Abstract
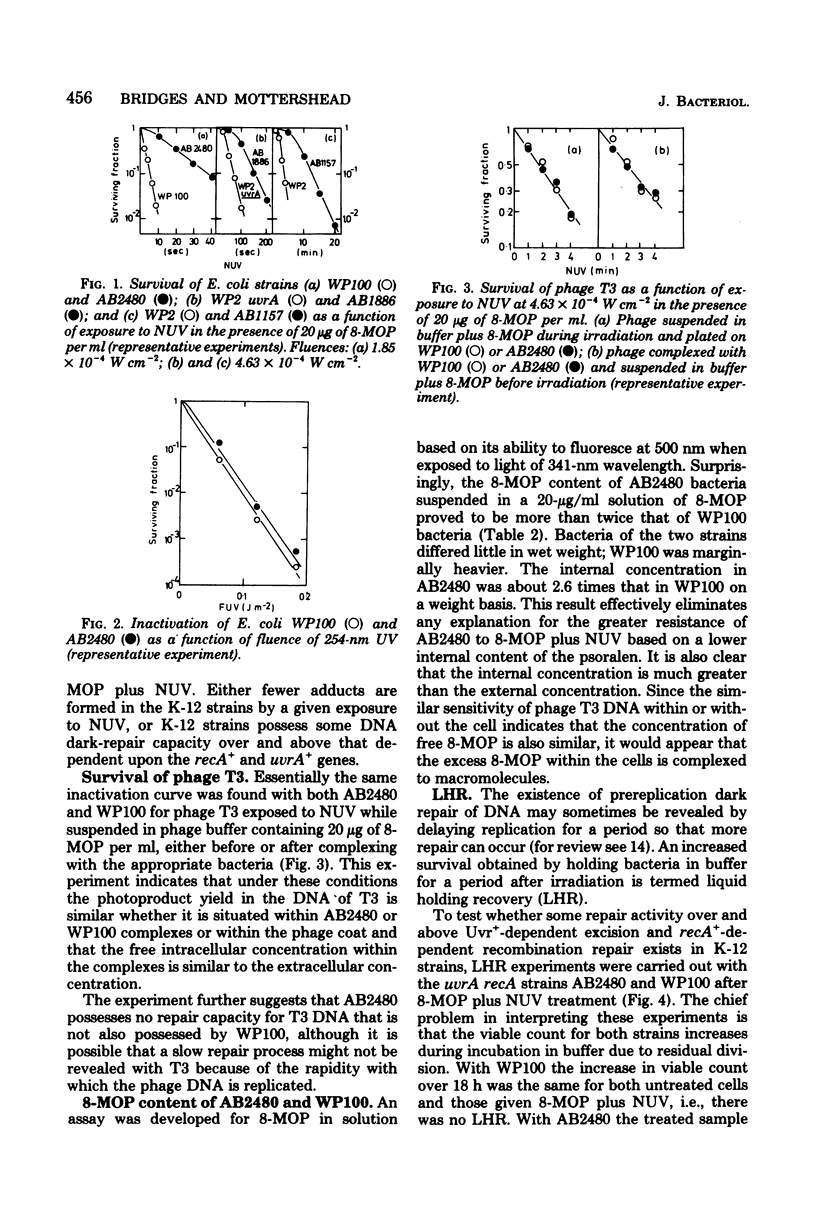
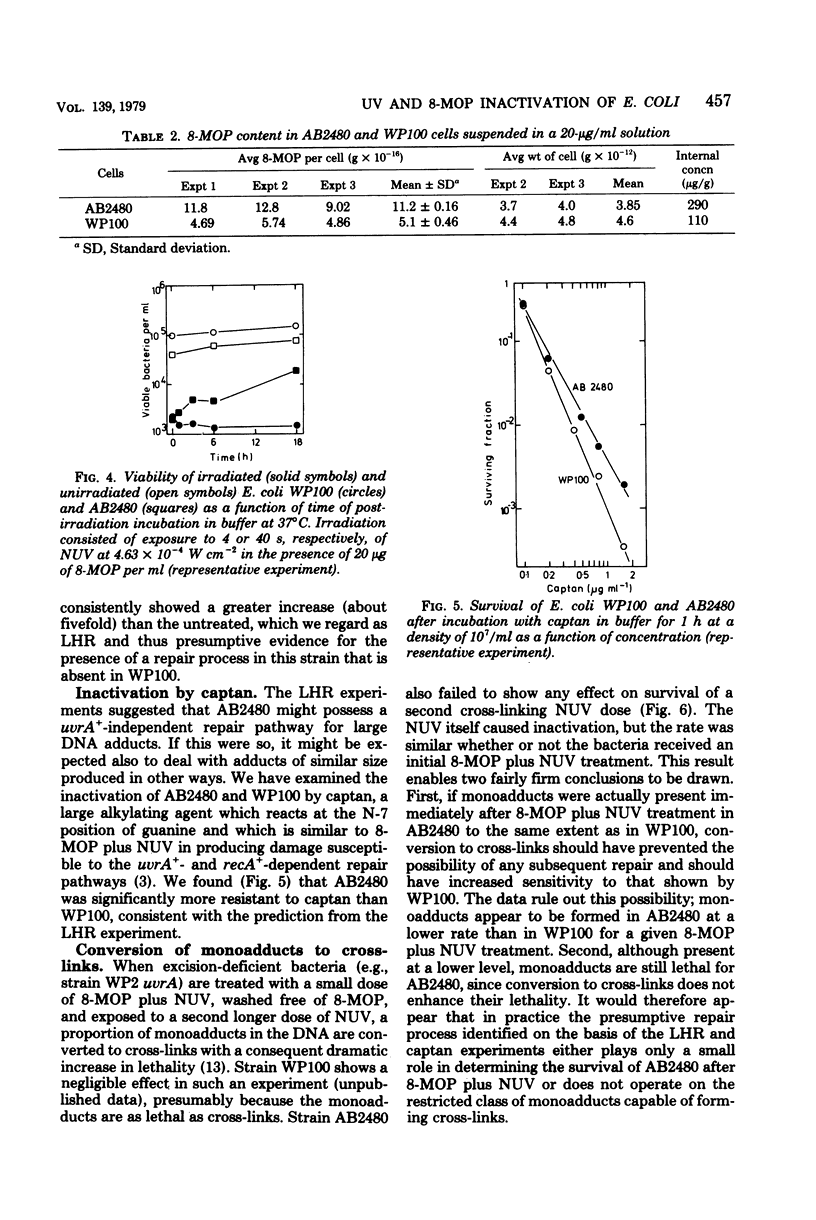
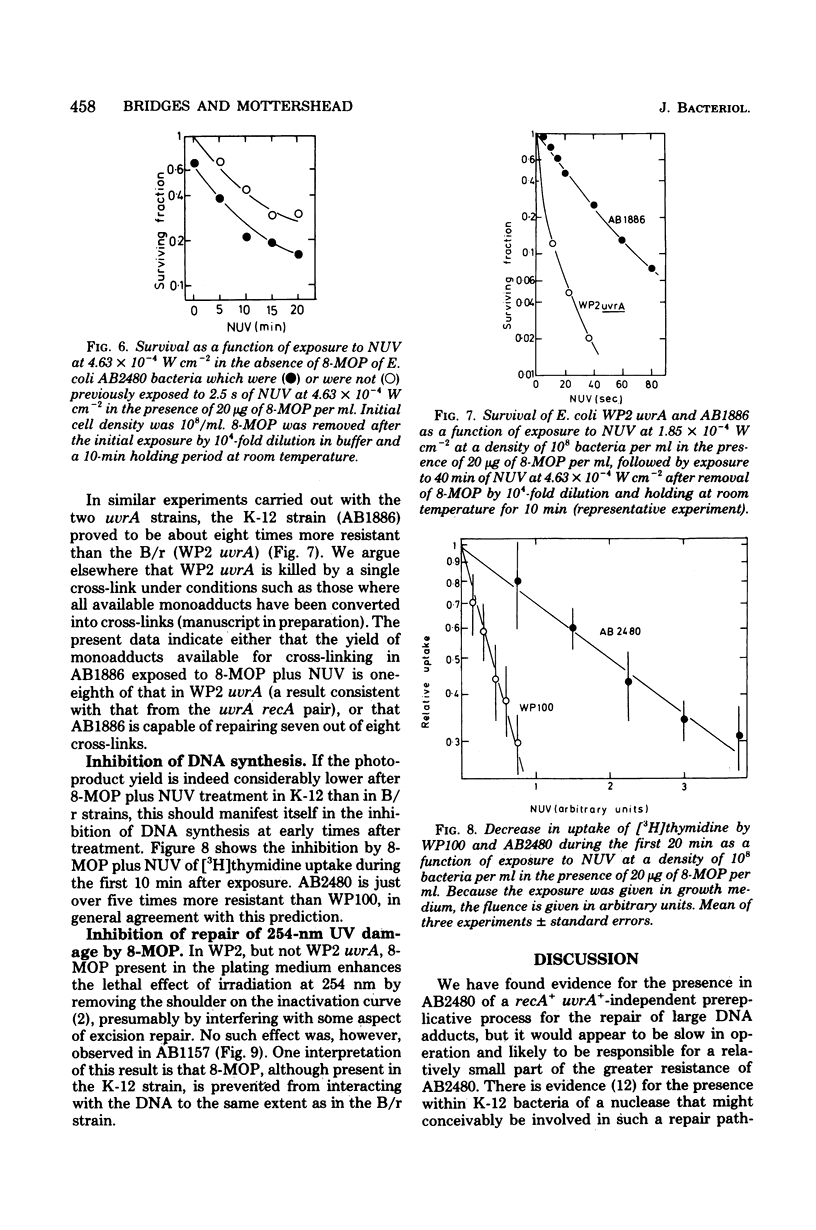
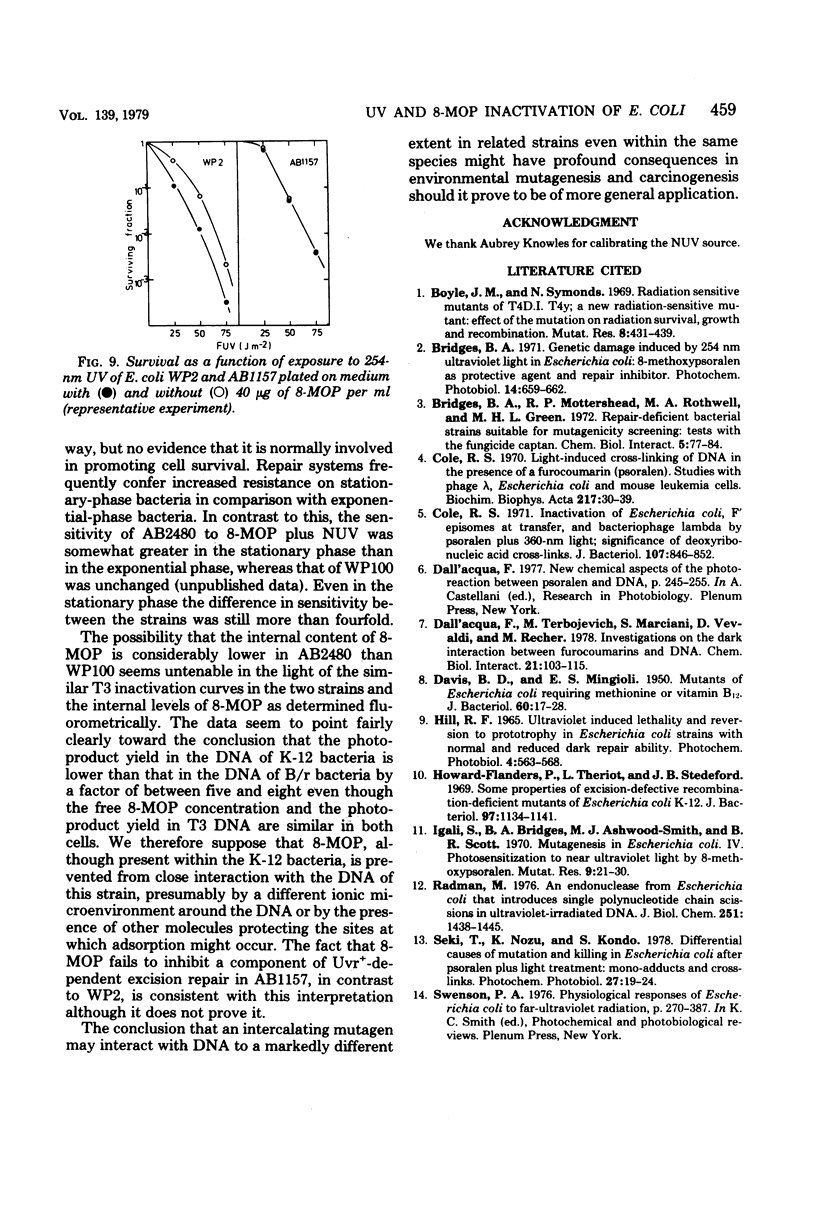
A series of Escherichia coli K-12 AB1157 strains with normal and defective deoxyribonucleic acid repair capacity were more resistant to treatment with 8-methoxypsoralen (8-MOP) and near-ultraviolet light (NUV) than a comparable series of strains from the B/r WP2 family although sensitivities to 254-nm ultraviolet light were closely similar. The difference was most marked with strains deficient in both excision and postreplication repair (uvrA recA). The hypothesis that the internal level of 8-MOP was lower in K-12 than B/r uvrA recA derivatives was ruled out on the basis of fluorometric determinations of 8-MOP content and the similar inactivation curves for phage T3 treated intracellularly within the two strains. The demonstration of liquid holding recovery with AB2480 but not WP100 (both recA uvrA strains) and the somewhat greater resistance of the former strain to inactivation by captan revealed the presence in the K-12 strain of a deoxyribonucleic acid repair system independent of the recA+ and uvrA+ genes. The presence of this repair system did not, however, affect the survival of T3 phage treated with 8-MOP plus NUV and probably has a relatively small effect on survival of AB2480 under normal conditions. Experiments in which 8-MOP monoadducts were converted to cross-links by a second NUV exposure in the absence of 8-MOP indicated that the level of potentially cross-linkable monoadducts immediately after 8-MOP + NUV is about eightfold lower in K-12-than in B/r-derived strains. It is therefore suggested that the photoproduct yield in the former is well below that in the latter. In agreement with this is the observation that, during the first 10 min after treatment, deoxyribonucleic acid synthesis was just over five times more sensitive to inhibition by 8-MOP plus NUV in WP100 than in AB2480. We assume that 8-MOP in K-12 bacteria is hindered in some way from adsorbing to cellular (though not to phage T3) deoxyribonucleic acid. Consistent with this, 8-MOP has been shown to act as an inhibitor of a component of repair of 254-nm ultraviolet light damage in WP2 but not in AB1157.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyle J. M., Symonds N. Radiation-sensitive mutants of T4D. I. T4y: a new radiation-sensitive mutant; effect of the mutation on radiation survival, growth and recombination. Mutat Res. 1969 Nov-Dec;8(3):431–439. doi: 10.1016/0027-5107(69)90060-8. [DOI] [PubMed] [Google Scholar]
- Bridges B. A. Genetic damage induced by 254 nm ultraviolet light in Escherichia coli: 8-methoxypsoralen as protective agent and repair inhibitor. Photochem Photobiol. 1971 Nov;14(5):659–662. doi: 10.1111/j.1751-1097.1971.tb06204.x. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Mottershead R. P., Rothwell M. A., Green M. H. Repair-deficient bacterial strains suitable for mutagenicity screening: tests with the fungicide captain. Chem Biol Interact. 1972 Jul;5(2):77–84. doi: 10.1016/0009-2797(72)90034-8. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Inactivation of Escherichia coli, F' episomes at transfer, and bacteriophage lambda by psoralen plus 360-nm light: significance of deoxyribonucleic acid cross-links. J Bacteriol. 1971 Sep;107(3):846–852. doi: 10.1128/jb.107.3.846-852.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S. Light-induced cross-linking of DNA in the presence of a furocoumarin (psoralen). Studies with phage lambda, Escherichia coli, and mouse leukemia cells. Biochim Biophys Acta. 1970 Sep 17;217(1):30–39. doi: 10.1016/0005-2787(70)90119-x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Acqua F., Terbojevich M., Marciani S., Vedaldi D., Recher M. Investigation of the dark interaction between furocoumarins and DNA. Chem Biol Interact. 1978 Apr;21(1):103–115. doi: 10.1016/0009-2797(78)90071-6. [DOI] [PubMed] [Google Scholar]
- Hill R. F. Ultraviolet-induced lethality and reversion to prototrophy in Escherichia coli strains with normal and reduced dark repair ability. Photochem Photobiol. 1965 Jun;4(3):563–568. doi: 10.1111/j.1751-1097.1965.tb09774.x. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L., Stedeford J. B. Some properties of excision-defective recombination-deficient mutants of Escherichia coli K-12. J Bacteriol. 1969 Mar;97(3):1134–1141. doi: 10.1128/jb.97.3.1134-1141.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igali S., Bridges B. A., Ashwood-Smith M. J., Scott B. R. Mutagenesis in Escherichia coli. IV. Photosensitization to near ultraviolet light by 8-methoxypsoralen. Mutat Res. 1970 Jan;9(1):21–30. doi: 10.1016/0027-5107(70)90067-9. [DOI] [PubMed] [Google Scholar]
- Radman M. An endonuclease from Escherichia coli that introduces single polynucleotide chain scissions in ultraviolet-irradiated DNA. J Biol Chem. 1976 Mar 10;251(5):1438–1445. [PubMed] [Google Scholar]
- Seki T., Nozu K., Kondo S. Differential causes of mutation and killing in Escherichia coli after psoralen plus light treatment: monoadducts and cross-links. Photochem Photobiol. 1978 Jan;27(1):19–24. doi: 10.1111/j.1751-1097.1978.tb07559.x. [DOI] [PubMed] [Google Scholar]



