Abstract
Ubiquitination of the human T-cell leukemia virus 1 Tax oncoprotein provides an important regulatory mechanism that promotes the Tax-mediated activation of NF-κB. However, the type of polyubiquitin chain linkages and the host factors that are required for Tax ubiquitination have not been identified. Here, we demonstrate that Tax polyubiquitin chains are composed predominantly of lysine 63-linked chains. Furthermore, the ubiquitination of Tax is critically dependent on the E2 ubiquitin-conjugating enzyme Ubc13. Tax interacts with Ubc13, and small interfering RNA-mediated knockdown of Ubc13 expression abrogates Tax ubiquitination and the activation of NF-κB. Mouse fibroblasts lacking Ubc13 exhibit impaired Tax activation of NF-κB despite normal tumor necrosis factor- and interleukin-1-mediated NF-κB activation. Finally, the interaction of Tax with NEMO is disrupted in the absence of Tax ubiquitination and Ubc13 expression, suggesting that Tax ubiquitination is critical for NEMO binding. Collectively, our results reveal that Ubc13 is essential for Tax ubiquitination, its interaction with NEMO, and Tax-mediated NF-κB activation.
Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia and a neuroinflammatory disease termed HTLV-1-associated myelopathy/tropical spastic paraparesis (16, 40, 60). HTLV-1 encodes a regulatory protein, Tax, that plays an essential role in regulating viral gene expression (59). Tax also functions as a potent oncogene by interacting with and modulating the function of components of signaling pathways and the cell cycle machinery (29, 36). One of the key signaling pathways targeted by Tax to facilitate cell transformation is NF-κB (21, 50). Tax mutants defective in NF-κB activation are unable to immortalize primary T cells (44). Furthermore, pharmacological inhibition of NF-κB in HTLV-1-transformed cell lines and leukemic cells from adult T-cell leukemia patients provokes an apoptotic response (37). Therefore, NF-κB is required for the Tax-mediated transformation of T cells and the survival of HTLV-1-transformed cells.
NF-κB is a family of transcription factors that play diverse roles in innate and adaptive immunity. NF-κB serves as a paradigm of how latent transcription factors in the cytoplasm can be rapidly activated and mobilized to the nucleus to activate target genes. In the canonical NF-κB pathway, the IκB family member, IκBα, interacts with NF-κB dimers and prevents nuclear translocation and DNA binding (45). In response to specific signals, such as inflammatory cytokines, or infection with pathogens, IκBα is phosphorylated by a multisubunit kinase complex, IκB kinase (IKK), consisting of two catalytic subunits, IKKα and IKKβ, and a regulatory subunit, NF-κB essential modulator (NEMO) or IKKγ (18, 26). The phosphorylation of IκBα by IKK triggers polyubiquitination and degradation by the 26S proteasome, thus allowing NF-κB to enter the nucleus. An important regulatory mechanism for NF-κB activation may be provided by lysine 63 (K63)-linked polyubiquitination of signaling proteins, such as tumor necrosis factor (TNF)-associated factor 6 (TRAF6) (1). TRAF6 functions as an E3 ligase and requires a heterodimeric ubiquitin-conjugating enzyme complex composed of Ubc13 and the Ubc13-related protein Uev1A (8). TRAF6 plays an essential role in interleukin-1 (IL-1), lipopolysaccharide, and CD40-mediated NF-κB activation (35). However, gene-targeting studies in mice have revealed that Ubc13 is dispensable for NF-κB activation, at least in fibroblasts and B lymphocytes (12, 56). Ubc13-deficient thymocytes displayed a modest reduction in NF-κB activation in response to T-cell receptor stimulation (57).
In the noncanonical pathway, a more restrictive set of signals provided by TNF receptor family members leads to the activation of the NF-κB-inducing kinase and IKKα, resulting in the limited degradation or processing of the p100 IκB family member to p52 (47, 55). The noncanonical pathway plays important roles in lymphoid organogenesis and B-cell maturation and survival (2, 7).
Tax is a potent activator of both the canonical and noncanonical NF-κB pathways. Tax activates the IKK complex by directly interacting with NEMO, an event that is central to the activation of both canonical and noncanonical pathways by Tax (23, 30). Although the exact mechanism by which Tax activates IKK remains unclear, Tax may recruit upstream kinases, such as transforming growth factor β-activated kinase 1 (15, 51, 52). In addition to providing an intracellular stimulus for IKK activation, Tax also opposes IKK-negative regulatory mechanisms, such as that exerted by the phosphatase PP2A (11). Recent studies have also demonstrated that the Tax activation of NF-κB is regulated by Tax mono- and polyubiquitination (6, 25, 34, 39, 41). Mutation of the lysine ubiquitin acceptor sites within Tax renders Tax unable to activate both canonical and noncanonical NF-κB pathways (25). Although the exact linkages of the Tax polyubiquitin chains have not been established, Tax ubiquitination does not promote the destabilization of Tax, suggesting that it plays a regulatory role.
In this paper, we have identified Tax polyubiquitin chains as predominantly K63-linked. The E2 ubiquitin-conjugating enzyme Ubc13 was found to be required for the ubiquitination of Tax. Interestingly, Tax interacted with Ubc13 in transfected cells and HTLV-1-transformed cell lines. NF-κB activation was disrupted by small interfering RNA (siRNA)-mediated knockdown of Ubc13 in Tax-expressing cells and in an HTLV-1-transformed cell line. Consistent with these results, Tax was deficient in NF-κB activation in Ubc13 knockout fibroblasts, despite normal TNF-α and IL-1-mediated activation of NF-κB in these cells. Finally, in the absence of Ubc13, Tax interaction with NEMO was abrogated, indicating that Tax ubiquitination is essential for NEMO interaction and NF-κB activation.
MATERIALS AND METHODS
Biological reagents and antibodies.
Jurkat E6-1 and 293T cells were obtained from ATCC (Manassas, VA). The HTLV-1-transformed cell line C8166 was described previously (25). Jurkat and C8166 cells were cultured in RPMI medium (Mediatech, Inc., Herndon, VA) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA). 293T cells and mouse embryonic fibroblasts (MEFs) were cultured in Dulbecco's modified Eagle's medium (Mediatech, Inc.) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. The monoclonal anti-Tax antibody was prepared from a Tax hybridoma (168B17-46-34) that was obtained from the AIDS Research and Reference Program, NIAID, National Institutes of Health. The phospho-IκBα antibody (14D4) was obtained from Cell Signaling Technology (Beverly, MA). The monoclonal p100 antibody was purchased from Upstate/Millipore (Charlottesville, VA). Normal rabbit immunoglobulin G, anti-IκBα (C-21), and NEMO (FL-419) antibodies were purchased from Santa Cruz (Santa Cruz, CA). The anti-Ubc13 antibody (clone 4E11) was obtained from Invitrogen/Zymed. Anti-hemagglutinin (HA) antibody (clone 12CA5) was purchased from Roche (Indianapolis, IN). Anti-ubiquitin antibody was purchased from Stressgen/Assay Designs (San Diego, CA). The β-actin antibody was purchased from Abcam (Cambridge, MA). Recombinant TNF-α and IL-1β were purchased from R&D Systems (Minneapolis, MN). Control siRNA (siControl nontargeting siRNA no. 1) and siGenome SMARTpool Ubc13 siRNA were purchased from Dharmacon (Lafayette, CO).
Plasmids.
pCMV4-Tax, pCLXSN-Tax, pCMV4-p100, HA-ubiquitin (HA-Ub) and HA-NEMO have all been described previously (23, 25, 53). Flag-TRAF6 was provided by Khaled Tolba. HA-Ubc13 and HA-Ubc13 C87A were kindly provided by James Chen (8). The pSG-5 Tax wild type and Tax K10R plasmids were gifts from Claudine Pique (6). The retroviral vector expressing Cre (pMRXP-Cre) has been previously described (56). HA-Ub K63-only and HA-Ub K48-only were constructed by replacing all lysines in HA-Ub with arginines, except for lysine 48 (K48-only) and lysine 63 (K63-only), using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). All mutations were confirmed by DNA sequencing. pSuppressor Retro-GFP siRNA and pSuppressor Retro-Ubc13 siRNA plasmids were generated by cloning siRNA sequences specific for green fluorescent protein (GFP) and Ubc13 into the Xho and XbaI sites in pSuppressor Retro (Imgenex, San Diego, CA).
Transfections and luciferase assays.
Transfections in 293T cells were performed with FuGENE 6 (Roche) according to the manufacturer's instructions. For siRNA transfections, cells were transfected with 60 pmol of control or Ubc13 siRNA using Lipofectamine 2000 (Invitrogen). Plasmid DNAs were transfected using FuGENE 6 the next day after siRNA transfections. Cells were harvested 4 days after the siRNA transfection. Luciferase assays were performed by using a dual luciferase assay kit (Promega, Madison, WI). All luciferase transfections included the Renilla luciferase reporter pRL-tk to normalize for transfection efficiency. Transfections for luciferase assays were performed in triplicate, and error bars represent the standard errors of the means. Transient transfections in MEFs were performed using FuGENE HD (Roche) according to the manufacturer's instructions.
EMSA.
The NF-κB electrophoretic mobility shift assay (EMSA) was done as described previously (22, 48). The Oct-1 EMSA probe was generated by annealing the following oligonucleotides: forward 5′-TGTCGAATGCAAATCACTAGAA and reverse 5′-TTCTAGTGAT. The annealed oligonucleotides were labeled with [32P]dTTP in a fill-in reaction with Klenow fragment (Promega). Nuclear extract (4 μg) was incubated with buffer containing 1 mM dithiothreitol, 1 μg poly(dI-dC), dialysis buffer (25 mM HEPES, pH 7.9, 10% glycerol, 100 mM KCl, and 0.1 mM EDTA), and 32P-labeled probe for 15 min. The reaction was terminated by the addition of 5× loading dye, and the reaction mixture was run on 5% polyacrylamide gels in 0.25× Tris-borate-EDTA buffer, dried under vacuum, and subjected to autoradiography.
Generation of Ubc13−/− MEFs.
Primary MEFs from control and Ubc13fl/fl embryos were immortalized with simian virus 40 large T antigen. T-antigen expression was confirmed by reverse transcription (RT)-PCR. Control and Ubc13fl/fl immortalized MEFs were infected with Cre-expressing recombinant retroviruses as described below.
Retroviral infections.
Retrovirus-mediated transfer of Cre into MEFs was performed by transfecting pMRXP-Cre into the Plat-E packaging cell line (38). For all other retroviral infections, retroviral vectors (pCLXSN, pCLXSN-Tax, pSuppressor Retro-GFP siRNA, and pSuppressor Retro-Ubc13 siRNA) were transfected into 293T cells together with pCL-Ampho and vesicular stomatitis virus glycoprotein. The supernatants were filtered and used to infect MEFs or Jurkat or C8166 cells in the presence of Polybrene (8 μg/ml). Jurkat and C8166 cells were centrifuged at 1,800 rpm for 45 min after resuspension in viral supernatant, to increase the infection efficiency. After 72 h, cells were selected in medium containing G418 (Invitrogen) to obtain stable, bulk cell lines. Stable Jurkat and C8166 cell lines were selected with 1 μg/ml G418. Stable MEFs were selected with 0.4 μg/ml G418.
RT-PCR.
RT-PCR was done as described previously (24). Total RNA was obtained from cells by using an RNeasy kit (QIAGEN, Valencia, CA) and converted to cDNA by using a first-strand cDNA synthesis kit (Roche). The following sets of primers were used to amplify gene products for PCRs: glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (263 bp) forward-5′-CCACAGTCCATGCCATCAC and reverse-5′-GCTTCACCACCTTCTTGATG; Tax (429 bp) forward-5′-CGGATACCCAGTCTACGTC and reverse-5′-GAGGTACATGCAGACAACGG; IL-2 (470 bp) forward-5′-GTACAGGATGCAACTCCTGTC and reverse-5′-CAAGTTAGTGTTGAGATGATGC; IL-2Rα (818 bp) forward-5′-GATGGATTCATACCTGCTGATG and reverse-5′-CTAGATTGTTCTTCTACTCTTCC; IL-6 (460 bp) forward-5′-GACTTCACAGAGGATACCACTC and reverse-5′-GTCCTTAGCCACTCCTTCTG; and A20 (560 bp) forward-5′-GACAGAAGTGTCCAGGCTTC and reverse-5′-GTGCTGGCTGTCATAGCCTAG.
Coimmunoprecipitation and ubiquitination assays.
Ubiquitination assays were performed essentially as described previously (55). In 293T cells, ubiquitination assays were performed by transfecting pCMV4-Tax and Flag-TRAF6 together with HA-Ub, HA-Ub K63-only, or HA-Ub K48-only plasmids. Approximately 40 h after transfection, cells were lysed in radioimmunoprecipitation assay (RIPA) buffer and subjected to immunoprecipitation with anti-Tax or anti-FLAG monoclonal antibodies. The agarose beads were washed three times with RIPA buffer, followed by an additional wash with RIPA buffer containing 1 M urea. Proteins were eluted from the beads, subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and detected by immunoblotting with anti-HA monoclonal antibody. For Tax ubiquitination assays in MEFs, Tax was immunoprecipitated with anti-Tax and immunoblotted with an antiubiquitin antibody.
Western blotting.
Western blotting was done as described previously (22). Cells were lysed in RIPA buffer, and the lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, blocked in 5% milk, incubated with appropriate primary and secondary antibodies, and detected by Western Lightning enhanced chemiluminescence reagent (Perkin Elmer, Boston, MA).
RESULTS
Tax polyubiquitination is predominantly K63-linked.
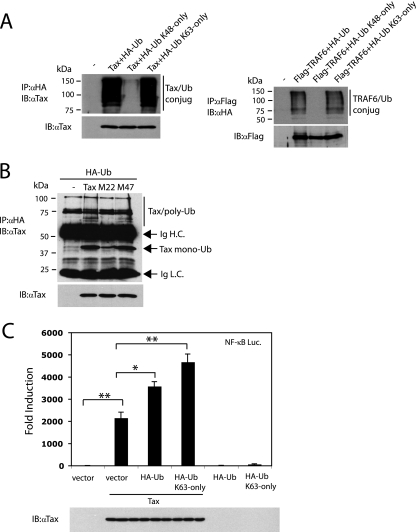
Although several reports have documented Tax ubiquitination, the exact biochemical nature of the types of ubiquitin linkages has remained elusive. Ubiquitin molecules harbor 7 lysine residues, each of which may mediate polyubiquitin linkages; however, the best characterized are K48- and K63-linked polyubiquitin chains. Polyubiquitin chains linked to one another via K48 target proteins for degradation by the proteasome (4). In contrast, the K63-linked polyubiquitin chains do not target proteins for degradation but rather modulate the subcellular locations or signaling functions of the ubiquitinated proteins (5, 19). Thus, we performed Tax ubiquitination assays with either wild-type ubiquitin, a ubiquitin variant with all of the lysines except for lysine 63 mutated to arginine (K63-only), or a ubiquitin variant with all of the lysines except for lysine 48 mutated to arginine (K48-only). We transfected 293T cells with a Tax expression plasmid together with each of the HA-tagged ubiquitin constructs and performed ubiquitination assays. As a control for K63 ubiquitination, we also transfected Flag-TRAF6 with each of the ubiquitin constructs. In agreement with the results of published studies (6, 34, 39, 41), polyubiquitination of Tax was readily detected in cells expressing the wild-type ubiquitin (Fig. 1A). More importantly, Tax ubiquitination also occurred efficiently in cells expressing K63-only ubiquitin, but poorly in cells expressing the K48-only ubiquitin (Fig. 1A). As expected, TRAF6 was also ubiquitinated, and the polyubiquitin chains were mainly K63-linked (Fig. 1A). These results suggest that Tax ubiquitination is predominantly composed of K63-linked polyubiquitin chains, although we cannot rule out polyubiquitin chains linked via lysines independent of K48 and K63.
FIG. 1.
Tax polyubiquitination is K63-linked. (A) 293T cells were transfected with 2 μg pCMV4-Tax or 1 μg Flag-TRAF6 together with 0.5 μg of HA-Ub, HA-Ub K48-only, or HA-Ub K63-only. After 36 h, cells were lysed and immunoprecipitated (IP) with either anti-Tax or anti-Flag antibodies, followed by immunoblotting (IB) with anti-HA. Lysates were examined for Tax and TRAF6 expression by immunoblotting with anti-Tax and anti-Flag, respectively. −, pCMV4 only. (B) 293T cells were transfected with pCMV4, 0.5 μg of HA-Ub together with 2 μg of pCMV4-Tax, pCMV4-Tax M22, or pCMV4-Tax M47. After 36 h, cells were lysed and immunoprecipitated with anti-HA, followed by immunoblotting with anti-Tax. Ig H.C., immunoglobulin heavy chain; Ig L.C., immunoglobulin light chain; −, pCMV4 only. (C) 293T cells were transfected with κB-TATA luciferase (0.1 μg), pRL-tk (0.01 μg), and pCMV4-Tax (1 μg), HA-Ub (0.25 μg), or HA-Ub K63-only (0.25 μg) as indicated. Cells were harvested after 36 h and dual luciferase assays were performed. NF-κB induction (n-fold) for each sample compared to vector alone was calculated. Statistical analysis was performed by one-way analysis of variance, followed by the Tukey-Kramer test for multiple comparisons (*, P < 0.01; **, P < 0.001). Tax expression in lysates was determined by immunoblotting with anti-Tax. Molecular sizes are shown to the left of the panels. Luc., luciferase; α, anti.
To confirm that the polyubiquitinated Tax was indeed Tax and not an associated protein, we performed a reciprocal immunoprecipitation in which HA-Ub was immunoprecipitated, followed by immunoblotting with Tax antibody. 293T cells were transfected with HA-Ub together with wild-type Tax, Tax M22 (a point mutant deficient in NF-κB activation), and Tax M47 (a point mutant deficient in CREB activation) (49). Monoubiquitinated forms of Tax, Tax M22, and Tax M47 were detected, although lower levels of monoubiquitinated Tax M22 were observed (Fig. 1B). A ladder of bands representing Tax and Tax M47 ubiquitin adducts were also detected; however, polyubiquitinated M22 was not observed, in agreement with the results of another study (Fig. 1B) (6). Therefore, these results indicate that Tax is polyubiquitinated and that Tax M22 displays a defect in polyubiquitination.
Next, we examined a potential functional role for K63-linked ubiquitin chains in Tax-mediated NF-κB activation. 293T cells were transfected with Tax in combination with either the HA-Ub or HA-Ub K63-only plasmids, and NF-κB luciferase assays were performed. As expected, Tax expression resulted in potent activation of the NF-κB luciferase reporter (Fig. 1C). Transfection of HA-Ub and HA-Ub K63-only plasmids led to significant increases in Tax-mediated NF-κB activation, although they had little effect on NF-κB activation in the absence of Tax (Fig. 1C). Immunoblotting confirmed that Tax was expressed at similar levels in transfected cells (Fig. 1C). Thus, ubiquitination, and specifically K63-linked polyubiquitination, strongly promotes the Tax activation of NF-κB.
The E2 ubiquitin-conjugating enzyme Ubc13 is required for Tax ubiquitination.
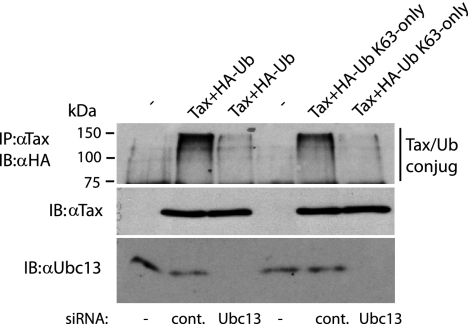
Ubiquitination is a reversible covalent modification that occurs on lysine residues in target proteins. Ubiquitination occurs in an ordered three-step process that is dependent on a ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzymes (E2), and ubiquitin ligases (E3) that provide specificity for ubiquitination (27). A number of E3 ligases, including TRAF6, RNF5, RNF8, and CHFR (3, 9, 33, 43), have been identified that require Ubc13 to catalyze the formation of K63-linked polyubiquitin chains. Because Tax ubiquitination was predominantly K63-linked, we examined the role of Ubc13 in Tax ubiquitination. We performed Tax ubiquitination assays in the presence of control or Ubc13-specific siRNA. As expected, Tax was polyubiquitinated in the presence of HA-Ub or HA-Ub K63-only and control siRNA (Fig. 2). However, knockdown of Ubc13 with siRNA led to a significant reduction in Tax ubiquitination (Fig. 2) suggesting that Ubc13 plays an important role in Tax ubiquitination.
FIG. 2.
Tax ubiquitination is dependent on Ubc13. 293T cells were transfected with control siRNA or Ubc13 siRNA. The next day, the same cells were transfected with vector alone (first and fourth lanes) or pCMV4-Tax (2 μg) together with either 0.5 μg of HA-Ub or HA-Ub K63-only. After 36 h, cells were lysed and immunoprecipitated (IP) with anti-Tax, followed by immunoblotting (IB) with anti-HA. Lysates were examined for Tax and Ubc13 expression. Molecular sizes are shown to the left of the panel. α, anti; cont., control; −, pCMV4 only.
Tax interacts with Ubc13.
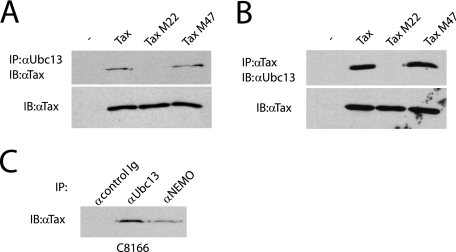
Since Ubc13 was required for Tax ubiquitination, we next examined if Tax interacted with Ubc13. 293T cells were transfected with wild-type Tax, Tax M22, and Tax M47. Cells expressing Tax and Tax mutants were then subjected to a coimmunoprecipitation assay for interaction with endogenous Ubc13. Surprisingly, wild-type Tax and Tax M47, but not Tax M22, interacted with Ubc13 (Fig. 3A). We also performed the reciprocal immunoprecipitation where Tax antibody was used for the immunoprecipitation, followed by immunoblotting with anti-Ubc13. Again, Tax and Tax M47, but not Tax M22, were found to interact with endogenous Ubc13 (Fig. 3B). The lack of binding of Tax M22 to Ubc13 may explain why M22 exhibits a defect in polyubiquitination (Fig. 1). Tax M22 was also previously shown to be defective for binding to NEMO (23). The interaction of Tax and Ubc13 was also confirmed in the HTLV-1-transformed cell lines C8166 (Fig. 3C) and MT-2 (data not shown). Given that E2 enzymes mainly interact with their cognate E3 ligases, these results indicate that Tax is likely tightly bound to an E3 ligase complex of which Ubc13 is a component.
FIG. 3.
Tax interacts with Ubc13. (A) 293T cells were transfected with 2 μg vector (first lane), pCMV4-Tax, pCMV4-Tax M22, or pCMV4-Tax M47. After 36 h, cells were lysed and immunoprecipitated (IP) with anti-Ubc13, followed by immunoblotting (IB) with anti-Tax. Lysates were examined for Tax expression. (B) 293T cells were transfected as described for panel A. Lysates were immunoprecipitated with anti-Tax, followed by immunoblotting with anti-Ubc13. (C) C8166 cells (1 × 107) were lysed and immunoprecipitated with control immunoglobulin (Ig), anti-Ubc13, or anti-NEMO, followed by immunoblotting with anti-Tax. α, anti; −, pCMV4 only.
Ubc13 is required for Tax activation of NF-κB.
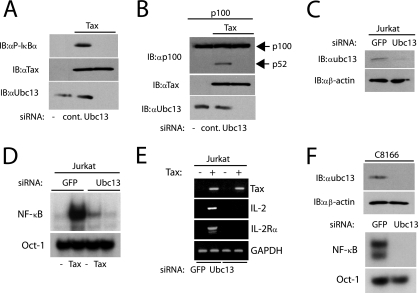
Prior studies have relied on Tax point mutants to establish a link between Tax ubiquitination and NF-κB activation. Since modification of Tax by mutation or deletion may potentially impair Tax function nonspecifically, it was important to confirm the role of Tax ubiquitination in NF-κB activation without manipulating Tax sequences. Therefore, we used siRNA to knock down Ubc13 expression and examined Tax activation of both the canonical and noncanonical NF-κB pathways. 293T cells were transfected with Tax together with control scrambled siRNA or Ubc13 siRNA. Knockdown of Ubc13 expression by siRNA resulted in defective NF-κB activation by Tax as assessed by phosphorylation of IκBα (Fig. 4A) and NF-κB DNA binding (data not shown). It has been previously shown that Tax is a potent activator of p100 processing to p52 in the noncanonical NF-κB pathway (53). Therefore, we examined if Ubc13 was required for Tax-mediated p100 processing. Indeed, knockdown of Ubc13 completely impaired the ability of Tax to induce p100 processing (Fig. 4B). Thus, Ubc13 is essential for Tax activation of both the canonical and noncanonical NF-κB pathway.
FIG. 4.
Tax requires Ubc13 for NF-κB activation. (A) 293T cells were transfected with control siRNA or Ubc13 siRNA. The next day, the same cells were transfected with 2 μg of vector (first lane) or pCMV4-Tax as indicated above the panel. After 36 h, cells were lysed and lysates were subjected to immunoblotting (IB) with anti-phospho-IκBα, anti-Tax, and anti-Ubc13. (B) 293T cells were transfected with control or Ubc13 siRNA. The next day, the same cells were transfected with pCMV4-p100 (0.25 μg) and pCMV4-Tax (2 μg) as indicated above the panel. After 36 h, lysates were immunoblotted with anti-p100, anti-Tax, and anti-Ubc13. (C) Jurkat GFP siRNA and Jurkat Ubc13 siRNA stable cell lines were lysed and subjected to immunoblotting with anti-Ubc13 and anti-β-actin. (D) Nuclear extracts from Jurkat GFP siRNA and Jurkat Ubc13 siRNA stable cell lines infected with pCLXSN (first and third lanes) or pCLXSN-Tax were subjected to NF-κB and Oct-1 EMSA. (E) RNA from Jurkat GFP siRNA and Jurkat Ubc13 siRNA stable cell lines infected with pCLXSN (first and third lanes) or pCLXSN-Tax (second and fourth lanes) was used for RT-PCR to examine mRNA levels of Tax, IL-2, IL-2Rα, and GAPDH. +, present; −, absent. (F) C8166 GFP siRNA and C8166 Ubc13 siRNA stable cell lines were lysed and subjected to immunoblotting with anti-Ubc13 and anti-β-actin (top panels). Nuclear extracts were used for NF-κB and Oct-1 EMSA (lower panels). α, anti; cont., control; −, pCMV4 only.
Since T lymphocytes are the natural target of HTLV-1 infection in vivo, we extended our studies to include T cells. To knock down Ubc13 expression in T cells, we infected Jurkat T cells with retroviral vectors expressing a control GFP siRNA or Ubc13 siRNA. Stable, bulk cell lines were derived by selection in medium containing G418. The absence of Ubc13 in the Ubc13 siRNA-expressing cell line was confirmed by immunoblotting (Fig. 4C). The tax gene was introduced into these cells by retroviral gene transfer to determine the effect of Ubc13 knockdown on Tax activation of NF-κB in T cells. Importantly, Tax was expressed in both control and Ubc13 knockdown Jurkat cells as determined by RT-PCR (Fig. 4E). Jurkat cells lacking Ubc13 expression were impaired in Tax-mediated NF-κB DNA binding (Fig. 4D). A control Oct-1 EMSA demonstrated similar DNA binding in all of the nuclear extracts (Fig. 4D). Thus, Ubc13 is required for Tax-mediated NF-κB activation in T cells. Moreover, the induction by Tax of T-cell-specific NF-κB-dependent target genes, including those for IL-2 and IL-2Rα, was abolished in the absence of Ubc13 expression (Fig. 4E). To determine the role of Ubc13 in NF-κB activation in a Tax-expressing HTLV-1-transformed cell line, we infected C8166 cells with retroviral vectors expressing control GFP or Ubc13 siRNA. Ubc13 knockdown in C8166 cells was confirmed by immunoblotting (Fig. 4F). As expected, constitutive NF-κB DNA binding was observed in C8166 GFP siRNA control cells; however, siRNA-mediated knockdown of Ubc13 in C8166 cells abolished NF-κB DNA binding (Fig. 4F). Oct-1 DNA binding was similar in both nuclear extracts (Fig. 4F). Ubc13 is therefore required for NF-κB activation in the context of a Tax-expressing HTLV-1-transformed cell line.
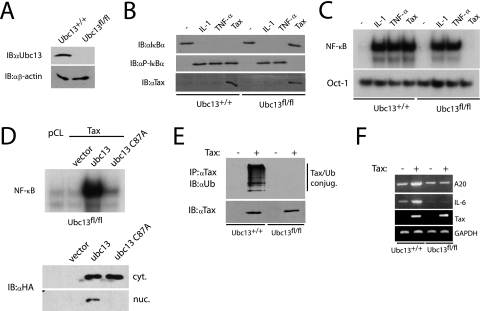
We next examined Tax activation of NF-κB in Ubc13-deficient MEFs. Primary MEFs were obtained from Ubc13+/+ and Ubc13fl/fl embryos engineered with loxP sites flanking exons 2 and 4 (56). Retrovirus-mediated delivery of Cre recombinase in Ubc13+/+ and Ubc13fl/fl effectively eliminated Ubc13 expression only in Ubc13fl/fl MEFs (Fig. 5A). Consistent with the results of previous studies, IL-1 and TNF-α treatment led to IκBα phosphorylation and degradation (Fig. 5B) and concomitant NF-κB DNA binding in Ubc13fl/fl MEFs (Fig. 5C) (12, 56). However, Tax was completely impaired in IκBα phosphorylation and degradation (Fig. 5B) and NF-κB DNA binding in Ubc13fl/fl MEFs (Fig. 5C). Reconstitution of Ubc13-deficient MEFs with wild-type Ubc13, but not a Ubc13 mutant (Ubc13 C87A) harboring a Cys→Ala mutation in its active site, rescued Tax-mediated NF-κB activation, as shown by an NF-κB EMSA (Fig. 5D). The ubiquitination of Tax was also impaired in Ubc13 knockout MEFs (Fig. 5E), consistent with a role for Tax ubiquitination regulating NF-κB activation. Finally, Tax induction of NF-κB-dependent target genes was examined by RT-PCR. Tax-mediated induction of A20 and IL-6 was defective in Ubc13fl/fl MEFs (Fig. 5F). Therefore, in MEFs lacking Ubc13, Tax is completely impaired in the ubiquitination and activation of NF-κB.
FIG. 5.
Tax-mediated NF-κB activation is impaired in Ubc13−/− MEFs. (A) Ubc13+/+ and Ubc13fl/fl MEFs infected with Cre-expressing retroviruses were lysed and subjected to immunoblotting (IB) with anti-Ubc13 and anti-β-actin. (B and C) Ubc13+/+ and Ubc13fl/fl MEFs expressing Cre were either stimulated with IL-1 (10 ng/ml) or TNF-α (20 ng/ml) for 30 min or infected with Tax-expressing retroviruses. −, no stimulation. (B) Lysates were subjected to immunoblotting with anti-IκBα, anti-phospho-IκBα and anti-Tax. (C) Nuclear extracts were used for NF-κB and Oct-1 EMSA. (D) Ubc13fl/fl MEFs expressing Tax were transiently transfected with pCMV4, HA-Ubc13, or HA-Ubc13 C87A. After 36 h, nuclear extracts were subjected to an NF-κB EMSA. The expression of ectopic Ubc13 was determined in cytoplasmic (cyt.) and nuclear (nuc.) extracts by immunoblotting with anti-HA. (E) Ubc13+/+ and Ubc13fl/fl MEFs expressing Cre were infected with retroviruses expressing Tax (+) or empty vector (−). After 72 h, cells were lysed and immunoprecipitated with anti-Tax, followed by immunoblotting with anti-ubiquitin. Lysates were examined for Tax expression. (F) Ubc13+/+ and Ubc13fl/fl MEFs expressing Cre were infected with Tax as described for panel D. After 72 h, RNA was harvested and subjected to RT-PCR analysis to examine the expression of A20, IL-6, Tax, and GAPDH. α, anti.
Ubc13 is required for Tax interaction with NEMO.
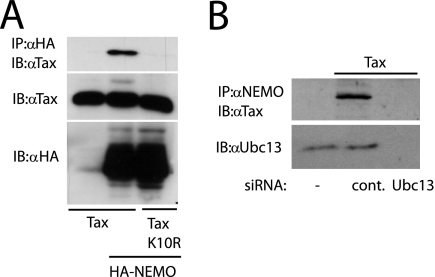
To further explore the mechanistic link between Tax ubiquitination and NF-κB activation, we examined the binding of Tax and NEMO in the absence of Ubc13 expression. First, we examined the binding of NEMO with a Tax ubiquitination-defective mutant that has all 10 lysine residues mutated to arginines (Tax K10R) (6). Tax interaction with NEMO was observed with wild-type Tax, but not the Tax K10R mutant (Fig. 6A). Tax binding to NEMO was also examined in the absence of Ubc13 expression. Tax interaction with endogenous NEMO occurred in the presence of control siRNA, but not Ubc13 siRNA (Fig. 6B). Therefore, Tax requires Ubc13 for interaction with NEMO.
FIG. 6.
Tax requires Ubc13 to interact with NEMO. (A) 293T cells were transfected with 2 μg of pSG-5-Tax or pSG-5-Tax K10R and HA-NEMO (50 ng). After 36 h, cells were lysed and subjected to immunoprecipitations with anti-HA followed by immunoblotting with anti-Tax. Lysates were examined for Tax and NEMO expression with anti-Tax and anti-HA, respectively. (B) 293T cells were transfected with control (cont.) or Ubc13 siRNA. The next day, the same cells were transfected with pCMV4-Tax (2 μg) or empty vector (first lane). After 36 h, cells were lysed and immunoprecipitated with anti-NEMO, followed by immunoblotting with anti-Tax. Lysates were examined for Ubc13 expression. α, anti; −, absent.
DISCUSSION
The HTLV-1 Tax oncoprotein is a potent intracellular activator of NF-κB by promotion of the constitutive activation of IKK. The stable ubiquitination of Tax has emerged as an important regulator of Tax-mediated NF-κB activation (39). However, previous studies have relied on Tax point mutants to demonstrate the importance of ubiquitination in NF-κB activation. In this study, we have determined that Tax polyubiquitin chains are primarily K63 linked and that the E2 ubiquitin-conjugating enzyme Ubc13 is essential for Tax ubiquitination. Tax interacts with Ubc13 both in transfected cells and in HTLV-1-transformed cell lines. Moreover, Tax requires Ubc13 for the activation of NF-κB and the induction of NF-κB-dependent target genes, including those for IL-2 and its high-affinity receptor IL-2Rα in T cells. Finally, in the absence of Ubc13, Tax is impaired in NEMO binding, suggesting that the role of Tax ubiquitination is to mediate protein-protein interactions.
The findings from this study suggest that Ubc13 is essential for the activation of NF-κB by Tax, but not by IL-1 or TNF-α stimulation. Ubc13 has previously been shown to be dispensable for IL-1- and TNF-α-mediated NF-κB activation in MEFs (56). Furthermore, the processing of p100 to p52 was normal in Ubc13-deficient B cells stimulated with α-CD40 or B-cell-activating factor of the TNF family (56). Thus, the mechanisms used by Tax to activate NF-κB are clearly different from the IL-1R and TNF receptor signaling pathways, although NEMO is required for NF-κB activation by Tax and cytokine stimulation (20, 58). In Tax-mediated NF-κB activation, the role of Ubc13 is to facilitate Tax K63-linked polyubiquitination (Fig. 2 and 5), which in turn mediates the interaction with NEMO (Fig. 6). The exact mechanism by which Tax stimulates IKK activity is poorly understood but may involve upstream kinases such as transforming growth factor β-activated kinase 1 (52). Interestingly, the Tax M22 mutant defective for NF-κB activation is unable to interact with either NEMO (23) or Ubc13 (Fig. 3), suggesting that an association of Tax with NEMO and/or Ubc13 is critical for NF-κB activation. Since the fusion of Tax M22 with NEMO restores NF-κB activation (54), it will be interesting to determine if the fusion of Ubc13 to Tax M22 will similarly activate NF-κB.
Our results are not in agreement with those of a recent study demonstrating that Tax activation of NF-κB is independent of Ubc13 (15). However, the conclusions by Gohda et al. were derived from a single experiment, an NF-κB luciferase assay with siRNA-mediated knockdown of Ubc13 in 293T cells (15). It is unclear to us why our results are inconsistent with the findings by Gohda et al. Our findings in four different cell types, 293T, Ubc13−/− MEFs, Jurkat, and C8166 cells, using a variety of different assays, consistently support a role for Ubc13 in Tax-mediated NF-κB activation. Furthermore, we have provided genetic evidence that Tax requires Ubc13 for the induction of NF-κB-dependent genes, such as those for IL-6 and IL-2. Consistent with the results of our studies, Kfoury et al. recently demonstrated that Tax undergoes K63-linked polyubiquitination (31).
Although previous studies have established Tax ubiquitination, the biochemical nature of the polyubiquitin chain linkages has remained elusive. Here, we describe Tax polyubiquitination as predominantly K63 linked. However, our findings do not rule out the ubiquitination of Tax through other types of ubiquitin linkages. K63-linked ubiquitination has emerged as an important regulator of a variety of different biological processes, including receptor trafficking and protein-protein interactions. The Kaposi's sarcoma-associated herpesvirus K3 gene product promotes the internalization of cell surface major histocompatibility complex class I molecules by K63-linked ubiquitination that is dependent on Ubc13 (10). Similarly, TRAF6-dependent K63-linked ubiquitination of the nerve growth factor receptor TrkA and neurotrophin receptor interacting factor regulates the trafficking and localization of these proteins (13, 14). K63-linked ubiquitination may also regulate protein function, as recently demonstrated for interferon regulatory factor 7, which exhibits enhanced transcriptional activity when ubiquitinated (28). In addition, p53 localization and activity are regulated by Ubc13- and K63-linked ubiquitination (32). To our knowledge, Tax is the first viral oncoprotein identified that is regulated by K63-linked ubiquitination.
K63-linked polyubiquitin chains may function as scaffolds that promote the assembly of complexes containing kinases and other signaling proteins (1). Thus, Tax polyubiquitination may nucleate a signaling complex that promotes the activation of IKK. Indeed, our results indicate that NEMO is likely an essential component of this complex. There are likely additional, yet-to-be-identified proteins within this complex, possibly including an E3 ligase specific for Tax. TRAF2, TRAF6, RNF5, RNF8, and CHFR are all E3 ligases capable of catalyzing K63-linked ubiquitination that is dependent on Ubc13 (3, 9, 17, 33, 43). Our preliminary data indicate that TRAF6 is not required for Tax-mediated NF-κB activation; therefore, TRAF6 may not serve as the Tax E3 ligase (data not shown). A more remote possibility is that Tax itself functions as an E3 ligase since Tax interacts with Ubc13. However, it is not clear if Tax interaction with Ubc13 is direct, and Tax lacks a conserved domain typical of E3 ligases, such as HECT (46) or RING (42). Future studies will more precisely determine the requirements for Tax ubiquitination and NF-κB activation.
Acknowledgments
We thank Shao-Cong Sun (University of Texas M. D. Anderson Cancer Center), Warner Greene (UCSF Gladstone), James Chen (University of Texas Southwestern Medical Center), Claudine Pique (Institut Cochin), Khaled Tolba (University of Miami), Matt Morrison (Cell Signaling Technologies), and Toshio Kitamura (University of Tokyo) for reagents.
These studies were supported in part by Public Health Service grant RO1 CA99926 to E.W.H. from the National Cancer Institute.
Footnotes
Published ahead of print on 17 October 2007.
REFERENCES
- 1.Adhikari, A., M. Xu, and Z. J. Chen. 2007. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene 26:3214-3226. [DOI] [PubMed] [Google Scholar]
- 2.Beinke, S., and S. C. Ley. 2004. Functions of NF-κB1 and NF-κB2 in immune cell biology. Biochem. J. 382:393-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bothos, J., M. K. Summers, M. Venere, D. M. Scolnick, and T. D. Halazonetis. 2003. The Chfr mitotic checkpoint protein functions with Ubc13-Mms2 to form Lys63-linked polyubiquitin chains. Oncogene 22:7101-7107. [DOI] [PubMed] [Google Scholar]
- 4.Chau, V., J. W. Tobias, A. Bachmair, D. Marriott, D. J. Ecker, D. K. Gonda, and A. Varshavsky. 1989. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science 243:1576-1583. [DOI] [PubMed] [Google Scholar]
- 5.Chen, Z. J. 2005. Ubiquitin signalling in the NF-κB pathway. Nat. Cell Biol. 7:758-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiari, E., I. Lamsoul, J. Lodewick, C. Chopin, F. Bex, and C. Pique. 2004. Stable ubiquitination of human T-cell leukemia virus type 1 Tax is required for proteasome binding. J. Virol. 78:11823-11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claudio, E., K. Brown, S. Park, H. Wang, and U. Siebenlist. 2002. BAFF-induced NEMO-independent processing of NF-κB2 in maturing B cells. Nat. Immunol. 3:958-965. [DOI] [PubMed] [Google Scholar]
- 8.Deng, L., C. Wang, E. Spencer, L. Yang, A. Braun, J. You, C. Slaughter, C. Pickart, and Z. J. Chen. 2000. Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103:351-361. [DOI] [PubMed] [Google Scholar]
- 9.Didier, C., L. Broday, A. Bhoumik, S. Israeli, S. Takahashi, K. Nakayama, S. M. Thomas, C. E. Turner, S. Henderson, H. Sabe, and Z. Ronai. 2003. RNF5, a RING finger protein that regulates cell motility by targeting paxillin ubiquitination and altered localization. Mol. Cell. Biol. 23:5331-5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan, L. M., S. Piper, R. B. Dodd, M. K. Saville, C. M. Sanderson, J. P. Luzio, and P. J. Lehner. 2006. Lysine-63-linked ubiquitination is required for endolysosomal degradation of class I molecules. EMBO J. 25:1635-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu, D. X., Y. L. Kuo, B. Y. Liu, K. T. Jeang, and C. Z. Giam. 2003. Human T-lymphotropic virus type I Tax activates IκB kinase by inhibiting IκB kinase-associated serine/threonine protein phosphatase 2A. J. Biol. Chem. 278:1487-1493. [DOI] [PubMed] [Google Scholar]
- 12.Fukushima, T., S. Matsuzawa, C. L. Kress, J. M. Bruey, M. Krajewska, S. Lefebvre, J. M. Zapata, Z. Ronai, and J. C. Reed. 2007. Ubiquitin-conjugating enzyme Ubc13 is a critical component of TNF receptor-associated factor (TRAF)-mediated inflammatory responses. Proc. Natl. Acad. Sci. USA 104:6371-6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geetha, T., J. Jiang, and M. W. Wooten. 2005. Lysine 63 polyubiquitination of the nerve growth factor receptor TrkA directs internalization and signaling. Mol. Cell 20:301-312. [DOI] [PubMed] [Google Scholar]
- 14.Geetha, T., R. S. Kenchappa, M. W. Wooten, and B. D. Carter. 2005. TRAF6-mediated ubiquitination regulates nuclear translocation of NRIF, the p75 receptor interactor. EMBO J. 24:3859-3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gohda, J., M. Irisawa, Y. Tanaka, S. Sato, K. Ohtani, J. Fujisawa, and J. Inoue. 2007. HTLV-1 Tax-induced NF-κB activation is independent of Lys-63-linked-type polyubiquitination. Biochem. Biophys. Res. Commun. 357:225-230. [DOI] [PubMed] [Google Scholar]
- 16.Grant, C., K. Barmak, T. Alefantis, J. Yao, S. Jacobson, and B. Wigdahl. 2002. Human T cell leukemia virus type I and neurologic disease: events in bone marrow, peripheral blood, and central nervous system during normal immune surveillance and neuroinflammation. J. Cell. Physiol. 190:133-159. [DOI] [PubMed] [Google Scholar]
- 17.Habelhah, H., S. Takahashi, S. G. Cho, T. Kadoya, T. Watanabe, and Z. Ronai. 2004. Ubiquitination and translocation of TRAF2 is required for activation of JNK but not of p38 or NF-κB. EMBO J. 23:322-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hacker, H., and M. Karin. 2006. Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006:re13. [DOI] [PubMed] [Google Scholar]
- 19.Haglund, K., and I. Dikic. 2005. Ubiquitylation and cell signaling. EMBO J. 24:3353-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harhaj, E. W., L. Good, G. Xiao, M. Uhlik, M. E. Cvijic, I. Rivera-Walsh, and S. C. Sun. 2000. Somatic mutagenesis studies of NF-κB signaling in human T cells: evidence for an essential role of IKKγ in NF-κB activation by T-cell costimulatory signals and HTLV-I Tax protein. Oncogene 19:1448-1456. [DOI] [PubMed] [Google Scholar]
- 21.Harhaj, E. W., and N. S. Harhaj. 2005. Mechanisms of persistent NF-κB activation by HTLV-I Tax. IUBMB Life 57:83-91. [DOI] [PubMed] [Google Scholar]
- 22.Harhaj, E. W., N. S. Harhaj, C. Grant, K. Mostoller, T. Alefantis, S. C. Sun, and B. Wigdahl. 2005. Human T cell leukemia virus type I Tax activates CD40 gene expression via the NF-κB pathway. Virology 333:145-158. [DOI] [PubMed] [Google Scholar]
- 23.Harhaj, E. W., and S. C. Sun. 1999. IKKγ serves as a docking subunit of the IκB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J. Biol. Chem. 274:22911-22914. [DOI] [PubMed] [Google Scholar]
- 24.Harhaj, N. S., B. Janic, J. C. Ramos, W. J. Harrington, Jr., and E. W. Harhaj. 2007. Deregulated expression of CD40 ligand in HTLV-I infection: distinct mechanisms of downregulation in HTLV-I-transformed cell lines and ATL patients. Virology 362:99-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harhaj, N. S., S. C. Sun, and E. W. Harhaj. 2007. Activation of NF-κB by the human T cell leukemia virus type I Tax oncoprotein is associated with ubiquitin-dependent relocalization of IκB kinase. J. Biol. Chem. 282:4185-4192. [DOI] [PubMed] [Google Scholar]
- 26.Hayden, M. S., and S. Ghosh. 2004. Signaling to NF-κB. Genes Dev. 18:2195-2224. [DOI] [PubMed] [Google Scholar]
- 27.Hershko, A., and A. Ciechanover. 1998. The ubiquitin system. Annu. Rev. Biochem. 67:425-479. [DOI] [PubMed] [Google Scholar]
- 28.Huye, L. E., S. Ning, M. Kelliher, and J. S. Pagano. 2007. Interferon regulatory factor 7 is activated by a viral oncoprotein through RIP-dependent ubiquitination. Mol. Cell. Biol. 27:2910-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeang, K. T., C. Z. Giam, F. Majone, and M. Aboud. 2004. Life, death, and Tax: role of HTLV-I oncoprotein in genetic instability and cellular transformation. J. Biol. Chem. 279:31991-31994. [DOI] [PubMed] [Google Scholar]
- 30.Jin, D. Y., V. Giordano, K. V. Kibler, H. Nakano, and K. T. Jeang. 1999. Role of adapter function in oncoprotein-mediated activation of NF-κB. Human T-cell leukemia virus type I Tax interacts directly with IKKγ. J. Biol. Chem. 274:17402-17405. [DOI] [PubMed] [Google Scholar]
- 31.Kfoury, Y., R. Nasr, A. Favre-Bonvin, M. El-Sabban, N. Renault, M. L. Giron, N. Setterblad, H. E. Hajj, E. Chiari, A. G. Mikati, O. Hermine, A. Saib, H. de The, C. Pique, and A. Bazarbachi. 24 September 2007. Ubiquitylated Tax targets and binds the IKK signalosome at the centrosome. Oncogene. doi: 10.1038/sj.onc.1210804. [DOI] [PubMed]
- 32.Laine, A., I. Topisirovic, D. Zhai, J. C. Reed, K. L. Borden, and Z. Ronai. 2006. Regulation of p53 localization and activity by Ubc13. Mol. Cell. Biol. 26:8901-8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamothe, B., A. Besse, A. D. Campos, W. K. Webster, H. Wu, and B. G. Darnay. 2007. Site-specific Lys-63-linked tumor necrosis factor receptor-associated factor 6 auto-ubiquitination is a critical determinant of IκB kinase activation. J. Biol. Chem. 282:4102-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamsoul, I., J. Lodewick, S. Lebrun, R. Brasseur, A. Burny, R. B. Gaynor, and F. Bex. 2005. Exclusive ubiquitination and sumoylation on overlapping lysine residues mediate NF-κB activation by the human T-cell leukemia virus Tax oncoprotein. Mol. Cell. Biol. 25:10391-10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lomaga, M. A., W. C. Yeh, I. Sarosi, G. S. Duncan, C. Furlonger, A. Ho, S. Morony, C. Capparelli, G. Van, S. Kaufman, A. van der Heiden, A. Itie, A. Wakeham, W. Khoo, T. Sasaki, Z. Cao, J. M. Penninger, C. J. Paige, D. L. Lacey, C. R. Dunstan, W. J. Boyle, D. V. Goeddel, and T. W. Mak. 1999. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 13:1015-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marriott, S. J., and O. J. Semmes. 2005. Impact of HTLV-I Tax on cell cycle progression and the cellular DNA damage repair response. Oncogene 24:5986-5995. [DOI] [PubMed] [Google Scholar]
- 37.Mori, N., Y. Yamada, S. Ikeda, Y. Yamasaki, K. Tsukasaki, Y. Tanaka, M. Tomonaga, N. Yamamoto, and M. Fujii. 2002. Bay 11-7082 inhibits transcription factor NF-κB and induces apoptosis of HTLV-I-infected T-cell lines and primary adult T-cell leukemia cells. Blood 100:1828-1834. [DOI] [PubMed] [Google Scholar]
- 38.Morita, S., T. Kojima, and T. Kitamura. 2000. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 7:1063-1066. [DOI] [PubMed] [Google Scholar]
- 39.Nasr, R., E. Chiari, M. El-Sabban, R. Mahieux, Y. Kfoury, M. Abdulhay, V. Yazbeck, O. Hermine, H. de The, C. Pique, and A. Bazarbachi. 2006. Tax ubiquitylation and sumoylation control critical cytoplasmic and nuclear steps of NF-κB activation. Blood 107:4021-4029. [DOI] [PubMed] [Google Scholar]
- 40.Osame, M., K. Usuku, S. Izumo, N. Ijichi, H. Amitani, A. Igata, M. Matsumoto, and M. Tara. 1986. HTLV-I associated myelopathy, a new clinical entity. Lancet 1:1031-1032. [DOI] [PubMed] [Google Scholar]
- 41.Peloponese, J. M., Jr., H. Iha, V. R. Yedavalli, A. Miyazato, Y. Li, K. Haller, M. Benkirane, and K. T. Jeang. 2004. Ubiquitination of human T-cell leukemia virus type 1 Tax modulates its activity. J. Virol. 78:11686-11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petroski, M. D., and R. J. Deshaies. 2005. Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 6:9-20. [DOI] [PubMed] [Google Scholar]
- 43.Plans, V., J. Scheper, M. Soler, N. Loukili, Y. Okano, and T. M. Thomson. 2006. The RING finger protein RNF8 recruits Ubc13 for lysine 63-based self polyubiquitylation. J. Cell. Biochem. 97:572-582. [DOI] [PubMed] [Google Scholar]
- 44.Robek, M. D., and L. Ratner. 1999. Immortalization of CD4+ and CD8+ T lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J. Virol. 73:4856-4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rothwarf, D. M., and M. Karin. 1999. The NF-κB activation pathway: a paradigm in information transfer from membrane to nucleus. Sci. STKE 1999:RE1. [DOI] [PubMed] [Google Scholar]
- 46.Scheffner, M., J. M. Huibregtse, and P. M. Howley. 1994. Identification of a human ubiquitin-conjugating enzyme that mediates the E6-AP-dependent ubiquitination of p53. Proc. Natl. Acad. Sci. USA 91:8797-8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Senftleben, U., Y. Cao, G. Xiao, F. R. Greten, G. Krahn, G. Bonizzi, Y. Chen, Y. Hu, A. Fong, S. C. Sun, and M. Karin. 2001. Activation by IKKα of a second, evolutionary conserved, NF-κB signaling pathway. Science 293:1495-1499. [DOI] [PubMed] [Google Scholar]
- 48.Shembade, N., N. S. Harhaj, D. J. Liebl, and E. W. Harhaj. 2007. Essential role for TAX1BP1 in the termination of TNF-α-, IL-1- and LPS-mediated NF-κB and JNK signaling. EMBO J. 26:3910-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith, M. R., and W. C. Greene. 1990. Identification of HTLV-I Tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 4:1875-1885. [DOI] [PubMed] [Google Scholar]
- 50.Sun, S. C., and S. Yamaoka. 2005. Activation of NF-κB by HTLV-I and implications for cell transformation. Oncogene 24:5952-5964. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki, S., P. Singhirunnusorn, A. Mori, S. Yamaoka, I. Kitajima, I. Saiki, and H. Sakurai. 2007. Constitutive activation of TAK1 by HTLV-1 Tax-dependent overexpression of TAB2 induces activation of JNK-ATF2 but not IKK-NF-κB. J. Biol. Chem. 282:25177-25181. [DOI] [PubMed] [Google Scholar]
- 52.Wu, X., and S. C. Sun. 2007. Retroviral oncoprotein Tax deregulates NF-κB by activating Tak1 and mediating the physical association of Tak1-IKK. EMBO Rep. 8:510-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao, G., M. E. Cvijic, A. Fong, E. W. Harhaj, M. T. Uhlik, M. Waterfield, and S. C. Sun. 2001. Retroviral oncoprotein Tax induces processing of NF-κB2/p100 in T cells: evidence for the involvement of IKKα. EMBO J. 20:6805-6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao, G., E. W. Harhaj, and S. C. Sun. 2000. Domain-specific interaction with the IκB kinase (IKK) regulatory subunit IKKγ is an essential step in Tax-mediated activation of IKK. J. Biol. Chem. 275:34060-34067. [DOI] [PubMed] [Google Scholar]
- 55.Xiao, G., E. W. Harhaj, and S. C. Sun. 2001. NF-κB-inducing kinase regulates the processing of NF-κB2 p100. Mol. Cell 7:401-409. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto, M., T. Okamoto, K. Takeda, S. Sato, H. Sanjo, S. Uematsu, T. Saitoh, N. Yamamoto, H. Sakurai, K. J. Ishii, S. Yamaoka, T. Kawai, Y. Matsuura, O. Takeuchi, and S. Akira. 2006. Key function for the Ubc13 E2 ubiquitin-conjugating enzyme in immune receptor signaling. Nat. Immunol. 7:962-970. [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto, M., S. Sato, T. Saitoh, H. Sakurai, S. Uematsu, T. Kawai, K. J. Ishii, O. Takeuchi, and S. Akira. 2006. Cutting edge: pivotal function of Ubc13 in thymocyte TCR signaling. J. Immunol. 177:7520-7524. [DOI] [PubMed] [Google Scholar]
- 58.Yamaoka, S., G. Courtois, C. Bessia, S. T. Whiteside, R. Weil, F. Agou, H. E. Kirk, R. J. Kay, and A. Israel. 1998. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κΒ activation. Cell 93:1231-1240. [DOI] [PubMed] [Google Scholar]
- 59.Yao, J., and B. Wigdahl. 2000. Human T cell lymphotropic virus type I genomic expression and impact on intracellular signaling pathways during neurodegenerative disease and leukemia. Front. Biosci. 5:D138-D168. [DOI] [PubMed] [Google Scholar]
- 60.Yoshida, M., I. Miyoshi, and Y. Hinuma. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 79:2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]