Abstract
The early immune response fails to prevent the establishment of chronic human immunodeficiency virus (HIV) infection but may influence viremia during primary infection, thereby possibly affecting long-term disease progression. CD25+ FoxP3+ regulatory T cells may contribute to HIV/simian immunodeficiency virus (SIV) pathogenesis by suppressing efficient antiviral responses during primary infection, favoring high levels of viral replication and the establishment of chronic infection. In contrast, they may decrease immune activation during chronic infection. CD4+ regulatory T cells have been studied in the most detail, but CD8+ CD25+ FoxP3+ T cells also have regulatory properties. We monitored the dynamics of CD25+ FoxP3+ T cells during primary and chronic SIVmac251 infection in cynomolgus macaques. The number of peripheral CD4+ CD25+ FoxP3+ T cells paralleled that of memory CD4+ T cells, with a rapid decline during primary infection followed by a rebound to levels just below baseline and gradual depletion during the course of infection. No change in the proportion of CD25+ FoxP3+ T cells was observed in peripheral lymph nodes. A small number of CD4+ CD25+ FoxP3+ T cells at set point was associated with a high plasma viral load. In contrast, peripheral CD8+ CD25+ FoxP3+ T cells were induced a few days after peak plasma viral load during primary infection. The number of these cells was positively correlated with viral load and negatively correlated with CD4+ T-cell activation, SIV antigen-specific proliferative responses during primary infection, and plasma viral load at set point, with large numbers of CD8+ CD25+ FoxP3+ T cells being indicative of a poor prognosis.
The early immune response fails to prevent the establishment of chronic human immunodeficiency virus (HIV) infection (43) but may influence viremia during primary infection, thereby possibly affecting long-term disease progression (44). An effective immune response is the result of a delicate balance between negative and positive immune stimulatory signals. Regulatory T cells, which inhibit the activation and effector functions of T cells, are important regulators of immune responses. Several subsets of regulatory T cells have been described, including natural CD25+ regulatory T cells (19, 56), type 1 T-regulatory (Tr1) cells (37), and T-helper 3 (Th3) cells (62). Natural CD25+ regulatory T cells exert their inhibitory effects principally through a cell-cell contact-dependent mechanism (6), whereas Tr1 and Th3 cells have been reported to suppress the immune response by secreting the immunosuppressive cytokines interleukin-10 and transforming growth factor β (TGF-β) (37, 62).
Natural regulatory T cells have been studied the most thoroughly. They limit the expansion and activation of CD4+ T-cell populations and have been shown to influence various inflammatory processes, including microbial infections (7) and autoimmune diseases (55). Natural regulatory T cells are characterized by the expression of various markers. The most widely used of these markers is CD25 both alone and, more recently, in combination with FoxP3. FoxP3 expression is associated with immunosuppression, and the expression of FoxP3 may render nonregulatory T cells suppressive (28, 32). Another well-described phenotypic and functionally important marker of regulatory T cells is CTLA-4 (54, 60). The blockade of CTLA-4 has been shown to reverse the suppressive effect of regulatory T cells (60).
Human CD8+ regulatory T cells have also been described but have been studied in less detail. They have regulatory properties that are similar to those of their CD4+ counterparts, inhibiting CD4+ T-cell activation and proliferation in a cell-cell contact-dependent manner (16, 23, 57). These regulatory, or suppressive, CD8+ T cells have been reported to have a wide range of phenotypic characteristics. Several groups have described CD8+ CD28− regulatory T cells (27, 33, 45, 57), whereas others have described CD8+ CD25+ regulatory T cells (10, 16, 33, 49). Some studies have detected FoxP3 expression in CD8+ regulatory T cells (10, 16, 33, 45).
CD4+ CD25+ regulatory T cells are thought to influence the pathogenesis of HIV infection (11). The number of peripheral regulatory T cells has been reported to decrease in patients with chronic HIV infection (3, 4, 21, 50) and rhesus macaques experimentally infected with simian immunodeficiency virus (SIV) (51). Direct infection and killing have been proposed as possible causes of the decrease in the number of these cells (50). The recovery and/or maintenance of CD4+ CD25+ regulatory T cells has been associated with low viral load and low immune activation in rhesus macaques with chronic SIV infection (51). Several studies have shown that CD4+ regulatory T cells from HIV-infected patients inhibit T-cell responses in vitro (1, 35, 63) and that this ability to suppress T-cell responses is negatively correlated with plasma viral load (35). In contrast to the decrease in the number of peripheral regulatory T cells observed during chronic HIV infection, regulatory T cells have been shown to accumulate in lymphoid tissue during HIV infection in humans (3, 47) and SIV infection in rhesus macaques (24). This increase in regulatory T-cell numbers in lymphoid tissue has been correlated with disease progression (3, 47).
We evaluated the influence of regulatory T cells during primary and chronic SIV infection in the cynomolgus macaque SIVmac251 infection model. The rate of progression in this model is variable, as is that in humans infected with HIV (20). FoxP3+ T cells in cynomolgus macaques were characterized, and the dynamics of these cells were monitored longitudinally during SIV infection. Peripheral FoxP3+ CD25+ CD8+ T cells were induced during primary infection. These cells had phenotypic features similar to those of regulatory T cells, and their numbers were positively correlated with viral load and negatively correlated with CD4+ T-cell activation and SIV antigen-specific proliferative responses.
MATERIALS AND METHODS
Animals and infections.
We studied 18 young adult male cynomolgus macaques (Macaca fascicularis), each weighing 2.7 to 4.5 kg, imported from Mauritius and kept according to European guidelines for animal care (Journal Officiel des Communautés Européennes, L358, 18 December 1986). Macaques were inoculated intravenously with pathogenic SIVmac251 (46) and divided into three groups of six animals. The animals in group 1 received fifty 50% animal infectious dose (50 AID50). The animals in group 2 received 50 AID50 and were treated with zidovudine (AZT) (4.5 mg/kg) and lamivudine (3TC) (2.5 mg/kg) combined with indinavir (20 mg/kg), as previously described (8), by the oral route twice per day from 4 h postinfection until 28 days postinfection. The animals in group 3 received 5,000 AID50 of the virus.
Determination of plasma SIV RNA load.
Viral RNA was prepared from 200 μl of cell-free plasma by using the High Pure viral RNA kit (Roche Diagnostics, Meylan, France) according to the manufacturer's instructions. RNA was eluted in 50 μl of nuclease-free water and frozen immediately at −80°C until analysis. SIVmac251 virus, titrated by the Branched-Chain DNA assay and diluted in EDTA-treated plasma sample from macaques not infected with SIV, was used to generate a standard curve (serial 10-fold dilutions). Three titrated SIVmac251-infected EDTA-treated plasma samples and two EDTA-treated plasma samples from SIV-negative macaques were used as positive and negative reverse transcription-PCR controls, respectively.
Standards, controls, and viral RNA samples were extracted and tested in parallel under the same conditions. The SIVmac251 gag cDNA sequence, ligated into the pCR4-TOPO (Invitrogen) plasmid and purified with the HiSpeed Maxiprep kit (Invitrogen), was used as a positive control for PCR.
One-tube reverse transcription-PCR was performed in an iCycler real-time thermocycler (Bio-Rad) under the following conditions. The 25-μl reaction mixture contained 50 mM KCl, 20 mM Tris-HCl (pH 8.3), 0.8 mM deoxynucleoside triphosphates, 3 mM MgCl2, 20 U RNasin (Promega), 25 U murine leukemia virus reverse transcriptase (Applied Biosystems), 0.625 U Hot-Start iTaq DNA polymerase (Bio-Rad), 450 nM of each primer, 250 nM fluorogenic probe, and 10 μl RNA elution samples. The probe and primers, as described previously by Hofmann-Lehmann et al. (31), were based on the sequence within the conserved SIV gag region. Samples were subjected to heating for 30 min at 46°C and 4 min at 95°C, followed by 50 cycles of 95°C for 15 s and 60°C for 1 min.
All amplifications were performed in duplicate, with the standard RNA template dilution, over 7 orders of magnitude. A correlation coefficient of up to 97% was obtained, with a sensitivity of at least 100 copies per ml.
CD4+ and CD8+ T-cell counts.
CD4+ and CD8+ T-cell counts were determined using 100 μl of whole blood by immunostaining with anti-CD3 fluorescein isothiocyanate (FITC) (clone FN18; CliniSciences, Montrouge, France), anti-CD4 phycoerythrin (PE) (clone L200; BD Biosciences, Grenoble, France), and anti-CD8 phycoerythrin-cyanin 5 (clone B9.11; Beckman Coulter, France) antibodies. Blood samples were incubated with antibodies for 15 min at room temperature, and the red blood cells were lysed with 1 ml fluorescence-activated cell sorter lysing solution (BD Biosciences). Stained cells were washed in phosphate-buffered saline (PBS) and fixed (Cell-Fix; BD Biosciences). Data were acquired with a BD FACScan instrument using CellQuest software (BD Biosciences). The proportions of CD3+ CD4+ and CD3+ CD8+ cells were determined in the lymphocyte gate, defined in terms of light-scattering properties, using CellQuest software. Absolute counts were calculated from the absolute blood count of lymphocytes obtained by automated cell counting (Coulter MDII; Coultronics, Margency, France).
Cell isolation.
Peripheral blood mononuclear cells (PBMC) were isolated from whole blood, either by Ficoll gradient centrifugation or using CPT tubes (BD Biosciences), according to the manufacturer's recommendations. Residual red blood cells were lysed by exposure to hypotonic shock for 5 min, followed by washing in PBS. Whole axillary or inguinal lymph nodes were mechanically disrupted and passed through a strainer with 40-μm pores (BD Biosciences), and cells were washed in PBS.
Quantification and phenotyping of regulatory and central memory T cells.
For the quantification of FoxP3+ CD25+ regulatory T cells, PBMC were immunostained with anti-CD3 FITC (clone FN18; CliniSciences), anti-CD4 PerCP (clone L200; BD Biosciences), anti-CD25 PE (clone 4E3; Miltenyi Biotec, Paris, France), and anti-FoxP3 allophycocyanin (APC) (clone PCH101; eBioscience, San Diego, CA) antibodies. For the characterization of FoxP3+ T cells, PBMC were immunostained with anti-CD3 FITC, anti-CD4 peridin chlorophyll protein (PerCP), and anti-FoxP3 APC antibodies together with anti-CTLA-4 PE (clone BN13; BD Biosciences), anti-CD127 PE (clone hIL-7R-M21; BD Biosciences), anti-CD28 PE (clone 28.2; BD Biosciences), anti-CD95 PE (clone DX2; BD Biosciences), anti-CCR5 PE (clone 3A9; BD Biosciences), or anti-CXCR4 (clone 12G5; BD Biosciences) antibody. FoxP3 Fix/Perm buffers were used for the intracellular staining of FoxP3 and CTLA-4 according to the manufacturer's protocol (eBioscience). Naive and memory T cells were quantified by immunostaining PBMC with anti-CD3 FITC, anti-CD4 PerCP, anti-CD28 PE, and anti-CD95 APC antibodies. Central memory cells were defined as CD28+ CD95+ cells (53). Data were acquired with a BD LSRI instrument using CellQuest software (BD Biosciences) and analyzed with FlowJo software (Tree Star, Ashland, OR).
T-cell proliferation assay.
PBMC (105 cells) were dispensed into the wells of flat-bottomed 96-well plates and stimulated with 2 μg/ml aldrithiol-2 (AT-2)-inactivated SIVmac239 or AT-2-treated SupT1 microvesicles as a negative control or with 5 μg/ml concanavalin A (Sigma-Aldrich, Saint-Quentin Fallavier, France) as a positive control. AT-2-inactivated SIVmac239 (ARP1018.1) and its negative control (ARP1018.2) were obtained from Jeff Lifson (Frederick, MA) through the European Union Program EVA Centralized Facility for AIDS Reagents (NIBSC, Potters Bar, United Kingdom). [3H]thymidine (1 μCi; Amersham Biosciences, Orsay, France) was added to the culture for the last 8 h before harvesting on day 3 for concanavalin A-treated PBMC and on day 5 for inactivated SIVmac239-activated PBMC. [3H]thymidine incorporation was measured using a Microbeta 450 liquid scintillation counter (EG&G Wallac, Turku, Finland). Results were expressed as net mean counts per minute (cpm) by subtracting the mean cpm of the negative control triplicate wells from the mean cpm of antigen- or mitogen-activated triplicate wells.
Statistical analysis.
We used nonparametric Spearman's rank correlation tests to investigate the relationship between different T-cell populations and viral load, immune activation, or proliferative response. The Mann-Whitney test was used to compare the viral loads of different groups of macaques during chronic infection. In some cases, we used the area under the curve for analysis of the amplitude of variation of a given parameter throughout primary infection. Statistical analyses were carried out with Statview software (SAS Institute, Inc., Cary, NC).
RESULTS
Characterization of FoxP3+ cells in cynomolgus macaques.
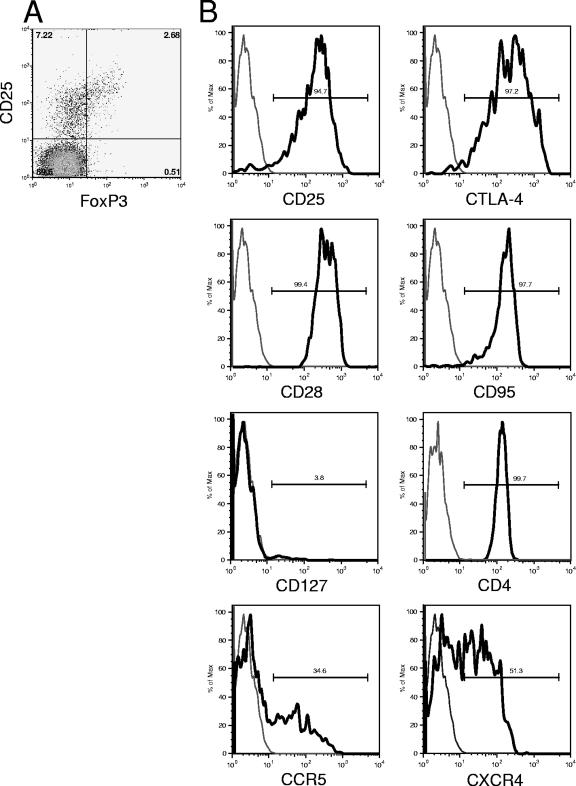
CD4+ FoxP3+ T cells were characterized by immunostaining and flow cytometry (Fig. 1) in healthy cynomolgus macaques (n = 5) and in cynomolgus macaques with chronic SIVmac251 infection (n = 5). The CD4+ FoxP3+ T cells expressed high levels of CD25 and CTLA-4, a typical feature of human regulatory T cells. A high percentage of CD4+ FoxP3+ T cells expressed CD28 and CD95 and therefore had a phenotype typical of central memory T cells. Low surface expression of CD127 in combination with CD25 has recently been shown to be an accurate means of identifying human regulatory T cells (40, 58). The CD4+ FoxP3+ T lymphocytes of cynomolgus macaques expressed no detectable CD127. They also displayed heterogeneous expression of the main HIV/SIV coreceptors CCR5 and CXCR4. Overall, the CD4+ FoxP3+ T lymphocytes of cynomolgus macaques had phenotypes similar to those of human and rhesus macaque regulatory T cells, with a memory phenotype and high CD25 and CTLA-4 expression (19, 51, 54, 60). The phenotypic characteristics of CD4+ FoxP3+ T cells did not change during SIV infection. The proportion of CD25+ FoxP3+ cells within the CD4+ T cells in uninfected animals was 1.5 to 4.0%.
FIG. 1.
Characterization of FoxP3+ T cells in cynomolgus macaques. (A) Plot showing CD25 and FoxP3 expression on CD3+ CD4+ lymphocytes. (B) SIV-negative cynomolgus macaque (gated on CD3+ CD4+ FoxP3+ lymphocytes). Data are representative of five different cynomolgus macaques tested. Gray line, isotype control; black line, antibody specific for the indicated marker.
Decrease in the number of peripheral CD4+ CD25+ FoxP3+ T cells during SIV infection.
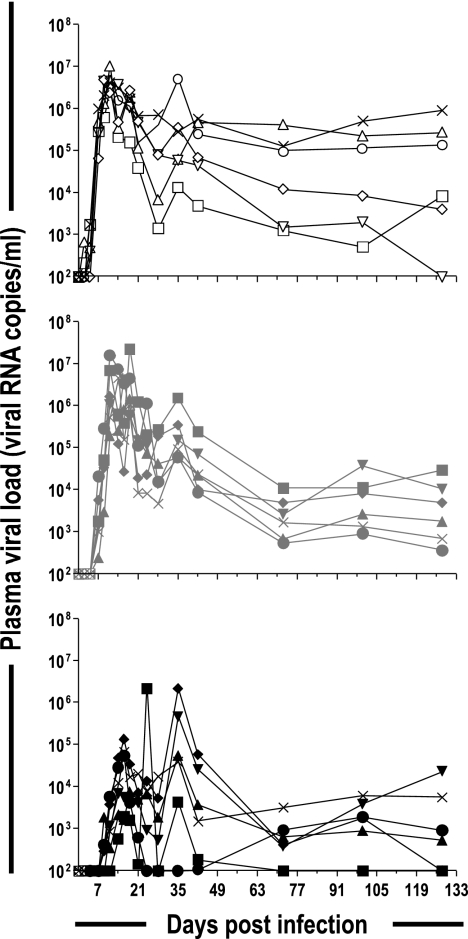
We monitored the dynamics of regulatory T cells in cynomolgus macaques during SIV infection by infecting 18 macaques intravenously with SIVmac251. The animals of one group (n = 6) were inoculated intravenously with a high dose of virus, 5,000 AID50, whereas those of another group (n = 6) were inoculated with a lower dose, 50 AID50. The animals of the third group (n = 6) received 50 AID50 and were treated with antiretroviral therapy from 4 h postinfection until 28 days postinfection. The treatment administered was previously demonstrated to lower the viral load during primary infection and to improve control over viremia in the long term (8). In this study, during chronic infection, 128 to 274 days after infection, viral load as a function of time (area under the curve) was significantly lower (P = 0.045) in the treated macaques than in the untreated macaques (data not shown). The changes in viral load during primary infection are shown in Fig. 2. Macaques infected with high doses of virus showed an earlier but not higher peak plasma viral load and did not differ from macaques infected with the lower dose at set point. Antiretroviral treatment (ART) delayed and lowered the plasma viral load peak. Surface labeling of CD3, CD4, and CD25 and intracellular labeling of FoxP3 followed by flow cytometry were used to monitor the number of CD25+ FoxP3+ T cells. Changes in the number of CD4+ CD25+ FoxP3+ T cells in blood paralleled those of central memory CD4+ T cells (Fig. 3), with a rapid decline during primary infection followed by a rebound to levels just below baseline and a gradual decrease during the course of the disease. At set point (day 128 postinfection), the plasma viral load was negatively correlated (P = 0.027; r2 = 0.261) with the number of CD4+ CD25+ FoxP3+ T cells. A negative correlation was also found between plasma viral load and the total number of central memory CD4+ cells (P = 0.007; r2 = 0.503) at set point, and this correlation remained during chronic infection.
FIG. 2.
Plasma viral RNA levels during SIVmac251 infection of cynomolgus macaques. Plasma viral loads of individual macaques are shown. (Top) Six cynomolgus macaques inoculated intravenously with 5,000 AID50 (open symbols). (Middle) Six cynomolgus macaques inoculated intravenously with 50 AID50 (gray symbols). (Bottom) Six cynomolgus macaques inoculated intravenously with 50 AID50 and treated (AZT, 3TC, and indinavir) from 4 h postinfection to 28 days postinfection (black symbols).
FIG. 3.
Number of CD4+ regulatory T cells and CD4+ central memory T cells during SIVmac251 infection of cynomolgus macaques is negatively correlated with plasma viral load at set point. CD4+ CD25+ FoxP3+ T cells (A) and central memory CD4+ T cells (B) of individual macaques are shown. (Top) Six cynomolgus macaques inoculated intravenously with 5,000 AID50 (open symbols). (Middle) Six cynomolgus macaques inoculated intravenously with 50 AID50 (gray symbols). (Bottom) Six cynomolgus macaques inoculated intravenously with 50 AID50 and treated (AZT, 3TC, and indinavir) from 4 h postinfection to 28 days postinfection (black symbols). There was an inverse correlation between the number of CD4+ CD25+ FoxP3+ T cells (P = 0.027 by Spearman correlation; r2 = 0.261) (C) and central memory T cells (P = 0.007 by Spearman correlation; r2 = 0.503) (D) and plasma viral load at set point, 128 days postinfection.
Increase in the number of peripheral CD8+ CD25+ FoxP3+ T cells during SIV infection.
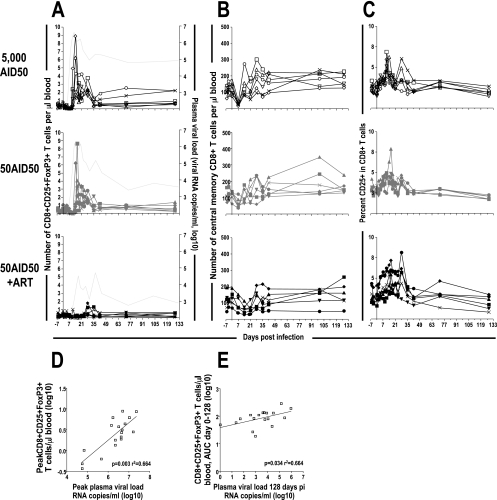
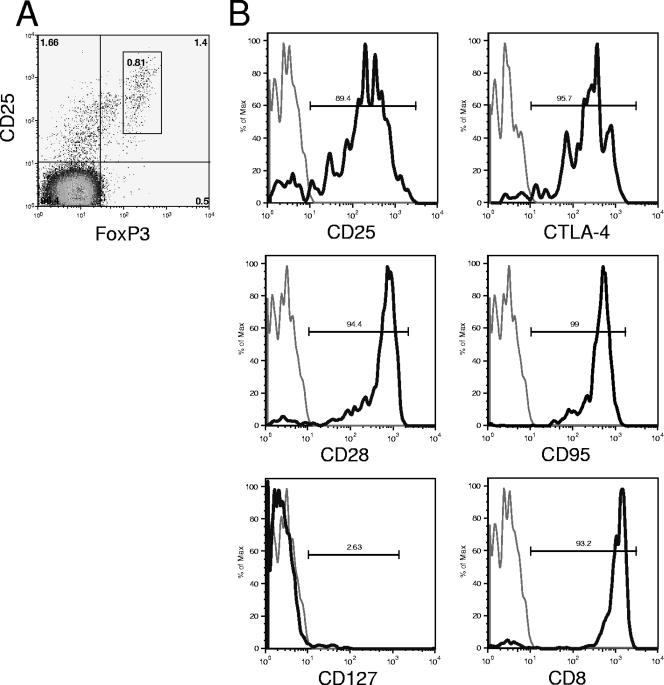
During acute infection, 3 to 5 days after peak plasma viral load, a large increase in the number of CD4− CD25+ FoxP3+ T cells in the blood of macaques not receiving ART was observed (Fig. 4A). This increase corresponded to up to 0.8% CD25+ FoxP3+ T cells within the CD4− T-cell population. These cells were found to have a pattern of cellular marker expression similar to that of CD4+ FoxP3+ T cells. These cells were characterized on days 18 (n = 4) and 190 (n = 2) after infection and were confirmed to be CD8+ T cells on the basis of their high levels of CD8 expression. They also expressed high levels of CTLA-4, which is typical of regulatory T cells (Fig. 5). As for CD4+ FoxP3+ T lymphocytes in cynomolgus macaques, no CD127 expression was detected on CD8+ FoxP3+ T cells. Furthermore, these cells expressed CD28 and CD95 and therefore had a phenotype typical of central memory cells, as their CD4+ counterparts, although this increase was not a reflection of all CD8+ central memory T cells (Fig. 4B). Moreover, the increase in the number of CD8+ CD25+ FoxP3+ T cells in the blood did not appear to reflect the activation of CD8+ T cells during primary infection, which was monitored using the frequency of CD25 expression on CD8+ T cells as a marker of activation (61). The proportion of CD8+ T cells expressing CD25 increased in all macaques, including those in the group receiving ART, and this increase was detected before the induction of CD8+ CD25+ FoxP3+ T cells (Fig. 4C). Before infection, there were few or undetectable CD8+ CD25+ FoxP3+ T cells. Within 4 weeks of infection, the number of these cells had returned to levels similar to those before infection in 16 of 18 macaques, but these cells remained detectable in two macaques. Interestingly, these two macaques were among the three identified as being progressors, with plasma viral loads of >105 copies per ml. The peak number of CD8+ CD25+ FoxP3+ T cells during primary infection was significantly correlated (P = 0.003; r2 = 0.664) with peak viral loads during the same period (Fig. 4D). Moreover, the number of CD8+ CD25+ FoxP3+ T cells during early infection (days 0 to 128 postinfection) was significantly correlated (P = 0.034; r2 = 0.251) with viral load at set point, 128 days after infection (Fig. 4E).
FIG. 4.
Induction of CD25+ FoxP3+ CD8+ T cells during SIVmac251 infection of cynomolgus macaques is correlated with plasma viral load. CD8+ CD25+ FoxP3+ T cells (A), CD8+ central memory T cells (B), and proportion of CD8+ T cells that were CD25+ (C) of individual macaques are shown. (Top) Six cynomolgus macaques inoculated intravenously with 5,000 AID50 (open symbols). (Middle) Six cynomolgus macaques inoculated intravenously with 50 AID50 (gray symbols). (Bottom) Six cynomolgus macaques inoculated intravenously with 50 AID50 and treated (AZT, 3TC, and indinavir) from 4 h postinfection to 28 days postinfection (black symbols). The light gray line without symbols indicates the median plasma viral load of the corresponding macaques (A). (D and E) Positive correlation between peak number of CD8+ CD25+ FoxP3+ T cells and peak viral load during primary infection (P = 0.003 by Spearman correlation; r2 = 0.664) (D) and between the total number of CD8+ CD25+ FoxP3+ T cells during primary infection (days 0 to 128), expressed as the area under the curve (AUC), and plasma viral load at 128 days postinfection (P = 0.034 by Spearman correlation; r2 = 0.251) (E).
FIG. 5.
Characterization of CD3+ CD8+ FoxP3+ T cells in SIVmac251-infected cynomolgus macaques. (A) Pseudocolor plot showing CD25 and FoxP3 expression on CD3+ CD4− lymphocytes 18 days after infection. (B) Gated CD3+ CD4− FoxP3+ lymphocytes 18 or 190 days after infection. Gray line, isotype control; black line, antibody specific for the indicated marker.
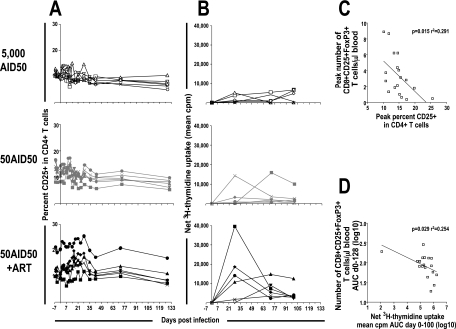
CD8+ CD25+ FoxP3+ T-cell numbers are negatively correlated with immune activation during primary infection.
The increase in the proportion of FoxP3+ cells within the peripheral CD8+ T-lymphocyte population during primary SIV infection was correlated with a lower proportion of activated CD4+ T cells and a lower SIV antigen-specific proliferative response during the same period (Fig. 6). The peak number of CD8+ CD25+ FoxP3+ T cells was also negatively correlated (P = 0.015; r2 = 0.291) with CD4+ T-cell activation, as reflected by the proportion of CD4+ T lymphocytes expressing CD25, including all CD25+ CD4+ T cells regardless of whether they also expressed FoxP3 (Fig. 6A and C). The number of CD8+ CD25+ FoxP3+ T cells was not correlated with activation (proportion of CD25+ cells) within CD8+ T lymphocytes (Fig. 4 and data not shown). The number of CD8+ CD25+ FoxP3+ T cells during early infection (days 0 to 128 postinfection) was also negatively correlated (P = 0.029; r2 = 0.254) with SIV antigen-specific proliferation during the same time period (Fig. 6B and D). Interestingly, the most significant CD4+ T-cell activation and proliferative response to AT-2-inactivated SIV were observed in ART-treated macaques with low viral loads during primary infection.
FIG. 6.
Correlation between CD8+ CD25+ FoxP3+ T cells and immune activation. The proportions of CD3+ CD4+ lymphocytes that were CD25+ (A) and the proliferative response to AT-2-inactivated SIV (B) of individual macaques are shown. (Top) Six cynomolgus macaques inoculated intravenously with 5,000 AID50 (open symbols). (Middle) Six cynomolgus macaques inoculated intravenously with 50 AID50 (gray symbols). (Bottom) Six cynomolgus macaques inoculated intravenously with 50 AID50 and treated (AZT, 3TC, and indinavir) from 4 h postinfection to 28 days postinfection (black symbols). (C and D) Inverse correlation between the peak number of CD8+ CD25+ FoxP3+ T cells and the peak proportion of CD25+ cells among CD4+ T cells during primary infection (P = 0.017 by Spearman correlation; r2 = 0.291). Shown is the inverse correlation between the total numbers of CD8+ CD25+ FoxP3+ T cells during primary infection (days 0 to 128) (C) and the total SIV antigen-specific proliferative response, days 0 to 100, expressed as the area under the curve (AUC) (P = 0.029 by Spearman correlation; r2 = 0.254) (D).
Regulatory T cells in lymphoid tissue.
Peripheral lymph node biopsies were performed before infection and 15, 38, and 245 days postinfection. The percentages of T cells with regulatory phenotypes were determined by immunostaining and flow cytometry in lymph node cells using the same method as that used for PBMC. At these time points, no significant changes in the proportion of CD25+ FoxP3+ cells among CD4+ cells, CD8+ T cells, or total lymphocytes was observed (data not shown). Moreover, there was no increase of the absolute number of cells with a regulatory phenotype per gram of tissue (Fig. 7). Rather, in some macaques, there was a decrease in the absolute number of CD25+ FoxP3+ T cells in peripheral lymphoid tissue.
FIG. 7.
Numbers of regulatory T cells in lymphoid tissue. The numbers of CD4+ CD25+ FoxP3+ (A) and CD8+ CD25+ FoxP3+ (B) T cells per gram of lymphoid tissue of individual macaques are shown. (Top) Six cynomolgus macaques inoculated intravenously with 5,000 AID50 (open symbols). (Middle) Six cynomolgus macaques inoculated intravenously with 50 AID50 (gray symbols). (Bottom) Six cynomolgus macaques inoculated intravenously with 50 AID50 and treated (AZT, 3TC, and indinavir) from 4 h postinfection to 28 days postinfection (black symbols).
DISCUSSION
The early immune events during HIV/SIV infection determine the extent to which the initial burst of viremia is controlled, which in turn shapes the development of disease during chronic infection (39, 41, 44). Recent studies on HIV/SIV pathogenesis suggested that regulatory T cells play a role in determining the outcome of disease (1, 3, 4, 11, 21, 35, 50, 51, 63). However, little is known about their influence during primary infection. An understanding of the early immunological events in HIV/SIV infection that make a significant contribution to disease progression is crucial for the development of effective HIV vaccines and prophylaxis. We therefore investigated the kinetics and influence of regulatory T cells during primary and chronic SIVmac251 infection of cynomolgus macaques. The progression rate is variable in this model as in HIV infection in humans. We show here that the number of CD4+ CD25+ FoxP3+ regulatory T cells decreased in the periphery during both primary and chronic infection. In contrast, a significant induction of peripheral CD8+ CD25+ FoxP3+ T cells was observed to correlate with plasma viral load during primary infection. These cells had phenotypic features typical of regulatory T cells, and the transient expansion of this cell population in the blood during primary infection was correlated with the viral load at set point. The number of these cells was also negatively correlated with CD4+ T-cell activation and the SIV antigen-specific proliferative response during primary infection.
The immune-suppressive effect of regulatory T cells in HIV/SIV pathogenesis is two sided. Exhaustion of the immune system, with the aberrant expression of inflammatory cytokines, upregulation of inhibitory T-cell surface markers, and decrease in T-cell proliferative capacity, has recently been identified as making a major contribution to disease progression during chronic infection (18, 29, 52). In this sense, immune suppression by regulatory T cells would be beneficial for the host, and the loss of regulatory T cells during chronic HIV infection would favor chronic immune activation and exhaustion, thereby accelerating disease progression. In contrast, it is thought that the establishment of chronic infection and the preservation of viral reservoirs are favored by insufficient immune responses during primary infection (43). In this context, immune suppression by regulatory T cells would be deleterious for the host. It is possible that the suppressive effect of regulatory T cells on immune activation and T-cell proliferation has different consequences at different stages of the disease. During primary infection, immune suppression may be deleterious, suppressing the host adaptive response and favoring a high initial burst of viremia and the establishment of chronic infection. During chronic infection, the suppressive effect may be beneficial, attenuating chronic immune activation and the exhaustion of the immune system.
A decrease in peripheral blood CD4+ CD25+ FoxP3+ regulatory T cells was observed during primary and chronic SIV infection of cynomolgus macaques, consistent with data from previous reports of HIV-infected patients (3, 4, 21, 50) and SIV-infected rhesus macaques (51). A small number of CD4+ CD25+ FoxP3+ regulatory T cells was associated with high plasma viral loads during early and asymptomatic chronic infection. This is consistent with the loss of regulatory T cells during chronic HIV/SIV infection favoring disease progression. However, the CD4+ CD25+ FoxP3+ regulatory T cells expressed the phenotypic markers of central memory CD4+ T cells, and the decrease in CD4+ CD25+ FoxP3+ regulatory T-cell numbers paralleled that of central memory CD4+ T-cell counts, which also decreased during SIV infection and which were correlated with a high viral load, as previously described (38, 42, 59). The expression of CCR5, the main coreceptor for SIV (14), by both memory CD4+ T cells and CD4+ CD25+ FoxP3+ regulatory T cells makes these cells susceptible to infection, probably resulting in a decrease in their numbers in peripheral blood.
During primary SIV infection, a few days after the peak of viral load, we observed an induction of CD8+ CD25+ FoxP3+ T cells. These cells had the same phenotypic markers as their CD4+ counterparts and CD8+ regulatory T cells, as previously reported (10, 16, 33, 45, 49). The increase in the number of CD8+ CD25+ FoxP3+ T cells during primary infection was correlated with viral load and persisted in two of the three macaques with sustained high plasma viral loads (>105 copies per microliter). This suggests that CD8+ CD25+ FoxP3+ T cells are directly or indirectly induced by the presence of virus. Other studies also suggested that CD8+ regulatory T cells are induced during viral infections (36, 49), including chronic and advanced HIV infection (22, 30). However, FoxP3 expression was not assessed in those studies. Consistent with the hypothesis that regulatory T cells are deleterious for the host during primary infection, the number of CD8+ CD25+ FoxP3+ T cells during primary infection was correlated with viral load at set point, which is indicative of disease progression (44). Moreover, the number of CD8+ CD25+ FoxP3+ T cells during primary infection was inversely correlated with the proportion of activated CD4+ T cells and the SIV antigen-specific proliferative response during the same period. This may be a consequence of the destruction of CCR5+ CD4+ memory T cells by the virus or of the impaired proliferative capacity of CD4+ T cells in the presence of a high viral load (48). However, it may also reflect a deleterious suppressive effect of CD8+ CD25+ FoxP3+ T cells, decreasing the efficiency of the immune response during primary infection and favoring high levels of viral replication and the establishment of chronic infection.
We could not confirm the regulatory properties of CD8+ CD25+ FoxP3+ T cells in this study due to the low abundance of these cells and the transient nature of their increase in numbers in peripheral blood. We cannot exclude the possibility that FoxP3 is a marker of activation, as recently described (2). However, FoxP3 expression was not correlated with an increase in the number of activated CD8+ T cells during primary infection. Moreover, the peak induction of CD8+ CD25+ FoxP3+ T cells did not coincide with peak proliferation in CD8+ T cells during primary infection, as demonstrated by bromodeoxyuridine incorporation in previous experiments using the same model and the same infection conditions (8).
Many factors are involved in controlling viral replication and disease progression; regulatory T cells are only part of the total picture. Disease progression is influenced by various host factors such as HLA subtype (12), chemokine receptor levels (9), and cytokine and chemokine production (34) and viral factors such as viral phenotype (5, 15), fitness, and replication capacity (26). Moreover, CD8+ regulatory T cells are not the only immune-suppressive mediator induced during HIV/SIV infection. Indoleamine-2,3-dioxygenase (IDO) is an immunosuppressive enzyme that inhibits T-cell proliferation by breaking down the essential amino acid tryptophan in the kynurenin pathway. IDO is induced during acute (24) and chronic (3, 47) HIV/SIV infection. It has been shown that regulatory T cells can induce IDO (25) and that IDO can induce CD4+ regulatory T cells (17). Regulatory T cells and IDO are found together in lymphoid tissues during both acute and chronic HIV/SIV infection (3, 24, 47), suggesting a close interaction between these two mediators of immune suppression that potentially participate in the suppression of efficient antiviral responses. The induction of IDO during primary SIV infection may create an optimal environment for the induction of CD8+ CD25+ FoxP3+ T cells, as demonstrated in vitro for CD4+ CD25+ FoxP3+ T cells (17).
In this study of SIV-infected cynomolgus macaques, we observed no significant difference in the proportion of CD25+ FoxP3+ T cells in peripheral lymph nodes. An increase in the numbers of regulatory T cells in the lymphoid tissues of HIV patients displaying progression was reported in previous studies (3, 47). In those studies, regulatory T cells were monitored by evaluating FoxP3 mRNA levels, whereas we analyzed CD25+ FoxP3+ T cells by flow cytometry. Pereira et al. (51) also carried out a flow cytometry study and found that the proportion of regulatory T cells in lymphoid tissues was lower in the terminal stages of disease in SIV-infected macaques than in uninfected controls. Similarly, Chase et al. (13) did not observe an increase of FoxP3+ in CD4+ T cells in peripheral lymph nodes during progressive SIV infection in pigtailed macaques using mRNA expression and flow cytometry. Estes et al. (24) previously demonstrated an increase in the proportion of FoxP3+ T cells in lymphoid tissue during early SIV infection in rhesus macaques by immunohistochemical staining. Discordance in the changes in regulatory T-cell populations in lymphoid tissues during HIV/SIV infection may be due to differences in the quantification methods and models used and/or differences in disease stage.
In conclusion, the outcome of the immune response during primary and chronic HIV/SIV infection is the result of a complex interplay between host and viral factors and positive and negative immune stimulatory signals in which regulatory T cells probably play a role. A decrease in CD4+ regulatory T-cell numbers during chronic infection was found to be correlated with a worsening of the prognosis for disease progression, possibly due to chronic activation and exhaustion of the immune system. We also observed the induction of CD8+ T cells with a regulatory phenotype during primary infection. The induction of these cells was correlated with high viral loads and low levels of immune activation together with an SIV antigen-specific proliferative response, suggesting that these cells are involved in the suppression of efficient antiviral responses, favoring the establishment of chronic infection.
Acknowledgments
This work was supported by the French National AIDS Agency, the Agence Nationale de Recherche sur le SIDA et les Hépatites Virales (ANRS). B.M. and I.K. were both supported by grants from the ANRS. We also thank the Lennanders Foundation, the Swedish Royal Academy of Sciences, and the Faculty of Medicine of Lund University, Lund, Sweden.
Footnotes
Published ahead of print on 26 September 2007.
REFERENCES
- 1.Aandahl, E. M., J. Michaelsson, W. J. Moretto, F. M. Hecht, and D. F. Nixon. 2004. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J. Virol. 78:2454-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allan, S. E., S. Q. Crome, N. K. Crellin, L. Passerini, T. S. Steiner, R. Bacchetta, M. G. Roncarolo, and M. K. Levings. 2007. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int. Immunol. 19:345-354. [DOI] [PubMed] [Google Scholar]
- 3.Andersson, J., A. Boasso, J. Nilsson, R. Zhang, N. J. Shire, S. Lindback, G. M. Shearer, and C. A. Chougnet. 2005. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J. Immunol. 174:3143-3147. [DOI] [PubMed] [Google Scholar]
- 4.Apoil, P. A., B. Puissant, F. Roubinet, M. Abbal, P. Massip, and A. Blancher. 2005. FOXP3 mRNA levels are decreased in peripheral blood CD4+ lymphocytes from HIV-positive patients. J. Acquir. Immune Defic. Syndr. 39:381-385. [DOI] [PubMed] [Google Scholar]
- 5.Asjo, B., L. Morfeldt-Manson, J. Albert, G. Biberfeld, A. Karlsson, K. Lidman, and E. M. Fenyo. 1986. Replicative capacity of human immunodeficiency virus from patients with varying severity of HIV infection. Lancet ii:660-662. [PubMed] [Google Scholar]
- 6.Baecher-Allan, C., J. A. Brown, G. J. Freeman, and D. A. Hafler. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167:1245-1253. [DOI] [PubMed] [Google Scholar]
- 7.Belkaid, Y., and B. T. Rouse. 2005. Natural regulatory T cells in infectious disease. Nat. Immunol. 6:353-360. [DOI] [PubMed] [Google Scholar]
- 8.Benlhassan-Chahour, K., C. Penit, V. Dioszeghy, F. Vasseur, G. Janvier, Y. Riviere, N. Dereuddre-Bosquet, D. Dormont, R. Le Grand, and B. Vaslin. 2003. Kinetics of lymphocyte proliferation during primary immune response in macaques infected with pathogenic simian immunodeficiency virus SIVmac251: preliminary report of the effect of early antiviral therapy. J. Virol. 77:12479-12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 10.Bisikirska, B., J. Colgan, J. Luban, J. A. Bluestone, and K. C. Herold. 2005. TCR stimulation with modified anti-CD3 mAb expands CD8+ T cell population and induces CD8+CD25+ Tregs. J. Clin. Investig. 115:2904-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boasso, A., M. Vaccari, J. Nilsson, G. M. Shearer, J. Andersson, V. Cecchinato, C. Chougnet, and G. Franchini. 2006. Do regulatory T-cells play a role in AIDS pathogenesis? AIDS Rev. 8:141-147. [PubMed] [Google Scholar]
- 12.Carrington, M., and S. J. O'Brien. 2003. The influence of HLA genotype on AIDS. Annu. Rev. Med. 54:535-551. [DOI] [PubMed] [Google Scholar]
- 13.Chase, A. J., A. R. Sedaghat, J. R. German, L. Gama, M. C. Zink, J. E. Clements, and R. F. Siliciano. 12 September 2007. Severe depletion of CD4+ CD25+ regulatory T cells from the intestinal lamina propria but not peripheral blood or lymph nodes during acute simian immunodeficiency virus infection. J. Virol. doi: 10.1128/JVI.00841-07. [DOI] [PMC free article] [PubMed]
- 14.Chen, Z., P. Zhou, D. D. Ho, N. R. Landau, and P. A. Marx. 1997. Genetically divergent strains of simian immunodeficiency virus use CCR5 as a coreceptor for entry. J. Virol. 71:2705-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng-Mayer, C., D. Seto, M. Tateno, and J. A. Levy. 1988. Biologic features of HIV-1 that correlate with virulence in the host. Science 240:80-82. [DOI] [PubMed] [Google Scholar]
- 16.Cosmi, L., F. Liotta, E. Lazzeri, M. Francalanci, R. Angeli, B. Mazzinghi, V. Santarlasci, R. Manetti, V. Vanini, P. Romagnani, E. Maggi, S. Romagnani, and F. Annunziato. 2003. Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood 102:4107-4114. [DOI] [PubMed] [Google Scholar]
- 17.Curti, A., S. Pandolfi, B. Valzasina, M. Aluigi, A. Isidori, E. Ferri, V. Salvestrini, G. Bonanno, S. Rutella, I. Durelli, A. L. Horenstein, F. Fiore, M. Massaia, M. P. Colombo, M. Baccarani, and R. M. Lemoli. 2007. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25− into CD25+ T regulatory cells. Blood 109:2871-2877. [DOI] [PubMed] [Google Scholar]
- 18.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350-354. [DOI] [PubMed] [Google Scholar]
- 19.Dieckmann, D., H. Plottner, S. Berchtold, T. Berger, and G. Schuler. 2001. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J. Exp. Med. 193:1303-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dioszeghy, V., K. Benlhassan-Chahour, B. Delache, N. Dereuddre-Bosquet, C. Aubenque, G. Gras, R. Le Grand, and B. Vaslin. 2006. Changes in soluble factor-mediated CD8+ cell-derived antiviral activity in cynomolgus macaques infected with simian immunodeficiency virus SIVmac251: relationship to biological markers of progression. J. Virol. 80:236-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eggena, M. P., B. Barugahare, N. Jones, M. Okello, S. Mutalya, C. Kityo, P. Mugyenyi, and H. Cao. 2005. Depletion of regulatory T cells in HIV infection is associated with immune activation. J. Immunol. 174:4407-4414. [DOI] [PubMed] [Google Scholar]
- 22.Elrefaei, M., B. Barugahare, F. Ssali, P. Mugyenyi, and H. Cao. 2006. HIV-specific IL-10-positive CD8+ T cells are increased in advanced disease and are associated with decreased HIV-specific cytolysis. J. Immunol. 176:1274-1280. [DOI] [PubMed] [Google Scholar]
- 23.Elrefaei, M., F. L. Ventura, C. A. Baker, R. Clark, D. R. Bangsberg, and H. Cao. 2007. HIV-specific IL-10-positive CD8+ T cells suppress cytolysis and IL-2 production by CD8+ T cells. J. Immunol. 178:3265-3271. [DOI] [PubMed] [Google Scholar]
- 24.Estes, J. D., Q. Li, M. R. Reynolds, S. Wietgrefe, L. Duan, T. Schacker, L. J. Picker, D. I. Watkins, J. D. Lifson, C. Reilly, J. Carlis, and A. T. Haase. 2006. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J. Infect. Dis. 193:703-712. [DOI] [PubMed] [Google Scholar]
- 25.Fallarino, F., U. Grohmann, K. W. Hwang, C. Orabona, C. Vacca, R. Bianchi, M. L. Belladonna, M. C. Fioretti, M. L. Alegre, and P. Puccetti. 2003. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 4:1206-1212. [DOI] [PubMed] [Google Scholar]
- 26.Fenyo, E. M., L. Morfeldt-Manson, F. Chiodi, B. Lind, A. von Gegerfelt, J. Albert, E. Olausson, and B. Asjo. 1988. Distinct replicative and cytopathic characteristics of human immunodeficiency virus isolates. J. Virol. 62:4414-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filaci, G., M. Fravega, S. Negrini, F. Procopio, D. Fenoglio, M. Rizzi, S. Brenci, P. Contini, D. Olive, M. Ghio, M. Setti, R. S. Accolla, F. Puppo, and F. Indiveri. 2004. Nonantigen specific CD8+ T suppressor lymphocytes originate from CD8+CD28− T cells and inhibit both T-cell proliferation and CTL function. Hum. Immunol. 65:142-156. [DOI] [PubMed] [Google Scholar]
- 28.Fontenot, J. D., M. A. Gavin, and A. Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330-336. [DOI] [PubMed] [Google Scholar]
- 29.Freeman, G. J., E. J. Wherry, R. Ahmed, and A. H. Sharpe. 2006. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J. Exp. Med. 203:2223-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garba, M. L., C. D. Pilcher, A. L. Bingham, J. Eron, and J. A. Frelinger. 2002. HIV antigens can induce TGF-beta(1)-producing immunoregulatory CD8+ T cells. J. Immunol. 168:2247-2254. [DOI] [PubMed] [Google Scholar]
- 31.Hofmann-Lehmann, R., R. K. Swenerton, V. Liska, C. M. Leutenegger, H. Lutz, H. M. McClure, and R. M. Ruprecht. 2000. Sensitive and robust one-tube real-time reverse transcriptase-polymerase chain reaction to quantify SIV RNA load: comparison of one- versus two-enzyme systems. AIDS Res. Hum. Retrovir. 16:1247-1257. [DOI] [PubMed] [Google Scholar]
- 32.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057-1061. [DOI] [PubMed] [Google Scholar]
- 33.Jarvis, L. B., M. K. Matyszak, R. C. Duggleby, J. C. Goodall, F. C. Hall, and J. S. Gaston. 2005. Autoreactive human peripheral blood CD8+ T cells with a regulatory phenotype and function. Eur. J. Immunol. 35:2896-2908. [DOI] [PubMed] [Google Scholar]
- 34.Kedzierska, K., S. M. Crowe, S. Turville, and A. L. Cunningham. 2003. The influence of cytokines, chemokines and their receptors on HIV-1 replication in monocytes and macrophages. Rev. Med. Virol. 13:39-56. [DOI] [PubMed] [Google Scholar]
- 35.Kinter, A. L., M. Hennessey, A. Bell, S. Kern, Y. Lin, M. Daucher, M. Planta, M. McGlaughlin, R. Jackson, S. F. Ziegler, and A. S. Fauci. 2004. CD25(+)CD4(+) regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4(+) and CD8(+) HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J. Exp. Med. 200:331-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kornfeld, C., M. J. Ploquin, I. Pandrea, A. Faye, R. Onanga, C. Apetrei, V. Poaty-Mavoungou, P. Rouquet, J. Estaquier, L. Mortara, J. F. Desoutter, C. Butor, R. Le Grand, P. Roques, F. Simon, F. Barre-Sinoussi, O. M. Diop, and M. C. Muller-Trutwin. 2005. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J. Clin. Investig. 115:1082-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levings, M. K., R. Sangregorio, C. Sartirana, A. L. Moschin, M. Battaglia, P. C. Orban, and M. G. Roncarolo. 2002. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. J. Exp. Med. 196:1335-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148-1152. [DOI] [PubMed] [Google Scholar]
- 39.Lifson, J. D., M. A. Nowak, S. Goldstein, J. L. Rossio, A. Kinter, G. Vasquez, T. A. Wiltrout, C. Brown, D. Schneider, L. Wahl, A. L. Lloyd, J. Williams, W. R. Elkins, A. S. Fauci, and V. M. Hirsch. 1997. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J. Virol. 71:9508-9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu, W., A. L. Putnam, Z. Xu-Yu, G. L. Szot, M. R. Lee, S. Zhu, P. A. Gottlieb, P. Kapranov, T. R. Gingeras, B. Fazekas de St. Groth, C. Clayberger, D. M. Soper, S. F. Ziegler, and J. A. Bluestone. 2006. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 203:1701-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyles, R. H., A. Munoz, T. E. Yamashita, H. Bazmi, R. Detels, C. R. Rinaldo, J. B. Margolick, J. P. Phair, J. W. Mellors, et al. 2000. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. J. Infect. Dis. 181:872-880. [DOI] [PubMed] [Google Scholar]
- 42.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 43.McMichael, A. J., and S. L. Rowland-Jones. 2001. Cellular immune responses to HIV. Nature 410:980-987. [DOI] [PubMed] [Google Scholar]
- 44.Mellors, J. W., C. R. Rinaldo, Jr., P. Gupta, R. M. White, J. A. Todd, and L. A. Kingsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272:1167-1170. [DOI] [PubMed] [Google Scholar]
- 45.Meloni, F., M. Morosini, N. Solari, I. Passadore, C. Nascimbene, M. Novo, M. Ferrari, M. Cosentino, F. Marino, E. Pozzi, and A. M. Fietta. 2006. Foxp3 expressing CD4+ CD25+ and CD8+CD28− T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Hum. Immunol. 67:1-12. [DOI] [PubMed] [Google Scholar]
- 46.Neildez, O., R. Le Grand, A. Cheret, P. Caufour, B. Vaslin, F. Matheux, F. Theodoro, P. Roques, and D. Dormont. 1998. Variation in virological parameters and antibody responses in macaques after atraumatic vaginal exposure to a pathogenic primary isolate of SIVmac251. Res. Virol. 149:53-68. [DOI] [PubMed] [Google Scholar]
- 47.Nilsson, J., A. Boasso, P. A. Velilla, R. Zhang, M. Vaccari, G. Franchini, G. M. Shearer, J. Andersson, and C. Chougnet. 2006. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood 108:3808-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishimura, Y., T. Igarashi, A. Buckler-White, C. Buckler, H. Imamichi, R. M. Goeken, W. R. Lee, B. A. Lafont, R. Byrum, H. C. Lane, V. M. Hirsch, and M. A. Martin. 2007. Loss of naive cells accompanies memory CD4+ T-cell depletion during long-term progression to AIDS in simian immunodeficiency virus-infected macaques. J. Virol. 81:893-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nordstrom, I., M. Nurkkala, L. V. Collins, and K. Eriksson. 2005. CD8+ T-cells suppress antigen-specific and allogeneic CD4+ T-cell responses to herpes simplex virus type 2-infected human dendritic cells. Viral Immunol. 18:616-626. [DOI] [PubMed] [Google Scholar]
- 50.Oswald-Richter, K., S. M. Grill, N. Shariat, M. Leelawong, M. S. Sundrud, D. W. Haas, and D. Unutmaz. 2004. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2:E198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pereira, L. E., F. Villinger, N. Onlamoon, P. Bryan, A. Cardona, K. Pattanapanysat, K. Mori, S. Hagen, L. Picker, and A. A. Ansari. 2007. Simian immunodeficiency virus (SIV) infection influences the level and function of regulatory T cells in SIV-infected rhesus macaques but not SIV-infected sooty mangabeys. J. Virol. 81:4445-4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrovas, C., J. P. Casazza, J. M. Brenchley, D. A. Price, E. Gostick, W. C. Adams, M. L. Precopio, T. Schacker, M. Roederer, D. C. Douek, and R. A. Koup. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 203:2281-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pitcher, C. J., S. I. Hagen, J. M. Walker, R. Lum, B. L. Mitchell, V. C. Maino, M. K. Axthelm, and L. J. Picker. 2002. Development and homeostasis of T cell memory in rhesus macaque. J. Immunol. 168:29-43. [DOI] [PubMed] [Google Scholar]
- 54.Read, S., V. Malmstrom, and F. Powrie. 2000. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakaguchi, S., M. Ono, R. Setoguchi, H. Yagi, S. Hori, Z. Fehervari, J. Shimizu, T. Takahashi, and T. Nomura. 2006. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 212:8-27. [DOI] [PubMed] [Google Scholar]
- 56.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151-1164. [PubMed] [Google Scholar]
- 57.Scotto, L., A. J. Naiyer, S. Galluzzo, P. Rossi, J. S. Manavalan, S. Kim-Schulze, J. Fang, R. D. Favera, R. Cortesini, and N. Suciu-Foca. 2004. Overlap between molecular markers expressed by naturally occurring CD4+CD25+ regulatory T cells and antigen specific CD4+CD25+ and CD8+CD28− T suppressor cells. Hum. Immunol. 65:1297-1306. [DOI] [PubMed] [Google Scholar]
- 58.Seddiki, N., B. Santner-Nanan, J. Martinson, J. Zaunders, S. Sasson, A. Landay, M. Solomon, W. Selby, S. I. Alexander, R. Nanan, A. Kelleher, and B. F. de St. Groth. 2006. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 203:1693-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun, Y., S. R. Permar, A. P. Buzby, and N. L. Letvin. 2007. Memory CD4+ T lymphocyte loss and dysfunction during primary simian immunodeficiency virus infection. J. Virol. 81:8009-8015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takahashi, T., T. Tagami, S. Yamazaki, T. Uede, J. Shimizu, N. Sakaguchi, T. W. Mak, and S. Sakaguchi. 2000. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 192:303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uchiyama, T., S. Broder, and T. A. Waldmann. 1981. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac(+) cells. J. Immunol. 126:1393-1397. [PubMed] [Google Scholar]
- 62.Weiner, H. L. 2001. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol. Rev. 182:207-214. [DOI] [PubMed] [Google Scholar]
- 63.Weiss, L., V. Donkova-Petrini, L. Caccavelli, M. Balbo, C. Carbonneil, and Y. Levy. 2004. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood 104:3249-3256. [DOI] [PubMed] [Google Scholar]