Abstract
Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN) is expressed by dendritic cells (DCs) at mucosal surfaces and appears to play an important role in the dissemination of human immunodeficiency virus type 1 (HIV-1) infection. DC-SIGN binds HIV-1 gp120 and efficiently transmits the virus to T CD4+ cells, which become the center of viral replication. Semen represents the main vector for HIV-1 dissemination worldwide. In the present study we show that human seminal plasma (SP), even when used at very high dilutions (1:104 to 1:105), markedly inhibits the capture and transmission of HIV-1 to T CD4+ cells mediated by both DCs and B-THP-1-DC-SIGN cells. In contrast, SP does not inhibit the capture of HIV-1 by DC-SIGN-negative target cells, such as the T-cell line SupT-1, monocytes, and activated peripheral blood mononuclear cells. The SP inhibitor has a high molecular mass (>100 kDa) and directly interacts with DC-SIGN-positive target cells but not with HIV-1. Moreover, the inhibitor binds to concanavalin A, suggesting that it contains high-mannose N-linked carbohydrates. Of note, using biotin-labeled SP we found that the binding of SP components to DCs was abrogated by mannan, while their interaction with B-THP-1 cells was almost completely dependent on the expression of DC-SIGN. Since epithelium integrity is often compromised after vaginal or anal intercourse, as well as in the presence of ulcerative-sexually transmitted diseases, our results support the notion that components of the SP might be able to access to the subepithelium, inhibiting the recognition of HIV-1 gp120 by DC-SIGN-positive DCs.
Unprotected sexual intercourse between discordant couples is by far the most common mode of human immunodeficiency virus type 1 (HIV-1) transmission (29, 51). Epidemiologic studies show, however, that HIV-1 is not particularly easy to acquire by sexual contact. The incidence of sexual transmission of HIV-1 is relatively low and appears to vary by anatomical site. Anal sex has the highest risk (1:100 to 1:1,000 for each sexual act), while vaginal sex has a lower risk (1:1,000 to 1:10,000) (16, 41, 59). As expected, the risk of infection is strongly dependent on the phase of the infection and is almost 10-fold higher during acute infection (14, 61). Many other sexually transmitted diseases are more efficiently transmitted. For example, hepatitis B is transmitted in 20 to 30% of exposures (24).
The epithelial surface acts as an effective barrier against HIV-1. After deposition of HIV-1 on the recipient mucosa, infectious virus must cross the mucosal epithelium and interact with T CD4+ lymphocytes, macrophages, and dendritic cells (DCs), which are the initial targets of infection (19, 29, 35, 51, 53). These cells express the HIV-1 receptor CD4 and the coreceptors CCR5 or CXCR4 that are required for infection. Although the productive infection of certain subsets of DCs by HIV-1 in vivo is controversial, it is now clear that DCs are able to capture HIV-1 at entry sites and transport the virus to draining lymph nodes, where HIV-1 is efficiently transmitted to T CD4+ cells, which become the center of viral replication (28, 62). The capacity of HIV-1 to hijack DCs for viral dissemination appears to be crucial in early HIV-1 pathogenesis (55, 64). It was not until the discovery of DC-SIGN (for DC-specific intercellular-adhesion-molecule-3-grabbing nonintegrin) that the molecular basis of this mechanism became clear (15). DC-SIGN is a 44-kDa C-type (Ca2+-dependent) lectin that binds to the mannose and fucose moieties on the HIV-1 envelope glycoprotein gp120 (2). DC-SIGN does not facilitate HIV-1 processing by DCs, but rather it appears to protect the virus from intracellular degradation and efficiently promotes infection in trans of T CD4+ cells (2, 15, 55, 62). Interestingly, DC-SIGN appears to be a universal pathogen receptor. It not only interacts with the envelope glycoprotein gp120 of HIV-1, HIV-2, and simian immunodeficiency virus (SIV) but also functions as a receptor for several viruses, such as Ebola virus, cytomegalovirus, hepatitis C virus, and dengue virus. Moreover, DC-SIGN also interacts with nonviral pathogens such as Mycobacterium spp., Leishmania spp., Candida albicans, and Helicobacter pylori (27, 58).
The mechanisms through which HIV-1 cross the mucosal epithelium and gain access to target cells are not well characterized and might differ at distinct tissue sites (20, 51). It is clear, however, that some degree of breakdown in epithelial integrity heightens the risk of HIV-1 transmission (17, 49). This is not an unusual scenario; in fact, epithelial micro-abrasions in the vagina are usually detected in 60% of healthy women after consensual intercourse (39). This would also explain the enhanced risk of HIV-1 transmission associated with the presence of concurrent infections that increase local inflammation or create lesions (14, 44). Anal intercourse is also often associated with mucosal trauma and since the rectal epithelium is only one cell layer thick, unlike the vaginal counterpart, it provides little protection against potential trauma, facilitating HIV-1 access to the underlying target cells (51). The access of virus to target cells may also be facilitated by an alternative mechanism: the binding of HIV-1 to DC projections that extend to, or near, the luminal surface, with subsequent presentation to subepithelial target cells (7, 45, 51).
Semen represents the main vector for HIV-1 dissemination worldwide. Usually, it is considered merely as a vehicle for HIV-1 transmission. However, though little is known about the early events involved in HIV-1 infection in vivo, semen is able to induce a variety of changes at HIV-1 entry sites that might favor HIV transmission. Among them, neutralization of vaginal mucous pH (5, 18, 56), opsonization of HIV-1 (4), stimulation of the expression of a range of inflammatory cytokines and chemokines in the ectocervix (50), induction of a transient infiltration of the cervix mucosa by leukocytes (42, 57), and the promotion of the chemotaxis of Langerhans cells via the secretion of CCL20 by vaginal epithelial cells (3). In the present study we analyze whether seminal plasma (SP) may be able to modulate the interaction of HIV-1 with DCs. This point seems to be relevant in the sexual transmission of HIV-1 since the existence of inflammatory processes or epithelial breaks in the mucosa, as well as the presence of DC projections that extend to, or near, the luminal surface may enable SP components to gain access to target cells. We show here that human SP abrogates the capture and transmission of HIV-1 to T CD4+ cells mediated by DC-SIGN.
MATERIALS AND METHODS
Reagents.
Lipopolysaccharide from Escherichia coli, recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF), trypsin, phytohemagglutinin (PHA), LewisX-bovine serum albumin conjugate (LeX-BSA), and mannan were from Sigma-Aldrich (St. Louis, MO). Recombinant human interleukin-4 (IL-4) was from Preprotech (Rocky Hill, NJ) or R&D Systems (Minneapolis, MN). Ficoll-Hypaque and Percoll were from Amersham Pharmacia Biotech (Piscataway, NJ). Mouse immunoglobulin M (IgM), anti-human LeX, and a control isotype antibody were from Calbiochem (San Diego, CA). Anti-DC-SIGN blocking antibodies (clone 120526) were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Disease, National Institutes of Health.
Preparation of human DCs.
Peripheral blood mononuclear cells (PBMC) were isolated from healthy volunteers by standard density gradient centrifugation on Ficoll-Hypaque. Monocytes were purified by centrifugation on a discontinuous Percoll gradient with modifications of a previously described method (8). Briefly, PBMC were suspended in Ca2+/Mg2+-free Tyrode solution supplemented with 0.2% EDTA and incubated during 30 min at 37°C. During this incubation, the osmolarity of the medium was gradually increased from 290 to 360 osmol/liter by the addition of 9% NaCl. Three different Percoll fractions were layered in polypropylene tubes: 50% at the bottom, followed by 46 and 40%. PBMC (5.10 × 106/ml) were layered at the top, and they were centrifuged at 400 × g for 20 min at 4°C. Monocytes were recovered at the 50/46% interface. The purity was checked by fluorescence-activated cell sorting (FACS) analysis using an anti-CD14 monoclonal antibody (MAb) and was found to be >85%. To obtain DCs, monocytes were cultured in RPMI 1640 medium (Life Technologies, Grand Island, NY) supplemented with 10% fetal calf serum, 50 U of penicillin/ml, 50 μg of streptomycin/ml, and 0.1 mM nonessential amino acids (all from Life Technologies) (complete culture medium) at 106 cells/ml with 10 ng of IL-4/ml and 10 ng of GM-CSF/ml, as described by Sallusto and Lanzavecchia (48). On day 6, the cells were analyzed by FACS.
Cell lines and virus.
Cell lines and virus were obtained from the AIDS Research and Reference Reagent Program. The following cell lines were used: B-THP-1-DC-SIGN, matched parental B-THP-1 cells (THP-1 are Raji B cells misidentified as THP-1) (65), the T-cell line SupT-1 (52), and GHOST cells (clone 3) (60). GHOST cells express CD4 and CCR5, as well as CXCR4, coreceptors, and a Tat-dependent green fluorescent protein reporter cassette. All cell lines were grown in RPMI 1640 medium supplemented with 10% fetal calf serum, 50 U of penicillin/ml, 50 μg of streptomycin/ml, and 0.1 mM nonessential amino acids (Life Technologies) (complete medium), except GHOST cells, which were grown as described previously (60). The CCR5-using HIV-1BaL isolate was grown on IL-2 (10 U/ml; R&D Systems) plus PHA (10 μg/ml)-stimulated PBMC. The CXCR4-using HIV-1IIIB was obtained from H9HTLV-1IIB supernatants. The viruses were concentrated by ultracentrifugation at 28,000 rpm for 90 min at 4°C (L2-65B ultracentrifuge; Beckman Instruments, Irvine, CA), and the virus pellet was suspended in RPMI 1640 medium. p24 antigen levels were determined by enzyme-linked immunosorbent assay (ELISA; Abbot Murex, Chicago, IL), and virus input into assays was a function of p24 antigen concentration.
SP samples.
Semen samples were collected from healthy donors (aged 25 to 45 years). Informed consent was obtained from each patient before sperm collection. Ejaculates were obtained by masturbation under hygienic conditions, after a period of 2 to 4 days of sexual abstinence, and were collected in sterile containers. The samples were allowed to liquefy for 30 min at 37°C. SP was separated from the cell fraction by centrifugation (1,000 × g, 30 min), and the supernatant was passed through a 0.22-μm-pore-size syringe filter (Millipore, Amsterdam, The Netherlands) and stored at −80°C until use.
Quantitation of cellular apoptosis and viability by fluorescence microscopy.
Quantitation was performed as previously described using the fluorescent DNA-binding dyes acridine orange (100 μg/ml) to determine the percentage of cells that had undergone apoptosis and ethidium bromide (100 μg/ml) to differentiate between viable and nonviable cells (9). With this method, nonapoptotic cell nuclei show “structure”: variations in fluorescence intensity that reflect the distribution of euchromatin and heterochromatin. In contrast, apoptotic nuclei exhibit highly condensed chromatin that is uniformly stained by acridine orange. In fact, the entire apoptotic nucleus is present as bright spherical beads. To assess the percentage of cells showing morphological features of apoptosis, at least 200 cells were scored in each experiment.
Analysis of cellular viability by the MTT reduction assay.
Cell viability was measured by using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) reduction assay, which measures the conversion of MTT into the purple-colored MTT formazan by the succinate-tetrazolium reductase system in living cells. Briefly, cells were incubated with MTT (Sigma) at 0.5 mg/ml for 2 h at 37°C. Cells were then washed, and the precipitated MTT formazan was solubilized with 10% Triton X-100-0.1 NHCl in anhydrous isopropanol. After 30 min of incubation at 37°C, the optical densities were measured at 570 nm in a conventional ELISA plate reader. Values for control cells were considered as 100% viability.
Flow cytometry.
Fluorescein isothiocyanate (FITC)- or phycoerythrin-conjugated MAbs directed to CD1a, CD14, CD4, CD80, CD86, HLA-DR, CD83, and CD209 (DC-SIGN) were from BD Pharmingen (San Diego, CA). In all cases isotype-matched control MAbs were used, and a gate (R1) was defined in the analysis to exclude all nonviable cells and debris, based on size and propidium iodine staining. Analysis was performed by using a FACS flow cytometer and CellQuest software (BD Biosciences, San Jose, CA). The results are expressed as the mean fluorescence intensity.
HIV-1 capture assays.
The ability of SP to inhibit the capture of HIV-1 was assessed in different cell types: DCs, B-THP-1 cells, B-THP-1-DC-SIGN cells, activated PBMC (10 U of IL-2/ml plus 10 μg of PHA/ml, 48 h at 37°C), monocytes, and SupT-1 cells. In all cases, 106 cells suspended in culture complete medium were incubated with or without the indicated dilutions of SP (prepared in RPMI 1640 medium) for 30 min at 37°C in a final volume of 200 μl and then cultured with HIV-1 stocks containing the indicated amounts of p24 for 90 min at 37°C. Cells were then washed thoroughly, pelleted, lysed, and assayed for HIV p24 antigen by ELISA.
HIV-1 infection assays.
Infection assays were carried out by incubating DCs or GHOST cells (105) with or without SP dilutions for 30 min at 37°C. Cells were then exposed to HIV (5 ng of p24) for 90 min at 37°C and then washed thoroughly. DCs were next cultured for 15 days in a final volume of 200 μl in 96-well flat-bottom plates in culture medium supplemented with 10 ng of IL-4/ml and 10 ng of GM-CSF/ml. Supernatants harvested at different times were assayed for HIV p24 antigen by ELISA. GHOST cells were cultured for 48 h in a final volume of 1 ml in 12-well flat-bottom plates. Infection was evaluated by flow cytometry as described previously (57).
HIV-1 trans-infection assays.
Trans-infection assays were carried out by incubating DCs, B-THP-1 cells, or B-THP-1-DC-SIGN cells (106 cells in 200 μl of complete medium) with or without SP dilutions for 30 min at 37°C. Cells were then exposed to HIV (5 ng of p24) for 90 min at 37°C. The cells were then washed thoroughly and suspended in culture complete medium. DC cultures were supplemented with 10 ng of IL-4/ml and 10 ng of GM-CSF/ml. Trans-infection of PBMC, previously activated by IL-2 (10 U/ml; R&D Systems) and PHA (10 μg/ml) for 2 days, was performed by incubating 5 × 104 DCs with 2 × 105 PMBC in a final volume of 200 μl in 96-well U-bottom plates. Supernatants, harvested at 3, 6, and 9 days of culture, were assayed for p24 antigen by ELISA. Trans-infection of SupT-1 cells was carried out by incubating 5 × 104 HIV-pulsed B-THP-1-DC-SIGN cells with 105 SupT-1 cells in a final volume of 200 μl in 96-well U-bottom plates. Supernatants, harvested at 3, 6, and 9 days of culture, were assayed for p24 antigen by ELISA. Trans-infection of GHOST cells was carried out by incubating 2 × 105 adherent GHOST cells with 3 × 105 HIV-treated B-THP-1-DC-SIGN cells in 12-well flat-bottom plates and in a final volume of 500 μl for 24 h at 37°C. Then, the B-THP-1-DC-SIGN cells were removed from the culture, and the infection of GHOST cells was evaluated after 24 h by flow cytometry as described previously (60).
Characterization of the inhibitory activity mediate by SP.
Samples of SP diluted 1:10 in RPMI 1640 medium were (i) heated at 95°C for 10 min; (ii) treated with trypsin (1,000 U/ml, 3 h at 37°C), after which the enzyme was inactivated by heating at 95°C for 10 min; and (iii) fractionated by using Microcon centrifugal filters (Millipore) with molecular weight cutoffs of 3,000, 10,000, 30,000, and 100,000.
Biotinylation of SP.
A dilution of SP of 1:10 (0.1 ml) was extensively dialyzed against phosphate-buffered saline (PBS), and biotinylation was performed by using a Sulfo-NHS-LC-Biotinylation kit (Pierce Biotechnology) according to the manufacturer's protocol. The biotinylated proteins were then dialyzed against RPMI 1640 culture medium to remove unincorporated biotin.
Isolation of a SP fraction by lectin affinity chromatography.
Chromatography on concanavalin A (ConA)-Sepharose column (22 by 1 cm; Amersham Pharmacia, Uppsala, Sweden) was performed as previously described (32, 34). The bed volume of the column was 5 ml, and the flow rate was 12 ml/h. An aliquot of 1 ml of SP diluted 1:10 with culture medium was applied to the column, which was then washed with RPMI 1640 medium until the absence of proteins and carbohydrates was achieved. The interacting compounds were eluted, in a first step, with PBS supplemented with 5 mM α-d-methyl glucoside to eliminate weakly bound compounds and then with PBS supplemented with 1 M α-methyl-d-mannoside. The eluate was dialyzed four times against 500 volumes of RPMI 1640 (12 h each time), and its ability to inhibit the interaction of HIV-1 with DC-SIGN-positive target cells was assessed.
Statistics.
All statistical comparisons were performed by using analysis of variance. P values of <0.01 and <0.05 were considered statistically significant.
RESULTS
SP inhibits the capture of HIV-1 by DCs.
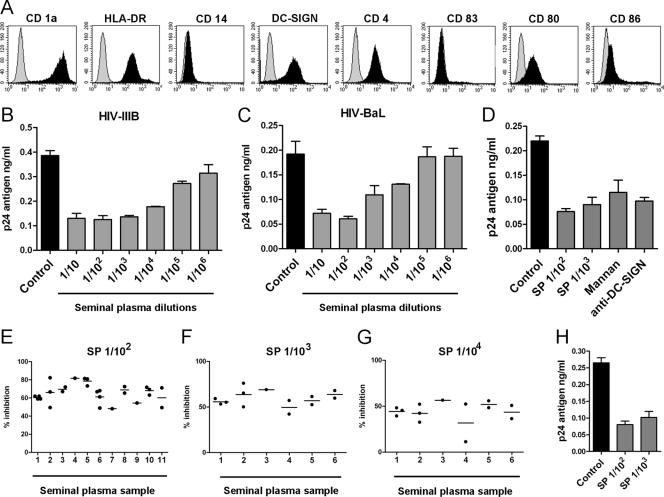
Figure 1A shows the phenotype of immature DCs used in our experiments. These cells were obtained from human monocytes cultured for 6 days with GM-CSF and IL-4. As expected, cells were CD1a positive, CD14 negative, DC-SIGN positive, CD4 positive, and CD83 negative and expressed low to intermediate levels of HLA-DR, CD80, and CD86. In a first set of experiments, we analyzed whether SP was able to inhibit the capture of HIV-1 by DCs. Cells were incubated for 30 min at 37°C with different dilutions of SP and then cultured with HIV-1 (5 ng of p24) for an additional period of 90 min at 37°C. DCs were then washed thoroughly, pelleted, lysed, and assayed for p24 antigen by ELISA. Figure 1B and C show that SP exerts a strong inhibitory effect on the capture of HIV-1 by DCs. A significant inhibition of HIV-1 capture was observed for both HIV-1IIIB (CXCR4) and HIV-1BaL (CCR5), using SP dilutions as high as 1:104 (P < 0.01, n = 9). Figure 1D shows that the inhibitory effect mediated by SP was similar to that induced by either blocking antibodies directed to DC-SIGN or mannan, supporting the notion that SP may inhibit DC-SIGN-dependent recognition of HIV-1 by DCs. Interestingly, Fig. 1E to G show that similar levels of inhibition were observed using SP and DCs from different donors, indicating that the ability of SP to inhibit the capture of HIV-1 by DCs is strongly conserved among healthy individuals.
FIG. 1.
Capture of HIV-1 by DCs is inhibited by SP. (A) Representative histograms of the phenotype of immature DCs. (B and C) DCs were incubated for 30 min at 37°C with different SP dilutions and then cultured with HIV-1IIIB (CXCR4) or HIV-1BaL (CCR5) (5 ng of p24 for 90 min at 37°C). Cells were then washed thoroughly, lysed, and assayed for p24 antigen by ELISA. The results from a representative experiment (n = 9) performed in triplicate are shown. (D) DCs were incubated for 30 min at 37°C in the absence or presence of different SP dilutions, mannan (1 mg/ml), or a blocking MAb directed to DC-SIGN used at a concentration three- to fivefold higher than those needed to saturate all binding sites, as determined by FACS analysis. DCs were then cultured with HIV-1BaL (5 ng of p24) for 90 min at 37°C. After this period, cells were washed thoroughly, lysed, and assayed for p24 antigen by ELISA. A representative experiments (n = 5) made by triplicate is shown. (E to G) SP and DCs from different donors were studied in capture assays. Each point represents a distinct DC donor. Experiments were carried out as described above, using SP dilutions of 1:102 (E), 1:103 (F), and 1:104 (G) and HIV-1BaL (5 ng of p24). The results are expressed as the percent inhibition of HIV-1 capture for each DC donor. (H) A 100-μl portion of an SP dilution of 1:5 was mixed with 100 μl of two HIV-1BAL stocks previously adjusted to 1 or 10 μg of p24/ml. Both mixtures were incubated for 15 min at 37°C. After this period, 10 μl of the first mixture and 1 μl of the second mixture were added to 106 DCs suspended in 90 and 99 μl of culture medium, respectively, to yield DC suspensions exposed to SP dilutions of 1:102 and 1:103 in the presence of 5 ng of p24. After incubation for 90 min at 37°C, the DCs were washed thoroughly, pelleted, lysed, and assayed for HIV p24 by ELISA. The results of representative experiments (n = 3) performed in triplicate are shown.
In all of the experiments described above, DCs were pretreated with SP for 30 min before the addition of HIV-1. Considering that during sexual transmission of HIV-1, virus and SP are deposited together in the genital mucosa, we performed additional studies to analyze how SP might modulate the capture of HIV-1 by DCs when SP and HIV-1 are added together to DC cultures. Figure 1H shows that, under this experimental condition, the inhibitory effect of SP was similar to those depicted in Fig. 1B to D. This suggests that prior exposure of DCs to SP does not result in an increased ability of SP to inhibit HIV-1 capture.
Since previous observations have shown that SP is able to induce cytotoxic effects on human lymphocytes in long-term cultures (1), we analyzed whether treatment of DCs with SP resulted in a loss of cell viability. DCs were incubated for 2 h at 37°C in the presence of SP (dilutions 1:10 and 1:100). The cells were then washed thoroughly, and quantitation of cellular apoptosis and viability was performed by fluorescence microscopy either immediately or after an additional period of culture for 48 h at 37°C. We observed that the percentages of cell viability were, in all cases, higher than 90%, whereas the percentages of apoptotic cells were lower than 10% in both untreated and SP-treated DCs. We also determined the absolute numbers of DCs recovered after 48 h of culture and found that cell recovery was always higher than 85%. Together, these observations indicate that transient exposure to SP did not induce deleterious effects on DCs.
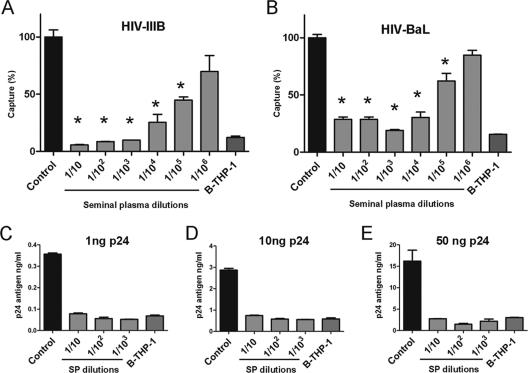
DC-SIGN plays an important role in the initial attachment of HIV-1 to immature DCs (2, 15, 55, 62). As mentioned above, our results showing that the inhibitory effect mediated by SP was similar to those induced by either blocking antibodies directed to DC-SIGN or to mannan (Fig. 1D) suggest that SP may inhibit the recognition of HIV-1 by DC-SIGN. This possibility was analyzed in a new set of experiments using B-THP-1-DC-SIGN cells. These cells capture HIV-1 mainly through DC-SIGN being the capture mediated by the matched parental B-THP-1 cells (DC-SIGN negative), almost 10 to 15% compared to DC-SIGN-positive cells. Figure 2A and B shows that SP markedly suppressed HIV-1 capture. Dilutions of SP of 1:103 diminished the capture of HIV-1IIIB and HIV-1BaL to the levels observed for DC-SIGN-negative cells, while significant levels of inhibition were observed even at SP dilutions of 1:105. Figure 2C to E illustrates the potency of SP to inhibit the capture of HIV-1 by B-THP-1-DC-SIGN cells. An SP dilution of 1:1,000 induced a complete inhibition of the capture of HIV-1 mediated by DC-SIGN when cells were exposed to viral suspensions containing amounts of p24 as high as 50 ng.
FIG. 2.
SP inhibits the capture of HIV-1 mediated by DC-SIGN. (A and B) B-THP-1-DC-SIGN cells were incubated for 30 min at 37°C without (controls) or with different SP dilutions and then were cultured with HIV-1IIIB or HIV-1BaL (5 ng of p24) for 90 min at 37°C. The cells were then washed thoroughly, lysed, and assayed for p24 antigen by ELISA. The capture mediated by B-THP-1 cells, which do not express DC-SIGN, is also shown. The results are expressed as the percentage of HIV-1 capture compared to control cells. The data represent the arithmetic means ± the SEM of five to six experiments carried out in triplicate. Asterisk represents statistical significance (P < 0.05) versus controls. (C to E) Capture assays by B-THP-1-DC-SIGN cells was performed as described above, using different amounts of HIV-1BaL as indicated for each panel. The results of representative experiments are shown (n = 3 to 4).
We then analyzed the effect of SP on the capture of HIV-1 by monocytes, activated PBMC, and the T-cell line SupT-1. These cells do not express DC-SIGN. Our results show that the capture of HIV-1 by these cell populations was not inhibited by SP, even when assessed at dilutions of 1:100 (Fig. 3), supporting that CD4-dependent mechanisms of HIV-1 capture are not inhibited by SP. To rule out the possibility that incubation with SP might result in the induction of deleterious effects on these cell populations, we performed additional experiments directed to evaluate cell viability. Activated PBMC (10 U of IL-2/ml plus 10 μg of PHA/ml for 48 h), resting monocytes, and SupT-1 cells were incubated for 2 h at 37°C with an SP dilution of 1:100. After a washing step, cell viability was analyzed for each cell population by using the MTT assay as described in Materials and Methods. Colorimetric analysis was performed at 570 nm, and values observed for cells preincubated in the absence of SP (controls), for each cellular population, were considered as 100% viability. The results obtained indicated that exposure to SP did not result in a loss of cell viability: % viability = 93 ± 8, 99 ± 7, and 94 ± 6 for activated PBMC, monocytes, and SupT-1 pretreated with SP, respectively (mean ± the standard error, n = 5).
FIG. 3.
SP does not inhibit the capture of HIV-1 mediated by DC-SIGN negative target cells. Activated PBMC (10 U of IL-2/ml plus 10 μg of PHA/ml for 48 h), monocytes, and the cell line SupT-1 were incubated for 30 min at 37°C without (controls) or with a SP dilution of 1:102 and then were exposed to HIV-1IIIB (CXCR4) (5 ng of p24) for 90 min at 37°C. The cells were then washed thoroughly, lysed, and evaluated for p24 antigen by ELISA. The data represent the arithmetic means ± the SEM of three experiments carried out in triplicate.
Infection of DCs by HIV-1 is also inhibited by SP.
We next analyzed whether SP was also able to inhibit the infection of DCs by HIV-1. To this aim, DCs were treated with different dilutions of SP for 30 min at 37°C and then cultured with HIV-1 (5 ng of p24) for an additional period of 90 min at 37°C. The DCs were then washed thoroughly and cultured in 96-well flat-bottom plates, and the supernatants harvested after 3, 6, and 9 days of culture were assessed for p24 antigen by ELISA. Figure 4A shows that SP, at a dilution of 1:100, inhibits DC infection in a fashion similar to that of blocking antibodies directed to DC-SIGN or mannan. On the other hand, and contrasting with the results obtained in capture assays (Fig. 1B and C), no inhibition was observed with higher dilutions of SP, e.g., 1:103 and 1:104 (Fig. 4A and data not shown), indicating that infection of DCs is less sensitive to the inhibitory action exerted by SP. Since infection of DCs depends on the viability and metabolic activity of the cells and also considering that inhibition of the infection of DCs by HIV-1 was only observed at a relatively high concentration of SP (dilution 1:102), we performed additional experiments to rule out that exposure to SP might result in a loss of DC viability. DCs were incubated in the absence or presence of SP dilutions of 1:102 for 2 h at 37°C, washed, and cultured for different periods at 37°C (0 h to 9 days). Cell viability was then analyzed by using the MTT assay. Colorimetric analysis was performed at 570 nm, and values observed for control DCs (i.e., cells not pretreated with SP) at each time point were considered as 100% viability. The results obtained (Fig. 4B) show that transient exposure to SP does not result in a loss of DC viability, even when it was assessed after long periods of culture. We also analyzed whether SP might be able to inhibit the infection of GHOST cells (DC-SIGN negative, CD4+, CXCR4+, and CCR5+). Consistent with the inability of SP to inhibit CD4-dependent mechanisms of HIV-1 capture, Fig. 4C shows that infection of GHOST cells was not affected by SP.
FIG. 4.
Infection of DCs by HIV-1 is inhibited by SP. (A) Infection of DCs was performed by incubating cells for 30 min at 37°C without (controls) or with SP dilutions of 1:102 or 1:103, mannan (1 mg/ml), or saturating concentrations of a blocking MAb directed to DC-SIGN. Cells were then exposed to HIV-1BaL (5 ng of p24) for 90 min at 37°C, washed thoroughly, and cultured for several days. Supernatants harvested at days 3, 6, and 9 were assayed for HIV p24 antigen by ELISA. The results of a representative experiment (n = 4) performed in triplicate are shown. (B) Transient exposure to SP does not result in a loss of DC viability. DCs were incubated in the absence or presence of a SP dilution of 1:102 for 2 h at 37°C. Cells were then washed and cultured for different periods at 37°C (0 h to 9 days). Cell viability was then analyzed using the MTT assay as described in Materials and Methods. Optical densities were measured at 570 nm. Values for control cells (i.e., cells not pretreated with SP) were considered, at each time point, as 100% viability. The results of a representative experiment (n = 3) performed in triplicate are shown. (C) Infection of GHOST cells is not inhibited by SP. GHOST cells (DC-SIGN negative, CD4+, CXCR4+, and CCR5+) were exposed to HIV-1BaL (5 ng of p24) for 90 min at 37°C, washed thoroughly, and cultured for 48 h at 37°C. Infection of GHOST cells was analyzed by flow cytometry as described in Materials and Methods. The results of a representative experiment (n = 5) are shown.
SP strongly inhibits trans-infection of HIV-1 mediated by DCs.
The capacity of HIV-1 to hijack DCs, promoting infection in trans of T CD4+ cells appears to be crucial in early HIV-1 pathogenesis (15, 64). To analyze whether SP interfered with this mechanism, we first studied trans-infection of activated PBMC mediated by DCs. DCs were incubated with or without SP dilutions for 30 min at 37°C and then were exposed to HIV-1BaL (5 ng of p24) for 90 min at 37°C. Cells were then washed thoroughly, and trans-infection of activated PBMC was assessed as described in Materials and Methods after 9 days of culture. Figure 5A shows that SP dilutions of 1:102 and 1:103 induced a strong inhibitory effect (P < 0.01, n = 5) that was similar to those induced by blocking antibodies directed to DC-SIGN or mannan. No inhibition was observed using SP at a dilution of 1:104. We also examined the trans-infection of T CD4+ cells mediated by B-THP-1-DC-SIGN cells. These cells were incubated with or without SP dilutions for 30 min at 37°C and then were exposed to HIV-1 for 90 min at 37°C. Cells were then washed thoroughly, and trans-infection of both the T-cell line SupT-1 and GHOST cells was assessed as described in Materials and Methods. Consistent with the observed ability of SP to inhibit the capture of HIV-1 by B-THP-1-DC-SIGN cells, we found that SP markedly suppressed the trans-infection of both SupT-1 and GHOST cells (Fig. 5B and C). In fact, in both cases, dilutions of SP of 1:104 diminished the trans-infection of HIV-1 to the levels observed for B-THP-1 cells.
FIG. 5.
SP inhibits trans-infection of T CD4+ cells. DCs (A) or B-THP-1-DC-SIGN cells (B and C) were incubated for 30 min at 37°C without (controls) or with different SP dilutions, mannan (1 mg/ml), or saturating concentrations of a blocking MAb directed to DC-SIGN. Cells were then exposed to HIV-1BaL (5 ng of p24) for 90 min at 37°C. After this period, cells were thoroughly washed, and trans-infection of activated PBMC (A), the T-cell line SupT-1 (B), and GHOST cells (DC-SIGN negative, CD4+, CXCR4+, and CCR5+) (C) was assessed as described in Materials and Methods. The results of representative experiments (n = 3 to 6) are shown. Trans-infection mediated by B-THP-1 cells, which do not express DC-SIGN, is also shown in panels B and C.
Characterization of the inhibitory activity of SP.
We first analyzed whether the inhibitory activity of SP was mediated by the interaction of the putative inhibitor with HIV-1 or DCs. To do this, DCs (106/ml) or the viral stock (HIV-1BaL, 5 ng of p24) were incubated with or without different SP dilutions for 90 min at 37°C. DCs were then washed four times by centrifugation at 1,500 rpm (10 min, 4°C), while HIV-1 stocks were washed three times by ultracentrifugation (28,000 rpm, 90 min, 4°C). Capture assays were carried out by incubating SP-pretreated DCs with untreated HIV-1 or, alternatively, by incubating untreated DCs with SP-pretreated HIV-1. The results obtained (Fig. 6A and B) show that pretreatment of DCs with SP markedly inhibited HIV-1 capture (P < 0.05, n = 4, for SP dilutions of 1:103 and 1:104). In contrast, pretreatment of HIV-1 with SP did not result in any inhibition, supporting the view that the inhibitor actually interacts with DCs. We then examined whether the inhibition of HIV-1 capture by SP could be related to a diminished expression of DC-SIGN. Figure 6C and D show that neither the expression of DC-SIGN nor the expression of CD4 was affected by SP. Similar results were observed for DCs cultured for 2 h with mannan.
FIG. 6.
Analysis of the inhibitory activity mediated by SP. (A) B-THP-1-DC-SIGN cells were incubated without (controls) or with different SP dilutions, for 30 min at 37°C. Cells were then washed four times and cultured with HIV-1BaL (5 ng of p24) for 90 min at 37°C. Cells were then washed thoroughly, lysed, and assayed for p24 antigen by ELISA. (B) Viral stocks (HIV-1BaL) were incubated without or with different SP dilutions for 30 min at 37°C. HIV-1 stocks were then washed three times by ultracentrifugation. B-THP-1-DC-SIGN cells were exposed to untreated (controls) or SP-pretreated HIV-1 (5 ng of p24) for 90 min at 37°C. Cells were then washed thoroughly, lysed, and assayed for p24 antigen by ELISA. (C and D) DCs were cultured for different periods in the absence or presence of SP (dilution 1:102) or mannan (1 mg/ml). After the cells were washed, the expression of DC-SIGN (C) and CD4 (D) was analyzed by flow cytometry. Dotted histograms represent the expression of DC-SIGN (C) or CD4 (D) by control DCs. The results of a representative experiment (n = 3) are shown. (E) Aliquots of SP (dilution 1:10) were subjected to different treatments. They were heated for 10 min at 95°C, treated with trypsin (3 h at 37°C and 10 min at 95°C), treated with an antibody directed to LeX, or filtered in centrifugal filter devices with 100-kDa cutoffs and then diluted with culture medium to its original volume. The ability of each SP sample to inhibit the capture of HIV-1BaL (5 ng of p24) by B-THP-1-DC-SIGN cells was then assessed by using a final dilution of 1:103. (F) ConA-Sepharose chromatography of SP (1 ml of SP diluted 1:10 with culture medium) was performed as described in Materials and Methods. The ConA-interacting fraction was eluted using 5% α-methyl-d-mannoside, thoroughly dialyzed, and used at final dilutions of 1:103, 1:104, or 1:105. The ability to inhibit the capture of HIV-1 by B-THP-1-DC-SIGN cells was then assessed. We also studied, as a control, the ability of the same SP sample, not subjected to chromatography, to inhibit HIV-1 capture. In panels A, B, E, and F the data represent the arithmetic means ± the SEM of three to five experiments carried out in triplicate. Asterisk represents statistical significance (P < 0.05) versus controls.
We then performed a number of experiments directed to characterize the properties of the SP inhibitor. Aliquots of SP were subjected to different treatments and then were assessed for their ability to inhibit the capture of HIV-1 by DCs. The results obtained are shown in Fig. 6E. First, we analyzed the molecular mass of the putative inhibitor. A dilution of 1:10 of SP was filtered in centrifugal filter devices with 100-kDa cutoffs. The inhibitory activity was almost fully retained by the filters. This indicates that the inhibitor is a compound with a molecular mass higher than 100 kDa. We also observed that the inhibitory activity was heat stable (95°C, 10 min) and resistant to the action of trypsin. On the other hand, taking into account previous studies showing that a multimeric LeX component in human milk binds to DC-SIGN, preventing HIV-1 from interacting with this receptor (37, 38) and also considering that SP contains LeX-carrying glycoprotein (6), we studied whether a similar inhibitor may account for our results. We preincubated SP with a mouse IgM antibody directed to LeX that has been shown to be able to prevent the inhibitory effect induced by human milk on the interaction of HIV-1 gp120 and DC-SIGN (37, 38). In contrast to the observations made in human milk, we found that this antibody did not exert any effect on the inhibition mediated by SP (Fig. 6E). Additional experiments were performed to demonstrate that this anti-LeX antibody was indeed capable of blocking the binding of LeX to DCs. To do this, we used LeX-BSA, previously biotinylated as described in Materials and Methods. Biotin-labeled LeX-BSA (25 μg/ml) was incubated for 15 min at room temperature with the IgM anti-human LeX or with a control isotype, used at concentrations of 5 μg/ml. Then, 50 μl of each of these solutions or 50 μl of saline was added to 2 × 105 DCs contained in 50 μl of culture medium. Cells were incubated for an additional period of 30 min at 4°C and, after the cells were washed, the binding of biotin-labeled LeX-BSA was evaluated by flow cytometry using FITC-avidin. The results obtained showed that the anti-LeX antibody almost completely abrogates de binding of biotin-labeled LeX-BSA to DCs: the mean fluorescence intensities were 26 ± 12, 93 ± 22, and 37 ± 13 for DCs cultured without biotin-labeled LeX-BSA and DCs cultured with biotin-labeled LeX-BSA in the presence of the isotype control or the anti-LeX antibody, respectively (mean ± the standard error of the mean [SEM], n = 3). Together, these results support the notion that inhibition of HIV-1 capture by SP does not involve LeX carbohydrate structures.
High-mannose N-linked carbohydrates are one of the most important ligands recognized by DC-SIGN (2, 27, 58). Previous reports in the field of sperm fertility have shown that human SP contains substantial amounts of high-mannose N-linked carbohydrates (30, 31, 33). Taking this into consideration, we studied whether these compounds might account for the inhibitory effect of SP. High-mannose glycoproteins from SP were isolated by virtue of their ability to strongly bind to ConA (32-34), using ConA-Sepharose chromatography. As shown in Fig. 6F, the ConA interacting fraction of SP, eluted by 1 M of α-methyl-d-mannoside, effectively inhibited the binding of HIV-1 to DC-SIGN-positive cells. Moreover, it showed an inhibition profile very similar to that induced by whole SP. These results suggest that the inhibitory action exerted by SP could be accounted for, at least in part, by high-mannose N-linked carbohydrates.
Finally, we performed additional studies to demonstrate that SP components actually interact with DCs. Experiments were carried out with biotin-labeled SP. Figure 7A shows that the binding of SP components to DCs was detected even when SP was used at dilutions as high as 1:105. Strikingly, data from Fig. 7B show that mannan almost complete abrogates the binding of biotin-labeled SP to DCs, supporting a role for DC-SIGN in the recognition of SP components by DCs. To further examine this point, additional experiments were performed with B-THP-1 and B-THP-1-DC-SIGN-positive cells. Of note, the results depicted in Fig. 7C and D show that the binding of SP components is almost completely dependent on the expression of DC-SIGN.
FIG. 7.
Analysis of the role of DC-SIGN in the binding of biotin-labeled SP to DCs and B-THP-1-DC-SIGN cells. Biotinylation of SP was performed as described under Materials and Methods. (A) DCs (1.5 × 106 cells/ml) were incubated for 30 min at 4°C with different dilutions of biotin-labeled SP. Cells were then washed, and the binding of biotin-labeled SP to DCs was evaluated by flow cytometry using FITC-avidin. The results of a representative experiment (n = 4) are shown. (B) DCs (1.5 × 106 cells/ml) were incubated for 30 min at room temperature with or without mannan (1 mg/ml). The cells were then incubated with dilutions of biotin-labeled SP of 1:102 or 1:103 for 30 min at 4°C. The cells were then washed, and the binding of biotin-labeled SP to DCs was evaluated by flow cytometry using FITC-avidin. The results of a representative experiment (n = 3) are shown. (C and D) The expression of DC-SIGN (C) and the binding of biotin-labeled SP (1:102) (D) were measured by flow cytometry in B-THP-1 cells, B-THP-1-DC-SIGN cells, and mixed cell suspensions containing equal numbers of each cell type. The results of a representative experiment (n = 4) are shown.
DISCUSSION
The UNAIDS/WHO AIDS epidemic update estimated that almost 40 million people were living with HIV at the end of 2006. New infections have been occurring in the past few years at a rate of 5 million per year, and almost 3 million individuals succumb due to AIDS-related diseases each year (29, 51). Most infections are acquired through sexual transmission during vaginal or anal intercourse, with semen being the major transmission vector for HIV-1 (29, 51). Surprisingly, no previous studies have analyzed whether semen might modulate the interaction of HIV-1 with target cells. This raises a first question: might SP components gain access to the subepithelium in which the interaction of HIV-1 with target cells occurs? Disruption of the epithelium integrity is usually associated with vaginal or anal intercourse, as well as with the concurrent presence of ulcerative-sexually transmitted diseases (14, 17, 20, 39, 44, 49). This supports the notion that SP components may often gain access to target cells together with HIV-1. Moreover, SP components might gain access to DCs through an alternative route, e.g., by binding to DC projections that extend to, or near, the luminal surface of the challenged epithelium (7, 45, 51).
Our results demonstrate that SP abrogates the recognition of HIV-1 by DC-SIGN. The expression of DC-SIGN by DCs varies in different mucosal tissues. Subepithelial DCs in the lamina propria express DC-SIGN, while Langerhans cells localized within the squamous vaginal epithelium express langerin (CD207) (18, 20-22). In contrast to the well-established role of DC-SIGN in the dissemination of HIV-1 infection (21, 22), the role of langerin is controversial. It was generally assumed that Langerhans cells are able to mediate the transmission of HIV-1 to T CD4+ cells through langerin in a fashion similar to that of DC-SIGN (25, 46, 47). However, in a very recent report this view was challenged. de Witte et al. (10a) showed that, in stark contrast to DC-SIGN, langerin does not promote, but instead effectively prevents the infection of Langerhans cells and the subsequent transmission of HIV-1 to T CD4+ cells. This finding supports the notion that different populations of DCs in mucosal tissues, i.e., subepithelial DCs and Langerhans cells, may exert opposite effects on the dissemination of HIV infection. Further studies are needed to define whether SP might also be able to modulate the function of langerin expressed by Langerhans cells, which represent the first DC subset to encounter HIV-1 at the vaginal mucosa (29, 51).
Interestingly, the most remarkable expression of DC-SIGN in mucosal surfaces is found in DCs localized in the rectal mucosa that, unlike its vaginal counterpart, is separated from the lumen by only a single columnar epithelium, allowing virus access to DC-SIGN-positive DCs more easily (19, 22). This could explain, at least in part, why receptive anal intercourse is associated with the highest risk of HIV-1 transmission (29, 51). Supporting a role of DC-SIGN in HIV transmission through the rectal mucosa, Gurney et al. have recently shown that rectal DC-SIGN-positive DCs bind ∼15-fold more virus than DC-SIGN-negative DCs. Moreover, these authors also showed that DC-SIGN-positive DCs account for >90% of the virus bound to total mononuclear cells from the rectal mucosa, in spite of the fact that DC-SIGN-positive DCs only represent 1 to 5% of these total mononuclear cells (19). Together, these observations support the notion that DC-SIGN-dependent mechanisms might play an important role in the transmission of HIV-1 associated with anal sex.
Previous reports have shown that human fluids contain HIV-1 inhibitors. Naarding et al. (38) showed that human milk contains LeX-bearing glycoprotein(s) able to bind to DC-SIGN, preventing the capture and subsequent transfer of HIV-1 to T CD4+ lymphocytes. Subsequent observations indicated that the inhibitory activity of human milk could be attributed, at least in part, to bile salt-stimulated lipase, an LeX-carrying glycoprotein found in human milk at concentrations of 100 to 200 μg/ml (37). This inhibitor might interfere with HIV-1 transfer in breast-fed infants. On the other hand, Jendrysik et al. (23) showed that human cervicovaginal lavage fluid from healthy donors also contains an inhibitor of HIV-1 binding to DC-SIGN. By analyzing samples of cervicovaginal fluids, these authors found that 12.6% of these samples were able to inhibit the interaction of HIV-1 to B-THP-1-DC-SIGN cells and that the inhibitory activity is mediated by a high-molecular-weight glycoprotein. Endogenous anti-HIV-1 activity has been also demonstrated in whole, parotid, and submandibular/sublingual saliva, with lactoferrin, secretory leukocyte protease, and high-molecular-weight mucinous glycoprotein being the three major compounds responsible for this effect (26). Another natural HIV-1 inhibitor was recently described by Munch et al. (36). By screening a comprehensive peptide library generated from human hemofiltrates, these researchers identified a natural 20-residue C-proximal fragment of α1-antitrypsin as a broad-based inhibitor of HIV-1 that blocks HIV-1 entry by specific binding to the gp41 fusion peptide.
We show here for the first time that SP contains a potent inhibitor of the attachment of HIV-1 to DC-SIGN. In fact, this inhibitor almost completely prevented the capture and transmission of HIV-1 to T CD4+ cells mediated by DC-SIGN, even when it was assessed at very high dilutions of SP such as 1:104. It should be considered, however, that our experimental conditions do not reflect what occurs in vivo. In fact, while our experiments were performed with relatively high HIV-1 doses, infection in vivo seems to involve a low virus inoculum (51).
Human SP contains a diverse array of components, including lipids, carbohydrates, peptides, proteins, cytokines, and chemokines. These components are secreted from different sources: the testis, epididymis, and accessory glands (12, 40). The average protein concentration of SP ranges from 35 to 55 g/liter, and proteomic analysis leads to the identification of more than 900 proteins (13, 43, 54). We have not yet identified the inhibitor of HIV-1 attachment to DC-SIGN. Of note, experiments performed with biotin-labeled SP showed that DC-SIGN has a strong ability to recognize SP component(s). In fact, not only was the binding of biotin-labeled SP to DCs abrogated by mannan, but also its interaction with B-THP-1 cells was shown to be almost completely dependent on DC-SIGN expression. Interestingly, while the recognition of ligands by DC-SIGN usually leads to the internalization of the ligand and the downregulation of DC-SIGN expression, the recognition of SP components does not result in a reduced expression of DC-SIGN, suggesting that it might function as an attachment receptor for SP components rather than as an entry receptor. Similar observations were made regarding the interaction of DC-SIGN with zymosan particles (10), as well as with measles virus (11) and dengue virus (29).
Preliminary characterization of the SP inhibitor(s) indicates that it has a molecular mass greater than 100 kDa. The inhibitor(s) binds to ConA-Sepharose and can be eluted by high concentrations of α-methyl-d-glucopyranoside, suggesting that it contains high-mannose N-linked carbohydrates, which could be recognized by DC-SIGN. In fact, mannose- and fucose-containing carbohydrates are the two most important motifs recognized by DC-SIGN (2, 27, 58).
Interestingly, previous studies related to the field of fertility have shown that SP contains substantial amounts of high-mannose N-linked carbohydrates and that these compounds play an important role in the control of sperm function by virtue of their ability to avoid the acrosomal reaction (6, 30, 33). This suggests that a similar family of SP glycoproteins, characterized by the presence of high-mannose N-linked carbohydrates, might be able to control both sperm function and the attachment of HIV-1 to DCs. Compounds able to display contraceptive and anti-HIV-1 activity might represent an attractive therapeutic approach in the field of microbicides. Further studies are required to define the structural requirements of N-linked carbohydrates that enable them to efficiently impair both the attachment of HIV-1 to DCs and the fertilization ability of spermatozoa.
Acknowledgments
This study was supported by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas, the Buenos Aires University School of Medicine, and the Agencia Nacional de Promoción Científica y Tecnológica (Argentina).
We thank Selma Tolosa and Evelia López for their technical assistance.
Footnotes
Published ahead of print on 3 October 2007.
REFERENCES
- 1.Allen, R. D., and T. K. Roberts. 1986. The relationship between the immunosuppressive and cytotoxic effect of human seminal plasma. Am. J. Reprod. Immunol. Microbiol. 11:59-64. [DOI] [PubMed] [Google Scholar]
- 2.Appelmelk, B. J., I. van Die, S. J. van Vliet, C. M. Vandenbroucke-Grauls, T. B. Geijtenbeek, and Y. van Kooyk. 2003. Carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J. Immunol. 170:1635-1639. [DOI] [PubMed] [Google Scholar]
- 3.Berlier, W., M. Cremel, H. Hamzeh, R. Lévy, F. Lucht, T. Bourlet, B. Pozzetto, and O. Delézayi. 2006. Seminal plasma promotes the attraction of Langerhans cells via the secretion of CCL20 by vaginal epithelial cells: involvement in the sexual transmission of HIV. Hum. Reprod. 21:1135-1142. [DOI] [PubMed] [Google Scholar]
- 4.Bouhlal, H., N. Chomont, N. Haeffner-Cavaillon, M. D. Kazatchkine, L. Belec, and H. Hocini. 2002. Opsonization of HIV-1 by semen complement enhances infection of human epithelial cells. J. Immunol. 169:3301-3306. [DOI] [PubMed] [Google Scholar]
- 5.Bouvet, J. P., G. Gresenguet, and L. Belec. 1997. Vaginal pH neutralization by semen as a cofactor of HIV transmission. Clin. Microbiol. Infect. 3:19. [DOI] [PubMed] [Google Scholar]
- 6.Chalabi, S., R. L. Easton, M. S. Patankar, F. A. Lattanzio, J. C. Morrison, M. Panico, H. R. Morris, A. Dell, and G. F. Clark. 2002. The expression of free oligosaccharides in human seminal plasma. J. Biol. Chem. 277:32562-32570. [DOI] [PubMed] [Google Scholar]
- 7.Chieppa, M., M. Rescigno, A. Y. Huang, and R. N. Germain. 2006. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J. Exp. Med. 203:2841-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuluyan, H. E., and A. C. Issekutz. 1993. VLA-4 integrin can mediate CD11/CD18-independent transendothelial migration of human monocytes. J. Clin. Investig. 92:2768-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coligan, J. E., A. M. Kruibeek, D. H. Margulies, E. M. Shevach, and W. Strober. 1994. Morphological and biochemical assays of apoptosis, p. 3.17. 1-3.17.16. In Current protocols in immunology. Wiley, New York, NY.
- 10.de la Rosa, G., M. Yanez-Mo, R. Samaneigo, D. Serrano-Gomez, L. Martinez-Munoz, E. Fernandez-Ruiz, N. Longo, F. Sanchez-Madrid, A. L. Corbi, and P. Sanchez-Mateos. 2005. Regulated recruitment of DC-SIGN to cell-cell contact regions during zymosan-induced human dendritic cell aggregation. J. Leukoc. Biol. 77:699-709. [DOI] [PubMed] [Google Scholar]
- 10a.de Witte, L., A. Nabatov, M. Pion, D. Fluitsma, M. A. W. P. de Jong, T. de Gruijl, V. Piguet, Y. van Kooyk, and T. B. H. Geijtenbeek. 2007. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat. Med. 12:367-371. [DOI] [PubMed] [Google Scholar]
- 11.de Witte, L., M. Abt, S. Schneider-Schaulies, Y. van Kooyk, and T. B. Geijtenbeek. 2006. Measles virus targets DC-SIGN to enhance dendritic cell infection. J. Virol. 80:3477-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser, L. R., S. A. Adeoya-Osiguwa, R. W. Baxendale, and R. Gibbons. 2006. Regulation of mammalian sperm capacitation by endogenous molecules. Front. Biosci. 11:1636-1645. [DOI] [PubMed] [Google Scholar]
- 13.Fung, K. Y., L. M. Glode, S. Green, and M. W. Duncan. 2004. A comprehensive characterization of the peptide and protein constituents of human seminal fluid. Prostate 61:171-181. [DOI] [PubMed] [Google Scholar]
- 14.Galvin, S. R., and M. S. Cohen. 2004. The role of sexually transmitted diseases in HIV transmission. Nat. Rev. Microbiol. 2:33-42. [DOI] [PubMed] [Google Scholar]
- 15.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1 binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 16.Gray, R. H., M. J. Wawer, R. Brookmeyer, N. K. Sawankambo, D. Serwadda, F. Wabwire-Mangen, T. Lutalo, X. Li, T. vanCott, T. C. Quinn, et al. 2001. Probability of HIV-1 transmission per coital act in monogamous heterosexual HIV-1 discordant couples in Rakai, Uganda. Lancet 357:1149-1153. [DOI] [PubMed] [Google Scholar]
- 17.Guimaraes, M. D., D. Vlahov, E. A. Castillo, et al. 1997. Postcoital vaginal bleeding as a risk factor for transmission of the human immunodeficiency virus in a heterosexual partner study in Brazil. Arch. Intern. Med. 157:1362-1368. [DOI] [PubMed] [Google Scholar]
- 18.Gupta, K., and P. J. Klasse. 2006. How do viral and host factors modulate the sexual transmission of HIV? PLoS Med. 3:181-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurney, K. B., J. Elliott, H. Nassanian, C. Song, E. Soilleux, I. McGowan, P. A. Anton, and B. Lee. 2005. Binding and transfer of human immunodeficiency virus by DC-SIGN+ cells in human rectal mucosa. J. Virol. 79:5762-5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haase, A. T. 2005. Perils at mucosal front lines for HIV and SIV and their hosts. Nat. Rev. Immunol. 5:783-792. [DOI] [PubMed] [Google Scholar]
- 21.Hu, Q., I. Frank, V. Williams, W. J. Santos, P. Watts, G. E. Griffin, J. P. Moore, M. Pope, and J. R. Shattock. 2004. Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J. Exp. Med. 199:1065-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jameson, B., F. Baribaud, S. Pohlmann, D. Ghavimi, F. Mortari, R. W. Doms, and A. Iwasaki. 2002. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J. Virol. 76:1866-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jendrysik, M. A., M. Ghassemi, P. J. Graham, L. A. Boksa, P. R. Williamson, and R. M. Novak. 2005. Human cervicovaginal lavage fluid contains an inhibitor of HIV binding to dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J. Infect. Dis. 192:630-639. [DOI] [PubMed] [Google Scholar]
- 24.Judson, F. N. 1981. Epidemiology of sexually transmitted hepatitis B infection in heterosexuals: a review. Sex. Transm. Dis. 8(Suppl.):336-343. [PubMed] [Google Scholar]
- 25.Kawamura, T., S. E. Kurtz, A. Blauvelt, and S. Shimada. 2005. The role of Langerhans cells in the sexual transmission of HIV. J. Dermatol. Sci. 40:147-155. [DOI] [PubMed] [Google Scholar]
- 26.Kazmi, S. H., J. R. Naglik, S. P. Sweet, R. W. Evans, S. O′Shea, J. E. Banatvala, and S. J. Challacombei. 2006. Comparison of human immunodeficiency virus type 1-specific inhibitory activities in saliva and other human mucosal fluids. Clin. Vaccine Immunol. 13:1111-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koppel, E. A., K. P. van Gisbergen, T. B. Geijtenbeek, and I. van Kooyk. 2005. Distinct functions of DC-SIGN and its homologues L-SIGN (DC-SIGNR) and mSIGNR1 in pathogen recognition and immune regulation. Cell Microbiol. 7:157-165. [DOI] [PubMed] [Google Scholar]
- 28.Larsson, M. 2005. HIV-1 and the hijacking of dendritic cells: a tug of war. Springer Semin. Immunol. 26:309-328. [DOI] [PubMed] [Google Scholar]
- 29.Lederman, M. M., R. E. Offord, and O. Hartley. 2006. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nat. Rev. Immunol. 6:371-382. [DOI] [PubMed] [Google Scholar]
- 30.Lopes, C. H. G. L., M. N. Mazzini, H. Tortorella, R. A. Konrath, and A. Brandelli. 1998. Isolation, partial characterization and biological activity of mannosyl glycopeptides from seminal plasma. Glycoconj. J. 15:477-481. [DOI] [PubMed] [Google Scholar]
- 31.Lozach, P. Y., L. Burleigh, I. Staropoli, E. Navarro-Sanchez, J. Harriague, J. L. Virelizier, F. A. Rey, P. Desprès, F. Arenzana-Seisdedos, and A. Amara. 2005. Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN)-mediated enhancement of dengue virus infection is independent of DC-SIGN internalization signals. J. Biol. Chem. 280:23698-23708. [DOI] [PubMed] [Google Scholar]
- 32.Marquínez, A. C., A. M. Andreetta, N. González, C. Wolfenstein-Todel, and J. M. Cerezo. 2003. Identification of gp17 glycoprotein and characterization of prostatic acid phosphatase (PAP) and carboxipeptidase E (CPE) fragments in a human seminal plasma fraction interacting with concanavalin A. J. Prot. Chem. 22:423-429. [DOI] [PubMed] [Google Scholar]
- 33.Martins, S. G., P. V. Miranda, and A. Brandelli. 2003. Acrosome reaction inhibitor released during in vitro sperm capacitation. Int. J. Androl. 26:296-304. [DOI] [PubMed] [Google Scholar]
- 34.Mazzini, M. N., A. S. Cerezo, and J. M. de Cerezo. 1979. Affinity chromatography on concanavalin A-Sepharose of antigenic fractions of human seminal plasma. J. Chromatogr. 173:365-371. [DOI] [PubMed] [Google Scholar]
- 35.Miller, C., and R. J. Shattock. 2003. Target cells in vaginal HIV transmission. Microbes Infect. 5:59-67. [DOI] [PubMed] [Google Scholar]
- 36.Munch, J., L. Standker, K. Adermann, A. Schulz, M. Schibdler, R. Chinnadurai, S. Pohlmann, C. Chaipan, T. Biet, T. Peters, B. Meyer, D. Wilhelm, H. Lu, W. Jing, S. Jiang, W. G. Forssmann, and F. Kirchhoff. 2007. Discovery and optimization of a natural HIV-1 entry inhibitor targeting the gp41 fusion peptide. Cell 129:263-275. [DOI] [PubMed] [Google Scholar]
- 37.Naarding, M. A., A. M. Dirac, I. S. Ludwig, D. Speijer, S. Lindquist, E. L. Vestman, M. J. Stax, T. B. H. Geijtenbeek, and G. Pollakis. 2006. Bile salt-stimulated lipase from human milk binds DC-SIGN and inhibits human immunodeficiency virus type I. Antimicrob. Agents Chemother. 50:3367-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naarding, M. A., I. S. Ludwig, F. Groot, B. Berkhout, T. B. H. Geijtenbeek, G. Pollakis, and W. A. Paxton. 2005. Lewis C component in human milk binds DC-SIGN and inhibits HIV-1 transfer to CD4+ T lymphocytes. J. Clin. Investig. 115:3256-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novell, M. K., G. I. Benrudi, and R. J. Thompson. 1984. Investigation of microtrauma after sexual intercourse. J. Reprod. Med. 29:269-271. [PubMed] [Google Scholar]
- 40.Owen, D. H., and D. F. Katz. 2005. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J. Androl. 26:459-469. [DOI] [PubMed] [Google Scholar]
- 41.Padian, N., S. Shiboski, S. Glass, and E. Vittinghoff. 1997. Heterosexual transmission of human immunodeficiency virus (HIV) in northern California: results of a ten-year study. Am. J. Epidemiol. 146:350-357. [DOI] [PubMed] [Google Scholar]
- 42.Pandya, I. J., and J. Cohen. 1985. The leukocytic reaction of the human uterine cervix to spermatozoa. Fertil. Steril. 43:417-421. [DOI] [PubMed] [Google Scholar]
- 43.Pilch, B., and M. Mann. 2006. Large-scale and high confidence proteomic analysis of human seminal plasma. Genome Biol. 7:R40.0-R40.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piot, P., and M. Laga. 1989. Genital ulcers, other sexually transmitted diseases, and the transmission of HIV. BMJ 298:623-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pope, M., and A. T. Haase. 2003. Transmission, acute HIV infection and the quest for strategies to prevent infection. Nat. Med. 9:847-852. [DOI] [PubMed] [Google Scholar]
- 46.Pope, M., M. G. Betjes, N. Romani, H. Hirmand, P. U. Cameron, L. Hoffman, S. Gezelter, G. Schuler, and R. M. Steinmann. 1994. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell 78:389-398. [DOI] [PubMed] [Google Scholar]
- 47.Reece, J. C., A. J. Handley, E. J. Anstee, W. A. Morrison, S. M. Crowe, and P. U. Cameron. 1998. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J. Exp. Med. 187:1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serwadda, D., R. H. Gray, N. K. Sewankambo, F. Wabwire-Mangen, M. Z. Chen, T. C. Quinn, T. Lutalo, N. Kiwanuka, G. Kigozi, F. Nalugoda, M. P. Meehan, R. Ashley-Morrow, and M. J. Wawer. 2003. Human Immunodeficiency virus acquisition associated with genital ulcer disease and herpes simplex type 2 infection: a nested case-control study in Rakai, Uganda. J. Infect. Dis. 188:1492-1497. [DOI] [PubMed] [Google Scholar]
- 50.Sharkey, D. J., A. M. Macpherson, K. P. Tremellen, and S. A. Robertson. 2007. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol. Hum. Reprod. 13:491-501. [DOI] [PubMed] [Google Scholar]
- 51.Shattock, R. J., and J. P. Moore. 2003. Inhibiting sexual transmission of HIV-1 infection. Nat. Rev. Microbiol. 1:25-34. [DOI] [PubMed] [Google Scholar]
- 52.Sorice, M., T. Garofalo, R. Misasi, A. Longo, V. Mattei, O. Sale, V. Dolo, R. Gradini, and A. Pavan. 2001. Evidence for cell surface association between CXCR4 and ganglioside GM3 after gp120 binding in SupT1 lymphoblastoid cells. FEBS Lett. 506:55-60. [DOI] [PubMed] [Google Scholar]
- 53.Spira, A. I., P. A. Marx, B. K. Patterson, J. Mahoney, R. A. Koup, S. M. Wolinsky, and D. D. Ho. 1996. Cellular targets of infection and route of viral dissemination after and intravaginal inoculation of simian immunodeficiency virus entry into rhesus macaques. J. Exp. Med. 183:215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Starita-Geribaldi, M., S. Poggioli, M. Zucchini, J. Garin, D. Chevallier, P. Fenichel, and G. Pointis. 2001. Mapping of seminal plasma proteins by two-dimensional gel electrophoresis in men with normal and impaired spermatogenesis. Mol. Hum. Reprod. 7:715-722. [DOI] [PubMed] [Google Scholar]
- 55.Steinman, R. M., A. Granelli-Piperno, M. Pope, C. Trumpfhellerr, R. Ignatius, G. Arrode, P. Racz, and K. Tenner-Racz. 2003. The interaction of immunodeficiency viruses with dendritic cells. Curr. Top. Microbiol. Immunol. 276:1-30. [DOI] [PubMed] [Google Scholar]
- 56.Tevi-Benissan, C., L. Belec, M. Levy, V. Schneider-Fauveau, A. Si Mohamed, M. C. Hallouin, M. Matta, and G. Gresenguet. 1997. In vivo semen-associated pH neutralization of cervicovaginal secretions. Clin. Diagn. Lab. Immunol. 4:367-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson, L. A., C. L. Barratt, A. E. Bolton, and I. D. Cooke. 1992. The leukocyte reaction of the human uterine cervix. Am. J. Reprod. Immunol. 28:85-89. [DOI] [PubMed] [Google Scholar]
- 58.Van Kooyk, Y., and T. B. Geijtenbeek. 2003. DC-SIGN: escape mechanisms for pathogens. Nat. Rev. Immunol. 3:697-709. [DOI] [PubMed] [Google Scholar]
- 59.Vittinghoff, E., J. Douglas, F. Judson, D. McKiman, K. MacQueen, and S. P. Buchbinder. 1999. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am. J. Epidemiol. 150:306-311. [DOI] [PubMed] [Google Scholar]
- 60.Vodros, D., C. Tscherning-Casper, L. Navea, D. Schols, E. De Clercq, and E. M. Fenyo. 2001. Quantitative evaluation of HIV-1 coreceptor use in the GHOST3 cell assay. Virology 291:1-11. [DOI] [PubMed] [Google Scholar]
- 61.Waver, M. J., R. H. Gray, N. K. Sewankambo, D. Serwadda, X. Li, O. Laeyendecker, N. Kiwanuka, G. Kigozi, M. Kiddugavu, T. Lutalo, F. Nalugoda, F. Wabwire-Mangen, M. P. Meehan, and T. C. Quinn. 2005. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J. Infect. Dis. 191:1403-1409. [DOI] [PubMed] [Google Scholar]
- 62.Wilkinson, J., and A. L. Cunningham. 2006. Mucosal transmission of HIV-1: first stop dendritic cells. Curr. Drug Targets 7:1563-1569. [DOI] [PubMed] [Google Scholar]
- 63.Reference deleted.
- 64.Wu, L., and V. N. KewalRamani. 2006. Dendritic cell interactions with HIV: infection and viral dissemination. Nat. Rev. Immunol. 6:859-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu, L., T. D. Martin, M. Carrington, and V. N. KewalRamani. 2004. Raji B cells misidentified as THP-1 cells stimulate DC-SIGN-mediated HIV transmission. Virology 318:17-23. [DOI] [PubMed] [Google Scholar]