Abstract
Transmissible spongiform encephalopathies (TSE) arise as a consequence of infection of the central nervous system by prions and are incurable. To date, most antiprion compounds identified by in vitro screening failed to exhibit therapeutic activity in animals, thus calling for new assays that could more accurately predict their in vivo potency. Primary nerve cell cultures are routinely used to assess neurotoxicity of chemical compounds. Here, we report that prion strains from different species can propagate in primary neuronal cultures derived from transgenic mouse lines overexpressing ovine, murine, hamster, or human prion protein. Using this newly developed cell system, the activity of three generic compounds known to cure prion-infected cell lines was evaluated. We show that the antiprion activity observed in neuronal cultures is species or strain dependent and recapitulates to some extent the activity reported in vivo in rodent models. Therefore, infected primary neuronal cultures may be a relevant system in which to investigate the efficacy and mode of action of antiprion drugs, including toward human transmissible spongiform encephalopathy agents.
Transmissible spongiform encephalopathies (TSE), which include Creutzfeldt-Jakob disease (CJD) in humans, bovine spongiform encephalopathy in cattle, and scrapie in sheep, are fatal, incurable neurodegenerative disorders caused by prions, a class of unconventional agents that target predominantly the central nervous system (14, 39). The only known, specific molecular marker of prion diseases is the abnormal prion protein (PrPSc), a misfolded form of the cellular PrP (PrPC). Transmissibility is believed to stem from the ability of the prion isoform to promote the conformational transition from PrPC to PrPSc (39, 40). Biologically distinct prion strains can propagate in the same host (for a review, see reference 9), presumably through the perpetuation of different, specific PrPSc conformers (47).
The search for drugs able to impede infection or prion-induced neuropathology currently relies on various experimental models, including an acellular PrP transconformation assay (27, 46), yeast prion systems (2), PrPSc accumulation in chronically infected mammalian cell lines (28), and assay in TSE animal models (for a review, see reference 51). Among many compounds selected for their ability to prevent PrPSc accumulation in cultured cells, only some of the most potent inhibitors significantly delay disease onset in prion-infected rodents. A few of them showed a therapeutic activity sensu stricto, and none was effective in clinically affected human patients (56). The reasons for these discrepancies remain unclear but probably include pharmacokinetic limitations (reviewed in reference 51). However, compounds known to cross the blood-brain barrier such as quinacrine and chlorpromazine proved to be ineffective in vivo (4, 5, 21). It is conceivable that biological differences between the available permissive cell lines and postmitotic neurons, the primary target of prions, may account for the disparity between in vitro and in vivo results. In addition, there is evidence to suggest that drug efficacy may depend upon the infecting prion strain (18, 28). Thus, there is a need for in vitro screening systems able to replicate different strains in a congruent cellular context and to predict more accurately the in vivo potency of antiprion drugs.
Dissociated primary neurons can be explanted from various brain regions from a wide range of organisms, thus allowing the growth of highly differentiated neuronal subtypes. These systems have several advantages for in vitro studies. They make an individual living cell with a phenotype very close to the in vivo one accessible for local application of pharmacological compounds or neurotropic infectious agents and allow morphological studies of, for example, neuronal connectivity and viability. As such, primary neuronal cultures are valuable tools routinely used for neurotrophic and antiapoptotic drug evaluation in neurodegenerative as well as infectious diseases (15, 35, 52, 57).
The propagation of sheep prions in primary nerve cell cultures derived from transgenic mice overexpressing ovine PrP has been recently reported (16). We show here that it is feasible to propagate rodent and human prions in cultures derived from transgenic mouse lines expressing the cognate PrPC. Using this cell system, we assayed the antiprion activity of three generic compounds that are known to cure prion-infected cell lines and for which efficacy has been evaluated in vivo (see Discussion). In primary cell culture, clear differences in the efficacy of these compounds were observed depending on the prion strain and/or species combination.
MATERIALS AND METHODS
Mouse lines.
Primary neuronal cultures were established from the following transgenic mouse lines: PrP0/0 (PrP knockout mice) (Zurich I) (10), tga20 (mouse Prnpa allele [20]), tg7 (hamster PrP [40]), tg338 (ovine PrP, V136 R154 Q171 allele [31]), tg650 (human PrP, M129 allele [unpublished data]). The tg7 line used in this study was kindly provided by CSL-Behring (Marburg); it originates from the S. Prusiner laboratory, where subsequent to publication (40) the line has been bred onto a PrP0/0 background. All mouse lines are homozygous for the transgene array and have been established on the same Zurich I PrP0/0 background. All experiments were performed according to national guidelines.
Primary cell cultures.
Primary cultures of cerebellar granule neurons (CGN) were established as previously described (16). Briefly, CGN cells were extracted from 6- or 7-day-old mice by enzymatic and mechanical dissociation. They were plated at a density of ∼2,000 cells/mm2 in 12- or 48-well plastic culture plates coated with 10 μg/ml poly-d-lysine and cultivated in Dulbecco's modified Eagle's medium-glutamax I high glucose (Gibco) containing 10% fetal calf serum (BioWhittaker), 20 mM KCl, penicillin and streptomycin (Gibco), and completed with N2 and B27 supplement (Gibco). The medium was complemented weekly with 1 mg/ml glucose and 10 μM concentrations of the antimitotics uridine and fluorodeoxiuridin (Sigma) to control astrocyte proliferation.
Prion infection.
Primary neuronal cultures were exposed to prion as previously described (16). Briefly, brains of terminally ill prion-infected mice were homogenized and adjusted to 20% (wt/vol) with 5% (wt/vol) glucose and stored at −80°C until use. Brain homogenates were then sonicated and added at final concentrations between 0.002% and 0.1% to primary cultures 2 or 3 days after plating (unless stated otherwise) and left for the whole experiment without washes. In one series of experiments performed with human CJD agent (see Results), PrPSc was purified from brain homogenate by sodium phosphotungstic acid precipitation (55), further diluted in phosphate-buffered saline as necessary, and applied to cultures similarly to homogenate. The final concentration of PrPSc was calculated as an equivalent of the initial homogenate. Prion infectious sources consisted of sheep scrapie strain 127S (54); the mouse strains 139A, 22L, ME7 (originating from the R. Carp Laboratory, Staten Island, NY) and Fukuoka-1 (originating from the S. Katamine Laboratory, Nagasaki, Japan); the hamster strains Sc237 (subclone of 263K strain, [24]) and 139H (26) (provided by R. Carp); and human type 1 CJD (WHO reference sporadic CJD brain sample NHBY0/0001; National Institute Biological Standards and Control, Potters Bar, United Kingdom). All strains were propagated in transgenic mice expressing PrPC of the corresponding species.
PrPSc detection.
The accumulation of proteinase K (PK)-resistant PrP (PrPres) was assessed by immunoblotting in cells lysed at various times postexposure as described previously (16). In short, lysates were clarified with a rapid centrifugation of 2,400 × g for 1 min. Then 50 μg of cell lysate proteins (measured by the bicinchoninic protein assay; Pierce) was treated with PK (7.5 μg/mg of protein; Euromedex) for 30 min at 37°C, and digestion was stopped by addition of 1 mM Pefabloc. Proteins were methanol precipitated for 1 h at −20°C and then centrifuged for 30 min at 16,000 × g. Pellets were resuspended in sample buffer, denatured, and loaded on 12% acrylamide precast gels (Invitrogen). Proteins were then electrotransferred on nitrocellulose membranes, and PrP was detected by incubation with biotinylated monoclonal anti-PrP antibodies ICSM18 (0.2 μg/ml) or Sha31 (0.1 μg/ml), followed with horseradish peroxidase-conjugated streptavidin (0.8 μg/ml; Pierce). Immunoreactivity was visualized by enhanced chemiluminescence (ECL kit; Pierce). Densitometric image analysis was performed by using Scion Image software.
Drug treatment.
The following drugs were used at the indicated final concentrations: chlorpromazine (1 and 5 μM), Congo red (1.5 and 7 μM), and MS-8209 (3.5 and 7.5 μM). Stock solutions were aliquoted and stored at −20°C until use. Unless stated otherwise, the drug or vehicle (0.1% dimethyl sulfoxide) was first added to the cultures at 3 days after prion exposure and then twice a week. Two or three independent experiments were performed for each treatment, as specified in the Results section. Each experiment was performed using duplicate or triplicate cultures.
RESULTS
Primary neuronal cultures transgenic for PrP allow the propagation of prions from various species.
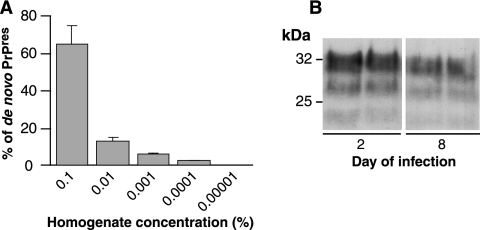
As reported in our previous study (16), CGN primary cultures derived from transgenic mice expressing ovine PrPC (CGNOv) and exposed to infectious inoculum at 2 to 3 days after plating are able to replicate the sheep scrapie agent. In such cultures, infection is effective at an inoculum dilution up to 0.0001% of brain homogenate, corresponding to a multiplicity of infection of ∼1 infectious dose/500 cells (Fig. 1A). The permissiveness to infection of CGNOv cultures exposed at 8 days postplating, i.e., after phenotypical and functional differentiation of the neurons (38), was also examined. Such cultures were composed of 85% MAP2- and β3 tubulin-positive cells and less than 1% nestin-positive cells (data not shown). As shown in Fig. 1B, PrPres accumulated at levels approaching those in cultures exposed at 2 days postplating (despite a 6-day-shorter incubation). This observation is of interest as it suggests that prion replication may initiate and take place in postmitotic, differentiated neurons.
FIG. 1.
High sensitivity of postmitotic neurons to prion infection. (A) Accumulation of PrPres in CGNOv cultures following infection with serial dilutions of 127S brain homogenate (final concentrations as indicated). De novo PrPres was quantified at 28 days postexposure by densitometric image analysis of immunoblots in two independent experiments (mean ± standard deviation) and is expressed as a percentage of total PrPres detected by immunoblotting of 1 mg of mouse brain from terminally ill 127S-infected tg338 mice. (B) CGNOv cultures were exposed to infectious prions either 2 or 8 days after plating, i.e., before or after their full differentiation. PrPres accumulation was assessed at 30 days postplating by immunoblotting of PK-treated lysate using biotinylated monoclonal antibody ICSM18 (see methods).
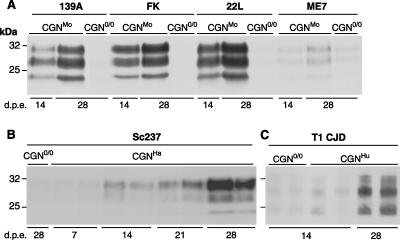
Deriving primary cell cultures from transgenic mice provided access to various PrP genotypes and allowed us to assess whether prions from other species could be propagated in CGN cells expressing the cognate PrPC. Figure 2 shows the results obtained with well-characterized strains of rodent-adapted prions. Cultures of CGN cells expressing mouse and hamster PrP (CGNMo and CGNHa, respectively) were established from the mouse lines tga20 and tg7 overexpressing mouse and Syrian hamster PrP, respectively (see Materials and Methods). Neuronal differentiation and survival as well as PrPC expression levels were found to be similar to levels in CGNOv cultures (data not shown). To infect the CGNMo cells, we used four mouse scrapie strains, 139A, 22L, ME7, and Fukuoka-1, which kill tga20 mice within 60 to 80 days postinfection (20; also our own data). For CGNHa cells, we used the hamster strain Sc237, a subclone of the 263K strain that kills tg7 mice within 50 days (40). Following exposure to diluted brain homogenate, a steady increase in PrPres was consistently observed in PrP-expressing CGN cultures but not in CGN cultures established from nonpermissive PrP0/0 mice (CGN0/0) (Fig. 2A and B) (n = 3 independent experiments). PrPres accumulation was detected at 2 weeks postexposure except with the ME7 strain, which multiplied less efficiently than the other mouse strains, as previously observed in different neuronal cell lines (3, 8, 44).
FIG. 2.
Accumulation of PrPres in CGN cultures upon exposure to rodent and human prions. CGN cultures were established from transgenic mice expressing mouse (A), hamster (B) or human (C) PrP and were exposed to brain homogenates (A and B) or purified PrPSc (C) from terminally ill mice infected with prions. CGN cultures established from PrP0/0 transgenic mice (CGN0/0) were also exposed to infectious prions in parallel. (A) CGNMo cultures exposed to mouse strain 139A, Fukuoka-1 (FK), 22L, or ME7 at a final concentration of 0.1% (wt/vol). (B) CGNHa cultures exposed to hamster strain Sc237 at a final concentration of 0.002%. (C) CGNHu cultures exposed to type 1 CJD (T1 CJD) at a final concentration equivalent to 0.1% of brain homogenate. The data shown in panels B and C correspond to duplicate culture wells within a representative experiment. In all PrP-expressing cultures, PrPres accumulation increased from 14 to 28 days postexposure (d.p.e.) and was weak or absent in nonpermissive CGN0/0 cultures. Cell lysates were PK treated, and PrPres was detected by immunoblotting using biotinylated monoclonal antibody ICSM18 (A and C) or Sha31 (B).
There is currently no available cellular model in which prions affecting humans can be propagated. To examine whether CGN cells would give access to a system permissive to a human agent, cultures derived from tg650 transgenic mice expressing human PrP Met129 were exposed to human CJD agent type 1. tg650 mice develop a TSE disease within ∼150 days when inoculated with this TSE agent (unpublished data). As a result, a specific and reproducible (n = 4) accumulation of PrPres was detected in CGN cells expressing human PrP (CGNHu) at 28 days postexposure (Fig. 2C) and as early as 14 days postexposure in half of the experiments (see also Fig. 4C). Collectively, these data demonstrate that primary cultured, postmitotic neurons are permissive to infection by prions from various species.
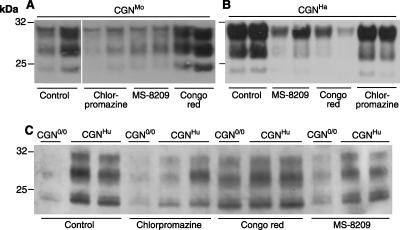
FIG. 4.
Inhibition of PrPres accumulation by chlorpromazine, Congo red, and MS-8209 in CGN cultures infected by prions from different species. CGN cultures expressing mouse (A), hamster (B), or human (C) PrPC were infected by brain homogenates on day 2 with prions from the corresponding species: 139A (0.01% final concentration), Sc237 (0.01%), and type 1 CJD (T1 CJD; 0.05%). (C) Nonpermissive CGN0/0 cultures were also exposed to T1 CJD prions. Three treatments were performed on days 5, 8, and 11 with chlorpromazine at a concentration of 1 μM (B) or 5 μM (A and C), Congo red (7 μM), or MS-8209 at a concentration of 3.5 μM (B) or 7.5 μM (A and C), and cells were lysed on day 15. PrPres was revealed by immunoblotting of PK-treated lysates using biotinylated monoclonal antibody Sha31 (A and B) or ICSM18 (C).
Congo red and MS-8209 efficiently inhibit PrPSc formation in sheep scrapie-infected neuronal cultures.
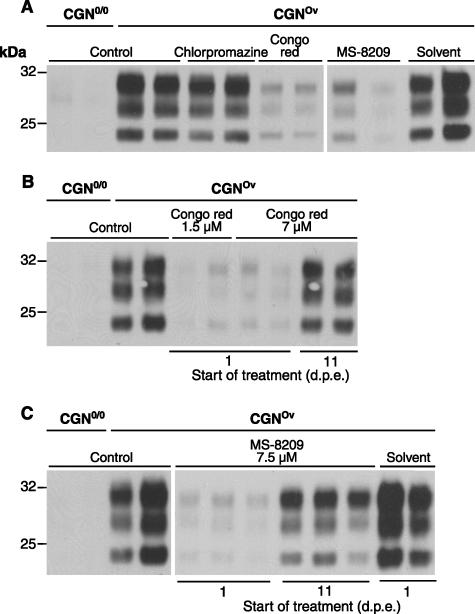
Next, we asked whether this novel TSE cell model would be suitable to assess the effect of compounds with antiprion activities. To this end, we selected three molecules known to be effective in prion-infected cell lines: chlorpromazine, Congo red, and MS-8209, a less-toxic derivative of amphotericin B. After their neurotoxicity levels in noninfected CGNOv cultures were estimated through a cell survival assay (data not shown), the following concentrations were retained for subsequent experiments: 5 μM chlorpromazine (subtoxic concentration), 7 μM Congo red, and 7.5 μM MS-8209; these concentrations are close to the effective concentrations for 50% inhibition of PrPres formation in chronically infected cell lines (4, 7, 29, 45). In a first series of experiments, drug treatments were started 3 days after exposure of CGNOv cultures to infectious brain homogenate (Fig. 3A). Both Congo red and MS-8209 reproducibly impaired prion propagation, based on the markedly lowered PrPres accumulation observed in treated versus untreated cultures (inhibition ≥80% as quantified by densitometric image analysis; n = 3). In contrast, chlorpromazine treatment had no effect on PrPres accumulation in infected CGNOv cells.
FIG. 3.
Effect of chlorpromazine, Congo red, and MS-8209 on sheep prion propagation in CGNOv cultures at an early or advanced stage of infection. (A) CGNOv cultures were infected on day 2, two treatments (chlorpromazine, 5 μM; Congo red, 7 μM; MS-8209, 7.5 μM; or solvent, 0.1%) were performed on days 5 and 8, and cells were lysed on day 12. Control wells of CGNOv and nonpermissive CGN0/0 cultures were infected similarly and left untreated (Control). (B and C) CGNOv cultures were infected on day 3, drugs (B, Congo red at 1.5 μM and 7 μM; C, 7.5 μM MS-8209 or 0.1% solvent) were added 1 day or 11 days after prion exposure and then twice a week; cells were lysed on day 21 (respectively, after 5 or 2 treatments). PrPres was revealed by immunoblotting of PK-treated lysates using biotinylated monoclonal antibody ICSM18. d.p.e., days postexposure.
In the brain of prion-infected individuals, propagation of the infectious agent is likely to induce infection of new neurons while PrPSc accumulation continues in already infected neurons. Therefore, we questioned whether Congo red or MS-8209 would show a curative activity in infected CGNOv cultures once PrPres had accumulated in substantial amounts. CGNOv cultures were treated 11 days after exposure to infectious inoculum (Fig. 3B and C). Contrary to early postexposure treatment, late treatment with Congo red was inefficient. However, a moderate inhibitory effect could be observed with MS-8209 (approximately 40% PrPres decrease in comparison to untreated, infected cells).
Drug efficiency in primary neuronal cultures can vary according to the infecting prion.
The cell lines currently used for antiprion drug screening propagate only mouse-adapted strains or sheep scrapie agent (28), and the data thus generated might not be fully transposable to prions infecting other species. Since primary cultured neurons are able to propagate prions from different species within a comparable environment, we sought to investigate whether any species-related effect of antiprion drugs would be observed. CGNMo, CGNHa, and CGNHu cultures were exposed to diluted homogenates from brains infected with one of the above-mentioned prion strains and then submitted to early postexposure treatment with one each of the three antiprion compounds (Fig. 4). PrPres accumulation levels reproducibly showed clear disparities of drug efficacy according to the host PrP species (n = 2). Congo red markedly inhibited PrPres accumulation in CGNHa cultures (Fig. 4B), as in CGNOv cultures (Fig. 3A), but had little or no effect in CGNMo and CGNHu cultures compared to untreated, control cultures (Fig. 4A and C). Notably, the PrPres levels in CJD-exposed CGNHu and nonpermissive CGN0/0 cultures were similar following Congo red treatment (Fig. 4C), indicating that input PrPSc present in the inoculum was actually stabilized by the drug. Chlorpromazine presented a relatively modest antiprion efficacy overall, except in CGNMo cultures. MS-8209 proved to be the only drug to be efficient across the range of prion species in these experiments, although with variable efficacy.
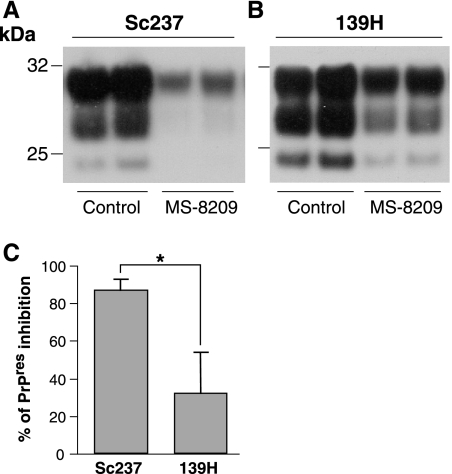
Earlier studies mainly in hamster TSE models have revealed a variable effect of amphotericin B and its analogue MS-8209 on the survival of the infected host according to the strain of prion (18, 33, 58). To see whether such a differential effect could also be visualized in cell culture, we compared the effects of MS-8209 in CGNHa cultures infected in parallel with either Sc237 or 139H, another well-characterized Syrian hamster strain. Upon early postexposure treatment of these cultures, a weaker inhibitory effect on PrPres accumulation was consistently found with 139H than with Sc237 prion (Fig. 5) (n = 3), in accordance with in vivo observations (see Discussion).
FIG. 5.
Comparison of antiprion activity of polyene antibiotic MS-8209 according to hamster prion strains. CGN cultures expressing hamster PrP were infected with hamster prion strains Sc237 (A; 0.01% final concentration) or 139H (B; 0.002%) on day 2. Three treatments with MS-8209 (3.5 μM) were performed, starting at the early stage of infection, and cells were lysed shortly after the last treatment. PrPres was revealed by immunoblotting of PK-treated lysates using biotinylated monoclonal antibody Sha31. (C) PrPres inhibition was quantified by densitometric image analysis of immunoblots (n = 3 independent experiments) and is expressed as a percentage of controls (mean ± standard error of the mean). MS-8209 treatment was significantly more effective toward Sc237 hamster prions than the 139H strain. *, P< 0.05 (Student's t test).
DISCUSSION
Despite the efforts of many research groups, only a few cell systems susceptible to prion infection have been developed, offering limited genetic diversity and susceptibility to a small number of strains. While several cell lines currently provide robust systems in which a few mouse-adapted strains (3, 48) or sheep scrapie (1, 54) and, recently, a deer TSE agent (42) can be cultivated, no cell system enabling routine propagation of prions affecting other species is available. Although a hamster TSE-infected hamster cell line and a CJD-infected human cell line were reported in earlier publications (30, 50), they have not been mentioned for more than a decade. More recently, there have been descriptions of alternative approaches utilizing mouse brain fetal stem cells or neurospheres that enabled the propagation of mouse-adapted prions (22, 34).
In this study, we report that primary cultured, differentiated neurons are susceptible to infection by a range of prion strains from four different species: sheep, mouse, hamster, and human. Modeling our previously described approach (16), we employed CGN cultures established from transgenic mice expressing PrP proteins of different species. Upon exposure, these cells were found to be susceptible to infection by prions propagated in the corresponding species, based on the accumulation of abnormal PrP. This newly introduced cell system thus offers a common, biologically relevant cellular environment in which prion agents from different species can be studied comparatively. While CGN cells may not provide a universal, susceptible system, one can expect that extending this approach to other neuron populations—as done successfully with cortical cells expressing ovine PrP (11)—might enlarge the spectrum of permissiveness. This would also pave the way for future studies of the determinism of the apparent, strain-dependent neuronal selectivity exhibited by these agents (17).
Primary nerve cell cultures have proved their usefulness for the evaluation of therapeutic compounds in neurodegenerative or infectious diseases (15, 52, 57). Here, we addressed the relevance of scrapie-infected primary nerve cell cultures as a potential model for the evaluation of antiprion molecules by using three different compounds that were previously described to clear PrPres in chronically infected cell lines but presented inconsistent prophylactic and therapeutic activities in vivo (51). As a main finding, these compounds markedly differed in their antiprion efficacy according to the species and/or prion strain, based on their effect on the accumulation of PrPres. In several instances, the differential effects observed in CGN cultures paralleled those documented in vivo for the same compound.
As a striking example, Congo red was shown here to exhibit a marked inhibitory activity toward ovine and hamster prions but not human and mouse prions. Such a result was not particularly expected in the case of the mouse prion, since this compound manifested clear antiprion activity when tested in the mouse cell lines ScN2a (12, 13) and ScSMB (45) infected by the Chandler strain, which is closely related to the 139A strain used in this study. The reason for these apparent discrepancies is uncertain but might reflect the limited effect of Congo red on preexisting PrPres, since several subpassages under treatment (12, 13) or high doses of the drugs (100 μM) (45) were required to inhibit PrPres accumulation in these cell lines. Of interest, the results obtained in CGN cultures infected with either mouse or hamster prions were in line with previous observations in both cell-free and animal models, where the effects of Congo red appear to differ markedly between these two rodents. In hamster, it was reported to disrupt inoculum-associated PrPres and to delay disease onset in scrapie-infected animals (6, 25), whereas it overstabilized murine PrPres and did not prevent PrPres accumulation in the spleen (6) or increase mouse survival time in vivo (6; also R. Race, unpublished data). Our experiments revealed a persistence of the inoculum-derived PrPres signal in both permissive and nonpermissive CJD-exposed cultures following Congo red treatment, thus suggesting similar interactions with the drug of the human and mouse prions.
Chlorpromazine was identified as an inhibitor of PrPres accumulation after screening on chronically infected ScN2a cells (29); it also proved active in prion clearance in mouse ScGT1 cells (4), as well as in a Saccharomyces cerevisiae prion model (2). However, this drug failed to delay significantly the outcome of the disease in compassionate treatments of human TSE (5), despite its ability to cross the blood-brain barrier. In primary cultured neurons, long-lasting application of chlorpromazine was more toxic than in stable cell cultures, which is consistent with its reported neurotoxic activity in cerebellum cell culture (43). At the maximal drug concentration tolerated in CGN cultures, this compound hampered PrPres accumulation to various degrees according to the prion agent. While chlorpromazine showed some inhibitory effect in CGNMo and in CGNHu cultures, it scarcely prevented PrPres accumulation in CGNHa and CGNOv cultures. In Rov cell cultures (53) infected by the same sheep strain as CGNOv cells, this drug showed a clear PrPres-inhibitory effect (data not shown). In a clinical trial on scrapie-infected ewes, however, it failed to produce any therapeutic benefit in combination with quinacrine, another tricyclic derivative (21). Altogether, these observations suggest that this antiprion drug assay involving primary neuronal cells could lead to a more accurate prediction of the in vivo effect than in a cell line, possibly due to a greater phenotypic proximity with postmitotic neurons. In such cells, the dynamics of PrPSc synthesis or accumulation differs from that in actively dividing cells. Moreover, the biogenesis of PrPC in primary and immortalized neuronal cells exhibits notable differences in terms of turnover and endocytosis (37, 49), which might also affect drug activity.
Of the three compounds assayed in our CGN cell model, the amphotericin B derivative MS-8209 showed the broadest spectrum of antiprion efficacy in terms of species or prion strain, yet variations in its PrPres inhibitory potency were noticeable. In CGN cells expressing hamster PrP, MS-8209 was found to be more effective against the Sc237 than the 139H strain. This is in keeping with previous in vivo observations showing an apparent strain-dependent effect of polyene antibiotics in the absence of PrP sequence variation. In hamsters, amphotericin B could significantly delay clinical phase onset following inoculation with the prion strain 263K (equivalent to Sc237) but not with the strains 139H (58) and DY (33), which have a longer incubation period. MS-8209 also exhibited some specificity, extending the life spans of transgenic mice with neuron-restricted hamster PrP expression when infected with the 263K strain but not with the DY strain (18). Thus, the strain-specific effect observed in CGN cells recapitulated to some extent those reported in vivo for this class of compounds. Such cells may therefore provide a relevant tissue culture system in which to investigate the mechanisms underlying the antiprion activity.
The cell system developed in this study made it possible for the first time to test the relative potency of several drugs toward a human prion. Thus far, intraventricular infusion of pentosan polysulfate in a variant CJD patient has been the only treatment that might slow the disease progression in human (41). Failure of compassionate treatments with other compounds was attributed to the late intervention and/or a poor penetration of the blood-brain barrier (19, 23, 32, 36). Another issue that is also supported by our findings is that screening in rodent TSE models might be inaccurate and select molecules that may not be particularly active against human agents. In this regard, this ex vivo assay against a CJD agent in primary nerve cells derived from transgenic mice expressing human PrP may provide new opportunities for the selection of compounds active against human prions.
Acknowledgments
We thank R. Carp and S. Lehmann for kindly providing rodent strain materials, J. Grassi and S. Simon (CEA, Saclay, France) for Sha31 antibody, S. Hawke (Imperial College, London and now at the University of Sydney) for ICSM18 antibody, and M. Fontes (INSERM U491, Marseille, France) and C. Weissmann (now at the Scripps Research Institute, Florida) for authorizing us to include tg650, tga20, and PrP0/0 mice in this study. We thank C. Trevitt for careful reading of the manuscript and R. Young for preparation of the figures.
This project was supported by a grant from the French Ministry of Research (GIS-Infections à Prions). S. Cronier was a recipient of an MRE fellowship.
Footnotes
Published ahead of print on 3 October 2007.
REFERENCES
- 1.Archer, F., C. Bachelin, O. Andreoletti, N. Besnard, G. Perrot, C. Langevin, A. Le Dur, D. Vilette, A. Baron-Van Evercooren, J. L. Vilotte, and H. Laude. 2004. Cultured peripheral neuroglial cells are highly permissive to sheep prion infection. J. Virol. 78:482-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bach, S., N. Talarek, T. Andrieu, J. M. Vierfond, Y. Mettey, H. Galons, D. Dormont, L. Meijer, C. Cullin, and M. Blondel. 2003. Isolation of drugs active against mammalian prions using a yeast-based screening assay. Nat. Biotechnol. 21:1075-1081. [DOI] [PubMed] [Google Scholar]
- 3.Baron, G. S., A. C. Magalhaes, M. A. Prado, and B. Caughey. 2006. Mouse-adapted scrapie infection of SN56 cells: greater efficiency with microsome-associated versus purified PrP-res. J. Virol. 80:2106-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barret, A., F. Tagliavini, G. Forloni, C. Bate, M. Salmona, L. Colombo, A. De Luigi, L. Limido, S. Suardi, G. Rossi, F. Auvre, K. T. Adjou, N. Sales, A. Williams, C. Lasmezas, and J. P. Deslys. 2003. Evaluation of quinacrine treatment for prion diseases. J. Virol. 77:8462-8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benito-Leon, J. 2004. Combined quinacrine and chlorpromazine therapy in fatal familial insomnia. Clin. Neuropharmacol. 27:201-203. [DOI] [PubMed] [Google Scholar]
- 6.Beringue, V., K. T. Adjou, F. Lamoury, T. Maignien, J. P. Deslys, R. Race, and D. Dormont. 2000. Opposite effects of dextran sulfate 500, the polyene antibiotic MS-8209, and Congo red on accumulation of the protease-resistant isoform of PrP in the spleens of mice inoculated intraperitoneally with the scrapie agent. J. Virol. 74:5432-5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beringue, V., D. Vilette, G. Mallinson, J. Solassol, S. Lehmann, J. P. Majoral, J. Collinge, S. Hawke, and H. Laude. 2003. Screening molecules that inhibit prion replication in scrapie-infected epithelial Rov cells, p. 97-106. In S. Lehmann (ed.), New perspectives for prion therapeutics. Editions de Condé, Paris, France.
- 8.Bosque, P. J., and S. B. Prusiner. 2000. Cultured cell sublines highly susceptible to prion infection. J. Virol. 74:4377-4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruce, M. E. 2003. TSE strain variation. Br. Med. Bull. 66:99-108. [DOI] [PubMed] [Google Scholar]
- 10.Bueler, H., M. Fischer, Y. Lang, H. Bluethmann, H. P. Lipp, S. J. DeArmond, S. B. Prusiner, M. Aguet, and C. Weissmann. 1992. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 356:577-582. [DOI] [PubMed] [Google Scholar]
- 11.Carimalo, J., S. Cronier, G. Petit, J. M. Peyrin, F. Boukhtouche, N. Arbez, Y. Lemaigre-Dubreuil, B. Brugg, and M. C. Miquel. 2005. Activation of the JNK-c-Jun pathway during the early phase of neuronal apoptosis induced by PrP106-126 and prion infection. Eur. J. Neurosci. 21:2311-2319. [DOI] [PubMed] [Google Scholar]
- 12.Caughey, B., D. Ernst, and R. E. Race. 1993. Congo red inhibition of scrapie agent replication. J. Virol. 67:6270-6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caughey, B., and G. J. Raymond. 1993. Sulfated polyanion inhibition of scrapie-associated PrP accumulation in cultured cells. J. Virol. 67:643-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collinge, J. 2001. Prion diseases of humans and animals: their causes and molecular basis. Annu. Rev. Neurosci. 24:519-550. [DOI] [PubMed] [Google Scholar]
- 15.Contestabile, A. 2002. Cerebellar granule cells as a model to study mechanisms of neuronal apoptosis or survival in vivo and in vitro. Cerebellum 1:41-55. [DOI] [PubMed] [Google Scholar]
- 16.Cronier, S., H. Laude, and J. M. Peyrin. 2004. Prions can infect primary cultured neurons and astrocytes and promote neuronal cell death. Proc. Natl. Acad. Sci. USA 101:12271-12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeArmond, S. J., H. Sanchez, F. Yehiely, Y. Qiu, A. Ninchak-Casey, V. Daggett, A. P. Camerino, J. Cayetano, M. Rogers, D. Groth, M. Torchia, P. Tremblay, M. R. Scott, F. E. Cohen, and S. B. Prusiner. 1997. Selective neuronal targeting in prion disease. Neuron 19:1337-1348. [DOI] [PubMed] [Google Scholar]
- 18.Demaimay, R., R. Race, and B. Chesebro. 1999. Effectiveness of polyene antibiotics in treatment of transmissible spongiform encephalopathy in transgenic mice expressing Syrian hamster PrP only in neurons. J. Virol. 73:3511-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dormont, D., P. Yeramian, P. Lambert, F. Cathala, B. Spire, F. C. Barre-Sinoussi, L. Court, and J. C. Chermann. 1989. In vitro and in vivo antiviral effects of HPA 23, p. 324-337. In L. A. Court, D. Dormont, P. Brown, and D. T. Kingsbury (ed.), Unconventional virus diseases of the central nervous system. C. E. A. Diffusion, Paris, France.
- 20.Fischer, M., T. Rulicke, A. Raeber, A. Sailer, M. Moser, B. Oesch, S. Brandner, A. Aguzzi, and C. Weissmann. 1996. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 15:1255-1264. [PMC free article] [PubMed] [Google Scholar]
- 21.Gayrard, V., N. Picard-Hagen, C. Viguie, V. Laroute, O. Andreoletti, and P. L. Toutain. 2005. A possible pharmacological explanation for quinacrine failure to treat prion diseases: pharmacokinetic investigations in a ovine model of scrapie. Br. J. Pharmacol. 144:386-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giri, R. K., R. Young, R. Pitstick, S. J. DeArmond, S. B. Prusiner, and G. A. Carlson. 2006. Prion infection of mouse neurospheres. Proc. Natl. Acad. Sci. USA 103:3875-3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haik, S., J. P. Brandel, D. Salomon, V. Sazdovitch, N. Delasnerie-Laupretre, J. L. Laplanche, B. A. Faucheux, C. Soubrie, E. Boher, C. Belorgey, J. J. Hauw, and A. Alperovitch. 2004. Compassionate use of quinacrine in Creutzfeldt-Jakob disease fails to show significant effects. Neurology 63:2413-2415. [DOI] [PubMed] [Google Scholar]
- 24.Hecker, R., A. Taraboulos, M. Scott, K. M. Pan, S. L. Yang, M. Torchia, K. Jendroska, S. J. DeArmond, and S. B. Prusiner. 1992. Replication of distinct scrapie prion isolates is region specific in brains of transgenic mice and hamsters. Genes Dev. 6:1213-1228. [DOI] [PubMed] [Google Scholar]
- 25.Ingrosso, L., A. Ladogana, and M. Pocchiari. 1995. Congo red prolongs the incubation period in scrapie-infected hamsters. J. Virol. 69:506-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimberlin, R. H., C. A. Walker, and H. Fraser. 1989. The genomic identity of different strains of mouse scrapie is expressed in hamsters and preserved on reisolation in mice. J. Gen. Virol. 70:2017-2025. [DOI] [PubMed] [Google Scholar]
- 27.Kocisko, D. A., J. H. Come, S. A. Priola, B. Chesebro, G. J. Raymond, P. T. Lansbury, and B. Caughey. 1994. Cell-free formation of protease-resistant prion protein. Nature 370:471-474. [DOI] [PubMed] [Google Scholar]
- 28.Kocisko, D. A., A. L. Engel, K. Harbuck, K. M. Arnold, E. A. Olsen, L. D. Raymond, D. Vilette, and B. Caughey. 2005. Comparison of protease-resistant prion protein inhibitors in cell cultures infected with two strains of mouse and sheep scrapie. Neurosci. Lett. 388:106-111. [DOI] [PubMed] [Google Scholar]
- 29.Korth, C., B. C. May, F. E. Cohen, and S. B. Prusiner. 2001. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc. Natl. Acad. Sci. USA 98:9836-9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ladogana, A., Q. Liu, Y. G. Xi, and M. Pocchiari. 1995. Proteinase-resistant protein in human neuroblastoma cells infected with brain material from Creutzfeldt-Jakob patient. Lancet 345:594-595. [DOI] [PubMed] [Google Scholar]
- 31.Laude, H., D. Vilette, A. Le Dur, F. Archer, S. Soulier, N. Besnard, R. Essalmani, and J. L. Vilotte. 2002. New in vivo and ex vivo models for the experimental study of sheep scrapie: development and perspectives. C. R. Biol. 325:49-57. [DOI] [PubMed] [Google Scholar]
- 32.Masullo, C., G. Macchi, Y. G. Xi, and M. Pocchiari. 1992. Failure to ameliorate Creutzfeldt-Jakob disease with amphotericin B therapy. J. Infect. Dis. 165:784-785. [DOI] [PubMed] [Google Scholar]
- 33.McKenzie, D., J. Kaczkowski, R. Marsh, and J. Aiken. 1994. Amphotericin B delays both scrapie agent replication and PrP-res accumulation early in infection. J. Virol. 68:7534-7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milhavet, O., D. Casanova, N. Chevallier, R. D. McKay, and S. Lehmann. 2006. Neural stem cell model for prion propagation. Stem Cells 24:2284-2291. [DOI] [PubMed] [Google Scholar]
- 35.Oberpichler-Schwenk, H., and J. Krieglstein. 1994. Primary cultures of neurons for testing neuroprotective drug effects. J. Neural Transm. Suppl. 44:1-20. [DOI] [PubMed] [Google Scholar]
- 36.Otto, M., L. Cepek, P. Ratzka, S. Doehlinger, I. Boekhoff, J. Wiltfang, E. Irle, G. Pergande, B. Ellers-Lenz, O. Windl, H. A. Kretzschmar, S. Poser, and H. Prange. 2004. Efficacy of flupirtine on cognitive function in patients with CJD: a double-blind study. Neurology 62:714-718. [DOI] [PubMed] [Google Scholar]
- 37.Parizek, P., C. Roeckl, J. Weber, E. Flechsig, A. Aguzzi, and A. J. Raeber. 2001. Similar turnover and shedding of the cellular prion protein in primary lymphoid and neuronal cells. J. Biol. Chem. 276:44627-44632. [DOI] [PubMed] [Google Scholar]
- 38.Powell, S. K., R. J. Rivas, E. Rodriguez-Boulan, and M. E. Hatten. 1997. Development of polarity in cerebellar granule neurons. J. Neurobiol. 32:223-236. [DOI] [PubMed] [Google Scholar]
- 39.Prusiner, S. B. 1998. Prions. Proc. Natl. Acad. Sci. USA 95:13363-13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prusiner, S. B., M. Scott, D. Foster, K. M. Pan, D. Groth, C. Mirenda, M. Torchia, S. L. Yang, D. Serban, G. A. Carlson, et al. 1990. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell 63:673-686. [DOI] [PubMed] [Google Scholar]
- 41.Rainov, N. G., Y. Tsuboi, P. Krolak-Salmon, A. Vighetto, and K. Doh-Ura. 2007. Experimental treatments for human transmissible spongiform encephalopathies: is there a role for pentosan polysulfate? Expert. Opin. Biol. Ther. 7:713-726. [DOI] [PubMed] [Google Scholar]
- 42.Raymond, G. J., E. A. Olsen, K. S. Lee, L. D. Raymond, P. K. Bryant, 3rd, G. S. Baron, W. S. Caughey, D. A. Kocisko, L. E. McHolland, C. Favara, J. P. Langeveld, F. G. van Zijderveld, R. T. Mayer, M. W. Miller, E. S. Williams, and B. Caughey. 2006. Inhibition of protease-resistant prion protein formation in a transformed deer cell line infected with chronic wasting disease. J. Virol. 80:596-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roubein, I. F., M. Samuelly, and W. Keup. 1973. The toxicity of chlorpromazine and mescaline on mouse cerebellum and fibroblast cells in culture. Acta Pharmacol. Toxicol. 33:326-329. [DOI] [PubMed] [Google Scholar]
- 44.Rubenstein, R., H. Deng, R. E. Race, W. Ju, C. L. Scalici, M. C. Papini, R. J. Kascsak, and R. I. Carp. 1992. Demonstration of scrapie strain diversity in infected PC12 cells. J. Gen. Virol. 73:3027-3031. [DOI] [PubMed] [Google Scholar]
- 45.Rudyk, H., S. Vasiljevic, R. M. Hennion, C. R. Birkett, J. Hope, and I. H. Gilbert. 2000. Screening Congo Red and its analogues for their ability to prevent the formation of PrP-res in scrapie-infected cells. J. Gen. Virol. 81:1155-1164. [DOI] [PubMed] [Google Scholar]
- 46.Saborio, G. P., B. Permanne, and C. Soto. 2001. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 411:810-813. [DOI] [PubMed] [Google Scholar]
- 47.Safar, J., H. Wille, V. Itri, D. Groth, H. Serban, M. Torchia, F. E. Cohen, and S. B. Prusiner. 1998. Eight prion strains have PrP(Sc) molecules with different conformations. Nat. Med. 4:1157-1165. [DOI] [PubMed] [Google Scholar]
- 48.Solassol, J., C. Crozet, and S. Lehmann. 2003. Prion propagation in cultured cells. Br. Med. Bull. 66:87-97. [DOI] [PubMed] [Google Scholar]
- 49.Sunyach, C., A. Jen, J. Deng, K. T. Fitzgerald, Y. Frobert, J. Grassi, M. W. McCaffrey, and R. Morris. 2003. The mechanism of internalization of glycosylphosphatidylinositol-anchored prion protein. EMBO J. 22:3591-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taraboulos, A., D. Serban, and S. B. Prusiner. 1990. Scrapie prion proteins accumulate in the cytoplasm of persistently infected cultured cells. J. Cell Biol. 110:2117-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trevitt, C. R., and J. Collinge. 2006. A systematic review of prion therapeutics in experimental models. Brain 129:2241-2265. [DOI] [PubMed] [Google Scholar]
- 52.Trinchese, F., S. Liu, I. Ninan, D. Puzzo, J. P. Jacob, and O. Arancio. 2004. Cell cultures from animal models of Alzheimer's disease as a tool for faster screening and testing of drug efficacy. J. Mol. Neurosci. 24:15-21. [DOI] [PubMed] [Google Scholar]
- 53.Vilette, D., O. Andreoletti, F. Archer, M. F. Madelaine, J. L. Vilotte, S. Lehmann, and H. Laude. 2001. Ex vivo propagation of infectious sheep scrapie agent in heterologous epithelial cells expressing ovine prion protein. Proc. Natl. Acad. Sci. USA 98:4055-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vilotte, J. L., S. Soulier, R. Essalmani, M. G. Stinnakre, D. Vaiman, L. Lepourry, J. C. Da Silva, N. Besnard, M. Dawson, A. Buschmann, M. Groschup, S. Petit, M. F. Madelaine, S. Rakatobe, A. Le Dur, D. Vilette, and H. Laude. 2001. Markedly increased susceptibility to natural sheep scrapie of transgenic mice expressing ovine PrP. J. Virol. 75:5977-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wadsworth, J. D., S. Joiner, A. F. Hill, T. A. Campbell, M. Desbruslais, P. J. Luthert, and J. Collinge. 2001. Tissue distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay. Lancet 358:171-180. [DOI] [PubMed] [Google Scholar]
- 56.Weissmann, C., and A. Aguzzi. 2005. Approaches to therapy of prion diseases. Annu. Rev. Med. 56:321-344. [DOI] [PubMed] [Google Scholar]
- 57.Weli, S. C., C. A. Scott, C. A. Ward, and A. C. Jackson. 2006. Rabies virus infection of primary neuronal cultures and adult mice: failure to demonstrate evidence of excitotoxicity. J. Virol. 80:10270-10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xi, Y. G., L. Ingrosso, A. Ladogana, C. Masullo, and M. Pocchiari. 1992. Amphotericin B treatment dissociates in vivo replication of the scrapie agent from PrP accumulation. Nature 356:598-601. [DOI] [PubMed] [Google Scholar]