Abstract
Dendritic cells (DCs) play a central role in innate immunity and antiviral responses. In this study, we investigated the production of alpha interferon (IFN-α) and inducible chemokines by human monocyte-derived dendritic cells (mDCs) and plasmacytoid dendritic cells (pDCs) infected with West Nile virus (WNV), an emergent pathogen whose infection can lead to severe cases of encephalitis in the elderly, children, and immunocompromised individuals. Our experiments demonstrated that WNV grown in mammalian cells (WNVVero) was a potent inducer of IFN-α secretion in pDCs and, to a lesser degree, in mDCs. The ability of WNVVero to induce IFN-α in pDCs did not require viral replication and was prevented by the treatment of cells with bafilomycin A1 and chloroquine, suggesting that it was dependent on endosomal Toll-like receptor recognition. On the other hand, IFN-α production in mDCs required viral replication and was associated with the nuclear translocation of IRF3 and viral antigen expression. Strikingly, pDCs failed to produce IFN-α when stimulated with WNV grown in mosquito cells (WNVC7/10), while mDCs responded similarly to WNVVero or WNVC7/10. Moreover, the IFN-dependent chemokine IP-10 was produced in substantial amounts by pDCs in response to WNVVero but not WNVC7/10, while interleukin-8 was produced in greater amounts by mDCs infected with WNVC7/10 than in those infected with WNVVero. These findings suggest that cell-specific mechanisms of WNV recognition leading to the production of type I IFN and inflammatory chemokines by DCs may contribute to both the innate immune response and disease pathogenesis in human infections.
West Nile virus (WNV) is an arthropod-borne pathogen that belongs to the genus Flavivirus of the family Flaviviridae, a genus that includes dengue virus (DENV), Japanese encephalitis virus, yellow fever virus (YFV), and tick-borne encephalitis virus (8). The flavivirus virion is composed of a positive-sense, single-stranded RNA encased in a protein capsid and surrounded by a spherical envelope. The genomic RNA encodes seven nonstructural proteins as well as three structural proteins, capsid (C), premembrane (prM), and envelope (E), the latter of which has been shown to mediate the entry of the virus into cells (43).
WNV was first isolated in Uganda in 1937 and is widely spread across the Eastern Hemisphere. Since its appearance in the Western Hemisphere in 1999, WNV has spread throughout North America, Latin America, and the Caribbean (28). The majority of human WNV infections produce either asymptomatic cases or a mild febrile disease. However, in some cases (∼1%), WNV infection can lead to encephalitis, meningitis, or other neurological diseases that can culminate in death. Severe symptoms are more common in the immunosuppressed and the elderly, suggesting that the immune system plays a critical role in the outcome of the disease (56, 58).
The precise role of different cells and organs in the initial immune response to flavivirus infection is not completely understood. Several lines of evidence indicate that the type I interferon (IFN) (alpha/beta IFN [IFN-α/β]) system plays a critical role determining the outcome of infection. In the case of WNV, IFN-α/β has been shown to be a potent inhibitor of replication in vitro (14). In addition, it has been demonstrated that pretreatment with IFN can protect mice and hamsters from morbidity and mortality following WNV infection (54). Furthermore, studies with IFN receptor-deficient mice have demonstrated that these animals display enhanced susceptibility to a variety of flaviviruses (31, 46, 63). The importance of IFN in controlling flavivirus infection can also be inferred from studies with multiple viruses, demonstrating that flavivirus-infected cells block the JAK-STAT signal transduction pathway triggered by IFN binding to its receptor (7, 26, 32, 42, 44, 55, 65).
Dendritic cells (DCs) play a pivotal role in the recognition of pathogens and in the linkage between innate and adaptive immunity. Different DC subsets have specialized functions including antigen presentation to T cells or secretion of proinflammatory cytokines. Immature myeloid-derived DCs are remarkably efficient in antigen capture in peripheral tissues. Following antigen capture, these cells become activated and migrate to secondary lymphoid organs, where they present their antigens to other immune system cells (13). Unlike myeloid-derived DCs, plasmacytoid DCs (pDCs) are not experts in antigen capture; rather, pDCs are able to secrete large amounts of IFN-α following the recognition of pathogens (2, 9, 67).
In many cell types, IFN synthesis is induced following the recognition of a pathogen-associated molecular pattern (PAMP) molecule, such as double-stranded RNA. This particular PAMP has been shown to induce IFN following binding to pathogen recognition receptors such as Toll-like receptor 3 (TLR3) or the RNA helicases RIG-I and mda5 (35). Following the ligation of double-stranded RNA, these pathogen recognition receptors instigate a signaling pathway that results in the phosphorylation of the IFN regulatory factor 3 (IRF3) transcription factor. The phosphorylation of IRF3, which is constitutively expressed in the cytoplasm of most cells, results in dimerization and translocation to the nucleus, where it activates the transcription of multiple genes, including those for IFN-β and IFN-α4 (50). When these IFNs are secreted from the activated cell, they can then bind to type I IFN receptors on the surface of the activated cells or surrounding cells. Receptor binding activates the JAK-STAT pathway, inducing the expression of a wide variety of IFN-stimulated genes, which can establish an antiviral state. One of these IFN-stimulated genes is IRF7, which is not constitutively expressed in most cell types. Like IRF3, IRF7 is phosphorylated in cells that are exposed to PAMPs, such as single-stranded RNA that binds to TLR7/TLR8 and unmethylated CpG DNA that binds to TLR9 (5). Following phosphorylation, IRF7 translocates to the nucleus, where it is able to activate large numbers of IFN-α genes. Thus, an initial IRF3-mediated IFN response can be potently amplified by an IRF7-amplified response (27, 64).
Unlike many cells, pDCs constitutively express IRF7 as well as high levels of TLR7, TLR8, and TLR9 (3, 34, 45). Thus, the recognition of PAMPs by these cells can lead directly to the activation of a large number of IFN-α genes. This functional capability is also supported by the fact that pDCs, also called IFN-producing cells, have the ability to secrete up to 1,000 times more type I IFN than other cell types (38, 45, 67). Unlike other DC subtypes, pDCs are not good antigen presenters due to their low capacities for phagocytosis and antigen loading onto major histocompatibility complex molecules (5, 45). Following activation, pDCs secrete IFN for a limited amount of time and then differentiate into antigen-presenting mature DCs that activate and modulate T-cell responses (33, 45). Therefore, pDCs first play a critical role as effector cells in antimicrobial innate immune responses and subsequently differentiate into antigen-presenting cells to initiate adaptive immune responses.
The production of high levels of IFN in response to initial PAMP recognition (rather than in a secondary amplification following IRF7 expression) may be particularly important for controlling flavivirus infections, since a number of studies demonstrated that flaviviruses block JAK-STAT signal transduction pathways (see above), and flavivirus-infected cells may suppress PAMP-activated signaling pathways (22, 23, 65).
DCs have been shown to be targets of flavivirus infection. DENV has been shown to be able to infect and activate DCs in vitro, resulting in cell maturation and cytokine production (37, 38, 40, 51, 59, 71). More recently, it was reported that the YFV vaccine strain (YF-17D) is able to replicate in both immature and mature DCs obtained from human peripheral blood cells (4), and YF-17D has been shown to induce the expression of multiple costimulatory molecules and cytokines in myeloid DCs obtained from peripheral blood monocytes (monocyte-derived DCs, referred to as mDCs throughout the rest of this paper) (4, 60) and pDCs in a TLR-dependent manner (60).
In this paper, we have compared levels of production of IFN-α (and other cytokines) in cultures of human pDCs and mDCs infected with WNV. WNV triggers the expression of IFN-α in both of these subsets of human DCs. However, we have noted important differences in the mechanisms of IFN gene activation. In pDCs, IFN-α production does not require viral replication and is blocked by chloroquine and bafilomycin A1, which prevent endosome acidification/maturation, indicating that IFN-α gene activation is likely triggered in a TLR-dependent manner, as has been observed for several other viruses. In contrast, IFN-α production by mDCs requires viral replication and is associated with IRF3 translocation to the nucleus, suggesting a role for an intracellular signaling pathway leading to the induction of IFN. Additionally, we compared the ability of mammalian cell- and mosquito cell-derived viruses to activate the synthesis of IFN-α and other cytokines in pDCs and mDCs. Our results demonstrate that mDCs synthesize IFN-α upon stimulation with both viruses, whereas pDCs are unable to synthesize IFN-α upon stimulation with the mosquito cell-derived viruses. These studies produce important new information about how WNV engages this important set of immune cells, which drive the initial innate response to infection and direct the adaptive responses as well.
MATERIALS AND METHODS
DC cultures.
This study was approved by the Institutional Review Board of the University of Texas Medical Branch (UTMB). Human DCs were obtained as previously described (25). Briefly, peripheral blood mononuclear cells (PBMC) were obtained by Ficoll-Hypaque (GE Healthcare, Bio-sciences, Piscataway, NJ) gradient centrifugation from 100 ml of peripheral blood collected from healthy donors. Human pDCs were isolated by using magnetic microbeads coated with anti-BDCA-4 monoclonal antibody (Miltenyi Biotec, Auburn, CA). The resulting pDC population had a purity of >97% based on the positive expression of BDCA-2 and CD123. Cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), antibiotics, 1 mM sodium pyruvate, and 10 ng/ml of interleukin-3 (IL-3) (R&D Systems). mDCs were generated from PBMC (obtained as mentioned above for pDCs) by selecting adherent cells. The mononuclear cells were laid on 25-cm2 flasks for 60 to 90 min at 37°C, after which nonadherent cells were removed by five washes with plain RPMI medium. Adherent cells were cultured for 7 days in RPMI 1640 supplemented with 10% FBS, antibiotics, 50 μM 2-mercaptoethanol, 100 ng/ml granulocyte-macrophage colony-stimulating factor (PeproTech, Rocky Hill, NJ), and 20 ng/ml IL-4 (R&D Systems, Minneapolis, MN).
Cell lines.
MRC-5 cells (ATCC) were grown in Dulbecco's modified Eagle's medium (DMEM) with 10% FBS, 1% nonessential amino acids, 1% sodium pyruvate, and antibiotics. K-562 cells (provided by T. Shenk, Princeton University) were maintained in DMEM with 10% FBS and antibiotics. Huh-7 cells and Vero cells were grown as previously described (62). Aedes albopictus C7/10 mosquito cells (provided by I. Frolov, UTMB) were grown at 30°C in L15 medium with 10% FBS, 10% tryptose phosphate broth, and antibiotics.
Viruses.
The WNV used in this study was a low-passage virus recovered from BHK cells transfected with a synthetic RNA derived from an infectious cDNA clone of a human 2002 isolate from Texas (62). This virus seed was initially grown in Vero cells and then amplified in either Vero or C7/10 cells in modified Eagle's medium (Vero) or L15 medium (C7/10) containing 1% FBS. Supernatants were collected when cytopathic effect was evident, clarified, and then concentrated by precipitation with 10% (wt/vol) polyethylene glycol (8,000 molecular weight) and 0.64 M NaCl (final concentration), followed by collection by centrifugation and solubilization in tissue culture medium. These viruses are referred as WNVVero and WNVC7/10. “WNV” used without a superscript refers to WNVVero. The titer of the WNV stocks was determined by focus formation assay in Vero and K562 cells as previously described (62). Briefly, serial dilutions of virus were added to the cells, and after a 1-h adsorption at 37°C, the virus inoculum was removed, and cells were overlaid with a semisolid overlay. Twenty-four hours later, the foci of infection were stained by immunohistochemistry using WNV-specific murine hyperimmune ascitic fluid (MHIAF). For some experiments, WNV was inactivated by subjecting virus preparations to UV light (4 W at a 254-nm wavelength and at a distance of 10 cm for 2 min); virus inactivation was confirmed by the detection of WNV antigen-positive focus-forming units in the treated preparations as described above. The vesicular stomatitis virus (VSV) used in all experiments was the Hazelhurst strain of the New Jersey serotype (obtained from R. B. Tesh, UTMB). VSV was grown in Vero cells, and titers were determined using methods similar to those described above by using VSV-specific MHIAF (provided by R. B. Tesh). The Sendai virus (SeV) preparation used for all studies consisted of allantoic fluid harvested from SeV-infected embryonated eggs (obtained from Charles River Laboratories), which is known to be a strong stimulator of IFN production.
Cell stimulation.
pDCs, mDCs, or MRC5 cells were seeded in 96-well plates at density of 5 × 104 to 2 × 105 cells per/well in a total volume of 200 μl of medium. Cells were mock treated or exposed to WNVVero, UV-inactivated WNVVero, WNVC7/10, or VSV at the multiplicities of infection (MOIs) indicated in the figure legends and text. All MOIs used for WNV and VSV studies were based on focus-forming units determined on Vero cells as described above. All infections with SeV were performed at a dose of 16 hemagglutinating units (HAU)/ml, and poly(I:C) was always used at 25 μg/ml. For some experiments, cells were preincubated with chloroquine (Sigma, St. Louis, MO) or bafilomycin A1 (Sigma, St. Louis, MO) for 30 min before virus addition. Twenty-four hours after virus [or poly(I:C)] addition, supernatants were collected from the cells and frozen at −80°C for subsequent assays.
IFN measurement.
IFN-α (isoforms IFN-αA, -α2, -αA/D, -αD, -αK, and -α4b) was measured in the supernatants by using a human IFN-α enzyme-linked immunosorbent assay (ELISA) kit (PBL Biomedical Laboratories, New Brunswick, NJ) according to the manufacturer's instructions. At the time of the assay, supernatants were thawed, and the residual virus was inactivated with Triton X-100 (0.1% final concentration). In some experiments, IFN was measured by bioassay. For these assays, the supernatants collected from virus-treated cells were inactivated with UV as described above to ensure that residual virus input did not confound the results; complete viral inactivation was checked by immunohistochemistry (as described above). To detect IFN activity in these samples, Huh7 cells (1 × 104 cells/well) were seeded in 96-well, black-wall plates in DMEM with 10% FBS and antibiotics. The next day, the medium was removed, and fourfold serial dilutions of the test samples or a human IFN-β standard (from the NIAID Reference Reagent Repository, which is operated by Braton Biotech, Gaithersburg, MD) was added to the monolayers. After 12 to 16 h, the medium was removed, and WNV-like particles expressing a firefly luciferase reporter gene (19) were added to the cells (1 × 104 to 3 × 104 infectious units/well) in a final volume of 50 μl. After a 24-h incubation time, 50 μl of reconstituted Steady-Glo luciferase assay substrate (Promega, Madison, WI) diluted with 3 volumes of lysis buffer (25 mM Tricine, 15 mM MgSO4, 4 mM EDTA, 0.1% Triton X-100 [pH 7.8]) was added to the wells. The plate was shaken for 5 min, and light output was measured in a TR717 microplate luminometer (Applied Biosystems). The amount of sample needed to reduce luciferase activity by 50% and the amount of NIAID standard required to inhibit activity by 50% (2 to 5 U/ml, determined in each assay) were used to calculate the U/ml of IFN in the test samples.
Cytokine measurement.
To assess the production of cytokines, Triton-treated supernatants from mock- or virus-infected mDCs or pDCs (prepared as described above) were tested using the Luminex-based Bio-Plex system (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. Samples were diluted up to 10-fold prior to analysis to obtain values within the dynamic range of the assay. Data were collected from samples obtained from four different donors for each cell type (three of these donors provided cells for both subtypes of DCs); cytokines showing consistently low activity or no induction were not reported.
Indirect immunofluorescence.
For immunofluorescence microscopy, mDCs were plated in LabTek chamber slides (5 × 104 cells/well) and infected with WNV or SeV (16 HAU/ml) for 8, 12, and 24 h. After infection, cells were washed once with 1× phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde for 10 min, and blocked with a solution containing 2% bovine serum albumin, 5% normal horse serum, and 10 mM glycine in PBS for 10 min. After blocking, cells were permeabilized with 0.1% Triton X-100 and incubated with a rabbit polyclonal anti-IRF3 antibody (1:200) (Santa Cruz Biotechnology, Santa Cruz, CA) and WNV-specific MHIAF. Samples infected with SeV were incubated only with anti-IRF3 antibody. After 1 h, cells were washed two times with PBS and incubated with a fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit antibody (1:100) (Southern Biotechnology, Birmingham, AL) and Texas Red-labeled goat anti-mouse antibody (1:100) (KPL, Gaithersburg, MD) for the double-labeled WNV-infected samples. Single-labeled SeV-infected samples were incubated with FITC-labeled goat anti-rabbit antibody only. After secondary antibody incubation, the cells were washed two times with PBS and incubated with DAPI (4′,6′-diamidino-2-phenylindole) (500 ng/ml) for 5 min for nucleus counterstaining. Stained cells were analyzed with a 1.0 Zeiss LSM 510 UV META laser scanning confocal microscope at the UTMB Infectious Disease and Toxicology Optical Imaging Core Facility.
Statistical analysis.
Statistical analyses were performed by the Mann-Whitney U test using the InStat 3.05 biostatistics package (GraphPad, San Diego, CA). Unless otherwise indicated, means ± standard errors of the means are shown.
RESULTS
WNV induction of IFN-α is replication independent in pDCs and replication dependent in mDCs.
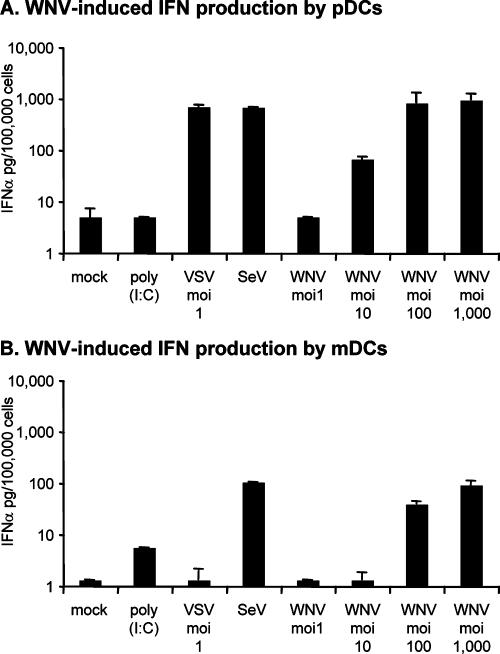
To investigate the ability of WNV to induce IFN-α production in human DCs, pDC and mDC cultures obtained from PBMC of healthy donors were incubated with WNV for 24 h, and IFN-α protein concentration in the cell culture supernatants was determined by ELISA. WNV elicited the expression of IFN-α in both pDCs (Fig. 1A) and mDCs (Fig. 1B) in an MOI-dependent manner. As expected, the pDC cultures produced about 10 times more IFN-α on a per-cell basis than the mDC cultures (Fig. 1). To further characterize the responses of our cultures to other stimuli, we treated these cells with poly(I:C), VSV, or SeV. These studies showed that mDCs produced some IFN-α in response to extracellular poly(I:C) and, in agreement with a previous report (47), we did not detect any increase in IFN-α production following poly(I:C) treatment of pDCs. However, both cultures produced high levels of IFN-α when exposed to SeV, but only the pDCs produced IFN-α when treated with VSV (Fig. 1). Microscopic examination of our WNV-treated DC cultures failed to detect any cytopathology at any dose used, and immunofluorescent antibody staining with a very potent polyclonal MHIAF failed to reveal any evidence of infection in the pDC cultures 24 h after the addition of WNV. However, this same MHIAF readily stained WNV antigen in mDCs at 24 h postinfection, with approximately 5% of the cells displaying antigen at this time point when infected with WNV at an MOI of 10, 50% of the cells displaying antigen at an MOI of 100, and 100% of the cells displaying antigen at an MOI of 1,000 (all MOIs were based on Vero titration) (see Materials and Methods) (data not shown). We were surprised at the large amount of WNV that we needed to use to obtain significant infection of mDC cultures, since Davis and coworkers reported previously that they obtained high levels of infection of mDC cultures at an MOI of 1 (15). However, they used K562 cells to quantitate their infectious dose of WNV (15), whereas we used Vero cells. In side-by-side titrations on these two cell types, we found that our WNV was 100 times more infectious on Vero cells than on K562 cells (data not shown), indicating that our mDC cultures were as susceptible to infection as the cultures utilized in previously reported studies (15). We did not have access to serum to help us determine the number of cells infected with SeV, but the egg-derived preparation used for our studies is known to be highly effective at inducing IFN in many cell types (see Materials and Methods). In the case of VSV infection, we did note a small number (approximately 5%) (results not shown) of VSV antigen-positive cells in VSV-treated preparations of both pDCs and mDCs, and VSV infection in the absence of IFN induction in murine mDCs was previously reported by others (1).
FIG. 1.
WNV induces IFN-α production in mDC and pDC cultures. IFN-α production was assessed in pDC (A) and mDC (B) cultures treated with the indicated virus or poly(I:C) as described in Materials and Methods. MOIs are based on WNV and VSV titration in Vero cells. SeV was added at 16 HAU/ml, and poly(I:C) was added at 25 μg/ml. IFN-α production was measured by ELISA and is presented as picograms of IFN-α produced by 100,000 cells. Bar graphs represent means from two different donors (a single blood collection from each of these two donors was used to generate both pDCs and mDCs) ± standard deviations (SD), except for SeV and poly(I:C) treatments, which were performed only on cultures from a single donor. Limits of detection (LODs) for IFN-α in these assays were 5 pg/100,000 pDCs and 1.25 pg/100,000 mDCs.
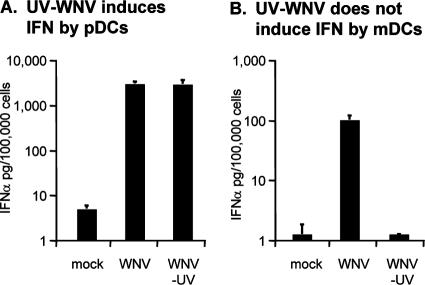
To determine if WNV replication was required for the induction of IFN-α in cultures of pDCs and mDCs, these cultures were exposed to UV-inactivated WNV. As shown in Fig. 2A, pDCs exposed to the UV-inactivated WNV or untreated WNV produced similar levels of IFN-α. However, no IFN-α was detected in supernatants of mDCs incubated with UV-inactivated WNV (Fig. 2B), indicating that WNV replication is not required for IFN-α production in pDCs but is required for IFN-α production by mDCs.
FIG. 2.
UV inactivation blocks the ability of WNV to induce IFN-α production by mDC but not by pDC cultures. Production of IFN-α was determined in pDC (A) and mDC (B) cultures treated with WNV or UV-treated WNV. Incubations were performed at an MOI of 1,000 (based on Vero titrations). IFN-α production was measured by ELISA and is presented as picograms of IFN-α produced by 100,000 cells. Bar graphs represent means from two or three different donors ± SD. LODs for IFN-α in these assays were 5 pg/100,000 pDCs and 1.25 pg/100,000 mDCs.
Endosome acidification is necessary for the production of IFN-α by WNV in human pDCs.
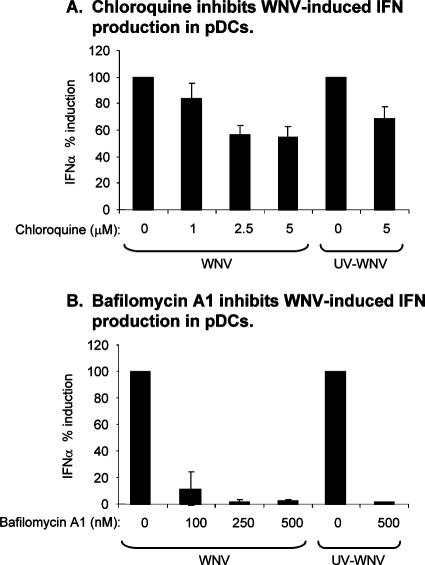
Multiple studies reported that the recognition of viruses or viral products by pDCs occurs through a TLR-dependent pathway that requires endosome maturation and acidification (6, 18, 48, 49, 60, 70). To verify the involvement of endosome maturation and acidification in IFN-α induction in WNV-treated pDCs, we examined WNV-induced IFN-α production in pDCs pretreated with either chloroquine or bafilomycin A1, two well-characterized inhibitors of endosomal acidification. As shown in Fig. 3A, treatment of pDCs with low levels of chloroquine partially inhibited WNV-induced IFN-α production by pDC cultures, and the more potent acidification inhibitor, bafilomycin A1, completely blocked WNV-induced IFN-α synthesis (Fig. 3B). Taken together, these data argue that the endosomal uptake of viral PAMPs present in these WNV preparations was required for WNV-induced IFN synthesis in these pDC cultures. Consistent with these findings, both drugs had a similar effect on IFN-α synthesis induced by treatment with UV-inactivated WNV (Fig. 3). Since the disruption of endosomal acidification has been shown to block WNV infection (11) and we have shown that WNV replication was required for IFN-α production by mDCs (Fig. 2B), data from chloroquine/bafilomycin A1-treated mDCs could not be unambiguously interpreted.
FIG. 3.
Chloroquine and bafilomycin A1 inhibit the ability of WNV to induce IFN-α in pDC cultures. Cultures of pDCs were either untreated or pretreated with chloroquine (A) or bafilomycin A1 (B) and then exposed to WNV or UV-inactivated WNV at an MOI of 1,000 (based on Vero titration). Each bar represents the average of the IFN-α ELISA values obtained from two different donors expressed as a percentage of the amount of IFN-α produced by that donor's cells in the absence of drug treatment; error bars show the SD between these percentages.
IRF-3 is translocated to the nucleus at late times of WNV infection in human mDCs.
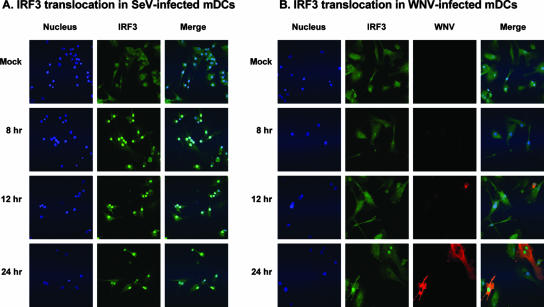
The fact that WNV replication was required for the induction of IFN-α synthesis in mDCs (Fig. 2B) suggested that a pathway requiring the intracellular production of a PAMP was responsible for IFN-α induction in these cells. Several of these pathways include IRF3, which has been implicated in the initiation of the antiviral response in other types of cells infected with WNV (23, 65). Therefore, we compared the nuclear translocation of IRF3 in mDCs treated with WNV to that in mDCs treated with SeV, a well-known activator of this pathway (41). As expected, IRF3 was detected in the nucleus of mDCs treated with SeV at 8 h posttreatment (Fig. 4A). In the case of WNV, translocation appeared to be slower, consistent with the replication of kinetics of this virus and the slow accumulation of antigen in cells infected with WNV (Fig. 4B.) However, by 24 h postinfection, when many of the cells were strongly immunopositive for WNV antigen, there was readily detectable IRF3 translocation in cells expressing high levels of WNV antigen (Fig. 4B), demonstrating an association of infection with signaling through an IRF3 pathway.
FIG. 4.
Infection with WNV or SeV results in IRF3 translocation in mDC. Micrographs of mDC cultures treated with SeV (16 HAU/ml) (A) or WNV (MOI of 100, based on titration on Vero cells) (B) were exposed for the indicated times and then fixed and subjected to indirect immunofluorescent staining as described in Materials and Methods. Nuclei were stained with DAPI (500 ng/ml); IRF3 was detected with rabbit polyclonal anti-IRF3 decorated with an FITC label, and WNV antigen was detected with anti-WNV MHIAF decorated with a Texas Red label.
Mosquito cell-derived WNV does not induce IFN-α production in pDCs.
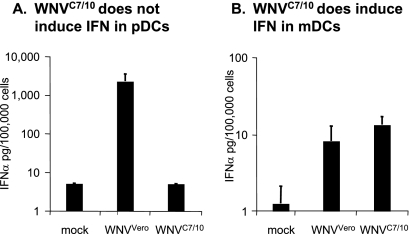
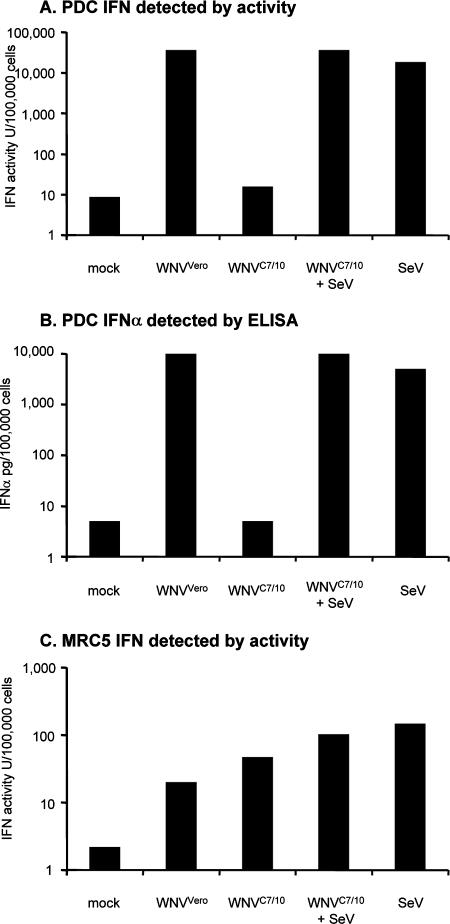
Recently, Davis and coworkers reported that mosquito cell-derived WNV and mammalian cell-derived WNV displayed different infectivities in mDC cultures (15). Thus, we were interested in comparing the abilities of WNV derived from mammalian cells (WNVVero) and mosquito cells (WNVC7/10) to induce IFN-α production in pDCs and mDCs. The infection of mDCs with WNVC7/10 resulted in approximately twofold-higher levels of IFN-α than those detected in parallel cultures infected with WNVVero (Fig. 5B). The higher level of IFN-α produced by the WNVC7/10-infected mDCs, although not statistically significant, is consistent with the results of Davis and coworkers, who reported previously that mosquito cell-grown WNV showed greater infectivity than mammalian cell-grown WNV on mDCs and other DC-SIGN-expressing cells (15). Surprisingly, no IFN-α was detected in the supernatants of pDCs treated with WNVC7/10 (Fig. 5A). To confirm this observation and determine if something in the mosquito cell-propagated virus preparations was inhibiting IFN-α induction by WNVC7/10, we compared WNV-induced IFN production in a side-by-side experiment with pDCs and the MRC-5 human cell line. In these experiments, we also included SeV challenge in the presence and absence of WNVC7/10 treatment. Both an IFN bioassay (Fig. 6A) and an IFN-α ELISA (Fig. 6B) confirmed that WNVC7/10 was unable to stimulate the production of IFN-α in pDC cultures, and these same experiments demonstrated that WNVC7/10 did not interfere with the ability of SeV to induce IFN synthesis by our pDC cultures. Furthermore, MRC-5 cells produced IFN when stimulated with either WNVC7/10 or WNVVero, and WNVC7/10 treatment did not appear to inhibit the ability of SeV to produce IFN in these cells either (Fig. 6C). As expected, no IFN-α was detected in supernatants from the MRC-5 cells by ELISA (data not shown), consistent with the fact that these cells, which do not constitutively express IRF7, would produce IFN-α at late times postinfection following IFN-β-mediated induction of IRF7 only.
FIG. 5.
Differential ability of mosquito cell-derived and mammalian cell-derived WNV to stimulate IFN-α production by mDC and pDC cultures. IFN-α production was determined in pDC (A) and mDC (B) cultures treated with WNVVero or WNVC7/10 at an MOI of 10 (based on Vero titration). Bar graphs represent means from two different donors ± SD. LODs for IFN-α in these assays were 5 pg/100,000 pDCs and 1.25 pg/100,000 mDCs.
FIG. 6.
Comparison of levels of IFN induced by treatment of pDC cultures or MRC-5 cells by mosquito cell-derived and mammalian cell-derived WNV in the absence or presence of SeV. (A) IFN activity detected by bioassay (see Materials and Methods) in pDC cultures treated with WNVVero, WNVC7/10, SeV, or WNVC7/10 and SeV. (B) IFN-α detected by ELISA in the same pDC-derived samples described above (A). (C) IFN activity detected by bioassay (see Materials and Methods) in MRC-5 cells treated with the indicated preparations of WNVVero, WNVC7/10, SeV, or WNVC7/10 and SeV. WNV treatments were done at an MOI of 100 (based on Vero titration), and SeV treatment was performed at 16 HAU/ml. IFN activities or IFN-α protein concentrations are presented as units or picograms produced by 100,000 cells. LODs for IFN in pDCs were 9 U/100,000 cells (A) and 5 pg/100,000 cells (B). The LOD for IFN in MRC-5 cells was 2.2 U/100,000 cells (C).
Evaluation of selected chemokines and cytokines produced by pDCs and mDCs treated with mammalian cell- and mosquito cell-derived WNV.
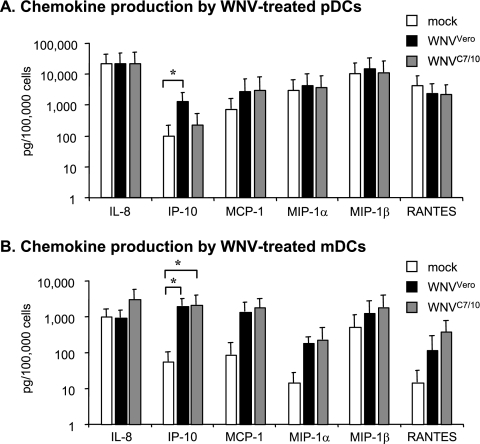
DCs have been shown to produce a number of cytokines that affect both humoral and T-cell-mediated immune responses. To examine whether the difference in the response of DC cultures to WNVVero and WNVC710 could be extended to chemokines and cytokines other than IFN-α, we used a Luminex-based Bio-Plex assay to measure cytokines in these samples. From the panel of 27 chemokines and cytokines analyzed, most of the cytokines were expressed below detection levels in cultures of both cell types exposed to the two viruses (data not shown). On the other hand, several chemokines appeared to be induced after exposure of mDCs to either WNVVero or WNVC7/10 (Fig. 7). Interestingly, in pDCs, we observed that only IP-10, a known IFN-dependent chemokine, was induced by WNVVero. This cytokine was not induced by exposure to WNVC7/10 (Fig. 7A), in agreement with our observations of the differential abilities of viruses from these two sources to stimulate IFN-α production (see above). On the other hand, in mDCs, infection with WNVVero or WNVC7/10 resulted in the production of comparable amounts of IP-10 (Fig. 7B). Although not statistically significant, we consistently observed (all four donors) that only WNVC7/10 was able to induce IL-8 in mDC cultures (Fig. 7B and results not shown), a finding that may have implications for disease pathogenesis.
FIG. 7.
Comparison of the levels of chemokines to be induced by treatment of pDC or mDC cultures with mosquito cell-derived and mammalian cell-derived WNV. Chemokine production was detected in pDC (A) and mDC (B) cultures treated with WNVVero or WNVC7/10 at an MOI of 10 (based on Vero titration). Chemokine concentrations were determined by Bio-Plex assay as described in Materials and Methods. For all values shown, a single treated sample from four different donors was assayed (three of these donors provided cells for both subtypes of DCs). Bar graphs represent means ± standard errors of the means. *, P < 0.05. MCP-1, monocyte chemoattractant protein 1; MIP-1α, macrophage inflammatory protein 1α.
DISCUSSION
The production of IFN in response to viral infections is crucial for the development of an appropriate innate immune response and likely plays a role in directing the adaptive immune response. The goal of this study was to investigate the induction of IFN and other cytokines during exposure of human DCs to WNV derived from different hosts. We demonstrate here that mammalian cell-derived WNV stimulates the production of IFN-α in human pDCs and mDCs, whereas the mosquito cell-derived virus is able to promote IFN-α induction only in mDCs. Furthermore, in mDCs, IFN-α induction required viral replication, whereas in pDCs, inactivated virus preparations were sufficient to induce IFN synthesis. Analysis of the pathways to induction via IRF3 translocation (in the case of mDCs) and through an endocytic pathway, likely involving TLRs (in the case of pDCs), was consistent with these differences in replication activity needed to induce IFN synthesis in these two important classes of DCs.
This report is the first to demonstrate that pDCs produce high levels of IFN when exposed to WNV. We detected approximately 10-fold more IFN in pDC cultures treated with WNV than in mDC cultures treated with WNV (on a per-cell basis). This high level of IFN production by pDCs that we noted following exposure to WNV is consistent with data reported previously for other viruses including human immunodeficiency virus (HIV) (20), herpes simplex virus type 2 (HSV-2) (24, 48), DENV (59), influenza virus (18), VSV (49), and YFV (60).
Virus-induced production of IFN-α by pDCs results from the cellular recognition of single-stranded RNA or double-stranded DNA by TLR7/TLR8 and TLR9, respectively, which are located in the endosomal compartment and trigger IFN gene induction through the phosphorylation of IRF7 (5, 53). However, there appear to be multiple mechanisms by which these components can be delivered to the endosomal TLRs. Previous work has shown that inactivated preparations of HSV (36, 48), influenza virus (18), or HIV (72) were capable of stimulating pDCs, whereas in the case of respiratory syncytial virus (30), viral replication was required to stimulate pDCs. Recent reports on investigations with flaviviruses have shown that an antagonist of TLR7 signaling prevented DENV-induced IFN-α synthesis in pDCs and that DENV was detected within endosomal vacuoles of pDCs shortly after exposure (70). That work also demonstrated that endosome acidification was necessary for DENV-induced IFN-α synthesis (70), which is consistent with our studies showing that endosomal acidification was necessary for the induction of IFN by both live and UV-inactivated WNV. Recently, Lee et al. provided a critical clue to this conundrum by demonstrating that autophagic delivery of RNAs synthesized in the cytoplasm of virus-infected cells was required for IFN stimulation by viruses that must be replicationally active to stimulate IFN production in pDCs (39).
Our finding demonstrating that UV-inactivated WNV is able to induce IFN-α synthesis in pDCs in the absence of genome replication is consistent with work on many other viruses, including HSV (36, 48), influenza virus (18), and HIV (21), that do not require infection to stimulate IFN production in pDCs. In addition, recent work has shown that for YFV, viral replication was also dispensable for the induction of IFN-α by pDCs (60). Those findings with WNV and YFV are particularly interesting in light of the work of Pichyangkul et al., who reported that UV-inactivated DENV was incapable of inducing IFN-α in human pDC cultures (59). The contrasts between work on DENV infection reported in their study and that reported in our study are made even more interesting by the fact that productive infection was not detected in cultures of pDCs treated with either DENV or WNV and that our work failed to detect pDC stimulation by mosquito cell-propagated WNV, whereas mosquito cell-propagated DENV elicited strong IFN responses from pDC cultures (59).
Several groups reported previously that human mDCs can be infected with DENV (10, 12, 17, 29, 57, 69), YFV (4, 60), or WNV (15, 61). Furthermore, several of those studies showed that mDCs can produce type I IFN following DENV infection (10, 17, 29); however, we are the first to report type I IFN production by mDCs infected with WNV. In our hands, replicationally active WNV was required for type I IFN induction, and the amount of IFN produced by mDC cultures was approximately 10-fold lower (on a per-cell basis) than that produced by pDCs. We also demonstrated that WNV infection resulted in the translocation of IRF3 to the nuclei of infected cells in mDC cultures, suggesting that IFN-α induction by mDCs was occurring via the RIG-I/mda5 or TLR3 pathway. Interestingly, Shabman et al. recently reported that a mosquito cell-derived alphavirus was slightly more efficient in infecting murine bone marrow-derived DCs than mammalian cell-derived alphavirus, but the mammalian cell-derived alphavirus induced much higher levels of IFN than mosquito cell-derived alphavirus in these murine cell cultures (66). Furthermore, UV inactivation was shown to block IFN induction in these murine bone marrow-derived DCs by the mosquito-derived alphavirus but not the mammalian cell-derived alphavirus (66).
Our finding that WNV derived from insect cells was unable to stimulate pDCs to produce IFN, whereas mammalian cell-derived WNV strongly stimulated these cells to produce IFN, is provocative, especially in light of studies showing that the mosquito-derived virus induced IFN in mDCs and non-immune-system cells and that the mosquito-derived WNV did not prevent SeV from inducing IFN in pDCs. These puzzling findings led us to perform experiments to confirm that adventitious materials present in WNVVero were not responsible for IFN production in pDC cultures. Specifically, we demonstrated that polyethylene glycol-precipitated culture fluids harvested from mock-infected Vero cells did not induce IFN in pDC cultures (result not shown). In addition, we tested the ability of the WNV NS1 protein to induce IFN in pDC cultures. The latter studies were undertaken based on the findings that Japanese encephalitis virus-infected Vero cells, but not Aedes albopictus cells, secrete large amounts of NS1 (52), a finding which we have confirmed for WNV-infected Vero and C7/10 cells (F. D. Gilfoy and P. W. Mason, unpublished data). However, studies that treated pDCs with Vero cell-produced NS1 in the presence or absence of WNV prepared in C7/10 cells failed to demonstrate any IFN induction. However, it remains possible that differences in the modification of the N-linked glycans on the E protein found in viruses produced in these two cell types may alter their ability to access the endocytic compartment where they initiate the IFN induction pathway.
Although we were unable to determine the mechanism responsible for the inability of WNVC7/10 to induce IFN, this finding led us to examine chemokine synthesis in pDCs and mDCs challenged with WNV from these two sources. These studies showed that in pDC cultures, the observation that WNVVero was a better inducer of IFN-α than WNVC7/10 extended to an IFN-dependent chemokine, IP-10. Interestingly, WNVC7/10 appeared to be a slightly better inducer of IL-8 than WNVVero in mDCs. These findings suggest that distinct pathways in DCs could be affected by virus-specific components, leading to differential induction of cytokines (including IFN) and chemokines by virus produced by different hosts. Future studies will be directed towards an understanding of how the transcriptional machinery that controls IFN or IL-8 gene expression in these cell populations is affected by WNV intermediate products that are expressed during the process of viral replication in pDCs or mDCs. Our finding that IL-8 is induced by WNV suggests that this chemokine could have an important role in the pathogenesis of natural infection in humans. The latter point is supported by two recent reports showing that neutrophilia in the central nervous system is an important feature of WNV infection in humans (16, 68), thus implicating a possible role for WNV-induced chemokines such as IL-8 in the pathogenesis of WNV infection in humans.
The mechanisms by which flavivirus infections are recognized by a mammalian host and the resulting production of a protective immune response are still incompletely understood. Here, we demonstrate that two important subclasses of DCs are activated by WNV infection and that activation is dependent, in some cases, on the species of host cell used to produce the virus. These studies provide important information on pathogenesis and protection from flavivirus diseases.
Acknowledgments
We thank R. B. Tesh, UTMB, for supplying VSV and VSV-specific MHIAF.
F.D.G. was supported by an NIH training grant in emerging and tropical infectious diseases (grant AI07526). This work was supported by a grant from the NIAID to P.W.M. through the Western Regional Center of Excellence for Biodefense and Emerging Infectious Disease Research (NIH grant U54 AI057156), grant AI061441, and the Sealy Center for Vaccine Development. This work was supported in part by a grant from the NIAID to R.P.G. (NIH grant P01 AI062885).
Footnotes
Published ahead of print on 3 October 2007.
REFERENCES
- 1.Ahmed, M., K. L. Brzoza, and E. M. Hiltbold. 2006. Matrix protein mutant of vesicular stomatitis virus stimulates maturation of myeloid dendritic cells. J. Virol. 80:2194-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asselin-Paturel, C., G. Brizard, K. Chemin, A. Boonstra, A. O'Garra, A. Vicari, and G. Trinchieri. 2005. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J. Exp. Med. 201:1157-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asselin-Paturel, C., and G. Trinchieri. 2005. Production of type I interferons: plasmacytoid dendritic cells and beyond. J. Exp. Med. 202:461-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barba-Spaeth, G., R. S. Longman, M. L. Albert, and C. M. Rice. 2005. Live attenuated yellow fever 17D infects human DCs and allows for presentation of endogenous and recombinant T cell epitopes. J. Exp. Med. 202:1179-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barchet, W., M. Cella, and M. Colonna. 2005. Plasmacytoid dendritic cells—virus experts of innate immunity. Semin. Immunol. 17:253-261. [DOI] [PubMed] [Google Scholar]
- 6.Beignon, A. S., K. McKenna, M. Skoberne, O. Manches, I. DaSilva, D. G. Kavanagh, M. Larsson, R. J. Gorelick, J. D. Lifson, and N. Bhardwaj. 2005. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J. Clin. Investig. 115:3265-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Best, S. M., K. L. Morris, J. G. Shannon, S. J. Robertson, D. N. Mitzel, G. S. Park, E. Boer, J. B. Wolfinbarger, and M. E. Bloom. 2005. Inhibition of interferon-stimulated JAK-STAT signaling by a tick-borne flavivirus and identification of NS5 as an interferon antagonist. J. Virol. 79:12828-12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke, D. S., and T. P. Monath. 2001. Flaviviruses, p. 1043-1125. In D. M. Knipe, P. M. Howley, D. G. Griffin, R. A. Lamb, M. A. Martin, and B. Roizman (ed.), Fields virology, 4th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 9.Cella, M., D. Jarrossay, F. Facchetti, O. Alebardi, H. Nakajima, A. Lanzavecchia, and M. Colonna. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5:919-923. [DOI] [PubMed] [Google Scholar]
- 10.Chen, Y. C., and S. Y. Wang. 2002. Activation of terminally differentiated human monocytes/macrophages by dengue virus: productive infection, hierarchical production of innate cytokines and chemokines, and the synergistic effect of lipopolysaccharide. J. Virol. 76:9877-9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu, J. J., and M. L. Ng. 2004. Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway. J. Virol. 78:10543-10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cologna, R., and R. Rico-Hesse. 2003. American genotype structures decrease dengue virus output from human monocytes and dendritic cells. J. Virol. 77:3929-3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colonna, M., B. Pulendran, and A. Iwasaki. 2006. Dendritic cells at the host-pathogen interface. Nat. Immunol. 7:117-120. [DOI] [PubMed] [Google Scholar]
- 14.Crance, J. M., N. Scaramozzino, A. Jouan, and D. Garin. 2003. Interferon, ribavirin, 6-azauridine and glycyrrhizin: antiviral compounds active against pathogenic flaviviruses. Antiviral Res. 58:73-79. [DOI] [PubMed] [Google Scholar]
- 15.Davis, C. W., H. Y. Nguyen, S. L. Hanna, M. D. Sanchez, R. W. Doms, and T. C. Pierson. 2006. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J. Virol. 80:1290-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis, L. E., R. DeBiasi, D. E. Goade, K. Y. Haaland, J. A. Harrington, J. B. Harnar, S. A. Pergam, M. K. King, B. K. DeMasters, and K. L. Tyler. 2006. West Nile virus neuroinvasive disease. Ann. Neurol. 60:286-300. [DOI] [PubMed] [Google Scholar]
- 17.Deauvieau, F., V. Sanchez, C. Balas, A. Kennel, A. De Monfort, J. Lang, and B. Guy. 2007. Innate immune responses in human dendritic cells upon infection by chimeric yellow-fever dengue vaccine serotypes 1-4. Am. J. Trop. Med. Hyg. 76:144-154. [PubMed] [Google Scholar]
- 18.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 19.Fayzulin, R., F. Scholle, O. Petrakova, I. Frolov, and P. W. Mason. 2006. Evaluation of replicative capacity and genetic stability of West Nile virus replicons using highly efficient packaging cell lines. Virology 351:196-209. [DOI] [PubMed] [Google Scholar]
- 20.Ferbas, J. J., J. F. Toso, A. J. Logar, J. S. Navratil, and C. R. Rinaldo, Jr. 1994. CD4+ blood dendritic cells are potent producers of IFN-alpha in response to in vitro HIV-1 infection. J. Immunol. 152:4649-4662. [PubMed] [Google Scholar]
- 21.Fonteneau, J. F., M. Larsson, A. S. Beignon, K. McKenna, I. Dasilva, A. Amara, Y. J. Liu, J. D. Lifson, D. R. Littman, and N. Bhardwaj. 2004. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J. Virol. 78:5223-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fredericksen, B. L., and M. Gale, Jr. 2006. West Nile virus evades activation of interferon regulatory factor 3 through RIG-I-dependent and -independent pathways without antagonizing host defense signaling. J. Virol. 80:2913-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fredericksen, B. L., M. Smith, M. G. Katze, P. Y. Shi, and M. Gale, Jr. 2004. The host response to West Nile virus infection limits viral spread through the activation of the interferon regulatory factor 3 pathway. J. Virol. 78:7737-7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gary-Gouy, H., P. Lebon, and A. H. Dalloul. 2002. Type I interferon production by plasmacytoid dendritic cells and monocytes is triggered by viruses, but the level of production is controlled by distinct cytokines. J. Interf. Cytok. Res. 22:653-659. [DOI] [PubMed] [Google Scholar]
- 25.Guerrero-Plata, A., A. Casola, G. Suarez, X. Yu, L. Spetch, M. E. Peeples, and R. P. Garofalo. 2006. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 34:320-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo, J. T., J. Hayashi, and C. Seeger. 2005. West Nile virus inhibits the signal transduction pathway of alpha interferon. J. Virol. 79:1343-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haller, O., G. Kochs, and F. Weber. 2006. The interferon response circuit: induction and suppression by pathogenic viruses. Virology 344:119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayes, E. B., and D. J. Gubler. 2006. West Nile virus: epidemiology and clinical features of an emerging epidemic in the United States. Annu. Rev. Med. 57:181-194. [DOI] [PubMed] [Google Scholar]
- 29.Ho, L. J., J. J. Wang, M. F. Shaio, C. L. Kao, D. M. Chang, S. W. Han, and J. H. Lai. 2001. Infection of human dendritic cells by dengue virus causes cell maturation and cytokine production. J. Immunol. 166:1499-1506. [DOI] [PubMed] [Google Scholar]
- 30.Hornung, V., J. Schlender, M. Guenthner-Biller, S. Rothenfusser, S. Endres, K. K. Conzelmann, and G. Hartmann. 2004. Replication-dependent potent IFN-alpha induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J. Immunol. 173:5935-5943. [DOI] [PubMed] [Google Scholar]
- 31.Johnson, A. J., and J. T. Roehrig. 1999. New mouse model for dengue virus vaccine testing. J. Virol. 73:783-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones, M., A. Davidson, L. Hibbert, P. Gruenwald, J. Schlaak, S. Ball, G. R. Foster, and M. Jacobs. 2005. Dengue virus inhibits alpha interferon signaling by reducing STAT2 expression. J. Virol. 79:5414-5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kadowaki, N., S. Antonenko, J. Y. Lau, and Y. J. Liu. 2000. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J. Exp. Med. 192:219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadowaki, N., and Y. J. Liu. 2002. Natural type I interferon-producing cells as a link between innate and adaptive immunity. Hum. Immunol. 63:1126-1132. [DOI] [PubMed] [Google Scholar]
- 35.Kawai, T., and S. Akira. 2006. Innate immune recognition of viral infection. Nat. Immunol. 7:131-137. [DOI] [PubMed] [Google Scholar]
- 36.Krug, A., G. D. Luker, W. Barchet, D. A. Leib, S. Akira, and M. Colonna. 2004. Herpes simplex virus type 1 activates murine natural interferon-producing cells through Toll-like receptor 9. Blood 103:1433-1437. [DOI] [PubMed] [Google Scholar]
- 37.Kwan, W. H., A. M. Helt, C. Maranon, J. B. Barbaroux, A. Hosmalin, E. Harris, W. H. Fridman, and C. G. Mueller. 2005. Dendritic cell precursors are permissive to dengue virus and human immunodeficiency virus infection. J. Virol. 79:7291-7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Bon, A., and D. F. Tough. 2002. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 14:432-436. [DOI] [PubMed] [Google Scholar]
- 39.Lee, H. K., J. M. Lund, B. Ramanathan, N. Mizushima, and A. Iwasaki. 2007. Autophagy-dependent viral recognition by plasmacytoid dendritic cell. Science 315:1398-1401. [DOI] [PubMed] [Google Scholar]
- 40.Libraty, D. H., S. Pichyangkul, C. Ajariyakhajorn, T. P. Endy, and F. A. Ennis. 2001. Human dendritic cells are activated by dengue virus infection: enhancement by gamma interferon and implications for disease pathogenesis. J. Virol. 75:3501-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin, R., C. Heylbroeck, P. M. Pitha, and J. Hiscott. 1998. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell. Biol. 18:2986-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin, R. J., C. L. Liao, E. Lin, and Y. L. Lin. 2004. Blocking of the alpha interferon-induced Jak-Stat signaling pathway by Japanese encephalitis virus infection. J. Virol. 78:9285-9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindenbach, B. D., and C. M. Rice. 2001. Flaviviridae: the viruses and their replication, p. 991-1041. In D. M. Knipe, P. M. Howley, D. G. Griffin, R. A. Lamb, M. A. Martin, and B. Roizman (ed.), Fields virology, 4th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 44.Liu, W. J., X. J. Wang, V. V. Mokhonov, P. Y. Shi, R. Randall, and A. A. Khromykh. 2005. Inhibition of interferon signaling by the New York 99 strain and Kunjin subtype of West Nile virus involves blockage of STAT1 and STAT2 activation by nonstructural proteins. J. Virol. 79:1934-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu, Y. J. 2005. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 23:275-306. [DOI] [PubMed] [Google Scholar]
- 46.Lobigs, M., A. Mullbacher, Y. Wang, M. Pavy, and E. Lee. 2003. Role of type I and type II interferon responses in recovery from infection with an encephalitic flavivirus. J. Gen. Virol. 84:567-572. [DOI] [PubMed] [Google Scholar]
- 47.Loseke, S., E. Grage-Griebenow, H. Heine, A. Wagner, S. Akira, S. Bauer, and A. Bufe. 2006. In vitro-generated viral double-stranded RNA in contrast to polyinosinic:polycytidylic acid induces interferon-alpha in human plasmacytoid dendritic cells. Scand. J. Immunol. 63:264-274. [DOI] [PubMed] [Google Scholar]
- 48.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lund, J. M., L. Alexopoulou, A. Sato, M. Karow, N. C. Adams, N. W. Gale, A. Iwasaki, and R. A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 101:5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marie, I., J. E. Durbin, and D. E. Levy. 1998. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 17:6660-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marovich, M., G. Grouard-Vogel, M. Louder, M. Eller, W. Sun, S. J. Wu, R. Putvatana, G. Murphy, B. Tassaneetrithep, T. Burgess, D. Birx, C. Hayes, S. Schlesinger-Frankel, and J. Mascola. 2001. Human dendritic cells as targets of dengue virus infection. J. Investig. Dermatol. Symp. Proc. 6:219-224. [DOI] [PubMed] [Google Scholar]
- 52.Mason, P. W. 1989. Maturation of Japanese encephalitis virus glycoproteins produced by infected mammalian and mosquito cells. Virology 169:354-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McKenna, K., A. S. Beignon, and N. Bhardwaj. 2005. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J. Virol. 79:17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morrey, J. D., C. W. Day, J. G. Julander, L. M. Blatt, D. F. Smee, and R. W. Sidwell. 2004. Effect of interferon-alpha and interferon-inducers on West Nile virus in mouse and hamster animal models. Antivir. Chem. Chemother. 15:101-109. [DOI] [PubMed] [Google Scholar]
- 55.Munoz-Jordan, J. L., G. G. Sanchez-Burgos, M. Laurent-Rolle, and A. Garcia-Sastre. 2003. Inhibition of interferon signaling by dengue virus. Proc. Natl. Acad. Sci. USA 100:14333-14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nash, D., F. Mostashari, A. Fine, J. Miller, D. O'Leary, K. Murray, A. Huang, A. Rosenberg, A. Greenberg, M. Sherman, S. Wong, and M. Layton. 2001. The outbreak of West Nile virus infection in the New York City area in 1999. N. Engl. J. Med. 344:1807-1814. [DOI] [PubMed] [Google Scholar]
- 57.Navarro-Sanchez, E., R. Altmeyer, A. Amara, O. Schwartz, F. Fieschi, J. L. Virelizier, F. Arenzana-Seisdedos, and P. Despres. 2003. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 4:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petersen, L. R., A. A. Marfin, and D. J. Gubler. 2003. West Nile virus. JAMA 290:524-528. [DOI] [PubMed] [Google Scholar]
- 59.Pichyangkul, S., T. P. Endy, S. Kalayanarooj, A. Nisalak, K. Yongvanitchit, S. Green, A. L. Rothman, F. A. Ennis, and D. H. Libraty. 2003. A blunted blood plasmacytoid dendritic cell response to an acute systemic viral infection is associated with increased disease severity. J. Immunol. 171:5571-5578. [DOI] [PubMed] [Google Scholar]
- 60.Querec, T., S. Bennouna, S. Alkan, Y. Laouar, K. Gorden, R. Flavell, S. Akira, R. Ahmed, and B. Pulendran. 2006. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J. Exp. Med. 203:413-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rios, M., M. J. Zhang, A. Grinev, K. Srinivasan, S. Daniel, O. Wood, I. K. Hewlett, and A. I. Dayton. 2006. Monocytes-macrophages are a potential target in human infection with West Nile virus through blood transfusion. Transfusion 46:659-667. [DOI] [PubMed] [Google Scholar]
- 62.Rossi, S. L., Q. Zhao, V. K. O'Donnell, and P. W. Mason. 2005. Adaptation of West Nile virus replicons to cells in culture and use of replicon-bearing cells to probe antiviral action. Virology 331:457-470. [DOI] [PubMed] [Google Scholar]
- 63.Samuel, M. A., and M. S. Diamond. 2005. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J. Virol. 79:13350-13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sato, M., H. Suemori, N. Hata, M. Asagiri, K. Ogasawara, K. Nakao, T. Nakaya, M. Katsuki, S. Noguchi, N. Tanaka, and T. Taniguchi. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13:539-548. [DOI] [PubMed] [Google Scholar]
- 65.Scholle, F., and P. W. Mason. 2005. West Nile virus replication interferes with both poly(I:C)-induced interferon gene transcription and response to interferon treatment. Virology 342:77-87. [DOI] [PubMed] [Google Scholar]
- 66.Shabman, R. S., T. E. Morrison, C. Moore, L. White, M. S. Suthar, L. Hueston, N. Rulli, B. Lidbury, J. P. Ting, S. Mahalingam, and M. T. Heise. 2007. Differential induction of type I interferon responses in myeloid dendritic cells by mosquito and mammalian-cell-derived alphaviruses. J. Virol. 81:237-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siegal, F. P., N. Kadowaki, M. Shodell, P. A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y. J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science 284:1835-1837. [DOI] [PubMed] [Google Scholar]
- 68.Tyler, K. L., J. Pape, R. J. Goody, M. Corkill, and B. K. Kleinschmidt-DeMasters. 2006. CSF findings in 250 patients with serologically confirmed West Nile virus meningitis and encephalitis. Neurology 66:361-365. [DOI] [PubMed] [Google Scholar]
- 69.Vasilakis, N., E. J. Shell, E. B. Fokam, P. W. Mason, K. A. Hanley, D. M. Estes, and S. C. Weaver. 2007. Potential of ancestral sylvatic dengue-2 viruses to re-emerge. Virology 358:402-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang, J. P., P. Liu, E. Latz, D. T. Golenbock, R. W. Finberg, and D. H. Libraty. 2006. Flavivirus activation of plasmacytoid dendritic cells delineates key elements of TLR7 signaling beyond endosomal recognition. J. Immunol. 177:7114-7121. [DOI] [PubMed] [Google Scholar]
- 71.Wu, S. J., G. Grouard-Vogel, W. Sun, J. R. Mascola, E. Brachtel, R. Putvatana, M. K. Louder, L. Filgueira, M. A. Marovich, H. K. Wong, A. Blauvelt, G. S. Murphy, M. L. Robb, B. L. Innes, D. L. Birx, C. G. Hayes, and S. S. Frankel. 2000. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 6:816-820. [DOI] [PubMed] [Google Scholar]
- 72.Yonezawa, A., R. Morita, A. Takaori-Kondo, N. Kadowaki, T. Kitawaki, T. Hori, and T. Uchiyama. 2003. Natural alpha interferon-producing cells respond to human immunodeficiency virus type 1 with alpha interferon production and maturation into dendritic cells. J. Virol. 77:3777-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]