Abstract
This study focuses on the development of a new clinical vaccine candidate (AdOprF.RGD.Epi8) against Pseudomonas aeruginosa using an E1− E3− adenovirus (Ad) vector expressing OprF (AdOprF.RGD.Epi8) and modifications of the Ad genome providing two capsid changes: (i) modification of the Ad hexon gene to incorporate an immune-dominant OprF epitope (Epi8) into loop 1 of the hexon, enabling repeat administration to boost the anti-OprF immune response, and (ii) modification of the fiber gene to incorporate an integrin-binding RGD sequence to enhance gene delivery to antigen-presenting cells. Western analysis confirmed that AdOprF.RGD.Epi8 expresses OprF, contains Epi8 in the hexon protein, and enhances gene transfer to dendritic cells compared to AdOprF, a comparable Ad vector expressing OprF with an unmodified capsid. Intramuscular immunization of C57BL/6 mice with AdOprF.RGD.Epi8 resulted in the generation of anti-OprF antibodies at comparable levels to those induced following immunization with AdOprF, but immunization with AdOprF.RGD.Epi8 was associated with increased CD4 and CD8 gamma interferon T-cell responses against OprF as well as increased survival against lethal pulmonary challenge with agar-encapsulated P. aeruginosa. Importantly, repeat administration of AdOprF.RGD.Epi8 resulted in boosting of the humoral anti-OprF response as well as increased protection, whereas no boosting could be achieved with repeat administration of AdOprF. This suggests that the capsid-modified AdOprF.RGD.Epi8 vector is a more effective immunogen compared to a comparable wild-type Ad capsid, making it a good candidate for an anti-P. aeruginosa vaccine.
Pulmonary infections with the gram-negative ubiquitous organism Pseudomonas aeruginosa are frequent in patients with cystic fibrosis, immunodeficiency, and bronchiectasis (14, 15). There is currently no vaccine against P. aeruginosa. One P. aeruginosa component considered to be a promising candidate for an anti-Pseudomonas vaccine is the P. aeruginosa major surface-exposed outer membrane protein F (OprF) (13, 17, 20, 25, 26, 29). OprF is antigenically conserved in wild-type strains of P. aeruginosa (31, 32) and appears to be invariant among P. aeruginosa clinical isolates (31, 32). Antibodies against OprF are associated with protection against Pseudomonas in animal models and are induced by immunization with recombinant OprF in experimental animals and humans (13, 17, 20, 25, 26, 29). Various immunogenic peptides have been identified in the outer loops of the OprF protein, including the immune-dominant 14-mer peptide Epi8 (16, 22, 55).
The present study evaluates a novel capsid-modified adenovirus (Ad) vector (AdOprF.RGD.Epi8) that expresses the gene for OprF to induce protective immunity against P. aeruginosa. The two capsid modifications of the AdOprF. RGD.Epi8 vector include the insertion of RGD into the fiber to enhance the infection of dendritic cells (DCs) (34, 54) as well as the insertion of the 14-mer OprF epitope Epi8 into hypervariable region 5 of the Ad hexon to enable repeat administration of the same vector to boost the anti-OprF humoral response (55). Anti-Ad capsid immune responses are usually augmented by repeated administration (12, 16, 17), and thus immune responses against epitopes that are part of the Ad capsid should be augmented with repeated administration and would thus allow boosting. In a murine model, AdOprF.RGD.Epi8 induced increased anti-OprF cellular and protective immunity compared to a non-capsid-modified vector expressing only OprF (AdOprF). Furthermore, repeat administration of AdOprF.RGD.Epi8 led to boosting of the anti-OprF humoral immunity and resulted in increased survival following pulmonary challenge with P. aeruginosa. Together, these data suggest that AdOprF.RGD.Epi8 may be a valuable candidate for an anti-P. aeruginosa vaccine.
MATERIALS AND METHODS
Adenovirus vectors.
The recombinant Ad vectors AdOprf and AdOprF.RGD.Epi8 used in this study are E1a, partial E1b, and partial E3 vectors and are based on the Ad5 genome. In both vectors, an OprF expression cassette was inserted into the E1 region, containing the human cytomegalovirus intermediate-early enhancer/promoter, the OprF cDNA, and a simian virus 40 poly(A) stop signal. A non-capsid-modified vector with no transgene (AdNull) was used as a control (21). In addition to AdOprF, AdOprF.RGD.Epi8 contains the OprF epitope Epi8 (NATAEGRAINRRVE) inserted into loop 1 of the hypervariable region 5 at residues 268 to 269 of the Ad5 hexon gene (the Epi8 insertion was derived from the previously published AdZ.Epi8 vector [55]) and the high-affinity RGD sequence GCDCRGDCFCA incorporated between the last codon (residue 585) and the stop codon at the COOH-terminal end of the Ad5 fiber protein, as previously described for AdZ.F.RGD (52, 54). The vectors were used on the basis of equal number of particle units (pu) and were propagated and purified as described previously (40, 41, 52, 54).
Production of recombinant OprF.
The recombinant vector pSUMO-OprF with an N-terminal His tag was constructed by cloning the PCR-amplified OprF gene (forward primer, 5′-CCCGGATCCAGAATGCAGGGCCAGAAC-3′; reverse primer, 5′-CCCAAGCTTTTTACTTGGCCTCAGCCTCC-3′) into the expression vector pET SUMO (Invitrogen, Carlsbad, CA). The recombinant plasmid pSUMO-His-OprF was transformed into Escherichia coli BL21(DE3), and the recombinant protein was purified by Ni-chelating affinity chromatography from a single transformant under native conditions. Briefly, the cultures were grown to an optical density at 600 nm of 0.8, stimulated with 0.5 mM isopropyl thiogalactoside for 3 h at 27°C, and collected by centrifugation. The cell pellet was washed and resuspended in TBS buffer I (50 mM Tris, 0.5 mM EDTA, 50 mM NaCl, pH 7.4). Cell lysis was induced by sonification, and the lysate was cleared by centrifugation (18,000 × g, 4°C). Imidazole (10 mM) was added, and the crude extract was placed on Ni-nitrilotriacetic acid-agarose (prebound; Qiagen, Valencia, CA) equilibrated with TBS buffer I. Unbound material was washed out successively with 10 column volumes of TBS buffer I. The specific protein was eluted with five column volumes of TBS buffer II (50 mM Tris, 0.5 mM EDTA, 50 mM NaCl, 300 mM imidazole, pH 7.4), and the positive fractions were dialyzed against phosphate-buffered saline (PBS), pH 7.4.
Bacteria.
The P. aeruginosa strain used in this study was the laboratory strain PAO1 (48). Bacteria were grown from frozen stocks in tryptic soy broth (Difco, Detroit, MI) at 37°C to mid-log phase, washed three times with PBS, and resuspended in PBS at the desired concentration as determined by spectrophotometry. Numbers of bacteria were confirmed by determining the CFU of diluted aliquots on MacConkey agar plates (Difco). P. aeruginosa-containing agar beads were prepared based on the method of Stevenson et al. (47) and used in a lethal respiratory infection with P. aeruginosa as previously described (55). Briefly, a log-phase culture of P. aeruginosa suspended in warm tryptic soy agar (52°C) was added to mineral oil with vigorous stirring and cooled with ice. The P. aeruginosa-impregnated beads were washed extensively with PBS, and the density of viable bacteria enmeshed in agar beads was determined by plating serial dilutions of homogenized beads.
Mice.
Female C57BL/6 mice were obtained from Taconic Farms (Tarrytown, NY). The animals were housed under specific-pathogen-free conditions and used at 6 to 8 weeks of age. If not noted otherwise, the mice were immunized once subcutaneously with the Ad vectors diluted in 50 ml PBS.
Infection of A549 and dendritic cells.
To confirm the expression of OprF, A549 cells were infected with 1,000 pu/cell of AdOprF.RGD.Epi8 or AdNull. A549 cells (CCL185; American Type Culture Collection, Rockville, MD) were maintained in complete Dulbecco's modified essential medium (10% fetal bovine serum, 100 U of penicillin/ml, 100 mg of streptomycin/ml), and expression of OprF was evaluated by Western analysis after 24 h.
Bone marrow-derived DC were generated from bone marrow precursors as described previously (46). In brief, bone marrow cells harvested from C57BL/6 mice were grown in complete RPMI 1640 medium (10% fetal bovine serum, 100 U of penicillin/ml, 100 μg of streptomycin/ml; GIBCO BRL, Gaithersburg, MD) supplemented with 10 ng/ml of recombinant mouse granulocyte-macrophage colony-stimulating factor and 2 ng/ml of recombinant mouse interleukin-4 (IL-4) (both from R&D Systems) for 8 days. The DC were then washed and resuspended in RPMI 1640 medium (2% fetal bovine serum, 100 U of penicillin/ml, 100 μg of streptomycin/ml) and infected with AdOprF, AdOprF.RGD.Epi8, or AdNull (104 pu/cell) for 4 h, washed, and maintained in complete medium. Cells were then harvested after 24 h.
The expression of OprF in the cell lysates was determined by Western analysis using sera from C57BL/6 mice collected 4 weeks after immunization with 50 mg of recombinant OprF (rOprRF) in Freund's adjuvant. Equal amounts of protein per lane were confirmed by using an anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Abcam, Cambridge, MA).
Detection of Epi8 in Ad virions by Western analysis.
To assess the presence of the Epi8 epitopes in the hexon protein, AdOprF.RGD.Epi8, AdOprF, and AdNull (1010 pu) were denatured by heating at 95°C for 5 min in NuPAGE sample buffer (Invitrogen, Carlsbad, CA), separated on a 4 to 20% polyacrylamide gradient sodium dodecyl sulfate (SDS)-polyacrylamide gel (NuPAGE System; Invitrogen) by electrophoresis, and transferred to a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Hercules, CA). Epi8 was detected by 1 h of incubation with anti-Epi8 serum. A peroxidase-conjugated goat anti-mouse antibody (Sigma-Aldrich) was then added for 1 h, followed by detection with chemiluminescent peroxidase substrate (ECL+ reagent; Amersham Biosciences, Piscataway, NJ).
Anti-OprF and anti-Epi8 antibodies.
To evaluate the anti-OprF humoral response, C57BL/6 mice were immunized intramuscularly with AdNull, AdOprF, or AdOprF.RGD.Epi8 at 2 × 1010 pu/mouse. Serum was collected from the tail vein 14 and 28 days after immunization. Anti-OprF and anti-Ad antibodies were determined in an enzyme-linked immunosorbent assay (ELISA). Microtiter plates (Nunc, Roskilde, Denmark) were coated with 0.5 mg/well of purified pSUMO-OprF or 109 AdNull particles in 0.05 M carbonate-bicarbonate buffer, pH 9.6 (Sigma-Aldrich) at 4°C for 12 h. The plates were washed three times with PBS and blocked with 5% fat-free milk (Bio-Rad Laboratories) in PBS. After three washes with 0.05% Tween 20 in PBS (PBST), the sera were added in sequential twofold dilutions starting at 1:20 and incubated for 1 h. After three washes with PBST, anti-mouse immunoglobulin G (IgG)-horseradish peroxidase (Sigma-Aldrich) was added for 1 h. Detection was accomplished using the peroxidase substrate kit (Bio-Rad Laboratories), and absorbance was determined at 415 nm. Titers were calculated as reciprocal dilutions twofold above background values (substrate only). For titer determinations, the absorbance values of all dilutions were extrapolated to the twofold background value by using a linear fit function.
OprF-specific cellular response.
To assess the OprF- and Epi8-specific cellular immune responses, C57BL/6 mice were immunized subcutaneously with 1010 pu of AdNull, AdOprF, or AdOprF.RGD.Epi8. The frequency of OprF- or Epi8-specific CD4 and CD8 T cells was determined with a gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assay. CD4 and CD8 T cells were purified from the spleen by negative depletion using SpinSep T-cell subset purification kits (StemCell Technologies). Splenic DC, to serve as antigen-presenting cells, were purified from naive syngeneic animals by positive selection using anti-CD11c magnetic-activated cell sorting (MACS) beads (Miltenyi Biotec) and double purification over two consecutive MACS LS+ columns (Miltenyi Biotec). Purity for CD4 and CD8 T cells was generally >95% and for DC it was >90%, as determined by flow cytometry. DC (5 × 106/ml) were incubated for 2 h with purified pSUMO-OprF protein (100 μg/ml) in RPMI medium supplemented with 2% fetal calf serum (HyClone, Logan, UT), 10 mM HEPES pH 7.5 (BioSource International, Camarillo, CA), and 10−5 M β-mercaptoethanol (Sigma-Aldrich). T cells (2 ×105) were incubated with splenic DC at a ratio of 4:1, with or without pSUMO-OprF protein, on commercially available anti-IL-4- and anti-IFN-γ-coated plates (parts 890906 and 890894, respectively; R&D Systems) for 48 h. The plates were then incubated with the biotinylated anti-IFN-γ or anti-IL-4 (both from R&D) detection antibodies for 14 h at 4°C. Following incubation with streptavidin-alkaline phosphatase conjugate (R&D) and the 3-amino-9-ethylcarbazole substrate (R&D), the spots were counted by computer-assisted ELISPOT image analysis (Zellnet Consulting, New York, NY).
Protection against pulmonary challenge with P. aeruginosa.
To determine if immunization with AdOprF.RGD.Epi8 resulted in protective immunity against a lethal pulmonary challenge with P. aeruginosa, C57BL/6 mice were immunized subcutaneously with AdOprF.RGD.Epi8, AdOprF, or AdNull at 1010 pu/mouse. Five weeks after immunization, the mice were challenged with P. aeruginosa encapsulated in agar beads. Fifty μl of agar beads containing the laboratory strain PAO1 (5 × 106 CFU) was intratracheally inoculated into the lungs. All mice were monitored daily for 14 days after the infection. Animals that appeared moribund were sacrificed, and this was recorded as the date of death.
Statistical analysis.
The data are presented as means ± standard errors of the means (SEM). Statistical analysis was performed using the nonpaired two-tailed Student's t test assuming equal variance. Statistical significance was determined at a P level of <0.05. Survival estimates and median survivals were determined using the method of Kaplan and Meier.
RESULTS
Expression and presentation of OprF and Epi8 epitope in AdOprF.RGD.Epi8.
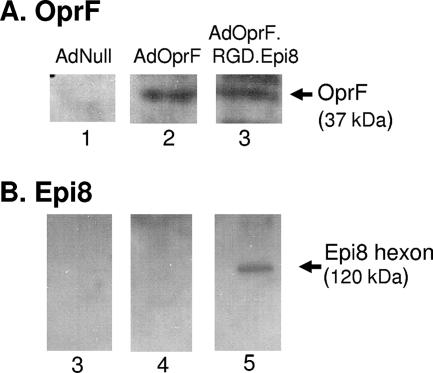
To verify the expression of OprF between AdOprf and AdOprF.RGD.Epi8 and the presence of the Epi8 epitope in an intact hexon protein of the modified capsid, Western analysis was performed. Serum from OprF-immunized mice confirmed the expression of the OprF transgene in cell lysates of A549 cells infected with AdOprf and AdOprF.RGD.Epi8 (Fig. 1A, lanes 2 and 3, respectively); no signal was detected in the AdNull-infected cell lysates (Fig. 1A, lane 1). Western analysis of AdOprF.RGD.Epi8 viral particles using serum from OprF-immunized mice confirmed the position of the Epi8 epitope at the corresponding size of the hexon protein (120 kDa) (Fig. 1B, lane 5), with no detectable signal for AdNull and AdOprf (Fig. 1B, lanes 3 and 4, respectively).
FIG. 1.
OprF expression following infection with AdOprF and AdOprF.RGD.Epi8 in A549 cells and detection of Epi8 in the AdOprF.RGD.Epi8 hexon. (A) OprF expression. A549 cells were infected with AdNull, AdOprF, or AdOprF.RGD.Epi8 (1,000 pu/cell, 24 h). Cell lysates were separated in a 10% SDS gel, and expression of OprF was assessed by Western analysis using sera from C57BL/6 mice immunized with recombinant OprF. Lane 1, AdNull; lane 2, AdOprF; lane 3, AdOprF.RGD.Epi8. The band representing OprF is indicated. (B) Epi8 in the AdOprF.RGD.Epi8 hexon. AdNull, AdOprF, and AdOprF.RGD.Epi8 (both 1010 particles) were assessed in a 4 to 12% gradient SDS gel, and the Epi8 epitope in the capsid was assessed by Western analysis using sera from C57BL/6 mice immunized with OprF. Lane 3, AdNull; lane 4, AdOprF; lane 5, AdOrpF.RGD.Epi8. The band visualized in lane 4 represents the 130-kDa hexon containing the Epi8 epitope.
Enhanced expression of dendritic cells with AdOprF.RGD.Epi8.
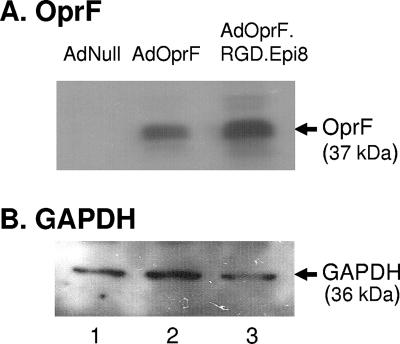
Comparison between AdOprF.RGD.Epi8 and AdOprf revealed the same OprF expression levels after infection in A549 cells, a cell line that is easily infectible in an integrin-independent manner (Fig. 1A). To evaluate if the RGD modification to the fiber of AdOprF.RGD.Epi8 enhances the expression of the OprF transgene in DC in vitro, murine bone marrow-derived DC infected with either AdOprF.RGD.Epi8 or AdOprF were analyzed for OprF expression (Fig. 2A). Western analysis of the cell lysates of the infected DC showed expression of OprF for DC infected with either AdOprF or AdOprF.RGD.Epi8 (Fig. 2A, lanes 2 and 3) and no OprF expression in the DC infected with AdNull (Fig. 2A, lane 1). Interestingly, the DC infected with AdOprF.RGD.Epi8 showed higher expression of OprF than those infected with AdOprF, indicating that the gene transfer to DC and therefore the expression of the transgene OprF was increased for AdOprF.RGD.Epi8 compared to the vector with the wild-type fiber AdOpF. GAPDH expression was equal for all groups (Fig. 2B).
FIG. 2.
Enhanced OprF expression in dendritic cells by AdOprF.RGD.Epi8 compared to AdOprF (shown are the results of one out of three experiments). Murine bone marrow-derived dendritic cells were infected with AdNull, AdOprF, or AdOprF.RGD.Epi8 at 104 pu per DC for 24 h. Lysates of infected DC were separated in a 10% SDS gel, and OprF expression was analyzed by Western analysis using sera from C57BL/6 immunized with recombinant OprF protein. (A) OprF expression; (B) expression of cellular GAPDH. Lanes in both panels: 1, AdNull; 2, AdOprF; 3, Ad OprF.RGD.Epi8.
Humoral immune responses.
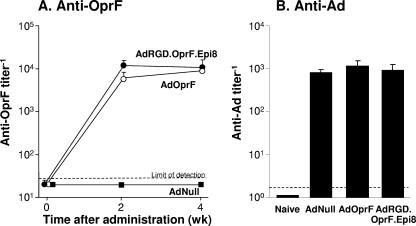
To assess the humoral response against OprF following immunization with the capsid-modified AdOprF.RGD.Epi8 vector, mice were immunized with either AdOprF, AdOprF.RGD.Epi8, or AdNull, and the serum anti-OprF and anti-Ad IgG responses were determined. Mice immunized with AdOprF and AdOprF.RGD.Epi8 had anti-OprF IgG antibodies present 2 and 4 weeks after immunization; no anti-OprF antibodies were detected in the serum of the AdNull group (Fig. 3A). The levels of the anti-OprF antibodies were comparable between animals immunized with either AdOprF or AdOprF.RGD.Epi8 at both time points (P > 0.2). The humoral response against the Ad capsid was also determined in these mice. Anti-Ad antibody titers were comparable in the AdOprF, AdOprF.RGD.Epi8, and AdNull groups at 4 weeks (P > 0.4, all comparisons), with no anti-Ad antibodies detected in naive mice (Fig. 3B). These data suggest that immunization with the capsid-modified AdOprF.RGD.Epi8 leads to anti-Ad capsid and antitransgene humoral responses comparable to those of a non-capsid-modified vector.
FIG. 3.
Humoral response to OprF and Ad capsid following immunization with AdOprF.RGD.Epi8 compared to immunization with AdOprF. C57BL/6 mice were immunized via the intramuscular route with AdNull, AdOprF, or AdOprF.RGD.Epi8 at a dose of 1010 pu/mouse. (A) Total IgG antibodies against OprF were determined by ELISA at 0, 2, and 4 weeks after administration, using purified recombinant OprF protein as the antigen. (B) Antiadenovirus response. Total IgG antibodies against the Ad capsid were determined by ELISA 4 weeks after administration, using AdNull capsids as the antigen. Data are shown as means ± SEM from five mice per group.
Cellular immune responses.
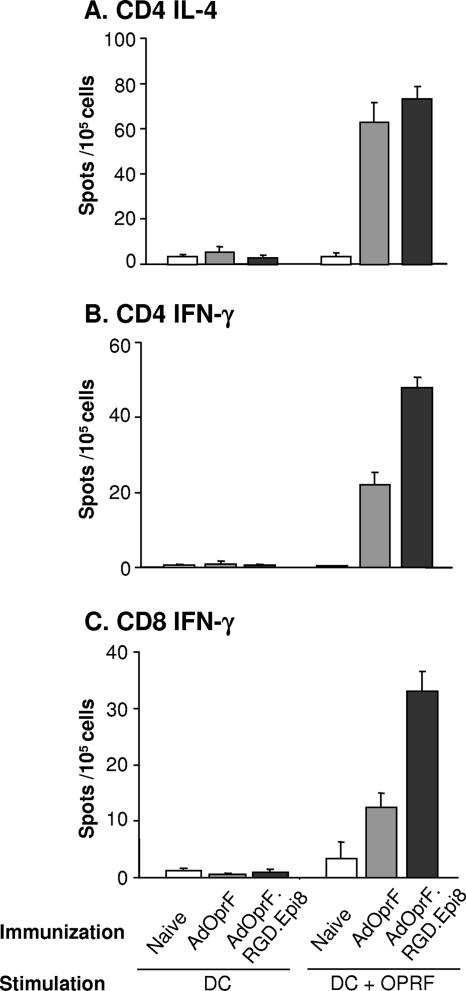
To assess the cellular immune responses, the frequencies of CD4 and CD8 T-cell responses to OprF in mice immunized with AdOprF.RGD.Epi8 or AdOprF were analyzed in an ELISPOT assay (Fig. 4). Ten days after immunization, purified splenic CD4 and CD8 T cells from vaccinated mice were stimulated with syngeneic DC pulsed with recombinant OprF protein. As the level of humoral immune response by B cells is dominantly supported by IL-4 secretion of CD4 Th2 helper cells in an antigen-dependent manner, we analyzed if CD4 IL-4 responses against OprF were increased in the mice immunized with AdOprF.RGD.Epi8 or AdOprF compared to the naive controls. AdOprF.RGD.Epi8 and AdOprF did induce IL-4 secretion in CD4 cells compared to the naive controls (P < 0.01) (Fig. 4A); however, they were not significantly different between the AdOprF.RGD.Epi8- and AdOprF-immunized animals (P > 0.2). No IL-4 was stimulated following exposure to unstimulated DC or in the AdNull controls. This is consistent with the humoral response against OprF following immunization with AdOprF.RGD.Epi8 and AdOprF as described above. The IFN-γ CD4 (Fig. 4B) and CD8 (Fig. 4C) responses were increased in the animals immunized with AdOprF or AdOprF.RGD.Epi8 compared to the AdNull group (P < 0.01 for CD4 and P < 0.03 for CD8). Interestingly, there was a significant increase in the IFN-γ CD4 and CD8 responses against OprF in the animals immunized with AdOprF.RGD.Epi8 compared to AdOprF (P < 0.01 for CD4 and P < 0.01for CD8). Taken together, these data suggest that immunization with the capsid-modified AdOprF.RGD.Epi8 vector leads to increased anti-OprF cellular Th1 immune responses due to enhanced presentation of the transgene protein and/or the Epi8 peptide.
FIG. 4.
CD4 and CD8 T-cell IL-4 and IFN-γ responses following immunization with AdOprF.RGD.Epi8. C57BL/6 mice were immunized with AdOprF and AdOprF.RGD.Epi8 at a dose of 1010 pu/mouse via the subcutaneous route. Ten days after immunization, CD4 and CD8 cells were isolated from spleens and incubated in vitro with DC alone or DC pulsed with recombinant OprF (DC+OprF), and the IL-4 and IFN-γ responses were determined in an ELISPOT assay. (A) CD4 IL-4 production; (B) CD8 IFN-γ production; (C) CD8 IFN-γ production. The data represent the means of pooled cells from five mice per group (n = 5) from three separate experiments.
Protection against pulmonary infection with P. aeruginosa.
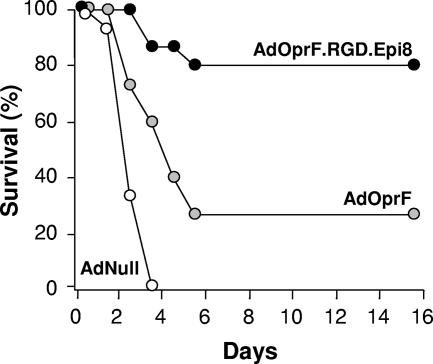
To evaluate the protective effect of immunization with AdOprF.RGD.Epi8 against pulmonary infection with P. aeruginosa, immunized mice were challenged with a lethal dose of agar-encapsulated PAO1 (106 CFU) 5 weeks after immunization. This model usually results in death in unimmunized mice 2 to 4 days after challenge and has been used to evaluate the protective effects of immunization (47, 55). All mice infected with AdNull died within the first 5 days after challenge (Fig. 5). In contrast, more mice immunized with AdOprF.RGD.Epi8 (80%) survived than those immunized with AdOprF (22%; P < 0.05). These data suggest that immunization with AdOprF.RGD.Epi8 induced enhanced protective immunity compared to immunization with the wild-type capsid AdOprF vector.
FIG. 5.
Ability of immunization with AdOprF.RGD.Epi8 to protect against pulmonary challenge with P. aeruginosa. C57BL/6 mice were immunized via the subcutaneous route with AdOprF.RGD.Epi8 or AdOprF at 1010 pu/mouse (n = 9 to 10 animals per group). Immunization with AdNull at equal doses or PBS served as negative controls. Five weeks after immunization, the mice were challenged with a lethal intratracheal dose of agar-encapsulated P. aeruginosa, and survival was monitored for 15 days.
Boosting of the AdOprF immune response by repeated administration.
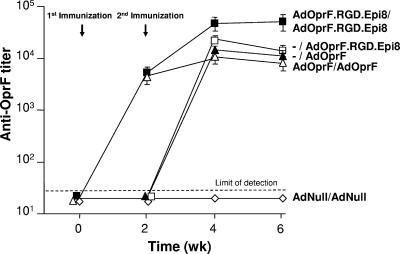
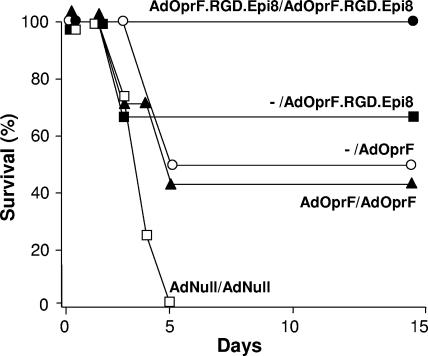
A second administration with AdOprF.RGD.Epi8 resulted in boosting of the anti-OprF humoral immune response. Mice immunized twice with AdOprF.RGD.Epi8 2 weeks apart had higher anti-Oprf IgG titers detectable at 2 weeks, and this titer reached an eightfold increase at 4 weeks after the second immunization compared to mice immunized once with AdOprF.RGD.Epi8 or AdOprF or twice with AdOprF (P < 0.05, all comparisons) (Fig. 6). No anti-OprF titers were detected in mice immunized twice with AdNull. Compared to the anti-Ad IgG titer after a single immunization (Fig. 3B), the anti-Ad IgG titer after the second immunization was increased in all groups (data not shown). To evaluate if repeat administration of AdOprF.RGD.Epi8 resulted in improved survival following pulmonary challenge with P. aeruginosa, the immunized mice were challenged 5 weeks after the second immunization with a lethal intratracheal dose of PAO1 (Fig. 7). Mice that had been immunized twice with the control AdNull vector died within the first 5 days. In contrast, mice that received AdOprF.RGD.Epi8 twice all survived the challenge, whereas mice that received AdOprF.RGD.Epi8 only once showed 67% survival (P < 0.05 compared to two immunizations with AdOprF.RGD.Epi8). Mice that had been immunized with AdOprF once or twice showed decreased survival compared to the AdOprF.RGD.Epi8 (single immunization, P < 0.05) or AdOprF.RGD.Epi8 (repeat immunization, P < 0.01) group. These data suggest that repeat administration of the capsid-modified AdOprF.RGD.Epi8 Ad vector results in boosting of the anti-OprF immune response and results in enhanced protective anti-P. aeruginosa immunity.
FIG. 6.
Anti-OprF humoral response after a single administration of AdOprF.RGD.Epi8 or AdOprF compared to boost administrations of the same vectors. C57BL/6 mice were immunized via the subcutaneous route with AdOprF.RGD.Epi8, AdOprF, AdNull (1010 pu/animal), or no vector, followed by a second immunization of the same vector at an equal dose 2 weeks later (or first immunization if no vector was given at week zero). Total IgG antibodies against OprF were assessed in an ELISA at 2, 4, and 6 weeks after the first immunization. Data are shown as means ± SEM from five mice per group. −, no vector given at the first immunization (vector administration at week 2 is labeled as the second immunization for these groups).
FIG. 7.
Comparison of abilities of boost immunizations with AdOprF.RGD.Epi8 or AdOprF to protect against a lethal pulmonary challenge with P. aeruginosa. C57BL/6 mice were immunized via the subcutaneous route with AdOprF.RGD.Epi8, AdOprF, AdNull (1010 pu/animal), or no vector, followed by a second immunization of the same vector at an equal dose 2 weeks later (n = 9 animals/group). Five weeks after immunization, the mice were challenged with a lethal intratracheal dose of agar-encapsulated P. aeruginosa, and survival was monitored for 15 days. −, no vector given at the first immunization.
DISCUSSION
The present study describes a novel Ad vaccine strategy using a dual capsid-modified Ad vector with an RGD peptide on the fiber to enhance OprF expression in dendritic cells and incorporation of an epitope of the transgene into the hexon to enable boosting of the antitransgene response with repeat administration. The dual capsid-modified AdOprF.RGD.Epi8 showed enhanced expression of the OprF transgene in dendritic cells in vitro compared to the wild-type capsid AdOprF, with both vectors expressing the same OprF transgene at equal levels in the integrin-negative cell line A549. A single immunization with AdOprF.RGD.Epi8 induced higher anti-OprF CD4-specific and CD8-specific IFN-γ responses and enhanced protection following pulmonary infection with P. aeruginosa compared to immunization with AdOprF, but in both vectors no difference was observed in the anti-OprF humoral and CD4-specific IL-4 responses following a single administration. Furthermore, repeat administration with AdOprF.RGD.Epi8 resulted in boosting of the anti-OprF humoral response and increased protection compared to repeat administration of AdOprF.
Adenovirus vaccines.
Ad vectors are suitable as platforms for vaccines due to their potential to induce robust antivector and antitransgene immunity in preclinical studies, and they are currently being evaluated in preclinical and clinical studies as vaccines against human immunodeficiency virus type 1 and other infectious organisms (1-4, 6, 8, 12, 18, 23, 33, 36, 39, 44, 45, 49, 53, 58). A potential limitation in the use of Ad vectors as vaccines, however, is the high prevalence of preexisting immunity to Ad in the human population (10, 11). Preexisting anti-Ad5 immunity has been shown to suppress the immunogenicity of Ad5 vaccines in both preclinical studies and clinical trials (8, 19, 28, 30). To overcome this problem, numerous Ad vector prime-protein boost regimens and combinations of plasmid DNA and Ad as immunogen have been assessed in a wide variety of animal models (5, 7, 27, 37, 42, 50, 56) and have entered phase I and II human testing (4, 8, 12). To further improve the potency or durability of the immune response to an antigen, several groups have developed novel Ad vaccine vectors with capsids derived from rare human or nonhuman Ad serotypes that evade anti-Ad5 immunity (35). All of these Ad capsid modification strategies, however, generate potent antivector immunity that diminishes the utility of vector readministration. Other strategies include heterologous Ad prime-boost regimens that include two different serotype Ad vectors to enhance antigen-specific responses, although the optimal combination of those vectors has yet to be defined (5, 27, 37, 50, 56).
Incorporation of RGD.
Although the exact mechanism of the induction of immune responses against foreign antigens expressed by Ad vectors is not fully understood, it likely involves infection of DC by the Ad facilitating antigen presentation and prolonging the immune response (24, 51, 54, 57). Ad vectors, however, do not productively infect DC well in vitro, probably due to the low numbers of the Ad receptor (CAR) on these cells (9, 51, 54). One strategy to enhance DC infection is to modify the Ad vector capsid proteins for targeting DC populations. One such capsid modification has been the addition of an integrin-binding motif RGD to the fiber knob (34, 52, 54). RGD-modified Ad vectors enhance infection of integrin-expressing cells such as DC and have been shown to enhance immune responses against the transgene in vivo in animals (34, 54). The AdOprF.RGD.Epi8 vector used in the present study led to enhanced expression of OprF in bone marrow-derived dendritic cells in vitro and in higher anti-OprF CD4 and CD8 responses following intramuscular immunization in vivo, suggesting that the addition of the RGD motif to the fiber enhanced antigen presentation of the OprF transgene and the Epi8 peptide. As the main focus of this study was to create an Ad vector with enhanced OprF expression in DC and anti-Epi8 immunity that enables repeat administration and boosting of the immune response, the individual contribution of the two capsid modifications was not individually studied, e.g., by creating an AdRGD.Epi8 vector as a additional control. The humoral anti-OprF or anti-Ad IgG titers were not significantly different following administration of AdOprF.RGD.Epi8 compared to the unmodified AdOprF vector, suggesting that the initial antitransgene and anticapsid humoral immune responses are not altered by the addition of RGD. This is consistent with previous observations using an RGD-modified vector expressing β-galactosidase, where no increase in the humoral anti-Ad antibody titers was observed (54). The present study demonstrates that RGD-modified Ad can be used to enhance cellular immunity against an infectious pathogen. Previous studies have demonstrated enhanced cellular immunity against model antigens (β-galactosidase and ovalbumin), resulting in enhanced antitumor immunity against tumors expressing that antigen on the cell surface (34, 54).
Incorporation of an epitope into the Ad capsid.
The incorporation of an epitope against the P. aeruginosa OprF protein into loop 1 of hypervariable region 5 of the Ad hexon protein has been shown to induce antiepitope humoral and cellular immunity to protect against infections with P. aeruginosa in a murine model (55). The advantage of incorporating an epitope into the Ad hexon is that this strategy enables repeat administration with the same vector to boost the antiepitope immunity (12, 16, 17). Another Ad capsid modification to circumvent preexisting anti-Ad immunity has been the use of hexon loops or fibers derived from different Ad serotypes (38, 43). Recently, a chimeric Ad vector with all extracellular loops of the hexon protein replaced with hexon loops of the rare human serotype 48 has been shown to be able to circumvent anti-Ad5 immunity. Incorporating the OprF epitope into the Ad hexon in addition to expression of the entire protein as transgene did not increase the anti-OprF humoral response. Consistent with this finding was the finding that the anti-OprF CD4 IL-4 response was not increased following immunization with AdOprF.RGD.Epi8 compared to AdOprF. However, the anti-OprF CD4 and CD8 IFN-γ responses were increased following administration of AdOprF.RGD.Epi8 compared to AdOprF. Whether this increased CD4 and CD8 IFN-γ response was due to the addition of RGD to enhance antigen presentation, the addition of Epi8 to the hexon, to both, or to other combinations cannot be stated so far. Most importantly, anti-OprF IgG titers and protective immunity as assessed by survival following challenge with P. aeruginosa were increased following repeat administration of AdOprF.RGD.Epi8 compared to single administration of AdOprF.RGD.Epi8 and AdOprF or repeat administration of AdOprF.
Taken together, addition of RGD to the Ad fiber to enhance transgene expression in antigen-presenting cells plus incorporation of an epitope of the antigen transgene into the Ad hexon in addition to expression of the antigen gene as a transgene creates a novel Ad vaccine vector that not only successfully induces robust anti-OprF humoral and cellular immunity but also allows repeat administration of the same vector to further boost the immune response, resulting in increased protection.
Acknowledgments
We thank N. Mohamed for help in preparing the manuscript.
These studies were supported, in part, by U01 AI069032-01, Will Rogers Memorial Fund, Los Angeles, CA, and R21 H77557.
Footnotes
Published ahead of print on 17 October 2007.
REFERENCES
- 1.Bangari, D. S., and S. K. Mittal. 2006. Development of nonhuman adenoviruses as vaccine vectors. Vaccine 24:849-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barouch, D. H. 2006. Rational design of gene-based vaccines. J. Pathol. 208:283-289. [DOI] [PubMed] [Google Scholar]
- 3.Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335-339. [DOI] [PubMed] [Google Scholar]
- 4.Barouch, D. H., and G. J. Nabel. 2005. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum. Gene Ther. 16:149-156. [DOI] [PubMed] [Google Scholar]
- 5.Barouch, D. H., M. G. Pau, J. H. Custers, W. Koudstaal, S. Kostense, M. J. Havenga, D. M. Truitt, S. M. Sumida, M. G. Kishko, J. C. Arthur, B. Korioth-Schmitz, M. H. Newberg, D. A. Gorgone, M. A. Lifton, D. L. Panicali, G. J. Nabel, N. L. Letvin, and J. Goudsmit. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 172:6290-6297. [DOI] [PubMed] [Google Scholar]
- 6.Boyer, J. L., G. Kobinger, J. M. Wilson, and R. G. Crystal. 2005. Adenovirus-based genetic vaccines for biodefense. Hum. Gene Ther. 16:157-168. [DOI] [PubMed] [Google Scholar]
- 7.Casimiro, D. R., A. Tang, L. Chen, T. M. Fu, R. K. Evans, M. E. Davies, D. C. Freed, W. Hurni, J. M. ste-Amezaga, L. Guan, R. Long, L. Huang, V. Harris, D. K. Nawrocki, H. Mach, R. D. Troutman, L. A. Isopi, K. K. Murthy, K. Rice, K. A. Wilson, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Vaccine-induced immunity in baboons by using DNA and replication-incompetent adenovirus type 5 vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:7663-7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catanzaro, A. T., R. A. Koup, M. Roederer, R. T. Bailer, M. E. Enama, Z. Moodie, L. Gu, J. E. Martin, L. Novik, B. K. Chakrabarti, B. T. Butman, J. G. Gall, C. R. King, C. A. Andrews, R. Sheets, P. L. Gomez, J. R. Mascola, G. J. Nabel, and B. S. Graham. 2006. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J. Infect. Dis. 194:1638-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng, C., J. G. Gall, W. P. Kong, R. L. Sheets, P. L. Gomez, C. R. King, and G. J. Nabel. 2007. Mechanism of Ad5 vaccine immunity and toxicity: fiber shaft targeting of dendritic cells. PLoS Pathog. 3:e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chirmule, N., K. Propert, S. Magosin, Y. Qian, R. Qian, and J. Wilson. 1999. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 6:1574-1583. [DOI] [PubMed] [Google Scholar]
- 11.D'Ambrosio, E., N. Del Grosso, A. Chicca, and M. Midulla. 1982. Neutralizing antibodies against 33 human adenoviruses in normal children in Rome. J. Hyg. 89:155-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duerr, A., J. N. Wasserheit, and L. Corey. 2006. HIV vaccines: new frontiers in vaccine development. Clin. Infect. Dis. 43:500-511. [DOI] [PubMed] [Google Scholar]
- 13.Fox, C. W., G. D. Campbell, Jr., W. M. Anderson, J. H. Zavecz, L. B. Gilleland, and H. E. Gilleland, Jr. 1994. Preservation of pulmonary function by an outer membrane protein F vaccine. A study in rats with chronic pulmonary infection caused by Pseudomonas aeruginosa. Chest 105:1545-1550. [DOI] [PubMed] [Google Scholar]
- 14.Garau, J., and L. Gomez. 2003. Pseudomonas aeruginosa pneumonia. Curr. Opin. Infect. Dis. 16:135-143. [DOI] [PubMed] [Google Scholar]
- 15.Gibson, R. L., J. L. Burns, and B. W. Ramsey. 2003. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 168:918-951. [DOI] [PubMed] [Google Scholar]
- 16.Gilleland, L. B., and H. E. Gilleland, Jr. 1995. Synthetic peptides representing two protective, linear B-cell epitopes of outer membrane protein F of Pseudomonas aeruginosa elicit whole-cell-reactive antibodies that are functionally pseudomonad specific. Infect. Immun. 63:2347-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gocke, K., U. Baumann, H. Hagemann, J. Gabelsberger, H. Hahn, J. Freihorst, and B. U. von Specht. 2003. Mucosal vaccination with a recombinant OprF-I vaccine of Pseudomonas aeruginosa in healthy volunteers: comparison of a systemic vs. a mucosal booster schedule. FEMS Immunol. Med. Microbiol. 37:167-171. [DOI] [PubMed] [Google Scholar]
- 18.Hackett, N. R., S. M. Kaminsky, D. Sondhi, and R. G. Crystal. 2000. Antivector and antitransgene host responses in gene therapy. Curr. Opin. Mol. Ther. 2:376-382. [PubMed] [Google Scholar]
- 19.Harvey, B. G., P. L. Leopold, N. R. Hackett, T. M. Grasso, P. M. Williams, A. L. Tucker, R. J. Kaner, B. Ferris, I. Gonda, T. D. Sweeney, R. Ramalingam, I. Kovesdi, S. Shak, and R. G. Crystal. 1999. Airway epithelial CFTR mRNA expression in cystic fibrosis patients after repetitive administration of a recombinant adenovirus. J. Clin. Investig. 104:1245-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedstrom, R. C., O. R. Pavlovskis, and D. R. Galloway. 1984. Antibody response of infected mice to outer membrane proteins of Pseudomonas aeruginosa. Infect. Immun. 43:49-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hersh, J., R. G. Crystal, and B. Bewig. 1995. Modulation of gene expression after replication-deficient, recombinant adenovirus-mediated gene transfer by the product of a second adenovirus vector. Gene Ther. 2:124-131. [PubMed] [Google Scholar]
- 22.Hughes, E. E., L. B. Gilleland, and H. E. Gilleland, Jr. 1992. Synthetic peptides representing epitopes of outer membrane protein F of Pseudomonas aeruginosa that elicit antibodies reactive with whole cells of heterologous immunotype strains of P. aeruginosa. Infect. Immun. 60:3497-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jooss, K., and N. Chirmule. 2003. Immunity to adenovirus and adeno-associated viral vectors: implications for gene therapy. Gene Ther. 10:955-963. [DOI] [PubMed] [Google Scholar]
- 24.Jooss, K., Y. Yang, K. J. Fisher, and J. M. Wilson. 1998. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J. Virol. 72:4212-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larbig, M., E. Mansouri, J. Freihorst, B. Tummler, G. Kohler, H. Domdey, B. Knapp, K. D. Hungerer, E. Hundt, J. Gabelsberger, and B. U. von Specht. 2001. Safety and immunogenicity of an intranasal Pseudomonas aeruginosa hybrid outer membrane protein F-I vaccine in human volunteers. Vaccine 19:2291-2297. [DOI] [PubMed] [Google Scholar]
- 26.Lee, N., B. Ahn, S. B. Jung, Y. G. Kim, H. Kim, and W. J. Park. 2000. Conformation-dependent antibody response to Pseudomonas aeruginosa outer membrane proteins induced by immunization in humans. FEMS Immunol. Med. Microbiol. 27:79-85. [DOI] [PubMed] [Google Scholar]
- 27.Lemckert, A. A., S. M. Sumida, L. Holterman, R. Vogels, D. M. Truitt, D. M. Lynch, A. Nanda, B. A. Ewald, D. A. Gorgone, M. A. Lifton, J. Goudsmit, M. J. Havenga, and D. H. Barouch. 2005. Immunogenicity of heterologous prime-boost regimens involving recombinant adenovirus serotype 11 (Ad11) and Ad35 vaccine vectors in the presence of anti-ad5 immunity. J. Virol. 79:9694-9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mack, C. A., W. R. Song, H. Carpenter, T. J. Wickham, I. Kovesdi, B. G. Harvey, C. J. Magovern, O. W. Isom, T. Rosengart, E. Falck-Pedersen, N. R. Hackett, R. G. Crystal, and A. Mastrangeli. 1997. Circumvention of anti-adenovirus neutralizing immunity by administration of an adenoviral vector of an alternate serotype. Hum. Gene Ther. 8:99-109. [DOI] [PubMed] [Google Scholar]
- 29.Mansouri, E., S. Blome-Eberwein, J. Gabelsberger, G. Germann, and B. U. von Specht. 2003. Clinical study to assess the immunogenicity and safety of a recombinant Pseudomonas aeruginosa OprF-OprI vaccine in burn patients. FEMS Immunol. Med. Microbiol. 37:161-166. [DOI] [PubMed] [Google Scholar]
- 30.Mastrangeli, A., B. G. Harvey, J. Yao, G. Wolff, I. Kovesdi, R. G. Crystal, and E. Falck-Pedersen. 1996. “Sero-switch” adenovirus-mediated in vivo gene transfer: circumvention of anti-adenovirus humoral immune defenses against repeat adenovirus vector administration by changing the adenovirus serotype. Hum. Gene Ther. 7:79-87. [DOI] [PubMed] [Google Scholar]
- 31.Mutharia, L. M., and R. E. Hancock. 1983. Surface localization of Pseudomonas aeruginosa outer membrane porin protein F by using monoclonal antibodies. Infect. Immun. 42:1027-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mutharia, L. M., T. I. Nicas, and R. E. Hancock. 1982. Outer membrane proteins of Pseudomonas aeruginosa serotype strains. J Infect. Dis. 146:770-779. [DOI] [PubMed] [Google Scholar]
- 33.Nabel, G. J. 2001. Challenges and opportunities for development of an AIDS vaccine. Nature 410:1002-1007. [DOI] [PubMed] [Google Scholar]
- 34.Okada, N., T. Saito, Y. Masunaga, Y. Tsukada, S. Nakagawa, H. Mizuguchi, K. Mori, Y. Okada, T. Fujita, T. Hayakawa, T. Mayumi, and A. Yamamoto. 2001. Efficient antigen gene transduction using Arg-Gly-Asp fiber-mutant adenovirus vectors can potentiate antitumor vaccine efficacy and maturation of murine dendritic cells. Cancer Res. 61:7913-7919. [PubMed] [Google Scholar]
- 35.Ophorst, O. J., K. Radosevic, M. J. Havenga, M. G. Pau, L. Holterman, B. Berkhout, J. Goudsmit, and M. Tsuji. 2006. Immunogenicity and protection of a recombinant human adenovirus serotype 35-based malaria vaccine against Plasmodium yoelii in mice. Infect. Immun. 74:313-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Randrianarison-Jewtoukoff, V., and M. Perricaudet. 1995. Recombinant adenoviruses as vaccines. Biologicals 23:145-157. [DOI] [PubMed] [Google Scholar]
- 37.Reyes-Sandoval, A., J. C. Fitzgerald, R. Grant, S. Roy, Z. Q. Xiang, Y. Li, G. P. Gao, J. M. Wilson, and H. C. Ertl. 2004. Human immunodeficiency virus type 1-specific immune responses in primates upon sequential immunization with adenoviral vaccine carriers of human and simian serotypes. J. Virol. 78:7392-7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts, D. M., A. Nanda, M. J. Havenga, P. Abbink, D. M. Lynch, B. A. Ewald, J. Liu, A. R. Thorner, P. E. Swanson, D. A. Gorgone, M. A. Lifton, A. A. Lemckert, L. Holterman, B. Chen, A. Dilraj, A. Carville, K. G. Mansfield, J. Goudsmit, and D. H. Barouch. 2006. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 441:239-243. [DOI] [PubMed] [Google Scholar]
- 39.Rodrigues, E. G., F. Zavala, R. S. Nussenzweig, J. M. Wilson, and M. Tsuji. 1998. Efficient induction of protective anti-malaria immunity by recombinant adenovirus. Vaccine 16:1812-1817. [DOI] [PubMed] [Google Scholar]
- 40.Rosenfeld, M. A., W. Siegfried, K. Yoshimura, K. Yoneyama, M. Fukayama, L. E. Stier, P. K. Paakko, P. Gilardi, L. D. Stratford-Perricaudet, M. Perricaudet, S. Jallat, A. Pavirani, J.-P. Lecocq, and R. G. Crystal. 1991. Adenovirus-mediated transfer of a recombinant α1-antitrypsin gene to the lung epithelium in vivo. Science 252:431-434. [DOI] [PubMed] [Google Scholar]
- 41.Rosenfeld, M. A., K. Yoshimura, B. C. Trapnell, K. Yoneyama, E. R. Rosenthal, W. Dalemans, M. Fukayama, J. Bargon, L. E. Stier, L. Stratford-Perricaudet, M. Perricaudet, W. B. Guggino, A. Pavirani, J.-P. Lecocq, and R. G. Crystal. 1992. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell 68:143-155. [DOI] [PubMed] [Google Scholar]
- 42.Santra, S., M. S. Seaman, L. Xu, D. H. Barouch, C. I. Lord, M. A. Lifton, D. A. Gorgone, K. R. Beaudry, K. Svehla, B. Welcher, B. K. Chakrabarti, Y. Huang, Z. Y. Yang, J. R. Mascola, G. J. Nabel, and N. L. Letvin. 2005. Replication-defective adenovirus serotype 5 vectors elicit durable cellular and humoral immune responses in nonhuman primates. J. Virol. 79:6516-6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schoggins, J. W., J. G. Gall, and E. Falck-Pedersen. 2003. Subgroup B and F fiber chimeras eliminate normal adenovirus type 5 vector transduction in vitro and in vivo. J. Virol. 77:1039-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiver, J. W., and E. A. Emini. 2004. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu. Rev. Med. 55:355-372. [DOI] [PubMed] [Google Scholar]
- 45.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 46.Song, W., H. L. Kong, H. Carpenter, H. Torii, R. Granstein, S. Rafii, M. A. Moore, and R. G. Crystal. 1997. Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. J. Exp. Med. 186:1247-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevenson, M. M., T. K. Kondratieva, A. S. Apt, M. F. Tam, and E. Skamene. 1995. In vitro and in vivo T cell responses in mice during bronchopulmonary infection with mucoid Pseudomonas aeruginosa. Clin. Exp. Immunol. 99:98-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 49.Sullivan, N. J., A. Sanchez, P. E. Rollin, Z. Y. Yang, and G. J. Nabel. 2000. Development of a preventive vaccine for Ebola virus infection in primates. Nature 408:605-609. [DOI] [PubMed] [Google Scholar]
- 50.Thorner, A. R., A. A. Lemckert, J. Goudsmit, D. M. Lynch, B. A. Ewald, M. Denholtz, M. J. Havenga, and D. H. Barouch. 2006. Immunogenicity of heterologous recombinant adenovirus prime-boost vaccine regimens is enhanced by circumventing vector cross-reactivity. J. Virol. 80:12009-12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tillman, B. W., T. D. de Gruijl, S. A. Luykx-de Bakker, R. J. Scheper, H. M. Pinedo, T. J. Curiel, W. R. Gerritsen, and D. T. Curiel. 1999. Maturation of dendritic cells accompanies high-efficiency gene transfer by a CD40-targeted adenoviral vector. J. Immunol. 162:6378-6383. [PubMed] [Google Scholar]
- 52.Wickham, T. J., E. Tzeng, L. L. Shears, P. W. Roelvink, Y. Li, G. M. Lee, D. E. Brough, A. Lizonova, and I. Kovesdi. 1997. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J. Virol. 71:8221-8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson, J. M. 1996. Adenoviruses as gene-delivery vehicles. N. Engl. J. Med. 334:1185-1187. [DOI] [PubMed] [Google Scholar]
- 54.Worgall, S., A. Busch, M. Rivara, D. Bonnyay, P. L. Leopold, R. Merritt, N. R. Hackett, P. W. Rovelink, J. T. Bruder, T. J. Wickham, I. Kovesdi, and R. G. Crystal. 2004. Modification to the capsid of the adenovirus vector that enhances dendritic cell infection and transgene-specific cellular immune responses. J. Virol. 78:2572-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Worgall, S., A. Krause, M. Rivara, K. K. Hee, E. V. Vintayen, N. R. Hackett, P. W. Roelvink, J. T. Bruder, T. J. Wickham, I. Kovesdi, and R. G. Crystal. 2005. Protection against P. aeruginosa with an adenovirus vector containing an OprF epitope in the capsid. J. Clin. Investig. 115:1281-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xin, K. Q., N. Jounai, K. Someya, K. Honma, H. Mizuguchi, S. Naganawa, K. Kitamura, T. Hayakawa, S. Saha, F. Takeshita, K. Okuda, M. Honda, D. M. Klinman, and K. Okuda. 2005. Prime-boost vaccination with plasmid DNA and a chimeric adenovirus type 5 vector with type 35 fiber induces protective immunity against HIV. Gene Ther. 12:1769-1777. [DOI] [PubMed] [Google Scholar]
- 57.Zhang, Y., N. Chirmule, G. P. Gao, R. Qian, M. Croyle, B. Joshi, J. Tazelaar, and J. M. Wilson. 2001. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol. Ther. 3:697-707. [DOI] [PubMed] [Google Scholar]
- 58.Zhi, Y., J. Figueredo, G. P. Kobinger, H. Hagan, R. Calcedo, J. R. Miller, G. Gao, and J. M. Wilson. 2006. Efficacy of severe acute respiratory syndrome vaccine based on a nonhuman primate adenovirus in the presence of immunity against human adenovirus. Hum. Gene Ther. 17:500-506. [DOI] [PubMed] [Google Scholar]