Abstract
A novel genetic vaccine that is based on a Venezuelan equine encephalitis virus (VEE) replicon launched from plasmid DNA is described. The plasmid encodes a VEE replicon under the transcriptional control of the cytomegalovirus immediate-early promoter (VEE DNA). The VEE DNA consistently expressed 3- to 15-fold more green fluorescent protein in vitro than did a conventional DNA vaccine. Furthermore, transfection with the DNA-launched VEE replicon induced apoptosis and type I interferon production. Inoculation of mice with VEE DNA encoding human immunodeficiency virus type 1 gp160 significantly increased humoral responses by several orders of magnitude compared to an equal dose of a conventional DNA vaccine. These increases were also observed at 10- and 100-fold-lower doses of the VEE DNA. Cellular immune responses measured by gamma interferon and interleukin 2 enzyme-linked immunospot assay were significantly higher in mice immunized with the VEE DNA at decreased doses. The immune responses induced by the VEE DNA-encoded antigen, however, were independent of an intact type I interferon signaling pathway. Moreover, the DNA-launched VEE replicon induced an efficient prime to a VEE replicon particle (VRP) boost, increasing humoral and cellular immunity by at least 1 order of magnitude compared to VEE DNA only. Importantly, immunization with VEE DNA, as opposed to VRP, did not induce any anti-VRP neutralizing antibodies. Increased potency of DNA vaccines and reduced vector immunity may ultimately have an impact on the design of vaccination strategies in humans.
To combat infectious diseases of great clinical importance, such as tuberculosis, AIDS, hepatitis C, and malaria, the development of new and efficient therapeutics and vaccines is highly desirable. DNA vaccination has shown great promise in small-animal models, especially at inducing cellular immunity, but has thus far failed to elicit robust immune responses in clinical trials. Effective dosage regimens and efficient adjuvants are required for efficient DNA immunization in humans. Numerous modalities to enhance immunogenicity are currently being investigated, aiming at the induction of both potent antibody and cellular responses.
In this quest for enhanced immunogenicity, several recombinant and plasmid-encoded cytokines, such as interleukin 2 (IL-2), granulocyte-macrophage colony-stimulating factor, IL-12, and IL-15, have been employed as molecular adjuvants with some success in both animal models and clinical trials (7, 39, 43). These cytokines may act by inducing an inflammatory response in the immunized tissue, thus recruiting immune cells to the site of immunization. Moreover, they may promote maturation of dendritic cells (DC) or modulate the immune response in other ways. For instance, IL-12 is a strong promoter of a Th1-type response, whereas IL-15 may contribute to induction of memory CD8-positive (CD8+) T cells (23). Furthermore, it has been shown that uptake of apoptotic bodies by DC is a major pathway for cross-priming and cross-presentation of major histocompatibility complex class I and class II peptides (1, 53). Therefore, proapoptotic genes have been used as molecular adjuvants in rodents (14, 40). Both B- and T-cell responses were stimulated, especially if apoptosis of the transfected cells was delayed, thus allowing substantial antigen expression. Infection with a pathogen often leads to a veritable cytokine storm in the host, and some recent strategies to increase the potency of gene-based vaccines focus on molecules capable of triggering innate immunity. Plasmid-encoded ligands to pathogen-recognition receptors, such as the bacterial Toll-like receptor 5 (TLR-5) ligand flagellin, can be used to potentiate genetic vaccines. Flagellin can increase humoral and cellular immune responses to influenza virus NP and confers increased protection against lethal influenza virus challenge in mice (4).
Another proposed strategy for increasing immune responses from DNA vectors involves optimization of expression levels. Currently, the cytomegalovirus (CMV) immediate-early promoter is the one most frequently used for DNA vaccines. However, expression in transfected cells rarely rivals that of alphavirus, flavivirus, or adenovirus vector-transduced cells.
Viral replicons based on alphaviruses, such as Venezuelan equine encephalitis virus (VEE), Sindbis virus (SIN), or Semliki Forest virus (SFV), are appealing vaccine vectors. Alphaviruses are arthropod-borne members of the family Togaviridae with a positive-sense single-stranded genome of approximately 11.7 kb. After infection, the 5′ end of the parental genome, including the viral replicase nonstructural proteins 1, 2, 3, and 4 (nsP1, nsP2, nsP3, and nsP4) genes, is translated in the cytoplasm. Next, the replicase catalyzes the transcription of a negative-sense copy of the genome, which serves as a template for progeny genomes and high-level transcription of subgenomic mRNA from the internal 26S promoter (44). Replacing the structural genes downstream of the 26S promoter with the gene to be expressed and supplying the alphavirus structural genes in trans produces replicon-containing particles capable of expressing copious amounts of the transgene product (31, 37). Moreover, alphavirus replicon-based vaccines target DC in vivo (17, 33), induce apoptosis of transduced cells (30), and activate innate inflammatory responses and release of type I interferons (41, 47). However, there remain theoretical safety issues and considerable practical impediments to cost-effective manufacture of alphavirus replicons. For these reasons, it may be desirable to launch a self-amplifying replicon RNA from a DNA plasmid in vivo. This strategy not only obviates recombinant virus production problems, but also provides a means to greatly enhance the efficacy of DNA-based vaccines. Previously, DNA-launched SIN- and SFV-based vectors have been proven effective in small-animal models (9, 20). Based on the properties of VEE, it is likely that a DNA-launched VEE replicon has properties different from those of other DNA-launched alphavirus vectors. For instance, VEE gene expression is considerably more tolerant of type I interferons than SIN, yet VEE induces high levels of alpha/beta interferons (IFN-α/β) (36, 50).
Mixed-modality immunization regimens, such as DNA prime followed by recombinant-virus boost, have repeatedly been described to increase immune responses to the vectored immunogen (2, 10, 15, 42, 46, 54). One obvious advantage of using a DNA prime versus priming with the recombinant vector is to avoid antivector immunity, which potentially could severely hamper the efficiency of the booster immunization (46). Moreover, priming with DNA is believed to effectively focus the immune response toward the vectored immunogen, thus eliminating potential anamnestic responses to the vector itself.
Here, we present a novel VEE-launched DNA-based immunogen that greatly increases the immunogenicity of an encoded human immunodeficiency virus type 1 (HIV-1) gp160 gene in immunized mice. VEE DNA induced apoptosis and expressed larger amounts of protein per plasmid transfected in vitro than the same gene driven by the CMV promoter. In addition, VEE DNA triggers type I interferon production and potentially other yet-to-be-determined immune-enhancing innate mediators. We hypothesize that these vector characteristics, either individually or combined, are responsible for the increased immune responses induced by VEE DNA. Notably, humoral immune responses to the HIV-1 gp160 antigen were greatly enhanced, as were cellular responses, at significantly reduced vaccine doses relative to a conventional DNA vaccine. Immune responses could be further enhanced by mixed-modality prime-boost immunizations using the DNA-launched VEE and VEE replicon particles (VRP) encoding the same antigen.
MATERIALS AND METHODS
Cloning.
A construct carrying the VEE genome with a multiple cloning site (MCS) downstream of the 26S promoter was constructed using conventional cloning techniques. Restriction enzymes, PCR polymerases, and alkaline phosphatase were provided by New England Biolabs. Reagents for DNA purification were purchased from Qiagen and used according to the manufacturer's recommendations. Briefly, the 5′ end of the VEE genome was joined to the CMV immediate-early promoter in such a way that the first nucleotide transcribed was the first nucleotide of the VEE genome 5′ untranslated region (UTR). The 3′ end of the CMV promoter includes a SacI site 14 nucleotides upstream of the transcription start site. This SacI site and its downstream sequence were incorporated in a PCR primer also including the first 18 nucleotides of the VEE genome. The reverse primer annealed to the genomic sequence between nucleotides 365 and 382 and also included a BsmBI site, leaving an EcoRI site downstream of the coding sequence. A PCR amplifying the 5′ end of the VEE genome through nucleotide 382 was performed, and the fragment was cloned into the pCI-neo plasmid (Promega) digested with SacI and EcoRI. Next, the plasmid was digested with AccIII and NotI, and an insert digested with the same enzymes derived from a replicon cDNA (pV5005) including a fragment of the nsP3 gene, the nsP4 gene, the 26S promoter, the green fluorescent protein (GFP) gene, the 3′ UTR, and the viral poly(A) was cloned into the vector. This plasmid was digested with AccIII, and an AccIII-digested fragment of the replicon cDNA including parts of the nsP1 gene, the nsP2 gene, and parts of the nsP3 gene was introduced. This vector expressed GFP, as detected by UV light microscopy.
The next step was to transfer the replicase-GFP-encoding unit to the pVAX1 vector (Invitrogen), a more versatile vector for DNA vaccination. pVAX1 is smaller than the pCI-neo vector and has been constructed to be consistent with the FDA document on plasmid DNA vaccines for preventive infectious-disease indications (16a). The vector contains the CMV immediate-early promoter, the bovine growth hormone (BGH) poly(A) signal, and the kanamycin resistance gene. We took advantage of the unique SnaBI site present in the CMV promoter and the NotI site downstream of the 3′ end of the VEE sequence, as well as in the pVAX1 MCS. Thereby, the whole VEE-GFP cassette was transferred to the pVAX1 backbone. The GFP gene was excised using ApaI and PmeI and replaced by an MCS. Four 73- to 80-mer primers with 20-nucleotide overlaps were designed to generate a 228-bp-long synthetic gene including the sequence from the ApaI site in the 3′ end of nsP4, the 26S promoter, an MCS, the 3′ UTR, and a PmeI site: ACTGGGGCCCCTATAACTCTCTACGGCTAACCTGAATGGACTACGACATAGTCTAGTCCGCCAAGATATGCGG, TAAGCAGCTTGCCAATTGCTGCTGTATTCTAGAGGCGCGCCTTAATTAAGGCCGGCCGCGGCCGCATATCTTGGCGGACT, CAGCAATTGGCAAGCTGCTTACATAGAACTCGCGGCGATTGGCATGCCGCTTTAAAATTTTTATTTTATTTTTCTTTTCT, and CAGTGTTTAAACGAAATATTAAAAACAAAATCCGATTCGGAAAAGAAAAGAAAAATAAAATAAAAATTTTAAAGCGGCAT.
The MCS was amplified by overlap extension PCR and cloned into the vector. To introduce the viral poly(A) sequence, two overlapping primers containing a minimal 17-nucleotide poly(A) signal from VEE were used. In an overlap extension PCR using an nsP4-specific and a BGH poly(A)-specific primer, the VEE poly(A) region was amplified and cloned into the vector digested with XbaI and PmeI by homologous recombination, thus finalizing the pCMVEE plasmid backbone (Fig. 1).
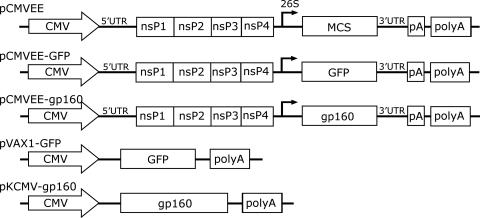
FIG. 1.
Constructs. A DNA-based expression system that encodes the VEE 5′ UTR; nsP1, nsP2, nsP3, and nsP4; the 26S subgenomic promoter followed by an MCS for cloning of transgenes; the 3′ UTR; and the vector-encoded poly(A) signal (polyA) the viral poly(A) signal (pA) under the control of the CMV immediate-early promoter was constructed and cloned into the pVAX1 plasmid vector (pCMVEE). The GFP gene and the HIV-1 gp160 genes were subsequently cloned into the MCS (pCMVEE-GFP and pCMVEE-gp160). For comparison, we used the GFP and gp160 genes expressed from a conventional expression plasmid (pVAX1-GFP and pKCMV-gp160).
Next, the GFP reporter gene was inserted into pCMVEE. GFP-specific primers were therefore constructed containing the SchI type II restriction enzyme cleavage site in the forward primer and an AscI site in the reverse primer. The GFP gene was amplified in a standard PCR, purified, and subcloned into PCR-script (Stratagene). The GFP gene was first excised using XhoI, which cuts the PCR-script vector upstream of the insert, and with AscI, which cuts in the 3′ end of the reverse primer. The GFP-encoding DNA fragment was purified on an agarose gel and subsequently treated with SchI (Fermentas). The GFP-encoding fragment was then ligated into pCMVEE treated with EcoRV and AscI to generate the pCMVEE-GFP construct (Fig. 1).
The gp160 gene derived from the HIV-1 subtype B laboratory-adapted Lai strain from the pKCMV-gp160 DNA vaccination vector (a kind gift from Britta Wahren, Swedish Institute for Infectious Disease Control) (Fig. 1) was amplified by PCR and cloned into pCMVEE using the EcoRV and NotI restriction sites. The sequence was confirmed by sequencing, and a schematic of the construct is presented in Fig. 1.
VRP.
A VRP expressing HIV gp160 was constructed by restriction digestion of the pCMVEE-gp160 construct with ApaI and AscI and cloning this fragment (including the 3′ end of the nsP4 gene, the 26S promoter, and the gp160 gene) into the T7-driven pVR21 vector backbone used for VRP production. VRP expressing GFP (VRP-GFP), HIV-1 gp160, or no transgene (null VRP) (45) were packaged by using a split helper system as previously described (37). Briefly, three RNA transcripts were coelectroporated into BHK-21 cells: the replicon genome, which contained the four VEE nonstructural genes and the heterologous gene expressed from the viral 26S promoter, and two defective helper RNAs, which provided either the wild-type capsid or the wild-type glycoprotein genes but lacked the virus-specific packaging signal. Since VRP replicon genomes lack the viral structural protein genes, infectious VRP undergo only one round of infection. After being packaged, VRP were harvested, concentrated through a sucrose cushion, and resuspended. Titers were determined on BHK-21 cells either by immunofluorescence (VRP-GFP) or immunocytochemistry using sera containing antibody to the VEE nonstructural proteins. Titers were expressed as infectious units (IU) per ml.
Expression.
Vero cells were transfected with Lipofectamine 2000 (Invitrogen), and expression of the cloned HIV-1 envelope gene was assessed in cell lysates by Galanthus nivalis agglutinin lectin precipitation, followed by Western blotting, as described previously (32). For gp160 detection, a cocktail of three mouse monoclonal antibodies (5F7, 1577, and Chessie 6) or human immune serum immune globulin was used. The NIH AIDS Research and Reference Reagent Program kindly provided all antibodies. GFP expression was quantified by flow cytometry (FACScan [Becton Dickinson] or Cyan flow cytometer [Dako]) and analyzed by establishing a region for GFP-positive cells on the untransfected cells that avoided the autofluorescent cells and was arranged to include 0.1% or fewer positive events in the negative control (untransfected) population. This positive region included both strongly and weakly positive cells. GFP expression was represented as the mean fluorescence intensity (MFI) over time. BHK-21, Vero, C2C12 myoblast, and NIH 3T3 cells were transfected using Lipofectamine 2000. Subsequently, the cells were washed with phosphate-buffered saline (PBS), harvested, and either fixed in 2% paraformaldehyde at various time points (6, 12, 18, 24, 36, 48, 72, and 96 h) and kept at +4°C until they were read or read unfixed immediately after being harvested.
Apoptosis.
C2C12 myoblasts or NIH 3T3 cells in six-well culture plates were transfected with pVAX-GFP and pCMVEE-GFP using Lipofectamine 2000 or infected with VRP-GFP. Camptothecin was utilized as a positive control for apoptosis induction. At the time of assay, nonadherent cells were collected and adherent cells were trypsinized. Fresh, serum-containing medium was added to each culture to quench the enzymatic activity of trypsin, and cells were collected and washed twice with PBS by centrifugation at +4°C. Then, the cells were resuspended to a final concentration of 1 × 106 cells ml−1 in 1× binding buffer (Becton-Dickinson [BD]). The cells were kept on ice, and annexin V-phycoerythrin (PE) and 7AAD (BD) were added according to the manufacturer's recommendations. The cells were stained for at least 15 min before being analyzed on a Cyan flow cytometer. Transfected cells were selected by gating on GFP-expressing cells in the FL1 channel and then analyzed for annexin V-PE binding in FL2 and for 7AAD staining in FL3.
Interferon bioassay.
The type I interferon bioassay was performed on collected supernatants from pCMVEE-GFP- and pVAX-GFP-transfected, as well as VRP-GFP-infected, C2C12 murine myoblasts essentially as described previously (3, 50). Briefly, L929 mouse fibroblasts (ATCC CCL-1) were seeded in 96-well plates. All supernatants were acidified to a pH of 2.0 for 24 h and then were neutralized to pH 7.4 prior to titration of twofold dilutions. Twenty-four hours after the addition of the supernatant, interferon-sensitive encephalomyocarditis virus was added to each plate. At 18 to 24 h postinfection, 3-[4,5-dimethylthiazol-2yl]-2,5-diphenyltetrazolium bromide (MTT) (Sigma), an indicator of viable cells, was added to each well. The MTT product was then dissolved in isopropanol-0.4% HCl, and the absorbance was read on a microplate reader at 570 nm. Each plate contained an interferon standard (Chemicon or R&D Systems) to determine the international units (IU/ml) of type I interferon in each culture.
Humoral immune responses.
Serum samples were collected by tail vein bleeds or terminal bleeds in microtainer tubes. Humoral immune responses were determined by enzyme-linked immunosorbent assay (ELISA) carried out as described previously (32). Briefly, ELISA plates were coated with 100 to 200 ng of recombinant gp160 (kindly provided by Britta Wahren) in 0.1 M NaCO3, pH 9.5, per well at room temperature overnight. Then, the plates were washed four times with ELISA wash buffer (EWB) (PBS containing 0.05% Tween 20). Sera were diluted in EWB supplemented with 10% Sigma block (EWB-Sigma), 100 μl was added to each well, and binding was allowed overnight at room temperature. The plates were washed as described above, and a horseradish peroxidase-anti-mouse immunoglobulin G (IgG) antibody (Santa Cruz) diluted 1:2,000 in EWB-Sigma was added and incubated for 1 h at 37°C. The plates were washed again, and 100 μl of substrate (0.05 M phosphate-citrate buffer, pH 5, containing 0.2 mg/ml o-phenylenediamine activated with 0.12‰ H2O2) was added per well. After 30 min, 0.1 M NaFl was added to stop the color reaction, and the optical density at 450 nm was determined. End point titers were determined as the greatest dilution with an optical density at 450 nm of ≥0.2. Neutralizing immune responses to HIV-1 were determined as described previously (29).
VEE neutralization was performed as follows. At day zero, 24-well plates were seeded with 105 BHK cells per well and incubated at 37°C and 5% CO2 overnight. At day 1, serum samples that were inactivated for 30 min at 56°C were serially diluted to the desired concentrations and mixed with GFP-VRP at a multiplicity of infection (MOI) of 0.1 in serum-free alpha-minimal essential medium (2 × 104 infectious units/50 μl). The neutralization reaction mixture was incubated for 1 h at 37°C and added to cells after the removal of cell culture medium. After 1 h of infection at 37°C-5% CO2, the inoculum was replaced with alpha-minimal essential medium containing 10% fetal bovine serum (FBS), and the infected cells were incubated overnight (≤18 h) at 37°C-5% CO2. At day 2, cells were harvested by trypsinization and centrifugation before fixation in 2% paraformaldehyde in PBS. The rate of infection was read by FACSscan as the number of GFP-expressing cells. Neutralization was calculated as the number of GFP-expressing cells in a given sample at a given dilution divided by the number of GFP-expressing cells incubated with a neutralization mixture consisting of serum from the corresponding prebleed at the same dilution. The neutralization titer was determined as the highest dilution of serum that reduced the number of GFP-VRP-infected cells by ≥50%.
Cellular immune responses.
Cellular immune responses were assessed in splenocytes by IFN-γ and IL-2 enzyme-linked immunospot (ELISPOT) assays or by intracellular cytokine staining (ICS) for IFN-γ and IL-2 in CD4+ and CD8+ T cells. For both assays, cells were prepared identically. Spleens were excised from anesthetized animals and ground with the plunger from a 10-ml syringe through a 40-μm cell grinder (BD Falcon) in a petri dish with 5 ml Hanks balanced salt solution (HBSS). The cells were transferred to a 15-ml polypropylene tube, and 5 ml of lympholyte was slowly underlaid with a 5-ml pipette. The cells were then centrifuged at room temperature at 2,500 rpm for 30 min without braking. The lymphocytes were subsequently collected and washed twice with HBSS. The cells were resuspended in RPMI 1640 containing 10% FBS and diluted to a working concentration of 2 million cells per ml. For ELISPOT analysis, 200,000 cells per well were seeded with the appropriate peptide stimulation (2 μg per well) or concanavalin A (5 μg per well) as a positive control in 96-well tissue culture dishes coated with anti-IFN-γ or anti-IL-2 antibodies. The cells were then cultured for 20 to 24 h at +37°C-5% CO2 and developed as recommended by the manufacturer (Mabtech).
For ICS, all reagents were obtained from BD Biosciences. Two million cells were seeded per well in 12-well plates, and 1 ml of stimulation peptide at 2 μg/ml or phorbol myristate acetate (100 ng/ml) plus ionomycin (1,000 ng/ml) were added to the cultures. Stimulation proceeded in the presence of 100 μl of 200-μg/ml brefeldin A per well at +37°C-5% CO2 for 12 h. Then, cells were harvested, transferred to 5-ml polystyrene tubes, and collected by centrifugation at +4°C at 1,000 rpm for 8 min. Subsequently, the cells were resuspended in 200 μl HBSS containing 1% FBS and 0.1% Na azide (HFA), and transferred to round-bottom 96-well plates. The plates were centrifuged at +4°C at 1,000 rpm for 4 min. The cells were resuspended in 75 μl of biotinylated CD4+ antibody diluted 1:1,600 in HFA and incubated on ice for 1.5 h. Then, 100 μl of HFA was added and the plates were centrifuged as before. The cells were washed once with 200 μl HFA and resuspended in 75 μl secondary antibodies in HFA—PerCP-SA (1:400), fluorescein isothiocyanate anti-CD3ɛ (1:200), and allophycocyanin anti-CD8 (1:800)—and incubated on ice for 1 h in the dark. The cells were then washed and collected as described above and resuspended in 100 μl Cytofix/Cytoperm (BD) for 20 min on ice in the dark. One hundred microliters of 1× Perm Wash was added, and the cells were centrifuged at +4°C at 1,000 rpm for 4 min and washed once in Perm Wash. The tertiary antibody (PE anti-IFN-γ) was added at a 1:800 dilution in Perm Wash. Then, the cells were incubated on ice in the dark for at least 1 h before they were collected and washed as before in HFA, and the samples were read on the FACSCALIBUR (Becton Dickinson).
Statistical analysis.
For pairwise analysis between two groups (same dose, different plasmid vaccine vector), we performed the nonparametric Mann-Whitney U test to analyze for significance. A P value of less than 0.05 was used as an indicator of statistical significance.
For multiple comparisons, we used the Kruskal-Wallis test for a priori analysis, followed by a post hoc analysis using Dunn's test. A P value of less than 0.05 was used as an indicator of statistical significance. All statistical analysis was performed with the GraphPad Prism 4 software package.
RESULTS
Construction of pCMVEE, pCMVEE-GFP, and pCMVEE-gp160.
A plasmid backbone (pCMVEE) was constructed that encoded the VEE 5′ UTR, nsP1 to -4, the 26S subgenomic promoter followed by an MCS for cloning of transgenes, the 3′ UTR, and the viral poly(A) signal, all under the control of the CMV immediate-early promoter (Fig. 1). The construct was engineered so that the transcription start site of the CMV promoter was positioned at the first nucleotide of the 5′ UTR. To clone transgenes into the vector, the first restriction site in the MCS is EcoRV, which cleaves after the AT nucleotides of the VEE capsid gene start codon. Using this site ensures reconstitution of the exact wild-type 26S promoter. Inserts can either be subcloned and excised using a type II restriction site compatible with EcoRV (e.g., SchI) and one of the downstream restriction sites in the MCS or be generated with PCR primers having the start codon G nucleotide as their first nucleotide. The GFP gene and the HIV-1 envelope gene were cloned into pCMVEE using these approaches, respectively, thus generating the pCMVEE-GFP and pCMVEE-gp160 plasmids (Fig. 1).
Expression.
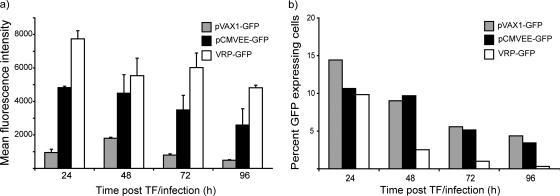
In order to determine expression levels from the pCMVEE vector, GFP expression was compared to CMV-driven GFP expression from the pVAX1 plasmid and from a VRP encoding GFP by flow cytometry-based analysis. In several independent experiments performed in various murine and primate cell lines, expression from the DNA-launched replicon was substantially increased compared to CMV-driven GFP expression and approached that of VRP-expressed GFP. These differences were also readily detectable under the UV microscope. Compared to CMV-driven GFP expression, the MFI was increased 3- to 17-fold, depending on the time point posttransfection and the cell line used. Cells infected with VRP-GFP expressed 1.5 to 2 times more protein than cells transfected with pCMVEE-GFP. In general, VRP-expressed GFP was first observed around 6 h posttransfection, whereas DNA-launched GFP expression appeared in the 6- to 12-h window. CMV-driven GFP expression could be detected at 18 to 24 h posttransfection (data not shown). In Fig. 2a, an average of two independent time course experiments in murine C2C12 myoblast cells is presented. We chose to use the C2C12 cells for two reasons. First, they are myoblasts and are relatively closely related to muscle cells transfected in vivo. Second, they have an intact interferon response system that may restrict replicon amplification similarly to muscle cells in vivo. In this particular cell line, the MFI from the pVAX-GFP was just below 1,000 at 24 h posttransfection. At the same time point, the MFI of pCMVEE-GFP-transfected cells was close to 5,000 and VRP-GFP-infected cells had an MFI of over 7,700. Over time, the MFI ratios shifted slightly, but the overall pattern persisted. CMV-driven GFP expression peaked at 48 h and then gradually decreased again through 96 h, whereas GFP expressed from VEE-DNA was already high at 24 h and decreased gradually from 72 h. VRP-encoded GFP expression remained high throughout the experiment on a per cell basis. We observed, however, that the number of GFP-positive cells in the VRP-GFP-infected cultures decreased much faster over time, whereas the number of GFP-positive cells in the DNA-transfected cultures declined at a much lower rate. In Fig. 2b, one representative experiment performed at a transfection efficiency of 10 to 15% and an MOI of 0.1 is presented.
FIG. 2.
Expression. Murine C2C12 myoblast cells were transfected with pCMVEE-GFP or pVAX1-GFP or infected with VRP-GFP. GFP expression was measured by flow cytometry at 24, 48, 72, and 96 h posttransfection (post TF)/infection. The expression, represented as an MFI of 1,000 to 20,000 recorded cells, is shown in panel a. An average of two independent experiments is shown. The error bars represent the standard errors of the mean. In these cell cultures, we followed the decrease in the number of green cells in proportion to the total number of cells over time, and the results from one representative experiment are presented in panel b.
HIV-1 gp160 expression from the pCMVEE-gp160 and pKCMV-gp160 plasmids also was analyzed by Western blotting (data not shown). Consistent with the results of flow cytometry of GFP expression, HIV-1 envelope expression from the DNA-launched replicon appeared to exceed that of the conventional expression plasmid. Thus, expression from the DNA-launched VEE replicon augmented expression levels considerably compared to conventional CMV-driven expression systems.
Induction of apoptosis.
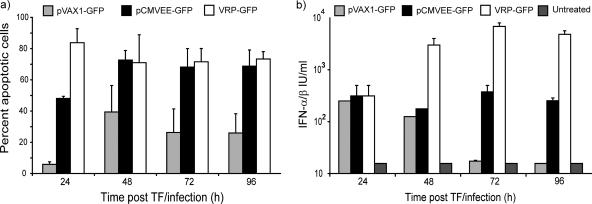
Apoptosis is an important mechanism for cross-presentation of viral antigens to DC (1). Alphavirus infections are known to induce apoptosis and/or necrosis in infected cells (reviewed in references 28 and 44). In addition, Leitner and colleagues have demonstrated that apoptosis is a crucial component for enhanced antitumor immunity induced by DNA-launched SIN replicons (25). Given the rapid decline in the number of GFP-expressing cells, we hypothesized that this could be due to apoptosis of the VRP-infected cells. Interestingly, NIH 3T3, C2C12, Vero, and BHK cells transfected with the pCMVEE plasmid observed over time in cell culture also acquired apoptotic morphology (data not shown). Therefore, NIH 3T3 and C2C12 cells were transfected or infected with pCMVEE-GFP, pVAX-GFP, or VRP-GFP. Cells were harvested daily over a 4-day period and stained with annexin V and 7AAD. By flow cytometry, we gated on GFP-expressing cells and studied the proportion of apoptotic and apoptotic/necrotic cells in the GFP-expressing population. VRP-GFP-infected cells showed extensive signs of apoptosis and necrosis at 24 h postinfection, corresponding to the observed decrease in the number of GFP-positive cells. Cells transfected with pCMVEE-GFP showed increased signs of apoptosis over time compared to cells transfected with pVAX-GFP plasmid in several independent experiments. An average of two representative independent experiments in C2C12 myoblasts are depicted in Fig. 3a, demonstrating the increase in the number of apoptotic cells in the VEE-DNA-transfected cell cultures compared to pVAX1-GFP-transfected cells. The number of apoptotic cells, however, was lower than the number of apoptotic cells in the VRP-GFP-infected cell cultures at early time points after infection/transfection, indicating slower kinetics for apoptosis induction in cells transfected with a DNA-encoded replicon than in VRP-infected cells. This also indicates that cell death is not solely responsible for the decrease in the number of GFP-positive cells over time in pVAX-GFP-transfected cells shown in Fig. 2b. It has previously been determined that expression from the CMV promoter is transient and may decrease by more than 60% over a 7-day period (52).
FIG. 3.
Induction of apoptosis and type I interferons. (a) To assess the amount of cell death induced in cells transfected (TF) with pVAX1-GFP or pCMVEE-GFP or infected with VRP-GFP over time, we performed annexin V and 7AAD staining. Using flow cytometry-based analysis, we gated on GFP-expressing cells and determined the number of annexin V-positive (apoptotic) and annexin V- and 7AAD-double-positive (apoptotic/necrotic) cells. The average proportion of apoptotic and apoptotic/necrotic (percent apoptotic) cells in two independent experiments in C2C12 myoblasts is represented. The error bars represent the standard errors of the mean (SEM). (b) Supernatants from the same cell cultures with an initial transfection/infection rate of approximately 10% were collected and tested for production of IFN-α/β in a functional type I interferon bioassay. The supernatants were used to treat L929 cells prior to challenge with encephalomyocarditis virus, and viability was determined and compared to that of a type I interferon control. Type I interferons are represented as IU per ml over time in one representative experiment. The error bars represent the SEM.
Induction of type I interferons.
Infection with many viruses, including alphaviruses, induces an antiviral response partly mediated by IFN-α/β. It has been reported that events upstream of type I interferon induction, such as recognition of double-stranded RNA by RNase L, can be induced by alphavirus DNA vaccines (26). Moreover, in certain models, a type I interferon response is essential for enhanced antitumor immunity using SIN-based DNA vaccine encoding a tumor antigen (24). We determined whether transfection of the pCMVEE plasmid was sufficient to induce IFN-α/β production in vitro. Supernatants were collected from DNA-transfected or VRP-infected C2C12 myoblast cultures and assayed for type I interferon production in a bioassay (3, 50). In an initial experiment, supernatants from several VRP-GFP- and pCMVEE-GFP-transfected C2C12 cell cultures clearly produced detectable amounts of interferon at 42 h postinfection/transfection, whereas untreated and pVAX-GFP-transfected cells did not (data not shown). The transfection efficiency and the rate of infection correlated with the amounts of type I interferons produced. Therefore, supernatants from cell cultures with a transfection efficiency of approximately 10% or an MOI of 0.1 were collected daily over a 96-h period and assayed for type I interferon production. Over time, VRP-GFP-infected cells produced about 10-fold more type I interferons than pCMVEE-GFP-transfected cells, which in turn produced more interferon at the later time points than pVAX-GFP-transfected cells (Fig. 3b).
Immunizations and immunity.
The gp160 gene used in this study has previously been thoroughly investigated in the context of a conventional DNA vaccine under transcriptional control of the immediate-early CMV promoter (pKCMV-gp160). It induces readily detectable T-cell responses but fails to induce potent antibody responses in small-animal models unless adjuvanted by recombinant granulocyte-macrophage colony-stimulating factor (10, 11, 32, 39).
In three independent experiments, mice were immunized twice intramuscularly in the gastrocnemius muscle at 6-week intervals. Here, we present results from a head-to-head comparison with eight BALB/c mice per group immunized with pCMVEE-gp160 (100 μg, 10 μg, and 1 μg, referred to as 100×, 10×, and 1×, respectively) or with equimolar amounts of pKCMV-gp160 (47 μg, 4.7 μg, and 0.47 μg, referred to as 100×, 10×, and 1×, respectively). Mice vaccinated with the DNA-launched VEE replicon expressing the HIV-1 env gene consistently elicited group mean serum IgG anti-envelope antibody titers above 103 at all doses tested. As has been shown previously, immunization with the conventional DNA vaccine with the gp160 gene under the direct control of the CMV promoter did not induce any serum IgG antibody responses above the level of detection at any dose in any of these studies (Fig. 4). The antibody responses were significantly higher (P < 0.05) in the mice immunized with VEE-DNA than in animals immunized with the same dose of pKCMV-gp160. The antibody subclass (IgG2a/IgG1) ratios were determined for the responders and were consistent with a Th1-type response (IgG2a/IgG1 ≥ 1.0) (data not shown).
FIG. 4.
Humoral and cellular immunity. Mice (BALB/c; n = 8/group) were immunized intramuscularly in the gastrocnemius muscle with PBS, pKCMV-gp160, or pCMVEE-gp160 at weeks 0 and 6. Three doses of pCMVEE-gp160 were evaluated—100 μg, 10 μg, and 1 μg (100×, 10×, and 1×, respectively)—and compared to equimolar amounts of pKCMV-gp160—47 μg, 4.7 μg, and 0.47 μg (100×, 10×, and 1×, respectively). (a) Serum IgG anti-gp160 end point titers were measured by ELISA 2 weeks after the boosting immunization. The level of detection was at an end point titer of 100. (b and c) IFN-γ (b) and IL-2 (c) ELISPOTs were performed on purified lymphocytes from the spleen and are expressed as spot-forming cells (SFC) per million lymphocytes. (d) We pooled lymphocytes from all animals within one group and stained for CD4, CD8, and IFN-γ in an ICS. The proportion of IFN-γ-positive CD8+ T cells in the total CD8+ T-cell population is presented. For all assays of the cellular immune response, we used the immunodominant P18 epitope from HIV-1 subtype B envelope for restimulation. The Mann-Whitney U test was used to test for statistical differences between groups immunized with the same dose. The error bars indicate the standard errors of the mean.
In addition to the significantly increased humoral immune response, cellular immunity was maintained in mice immunized with the DNA-launched VEE replicon expressing the HIV-1 env gene. Moreover, the lowest dose of the DNA-launched VEE DNA vaccine expressing the gp160 gene was equally immunogenic to the conventional DNA vaccine at a 100-fold-higher dose. Only 1 μg of pCMVEE-gp160 induced IFN-γ and IL-2 (Fig. 4b and c) responses, whereas 100 times more plasmid copies (47 μg) of the pKCMV-gp160 plasmid were needed to obtain a similar cellular response. At the decreased doses (pCMVEE-gp160 at 10× versus pKCMV-gp160 at 10× and pCMVEE-gp160 at 1× versus pKCMV-gp160 at 1×), significantly more IFN-γ and IL-2 were produced in the pCMVEE-gp160-vaccinated animals (P < 0.0002 and 0.002, respectively).
This result was confirmed by ICS on pooled splenocytes from each group staining for IFN-γ-positive CD8+ T cells (Fig. 4d). Thus, the DNA-launched VEE-based vaccine was considerably more immunogenic than a conventional DNA vaccine in terms of both humoral and cellular responses.
Immunization of IFN-α/βR KO mice.
In an attempt to answer why immunogenicity is increased after vaccination with the DNA-launched VEE plasmid over that of a conventional DNA vaccine, we immunized IFN-α/β receptor knockout (IFN-α/βR KO) mice on a 129 background, as well as wild-type 129 mice. The IFN-α/βR KO animals are capable of producing interferons on viral challenge with a VRP, but once interferons are released from the infected cells, they do not act in an autocrine or paracrine feedback loop. DNA-launched SIN-based replicon vaccines yield stronger humoral immune responses in the absence of type I IFN signaling, while T-cell-mediated antitumor effects are abolished (24).
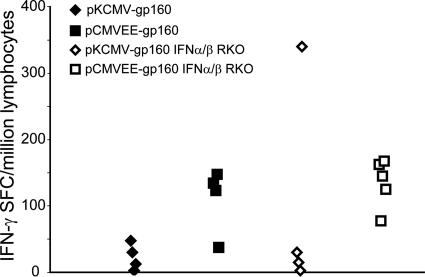
Mice from the two strains (n = 4 to 5/group) were immunized as before with the 100× dose of plasmid DNA, and splenocytes were prepared for IFN-γ ELISPOT assays. As shown in Fig. 5, there were no significant (P > 0.05) differences in the accumulated IFN-γ responses between the two strains with either DNA construct. An analogous result was obtained comparing the antibody responses in pCMVEE-gp160-immunized mice of the two different strains (data not shown). Thus, at the dose tested, it appears that IFN-α/β signaling may not be directly involved in the increased immunogenicity of DNA-launched VEE-based DNA vaccines.
FIG. 5.
Immune response in IFN-α/βR KO mice. IFN-α/βR KO mice on a 129 background (n = 4 or 5/group) and wild-type 129 mice (n = 4/group) were immunized as before with the 100× dose of pCMVEE-gp160 and pVAX1-gp160. A subtype B consensus peptide library of 15-mer peptides with an overlap of 11 amino acids was used to restimulate lymphocytes from the spleen in an IFN-γ ELISPOT assay. SFC, spot-forming cells.
Mixed-modality prime-boost.
To alleviate any effects of antivector immunity, we designed a mixed-modality prime-boost regimen in which mice were immunized at week zero with (i) a DNA-launched VEE replicon encoding HIV-1 gp160 (DNA), (ii) a VRP expressing HIV-1 gp160 (VRP), or (iii) a nonantigen-coding VRP (null VRP) (45). At week 6, these animals were boosted with either the homologous (DNA/DNA and VRP/VRP) or the heterologous (DNA/VRP and VRP/DNA) construct. Controls were boosted with null VRP (null VRP/null VRP). Immune responses were measured by anti-HIV envelope ELISA, IFN-γ and IL-2 ELISPOT, and HIV and VRP neutralization assays 2 weeks postboost.
End point serum IgG anti-gp160 titers were increased by VRP inoculation compared to DNA only. The highest end point titers were achieved in mice immunized twice with VRP-gp160, resulting in end point titers in the 106 range. The group of animals primed with DNA and boosted with VRP had an average titer of 3 × 105. The inverse modality (VRP/DNA) had an average end point titer of 1 × 105, while two DNA inoculations gave an anti-gp160 end point titer of 3 × 103 (Fig. 6a). Pairwise analysis using the Mann-Whitney U test showed that all VRP-immunized groups had significantly higher anti-gp160 antibody titers than the VEE-DNA-immunized animals (P < 0.05). However, when a more stringent test was used (Kruskal-Wallis, followed by Dunn's test for multiple comparisons), statistical differences were present only between the DNA/DNA group and the VRP/DNA and VRP/VRP groups (P < 0.05 and 0.001, respectively). Interestingly, the effect of the booster immunization was much greater in the group of animals primed with DNA and boosted with VRP, which led to a 450-fold increase in the end point titer compared to a 20-fold increase between the prime and the boost in the group immunized two times with VRP.
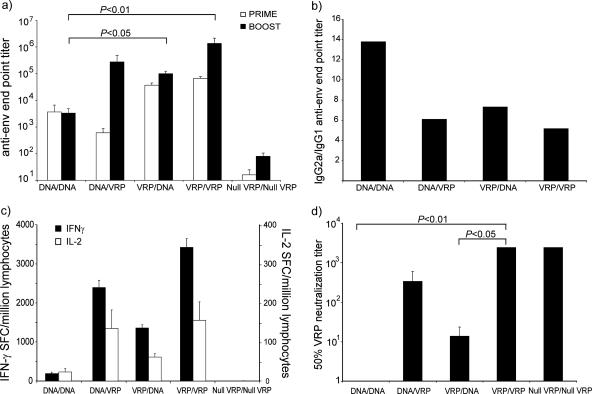
FIG. 6.
Mixed-modality prime-boost. Mice (BALB/c; n = 8/group) were immunized at week zero with a DNA-launched VEE replicon encoding HIV-1 gp160 (DNA), a VRP-expressing HIV-1 gp160 (VRP), or a noncoding VRP (null VRP). At week 6, the animals were boosted with either the homologous (DNA/DNA and VRP/VRP) or the heterologous (DNA/VRP and VRP/DNA) construct. Controls were boosted with null VRP (null VRP/null VRP). (a) Humoral immunity was measured as anti-HIV envelope IgG in serum 1 week preboost (Prime) and 2 weeks postboost (Boost). (b) The IgG isotype profile was determined by calculating the ratio between the IgG2a and IgG1 ELISA end point titers on the postboost sera. (c) IFN-γ and IL-2 ELISPOT assays were performed on purified lymphocytes from the spleen restimulated with the P18 peptide. Responses are expressed as spot-forming cells (SFC) per million lymphocytes. (d) To determine the degree of antivector immunity, serum samples from two animals were pooled and diluted in serial dilutions. VRP-GFP was added to the samples, and the neutralization mixture was used to infect BHK cells. The reduction in the number of green cells as measured by flow cytometry 20 h postinfection compared to controls was used to determine the 50% neutralization titers. The error bars indicate the standard errors of the mean. For a priori statistical analysis, we used the Kruskal-Wallis test, followed by Dunn's test for multiple comparisons for post hoc analysis.
A high (≥1) IgG2a/IgG1 isotype ratio is indicative of a Th1-type response. Therefore, the ratio between IgG2a and IgG1 titers was calculated. In all groups, average ratios were larger than 1, ranging between 6 and 14, and the responses were thus consistent with a Th1-type response (Fig. 6b).
All groups receiving gp160-expressing constructs responded with antigen-specific IFN-γ and IL-2 production in ELISPOT analysis. Both IFN-γ and IL-2 responses were markedly and significantly increased (P < 0.05 using a Kruskal-Wallis test, followed by Dunn's test for multiple comparison) in groups receiving VRP boosting compared to the single-modality DNA approach (Fig. 6c). In addition, priming with DNA and boosting with VRP was more efficient than priming with VRP and boosting with DNA. The most effective strategy in mice, however, was priming and boosting with VRP.
Antivector immunity can be assessed by measuring the VRP-neutralizing antibody titers in serum samples from immunized animals. Therefore, we used a flow cytometry-based VRP neutralization assay to measure the VRP-neutralizing titers in terminal bleeds. Animals that were immunized twice with VRP had very highly neutralizing sera, with mean 50% neutralization titers greater than 2,430 (VRP/VRP and null VRP/null VRP in Fig. 6d). Mice that had received only one immunization with VRP had considerably lower titers, and the time after immunization seemed to influence the level of anti-VRP antibodies. Comparing the anti-VRP titers in the groups that received only one VRP inoculation (VRP/DNA and DNA/VRP in Fig. 6d), the titer at 2 weeks postimmunization (DNA/VRP in Fig. 6d) was 340, and at 8 weeks post-VRP inoculation, the titers were only 14 (VRP/DNA in Fig. 6d). Animals immunized with VEE DNA did not have any detectable neutralizing antibodies to VEE (Fig. 6d), nor did they have any detectable antibodies to VEE nonstructural proteins as determined by Western blotting (data not shown). Animals immunized twice with VRP produced significantly higher (P < 0.05; Kruskal-Wallis test, followed by Dunn's test for multiple comparison) levels of anti-VRP neutralizing antibodies than DNA/DNA- and VRP/DNA-immunized animals. In humans, an 80% neutralization titer of 20 is considered protective and is required for work with VEE in the laboratory.
In addition to vector neutralization, the capabilities of these sera to neutralize HIV-1 were investigated (29). Unfortunately, background neutralization in prebleeds and mock-immunized controls was high and only a few truly neutralizing sera were found, all of them in the VRP/VRP group (data not shown).
DISCUSSION
Genetic vaccines have been considered a promising novel strategy for mass vaccination for the last decade, but there has been no considerable clinical success in humans. One potential explanation for this may be difficulty in obtaining an effective dosage of DNA in larger animals and human volunteers. A typical dose given to a 20-g mouse is 100 μg of plasmid DNA. If the same dosage were applied to an 80-kg human subject, it would mean administering 400 mg of DNA per immunization. To date, DNA doses in clinical trials have been limited to a maximum dose of up to 8 mg of DNA (13, 19, 34, 48).
Therefore, improvements in plasmid-based genetic vaccines would be highly attractive. Ideally, we would like to combine the safety profile and stability of naked-DNA vaccines with the immunity-enhancing properties of viral replicons. Previously, SFV and SIN-based DNA-launched replicons under the transcriptional control of a CMV promoter were engineered to express a foreign antigen from the subgenomic promoter. This concept has shown promise in small-animal models and nonhuman primates but has not been tested in humans. In this paper, we describe a newly developed DNA-launched replicon intended for genetic vaccination based on VEE. The VEE DNA replicon expresses substantially more protein than a conventional expression plasmid. This is consistent with several other findings (9, 16), even though some alphavirus-based DNA replicons do not increase reporter gene expression (27). The explanation for these differences in expression levels may be related to the cell line used. In general, we observed sustained and increased protein expression in cell lines that were incapable of a normal response to, or production of, type I interferons, such as BHK and Vero cells. It is likely that these cells are less affected by the downstream antiviral effects induced by type I interferons. Furthermore, the replicon plasmids are significantly larger than conventional expression plasmids, and investigators have reported decreased transfection levels of the replicon-encoding plasmids (24). This would potentially influence the estimation of antigen production unless it is performed on a per cell basis.
Consistent with previously described DNA-launched alphavirus replicons, the VEE DNA also induces cell death through apoptosis (9, 25). Arguably, this is an important feature of this vector, because apoptosis has been implicated in immune induction (1, 53). In the context of DNA-launched SIN replicons, apoptosis induction, and not expression levels, is key to breaking tolerance against a tumor antigen (25). Codelivery of an antiapoptotic gene did in fact abrogate the immune response in that study, albeit prolonging survival of the transfected cell and thus increasing overall antigen expression. Obviously, it would be interesting to perform similar studies in the VEE replicon system to establish whether active apoptosis induction actually plays a role in the increased immunogenicity of these vectors relative to a conventional DNA vaccine. In addition to potential immune-potentiating effects of apoptosis in this system, it also confers increased safety for the clinical use of DNA vaccines. One major concern for regulatory authorities is to establish that introduced plasmid DNA is not integrated into the genetic material of the host organism. A self-limiting suicidal DNA vaccine vector would certainly decrease such concerns.
The immunogenicity of VEE DNA was tested using an HIV-1 subtype B gp160 gene as a model antigen. This gene has been thoroughly investigated in the context of a conventional DNA vaccine and is currently in clinical trials in Tanzania in a multigene, multisubtype vaccine (12). Here, a total of 60 mice were immunized with the gp160 gene encoded by a conventional DNA vaccine plasmid, and none of them developed any demonstrable antibody titers, although IFN-γ- and IL-2-producing T cells were readily detectable at the highest dose, consistent with previous findings (32, 39). In contrast, VEE DNA elicited antibody responses at all doses tested, i.e., at as little as 1 μg of DNA. Moreover, robust IFN-γ and IL-2 cellular responses were also present at all doses. SIN- and SFV-based DNA-encoded replicons have also demonstrated vaccine efficiency at decreased doses in various infectious-disease and tumor challenge models (5, 18, 49, 51).
We hypothesize that the increased protein expression, apoptosis induction, and activation of innate immunity are all factors that increase the potency of VEE-DNA vaccines over conventional DNA vaccines. The relative contribution of each of these factors will be subject to further studies. It would also be interesting to test the available DNA-launched replicons in head-to-head comparisons to determine whether there are differences in immune induction between them that can be attributed to the original viruses and/or the individual antigens expressed. It seems plausible that different vector systems with similar but not identical vector characteristics may indeed be suitable for vaccination against different antigens.
During cytoplasmic amplification of alphavirus replicons, single-stranded and double-stranded RNA intermediates are likely to be recognized by one or several pathogen recognition receptors, such as PKR, RNase L, TLR-3, TLR-7/8, RIG-I, and MDA-5, leading to an innate immune response characterized by release of type I interferons (reviewed in reference 22). We report that transfection of interferon-competent murine myoblasts responds to VEE replicon amplification with sustained production of IFN-α/β, similar to what has been reported for SIN-based replicons (24). Therefore, we tested the immunogenicity of VEE-DNA in mice deficient for the IFN-α/β receptor. We predicted that the experimental outcome would be one of the following if type I interferons per se were also involved in the immune-potentiating mechanism for VEE-DNA: (i) the lack of IFN-α/β signaling would obviate type I IFN-mediated suppression of genome replication and expression, allowing increased antigen production and immunogenicity, or (ii) IFN-α/β would act locally and/or systemically as a mediator of inflammation and as a danger signal for activation of the adaptive immune system. If this hypothesis were true, the immune response in the IFN-α/βR KO mice would be lower than in the wild-type strain. A third possibility is that both outcomes would occur simultaneously, resulting in a response reflective of the algebraic sum of the two effects. Under the experimental conditions used here, no differences between wild-type and IFN-α/βR KO mice were detected after immunization with VEE DNA encoding HIV-1 gp160. In the SFV expression system, IFN-α/β responses appear to be crucial for immunity to a codelivered protein but not essential for eliciting immune responses to an expressed antigen (21).
However, we do not exclude the possibility that type I interferons may still play an essential role in eliciting an immune response to a VEE DNA-expressed antigen. First, the role of the interferon response may be antigen dependent, at least to some degree. Secondly, the role of the interferon response may be intimately connected to the dose of VEE DNA delivered. In this study, only the high dose of DNA was used, and it could be that the effects of intact type I interferon signaling would be detected only at lower doses. That would be consistent with the finding that the conventional DNA vaccine elicited T-cell responses comparable to those elicited by the VEE-DNA at the high dose in the dose-response studies, and this remains to be further investigated. Thirdly, it may be that the interferon response is a marker for, but not the direct mediator of, increased immune responses in the VEE DNA system. Further studies will be needed to elucidate the roles of activation of the interferon system and other innate immune mechanisms after vaccination with a DNA-launched VEE replicon.
One concern regarding the efficacy of viral vectors for vaccination is the presence of preexisting immunity. This has been extensively studied for adenovirus-based vaccine vehicles, and preexisting neutralizing immunity to adenovirus serotype 5 substantially blunted immune responses to immunization with a recombinant adenovirus serotype 5 vector expressing HIV gag (6). The effects of preexisting immunity could be overcome using a recombinant adenovirus of another serotype or by replacement of the antigen determinant regions of the adenovirus hexon protein with those of an adenovirus with very low prevalence in the human population (38). Although VEE is endemic only in certain areas and only sporadically circulates in the human population, the use of multiple doses of VRP-encoded antigens may indeed give rise to undesired antivector immunity. We demonstrate here that two immunizations with VRP give very high titers of neutralizing serum anti-VEE antibodies. We and others nevertheless have not observed any negative effects using recombinant alphavirus replicons in mice when a second round of immunizations to an unrelated antigen has been initiated (N. L. Davis and R. E. Johnston, unpublished data; 8). However, this may not be the case in nonhuman primates and human volunteers. In an unrelated study, one rhesus monkey with prevaccination neutralizing titers to VEE had a seriously abrogated response to the vectored immunogen (L. J. White and R. E. Johnston, unpublished data). Further studies to thoroughly investigate the potential effects of preexisting anti-VEE immunity are being planned.
One strategy to circumvent antivector immunity is based on heterologous prime-boost protocols, in which the vaccinee is primed with one vaccine modality and boosted with another. The anamnestic response thus elicited to the vectored antigen would then be much more efficient than the primary response to the vector itself. Mixed-modality prime-boost strategies increased the potencies of several gene-based vaccine strategies, including immunity to the gp160 gene described here (2, 12, 15, 35, 42). Here, we show that a mixed-modality regimen with a relatively low dose (10 μg) of VEE DNA delivered as a prime, followed by a VRP boost, induces very strong cellular and humoral immune responses to HIV-1 gp160. We believe that the improvement of the DNA vector gives a more potent prime for the prime-boost regimen, although this has not been directly tested.
Even though the antivector immunity waned rather rapidly after one dose of VRP, the effect of the boost on the anti-gp160 end point titers was much stronger in the VEE DNA-primed animals than in the mice primed with VRP. The group mean anti-gp160 titer was increased about 450-fold in the first case and only about 20-fold in the second. One possible explanation for this is that antivector immunity was present at the time of the boost in the mice immunized twice with VRP. Alternatively, the antibody response may already have reached close to a maximum level in this group and therefore could not be increased further.
In conclusion, the VEE-DNA replicon described here expresses high levels of protein encoded by the transgene under the VEE 26S promoter. Furthermore, it induces apoptosis and type I interferons in vitro and promotes Th1-type antibody responses while maintaining cellular immune responses to gp160 in mice. Importantly, it is effective at considerably lower doses than a state-of-the-art DNA vaccine vector encoding the same antigen. It also provides an efficient prime to a VRP boost without inducing any potentially interfering antivector immunity. Taken together, these improved vector characteristics may have significant clinical implications for DNA vaccines generally and HIV vaccines in particular.
Acknowledgments
This work was supported by postdoctoral fellowship grants from the Wenner-Gren Foundation and the Swedish Society for Medical Research (to K.L.) and by National Institutes of Health grant PO1-AI050246 (to R.E.J.).
We thank Laura White and Nancy Davis for their expertise with VEE neutralization assays.
Footnotes
Published ahead of print on 3 October 2007.
REFERENCES
- 1.Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86-89. [DOI] [PubMed] [Google Scholar]
- 2.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 3.Antalis, T. M., M. La Linn, K. Donnan, L. Mateo, J. Gardner, J. L. Dickinson, K. Buttigieg, and A. Suhrbier. 1998. The serine proteinase inhibitor (serpin) plasminogen activation inhibitor type 2 protects against viral cytopathic effects by constitutive interferon alpha/beta priming. J. Exp. Med. 187:1799-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Applequist, S. E., E. Rollman, M. D. Wareing, M. Liden, B. Rozell, J. Hinkula, and H. G. Ljunggren. 2005. Activation of innate immunity, inflammation, and potentiation of DNA vaccination through mammalian expression of the TLR5 agonist flagellin. J. Immunol. 175:3882-3891. [DOI] [PubMed] [Google Scholar]
- 5.Balasuriya, U. B., H. W. Heidner, N. L. Davis, H. M. Wagner, P. J. Hullinger, J. F. Hedges, J. C. Williams, R. E. Johnston, W. David Wilson, I. K. Liu, and N. James MacLachlan. 2002. Alphavirus replicon particles expressing the two major envelope proteins of equine arteritis virus induce high level protection against challenge with virulent virus in vaccinated horses. Vaccine 20:1609-1617. [DOI] [PubMed] [Google Scholar]
- 6.Barouch, D. H., M. G. Pau, J. H. Custers, W. Koudstaal, S. Kostense, M. J. Havenga, D. M. Truitt, S. M. Sumida, M. G. Kishko, J. C. Arthur, B. Korioth-Schmitz, M. H. Newberg, D. A. Gorgone, M. A. Lifton, D. L. Panicali, G. J. Nabel, N. L. Letvin, and J. Goudsmit. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 172:6290-6297. [DOI] [PubMed] [Google Scholar]
- 7.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 8.Berglund, P., M. N. Fleeton, C. Smerdou, and P. Liljeström. 1999. Immunization with recombinant Semliki Forest virus induces protection against influenza challenge in mice. Vaccine 17:497-507. [DOI] [PubMed] [Google Scholar]
- 9.Berglund, P., C. Smerdou, M. N. Fleeton, I. Tubulekas, and P. Liljeström. 1998. Enhancing immune responses using suicidal DNA vaccines. Nat. Biotechnol. 16:562-565. [DOI] [PubMed] [Google Scholar]
- 10.Bråve, A., A. Boberg, L. Gudmundsdotter, E. Rollman, K. Hallermalm, K. Ljungberg, P. Blomberg, R. Stout, S. Pauli, E. Sandström, G. Biberfeld, P. L. Earl, B. Moss, J. Cox, and B. Wahren. 2007. A new multi-clade DNA prime recombinant MVA boost vaccine induces broad and high levels of HIV-1 specific CD8+ T cell and humoral responses in mice. Mol. Ther. 15:1724-1733. [DOI] [PubMed] [Google Scholar]
- 11.Bråve, A., K. Ljungberg, A. Boberg, E. Rollman, G. Engström, J. Hinkula, and B. Wahren. 2006. Reduced cellular immune responses following immunization with a multi-gene HIV-1 vaccine. Vaccine 24:4524-4526. [DOI] [PubMed] [Google Scholar]
- 12.Bråve, A., K. Ljungberg, A. Boberg, E. Rollman, M. Isaguliants, B. Lundgren, P. Blomberg, J. Hinkula, and B. Wahren. 2005. Multigene/multisubtype HIV-1 vaccine induces potent cellular and humoral immune responses by needle-free intradermal delivery. Mol. Ther. 12:1197-1205. [DOI] [PubMed] [Google Scholar]
- 13.Calarota, S., G. Bratt, S. Nordlund, J. Hinkula, A. C. Leandersson, E. Sandström, and B. Wahren. 1998. Cellular cytotoxic response induced by DNA vaccination in HIV-1-infected patients. Lancet 351:1320-1325. [DOI] [PubMed] [Google Scholar]
- 14.Chattergoon, M. A., J. J. Kim, J. S. Yang, T. M. Robinson, D. J. Lee, T. Dentchev, D. M. Wilson, V. Ayyavoo, and D. B. Weiner. 2000. Targeted antigen delivery to antigen-presenting cells including dendritic cells by engineered Fas-mediated apoptosis. Nat. Biotechnol. 18:974-979. [DOI] [PubMed] [Google Scholar]
- 15.Degano, P., J. Schneider, C. M. Hannan, S. C. Gilbert, and A. V. Hill. 1999. Gene gun intradermal DNA immunization followed by boosting with modified vaccinia virus Ankara: enhanced CD8+ T cell immunogenicity and protective efficacy in the influenza and malaria models. Vaccine 18:623-632. [DOI] [PubMed] [Google Scholar]
- 16.DiCiommo, D. P., and R. Bremner. 1998. Rapid, high level protein production using DNA-based Semliki Forest virus vectors. J. Biol. Chem. 273:18060-18066. [DOI] [PubMed] [Google Scholar]
- 16a.FDA. 1996. Points to consider on plasmid DNA vaccines for preventive infectious disease indications. FDA, Washington, DC.
- 17.Gardner, J. P., I. Frolov, S. Perri, Y. Ji, M. L. MacKichan, J. zur Megede, M. Chen, B. A. Belli, D. A. Driver, S. Sherrill, C. E. Greer, G. R. Otten, S. W. Barnett, M. A. Liu, T. W. Dubensky, and J. M. Polo. 2000. Infection of human dendritic cells by a Sindbis virus replicon vector is determined by a single amino acid substitution in the E2 glycoprotein. J. Virol. 74:11849-11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg, S. M., S. M. Bartido, J. P. Gardner, J. A. Guevara-Patino, S. C. Montgomery, M. A. Perales, M. F. Maughan, J. Dempsey, G. P. Donovan, W. C. Olson, A. N. Houghton, and J. D. Wolchok. 2005. Comparison of two cancer vaccines targeting tyrosinase: plasmid DNA and recombinant alphavirus replicon particles. Clin. Cancer Res. 11:8114-8121. [DOI] [PubMed] [Google Scholar]
- 19.Graham, B. S., R. A. Koup, M. Roederer, R. T. Bailer, M. E. Enama, Z. Moodie, J. E. Martin, M. M. McCluskey, B. K. Chakrabarti, L. Lamoreaux, C. A. Andrews, P. L. Gomez, J. R. Mascola, and G. J. Nabel. 2006. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. J. Infect. Dis. 194:1650-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hariharan, M. J., D. A. Driver, K. Townsend, D. Brumm, J. M. Polo, B. A. Belli, D. J. Catton, D. Hsu, D. Mittelstaedt, J. E. McCormack, L. Karavodin, T. W. Dubensky, Jr., S. M. Chang, and T. A. Banks. 1998. DNA immunization against herpes simplex virus: enhanced efficacy using a Sindbis virus-based vector. J. Virol. 72:950-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hidmark, Å. S., E. K. Nordström, P. Dosenovic, M. N. Forsell, P. Liljeström, and G. B. Karlsson Hedestam. 2006. Humoral responses against coimmunized protein antigen but not against alphavirus-encoded antigens require alpha/beta interferon signaling. J. Virol. 80:7100-7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawai, T., and S. Akira. 2006. Innate immune recognition of viral infection. Nat. Immunol. 7:131-137. [DOI] [PubMed] [Google Scholar]
- 23.Kutzler, M. A., T. M. Robinson, M. A. Chattergoon, D. K. Choo, A. Y. Choo, P. Y. Choe, M. P. Ramanathan, R. Parkinson, S. Kudchodkar, Y. Tamura, M. Sidhu, V. Roopchand, J. J. Kim, G. N. Pavlakis, B. K. Felber, T. A. Waldmann, J. D. Boyer, and D. B. Weiner. 2005. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. J. Immunol. 175:112-123. [DOI] [PubMed] [Google Scholar]
- 24.Leitner, W. W., E. S. Bergmann-Leitner, L. N. Hwang, and N. P. Restifo. 2006. Type I interferons are essential for the efficacy of replicase-based DNA vaccines. Vaccine 24:5110-5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leitner, W. W., L. N. Hwang, E. S. Bergmann-Leitner, S. E. Finkelstein, S. Frank, and N. P. Restifo. 2004. Apoptosis is essential for the increased efficacy of alphaviral replicase-based DNA vaccines. Vaccine 22:1537-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leitner, W. W., L. N. Hwang, M. J. deVeer, A. Zhou, R. H. Silverman, B. R. Williams, T. W. Dubensky, H. Ying, and N. P. Restifo. 2003. Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways. Nat. Med. 9:33-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leitner, W. W., H. Ying, D. A. Driver, T. W. Dubensky, and N. P. Restifo. 2000. Enhancement of tumor-specific immune response with plasmid DNA replicon vectors. Cancer Res. 60:51-55. [PMC free article] [PubMed] [Google Scholar]
- 28.Levine, B., Q. Huang, J. T. Isaacs, J. C. Reed, D. E. Griffin, and J. M. Hardwick. 1993. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature 361:739-742. [DOI] [PubMed] [Google Scholar]
- 29.Li, M., F. Gao, J. R. Mascola, L. Stamatatos, V. R. Polonis, M. Koutsoukos, G. Voss, P. Goepfert, P. Gilbert, K. M. Greene, M. Bilska, D. L. Kothe, J. F. Salazar-Gonzalez, X. Wei, J. M. Decker, B. H. Hahn, and D. C. Montefiori. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108-10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, M. L., and V. Stollar. 2004. Alphaviruses and apoptosis. Int. Rev. Immunol. 23:7-24. [DOI] [PubMed] [Google Scholar]
- 31.Liljeström, P., and H. Garoff. 1991. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology 9:1356-1361. [DOI] [PubMed] [Google Scholar]
- 32.Ljungberg, K., E. Rollman, L. Eriksson, J. Hinkula, and B. Wahren. 2002. Enhanced immune responses after DNA vaccination with combined envelope genes from different HIV-1 subtypes. Virology 302:44-57. [DOI] [PubMed] [Google Scholar]
- 33.MacDonald, G. H., and R. E. Johnston. 2000. Role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J. Virol. 74:914-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacGregor, R. R., J. D. Boyer, K. E. Ugen, K. E. Lacy, S. J. Gluckman, M. L. Bagarazzi, M. A. Chattergoon, Y. Baine, T. J. Higgins, R. B. Ciccarelli, L. R. Coney, R. S. Ginsberg, and D. B. Weiner. 1998. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J. Infect. Dis. 178:92-100. [DOI] [PubMed] [Google Scholar]
- 35.McConkey, S. J., W. H. Reece, V. S. Moorthy, D. Webster, S. Dunachie, G. Butcher, J. M. Vuola, T. J. Blanchard, P. Gothard, K. Watkins, C. M. Hannan, S. Everaere, K. Brown, K. E. Kester, J. Cummings, J. Williams, D. G. Heppner, A. Pathan, K. Flanagan, N. Arulanantham, M. T. Roberts, M. Roy, G. L. Smith, J. Schneider, T. Peto, R. E. Sinden, S. C. Gilbert, and A. V. Hill. 2003. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat. Med. 9:729-735. [DOI] [PubMed] [Google Scholar]
- 36.Perri, S., C. E. Greer, K. Thudium, B. Doe, H. Legg, H. Liu, R. E. Romero, Z. Tang, Q. Bin, T. W. Dubensky, Jr., M. Vajdy, G. R. Otten, and J. M. Polo. 2003. An alphavirus replicon particle chimera derived from Venezuelan equine encephalitis and Sindbis viruses is a potent gene-based vaccine delivery vector. J. Virol. 77:10394-10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pushko, P., M. Parker, G. V. Ludwig, N. L. Davis, R. E. Johnston, and J. F. Smith. 1997. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology 239:389-401. [DOI] [PubMed] [Google Scholar]
- 38.Roberts, D. M., A. Nanda, M. J. Havenga, P. Abbink, D. M. Lynch, B. A. Ewald, J. Liu, A. R. Thorner, P. E. Swanson, D. A. Gorgone, M. A. Lifton, A. A. Lemckert, L. Holterman, B. Chen, A. Dilraj, A. Carville, K. G. Mansfield, J. Goudsmit, and D. H. Barouch. 2006. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 441:239-243. [DOI] [PubMed] [Google Scholar]
- 39.Rollman, E., J. Hinkula, J. Arteaga, B. Zuber, A. Kjerrström, M. Liu, B. Wahren, and K. Ljungberg. 2004. Multi-subtype gp160 DNA immunization induces broadly neutralizing anti-HIV antibodies. Gene Ther. 11:1146-1154. [DOI] [PubMed] [Google Scholar]
- 40.Sasaki, S., R. R. Amara, A. E. Oran, J. M. Smith, and H. L. Robinson. 2001. Apoptosis-mediated enhancement of DNA-raised immune responses by mutant caspases. Nat. Biotechnol. 19:543-547. [DOI] [PubMed] [Google Scholar]
- 41.Schulz, O., S. S. Diebold, M. Chen, T. I. Näslund, M. A. Nolte, L. Alexopoulou, Y. T. Azuma, R. A. Flavell, P. Liljeström, and C. Reis e Sousa. 2005. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433:887-892. [DOI] [PubMed] [Google Scholar]
- 42.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 43.Sin, J. I., J. J. Kim, J. D. Boyer, R. B. Ciccarelli, T. J. Higgins, and D. B. Weiner. 1999. In vivo modulation of vaccine-induced immune responses toward a Th1 phenotype increases potency and vaccine effectiveness in a herpes simplex virus type 2 mouse model. J. Virol. 73:501-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strauss, J. H., and E. G. Strauss. 1994. The alphaviruses: gene expression, replication, and evolution. Microbiol. Rev. 58:491-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson, J. M., A. C. Whitmore, J. L. Konopka, M. L. Collier, E. M. Richmond, N. L. Davis, H. F. Staats, and R. E. Johnston. 2006. Mucosal and systemic adjuvant activity of alphavirus replicon particles. Proc. Natl. Acad. Sci. USA 103:3722-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thorner, A. R., A. A. Lemckert, J. Goudsmit, D. M. Lynch, B. A. Ewald, M. Denholtz, M. J. Havenga, and D. H. Barouch. 2006. Immunogenicity of heterologous recombinant adenovirus prime-boost vaccine regimens is enhanced by circumventing vector cross-reactivity. J. Virol. 80:12009-12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trgovcich, J., J. F. Aronson, and R. E. Johnston. 1996. Fatal Sindbis virus infection of neonatal mice in the absence of encephalitis. Virology 224:73-83. [DOI] [PubMed] [Google Scholar]
- 48.Wang, R., D. L. Doolan, T. P. Le, R. C. Hedstrom, K. M. Coonan, Y. Charoenvit, T. R. Jones, P. Hobart, M. Margalith, J. Ng, W. R. Weiss, M. Sedegah, C. de Taisne, J. A. Norman, and S. L. Hoffman. 1998. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 282:476-480. [DOI] [PubMed] [Google Scholar]
- 49.Wang, X., J. P. Wang, M. F. Maughan, and L. B. Lachman. 2005. Alphavirus replicon particles containing the gene for HER2/neu inhibit breast cancer growth and tumorigenesis. Breast Cancer Res. 7:R145-R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.White, L. J., J. G. Wang, N. L. Davis, and R. E. Johnston. 2001. Role of alpha/beta interferon in Venezuelan equine encephalitis virus pathogenesis: effect of an attenuating mutation in the 5′ untranslated region. J. Virol. 75:3706-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao, S., H. Chen, L. Fang, C. Liu, H. Zhang, Y. Jiang, and W. Hong. 2004. Comparison of immune responses and protective efficacy of suicidal DNA vaccine and conventional DNA vaccine encoding glycoprotein C of pseudorabies virus in mice. Vaccine 22:345-351. [DOI] [PubMed] [Google Scholar]
- 52.Yew, N. S., D. M. Wysokenski, K. X. Wang, R. J. Ziegler, J. Marshall, D. McNeilly, M. Cherry, W. Osburn, and S. H. Cheng. 1997. Optimization of plasmid vectors for high-level expression in lung epithelial cells. Hum. Gene Ther. 8:575-584. [DOI] [PubMed] [Google Scholar]
- 53.Yrlid, U., and M. J. Wick. 2000. Salmonella-induced apoptosis of infected macrophages results in presentation of a bacteria-encoded antigen after uptake by bystander dendritic cells. J. Exp. Med. 191:613-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu, W., C. E. Thomas, and P. F. Sparling. 2004. DNA immunization of mice with a plasmid encoding Neisseria gonorrhoeae PorB protein by intramuscular injection and epidermal particle bombardment. Vaccine 22:660-669. [DOI] [PubMed] [Google Scholar]