Abstract
Box C/D snoRNPs, factors essential for ribosome biogenesis, are proposed to be assembled in the nucleoplasm before localizing to the nucleolus. However, recent work demonstrated the involvement of nuclear export factors in this process, suggesting that export may take place. Here we show that there are distinct distributions of U8 pre-snoRNAs and pre-snoRNP complexes in HeLa cell nuclear and cytoplasmic extracts. We observed differential association of nuclear export (PHAX, CRM1, and Ran) factors with complexes in the two extracts, consistent with nucleocytoplasmic transport. Furthermore, we show that the U8 pre-snoRNA in one of the cytoplasmic complexes contains an m3G cap and is associated with the nuclear import factor Snurportin1. Using RNA interference, we show that loss of either PHAX or Snurportin1 results in the incorrect localization of the U8 snoRNA. Our data therefore show that nuclear export and import factors are directly involved in U8 box C/D snoRNP biogenesis. The distinct distribution of U8 pre-snoRNP complexes between the two cellular compartments together with the association of both nuclear import and export factors with the precursor complex suggests that the mammalian U8 snoRNP is exported during biogenesis.
In the eukaryotic nucleolus, small nucleolar RNAs (snoRNAs) are essential for the production of functional ribosomes (2, 29, 47). The U3 and U8 box C/D snoRNAs are essential for rRNA processing and are believed to function as chaperones directing the folding and cleavage of the pre-rRNA (47). These two snoRNAs are stably associated with larger, pre-rRNA processing complexes in mammalian cell extracts (48). A subset of mammalian box C/D snoRNAs, which include U3, U8, and U13, are independently transcribed by RNA polymerase II, and the mature transcripts possess an m3G cap (24). The initial transcripts contain an m7G cap and a short 3′ extension. The precursors are matured by removal of the 3′ extension and hypermethylation of the m7G cap to m3G (10, 24, 54, 55). However, the majority of box C/D snoRNAs are encoded in introns, do not contain a 5′ cap structure, and, with one or two exceptions, direct the site-specific 2′ O methylation of the rRNA through base pairing to the target sequence (19). Box C/D snoRNAs contain a conserved box C/D motif to which the four core proteins, namely, 15.5K, NOP56, NOP58, and fibrillarin, bind (47). These proteins assemble onto the box C/D motif in a stepwise manner, with the initial binding of 15.5K necessary for recruitment of the three remaining core proteins (51).
The box C/D snoRNAs are transcribed and assembled into pre-snoRNPs in the nucleoplasm. We have shown recently that the nucleoplasmic U3 pre-snoRNA is present in a large dynamic multiprotein complex during snoRNP biogenesis (52). This complex contains proteins involved in assembly (TIP48 and TIP49) and RNA processing (La, LSm, the exosome, and TGS1) and mediates the maturation of the snoRNP. The U3 pre-snoRNP was also associated with export factors, namely, the phosphorylated export adaptor PHAX, the cap-binding complex, Ran, and the exportin CRM1 as well as the nucleocytoplasmic shuttle protein Nopp140 (5, 52). PHAX and CRM1 bind sequentially to the U3 pre-snoRNP and are required for Cajal body and nucleolar localization, respectively (5). In addition, PHAX is required for the maintenance of snoRNA levels, suggesting that this protein is also involved in snoRNP assembly (5, 52).
The m3G-capped U1, U2, U4, and U5 snRNAs, which are key components of the pre-mRNA splicing machinery, are associated with the seven core Sm proteins. The snRNAs are transcribed by RNA polymerase II, and the initial precursor contains an m7G cap and a short 3′ extension that is processed to form the mature RNA (55). In the nucleus, the nascent pre-snRNA is bound by a series of export factors, namely, the cap-binding complex, PHAX, CRM1, and Ran (19, 55). This complex is rapidly exported to the cytoplasm, where GTP hydrolysis by Ran results in the release of CRM1 and Ran (22). Dephosphorylation of PHAX and the binding of the import factors importin α and importin β to the cap-binding complex result in release of the remaining export factors (12, 33). In the cytoplasm, the core Sm proteins are assembled onto the snRNA, an event mediated by the SMN complex (8, 14). This in turn is a prerequisite for processing the 3′ extension and hypermethylation of the m7G to an m3G cap by TGS1. The assembled Sm core and the modified cap then function as independent nuclear localization signals for the subsequent reimport of the mature snRNP into the nucleus (55). The m3G cap is recognized by Snurportin1, an import adaptor that interacts with importin β (16). The Sm core-mediated import is linked to the nuclear import of SMN; however, the import adaptor has yet to be identified (28). After import, the snRNP complex localizes first to the Cajal body, where the RNA is modified, before localizing to the splicing speckles from where the complexes are recruited to sites of active splicing (43).
SnoRNP biogenesis in Xenopus laevis oocytes was originally proposed to include a cytoplasmic phase (3). Later work suggested that snoRNP biogenesis takes place in the nucleus and that the U3 snoRNA was not exported to the cytoplasm (45, 46). From this it was proposed that snoRNP biogenesis occurs solely in the nucleus. However, it has since been shown that U8 and U22 snoRNAs, injected into the cytoplasm, are imported into the nuclei of Xenopus oocytes (36). Furthermore, as noted above, the association of PHAX with mammalian U3, U8, and U13 snoRNAs and the complete export complex (i.e., PHAX, cap-binding complex, CRM1, and Ran) with the U3 box C/D snoRNA has recently been shown (5, 52). This suggested that either the capped mammalian pre-snoRNAs are exported to the cytoplasm or the export factors perform a novel nuclear role in box C/D snoRNP biogenesis. The question of whether nucleocytoplasmic transport of box C/D snoRNAs occurs in mammalian somatic cells had, however, not been addressed directly. In addition, almost all of the work to date analyzing snoRNP transport is based on the injection of RNAs into the cell, and little has been done to study the endogenous, naturally produced complexes. It was therefore important to further investigate the role of nuclear export factors in snoRNP biogenesis. In this paper we show that U8 snoRNP biogenesis involves both nuclear import and export factors and that pre-snoRNPs are present in both HeLa nuclear and cytoplasmic extracts, suggesting that these complexes undergo nucleocytoplasmic transport.
MATERIALS AND METHODS
Extract preparation and analysis.
HeLa nuclear extracts or cytoplasmic extracts were prepared as described previously (7) and fractionated on 10 to 30% glycerol gradients containing 150 mM KCl according to the method of Schneider et al. (41). As described previously (52), during this standard extract procedure, the nucleoli, containing the mature snoRNPs, are pelleted along with the cellular debris in the final centrifugation step. Immunoblot and immunoprecipitation analyses were performed as described previously (51). Antibodies recognizing NOP56, NOP58, TIP48, and the anticap antibodies R1131 and H20 were described previously (4, 21, 51, 53). TIP49 antibodies were provided by Stuart Maxwell (32). Fibrillarin antibodies were provided by Michael Pollard. La and hRrp46 antibodies were provided by Ger Pruijn (6, 40). PHAX antibodies were provided by Iain Mattaj (33) and TGS1 antibodies by Remy Bordonne (50). CRM1 antibodies were either provided by Achim Dickmanns and Ralf Kehlenbach (18) or purchased from Santa Cruz Biotech. Ran antibodies were purchased from Santa Cruz Biotech. Snurportin1 antibodies were described previously (16).
siRNA transfection and cell culture.
All small interfering RNA (siRNA) duplexes were designed as 21-mers with 3′dTdT overhangs (9). For the Snurportin1 knockdown, siRNA duplexes targeting the sequences CAGACTGATTTCCGATTCTACTG and GAGGTTCCCAGATTGCGTAGCAT in the cDNA (GenBank accession number NM_005701) were used. The siRNAs used for PHAX depletion were described previously (52). The GL2 siRNA (which targets the firefly luciferase gene) was used as a control (9). Sixty hours after transfection, cells were fixed and analyzed by in situ hybridization using fluorescent U8 and U2 antisense probes as described previously (13). The same exposure time was used for each fluorescent probe. Two distinct siRNA duplexes were required for the complete knockdown of PHAX and Snurportin1, as individual duplexes resulted in an incomplete knockdown of the protein. A weak effect on snoRNA localization was observed upon use of the individual siRNA duplexes (data not shown). This effect was strengthened significantly by the combined use of two siRNA duplexes in the knockdown experiments. The differential interference contrast image and the nucleoplasmic signal seen for the U2 snoRNA were both used to make sure that both the nuclear and cytoplasmic compartments were visible and in focus prior to image capture. Image analysis was performed using Axiovision software (Zeiss). A defined region of the nucleoplasm and one of the cytoplasm were selected based on differential interference contrast images (not shown) from 25 to 30 cells and the mean intensity of each area calculated. In order to avoid overexposed areas close to the nucleoli, care was taken to select nucleoplasmic regions in close proximity to the nuclear membrane and as far away from the nucleoli as possible. For nucleolar signals, the whole nucleolar region in each cell was analyzed.
RESULTS
U8 pre-snoRNAs are present in a large, dynamic multiprotein complex in nuclear extracts.
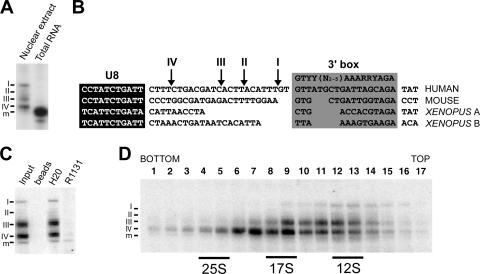
Previously, we have characterized the association of both core box C/D snoRNP proteins and biogenesis factors with the precursor and mature form of the U3 snoRNA in nuclear extracts (52). Analysis of the low-abundance pre-snoRNP complexes was facilitated by the fact that nucleoli, which contain the vast majority of the mature box C/D snoRNPs, were removed during the preparation of nuclear extract according to the method of Dignam et al. (7, 52). During this work, we noticed multiple U8 snoRNA species in nuclear extracts which we believe represent specific steps in the biogenesis of this complex. As shown in Fig. 1A, polyacrylamide gel electrophoresis and Northern blot analysis revealed a single U8 snoRNA species in RNA derived from whole cells. In contrast, the mature-length U8 snoRNA was largely underrepresented in nuclear extract (excludes nucleolar material), which primarily contains longer forms of the U8 snoRNA (intermediates I through IV) (Fig. 1A). It is important to note that, due to the low abundance of the pre-snoRNAs present in nuclear extract (about 1% of the total U8 in the cell), about 100× more nuclear RNA than total RNA was loaded. Primer extension revealed a single 5′ end for the precursor transcripts, indicating that the U8 snoRNAs differ in length of the 3′ extension (data not shown). The relative lengths of the 3′ extensions are indicated schematically in Fig. 1B and compared to the downstream sequences of the mouse and Xenopus U8 genes (37). As with the U3 snoRNA, the human U8 3′ extension contains U-rich stretches. Based on the position of the 3′ box in the U8 gene, the sequence element linked to 3′ processing and transcription termination in small RNA genes, it is likely that intermediate I represents the initial precursor transcript (49).
FIG. 1.
Nuclear extract contains multiple U8 pre-snoRNAs. (A) Northern blot analysis of U8 snoRNAs in RNA isolated from either nuclear extract or total RNA. Note that more nuclear extract RNA (100-fold) than total nuclear RNA was loaded, due to the different amounts of U8 snoRNA in each extract. The four U8 precursor transcripts (I to IV) and mature-length U8 snoRNA (m) are indicated on the left. (B) Comparison of human, mouse, and Xenopus U8 snoRNA 3′ extension sequences. DNA sequences are aligned with respect to the coding region (white text on black background) and the 3′ box (gray box). The 3′ box was identified based on previous works (15, 30, 31). The 3′ ends of the human U8 snoRNA precursor transcripts, as derived from RNA analysis, are indicated by arrows at the top of the alignment. (C) Analysis of the cap structure of U8 pre-snoRNAs present in nuclear extract. RNA was isolated from nuclear extract and then immunoprecipitated with antibodies that recognize either an m3G cap (R1131) or both an m7G and an m3G cap structure (H20) or with protein A-Sepharose alone (beads). Precipitated RNAs were then analyzed by polyacrylamide gel electrophoresis and Northern hybridization. The antibodies used are indicated at the top of each lane. Input, 10% of the starting material used for immunoprecipitation. The U8 precursors (I to IV) and mature-length transcript (m) are indicated on the left. (D) Sedimentation behaviors of snoRNPs present in HeLa nuclear extract separated on a 10 to 30% glycerol gradient are shown. RNAs present in each fraction were isolated and separated on an 8% polyacrylamide-7 M urea gel. The sedimentation coefficients of the major snRNP peaks (data not shown) are indicated at the bottom. Fraction numbers are indicated at the top. The U8 snoRNAs were detected by Northern blotting. The U8 precursor (I to IV) and mature-length transcript (m) are indicated. Note that different extracts show differences in the relative levels of the distinct U8 species (cf. Fig. 1D and 3). While the same-length U8 species are present in both extracts and each transcript has the same sedimentation behavior in glycerol gradients, the relative levels of precursors I and II and the mature-length RNA vary. The reduced levels of RNA seen with fraction 8 relative to levels of fractions 7 and 9 are due to a slight error in gel loading.
We next analyzed the status of the cap on the nucleoplasmic U8 snoRNAs. Antibodies that recognize either the m3G cap (R1131) or both the m7G and the m3G cap (H20) were used to immunoprecipitate RNA isolated from nuclear extract. The immunoprecipitated RNA was then analyzed by Northern hybridization (Fig. 1C). H20 antibodies precipitated all forms of the U8 snoRNA. The m3G-specific R1131 antibodies precipitated the mature-length U8 and a small amount of precursor IV.
We next characterized the various U8 pre-snoRNP complexes present in HeLa nuclear extracts by glycerol gradient centrifugation. The sedimentation behavior of the U8 box C/D snoRNAs in the gradient fractions was analyzed by Northern hybridization. The U8 precursors and the mature-length RNA exhibited different sedimentation behaviors in the glycerol gradient (Fig. 1D). The two longest intermediates (I and II) sedimented at 12S (see also Fig. 3 for intermediate II). In contrast, the shortest intermediates (III and IV) sedimented in a broad peak from 12S to 17S, with intermediate IV found predominantly in a 17S peak. Based on the glycerol gradient data as well as immunoprecipitation work (see below), we believe that the broad precursor U8 snoRNA peak is comprised of two complexes sedimenting at 12S (fractions 10 to 14) and 17S (fractions 6 to 11) in the glycerol gradient. The presence of multiple processing intermediates for the U8 pre-snoRNA suggests a more complicated process of U8 3′ maturation, possibly due to the length or sequence of the initial transcript, which results in the accumulation of more-distinct biogenesis intermediates than were observed for U3 snoRNA (52). In contrast to the pre-snoRNAs, the mature-length RNA present in the nuclear extract did not clearly resolve on the gradient and was commonly found in the majority of the gradient fractions (see also Fig. 3). This could imply that the mature transcript is present in multiple, diverse complexes. Importantly, due to the lack of significant quantities of nucleolar material, the 10S and 80S U8 snoRNP complexes identified by Tyc and Steitz (48) were not detected in our nuclear extracts (52; data not shown).
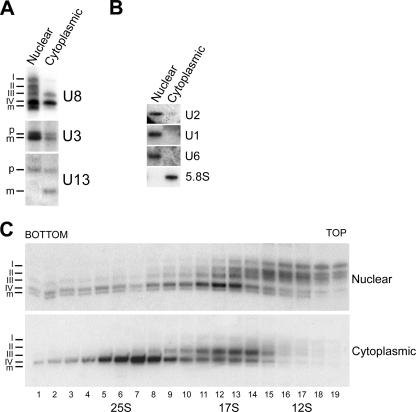
FIG. 3.
Comparison of snoRNP complexes present in nuclear and cytoplasmic extracts. (A) RNA was extracted from HeLa nuclear and cytoplasmic extracts, derived from an equivalent number of cells, and separated on an 8% polyacrylamide-7 M urea gel. U8, U3, and U13 snoRNAs were detected by Northern blotting. The probe used is indicated on the right of each panel. The source of the RNA is indicated above each lane. The positions of the U8 precursors (I to IV) and mature-length transcripts (m), as well as the precursor (p) and mature (m) U3 and U13 snoRNAs, are indicated on the left. (B) The nuclear and cytoplasmic RNAs were separated on an 8% polyacrylamide-7 M urea gel and analyzed by Northern blotting. The identities of the major nuclear and cytoplasmic RNAs analyzed are indicated on the left and right of the image, respectively. The source of the RNA is indicated above each lane. (C) HeLa nuclear and cytoplasmic extracts were separated on 10 to 30% glycerol gradients. RNAs present in each fraction of both gradients were isolated and separated on an 8% polyacrylamide-7 M urea gel. U8 snoRNAs were detected by Northern blotting. The sedimentation coefficients of the major snRNP peaks are indicated at the bottom, and the identities of the RNAs are marked on the left. The extract used is indicated on the right. Fraction numbers are indicated at the bottom. The U8 precursors (I to IV) and mature-length transcripts (m) are indicated.
To further characterize the distinct U8 pre-snoRNA intermediates, we next analyzed the association of a number of biogenesis factors with the U8 snRNP. Individual fractions from glycerol gradients encompassing the U8 snoRNP peak (fractions 8 through 14) were analyzed by immunoprecipitation using a collection of antibodies specific to the core box C/D snoRNP proteins (NOP56, NOP58, and fibrillarin), assembly factors (TIP48 and TIP49), RNA processing components (La, LSm4, and exosome component hRrp46), or nuclear export proteins (PHAX, CRM1, and Ran). The coprecipitated RNAs were analyzed by Northern hybridization.
Antibodies specific to NOP56, NOP58, and fibrillarin each coprecipitated all of the precursor transcripts as well as the mature-length U8 snoRNA present in nuclear extract (Fig. 2A; data not shown). In contrast, anti-TIP48, -hRrp46, -LSm4, -PHAX, and -Ran antibodies preferentially coprecipitated intermediates III and IV. Anti-CRM1 antibodies preferentially coprecipitated intermediate IV, although a clear signal for intermediate III was seen reproducibly. Antibodies specific for La and TIP49 coprecipitated only intermediates I and IV, respectively. As a comparison, we also analyzed the coprecipitation of the mature U8 snoRNP present in nucleolar extracts. Importantly, only antibodies specific for the core box C/D proteins, but not the biogenesis factors, coprecipitated the mature U8 snoRNA (Fig. 2B; data not shown). This strongly suggests that the biogenesis factors dissociate from the snoRNP prior to nucleolar localization.
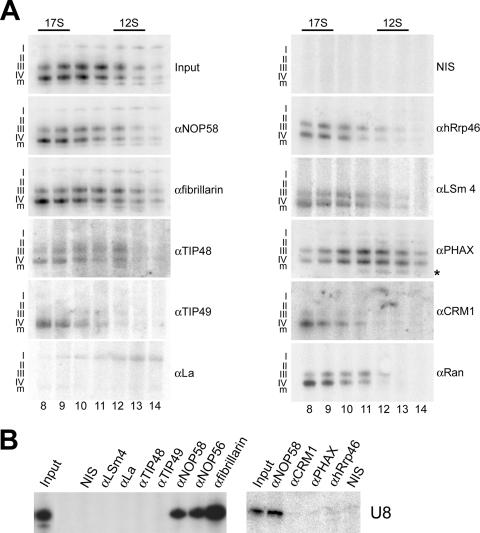
FIG. 2.
(A) Differential association of proteins with precursor and mature U8 snoRNAs. Nuclear extract was separated on a 10 to 30% glycerol gradient, and the gradient fractions containing the U8 snoRNA (Fig. 1D, fractions 8 through 14) were used for immunoprecipitation with either protein-specific antibodies or control nonimmune serum (NIS). The RNAs precipitated from each fraction were isolated and separated on an 8% polyacrylamide-7 M urea gel and the U8 snoRNA revealed by Northern blotting using a probe specific for the U8 snoRNA. The antibody used is indicated on the right of each panel. The gradient fraction numbers are indicated at the bottom. The sedimentation coefficients of the major snRNP peaks (data not shown) are indicated at the top. The U8 precursors (I to IV) and mature-length transcript (m) are indicated on the left of each panel. Input, RNA derived from 10% of the material used for immunoprecipitation. The asterisk indicates a degradation product that runs faster than the mature-length RNA in the anti-PHAX (αPHAX)-coprecipitated RNAs, which was seen only in this experiment. (B) Immunoprecipitations were performed using nucleolar extract, and precipitated RNAs were analyzed as described above. Antibodies used are indicated at the top of the panel. Input, RNA derived from nucleolar extract equivalent to 10% of the material used for immunoprecipitation.
Further analysis of the immunoprecipitation data obtained with nuclear extracts (Fig. 2A) revealed that the proteins are not only differentially associated with the various precursors but also differentially associated with the 12S and 17S U8 pre-snoRNP complexes. NOP58, NOP56, fibrillarin, TIP48, LSm4, and PHAX were associated with both the 12S and 17S complexes. TIP49, hRrp46, CRM1, and Ran were found primarily in the 17S U8 pre-snoRNP. Conversely, La was the only protein we have identified that was associated only with the 12S complex. Furthermore, La was associated just with intermediate I, while antibodies against other biogenesis factors present in the 12S complex did not coprecipitate this pre-snoRNA species. Taken together, this strongly suggests that there are two distinct 12S U8 pre-snoRNP complexes. One complex contains U8 snoRNA intermediate I, La, and the core box C/D proteins. The second complex contains primarily intermediates III and IV and is associated with the core box C/D proteins, LSm proteins, exosome, PHAX, and TIP48. These data clearly indicate that the formation of the U8 snoRNP is a dynamic process involving the specific recruitment and release of a variety of biogenesis factors. It is important to note that fractions 6 and 7 form a part of the 17S peak and that the RNA was associated with the same proteins as that found in fractions 8, 9, 10, and 11 (data not shown).
U8 pre-snoRNPs, containing snoRNA precursors III and IV, are present in cytoplasmic extracts.
As the U3, U8, and U13 snoRNAs are all associated with RNA export factors (5, 52; also this work), we next investigated whether these RNAs are present in the cytoplasm. Cells were fractionated and RNA was isolated from nuclear and cytoplasmic extracts, derived from equivalent numbers of cells. U3, U8, and U13 snoRNAs were detected by polyacrylamide gel electrophoresis and Northern hybridization.
Surprisingly, similar levels of U8 and U13 snoRNA were found in both the cytoplasmic and nuclear extracts (Fig. 3A). As seen previously (Fig. 1), the four precursors and the mature U8 snoRNA were detected in the nucleoplasmic extracts (Fig. 3A, left). In contrast, cytoplasmic extract contained almost exclusively U8 precursors III and IV (Fig. 3A, right). A single U13 pre-snoRNA was observed in nuclear extract, while both the precursor and mature snoRNAs were found in the cytoplasmic extracts. From this, we conclude that while both extracts contain similar amounts of U8 and U13 snoRNAs there are clearly different populations of precursor and mature RNAs in the nucleoplasm and cytoplasm. Our data indicate that approximately 1% of the total RNA for the U8 and U13 snoRNA is present in nuclear and cytoplasmic extracts. In contrast, about fourfold less U3 snoRNA was seen in the cytoplasm than in nuclear extract. Interestingly, the mature-length and pre-U3 snoRNA were present in both the nuclear and cytoplasmic extracts.
To control for leakage during extract preparation, nuclear and cytoplasmic extracts, derived from the same batch of cells, were separated by polyacrylamide gel electrophoresis and analyzed by Northern blotting for major nucleoplasmic and cytoplasmic RNAs. This revealed that the majority (greater than 90%) of the U1, U2, and U6 snRNAs were present in nuclear extracts (Fig. 3B; data not shown), while greater than 90% of the 5.8S rRNA was present in the cytoplasmic fraction. It is worth noting that only approximately 1% of snRNAs are found in cytoplasmic extracts and can be seen only with significantly longer exposures. These data therefore indicate that there was no significant leakage of material during the preparation of the cytoplasmic extracts.
We were next interested in characterizing the U8 pre-snoRNP complexes in cytoplasmic extracts. Nuclear and cytoplasmic extracts were separated by glycerol gradient centrifugation. RNA was isolated from the individual fractions and analyzed by Northern hybridization (Fig. 3C). As described above, the nuclear U8 snoRNA was found in 12S and 17S complexes in the glycerol gradient. Interestingly, U8 snoRNA precursors III and IV were found in 17S complexes in both the nuclear and cytoplasmic extracts. Precursor IV was also found in a significantly larger cytoplasmic-specific complex that sedimented at 25S. Therefore, the nuclear and cytoplasmic extracts each contain a distinct population of both pre-snoRNAs and pre-snoRNP complexes.
Association of import and export factors with the cytoplasmic snoRNPs.
The differential sedimentation behaviors of the two cytoplasmic U8 pre-snoRNPs in glycerol gradients suggest that each complex contains a distinct set of proteins. In particular, the larger 25S complex likely contains significantly more proteins than the 17S U8 pre-snoRNP. In order to identify proteins associated with the two distinct complexes, we analyzed fractions corresponding to the 17S and 25S cytoplasmic U8 snoRNP complexes by immunoprecipitation and Northern blotting as described for the nuclear complexes.
This revealed that the core box C/D proteins NOP58 and fibrillarin were associated with both the 17S and 25S cytoplasmic U8 pre-snoRNP complexes (Fig. 4). Anti-PHAX antibodies coprecipitated 17S but only background levels of 25S U8 pre-snoRNP (Fig. 4). In contrast, antibodies that recognize CRM1 and Ran coprecipitated only background levels of the U8 pre-snoRNAs present in the 17S or 25S complexes, suggesting that these two proteins were not associated with the cytoplasmic pre-snoRNP. We next tested the association of Snurportin1, the m3G cap-binding snRNP import factor, with the cytoplasmic pre-snoRNP complexes. Anti-Snurportin1 antibodies clearly and efficiently coprecipitated the U8 pre-snoRNA present in the 25S cytoplasmic complex (Fig. 4). In addition, very low levels of the U8 snoRNA in the 17S complex were coprecipitated by anti-Snurportin1 antibodies. Therefore, PHAX and Snurportin1 are differentially associated with the two U8 pre-snoRNP complexes present in cytoplasmic extracts. Taken together, these data suggest that the U8 pre-snoRNPs present in cytoplasmic extracts have undergone nuclear export. If this is the case, Snurportin1 likely functions in the reimport of these complexes into the nucleus.
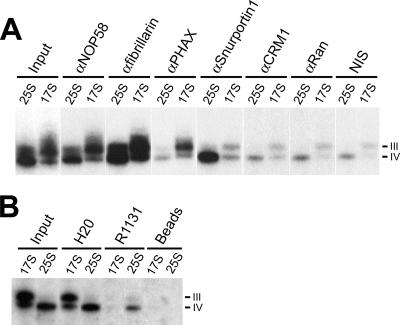
FIG. 4.
Differential association of nuclear export and import factors with cytoplasmic U8 pre-snoRNPs. (A) Cytoplasmic extract was separated on a 10 to 30% glycerol gradient, and the gradient fractions containing the 17S and 25S U8 pre-snoRNP (Fig. 3) were used for immunoprecipitation with either protein-specific antibodies (αNOP58, anti-NOP58) or control nonimmune serum (NIS). The RNAs precipitated from each complex were isolated and separated on an 8% polyacrylamide-7 M urea gel, and the U8 snoRNA was revealed by Northern blotting. Input, 10% of the material used for immunoprecipitation. (B) Analysis of the cap structure of the cytoplasmic U8 pre-snoRNAs. RNA was isolated from the 17S or 25S U8 pre-snoRNP peak fractions and then immunoprecipitated with antibodies that recognize either an m3G cap (R1131) or both an m7G and m3G cap structure (H20) or with protein A-Sepharose alone (beads). Precipitated RNAs were then analyzed by Northern hybridization. The antibody and complex (17S or 25S) used are indicated above each lane. The U8 precursors (III and IV) are indicated on the right. Input, RNA derived from 10% of the material used for immunoprecipitation.
The association of m3G cap-binding protein Snurportin1 with the 25S complex suggested that intermediate IV in the 25S complex contains an m3G cap. To investigate this, RNA isolated from 17S and 25S cytoplasmic complexes was immunoprecipitated with antibodies that recognize either the m3G cap (R1131) or both m7G and m3G caps (H20) and analyzed by Northern hybridization. The H20 antibodies precipitated the RNAs present in both complexes (Fig. 4). In contrast, the m3G-specific R1131 antibodies precipitated only precursor IV from the 25S complex (Fig. 4). This implies that cap hypermethylation occurs in the cytoplasm directly after the release of the export factors and coincides with the recruitment of Snurportin1.
Loss of PHAX and Snurportin1 results in the incorrect localization of the U8 snoRNA.
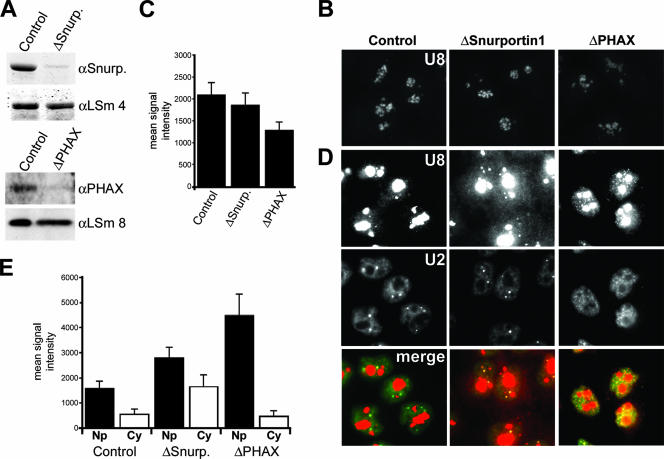
We next used RNA interference to specifically deplete Snurportin1 and PHAX in order to investigate the effect of the loss of these proteins on U8 snoRNP localization in vivo. HeLa cells were transfected with siRNAs, and after 60 h of incubation, cells were fixed and analyzed by fluorescence in situ hybridization using probes specific for the U8 snoRNA and the U2 snRNA. Specific depletion of the individual proteins was monitored by Western blot analysis (52) (Fig. 5A). As a control, cells were transfected with the functional duplex GL2 (targets transcripts derived from the firefly luciferase gene), which does not affect snoRNP biogenesis (52).
FIG. 5.
Snurportin1 and PHAX are required for the correct localization of the U8 snoRNA. (A) Western blot analysis of the depletion of Snurportin1 and PHAX. The siRNA duplex used is indicated above each lane. The antibodies used are indicated on the right (αSnurp., anti-Snurportin1). Proteins derived from equal numbers of cells were loaded. GL2 is the control siRNA targeting luciferase. (B and D) Protein requirement for U8 snoRNA localization. Cells were treated with siRNA duplexes targeting Snurportin1 or PHAX or the control siRNA targeting luciferase (GL2). After 60 h of incubation, the cells were hybridized with fluorescent oligonucleotides complementary to the U8 snoRNA (B and D) and the U2 snRNA (D). The same exposure time was used for each fluorescent probe to allow direct comparison of the subcellular distribution of the RNA after the depletion of specific factors. A short exposure of the U8 snoRNA signal from each of the knockdowns is shown in panel B. A longer exposure of the U8 snoRNA signal, needed to visualize nucleoplasmic and cytoplasmic U8 snoRNA signals, is shown in panel D. The protein targeted is indicated at the top. For each set of cells, the top, middle, and bottom rows of panels show the U8 snoRNA, the U2 snRNA, and an overlay of the two individual images, respectively. The mean levels of U8 snoRNA in the nucleolus (C), nucleoplasm (Np) (E), and cytoplasm (Cy) (E) from control and knockdown cells are represented graphically. The data shown for each knockdown represent the averages of levels derived from 25 to 30 cells. When selecting nucleoplasmic regions for quantitation, care was taken to pick areas close to the nuclear membrane and as far away from the nucleolus as possible to avoid the nucleolar signal. The horizontal axis, in each case, denotes the protein targeted. The vertical axis denotes the mean fluorescent intensity.
The majority of the U8 snoRNA was found primarily in the nucleolus (Fig. 5B) and localized primarily to the dense fibrillar component, as described previously (23), in the control and knockdown cells. We and others have shown previously that PHAX is essential for maintaining snoRNA levels (5, 52). The reduction in nucleolar signal upon knockdown of PHAX is consistent with our previous observation (52) (Fig. 5C). Loss of Snurportin1 had no effect on the levels of the U3 and U8 snoRNAs (data not shown). Longer-exposure images of this experiment revealed that depletion of PHAX resulted in the accumulation of the U8 snoRNA in the nucleoplasm but not in the cytoplasm (Fig. 5D). This is consistent with the earlier observation that loss of PHAX resulted in the accumulation of the box C/D proteins NOP58 and fibrillarin in the nucleoplasm (20). In contrast, knockdown of Snurportin1 resulted in a significant increase in U8 snoRNA levels in the cytoplasm, nucleoplasm, and Cajal bodies relative to levels in the control cells. Quantitation of these data showed that depletion of PHAX resulted in an approximately threefold increase in the average intensity of the nucleoplasmic U8 snoRNA relative to that for the control cells (Fig. 5E). It is important to note that care was taken to ensure that the nuclear and cytoplasmic regions measured were not overexposed. Knockdown of Snurportin1 resulted in twofold and fourfold increases in the average nuclear and cytoplasmic U8 snoRNA levels, respectively. This therefore suggests that PHAX and Snurportin1 are important for the correct subcellular localization of the U8 snoRNA. Importantly, the levels of incorrectly localized snoRNP are consistent with the effects seen upon depletion of the core protein fibrillarin on U3 and U8 localization (25; data not shown) and the loss of PHAX on NOP58 and fibrillarin localization (20). In comparison, knockdown of Snurportin1 and PHAX had no effect on the nucleocytoplasmic distribution of the U2 snRNA. Indeed, loss of Snurportin1 had no effect on the nucleocytoplasmic distribution of any of the snRNAs tested. Two distinct import adaptors mediate spliceosomal snRNP import (55), suggesting that Snurportin1 functions as part of a redundant mechanism. In PHAX knockdown cells, the observed change in subnuclear distribution of the U2 snRNA (Fig. 5B) is consistent with the block in snRNP biogenesis and the loss of Cajal bodies, as previously described (20).
DISCUSSION
Nuclear U8 pre-snoRNP complexes.
We have characterized native U8 box C/D pre-snoRNP complexes present in extracts derived from HeLa cells in order to investigate the biogenesis of this essential RNP. The four distinct U8 pre-snoRNAs present in nuclear extracts possess 4- to 23-nucleotide 3′ extensions and likely represent 3′ processing intermediates. The U8 pre-snoRNPs were associated both with the mature snoRNP proteins and with a diverse range of factors involved in snoRNP assembly (TIP48 and TIP49), pre-snoRNA processing (La, LSm4, and hRrp46), and nuclear export (PHAX, CRM1, and Ran). The association of these proteins with the nucleoplasmic but not the mature nucleolar complexes suggests that they dissociate prior to nucleolar localization. Based on this work and on our previous analysis of the U3 snoRNP, we believe that nuclear extract contains snoRNP biogenesis intermediates.
Glycerol gradient analysis of complexes in nuclear extract, coupled with immunoprecipitation analysis, revealed three distinct U8 pre-snoRNP complexes in nuclear extracts, indicating the dynamic nature of U8 snoRNP biogenesis. Our data indicate that the biogenesis of both U3 and U8, and presumably that of other box C/D snoRNPs, is a dynamic process mediated by large pre-snoRNP complexes. The distinct U8 precursors provide further resolution of the various stages of snoRNP biogenesis. La is associated primarily with the longer, initial transcript (intermediate I), while factors such as LSm4, hRrp46, PHAX, and TIP48 are preferentially bound to intermediates III and IV (Fig. 2A). NOP56, NOP58, and fibrillarin are associated with the initial pre-snoRNA transcript (intermediate I), indicating that the core snoRNP factors are recruited very early in the biogenesis pathway. The association of hRrp46 indicates that the exosome is stably associated with the U8 pre-snoRNP during biogenesis. The multiple precursors suggest either that the U8 pre-snoRNA is processed by more than one exonuclease activity or that the sequence of the 3′ extension may determine the kinetics of 3′ processing, i.e., in a single step or via multiple intermediate stages.
Two distinct U8 snoRNP complexes, which separated at 12S and 17S, were clearly separable by glycerol gradient centrifugation. Immunoprecipitation analysis revealed that CRM1 and Ran were preferentially associated with the 17S complex. In contrast, PHAX was associated with both the 12S and 17S nuclear U8 pre-snoRNP complexes, indicating that this export factor binds before CRM1 and Ran. The stepwise recruitment of the export factors in the nucleoplasmic extracts was observed previously for the U3 pre-snoRNP (5, 52) and is consistent with the previously reported assembly of m7G-capped small RNA export complexes (33).
U8 pre-snoRNPs in cytoplasmic extracts.
U8 pre-snoRNPs were found in cytoplasmic extracts at levels comparable to those found in nuclear extracts. Two distinct U8 pre-snoRNPs, namely, a 17S complex (containing intermediates III and IV) and a 25S complex specific to the cytoplasmic extracts that contained just intermediate IV, were present in the cytoplasm. Immunoprecipitation revealed that both complexes present in the cytoplasmic extracts were associated with the core box C/D proteins. However, neither cytoplasmic complex was associated with CRM1 or Ran. Interestingly, PHAX was bound to the 17S cytoplasmic complex while the 25S cytoplasmic-specific complex was associated with the m3G cap-binding import factor Snurportin1, consistent with the fact that the U8 pre-snoRNA present in this complex contains an m3G cap. Indeed, this is the first time that the nuclear import factor Snurportin1 has been shown to be involved in box C/D snoRNP biogenesis. Comparison of the processing statuses of the U8 pre-snoRNAs in the two complexes present in cytoplasmic extracts suggested that the 25S complex, which contains an m3G cap and just intermediate IV, occurs later in the biogenesis pathway than the 17S complex with the m7G cap and mixture of intermediate IV and the longer intermediate III. This therefore implies that cap hypermethylation coincides with the release of PHAX and that presumably the cap-binding complex would make the cap available for modification by the cap hypermethyltransferase TGS1. Unfortunately, due to the lack of suitable antibodies we were unable to test this. In addition, this suggests that recruitment of the import factor Snurportin1, which likely binds the recently hypermodified cap structure, occurs after release of export factors.
We have found a distinct population of pre-snoRNAs and pre-snoRNP complexes in nuclear and cytoplasmic extracts. Importantly, no significant leakage of similarly sized RNP complexes from the nucleus when preparing the cytoplasmic extract was observed. While we cannot completely rule out the possibility of selective nuclear leakage during extract preparation, our data lead us to suggest that these complexes are naturally present in the cytoplasm. The biochemical data presented here for the U8 snoRNP show strong similarities with the export and subsequent import of the spliceosomal snRNPs (see the introduction). In particular, the stepwise assembly and disassembly of the export factors on the pre-snoRNP complex are almost identical to those seen with the snRNPs. Therefore, given the involvement of nuclear import and export factors in box C/D snoRNP biogenesis our interpretation of these data is that the U8 pre-snoRNP is exported to the cytoplasm during the biogenesis pathway. Indeed, it is likely that the 17S cytoplasmic complex represents the recently exported particle (note the similarity in size to that of the nuclear complex primed for export), while the 25S complex corresponds to a complex that has undergone RNA maturation, has bound the import factor Snurportin1, and is ready for reimport into the nucleus.
RNA interference-mediated knockdown of Snurportin1 resulted in the accumulation of U8 snoRNA in the nucleoplasm and cytoplasm. Conversely, loss of PHAX caused the accumulation of U8 in the nucleoplasm but not the cytoplasm. This implies that these two proteins are important for the correct localization of the U8 snoRNP. While this approach does not directly test the function of the two proteins in nucleocytoplasmic transport, the data are consistent with the potential import and export roles for Snurportin1 and PHAX. Furthermore, the fact that we observe cytoplasmic accumulation of U8 snoRNA upon loss of Snurportin1 is consistent with a cytoplasmic phase to box C/D snoRNP biogenesis. The effect on snoRNA localization observed with these knockdowns is consistent with earlier work analyzing the role of PHAX in Cajal body maintenance (20). In this earlier work, both fibrillarin and NOP58 were shown to accumulate in the nucleoplasm upon the loss of PHAX. In addition, previous work has emphasized the importance of PHAX in maintaining box C/D snoRNA levels (5, 52). Furthermore, the levels of the effects on snoRNA localization seen upon depletion of either PHAX or Snurportin1 are consistent with that seen upon U3 and U8 localization upon the loss of the core box C/D protein fibrillarin (25; data not shown), supporting our proposal that the transport factors are important for snoRNA localization. Our analysis has shown that Snurportin1 is not essential for cell growth (data not shown) or snRNA localization (Fig. 5), consistent with the fact that this protein forms part of a redundant snRNP nuclear import mechanism. The accumulation of some but not all of the U8 snoRNA in the cytoplasm upon loss of Snurportin1 suggests that this protein may form part of a redundant import system for box C/D snoRNPs. Interestingly, the fact that we observe the nucleoplasmic accumulation of the U8 snoRNA in Snurportin1 knockdown cells also suggests a role for this protein in snoRNP biogenesis in the nucleus. Indeed, it is also possible that this protein could associate with the box C/D snoRNPs in the nucleoplasm. Snurportin1 has been linked to Cajal body localization of snRNPs (35) and could also play a role in subnuclear localization of snoRNPs.
Our analysis of the human U8 snoRNP in somatic cells has led us to suggest that this complex is exported to the cytoplasm during biogenesis. It was originally proposed that the U3 snoRNA undergoes nucleocytoplasmic transport in Xenopus oocytes (3). There are also many reports that box C/D snoRNAs are imported and exported from the nucleus in a variety of cell types, including both Xenopus oocytes and mammalian somatic cells (1, 3, 11, 26, 27, 36, 38, 39, 42). However, work with Xenopus oocytes has also suggested that these complexes do not leave the nucleus (45, 46) and are incapable of being imported into the nucleus (44). Two papers have described the localization of fluorescent m7G-capped snoRNAs injected into mammalian cells (5, 17). The snoRNAs were not observed to accumulate in the cytoplasm. However, since nuclear localization occurs in less than 20 s, it is clear that this assay is not suitable to detect a cytoplasmic phase (17). Our data suggest that the box C/D snoRNAs are exported to the cytoplasm during biogenesis. However, there are other normal situations during which these complexes may traverse the nuclear membrane. During serum starvation or at the end of mitosis, box C/D snoRNPs are exported from or imported into the nucleus, respectively (1, 42). The cells used to generate the extracts used in the current work were growing exponentially when harvested. The box C/D snoRNAs present in our cytoplasmic extracts could originate from cells undergoing mitosis. However, the cytoplasmic material contained significant levels of longer, precursor forms of the snoRNAs. If this material was derived from M-phase cells, this would suggest that a large proportion of the box C/D snoRNAs are modified during mitosis. Analysis of box C/D snoRNAs in M-phase cells revealed no significant increase in U3 and U8 pre-snoRNA levels or alterations to the snoRNA length (data not shown). This suggests that the snoRNAs present in our cytoplasmic extracts are not derived from mitotic cells and are therefore likely pre-snoRNAs that are intermediates in the snoRNP biogenesis pathway. However, based on our data we cannot rule out the possibility that, as previously proposed (5), the nuclear export factors have a novel nuclear function independent of nuclear export.
Leptomycin B (CRM1 export inhibitor) treatment of HeLa cells did not inhibit cap hypermethylation of the U3 snoRNA but did block the modification of HeLa snRNAs (5). All of the components necessary for RNA modification/processing (core proteins, TGS1, and exosome) are associated with the pre-snoRNP complex already in the nucleoplasm (52; also this work). Conversely, the snRNAs require nuclear export to associate with the SMN complex and the Sm proteins, components essential to the recruitment of TGS1 and the 3′ processing of the snRNA (55). By blocking CRM1 binding, leptomycin B could conceivably cause the dissociation of the other export factors from the box C/D snoRNP, thereby enabling nuclear maturation of these RNAs.
The U3, U8, and U13 pre-snoRNPs are each associated with nuclear export factors (5, 52; also this work and data not shown), and we find all three pre-snoRNPs in cytoplasmic extracts. If our interpretation is correct, this suggests that nuclear export may be a common aspect of m3G-capped box C/D snoRNP biogenesis. The intronic box C/D snoRNAs contain only a 5′ cap as part of a long pre-mRNA, a feature that would normally preclude the recruitment of PHAX (34). However, PHAX is important for intronic U14 box C/D snoRNA accumulation (5, 52), suggesting that this protein is recruited at some point during biogenesis by an alternative mechanism. However, further work is required to determine whether intronic box C/D snoRNP biogenesis involves nucleocytoplasmic transport.
Acknowledgments
We thank Iain Mattaj, Remy Bordonne, Stuart Maxwell, Ger Pruijn, Achim Dickmanns, Ralph Kehlenbach, and Michael Pollard for generously providing antibodies. We thank Peter Kempkes and Kami Kohansal for excellent technical assistance. We also thank Jeremy Brown and Claudia Schneider for critically reading the manuscript.
This work was supported by the BBSRC and Royal Society (N.J.W.) and by grants from the Deutsche Forschungsgemeinschaft (SFB523), the Fonds der Chemischen Industrie, and the Ernst-Jung-Stiftung (R.L.).
Footnotes
Published ahead of print on 20 August 2007.
REFERENCES
- 1.Azum-Gelade, M. C., J. Noaillac-Depeyre, M. Caizergues-Ferrer, and N. Gas. 1994. Cell cycle redistribution of U3 snRNA and fibrillarin. Presence in the cytoplasmic nucleolus remnant and in the prenucleolar bodies at telophase. J. Cell Sci. 107:463-475. [DOI] [PubMed] [Google Scholar]
- 2.Bachellerie, J. P., J. Cavaille, and A. Huttenhofer. 2002. The expanding snoRNA world. Biochimie 84:775-790. [DOI] [PubMed] [Google Scholar]
- 3.Baserga, S. J., M. Gilmore-Hebert, and X. W. Yang. 1992. Distinct molecular signals for nuclear import of the nucleolar snRNA, U3. Genes Dev. 6:1120-1130. [DOI] [PubMed] [Google Scholar]
- 4.Bochnig, P., R. Reuter, P. Bringmann, and R. Lührmann. 1987. A monoclonal antibody against 2,2,7-trimethylguanosine that reacts with intact, class U, small nuclear ribonucleoproteins as well as with 7-methylguanosine-capped RNAs. Eur. J. Biochem. 168:461-467. [DOI] [PubMed] [Google Scholar]
- 5.Boulon, S., C. Verheggen, B. E. Jady, C. Girard, C. Pescia, C. Paul, J. K. Ospina, T. Kiss, A. G. Matera, R. Bordonne, and E. Bertrand. 2004. PHAX and CRM1 are required sequentially to transport U3 snoRNA to nucleoli. Mol. Cell 16:777-787. [DOI] [PubMed] [Google Scholar]
- 6.Brouwer, R., C. Allmang, R. Raijmakers, Y. van Aarssen, W. V. Egberts, E. Petfalski, W. J. van Venrooij, D. Tollervey, and G. J. Pruijn. 2001. Three novel components of the human exosome. J. Biol. Chem. 276:6177-6184. [DOI] [PubMed] [Google Scholar]
- 7.Dignam, J. D., R. M. Lebovitz, and R. G. Roeder. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eggert, C., A. Chari, B. Laggerbauer, and U. Fischer. 2006. Spinal muscular atrophy: the RNP connection. Trends Mol. Med. 12:113-121. [DOI] [PubMed] [Google Scholar]
- 9.Elbashir, S. M., J. Harborth, K. Weber, and T. Tuschl. 2002. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods 26:199-213. [DOI] [PubMed] [Google Scholar]
- 10.Filipowicz, W., and V. Pogacic. 2002. Biogenesis of small nucleolar ribonucleoproteins. Curr. Opin. Cell Biol. 14:319-327. [DOI] [PubMed] [Google Scholar]
- 11.Glibetic, M., D. E. Larson, N. Sienna, J. P. Bachellerie, and B. H. Sells. 1992. Regulation of U3 snRNA expression during myoblast differentiation. Exp. Cell Res. 202:183-189. [DOI] [PubMed] [Google Scholar]
- 12.Görlich, D., R. Kraft, S. Kostka, F. Vogel, E. Hartmann, R. A. Laskey, I. W. Mattaj, and E. Izaurraide. 1996. Importin provides a link between nuclear protein import and U snRNA export. Cell 87:21-32. [DOI] [PubMed] [Google Scholar]
- 13.Granneman, S., J. Vogelzangs, R. Lührmann, W. J. van Venrooij, G. J. Pruijn, and N. J. Watkins. 2004. Role of pre-rRNA base pairing and 80S complex formation in subnucleolar localization of the U3 snoRNP. Mol. Cell. Biol. 24:8600-8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gubitz, A. K., W. Feng, and G. Dreyfuss. 2004. The SMN complex. Exp. Cell Res. 296:51-56. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez, N., and A. M. Weiner. 1986. Formation of the 3′ end of U1 snRNA requires compatible snRNA promoter elements. Cell 47:249-258. [DOI] [PubMed] [Google Scholar]
- 16.Huber, J., U. Cronshagen, M. Kadokura, C. Marshallsay, T. Wada, M. Sekine, and R. Lührmann. 1998. Snurportin1, an m3G-cap-specific nuclear import receptor with a novel domain structure. EMBO J. 17:4114-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobson, M. R., and T. Pederson. 1998. A 7-methylguanosine cap commits U3 and U8 small nuclear RNAs to the nucleolar localization pathway. Nucleic Acids Res. 26:756-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kehlenbach, R. H., A. Dickmanns, and L. Gerace. 1998. Nucleocytoplasmic shuttling factors including Ran and CRM1 mediate nuclear export of NFAT In vitro. J. Cell Biol. 141:863-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiss, T. 2004. Biogenesis of small nuclear RNPs. J. Cell Sci. 117:5949-5951. [DOI] [PubMed] [Google Scholar]
- 20.Lemm, I., C. Girard, A. N. Kuhn, N. J. Watkins, M. Schneider, R. Bordonne, and R. Lührmann. 2006. Ongoing U snRNP biogenesis is required for the integrity of Cajal bodies. Mol. Biol. Cell 17:3221-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lührmann, R., B. Appel, P. Bringmann, J. Rinke, R. Reuter, S. Rothe, and R. Bald. 1982. Isolation and characterization of rabbit anti-m3 2,2,7G antibodies. Nucleic Acids Res. 10:7103-7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massenet, S., L. Pellizzoni, S. Paushkin, I. W. Mattaj, and G. Dreyfuss. 2002. The SMN complex is associated with snRNPs throughout their cytoplasmic assembly pathway. Mol. Cell. Biol. 22:6533-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matera, A. G., K. T. Tycowski, J. A. Steitz, and D. C. Ward. 1994. Organization of small nucleolar ribonucleoproteins (snoRNPs) by fluorescence in situ hybridization and immunocytochemistry. Mol. Biol. Cell 5:1289-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maxwell, E. S., and M. J. Fournier. 1995. The small nucleolar RNAs. Annu. Rev. Biochem. 64:897-934. [DOI] [PubMed] [Google Scholar]
- 25.McKeegan, K. S., C. M. Debieux, S. Boulon, E. Bertrand, and N. J. Watkins. 2007. A dynamic scaffold of pre-snoRNP factors facilitates human box C/D snoRNP assembly. Mol. Cell. Biol. 27:6782-6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michaud, N., and D. Goldfarb. 1992. Microinjected U snRNAs are imported to oocyte nuclei via the nuclear pore complex by three distinguishable targeting pathways. J. Cell Biol. 116:851-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murdoch, K. J., and L. A. Allison. 1996. A role for ribosomal protein L5 in the nuclear import of 5S rRNA in Xenopus oocytes. Exp. Cell Res. 227:332-343. [DOI] [PubMed] [Google Scholar]
- 28.Narayanan, U., T. Achsel, R. Lührmann, and A. G. Matera. 2004. Coupled in vitro import of U snRNPs and SMN, the spinal muscular atrophy protein. Mol. Cell 16:223-234. [DOI] [PubMed] [Google Scholar]
- 29.Nazar, R. N. 2004. Ribosomal RNA processing and ribosome biogenesis in eukaryotes. IUBMB Life 56:457-465. [DOI] [PubMed] [Google Scholar]
- 30.Neuman de Vegvar, H. E., and J. E. Dahlberg. 1990. Nucleocytoplasmic transport and processing of small nuclear RNA precursors. Mol. Cell. Biol. 10:3365-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuman de Vegvar, H. E., E. Lund, and J. E. Dahlberg. 1986. 3′ end formation of U1 snRNA precursors is coupled to transcription from snRNA promoters. Cell 47:259-266. [DOI] [PubMed] [Google Scholar]
- 32.Newman, D. R., J. F. Kuhn, G. M. Shanab, and E. S. Maxwell. 2000. Box C/D snoRNA-associated proteins: two pairs of evolutionarily ancient proteins and possible links to replication and transcription. RNA 6:861-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohno, M., A. Segref, A. Bachi, M. Wilm, and I. W. Mattaj. 2000. PHAX, a mediator of U snRNA nuclear export whose activity is regulated by phosphorylation. Cell 101:187-198. [DOI] [PubMed] [Google Scholar]
- 34.Ohno, M., A. Segref, S. Kuersten, and I. W. Mattaj. 2002. Identity elements used in export of mRNAs. Mol. Cell 9:659-671. [DOI] [PubMed] [Google Scholar]
- 35.Ospina, J. K., G. B. Gonsalvez, J. Bednenko, E. Darzynkiewicz, L. Gerace, and A. G. Matera. 2005. Cross-talk between snurportin1 subdomains. Mol. Biol. Cell 16:4660-4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peculis, B. A. 2001. snoRNA nuclear import and potential for cotranscriptional function in pre-rRNA processing. RNA 7:207-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peculis, B. A., S. DeGregorio, and K. McDowell. 2001. The U8 snoRNA gene family: identification and characterization of distinct, functional U8 genes in Xenopus. Gene 274:83-92. [DOI] [PubMed] [Google Scholar]
- 38.Peculis, B. A., and J. A. Steitz. 1993. Disruption of U8 nucleolar snRNA inhibits 5.8S and 28S rRNA processing in the Xenopus oocyte. Cell 73:1233-1245. [DOI] [PubMed] [Google Scholar]
- 39.Peculis, B. A., and J. A. Steitz. 1994. Sequence and structural elements critical for U8 snRNP function in Xenopus oocytes are evolutionarily conserved. Genes Dev. 8:2241-2255. [DOI] [PubMed] [Google Scholar]
- 40.Pruijn, G. J., J. P. Thijssen, P. R. Smith, D. G. Williams, and W. J. Van Venrooij. 1995. Anti-La monoclonal antibodies recognizing epitopes within the RNA-binding domain of the La protein show differential capacities to immunoprecipitate RNA-associated La protein. Eur. J. Biochem. 232:611-619. [DOI] [PubMed] [Google Scholar]
- 41.Schneider, C., C. L. Will, O. V. Makarova, E. M. Makarov, and R. Lührmann. 2002. Human U4/U6.U5 and U4atac/U6atac.U5 tri-snRNPs exhibit similar protein compositions. Mol. Cell. Biol. 22:3219-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sienna, N., D. E. Larson, and B. H. Sells. 1996. Altered subcellular distribution of U3 snRNA in response to serum in mouse fibroblasts. Exp. Cell Res. 227:98-105. [DOI] [PubMed] [Google Scholar]
- 43.Sleeman, J. E., and A. I. Lamond. 1999. Newly assembled snRNPs associate with coiled bodies before speckles, suggesting a nuclear snRNP maturation pathway. Curr. Biol. 9:1065-1074. [DOI] [PubMed] [Google Scholar]
- 44.Speckmann, W., A. Narayanan, R. Terns, and M. P. Terns. 1999. Nuclear retention elements of U3 small nucleolar RNA. Mol. Cell. Biol. 19:8412-8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terns, M. P., and J. E. Dahlberg. 1994. Retention and 5′ cap trimethylation of U3 snRNA in the nucleus. Science 264:959-961. [DOI] [PubMed] [Google Scholar]
- 46.Terns, M. P., C. Grimm, E. Lund, and J. E. Dahlberg. 1995. A common maturation pathway for small nucleolar RNAs. EMBO J. 14:4860-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terns, M. P., and R. M. Terns. 2002. Small nucleolar RNAs: versatile trans-acting molecules of ancient evolutionary origin. Gene Expr. 10:17-39. [PMC free article] [PubMed] [Google Scholar]
- 48.Tyc, K., and J. A. Steitz. 1989. U3, U8 and U13 comprise a new class of mammalian snRNPs localized in the cell nucleolus. EMBO J. 8:3113-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uguen, P., and S. Murphy. 2003. The 3′ ends of human pre-snRNAs are produced by RNA polymerase II CTD-dependent RNA processing. EMBO J. 22:4544-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verheggen, C., D. L. Lafontaine, D. Samarsky, J. Mouaikel, J. M. Blanchard, R. Bordonne, and E. Bertrand. 2002. Mammalian and yeast U3 snoRNPs are matured in specific and related nuclear compartments. EMBO J. 21:2736-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watkins, N. J., A. Dickmanns, and R. Lührmann. 2002. Conserved stem II of the box C/D motif is essential for nucleolar localization and is required, along with the 15.5K protein, for the hierarchical assembly of the box C/D snoRNP. Mol. Cell. Biol. 22:8342-8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watkins, N. J., I. Lemm, D. Ingelfinger, C. Schneider, M. Hossbach, H. Urlaub, and R. Lührmann. 2004. Assembly and maturation of the U3 snoRNP in the nucleoplasm in a large dynamic multiprotein complex. Mol. Cell 16:789-798. [DOI] [PubMed] [Google Scholar]
- 53.Watkins, N. J., V. Segault, B. Charpentier, S. Nottrott, P. Fabrizio, A. Bachi, M. Wilm, M. Rosbash, C. Branlant, and R. Lührmann. 2000. A common core RNP structure shared between the small nucleoar box C/D RNPs and the spliceosomal U4 snRNP. Cell 103:457-466. [DOI] [PubMed] [Google Scholar]
- 54.Weinstein, L. B., and J. A. Steitz. 1999. Guided tours: from precursor snoRNA to functional snoRNP. Curr. Opin. Cell Biol. 11:378-384. [DOI] [PubMed] [Google Scholar]
- 55.Will, C. L., and R. Lührmann. 2001. Spliceosomal UsnRNP biogenesis, structure and function. Curr. Opin. Cell Biol. 13:290-301. [DOI] [PubMed] [Google Scholar]