Abstract
The Swi/Snf nucleosome-remodeling complex is recruited by DNA-binding activator proteins, whereupon it alters chromatin structure to increase preinitiation complex formation and transcription. At the SUC2 promoter, the Swi/Snf complex is required for histone eviction in a manner that is independent of transcriptional activity. Swi/Snf travels through coding regions with elongating RNA polymerase (Pol) II, and swi2 mutants exhibit sensitivity to drugs affecting Pol elongation. In FACT-depleted cells, Swi/Snf is important for internal initiation within coding regions, suggesting that Swi/Snf is important for histone eviction that occurs during Pol II elongation. Taken together, these observations suggest that Swi/Snf is important for histone eviction at enhancers and that it also functions as a Pol II elongation factor.
Eukaryotic organisms contain multiple ATP-dependent, nucleosome-remodeling complexes that play critical roles in transcription, histone exchange, DNA replication, double-strand break repair, and other processes that occur on chromatin templates (3, 6, 39, 54). By mobilizing and sliding nucleosomes along the DNA template, such complexes typically increase accessibility of the DNA to nuclear proteins. As such, nucleosome-remodeling complexes facilitate transcription and other chromatin-dependent processes. In principle, however, nucleosomes can be mobilized to positions that inhibit binding of a gene-specific factor and hence repress transcription.
In addition to sliding, nucleosome-remodeling complexes can also disassemble histone octamers from DNA. In vitro, such histone eviction is stimulated by histone chaperones, such as Asf1 and Nap1 (32), and acetylated nucleosomes (9). In vivo, histone eviction is mediated by DNA-binding transcriptional activators bound to their target sites in promoter regions (4, 5, 14, 50, 59). Histones are also evicted during the process of transcriptional elongation by RNA polymerase (Pol) II, and such eviction is critical for passage of Pol II through the coding region (27, 30, 53, 59). It is presumed that nucleosome-remodeling complexes directly mediate activator- and elongation-dependent histone eviction in vivo, but the specific complexes that perform these functions have not yet been identified.
Saccharomyces cerevisiae Swi/Snf, the first nucleosome-remodeling complex to be described, contains 11 subunits, each of which is required for Swi/Snf function in vivo (57). The Swi/Snf complex plays a direct role in the mechanism by which DNA-binding activator proteins stimulate transcriptional initiation by Pol II. In vitro, activator proteins directly interact with Swi/Snf (40-42, 47, 61, 62) and drive nucleosome eviction (18). Activator proteins recruit Swi/Snf to activator-binding sites in vivo (12, 15, 60). Upon recruitment, Swi/Snf locally alters nucleosome positioning and perhaps other features of chromatin structure (17, 20, 36), but its effect on nucleosome density has not been determined. Recruitment of Swi/Snf can also facilitate the association of SAGA histone acetylase complexes (12), and conversely, histone acetylation facilitates association of Swi/Snf to nucleosomes (19). When artificially recruited to promoters, Swi/Snf stimulates transcription in a manner that depends on the catalytic activity of the complex (29). Loss of Swi/Snf function reduces activator-dependent recruitment of the basic transcription machinery (12, 31, 37, 49) and hence activator-dependent induction of gene expression in response to appropriate environmental signals.
In addition to its role in transcriptional activation, there is some evidence suggesting the possibility that the Swi/Snf complex may have a role during Pol II elongation. Brg1 and Brm, the catalytic subunits of distinct human Swi/Snf complexes, have been observed in the coding regions of HSP70 and CD44 genes, respectively (2, 11). Yeast cells lacking Swi/Snf are sensitive to drugs that inhibit Pol II elongation. Swi/Snf also exhibits genetic and physical interactions with proteins that can function as Pol II elongation factors, including Asf1, Spt6, Spt16, and TFIIS (13, 33, 38, 43).
In this paper, we show that the Swi/Snf complex travels through coding regions with elongating Pol II. Further, loss of Swi/Snf reduces initiation within coding regions that is observed in FACT-depleted cells, suggesting that Swi/Snf is important for histone eviction during Pol II elongation. We also find that Swi2 is necessary for the activator-dependent histone eviction from the SUC2 promoter that is independent of transcriptional activity.
MATERIALS AND METHODS
Yeast strains.
The strain expressing Swi2-18xMyc (15), asf1 and spt16-197 mutant strains (33, 52), and isogenic wild-type, swi2, and suc2::ΔTATA mutant strains (20) have been previously described. The strains expressing Swi3- and Snf11-18xMyc were made in JDY51, an FT5 strain background, as previously described (15), and verified by Western blotting for the tagged protein. Occupancy of histone H2B was monitored with a strain expressing FLAG-tagged histone H2B (46, 53). The swi2 null mutant was made via one-step gene replacement with a KanMX cassette or URA3 gene and phenotypically tested (significant growth defect and poor growth on galactose). The GAL1-YLR454 strain has been previously described (35), and the GRE2-YLR454 construct has 900 bp of the GRE2 promoter proximal to the YLR454 coding region. GAL1-YLR454 and GRE2-YLR454 strains were constructed via one-step integration. The relevant genotypes of all newly made strains were verified by PCR.
Media.
All strains were grown in yeast extract-peptone (YP) containing 2% carbon source, except for the GAL1-YLR454 and GRE2-YLR454 strains, which were grown in medium containing 2% carbon source and Casamino Acids. For GRE2 induction experiments, cells were osmotically shocked with 0.4 M NaCl. For the galactose-to-glucose shifts, cells were removed from medium containing 2% galactose by rapid centrifugation and then quickly resuspended in new medium containing 2% glucose. For MET repression, cells were grown overnight in synthetic complete medium lacking methionine (activating conditions) and repressed by the addition of 5 mM methionine. For the heat shock experiments, cells were grown overnight in YP-dextrose (YPD) at 30°C and then heat shocked at 39°C. For SUC2 induction experiments, cells were grown overnight in YPD, washed once in H2O, and resuspended in YP containing 0.05% glucose. For GAL1-YLR454 experiments and experiments with swi2 mutants, cells were grown overnight in media containing 2% galactose and 0.025% glucose. Glucose was then added to a concentration of 4% to repress GAL genes. Plates with nucleotide-depleting drugs are YPD containing 45 μg/ml mycophenolic acid (MPA) or Casamino Acids lacking uracil plus 100 μg/ml 6-azauracil (6-AU). Cells were grown on YPD with or without MPA or Casamino Acids lacking 6-AU for 2 days and grown on Casamino Acids plus 6-AU for 5 days.
Chromatin immunoprecipitation.
Chromatin immunoprecipitation was carried out with a modified version of a procedure described previously (28). Cells (A600 of 0.7) were fixed in 1% formaldehyde for 20 min at room temperature, quenched for 5 min with glycine, and lysed with zirconia-silica beads (BioSpec Products) in a mini-bead beater (BioSpec Products). Chromatin was first pelleted by centrifugation and then solubilized by sonication (Branson sonifier 350, two times, 100% duty, power setting of 5, 30 s for each cycle). Cross-linked chromatin was immunoprecipitated with monoclonal antibodies to the Myc epitope (9E10; Upstate), Rbp1 (8WG16; Covance), or FLAG epitope (Sigma anti-FLAG M2) or with polyclonal antibodies to histone H3 (Abcam). Quantitative PCR analyses were performed in real time with an Applied Biosystems 7700 or 7000 sequence detector. Percent immunoprecipitation efficiency was determined by dividing the amount of PCR product in the immunoprecipitated sample by the amount of PCR product in the input sample and corrected for a dilution factor. Relative occupancy values were calculated by dividing the percent immunoprecipitation efficiency of the target DNA by the efficiency of an intergenic region of chromosome I, which was usually defined as 1.0. For all data shown, each experiment was performed independently at least twice.
RNA analysis.
DNase I-treated RNA was purified with the RNeasy QIAGEN kit, and cDNA was generated with a poly(dT) primer and Superscript III reverse transcriptase (Invitrogen). Real-time PCR was used to quantify cDNA levels using the level of POL1 RNA as an internal control.
RESULTS
The Swi/Snf complex associates with promoters and coding regions of transcriptionally active genes.
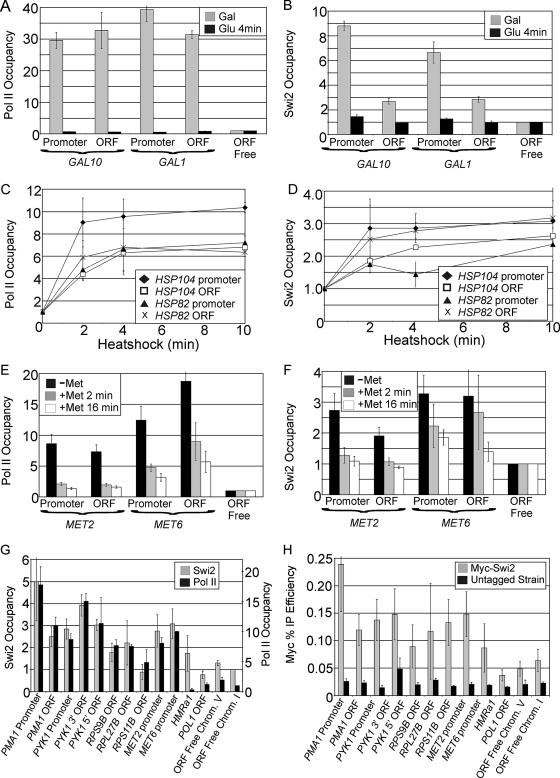
Using strains expressing Myc18-tagged versions of Swi2, Swi3, and Snf11 from their normal chromosomal locations, we examined the relationship between transcription and association of these three Swi/Snf subunits (data for Swi2 are presented in Fig. 1; data for Swi3 and Snf11 are presented in Fig. S1 in the supplemental material). To examine GAL transcription, cells were grown in galactose-containing medium (activating conditions) and then shifted into glucose-containing medium (repressing conditions) for 4 minutes. As expected, Pol II association at the GAL promoters and coding regions is virtually eliminated after this brief shift to repressing conditions (Fig. 1A). Swi2, Swi3, and Snf11 all associate with the GAL1/GAL10 promoters and coding regions during transcriptional activation, and they rapidly dissociate from these regions upon transcriptional repression (Fig. 1B; see also Fig. S1A and B in the supplemental material). Similarly, in response to heat shock, association of these three Swi/Snf subunits with the HSP104 and HSP82 promoters and coding regions increases with kinetics similar to the kinetics of Pol II (Fig. 1C and D; see also Fig. S1C and D in the supplemental material). Last, association with the MET2 and MET6 promoters and coding regions decreases in a manner that strongly correlates with Pol II dissociation when cells are treated with methionine (Fig. 1E and F; see also Fig. S1E and F in the supplemental material).
FIG. 1.
The Swi/Snf complex associates with transcriptionally active promoters and coding regions. (A and B) Pol II (A) and Swi2 (B) occupancy at the indicated GAL1/GAL10 regions in cells expressing Myc18-Swi2 grown in medium containing galactose (Gal) and then shifted into medium containing glucose (Glu) for 4 min. ORF, open reading frame. (C and D) Pol II (C) and Swi2 (D) occupancy at the indicated regions of HSP104 and HSP82 in cells grown in YPD medium and heat shocked at 39°C for the indicated times. (E and F) Pol II (E) and Swi2 (F) occupancy at the indicated regions of MET2 and MET6 in cells grown in medium lacking methionine (−Met) or treated by the addition of methionine (+Met) for the indicated times. (G) Pol II and Swi2 occupancy at the indicated locations in cells grown in synthetic complete medium lacking methionine. ORF Free Chrom. V, open reading frame-free chromosome V. (H) Percent immunoprecipitation (IP) efficiency using an antibody against the Myc epitope in cells that express or do not express Myc18-Swi2. Values in the figure are the means ± standard errors of the means (error bars) from two to four independent experiments and are expressed as the change from the value for the control ORF-free region, except for panel H.
Additional examination of a variety of well-expressed genes shows a strong relationship between Pol II and Swi2 occupancy at both promoters and coding regions (Fig. 1G). Control experiments comparing Myc18-tagged Swi2 versus untagged strains verify the association of Swi2 at these regions (Fig. 1H). Thus, the Swi/Snf complex associates with both promoters and coding regions in a manner that correlates with Pol II association and the transcriptional status of the gene.
The Swi/Snf complex travels with elongating Pol II.
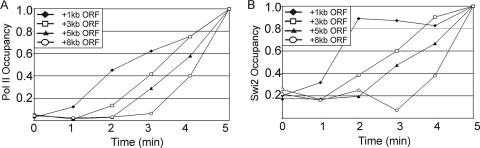
We addressed whether Swi/Snf travels with elongating Pol II using a modified version of a procedure that kinetically monitors the first wave of Pol II transcription (34, 35, 53, 55). Using a gene in which the large (8-kb) YLR454 coding region is controlled by the osmotically inducible GRE2 promoter (48), we determined the level of Swi2 and Pol II association at various positions within the YLR454 coding region following addition of NaCl. In the YLR454 coding region, the patterns of Swi2 and Pol II occupancy are indistinguishable during the first wave of Pol II transcription (Fig. 2A and B), indicating that Swi/Snf travels with elongating Pol II.
FIG. 2.
The Swi/Snf complex travels with elongating Pol II. Pol II and Swi2 occupancy at the indicated regions of GRE2-YLR454 in cells expressing Myc18-Swi2 osmotically shocked with NaCl is shown for the indicated times. The background (open reading frame [ORF]-free) values were subtracted from each time point, and the 5-min time point was set at 1.
The Swi/Snf complex is necessary for normal levels of Pol II occupancy at promoters and coding regions.
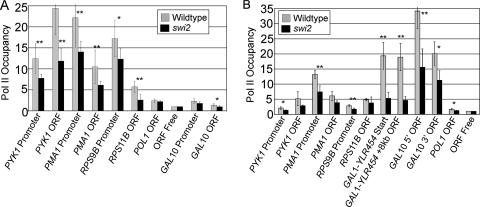
Whole-genome microarray analyses comparing RNAs in wild-type and swi/snf mutant strains have indicated positive and negative effects on gene expression for a relatively small proportion of genes (21, 56). However, due to normalization of the samples to total RNA, effects on RNA stability, and difficulty in measuring subtle effects on microarrays, these transcriptional profiling experiments do not address the possibility that Swi/Snf has a general effect on Pol II transcription. To address this possibility, we examined Pol II occupancy in a wild-type or swi2 null mutant strain. In two different strain backgrounds, there is a statistically significant decrease in Pol II occupancy at promoters and coding regions in the swi2 mutant compared to the wild type (Fig. 3). This decrease in Pol II association is not due to the reduced growth rate of swi2 mutant strains, because Pol II association is unaffected in wild-type cells grown in a nonoptimal carbon source, which results in a comparable reduction in growth rate (53). Thus, in addition to its selective transcriptional effects and its importance in activator-mediated transcription, Swi/Snf has a general role in Pol II transcription.
FIG. 3.
Swi2 is important for normal levels of Pol II occupancy at promoters and coding regions. (A) Pol II occupancy in wild-type or swi2 mutant cells expressing FLAG-tagged histone H2B as the only copy of histone H2B were grown in YPD medium. (B) Pol II occupancy in wild-type or swi2 mutant strains with the integrated GAL1-YLR454 construct (20) were grown in 2% galactose plus 0.025% glucose. Data are expressed as the change from the value for an open reading frame (ORF)-free region on chromosome I defined as 1 and are the means ± standard errors of the means (error bars) from three to five independent experiments. A t test was used to determine statistical significance, which is shown as follows: *, P < 0.025; **, P < 0.01.
The Swi/Snf complex is necessary for histone eviction at the SUC2 promoter.
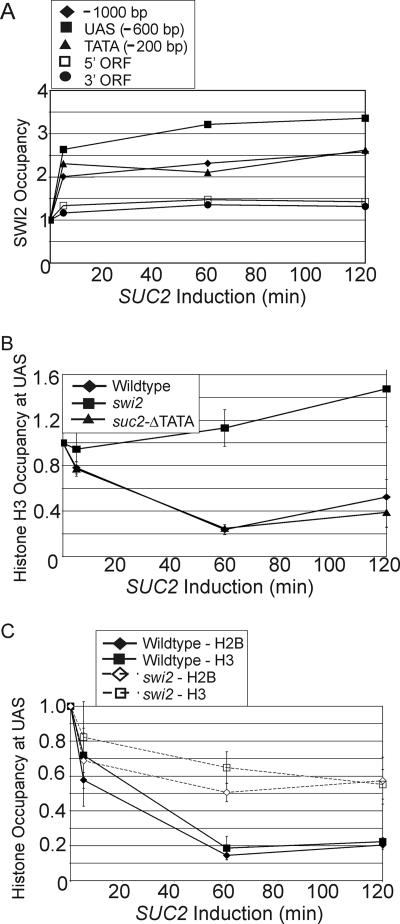
Transcriptional activator proteins are associated with histone eviction at promoter regions (4, 5, 14, 26, 50), but the factors responsible for such histone eviction have yet to be identified. Swi/Snf causes activator-dependent changes in chromatin structure, and it is important for histone deposition at the PHO5 promoter upon cessation of activation (51). We therefore examined Swi2 and histone occupancy at the well-characterized Swi/Snf-dependent SUC2 promoter in cells grown overnight in YPD (repressing conditions) and shifted into YP with limiting amounts of dextrose (activating conditions).
As expected, activation results in recruitment of Swi2 (Fig. 4A) and decreased H2B and H3 occupancy (Fig. 4B and C) at the SUC2 promoter region. Importantly, there is a significant defect in H2B and H3 eviction in the swi2 mutant strain (Fig. 4B and C). Histone eviction in a strain deleted for the SUC2 TATA region occurs as efficiently as in the wild-type strain (Fig. 4B), indicating that it is independent of transcription and recruitment of general initiation factors and Pol II. Thus, the Swi/Snf complex is necessary for activator-mediated eviction of histones at the SUC2 promoter during induction in vivo.
FIG. 4.
Swi2 binds the SUC2 upstream activation sequence (UAS) and mediates histone H2B and H3 eviction. (A) Swi2 occupancy at the indicated regions of SUC2 in cells expressing Myc18-Swi2 shifted to inducing conditions for the indicated times. (B) Histone H3 occupancy at the SUC2 UAS in wild-type, swi2, and suc2::ΔTATA strains. (C) Histone H2B and H3 occupancy at the SUC2 UAS in wild-type and swi2 strains. Data are expressed as the change in value from an open reading frame (ORF)-free region on chromosome I defined as 1, and the time zero value was set at 1. Standard errors of the means (error bars) (shown in panels B and C) are not shown in panel A for clarity but were generally less than 15% of the means.
The Swi/Snf complex is important for growth in the presence of elongation inhibitors, but not for Pol II elongation rate.
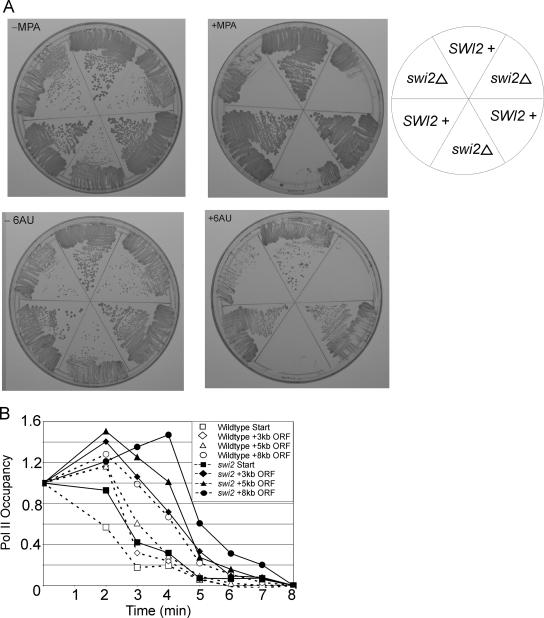
Treatment of yeast cells with the nucleotide-depleting drugs 6-AU and/or MPA results in a reduced rate of Pol II elongation and decreased processivity (34). Sensitivity to 6-AU and MPA is observed in cells lacking a variety of elongation-related factors, and a screen of the yeast deletion collection identified a swi2 strain as hypersensitive to these drugs (16). We confirmed hypersensitivity of swi2 strains to 6-AU and MPA in three different strain backgrounds (Fig. 5A).
FIG. 5.
swi2 mutants are sensitive to drugs that inhibit Pol II elongation, but they exhibit wild-type rates of Pol II elongation. (A) Wild-type or swi2 mutant cells in the BY4741, FY120, and FY406 Flag-tagged H2B backgrounds were grown on YPD with (+) or without (−) 45 μg/ml MPA or on Casamino Acids lacking uracil with or without 100 μg/ml 6-AU. (B) Pol II occupancy at the indicated regions of GAL1-YLR454 in wild-type and swi2 mutant strains subject to a glucose shift for the indicated times. Data were divided by the value for the control open reading frame (ORF)-free region, the 8-min (background) value was subtracted from each time point, and the time zero value was set at 1.
Given the hypersensitivity of swi2 mutants to 6-AU and MPA and the requirement of TFIIS for viability in a swi2 mutant background (13), we examined whether the Swi/Snf complex affected the rate of Pol II elongation in vivo. As described previously, the Pol II elongation rate was determined in a strain containing the GAL1 promoter upstream of the large (8-kb) YLR454 coding region. Galactose-grown cells were treated with glucose, and the kinetics of Pol II dissociation at different positions within the YLR454 coding region were monitored during the “last wave” of transcription. Unlike previous experiments, the wild-type and swi2 cells were initially grown in a mixture of 2% galactose and 0.025% glucose, because mutant cells lacking Swi/Snf grow very poorly when galactose is the sole carbon source.
The wild-type and swi2 mutant strain behave indistinguishably with respect to the rate of Pol II elongation (Fig. 5B). Specifically, the time difference between the Pol II decay curves near the promoter and 8 kb downstream are comparable in the two strains. Furthermore, the swi2 deletion strain does not show a defect in Pol II processivity (i.e., lower Pol II density at 3′ positions compared to 5′ positions), which is a consequence of a reduced Pol II elongation rate (34). It should be noted, however, that Pol II dissociation just downstream of the promoter takes about 1 minute longer in the swi2 mutant than in the wild-type strain, and this 1-minute difference persists at more downstream positions in the YLR454 coding region (Fig. 5B). This observation indicates that the glucose shutoff per se is slower in the swi2 mutant strain, either because of its reduced growth rate and/or some indirect effect on carbon source metabolism.
The Swi/Snf complex affects internal initiation and histone density in FACT-depleted cells.
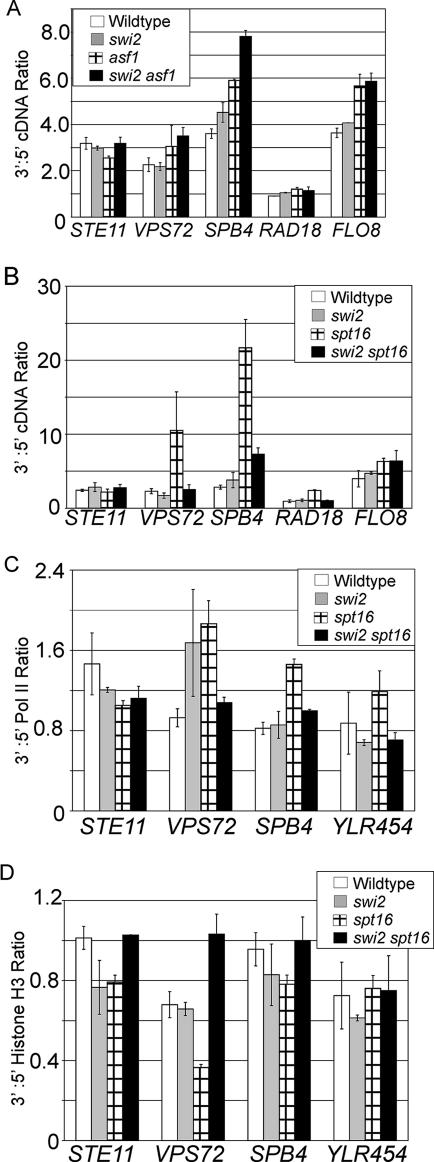
As Swi2 exhibits genetic and biochemical interactions with Asf1 and the FACT subunit Spt16 (33, 38), we examined the effects of Swi2 on histone and Pol II occupancy in asf1 and spt16 backgrounds. As shown previously, Spt16 (25, 35) and Asf1 (52) are important for histone deposition after passage of elongating Pol II and as a consequence, for inhibiting internal initiation from cryptic promoters within coding regions. In accord with these observations, spt16 and asf1 mutant strains display higher RNA levels and reduced histone density at the 3′ ends of certain genes than observed at the corresponding 5′ ends (Fig. 6A). In contrast, the swi2 strain shows equal levels of RNA at the 5′ and 3′ ends of these genes, indicating that Swi/Snf is not required to inhibit internal initiation. Interestingly, internal initiation due to the spt16 mutation is suppressed by the swi2 mutation (Fig. 6B and C). Furthermore, in comparison to the spt16 single mutant, the swi2 spt16 double mutant exhibits higher levels of histone H3 towards the 3′ end of STE11, VPS72, and SPB4 (Fig. 6D). This observation suggests that, in a FACT-depleted strain, Swi2 is important for histone eviction within coding regions.
FIG. 6.
Swi2 is important for internal initiation in FACT-depleted cells. (A) RNA levels of the indicated genes in the indicated strains grown in YPD. (B) RNA levels of the indicated strains grown in YPD and shifted to 37°C for 1 h. RNA levels of the 5′ and 3′ portions of the indicated genes were quantified, relative to a POL1 internal control, and expressed as a 3′-to-5′ (3′:5′) ratio. (C and D) Pol II (C) and histone H3 (D) occupancy levels at the 5′ and 3′ portions of the indicated genes were divided by the levels of control open reading frame (ORF)-free region and expressed as a 3′:5′ ratio. Values are means ± standard errors of the means (error bars).
DISCUSSION
The Swi/Snf complex mediates activator-dependent eviction of nucleosomes in vivo.
Nucleosomes are evicted at promoters in a manner that depends on activator proteins (4, 5, 14, 50, 59). Asf1, the H3/H4 chaperone, is required for activator-dependent eviction at the PHO5 and PHO8 promoters in vivo (1), but Asf1-dependent eviction of histones in vitro requires ATP-dependent nucleosome-remodeling complexes (32). The Swi/Snf (12, 15, 60) and RSC (44) complexes are recruited to promoters by activator proteins, but the roles of such complexes in activator-mediated eviction of nucleosomes have not been described.
Here, we demonstrate that the Swi/Snf complex is necessary for eviction of H3 and H2B, and hence nucleosomes, at the SUC2 promoter. Nucleosome eviction requires conditions of transcriptional activation, but not transcription per se, indicating that it is activator dependent. Although the activator(s) that mediates SUC2 induction is not clearly identified, we presume that Swi/Snf has a direct role in nucleosome eviction, because it is rapidly recruited to the SUC2 promoter in response to activating conditions. Last, we suggest that Swi/Snf is involved in nucleosome eviction by other activators that recruit this nucleosome-remodeling complex to enhancers.
As histone chaperones are required for nucleosome eviction in vitro (32) and Asf1 is important for Pho4-dependent eviction in vivo (1), it seems likely that Asf1 (or another histone chaperone) is important for Swi/Snf-dependent eviction of nucleosomes at the SUC2 promoter. There is no evidence that activator proteins interact directly with Asf1 or other histone chaperones. This strongly suggests that such histone chaperones are “recruited” to promoters by recognizing altered histone-DNA structures generated by the Swi/Snf complex and other nucleosome-remodeling activities. Such an indirect recruitment model is analogous to our previous suggestion for how Asf1 is important for histone eviction and deposition during transcriptional elongation by RNA Pol II (52).
In addition to its role in histone eviction, the Swi/Snf complex is necessary for histone deposition at the PHO5 promoter that occurs when cells are transferred to noninducing conditions (51). This seems paradoxical, in that Swi/Snf association with the PHO5 promoter is correlated to activation. Perhaps, the role of Swi/Snf in histone deposition occurs during the transition between inducing and noninducing conditions, when intermediate (and gradually decreasing) levels of Swi/Snf would be predicted to be associated with the PHO5 promoter. Alternatively, the role of Swi/Snf might not be connected to activator-mediated recruitment, but rather to its nonspecific and genome-wide activity.
Evidence that the Swi/Snf complex is a Pol II elongation factor.
Pol II elongation is mechanistically linked to posttranscriptional processes, such as 5′-end capping, splicing, 3′-end formation, polyadenylation, and nuclear export. As a consequence, many proteins travel with elongating Pol II throughout mRNA coding regions. Here, we show that in addition to its ability to be recruited to promoter regions by activator proteins, Swi/Snf also travels with elongating Pol II throughout mRNA coding regions. There are two possible mechanisms by which Swi/Snf travels with Pol II. First, Swi/Snf might associate with Pol II, either directly or indirectly by associating with one or more of the many factors that travel with elongating Pol II (e.g., FACT, Paf complex, Spt6, and TREX). In this regard, Swi/Snf has been reported to associate with a Pol II “holoenzyme” (58), although this result is controversial. Alternatively, as proposed for the histone chaperone Asf1 (52), Swi/Snf might recognize distorted chromatin that arises during the process of transcriptional elongation and permits passage of Pol II. In this view, Swi/Snf would dissociate from the mRNA coding region upon passage of Pol II and restoration of normal chromatin structure.
As is the case for many factors that travel with elongating Pol II (34), the Swi/Snf complex does not detectably affect either the Pol II elongation rate or processivity. However, a key feature of Pol II elongation in vivo is the dynamic cycle of nucleosome eviction and deposition that permits passage of Pol II and then restores normal chromatin structure. Proteins that travel with elongating Pol II and affect this dynamic cycle of histone eviction and deposition play a direct role in Pol II elongation and can be considered elongation factors. Thus, Asf1, Spt6, and FACT behave as elongation factors, because loss-of-function mutations cause decreased histone deposition and internal initiation within coding regions in vivo (8, 24, 25). Importantly, we show that Swi/Snf is important for internal initiation observed in cells depleted for the Spt16 subunit of FACT. Furthermore, the spt16 swi2 double mutant exhibits higher H3 occupancy towards the 3′ end of genes than the single spt16 mutant does. While we cannot exclude the formal possibility that Swi/Snf inhibits histone deposition, these results suggest that Swi/Snf is important for histone eviction within coding regions in a FACT-depleted background.
Pol II elongation on chromatin templates is virtually blocked by nucleosomes or histone H3/H4 tetramers (10, 22, 23), strongly suggesting that histone eviction is required for transcriptional elongation. As ATP-dependent nucleosome-remodeling complexes (together with histone chaperones) are the major (and perhaps only) biochemical entities capable of histone eviction, it is likely that they are essential for Pol II elongation. Our results provide strong evidence that the Swi/Snf complex plays a role in Pol II elongation via its effects on histone eviction. However, cells lacking Swi/Snf grow (albeit less well than wild-type cells), do not display a defect in Pol II elongation rate, and have only a modest effect on histone density. These observations strongly suggest that histone eviction during Pol II elongation is also mediated by other nucleosome-remodeling complexes (and perhaps other factors). The RSC complex is an attractive candidate in this regard, because it is the only nucleosome-remodeling complex essential for yeast cell growth (7). However, genome-wide analysis did not reveal a relationship between RSC occupancy and Pol II transcription (44), whereas such a relationship was observed in a comparable analysis with Set1 histone methylase (45). The relative contributions of the multiple nucleosome-remodeling complexes to histone eviction during Pol II elongation remain to be determined.
Supplementary Material
Acknowledgments
We thank Fred Winston for yeast strains and useful suggestions.
This work was supported by grants to K.S. from the National Institutes of Health (GM30186).
Footnotes
Published ahead of print on 20 August 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Adkins, M. W., S. R. Howar, and J. K. Tyler. 2004. Chromatin disassembly mediated by the histone chaperone Asf1 is essential for transcriptional activation of the yeast PHO5 and PHO8 genes. Mol. Cell 14:657-666. [DOI] [PubMed] [Google Scholar]
- 2.Batsche, E., M. Yaniv, and C. Muchardt. 2006. The human SWI/SNF subunit Brm is a regulator of alternative splicing. Nat. Struct. Mol. Biol. 13:22-29. [DOI] [PubMed] [Google Scholar]
- 3.Becker, P. B., and W. Horz. 2002. ATP-dependent nucleosome remodeling. Annu. Rev. Biochem. 71:247-273. [DOI] [PubMed] [Google Scholar]
- 4.Boeger, H., J. Griesenbeck, J. S. Strattan, and R. D. Kornberg. 2003. Nucleosomes unfold completely at a transcriptionally active promoter. Mol. Cell 11:1587-1598. [DOI] [PubMed] [Google Scholar]
- 5.Boeger, H., J. Griesenbeck, J. S. Strattan, and R. D. Kornberg. 2004. Removal of promoter nucleosomes by disassembly rather than sliding in vivo. Mol. Cell 14:667-673. [DOI] [PubMed] [Google Scholar]
- 6.Cairns, B. R. 2005. Chromatin remodeling complexes: strength in diversity, precision through specialization. Curr. Opin. Genet. Dev. 15:185-190. [DOI] [PubMed] [Google Scholar]
- 7.Cairns, B. R., Y. Lorch, Y. Li, M. Zhang, L. Lacomis, H. Erdjument-Bromage, P. Tempst, J. Du, B. Laurent, and R. D. Kornberg. 1996. RSC, an essential, abundant chromatin-remodeling complex. Cell 87:1249-1260. [DOI] [PubMed] [Google Scholar]
- 8.Carrozza, M. J., B. Li, L. Florens, T. Suganuma, S. K. Swanson, K. K. Lee, W. J. Shia, S. Anderson, J. Yates, M. P. Washburn, and J. L. Workman. 2005. Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell 123:581-592. [DOI] [PubMed] [Google Scholar]
- 9.Chandy, M., J. L. Gutierrez, P. Prochasson, and J. L. Workman. 2006. SWI/SNF displaces SAGA-acetylated nucleosomes. Eukaryot. Cell 5:1738-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, C. H., and D. S. Luse. 1997. The H3/H4 tetramer blocks transcript elongation by RNA polymerase II in vitro. J. Biol. Chem. 272:23427-23434. [DOI] [PubMed] [Google Scholar]
- 11.Corey, L. L., C. S. Weirich, I. J. Benjamin, and R. E. Kingston. 2003. Localized recruitment of a chromatin-remodeling activity by an activator in vivo drives transcriptional elongation. Genes Dev. 17:1392-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cosma, M. P., T. Tanaka, and K. Nasmyth. 1999. Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle- and developmentally regulated promoter. Cell 97:299-311. [DOI] [PubMed] [Google Scholar]
- 13.Davie, J. K., and C. M. Kane. 2000. Genetic interactions between TFIIS and the Swi/Snf chromatin remodeling complex. Mol. Cell. Biol. 20:5960-5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deckert, J., and K. Struhl. 2001. Histone acetylation at promoters is differentially affected by activators and repressors. Mol. Cell. Biol. 21:2726-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deckert, J., and K. Struhl. 2002. Targeted recruitment of Rpd3 histone deacetylase represses transcription by inhibiting recruitment of Swi/Snf, SAGA, and TATA binding protein. Mol. Cell. Biol. 22:6458-6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desmoucelles, C., B. Pinson, C. Saint-Marc, and B. Daignan-Fornier. 2002. Screening the yeast “disruptome” for mutants affecting resistance to the immunosuppressive drug, mycophenolic acid. J. Biol. Chem. 277:27036-27044. [DOI] [PubMed] [Google Scholar]
- 17.Gregory, P. D., A. Schmid, M. Zavari, M. Munsterkotter, and W. Horz. 1999. Chromatin remodeling at the PHO8 promoter requires SWI-SNF and SAGA at a step subsequent to activator binding. EMBO J. 18:6407-6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutierrez, J. L., M. Chandy, M. J. Carrozza, and J. L. Workman. 2007. Activation domains drive nucleosome eviction by SWI/SNF. EMBO J. 26:730-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassan, A. H., K. E. Neely, and J. L. Workman. 2001. Histone acetyltransferase complexes stabilize Swi/Snf binding to promoter nucleosomes. Cell 104:817-827. [DOI] [PubMed] [Google Scholar]
- 20.Hirschhorn, J. N., S. A. Brown, C. D. Clark, and F. Winston. 1992. Evidence that SNF2/SWI2 and SNF5 activate transcription in yeast by altering chromatin structure. Genes Dev. 6:2288-2298. [DOI] [PubMed] [Google Scholar]
- 21.Holstege, F. C., E. G. Jennings, J. J. Wyrick, T. I. Lee, C. J. Hengartner, M. R. Green, T. R. Golub, E. S. Lander, and R. A. Young. 1998. Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95:717-728. [DOI] [PubMed] [Google Scholar]
- 22.Izban, M. G., and D. S. Luse. 1992. Factor-stimulated RNA polymerase II transcribes at physiological elongation rates on naked DNA but very poorly on chromatin templates. J. Biol. Chem. 267:13647-13655. [PubMed] [Google Scholar]
- 23.Izban, M. G., and D. S. Luse. 1991. Transcription on nucleosomal templates by RNA polymerase II in vitro: inhibition of elongation with enhancement of sequence-specific pausing. Genes Dev. 5:683-686. [DOI] [PubMed] [Google Scholar]
- 24.Joshi, A. A., and K. Struhl. 2005. Interaction of the Eaf3 chromodomain with methylated histone H3-K36 mediates preferential histone deacetylation at mRNA coding regions. Mol. Cell 20:971-978. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan, C. D., L. Laprade, and F. Winston. 2003. Transcription elongation factors repress transcription initiation from cryptic sites. Science 301:1096-1099. [DOI] [PubMed] [Google Scholar]
- 26.Korber, P., T. Luckenbach, D. Blaschke, and W. Horz. 2004. Evidence for histone eviction in trans upon induction of the yeast PHO5 promoter. Mol. Cell. Biol. 24:10965-10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristjuhan, A., and J. Q. Svejstrup. 2004. Evidence for distinct mechanisms facilitating transcript elongation through chromatin in vivo. EMBO J. 23:4243-4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuras, L., and K. Struhl. 1999. Binding of TBP to promoters in vivo is stimulated by activators and requires Pol II holoenzyme. Nature 399:609-612. [DOI] [PubMed] [Google Scholar]
- 29.Laurent, B. C., M. A. Treitel, and M. Carlson. 1991. Functional interdependence of the yeast SNF2, SNF5, and SNF6 proteins in transcriptional activation. Proc. Natl. Acad. Sci. USA 88:2687-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, C. K., Y. Shibata, B. Rao, B. D. Strahl, and J. D. Lieb. 2004. Evidence for nucleosome depletion at active regulatory regions genome-wide. Nat. Genet. 36:900-905. [DOI] [PubMed] [Google Scholar]
- 31.Lomvardas, S., and D. Thanos. 2001. Nucleosome sliding via TBP DNA binding in vivo. Cell 106:685-696. [DOI] [PubMed] [Google Scholar]
- 32.Lorch, Y., B. Maier-Davis, and R. D. Kornberg. 2006. Chromatin remodeling by nucleosome disassembly in vitro. Proc. Natl. Acad. Sci. USA 103:3090-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malone, E. A., C. D. Clark, A. Chiang, and F. Winston. 1991. Mutations in the SPT16/CDC68 suppress cis- and trans-acting mutations that affect promoter function in Saccharomyces cerevisiae. Mol. Cell. Biol. 11:5710-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mason, P. B., and K. Struhl. 2005. Distinction and relationship between elongation rate and processivity of RNA polymerase II in vivo. Mol. Cell 17:831-840. [DOI] [PubMed] [Google Scholar]
- 35.Mason, P. B., and K. Struhl. 2003. The FACT complex travels with elongating RNA polymerase II and is important for the fidelity of transcriptional initiation in vivo. Mol. Cell. Biol. 23:8323-8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matallana, E., L. Franco, and J. E. Perez-Ortin. 1992. Chromatin structure of the yeast SUC2 promoter in regulatory mutants. Mol. Gen. Genet. 231:395-400. [DOI] [PubMed] [Google Scholar]
- 37.Mitra, D., E. J. Parnell, J. W. Landon, Y. Yu, and D. J. Stillman. 2006. SWI/SNF binding to the HO promoter requires histone acetylation and stimulates TATA-binding protein recruitment. Mol. Cell. Biol. 26:4095-4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moshkin, Y. M., J. A. Armstrong, R. K. Maeda, J. W. Tamkun, P. Verrijzer, J. A. Kennison, and F. Karch. 2002. Histone chaperone ASF1 cooperates with the Brahma chromatin-remodeling machinery. Genes Dev. 16:2621-2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Narlikar, G. J., H.-Y. Fan, and R. E. Kingston. 2002. Cooperation between complexes that regulate chromatin and transcription. Cell 108:475-487. [DOI] [PubMed] [Google Scholar]
- 40.Natarajan, K., B. M. Jackson, H. Zhou, F. Winston, and A. G. Hinnebusch. 1999. Transcriptional activation by Gcn4 involves independent interactions with the Swi/Snf complex and the Srb/mediator. Mol. Cell 4:657-664. [DOI] [PubMed] [Google Scholar]
- 41.Neely, K. E., A. H. Hassan, C. E. Brown, L. Howe, and J. L. Workman. 2002. Transcription activator interactions with multiple SWI/SNF subunits. Mol. Cell. Biol. 22:1615-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neely, K. E., A. H. Hassan, A. E. Wallberg, D. J. Steger, B. R. Cairns, A. P. Wright, and J. L. Workman. 1999. Activation domain-mediated targeting of the SWI/SNF complex to promoters stimulates transcription from nucleosome arrays. Mol. Cell 4:649-655. [DOI] [PubMed] [Google Scholar]
- 43.Neigeborn, L., K. Rubin, and M. Carlson. 1986. Suppressors of SNF2 mutations restore invertase derepression and cause temperature-sensitive lethality in yeast. Genetics. 112:741-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ng, H. H., F. Robert, R. A. Young, and K. Struhl. 2002. Genome-wide location and regulated recruitment of the RSC nucleosome-remodeling complex. Genes Dev. 16:806-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ng, H. H., F. Robert, R. A. Young, and K. Struhl. 2003. Targeted recruitment of Set1 histone methylase by elongating Pol II provides a localized mark and memory of recent transcriptional activity. Mol. Cell 11:709-719. [DOI] [PubMed] [Google Scholar]
- 46.Ng, H. H., R. M. Xu, Y. Zhang, and K. Struhl. 2002. Ubiquitination of histone H2B by Rad6 is required for efficient Dot1-mediated methylation of histone H3-lysine 79. J. Biol. Chem. 277:34655-34657. [DOI] [PubMed] [Google Scholar]
- 47.Prochasson, P., K. E. Neely, A. H. Hassan, B. Li, and J. L. Workman. 2003. Targeting activity is required for Swi/Snf function in vivo and is accomplished through two partially redundant activator-interaction domains. Mol. Cell 12:983-990. [DOI] [PubMed] [Google Scholar]
- 48.Proft, M., and K. Struhl. 2004. A MAP kinase-mediated stress relief response that precedes and regulates the timing of transcriptional induction. Cell 118:351-361. [DOI] [PubMed] [Google Scholar]
- 49.Qiu, H., C. Hu, S. Yoon, K. Natarajan, M. J. Swanson, and A. G. Hinnebusch. 2004. An array of coactivators is required for optimal recruitment of TATA binding protein and RNA polymerase II by promoter-bound Gcn4p. Mol. Cell. Biol. 24:4104-4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reinke, H., and W. Horz. 2003. Histones are first hyperacetylated and then lose contact with the activated PHO5 promoter. Mol. Cell 11:1599-1607. [DOI] [PubMed] [Google Scholar]
- 51.Schermer, U. J., P. Korber, and W. Horz. 2005. Histones are incorporated in trans during reassembly of the yeast PHO5 promoter. Mol. Cell 19:279-286. [DOI] [PubMed] [Google Scholar]
- 52.Schwabish, M. A., and K. Struhl. 2006. Asf1 mediates histone eviction and deposition during elongation by RNA polymerase II. Mol. Cell 22:415-422. [DOI] [PubMed] [Google Scholar]
- 53.Schwabish, M. A., and K. Struhl. 2004. Evidence for eviction and rapid deposition of histones upon transcriptional elongation by RNA polymerase II. Mol. Cell. Biol. 24:10111-10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith, C. L., and C. L. Peterson. 2005. ATP-dependent chromatin remodeling. Curr. Top. Dev. Biol. 65:115-148. [DOI] [PubMed] [Google Scholar]
- 55.Strasser, K., S. Masuda, P. Mason, J. Pfannstiel, M. Oppizzi, S. Rodriguez-Navarro, A. G. Rondon, A. A. Aguilera, K. Struhl, R. Reed, and E. Hurt. 2002. TREX is a conserved complex coupling transcription with mRNA export. Nature 417:304-307. [DOI] [PubMed] [Google Scholar]
- 56.Sudarsanam, P., V. R. Iyer, P. O. Brown, and F. Winston. 2000. Whole-genome expression analysis of snf/swi mutants of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 97:3364-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sudarsanam, P., and F. Winston. 2000. The Swi/Snf family of nucleosome-remodeling complexes and transcriptional control. Trends Genet. 16:345-351. [DOI] [PubMed] [Google Scholar]
- 58.Wilson, C. J., D. M. Chao, A. N. Imbalzano, G. R. Schnitzler, R. E. Kingston, and R. A. Young. 1996. RNA polymerase II holoenzyme contains SWI/SNF regulators involved in chromatin remodeling. Cell 84:235-244. [DOI] [PubMed] [Google Scholar]
- 59.Workman, J. L. 2006. Nucleosome displacement in transcription. Genes Dev. 20:2009-2017. [DOI] [PubMed] [Google Scholar]
- 60.Yoon, S., H. Qui, M. J. Swanson, and A. G. Hinnebusch. 2003. Recruitment of SWI/SNF by Gcn4p does not require Snf2p or Gcn5p but depends strongly on SWI/SNF integrity, SRB mediator, and SAGA. Mol. Cell. Biol. 23:8829-8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshinaga, S. K., C. L. Peterson, I. Herskowitz, and K. R. Yamamoto. 1992. Roles of SWI1, SWI2, and SWI3 proteins for transcriptional enhancement by steroid receptors. Science 258:1598-1604. [DOI] [PubMed] [Google Scholar]
- 62.Yudkovsky, N., C. Logie, S. Hahn, and C. L. Peterson. 1999. Recruitment of the SWI/SNF chromatin remodeling complex by transcriptional activators. Genes Dev. 13:2369-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.