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins, P. L., and G. Mottet. 1992. Oligomerization and posttranslational processing of glycoprotein G of human respiratory syncytial virus: altered O glycosylation in the presence of brefeldin A. J. Gen. Virol. 73(Pt. 4):849-863. [DOI] [PubMed] [Google Scholar]
- 17.Collins, P. L., and G. Mottet. 1991. Post-translational processing and oligomerization of the fusion glycoprotein of human respiratory syncytial virus. J. Gen. Virol. 72(Pt. 12):3095-3101. [DOI] [PubMed] [Google Scholar]
- 18.Connors, M., P. L. Collins, C. Y. Firestone, and B. R. Murphy. 1991. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J. Virol. 65:1634-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doms, R. W., G. Russ, and J. W. Yewdell. 1989. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J. Cell Biol. 109:61-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorner, A. J., and R. J. Kaufman. 1990. Analysis of synthesis, processing, and secretion of proteins expressed in mammalian cells. Methods Enzymol. 185:577-596. [DOI] [PubMed] [Google Scholar]
- 21.Esper, F., D. Boucher, C. Weibel, R. A. Martinello, and J. S. Kahn. 2003. Human metapneumovirus infection in the United States: clinical manifestations associated with a newly emerging respiratory infection in children. Pediatrics 111:1407-1410. [DOI] [PubMed] [Google Scholar]
- 22.Feldmann, H., C. Will, M. Schikore, W. Slenczka, and H. D. Klenk. 1991. Glycosylation and oligomerization of the spike protein of Marburg virus. Virology 182:353-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujiwara, T., K. Oda, S. Yokota, A. Takatsuki, and Y. Ikehara. 1988. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J. Biol. Chem. 263:18545-18552. [PubMed] [Google Scholar]
- 24.Griffiths, G., P. Quinn, and G. Warren. 1983. Dissection of the Golgi complex. I. Monensin inhibits the transport of viral membrane proteins from medial to trans Golgi cisternae in baby hamster kidney cells infected with Semliki Forest virus. J. Cell Biol. 96:835-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffiths, G., and K. Simons. 1986. The trans Golgi network: sorting at the exit site of the Golgi complex. Science 234:438-443. [DOI] [PubMed] [Google Scholar]
- 26.Gruber, C., and S. Levine. 1985. Respiratory syncytial virus polypeptides. IV. The oligosaccharides of the glycoproteins. J. Gen. Virol. 66(Pt. 3):417-432. [DOI] [PubMed] [Google Scholar]
- 27.Hamelin, M. E., Y. Abed, and G. Boivin. 2004. Human metapneumovirus: a new player among respiratory viruses. Clin. Infect. Dis. 38:983-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamelin, M. E., and G. Boivin. 2005. Human metapneumovirus: a ubiquitous and long-standing respiratory pathogen. Pediatr. Infect. Dis. J. 24:S203-S207. [DOI] [PubMed] [Google Scholar]
- 29.Herfst, S., M. de Graaf, J. H. Schickli, R. S. Tang, J. Kaur, C. F. Yang, R. R. Spaete, A. A. Haller, B. G. van den Hoogen, A. D. Osterhaus, and R. A. Fouchier. 2004. Recovery of human metapneumovirus genetic lineages a and B from cloned cDNA. J. Virol. 78:8264-8270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jentoft, N. 1990. Why are proteins O-glycosylated? Trends Biochem. Sci. 15:291-294. [DOI] [PubMed] [Google Scholar]
- 31.Johnson, P. R., M. K. Spriggs, R. A. Olmsted, and P. L. Collins. 1987. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc. Natl. Acad. Sci. USA 84:5625-5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kahn, J. S. 2006. Epidemiology of human metapneumovirus. Clin. Microbiol. Rev. 19:546-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kingsley, D. M., K. F. Kozarsky, L. Hobbie, and M. Krieger. 1986. Reversible defects in O-linked glycosylation and LDL receptor expression in a UDP-Gal/UDP-GalNAc 4-epimerase deficient mutant. Cell 44:749-759. [DOI] [PubMed] [Google Scholar]
- 34.Kornfeld, R., and S. Kornfeld. 1985. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 54:631-664. [DOI] [PubMed] [Google Scholar]
- 35.Kozarsky, K., D. Kingsley, and M. Krieger. 1988. Use of a mutant cell line to study the kinetics and function of O-linked glycosylation of low density lipoprotein receptors. Proc. Natl. Acad. Sci. USA 85:4335-4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozarsky, K., M. Penman, L. Basiripour, W. Haseltine, J. Sodroski, and M. Krieger. 1989. Glycosylation and processing of the human immunodeficiency virus type 1 envelope protein. J. Acquir. Immune Defic. Syndr. 2:163-169. [PubMed] [Google Scholar]
- 37.Kozarsky, K. F., S. M. Call, S. K. Dower, and M. Krieger. 1988. Abnormal intracellular sorting of O-linked carbohydrate-deficient interleukin-2 receptors. Mol. Cell. Biol. 8:3357-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krieger, M., P. Reddy, K. Kozarsky, D. Kingsley, L. Hobbie, and M. Penman. 1989. Analysis of the synthesis, intracellular sorting, and function of glycoproteins using a mammalian cell mutant with reversible glycosylation defects. Methods Cell Biol. 32:57-84. [DOI] [PubMed] [Google Scholar]
- 39.Lambert, D. M. 1988. Role of oligosaccharides in the structure and function of respiratory syncytial virus glycoproteins. Virology 164:458-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li, Y., L. Luo, N. Rasool, and C. Y. Kang. 1993. Glycosylation is necessary for the correct folding of human immunodeficiency virus gp120 in CD4 binding. J. Virol. 67:584-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lippincott-Schwartz, J., J. G. Donaldson, A. Schweizer, E. G. Berger, H. P. Hauri, L. C. Yuan, and R. D. Klausner. 1990. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell 60:821-836. [DOI] [PubMed] [Google Scholar]
- 42.Maggi, F., M. Pifferi, M. Vatteroni, C. Fornai, E. Tempestini, S. Anzilotti, L. Lanini, E. Andreoli, V. Ragazzo, M. Pistello, S. Specter, and M. Bendinelli. 2003. Human metapneumovirus associated with respiratory tract infections in a 3-year study of nasal swabs from infants in Italy. J. Clin. Microbiol. 41:2987-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Misumi, Y., Y. Misumi, K. Miki, A. Takatsuki, G. Tamura, and Y. Ikehara. 1986. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J. Biol. Chem. 261:11398-11403. [PubMed] [Google Scholar]
- 44.Oda, K., S. Hirose, N. Takami, Y. Misumi, A. Takatsuki, and Y. Ikehara. 1987. Brefeldin A arrests the intracellular transport of a precursor of complement C3 before its conversion site in rat hepatocytes. FEBS Lett. 214:135-138. [DOI] [PubMed] [Google Scholar]
- 45.Olmsted, R. A., B. R. Murphy, L. A. Lawrence, N. Elango, B. Moss, and P. L. Collins. 1989. Processing, surface expression, and immunogenicity of carboxy-terminally truncated mutants of G protein of human respiratory syncytial virus. J. Virol. 63:411-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osawa, T., and T. Tsuji. 1987. Fractionation and structural assessment of oligosaccharides and glycopeptides by use of immobilized lectins. Annu. Rev. Biochem. 56:21-42. [DOI] [PubMed] [Google Scholar]
- 47.Osterhaus, A., and R. Fouchier. 2003. Human metapneumovirus in the community. Lancet 361:890-891. [DOI] [PubMed] [Google Scholar]
- 48.Peiris, J. S., W. H. Tang, K. H. Chan, P. L. Khong, Y. Guan, Y. L. Lau, and S. S. Chiu. 2003. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg. Infect. Dis. 9:628-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pelletier, G., P. Dery, Y. Abed, and G. Boivin. 2002. Respiratory tract reinfections by the new human Metapneumovirus in an immunocompromised child. Emerg. Infect. Dis. 8:976-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peret, T. C., Y. Abed, L. J. Anderson, D. D. Erdman, and G. Boivin. 2004. Sequence polymorphism of the predicted human metapneumovirus G glycoprotein. J. Gen. Virol. 85:679-686. [DOI] [PubMed] [Google Scholar]
- 51.Peret, T. C., G. Boivin, Y. Li, M. Couillard, C. Humphrey, A. D. Osterhaus, D. D. Erdman, and L. J. Anderson. 2002. Characterization of human metapneumoviruses isolated from patients in North America. J. Infect. Dis. 185:1660-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perez-Vilar, J., J. Hidalgo, and A. Velasco. 1991. Presence of terminal N-acetylgalactosamine residues in subregions of the endoplasmic reticulum is influenced by cell differentiation in culture. J. Biol. Chem. 266:23967-23976. [PubMed] [Google Scholar]
- 53.Perkel, V. S., A. Y. Liu, Y. Miura, and J. A. Magner. 1988. The effects of brefeldin-A on the high mannose oligosaccharides of mouse thyrotropin, free alpha-subunits, and total glycoproteins. Endocrinology 123:310-318. [DOI] [PubMed] [Google Scholar]
- 54.Qiu, Z., F. Tufaro, and S. Gillam. 1995. Brefeldin A and monensin arrest cell surface expression of membrane glycoproteins and release of rubella virus. J. Gen. Virol. 76(Pt. 4):855-863. [DOI] [PubMed] [Google Scholar]
- 55.Roth, J., Y. Wang, A. E. Eckhardt, and R. L. Hill. 1994. Subcellular localization of the UDP-N-acetyl-d-galactosamine: polypeptide N-acetylgalactosaminyltransferase-mediated O-glycosylation reaction in the submaxillary gland. Proc. Natl. Acad. Sci. USA 91:8935-8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Satake, M., J. E. Coligan, N. Elango, E. Norrby, and S. Venkatesan. 1985. Respiratory syncytial virus envelope glycoprotein (G) has a novel structure. Nucleic Acids Res. 13:7795-7812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sato, K., H. Okamoto, S. Aihara, Y. Hoshi, T. Tanaka, and S. Mishiro. 1993. Demonstration of sugar moiety on the surface of hepatitis C virions recovered from the circulation of infected humans. Virology 196:354-357. [DOI] [PubMed] [Google Scholar]
- 58.Schowalter, R. M., S. E. Smith, and R. E. Dutch. 2006. Characterization of human metapneumovirus F protein-promoted membrane fusion: critical roles for proteolytic processing and low pH. J. Virol. 80:10931-10941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Segrest, J. P., and R. L. Jackson. 1972. Molecular weight determination of glycoproteins by polyacrylamide gel electrophoresis in sodium dodecyl sulfate. Methods Enzymol. 28:54-63. [Google Scholar]
- 60.Skiadopoulos, M. H., S. Biacchesi, U. J. Buchholz, E. Amaro-Carambot, S. R. Surman, P. L. Collins, and B. R. Murphy. 2006. Individual contributions of the human metapneumovirus F, G, and SH surface glycoproteins to the induction of neutralizing antibodies and protective immunity. Virology 345:492-501. [DOI] [PubMed] [Google Scholar]
- 61.Tartakoff, A. M. 1983. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell 32:1026-1028. [DOI] [PubMed] [Google Scholar]
- 62.Tooze, S. A., J. Tooze, and G. Warren. 1988. Site of addition of N-acetyl-galactosamine to the E1 glycoprotein of mouse hepatitis virus-A59. J. Cell Biol. 106:1475-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ulmer, J. B., and G. E. Palade. 1991. Effects of Brefeldin A on the Golgi complex, endoplasmic reticulum and viral envelope glycoproteins in murine erythroleukemia cells. Eur. J. Cell Biol. 54:38-54. [PubMed] [Google Scholar]
- 64.Ulmer, J. B., and G. E. Palade. 1989. Targeting and processing of glycophorins in murine erythroleukemia cells: use of brefeldin A as a perturbant of intracellular traffic. Proc. Natl. Acad. Sci. USA 86:6992-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van den Hoogen, B. G., T. M. Bestebroer, A. D. Osterhaus, and R. A. Fouchier. 2002. Analysis of the genomic sequence of a human metapneumovirus. Virology 295:119-132. [DOI] [PubMed] [Google Scholar]
- 66.van den Hoogen, B. G., J. C. de Jong, J. Groen, T. Kuiken, R. de Groot, R. A. Fouchier, and A. D. Osterhaus. 2001. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 7:719-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van den Hoogen, B. G., D. M. Osterhaus, and R. A. Fouchier. 2004. Clinical impact and diagnosis of human metapneumovirus infection. Pediatr. Infect. Dis. J. 23:S25-S32. [DOI] [PubMed] [Google Scholar]
- 68.van den Hoogen, B. G., G. J. van Doornum, J. C. Fockens, J. J. Cornelissen, W. E. Beyer, R. de Groot, A. D. Osterhaus, and R. A. Fouchier. 2003. Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J. Infect. Dis. 188:1571-1577. [DOI] [PubMed] [Google Scholar]
- 69.Van den Steen, P., P. M. Rudd, R. A. Dwek, and G. Opdenakker. 1998. Concepts and principles of O-linked glycosylation. Crit. Rev. Biochem. Mol. Biol. 33:151-208. [DOI] [PubMed] [Google Scholar]
- 70.Viazov, S., F. Ratjen, R. Scheidhauer, M. Fiedler, and M. Roggendorf. 2003. High prevalence of human metapneumovirus infection in young children and genetic heterogeneity of the viral isolates. J. Clin. Microbiol. 41:3043-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wertz, G. W., P. L. Collins, Y. Huang, C. Gruber, S. Levine, and L. A. Ball. 1985. Nucleotide sequence of the G protein gene of human respiratory syncytial virus reveals an unusual type of viral membrane protein. Proc. Natl. Acad. Sci. USA 82:4075-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wertz, G. W., M. Krieger, and L. A. Ball. 1989. Structure and cell surface maturation of the attachment glycoprotein of human respiratory syncytial virus in a cell line deficient in O glycosylation. J. Virol. 63:4767-4776. [DOI] [PMC free article] [PubMed] [Google Scholar]