Abstract
Recognition of viruses by germ line-encoded pattern recognition receptors of the innate immune system is essential for rapid production of type I interferon (IFN) and early antiviral defense. We investigated the mechanisms of viral recognition governing production of type I IFN during herpes simplex virus (HSV) infection. We show that early production of IFN in vivo is mediated through Toll-like receptor 9 (TLR9) and plasmacytoid dendritic cells, whereas the subsequent alpha/beta IFN (IFN-α/β) response is derived from several cell types and induced independently of TLR9. In conventional DCs, the IFN response occurred independently of viral replication but was dependent on viral entry. Moreover, using a HSV-1 UL15 mutant, which fails to package viral DNA into the virion, we found that entry-dependent IFN induction also required the presence of viral genomic DNA. In macrophages and fibroblasts, where the virus was able to replicate, HSV-induced IFN-α/β production was dependent on both viral entry and replication, and ablated in cells unable to signal through the mitochondrial antiviral signaling protein pathway. Thus, during an HSV infection in vivo, multiple mechanisms of pathogen recognition are active, which operate in cell-type- and time-dependent manners to trigger expression of type I IFN and coordinate the antiviral response.
Type I interferon (IFN) or alpha/beta IFN (IFN-α/β) is a key component of the innate immune response against virus infections and is produced as the first wave of antiviral defense (8). The antiviral activity exerted by IFN-α/β is transduced through the specific receptor chains IFN-α receptor (IFNAR) 1 and IFNAR 2, which are abundantly expressed. IFNAR engagement induces intracellular signaling, leading to the expression of an array of IFN-stimulated genes, which inhibit viral replication through a range of mechanisms (8). In addition to this classical antiviral activity, type I IFNs also contribute to the antiviral immune response by stimulating the cytotoxic activity of natural killer cells, maturation of dendritic cells (DCs), and promotion of various T-cell functions, including expansion of the memory population (8).
IFN-α/β can be expressed by all nucleated cells, but cell types differ with respect to the amount of IFN produced during infection. In particular, plasmacytoid DCs (pDCs) have been identified as the cell type responsible for the majority of the IFN produced during many, but not all, viral infections (2, 30, 37). Studies in cell culture and gene-modified mice, as well as the identification of human virus susceptibility genes, have underscored the important role of the IFN system in antiviral defense (7, 20).
Herpes simplex virus (HSV) is a DNA virus of the alphaherpesvirus subfamily (34). There are two types of HSV, which cause an overlapping set of clinical manifestations, primarily in immunocompromised individuals, including encephalitis, genital herpes, systemic infection, and various skin manifestations (44). HSV is able to enter most cell types due to the abundant expression of cellular receptors and can productively infect many but not all cell types (38). Although HSV is in possession of several mechanisms to evade IFN responses (19), type I IFN is known to block HSV replication at an early step in replication (27) and to be very important for resistance against this virus (7, 20).
Production of IFN-α/β is preceded by viral recognition through pattern recognition receptors (PRRs), which recognize conserved pathogen-associated molecular patterns (PAMPs) present in the virus particle or produced during viral replication (13, 28). PRRs are expressed in cell-type specific patterns, and recent work has shown that viruses can indeed be recognized by different PPRs during infection in vitro, depending on factors such as PRR expression and cellular tropism (12, 24).
For HSV, it has been demonstrated that Toll-like receptor 2 (TLR2) and TLR9 are involved in activation of the host response. TLR2 plays an important role in the inflammatory response and the immunopathology of HSV infection (18) but has not been ascribed a role in the expression of IFN-α/β. The PAMP triggering TLR2 signaling during HSV infection has not been identified. TLR9 recognizes unmethylated CpG DNA and potently induces type I expression in pDCs in response to HSV through recognition of viral DNA (16, 21). However, we and others have previously shown that HSV-induced expression of type I IFN in vitro occurs via both TLR9-dependent and -independent mechanisms (9, 23). As for the TLR-independent IFN response, it has been shown that transfection of DNA, including HSV DNA, into the cytoplasm of cells results in the activation of a strong IFN response (11, 39), and it was recently shown that a DNA-dependent activator of IFN regulatory factors (DAI) is a cytosolic DNA sensor (42). Furthermore, the retinoic acid-inducible gene I (RIG-I) family of helicases induce type I IFN expression through the mitochondrial antiviral signaling protein (MAVS) adaptor protein in response to recognition of RNA structures (12, 36, 48), and we have recently reported that infection of a permissive cell line with DNA viruses, including HSV-2, is associated with accumulation of double-stranded RNA (43). Finally, there is evidence that viral particle entry is sensed by the host to trigger expression of a subset of IFN-stimulated genes (32). Thus, a number of PAMPs and PRRs with the potential to trigger a type I IFN response are present during HSV infection, but their contribution to the IFN response in vivo is not known.
We show here that early expression of IFN-α/β after infection with HSV-1 or HSV-2 in vivo is largely performed by pDCs and is dependent on TLR9. In contrast, the later production of IFN-α/β is derived from several different cell types, which utilize various TLR9-independent mechanisms to recognize the virus and trigger IFN expression. Thus, during an HSV infection in vivo, multiple mechanisms of pathogen recognition operate in cell-type- and time-dependent manners to trigger expression of type I IFN and coordinate the antiviral response.
MATERIALS AND METHODS
Mice, virus, and cell lines.
The mice used in the present study were 4- to 5-week-old female C57BL/6, TLR9−/− (C57BL/6 background), TLR2−/− (C57BL/6 background), 129/sv, and, IFNAR−/− (129Sv background) mice. All animals were bred at Taconic M&B (Ry, Denmark). The TLR9−/− and TLR2−/− were obtained from Oriental Yeast Co., Ltd. (Shizuoka, Japan), and the IFNAR−/− mice were from B&K Universal Ltd (Hull, United Kingdom). The wild-type (WT) virus used was the 17+ and KOS strains of HSV-1 and the MS strain of HSV-2. The mutant HSV-1 viruses used were dl1403 (17+), d120 (ΔICP4, KOS), d22 (ΔICP22, KOS), d27-1 (ΔICP27, KOS) gL86 (Δglycoprotein L, gL) (KOS) (29), and the ΔUL15ExII (ΔUL15) mutant of HSV-1 (F) (3), which were grown in either complementing or noncomplementing cell lines to give mutant virus either containing or lacking the protein encoded by the mutant gene. For propagation of the entry deficient ΔgL virus, noncomplementing cells were infected with virus at a multiplicity of infection (MOI) of 10, and the virus produced by one replication cycle was harvested. For propagation of other viruses, the relevant cells were infected with virus at 0.01 MOI, and virus was harvested at >95% cytopathic effect. For purification of virus, the cell cultures were first freeze-thawed twice, and the supernatants were clarified by centrifugation at 3,000 × g for 1 h, followed by pelleting of the virus by ultracentrifugation at 45,000 × g for 1 h. The viruses were quantified by plaque assays on the appropriate cell line as previously described (23) or (for the replication-defective mutants) by Western blotting with mouse monoclonal anti-gD immunoglobulin G2a, clone 2C10 (Virusys), in which case “PFU/ml” was estimated by comparison to the WT virus with a known titer. UV inactivation was performed for a period of time sufficient to reduce virus titers by a factor of 107. The cell lines used for propagation of virus were Vero cells, a Vero-derived cell line stably expressing gL (29), rabbit skin cells, and a derived cell line stably expressing UL15 (3). For measurement of the IFN-α/β bioactivity we used L929 cells. Mouse embryonic fibroblasts (MEFs) from C57BL/6, MAVS−/−, and TLR9−/− mice were prepared from day 13.5 embryos. All of the above cells were maintained in minimal essential medium (MEM) supplemented with antibiotics and 10% fetal calf serum (FCS).
Virus infection in vivo.
For measurement of viral load in infected organs, mice were infected intraperitoneally (i.p.) with 106 PFU of virus. At 2 days postinfection (p.i.), the mice were sacrificed, and liver and spleens were harvested and snap-frozen. For determination of IFN-α/β production, the mice were infected i.p. with 2 × 107 PFU of virus. The animals were sacrificed at the indicated time points p.i., and sera and spleens were harvested either for direct measurement of IFN-α/β or for further purification of cells (see below).
Isolation of primary cells from mouse spleens.
Spleens were surgically removed and transferred to RPMI with 5% FCS. The spleens were then transferred to a 1-mg/ml suspension of collagenase D (Roche). The enzyme was injected into the organ, which was subsequently cut into small pieces, followed by incubation in the collagenase D suspension for 30 min at 37°C. The suspension was filtrated over a 70-μm-pore-size cell strainer (BD Falcon), spun down, and suspended in RPMI-5% FCS, and the cells were counted. After centrifugation, the cells were resuspended in phosphate-buffered saline with 2 mM EDTA-0.5% bovine serum albumin (MACS running buffer) in concentrations according to the manufacturer's instruction (MACS; Miltenyi Biotec). Anti-mPDCA-1 microbeads were added, and after incubation for 15 min at 4°C the suspension was spun down and suspended in running buffer. pDCs were then isolated in an Auto-MACS separator by positive selection. The negative selected cell fraction was incubated with anti-CD11b or anti-CD11c, and cells were isolated by positive selection. Isolated cell fractions were spun down, suspended in RPMI-5% FCS, and counted. The purity of the cells was determined by flow cytometry (pDCs, 73%; conventional DCs (cDCs), 90%; macrophages, 87%). For determination of ex vivo IFN-α/β production, the cells were cultured for 24 h at a concentration of 3.0 × 106 cells in 100 μl of RPMI plus 5% FCS, and supernatants were eventually harvested for measurement of IFN-α/β levels.
IFN-α/β bioassay.
IFN-α/β bioactivity was measured by an L929-cell-based bioassay. L929 cells (2 × 104 cells/well in 100 μl) in modified Eagle medium with 5% FCS were incubated overnight at 37°C in successive twofold dilutions of samples or murine IFN-α/β as the standard. Subsequently, vesicular stomatitis virus (VSV/V10) was added to the wells, and the cells were incubated for 2 to 3 days. The dilution mediating 50% protection was defined as 1 U of IFN-α/β/ml. The specificity was determined by neutralization with two polyclonal rabbit antibodies directed against IFN-α and IFN-β (PBL Biomedical Laboratories, Piscataway, NJ).
IL-12 p40 ELISA.
Murine interleukin-12 (IL-12) p40 was detected by enzyme-linked immunosorbent assay (ELISA) as previously described (24).
Virus plaque assay.
For quantification of virus in infected animals, organ samples were weighed, thawed, and homogenized in MEM supplemented with 2% FCS just before use. The homogenates were pelleted by centrifugation at 1,600 × g for 30 min, and the supernatants were used for plaque assay on monolayers of Vero cells seeded in 20-cm2 tissue culture plates at a density of 1.0 × 106 cells in MEM supplemented with 5% FCS. The cells were left overnight to settle and were infected by incubation for 1 h at 37°C with serial dilutions of organ suspensions and vaginal washes. The plates were rocked every 15 min to ensure even distribution of virus. After 1 h, 8 ml of medium containing 2% human immunoglobulin was added, and the plates were incubated for 2 days. For liver titration, the organ suspensions were removed before adding immunoglobulin-containing media. After incubation, the plates were stained with 0.03% methylene blue to allow quantification of plaques. The numbers of PFU were counted, and the results were expressed as PFU/gram of tissue.
Isolation of RNA and DNA and PCR.
Total RNA was isolated from cells using TRIzol according to the manufacturer's recommendations. The RNA was cleared of DNA contaminants by treatment with DNase I. Virus genomic DNA was isolated using the RTP virus kits for viral RNA and DNA purification (Westburg). Two micrograms of RNA was subjected to reverse transcription (RT) using oligo(dT)15 and Expand reverse transcriptase (Roche). The cDNA was amplified by PCR with the following primers (from DNA technology): ICP27(HSV-2), 5′-AGA TCG ACT ACA CGA CCG T-3′ (forward) and 5′-TGC CGT GCA CAT ATA AGG G-3′ (reverse); IFN-α, 5′-CGG TGA TGA GCT ACT GGC-3′ (forward) and 5′-TTT GTA CCA GGA GTG TCA AGG-3′ (reverse); IFN-β, 5′-GGT GGA ATG AGA CTA TTG TTG-3′ (forward) and 5′-AGG ACA TCT CCC ACG TC-3′ (reverse); β-actin-1, 5′-TAG CAC CAT GAA GAT CAA GAT-3′ (forward) and 5′-CCG ATC CAC ACA GAG TAC TT-3′ (reverse); and β-actin-2, 5′-CCA ACC GTG AAA AGA TGA CC-3′ (forward) and 5′-GCA GTA ATC TCC TTC TGC ATC C-3′ (reverse). Nested PCR was performed to amplify the target HSV genomic DNA (DNA polymerase gene) using two paired sets of primers. The outer primers were 5′-TGC CCG AGG GAC TGC AGG CGT T-3′ and 5′-CCT TGA TGG ACG GGA CCT GCG C-3′ with which to amplify a 201-bp DNA fragment. The inner primers, 5′-CAT CAC CGA CCC GGA GAG GGA C-3′ and 5′-GGG CCA GGC GCT TGT TGG TGT A-3′, were used to amplify a 92-bp DNA fragment. These regions are identical for HSV-1 and HSV-2. Virus genomic DNA, ICP27, and β-actin-2 were detected by ethidium bromide staining of agarose gels, whereas IFNs and β-actin-1 were measured real-time PCR using SYBR green I (QIAGEN).
Isolation of nuclear extracts.
To isolate nuclear proteins, the cell monolayer was washed twice with ice-cold phosphate-buffered saline, scraped off the plate, and spun down (2,000 × g for 1 min). The cells were resuspended in a hypotonic buffer (20 mM HEPES [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, 0.2 mM EDTA, 0.2 mM phenylmethylsulfonyl fluoride, 0.2 mM leupeptin, 0.2 mM pepstatin A, 0.1 mM Na3VO4) and left on ice for 15 min. NP-40 was added to 0.6%, and the mixture was vortex mixed for 15 s and centrifuged at 10,000 × g for 1 min. Extraction buffer (20 mM HEPES [pH 7.9], 20% glycerol, 1.5 mM MgCl2, 420 mM NaCl, 0.5 mM dithiothreitol, 0.2 mM EDTA, 0.2 mM phenylmethylsulfonyl fluoride, 0.2 mM leupeptin, 0.2 mM pepstatin A, 0.1 mM Na3VO4, 0.2% NP-40) was added to the nuclei, followed by incubation for 30 min at 4°C with rocking. The samples were centrifuged at 10,000 × g for 15 min at 4°C, and the supernatants were harvested as nuclear extracts.
Western blotting.
Virus particles or nuclear extracts were denatured in sample buffer (140 mM Tris-HCl [pH 8.5], 10% glycerol, 2% sodium dodecyl sulfate, 1 mM EDTA, 0.019% Serva Blue G250, 0.06% phenol red), heated to 80°C for 10 min, and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins were blotted onto a polyvinylidene difluoride membrane and blocked for 1 h with TBS (10 mM Tris, 140 mM NaCl) supplemented with 0.05% Tween 20 and 5% skim milk powder. Antibodies (rabbit polyclonal anti-VP16 [BD Clontech] and mouse monoclonal anti-gD [Virusys]) were added for overnight incubation at 4°C. The membrane was washed four times for 10 min each in washing buffer (TBS with 0.05% Tween 20), followed by incubation for 1 h at room temperature with a polyclonal horseradish peroxidase-conjugated antibody against mouse immunoglobulin. The membrane was washed as described above, and the horseradish peroxidase-conjugated antibody was visualized by using enhanced chemiluminescence. As a negative control for viral proteins, whole-cell extracts from uninfected Vero cells were used. As a positive control, a pool of extracts from Vero cells infected for 4.5, 7, and 16 h was used.
Statistical analysis.
The data are presented as means ± the standard deviations. The statistical significance was estimated with the Wilcoxon rank sum test. P values of <0.05 were considered statistically significant.
RESULTS
TLR9 is required for early but not late type I IFN expression during HSV infection in vivo.
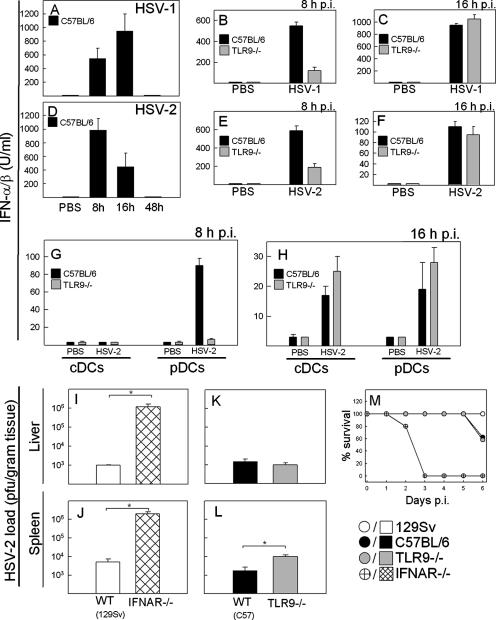
Previous work has shown that TLR9 is important for the production of type I IFN by pDCs in response to HSV (16, 21) and that TLR9-independent mechanisms of viral recognition also contribute to the IFN response in vitro (9, 23). To further study which recognition systems were involved in triggering IFN-α/β expression during HSV infection, we infected C57BL/6 and TLR9−/− mice with HSV-1 (Fig. 1A to C) or HSV-2 (Fig. 1D to F), and measured the levels of IFN-α/β in serum at different time points p.i. In response to HSV-1 infection, IFN-α/β was detectable after 8 h, peaked after 16 h, and was down to basal levels after 48 h (Fig. 1A). The IFN response to HSV-2 followed a more rapid kinetics (Fig. 1D). However, the IFN response to both viruses was reduced in TLR9−/− mice after 8 h but not after 16 h (Fig. 1B, C, E, and F). In addition to type IFN, we also measured serum levels of tumor necrosis factor α, IL-6, KC, and RANTES and observed that the levels of RANTES were reduced at 8 h but not at 16 h p.i., whereas the three other cytokines were produced independent of TLR9−/−, irrespective of the time point (data not shown). TLR2 is also involved in recognition of HSV (18), and TLR2−/− mice have been reported to produce reduced amounts of type I IFN after infection with other viruses (41, 49). However, we found that TLR2−/− mice responded to HSV-2 infection with a type I IFN response indistinguishable from that of WT mice (data not shown).
FIG. 1.
The pDC-TLR9 pathway is responsible for early but not for late IFN-α/β production during HSV infection in vivo. (A to H) C57BL/6 or TLR9−/− mice were infected i.p. with 2 × 107 PFU of HSV-1 or HSV-2 (five mice per group). At the indicated time points p.i., sera were harvested (A to F), or spleen cDCs and pDCs were isolated and cultured for ex vivo cytokine expression for 24 h (G to H). The levels of IFN-α/β were measured by bioassay. Similar results were obtained in three to four independent experiments. (I to L) WT mice (129sv in panels I and J; C57BL/6 in panels K and L), IFNAR−/− mice, and TLR9−/− mice were infected i.p. with 106 PFU of HSV-2 (five mice per group). At 2 days p.i., livers and spleens were harvested, and the viral loads in the organs were determined by plaque assay. Similar results were obtained in three independent experiments. (M) Mice treated as in panels I to L were monitored for 6 days. The mice were sacrificed when they displayed symptoms irreversibly associated with death. Error bars indicate the standard errors of the mean (SEM). *, P < 0.05.
To look into the cellular source of IFN-α/β after 8 h versus after 16 h, we isolated splenic cDCs and pDCs from infected mice and measured ex vivo cytokine production. As shown in Fig. 1G, we could not detect any IFN-α/β in cultures of cDCs harvested from mice at 8 h p.i. In contrast, pDCs harvested from infected WT mice produced significant amounts of IFN-α/β, whereas pDCs from TLR9−/− mice failed to produce IFN. This effect of TLR9 on IFN-α/β production was not due to decreased recruitment of pDCs to the spleen, since similar numbers of pDCs were observed before and after infection in WT and TLR9−/− mice (data not shown). When the cells were harvested from mice infected for 16 h, we observed that both cDCs and pDCs produced type I IFN in a manner independent of TLR9 (Fig. 1H). Thus, the early production of IFN-α/β in response to HSV infection in vivo is in part derived from pDCs through a TLR9-dependent mechanism, whereas the later IFN response also involves other cell types and is independent of TLR9.
To examine whether the first wave of IFN production was sufficient to mediate the innate antiviral activity of IFN-α/β, we compared the viral load in TLR9−/− and IFNAR−/− mice infected with HSV-2. Whereas IFNAR−/− mice displayed approximately 102- and 103-fold-higher viral loads than WT 129Sv mice in the spleen and liver, respectively (Fig. 1I and J) and succumbed to the infection several days before the WT mice (Fig. 1M), the TLR9−/− mice were indistinguishable from WT C57BL/6 mice with respect to viral load in the liver and harbored about six times more virus in the spleen (Fig. 1K and L). Thus, although TLR9 does contribute to control of the virus in the spleen, these data demonstrate that the early TLR9-dependent production of type I IFN is only a minor contributor to the antiviral function evoked by IFN-α/β during HSV infection.
HSV-induced IFN-α/β expression is regulated through cell-type-specific mechanisms with different requirements for TLR9 and viral replication.
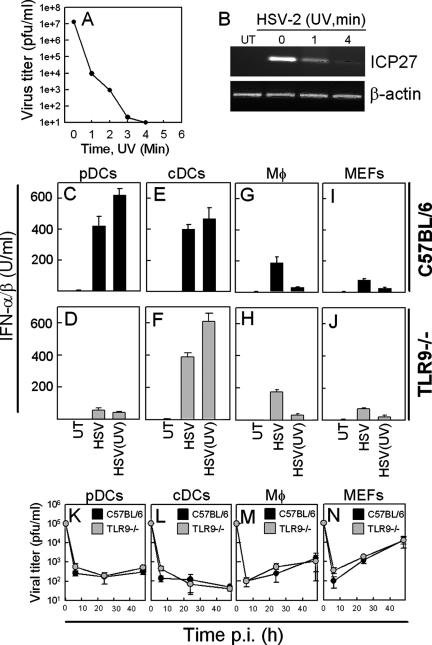
Recently, it has been shown that different cell types may use different mechanisms to recognize RNA viruses and that this involves both TLR-dependent and -independent mechanisms (12, 24). To examine the requirement for viral replication in HSV-induced IFN-α/β expression, we treated the virus with UV light prior to infection of cultures with cDCs, pDCs, macrophages, and fibroblasts harvested from C57BL/6 and TLR9−/− mice and examined them for IFN-α/β production. As seen in Fig. 2A, treatment of virus with UV light rapidly impaired the ability of the virus to replicate in Vero cells, and after 4 min of treatment no viral replication could be detected. This observation was associated with reduced expression of the viral immediate-early (IE) gene ICP27 (Fig. 2B). When we treated cells with this UV-treated virus, we found that pDCs produced IFN-α/β in a manner dependent on TLR9 but independent of viral replication (Fig. 2C and D), cDCs produced IFN-α/β independent of both TLR9 and viral replication (Fig. 2E and F), while macrophages and fibroblasts were dependent on viral replication but not TLR9 to evoke a type I IFN response (Fig. 2G to J). Thus, cDCs, pDCs, and macrophages/fibroblasts have different requirements for TLR9 and viral replication to induce production of IFN-α/β in vitro.
FIG. 2.
Cell-type-specific requirements for TLR9 and virus replication for induction of IFN-α/β by HSV-2 in vitro. HSV-2 was treated with UV light for the indicated time intervals before being subjected to plaque assay (A) or used for infection of Vero cells for 5 h at an MOI of 1 (B) before harvesting of total RNA and detection of ICP27 and β-actin by RT-PCR. Similar results were obtained in two independent experiments. (C to J) Cells were harvested from WT or TLR9−/− mice and cultured with medium alone or in the presence of 3 × 106 PFU of HSV-2/ml or an equivalent amount of virus UV inactivated for 4 min. Supernatants were harvested 24 h p.i., and the levels of IFN-α/β were determined by bioassay. Similar results were obtained in three independent experiments. (K to N) Splenic pDCs, cDCs, and macrophages, as well as MEFs, were cultured and infected with 105 PFU of HSV-2/ml. Supernatants were harvested 6, 24, and 48 h p.i., and the viral load was measured by plaque assay. Similar results were obtained in two independent experiments. Error bars indicate the SEM.
In order to correlate the above findings with viral replication in the cells, splenic pDCs, cDCs, and macrophages or MEFs were cultured. The cells were infected with 105 PFU of HSV-2, and infectious virus in the culture supernatants was quantified at different time points p.i. In cultures from all cells, we observed a rapid decline in the virus titer at 6 h p.i., suggesting that the virus had adsorbed to or entered the cells (Fig. 2K to N). In pDCs and cDCs the virus titer did not increase at later time points (Fig. 2K and L), whereas the levels of infectious virus in supernatants from macrophages and fibroblasts increased between 6 and 48 h p.i. (Fig. 2M and N). In support of this, we were able to detect ICP27 mRNA in the RNA harvested from infected macrophages and MEFs, whereas none or very little could be detected in the RNA from pDCs or cDCs (data not shown). The ability of HSV to replicate in the cell types under investigation was not dependent on the presence of TLR9 (Fig. 2K to N). To support these in vitro findings with in vivo data, we infected WT mice and isolated splenic pDCs, cDCs, and macrophages at 18 h p.i. The total RNA was isolated, and ICP27 mRNA was detected by RT-PCR. The macrophage RNA was positive for ICP27 mRNA in four of six mice, whereas the cDC RNA was positive for the viral transcript in only one of six mice (data not shown). None of the pDC RNA samples from six infected mice contained ICP27 mRNA. Thus, the observed dependency of HSV-induced IFN-α/β expression on viral replication is correlated with the ability of the virus to replicate in the different cell types.
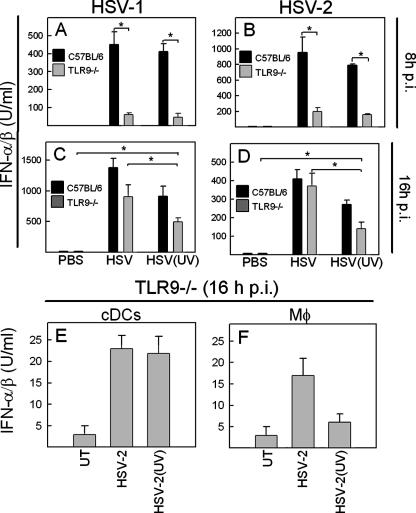
To determine whether these findings could be seen in vivo, we injected mice with infectious or UV-inactivated HSV-1 or HSV-2 and harvested sera at 8 and 16 h p.i. As expected, the early production of IFN-α/β was largely dependent on TLR9 and not dependent on viral replication (Fig. 3A and B). However, when we examined sera from mice treated for 16 h with the virus, we found significant differences between IFN-α/β levels in the sera from TLR9−/− mice receiving “live” or inactivated virus. Moreover, we observed that TLR9−/− mice treated with UV-inactivated virus produced significant levels of IFN-α/β (Fig. 3C and D). The TLR9-independent response to HSV-2 infection was also seen when ex vivo production of IFN-α/β was evaluated in cDCs and macrophages harvested from TLR9−/− mice at 16 h p.i. (Fig. 3E and F). These data demonstrate that the late IFN-α/β response during HSV infection in vivo involves at least two mechanisms independent of TLR9: one dependent on viral replication and one independent of this event.
FIG. 3.
IFN-α/β expression in vivo involves at least three mechanisms with differential requirements for TLR9 and virus replication. C57BL/6 or TLR9−/− mice were infected i.p. with 2 × 107 PFU of infectious or inactivated HSV-1 (A and C) or HSV-2 (B and D) (five mice per group). After 8 h (A and B) or 16 h (C and D) of treatment, serum was harvested, and the levels of IFN-α/β were measured by bioassay. (E and F) Splenic cDCs and macrophages were harvested from TLR9−/− mice infected as described above for 16 h. The cells were cultured for 24 h, and the ex vivo production of IFN was measured by bioassay. Similar results were obtained in three independent experiments. Error bars indicate the SEM. *, P < 0.05.
HSV-induced expression of IFN-α/β is dependent on virion DNA and displays a differential requirement for viral entry.
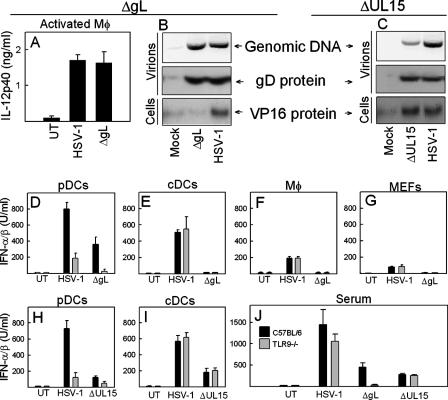
The data obtained with TLR9−/− mice and inactivated virus suggested that cell-type-specific mechanisms of viral recognition are operative during HSV infection. To look further into the TLR9-independent response, we used two different HSV-1 mutants with deletion of the genes encoding gL and UL15, respectively. The gL deletion mutant is unable to enter target cells due to the lack of the gL/gH dimer, which is required for virus-cell membrane fusion (29, 38). UL15 encodes a subunit of the viral terminase, which cleaves the concatameric DNA into genomic lengths. The UL15 deletion mutant is incapable of packing genomic viral DNA into capsids and is therefore unable to produce progeny virions containing DNA (3), although these virions can enter new cells. Before testing the viruses in our system, we wanted to determine whetehr the preparations of ΔgL and ΔUL15 behaved as expected. In a first set of experiments, we found that the ΔgL virus was able to stimulate production of IL-12 p40 in activated macrophages (Fig. 4A). The mutant virus contained the same amounts of virion DNA and gD protein as did the WT virus but, in contrast to what was seen after infection with the WT virus, no VP16 accumulated in cells infected with ΔgL (Fig. 4B). The virions in the preparation of the ΔUL15 virus, although having the same levels of gD protein, contained much less virus genomic DNA (Fig. 4C). The residual viral DNA in the ΔUL15 virus is unlikely to be due to contamination with WT virus, since no viral activity (as measured by IE gene expression or production of plaques) was found in the ΔUL15 preparations (data not shown). The ΔUL15 virus was able to enter cells, since VP16 was delivered to the nucleus of cells treated with the virus. Moreover, by Western blotting we further characterized the virus particles of the ΔUL15 virus grown in noncomplementing versus complementing cells and found no differences between these two virus preparations with respect to the levels of the capsid protein ICP5, the tegument protein VP16, and the glycoproteins gB and gD (data not shown and Fig. 4C). Thus, the virus preparations used in our experiments had the properties previously reported in the literature (3, 29).
FIG. 4.
HSV-induced expression of IFN-α/β is dependent on virion DNA and displays a differential requirement for viral entry. (A) Thioglycolate-activated macrophages were harvested from BALB/c mice and treated with 3 × 106 PFU of HSV-1 or ΔgL/ml for 24 h. IL-12p40 was measured by ELISA. Similar results were obtained in three independent experiments. (B and C) Genomic DNA or total lysates of the preparations of the indicated viruses were analyzed for content of DNA and gD protein, respectively (upper panels). Nuclear extracts were prepared from Vero cells treated for 3 h with the indicated virus at an MOI of 12. VP16 was detected in the extracts by Western blotting (lower panel). Similar results were obtained in three independent experiments. (D to I) Splenic pDCs, cDCs, and macrophages, together with MEFs from C57BL/6 and TLR9−/− mice, were cultured and treated for 20 h with 3 × 106 PFU of HSV-1, ΔgL, or ΔUL15 viruses/ml as indicated. The supernatants were harvested, and the IFN-α/β levels were measured. Similar results were obtained in three to four independent experiments. (J) C57BL/6 and TLR9−/− mice were injected i.p. with 2 × 107 PFU of HSV-1, ΔgL, or ΔUL15 viruses (five mice per group). After 16 h, sera were harvested, and the type I IFN levels were measured. Similar results were obtained in three independent experiments. Error bars indicate the SEM.
To explore the ability of these viruses to induce IFN production, we harvested pDCs, cDCs, macrophages, and MEFs from WT and TLR9−/− mice and first looked for IFN-α/β expression after treatment with WT and ΔgL HSV-1 virus. In pDCs from WT mice we observed that the IFN response was partly dependent on viral entry, whereas pDCs from TLR9−/− mice treated with WT virus, but not ΔgL, retained a low capacity to induce production of IFN-α/β (Fig. 4D). In cDCs, macrophages, and MEFs no ΔgL-induced IFN production was observed irrespective of the genetics of the cells (Fig. 4E to G).
When examining the IFN response to the ΔUL15 virus, which contained very little genomic DNA, we looked in splenic pDCs and cDCs, since both of these cell types produce IFN independently of HSV replication (Fig. 2C to E). The cells were treated with WT or ΔUL15 virus, followed by measurement of the type I IFN in the culture supernatant harvested at 20 h p.i. As seen in Fig. 4H, the pDCs produced IFN in response to HSV-1 in a manner dependent on TLR9, and this response was strongly reduced during infection with ΔUL15 virus. The cDCs responded to the infection by producing IFN-α/β independent of TLR9, but again the ΔUL15 virus failed to induce a full type I IFN response (Fig. 4I).
Finally, we examined the ability of the two mutant viruses to stimulate IFN-α/β expression in vivo in WT and TLR9−/− mice. We observed that the ΔgL virus induced less IFN in TLR9−/− than C57BL/6 mice, although a significant response was still observed (Fig. 4J). In the TLR9−/− mice, the ΔgL virus did not trigger a type I IFN response. The ΔUL15 virus was also impaired with respect to induction of IFN-α/β in serum, but after infection with this virus we observed comparable levels in WT and TLR9−/− mice (Fig. 4J).
Collectively, these data indicate that the IFN response to HSV infection in pDCs is dependent on TLR9, viral genomic DNA, and partly on viral entry. IFN-α/β production by cDCs occurred independently of TLR9 but required viral entry and virion DNA, although we cannot formally exclude that the low amount of DNA present in the ΔUL15 virus could be sufficient to trigger a cellular DNA sensor and that a separate mechanism is involved. Finally, macrophages and MEFs produce IFN in response to replicating HSV and hence rely on viral entry to initiate this response.
MAVS−/− fibroblasts are unable to produce type I IFN in response to HSV.
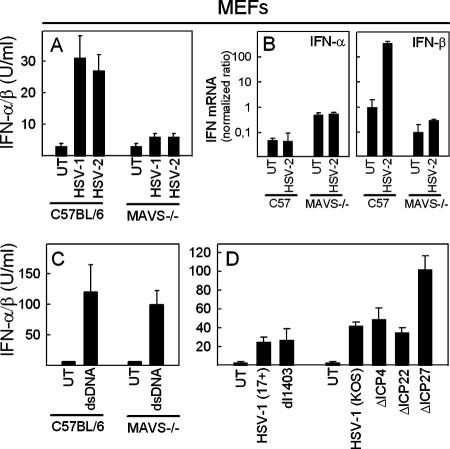
To address which recognition system could be responsible for the observed IFN production in macrophages and fibroblasts, we infected WT and MAVS−/− fibroblasts with HSV-1 and HSV-2 and looked for the accumulation of type I IFN in the culture supernatant. As observed in previous experiments, the fibroblasts induced a modest but reproducible IFN response to the infection, and this was absent in cells from MAVS−/− mice (Fig. 5A). Real-time PCR revealed that type I IFN was detectable at the mRNA level after 4 h of infection and that it consisted primarily of IFN-β (Fig. 5B).
FIG. 5.
MEFs use the MAVS pathway to induce type I IFN production in response to HSV infection. (A and B) MEFs from C57BL/6 or MAVS−/− mice were seeded and infected with 3 × 106 PFU of HSV-1 or HSV-2/ml. After 24 and 4 h, supernatants and total RNA were harvested for measurement of the IFN-α/β bioactivity (A) and mRNA (B), respectively. (C) MEFs from C57BL/6 or MAVS−/− mice were seeded and transfected with calf thymus-derived DNA using Lipofectamine 2000. Twenty-four hours later the supernatants were harvested for measurement of the IFN-α/β bioactivity. (D) MEFs from C57BL/6 mice were seeded and infected with 3 × 106 PFU/ml of the 17+ or KOS strains of HSV-1 and the indicated mutants. After 24 h, the supernatants were harvested, and the IFN-α/β levels were measured. For all data in this figure, similar results were obtained in three independent experiments. Error bars indicate the SEM.
The role of MAVS in double-stranded DNA (dsDNA) signaling is controversial. RNA interference studies first suggested MAVS to be involved in signaling in response to dsDNA (11), but studies in MAVS−/− mice subsequently led to the conclusion that MAVS is not involved in this process (17, 40). Recently, Chisari and coworkers further suggested that RIG-I/MAVS is involved in recognition of dsDNA and induction of IFN-β expression in the human hepatoma cell line Huh-7 (5). Therefore, in order to examine the role of MAVS in dsDNA signaling in our system, we transfected WT and MAVS−/− MEFs with calf-thymus-derived DNA and looked for production of IFN-α/β. This stimulus led to a robust induction of IFN-α/β production in both WT and MAVS−/− cells (Fig. 5C). Thus, MAVS is not involved in the induction of type I IFN production in MEFs in response to dsDNA.
The finding that the IFN-β transcript was detectable after 4 h of infection suggested that viral IE mRNA was the PAMP activating the MAVS pathway. Therefore, we infected C57BL/6 MEFs with HSV IE gene mutant viruses and looked for production of IFN-α/β. In the dl1403 strain, only the region encoding the N-terminal 105 amino acids are present, while the region encoding the C-terminal 670 residues have been deleted. The three other mutant HSV-1 strains used were deletion mutants for the genes ICP4, ICP22, and ICP27. As shown in Fig. 5D, none of the mutants displayed impaired ability to mount an IFN response, and the ICP27 mutant even led to enhanced IFN production, as reported previously (25). Thus, no single viral IE mRNA species seems to be essential for activation of MAVS-dependent IFN-α/β production, although a role for the 5′ end of the ICP0 mRNA cannot be excluded.
DISCUSSION
Type I IFN is produced as the first line of antiviral defense in response to innate immune recognition of viruses (8). In the present study we show that IFN production during infection with HSV involves a number of different recognition mechanisms, which work in cell-type-specific and time-dependent patterns. Immediate IFN production was mediated largely by pDCs through TLR9, while subsequent IFN production was independent of TLR9 and involved at least two different mechanisms: fibroblasts (and possibly also macrophages) replicating virus were detected through the MAVS pathway, and in cDCs the entering virus was recognized through a novel TLR9 independent mechanism dependent on viral entry and virion DNA.
pDCs are specialized IFN-producing cells and constitute an important source of type I IFN during viral infection (2, 4, 30, 37). These cells largely rely on TLRs to bring about this response (12, 15, 16, 21). An important finding in the present study is that although TLR9/pDCs were responsible for inducing the first wave of IFN-α/β production (16), TLR9 played only a minor role in the antiviral response. Therefore, it seems to be the TLR9-independent IFN response that confers the majority of the early protection. This is in contrast to what has been reported for murine cytomegalovirus, where type I IFN production also occurs in two waves (6). In that case, the initial TLR9/pDC-dependent IFN response was reported to be very important for control of the infection (6). It should be noted that our findings do not exclude a role for TLR9 in the host response against HSV, since it has been shown that pDCs help lymph node DCs to induce anti-HSV cytotoxic T cells (47), and TLR9−/− mice do display impaired host response to HSV-2 in a model for genital herpes (22). However, based on studies in IL-1R-associated kinase 4-deficient patients, Casanova and coworkers have suggested that unresponsiveness to TLR9 is not associated with enhanced susceptibility to HSV infections in humans (46).
Cytoplasmic recognition of viral RNA by the innate immune is mediated by the DExD/H-box helicases RIG-I and MDA5 (1, 48), which recognize RNA motifs including 5′-triphosphorylated RNA and double-stranded RNA (1, 10, 33). The signal transduction downstream of the helicases relies on the adaptor MAVS, which is located in the mitochondrial membrane (36). Activation of the MAVS pathway potently induces the production of type I IFN (14, 26, 36, 45). Several RNA viruses have been shown to be sensed by the innate immune system through the helicases, and two reports have also suggested this system to contribute to recognition of DNA viruses (17, 35). The antiviral activity of MEFs against the vaccinia virus mutant MVA is partially dependent on MAVS/IPS-1 (17), and the Epstein-Barr virus-encoded RNAs EBER interact with RIG-I and activate NF-κB and IRF-3 (35). Our work suggests that HSV is also recognized by a MAVS-dependent PRR in MEFs and possibly also macrophages. Interestingly, this correlated with the cell types where the virus was able to replicate, and we have previously found that HSV replication is associated with the accumulation of double-stranded RNA in the cytoplasm (43). Therefore, we suggest that HSV can activate the MAVS pathway only in permissive cells. We cannot exclude that other HSV strains or clinical isolates can replicate in a broader or more narrow spectrum of cell types and hence activate the MAVS pathway in a different cellular pattern. The induction of IFN mRNA expression in MEFs occurred in the early phase of infection, where the virus expresses IE genes. By using a panel of HSV mutants with deletions in viral IE genes, we tried to identify the viral mRNA specie(s) responsible for activating the MAVS pathway. Since, we found no single virus mutant to be impaired in inducing IFN production, this suggests that viral RNA derived from several genes triggers the MAVS pathway in permissive cells. In the present study we did not address which PRR upstream of MAVS is responsible for the recognition of HSV. This is currently being investigated in the laboratory.
A third mechanism of HSV recognition was found in cDCs that was independent of TLR9 and viral replication but dependent on viral entry and probably also virion DNA. The conclusion that recognition of HSV in cDCs is dependent on virion DNA is based on the use of the ΔUL15 virus, and it should be noted that we cannot formally exclude that the low amount of DNA present in the ΔUL15 virus could be sufficient to trigger a cellular DNA sensor and that a separate mechanism of viral recognition is involved. For instance, viral particle entry is sensed by the host to trigger the expression of a subset of IFN-stimulated genes (32). The detection of cytoplasmic localization of DNA including HSV DNA is known to trigger a type I IFN response through a mechanism involving the recently identified DNA sensor DAI (11, 39, 42), and this does indeed contribute to recognition of DNA viruses (31, 42). Although the mechanism of recognition of cytoplasmic DNA seems to be operative in a broad range of cell types, including MEFs, macrophages, cDCs, and pDCs (11, 39), we found that only in cDCs was this mechanism of HSV recognition essential for inducing a type I IFN response. If the recognition mechanism identified here is identical to the cytoplasmic DNA recognition mechanism reported by others (11, 31, 39, 42), this would suggest that factors other than receptor expression contribute to activation of the pathway in a given cell type. This could include making viral genomic DNA accessible for the receptor. The current understanding of herpesvirus replication does not support the idea of viral DNA being freely accessible for cytoplasmic PRRs (34). Therefore, in order for capsid-enclosed viral DNA to be recognized by a cytoplasmic PRR, the cells must have mechanisms to release the DNA into the cytoplasm during the early stages on infection. This could occur if viral capsids are degraded as they move toward the nucleus or if viral DNA can leak from the capsid. To our knowledge, none of these events has been shown to occur during infection with herpesviruses, and this issue awaits future investigations. However, since it has recently been shown that adenovirus is recognized through cellular detection of virus DNA, this mechanism is likely to play an important role in innate sensing of DNA viruses (31).
HSV is a complex virus, which displays a number of PAMPs to the host during infection (11, 16, 18, 21, 43). We have shown here that pathogen recognition by the innate immune system can involve several PAMP-PRR interactions that work in cell-type-specific patterns to orchestrate the innate immune response.
Acknowledgments
The technical assistance of Kirsten Stadel Petersen and Birthe Søby is greatly appreciated.
This study was supported by research grants from the Danish Medical Research Council (grant 271-06-0438), The Danish Natural Science Research Council (grant 272-05-0222), The Lundbeck Foundation (grants 104/04 and 116/06), the Aarhus University (AU) Research Foundation, and the Research Program in Molecular Medicine, The Faculty of Health Science, AU. L.N.S. and N.A. were supported by fellowships from the Faculty of Health Science, AU, and L.M. was the recipient of a postdoctoral fellowship from the Danish Medical Research Council (grant 271-05-0678).
Footnotes
Published ahead of print on 3 October 2007.
REFERENCES
- 1.Andrews, D. M., A. A. Scalzo, W. M. Yokoyama, M. J. Smyth, and M. A. Degli-Esposti. 2003. Functional interactions between dendritic cells and NK cells during viral infection. Nat. Immunol. 4:175-181. [DOI] [PubMed] [Google Scholar]
- 2.Asselin-Paturel, C., A. Boonstra, M. Dalod, I. Durand, N. Yessaad, C. Dezutter-Dambuyant, A. Vicari, A. O'Garra, C. Biron, F. Briere, and G. Trinchieri. 2001. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2:1144-1150. [DOI] [PubMed] [Google Scholar]
- 3.Baines, J. D., C. Cunningham, D. Nalwanga, and A. Davison. 1997. The UL15 gene of herpes simplex virus type 1 contains within its second exon a novel open reading frame that is translated in frame with the UL15 gene product. J. Virol. 71:2666-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorck, P. 2001. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood 98:3520-3526. [DOI] [PubMed] [Google Scholar]
- 5.Cheng, G., J. Zhong, J. Chung, and F. V. Chisari. 2007. Double-stranded DNA and double-stranded RNA induce a common antiviral signaling pathway in human cells. Proc. Natl. Acad. Sci. USA 104:9035-9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delale, T., A. Paquin, C. Asselin-Paturel, M. Dalod, G. Brizard, E. E. Bates, P. Kastner, S. Chan, S. Akira, A. Vicari, C. A. Biron, G. Trinchieri, and F. Briere. 2005. MyD88-dependent and -independent murine cytomegalovirus sensing for IFN-alpha release and initiation of immune responses in vivo. J. Immunol. 175:6723-6732. [DOI] [PubMed] [Google Scholar]
- 7.Dupuis, S., E. Jouanguy, S. Al Hajjar, C. Fieschi, I. Z. Al Mohsen, S. Al Jumaah, K. Yang, A. Chapgier, C. Eidenschenk, P. Eid, A. Al Ghonaium, H. Tufenkeji, H. Frayha, S. Al Gazlan, H. Al Rayes, R. D. Schreiber, I. Gresser, and J. L. Casanova. 2003. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat. Genet. 33:388-391. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Sastre, A., and C. A. Biron. 2006. Type 1 interferons and the virus-host relationship: a lesson in detente. Science 312:879-882. [DOI] [PubMed] [Google Scholar]
- 9.Hochrein, H., B. Schlatter, M. O'Keeffe, C. Wagner, F. Schmitz, M. Schiemann, S. Bauer, M. Suter, and H. Wagner. 2004. Herpes simplex virus type-1 induces IFN-α production via Toll-like receptor 9-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA 101:11416-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hornung, V., J. Ellegast, S. Kim, K. Brzozka, A. Jung, H. Kato, H. Poeck, S. Akira, K. K. Conzelmann, M. Schlee, S. Endres, and G. Hartmann. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314:994-997. [DOI] [PubMed] [Google Scholar]
- 11.Ishii, K. J., C. Coban, H. Kato, K. Takahashi, Y. Torii, F. Takeshita, H. Ludwig, G. Sutter, K. Suzuki, H. Hemmi, S. Sato, M. Yamamoto, S. Uematsu, T. Kawai, O. Takeuchi, and S. Akira. 2006. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat. Immunol. 7:40-48. [DOI] [PubMed] [Google Scholar]
- 12.Kato, H., S. Sato, M. Yoneyama, M. Yamamoto, S. Uematsu, K. Matsui, T. Tsujimura, K. Takeda, T. Fujita, O. Takeuchi, and S. Akira. 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity 23:19-28. [DOI] [PubMed] [Google Scholar]
- 13.Kawai, T., and S. Akira. 2006. Innate immune recognition of viral infection. Nat. Immunol. 7:131-137. [DOI] [PubMed] [Google Scholar]
- 14.Kawai, T., K. Takahashi, S. Sato, C. Coban, H. Kumar, H. Kato, K. J. Ishii, O. Takeuchi, and S. Akira. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6:981-988. [DOI] [PubMed] [Google Scholar]
- 15.Krug, A., A. R. French, W. Barchet, J. A. Fischer, A. Dzionek, J. T. Pingel, M. M. Orihuela, S. Akira, W. M. Yokoyama, and M. Colonna. 2004. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity 21:107-119. [DOI] [PubMed] [Google Scholar]
- 16.Krug, A., G. D. Luker, W. Barchet, D. A. Leib, S. Akira, and M. Colonna. 2004. Herpes simplex virus type 1 activates murine natural interferon-producing cells through Toll-like receptor 9. Blood 103:1433-1437. [DOI] [PubMed] [Google Scholar]
- 17.Kumar, H., T. Kawai, H. Kato, S. Sato, K. Takahashi, C. Coban, M. Yamamoto, S. Uematsu, K. J. Ishii, O. Takeuchi, and S. Akira. 2006. Essential role of IPS-1 in innate immune responses against RNA viruses. J. Exp. Med. 203:1795-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurt-Jones, E. A., M. Chan, S. Zhou, J. Wang, G. Reed, R. Bronson, M. M. Arnold, D. M. Knipe, and R. W. Finberg. 2004. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl. Acad. Sci. USA 101:1315-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leib, D. A. 2002. Counteraction of interferon-induced antiviral responses by herpes simplex viruses. Curr. Top. Microbiol. Immunol. 269:171-185. [DOI] [PubMed] [Google Scholar]
- 20.Leib, D. A., T. E. Harrison, K. M. Laslo, M. A. Machalek, N. J. Moorman, and H. W. Virgin. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189:663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lund, J. M., M. M. Linehan, N. Iijima, and A. Iwasaki. 2006. Cutting edge: plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J. Immunol. 177:7510-7514. [DOI] [PubMed] [Google Scholar]
- 23.Malmgaard, L., J. Melchjorsen, A. G. Bowie, S. C. Mogensen, and S. R. Paludan. 2004. Viral activation of macrophages through TLR-dependent and -independent pathways. J. Immunol. 173:6890-6898. [DOI] [PubMed] [Google Scholar]
- 24.Melchjorsen, J., S. B. Jensen, L. Malmgaard, S. B. Rasmussen, F. Weber, A. G. Bowie, S. Matikainen, and S. R. Paludan. 2005. Activation of innate defense against a paramyxovirus is mediated by RIG-I and TLR7 and TLR8 in a cell-type-specific manner. J. Virol. 79:12944-12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melchjorsen, J., J. Siren, I. Julkunen, S. R. Paludan, and S. Matikainen. 2006. Induction of cytokine expression by herpes simplex virus in human monocyte-derived macrophages and dendritic cells is dependent on virus replication and is counteracted by ICP27 targeting NF-κB and IRF-3. J. Gen. Virol. 87:1099-1108. [DOI] [PubMed] [Google Scholar]
- 26.Meylan, E., J. Curran, K. Hofmann, D. Moradpour, M. Binder, R. Bartenschlager, and J. Tschopp. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437:1167-1172. [DOI] [PubMed] [Google Scholar]
- 27.Mittnacht, S., P. Straub, H. Kirchner, and H. Jacobsen. 1988. Interferon treatment inhibits onset of herpes simplex virus immediate-early transcription. Virology 164:201-210. [DOI] [PubMed] [Google Scholar]
- 28.Mogensen, T. H., and S. R. Paludan. 2005. Reading the viral signature by Toll-like receptors and other pattern recognition receptors. J. Mol. Med. 83:180-192. [DOI] [PubMed] [Google Scholar]
- 29.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 30.Nakano, H., M. Yanagita, and M. D. Gunn. 2001. CD11c+ B220+ Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J. Exp. Med. 194:1171-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nociari, M., O. Ocheretina, J. W. Schoggins, and E. Falck-Pedersen. 2007. Sensing infection by adenovirus: Toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. J. Virol. 81:4145-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paladino, P., D. T. Cummings, R. S. Noyce, and K. L. Mossman. 2006. The IFN-independent response to virus particle entry provides a first line of antiviral defense that is independent of TLRs and retinoic acid-inducible gene I. J. Immunol. 177:8008-8016. [DOI] [PubMed] [Google Scholar]
- 33.Pichlmair, A., O. Schulz, C. P. Tan, T. I. Naslund, P. Liljestrom, F. Weber, and Reis e Sousa. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′ phosphates. Science 314:997-1001. [DOI] [PubMed] [Google Scholar]
- 34.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott/Williams & Wilkins, Philadelphia, PA.
- 35.Samanta, M., D. Iwakiri, T. Kanda, T. Imaizumi, and K. Takada. 2006. EB virus-encoded RNAs are recognized by RIG-I and activate signaling to induce type I IFN. EMBO J. 25:4207-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seth, R. B., L. Sun, C. K. Ea, and Z. J. Chen. 2005. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF 3. Cell 122:669-682. [DOI] [PubMed] [Google Scholar]
- 37.Siegal, F. P., N. Kadowaki, M. Shodell, P. A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y. J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science 284:1835-1837. [DOI] [PubMed] [Google Scholar]
- 38.Spear, P. G. 2004. Herpes simplex virus: receptors and ligands for cell entry. Cell Microbiol. 6:401-410. [DOI] [PubMed] [Google Scholar]
- 39.Stetson, D. B., and R. Medzhitov. 2006. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity 24:93-103. [DOI] [PubMed] [Google Scholar]
- 40.Sun, Q., L. Sun, H. H. Liu, X. Chen, R. B. Seth, J. Forman, and Z. J. Chen. 2006. The specific and essential role of MAVS in antiviral innate immune responses. Immunity 24:633-642. [DOI] [PubMed] [Google Scholar]
- 41.Szomolanyi-Tsuda, E., X. Liang, R. M. Welsh, E. A. Kurt-Jones, and R. W. Finberg. 2006. Role for TLR2 in NK cell-mediated control of murine cytomegalovirus in vivo. J. Virol. 80:4286-4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takaoka, A., Z. Wang, M. K. Choi, H. Yanai, H. Negishi, T. Ban, Y. Lu, M. Miyagishi, T. Kodama, K. Honda, Y. Ohba, and T. Taniguchi. 2007. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448:501-505. [DOI] [PubMed] [Google Scholar]
- 43.Weber, F., V. Wagner, S. B. Rasmussen, R. Hartmann, and S. R. Paludan. 2006. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 80:5059-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitley, R. J., and B. Roizman. 2001. Herpes simplex virus infections. Lancet 357:1513-1518. [DOI] [PubMed] [Google Scholar]
- 45.Xu, L. G., Y. Y. Wang, K. J. Han, L. Y. Li, Z. Zhai, and H. B. Shu. 2005. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol. Cell 19:727-740. [DOI] [PubMed] [Google Scholar]
- 46.Yang, K., A. Puel, S. Zhang, C. Eidenschenk, C. L. Ku, A. Casrouge, C. Picard, H. von Bernuth, B. Senechal, S. Plancoulaine, S. Al Hajjar, A. Al Ghonaium, L. Marodi, D. Davidson, D. Speert, C. Roifman, B. Z. Garty, A. Ozinsky, F. J. Barrat, R. L. Coffman, R. L. Miller, X. Li, P. Lebon, C. Rodriguez-Gallego, H. Chapel, F. Geissmann, E. Jouanguy, and J. L. Casanova. 2005. Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity. 23:465-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoneyama, H., K. Matsuno, E. Toda, T. Nishiwaki, N. Matsuo, A. Nakano, S. Narumi, B. Lu, C. Gerard, S. Ishikawa, and K. Matsushima. 2005. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J. Exp. Med. 202:425-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 49.Zhou, S., E. A. Kurt-Jones, L. Mandell, A. Cerny, M. Chan, D. T. Golenbock, and R. W. Finberg. 2005. MyD88 is critical for the development of innate and adaptive immunity during acute lymphocytic choriomeningitis virus infection. Eur. J. Immunol. 35:822-830. [DOI] [PubMed] [Google Scholar]