Abstract
Androgen receptor (AR) is essential for the maintenance of the male reproductive systems and is critical for the carcinogenesis of human prostate cancers (PCas). D-type cyclins are closely related to the repression of AR function. It has been well documented that cyclin D1 inhibits AR function through multiple mechanisms, but the mechanism of how cyclin D3 exerts its repressive role in the AR signaling pathway remains to be identified. In the present investigation, we demonstrate that cyclin D3 and the 58-kDa isoform of cyclin-dependent kinase 11 (CDK11p58) repressed AR transcriptional activity as measured by reporter assays of transformed cells and prostate-specific antigen expression in PCa cells. AR, cyclin D3, and CDK11p58 formed a ternary complex in cells and were colocalized in the luminal epithelial layer of the prostate. AR activity is controlled by phosphorylation at specific sites. We found that AR was phosphorylated at Ser-308 by cyclin D3/CDK11p58 in vitro and in vivo, leading to the repressed activity of AR transcriptional activation unit 1 (TAU1). Furthermore, androgen-dependent proliferation of PCa cells was inhibited by cyclin D3/CDK11p58 through AR repression. These data suggest that cyclin D3/CDK11p58 signaling is involved in the negative regulation of AR function.
Androgen receptor (AR), a member of the nuclear receptor family, directly regulates patterns of gene expression in response to the steroids testosterone and dihydrotestosterone (DHT) and is subsequently involved in the regulation of the development and differentiation of the male reproductive system (10). Similar to other steroid receptors, AR contains a transactivation domain (TAD), also named AF-1, in its N terminus, a ligand binding domain (LBD) in its C terminus, a DNA-binding domain (DBD), and a hinge region between the TAD and LBD. The transcriptional activation unit 1 (TAU1) and TAU5 motifs in the AR N-terminal domain (NTD) (residues 101 to 370 and 360 to 485, respectively) as well as the AF-2 motif in the AR LBD have been implicated in directly contacting p160 proteins and mediating transcription (1, 23, 31, 48).
AR is a phosphoprotein whose function is regulated by the modulation of its phosphorylation status at different sites (4). The consensus phosphorylation sites found in AR indicate that AR could be a substrate for DNA-dependent kinase, protein kinase A, protein kinase C, mitogen-activated protein kinase, and casein kinase 2 (4). Ser-16, Ser-81, Ser-94, Ser-256, Ser-308, Ser-424, and Ser-650 have been identified as being phosphorylation sites of AR by mutagenesis, peptide mapping, and mass spectrometry (6, 60). Recently, several Ser/Thr protein kinases have been found to phosphorylate AR at the above-mentioned sites in vitro and in vivo. For example, AR Ser-515 is phosphorylated by mitogen-activated protein kinase, Ser-213 and Ser-791 are phosphorylated by Akt, and Ser-650 is phosphorylated by p38α and JNK1 (17, 30, 57).
More and more studies suggest that cyclins and cyclin-dependent kinases (CDKs) are also involved in the regulation of AR-dependent transcription. Cyclin D1 functions as a corepressor to inhibit ligand-dependent AR activation (26). It directly binds to and represses AR independent of CDK4 and its cyclin function. Instead, the repressive role of cyclin D1 requires histone deacetylase (HDAC) activity and abrogates the ability of the AR NTD to interact with the C terminus (8, 37). Cyclin E is another cyclin that binds directly to the COOH-terminal portion of the AR TAD, enhancing the transactivation function of this domain (54). CDK6 associates with AR and enhances its transcriptional activity in a kinase-independent manner (29). Despite intensive efforts to reveal the independent roles of cyclins or CDKs in AR regulation, no evidence is provided to elucidate whether the kinase activities of cyclin D1/CDK4 and cyclin D1/CDK6 complexes are associated with AR phosphorylation.
Previous reports have indicated that the kinase activity of cyclin A/CDK2, a kinase that is active in the S phase, is required for the functions of estrogen receptor (ER) and progesterone receptor, two members of the nuclear receptor family (34, 43). Several sites of progesterone receptor are phosphorylated in vitro by cyclin A/CDK2 (24, 59). Cyclin A/CDK2 may activate ERα transcriptional activation through phosphorylating the Ser-104 and Ser-106 of ERα (43). Until now, CDK1 was the only CDK reported to mediate AR phosphorylation at Ser-81 and increase AR expression and stability (11). These data provide a new view of the cyclin/CDK complex in the regulation of steroid hormone receptors.
CDK11, which is encoded by two highly homologous p34cdc2-related genes, Cdc2L1 and Cdc2L2, and is known as PITSLRE protein kinase due to the conserved PITSLRE motif within the protein kinase domain, involves two major isoforms. CDK11p110 is a 779-amino-acid-containing protein representing the full length of the CDK11 gene product (53), and CDK11p58 is a polypeptide consisting of 440 amino acids, which maps to approximately amino acids 341 to 779 of CDK11p110, and is produced by cell cycle-dependent translation initiation from internal ribosome entry sites of the same transcript as CDK11 (12, 44). Structurally, CDK11p58 contains a conserved p34cdc2-related Ser/Thr protein kinase catalytic domain (amino acids 80 to 389) and N-terminal and C-terminal regions (7). Although CDK11p58 shares the same sequences including the kinase domain as the C terminus of CDK11p110, the two isoforms possess different functions. For example, CDK11p110 is associated with transcription and RNA processes, while CDK11p58 is closely related to cell cycle arrest and apoptosis in a kinase-dependent manner (7, 21, 28, 49). Recent studies also revealed that CDK11p58 promotes centrosome maturation and bipolar spindle formation (38). We have shown previously that cyclin D3 is vital for the kinase activity of CDK11p58 (58). Compared with the other D-type cyclins, cyclin D3 is ubiquitously expressed in a wide variety of cell types, but its function is poorly understood (2). Cyclin D3 functions not only in G1 phase as a regulatory subunit of CDK4 and CDK6 but also in G2/M phase as a partner of CDK11p58 during cell cycle progression. The histone acetyltransferase HBO1 was identified as being an interacting protein of CDK11p58 (62). Both cyclin D3 and HBO1 repress AR transcriptional activity through unknown mechanisms (26, 46). In the current study, we investigated whether cyclin D3 and CDK11p58 were involved in the regulation of AR-mediated transactivation. We report here that the cyclin D3/CDK11p58 holoenzyme kinase complex represses AR function through phosphorylating AR at Ser-308. These data contribute to a better understanding of the mechanism by which the cyclin/CDK complex regulates steroid signaling.
MATERIALS AND METHODS
Materials.
Dulbecco's modified Eagle's medium (DMEM), F12 medium, and bromodeoxyuridine (BrdU) were purchased from Sigma. Lipofectamine 2000 reagent, TRIzol, and rabbit polyclonal and mouse monoclonal anti-Myc antibodies were purchased from Invitrogen. Mouse antihemagglutinin (anti-HA) monoclonal antibody, HA peptide, polyvinylidene difluoride membranes, protein G-agarose, leupeptin, aprotinin, and phenylmethylsulfonyl fluoride (PMSF) were purchased from Roche Applied Science. Rabbit polyclonal anti-PITSLRE (C-17), anti-AR (N-20), anti-p300, and anti-HDAC1 antibodies; mouse monoclonal anti-AR (441), anti-CCND3 (D-7), and anti-BrdU antibodies; and rhodamine-conjugated goat anti-mouse immunoglobulin G (IgG), fluorescein isothiocyanate-conjugated mouse anti-goat IgG, horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG, and HRP-conjugated goat anti-mouse IgG secondary antibodies were purchased from Santa Cruz Biotechnology. The enhanced chemiluminescence (ECL) assay kit, [35S]methionine, and glutathione-Sepharose 4B beads were purchased from Amersham Biosciences. A dual luciferase reporter assay system, control pRL plasmid, and the TNT-coupled reticulocyte lysate system were purchased from Promega. β-Glycerolphosphate disodium and DHT were purchased from Fluka.
Experimental animals, cell culture, and transfections.
Eight-week-old male C-57 black mice, human PC-3 and LNCaP prostate cancer (PCa) cells, and COS-1 cells were obtained from the Institute of Cell Biology Academic Sinica. COS-1 cells were maintained in DMEM supplemented with 10% fetal calf serum (FCS), 100 units/ml of penicillin, and 50 μg/ml of streptomycin. PC-3 and LNCaP cells were cultured in F12 medium containing 10% FCS, 100 units/ml of penicillin, and 50 μg/ml of streptomycin. Transient transfection for luciferase assays was performed using 24-well plates (3 × 104 cells per well) with 500 ng of total plasmids using Lipofectamine 2000 reagent according to the manufacturer's instructions. Transfection for immunoprecipitation was performed in 100-mm dishes (approximately 3 × 106 cells) with 8 μg of total plasmids.
Plasmid construction and RNA interference.
The AR expression plasmid and mouse mammary tumor virus (MMTV)-LUC reporter plasmid were kindly provided by Zijie Sun (Department of Surgery and Genetics, Liem Sioe Liong Molecular Biology Laboratory, Stanford University School of Medicine, Stanford, CA) (46). Prostate-specific antigen (PSA)-LUC and androgen-responsive element (ARE)-LUC reporter plasmids were gifts from Roland Schüle (Universitäts-Frauenklinik und Zentrum für Klinische Forschung, Klinikum der Universität Freiburg, Freiburg, Germany) (32). HA-CDK11p58, pcDNA3-GST-CDK11p58, NTD (amino acids 1 to 100), PKD (amino acids 66 to 389), and C-terminal domain (CTD) (amino acids 374 to 439) expression plasmids were constructed as described previously (62). The D224N mutation was generated by site-directed mutagenesis using an MutanBEST kit (Takara) with primers described previously (28). The AR TAD (amino acids 1 to 556), DBD (amino acids 559 to 670), LBD (amino acids 712 to 919), TAU1 (amino acids 101 to 372), ΔTAU1 (amino acids 373 to 919), and TAU5 (amino acids 355 to 490) were PCR amplified and cloned into the pcDNA3-HA vector. Cyclin D3 N1 (amino acids 1 to 88), NTD (amino acids 1 to 153), and CTD (amino acids 154 to 292) were generated as described previously (47). Two complementary oligonucleotides targeted to the cyclin D3 gene were designed to knock down cyclin D3 expression: 5′-GATCC CCG ATG CTG GCT TAC TGG ATG TTC AAG AGA CAT CCA GTA AGC CAG CAT CTT TTT GGA AA-3′ and 5′-AGCTT TTC CAA AAA GAT GCT GGC TTA CTG GAT GTC TCT TGA ACA TCC AGT AAG CCA GCA TCG GG-3′. Plasmid pSi-CCND3 was constructed by inserting the annealed complementary oligonucleotides into the pSilencer 2.1-U6 neo vector (Ambion). The stealth small interfering RNA (siRNA) designed for CDK11 (siCDK11) was generated from the CDK11 cDNA sequence from bp 1664 to 1688: 5′-CCG GCA UCC UCA AGG UGG GUG ACU U-3′ and 5′-AAG TCA CCC ACC TTG AGG ATG CCG G-3′ (Invitrogen). The stealth siRNAs designed for AR were 5′-UAG AGA GCA AGG CUG CAA AGG AGU C-3′ and 5′-GAC UCC UUU GCA GCC UUG CUC UCU A-3′ (Invitrogen). To construct pSi-AR expression plasmids, two complementary oligonucleotides were synthesized and cloned into the pSilencer 2.1-U6 neo vector: 5′-AGCTTAAAAAAGTGGAAGATTCAGCCAAGCTCTCTTGAAGCTTGGCTGAATCTTCCACG-3′ and 5′-GATCCGTGGAAGATTCAGCCAAGCTTCAAGAGAGCTTGGCTGAATCTTCCACTTTTTTA-3′ (55).
Dual luciferase reporter gene assays.
LNCaP, PC-3, or COS-1 cells (3 × 104 cells per well in 24-well plates) were incubated in 5% charcoal-stripped fetal bovine serum-supplemented F12 medium or DMEM for 24 h prior to transfection. Cells were cotransfected with AR (20 ng), an androgen-responsive MMTV-luciferase reporter construct (200 ng), a control Renilla luciferase plasmid (pRL) (2 ng), and cyclin D3 or CDK11p58 in the indicated amounts. Total DNA was adjusted to 500 ng with empty pcDNA3 vector. At 16 h posttransfection, the culture medium was replaced with F12 medium or DMEM containing 5% charcoal-stripped fetal bovine serum and supplemented with ethanol (EtOH) or 10 nM DHT. After a further 24 h, cells were lysed using passive lysis buffer (Promega) according to the manufacturer's specifications and assayed immediately for reporter and control gene activities with the dual luciferase reporter gene assay (Promega) using a Lumat LB 9507 luminometer (EG&G Berthold, Bad Wildbad, Germany).
Immunoprecipitation and Western blotting.
COS-1 cells were transfected with 4 μg of HA-CDK11p58 and 4 μg of AR plasmids in 100-mm plates. Approximately 16 h after transfection, cells were cultured in medium containing 5% charcoal-stripped serum with EtOH or 10 nM DHT. After another 24 h, cells were washed with ice-cold phosphate-buffered saline and solubilized with 1 ml of coimmunoprecipitation (CoIP) buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.1% Nonidet P-40, 5 mM EDTA, 5 mM EGTA, 15 mM MgCl2, 60 mM β-glycerophosphate, 0.1 mM sodium orthovanadate, 0.1 mM NaF, 0.1 mM benzamide, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM PMSF). CoIP was performed as described previously (34). For the CoIP from mouse tissues, the testes and the prostates of 5-week-old male C-57 black mice were extracted in CoIP buffer. One milligram of total tissue lysates was subjected to CoIP as described above.
Sequential CoIP assays were performed as described previously, with minor modifications (56). HA peptide, bacterially expressed and purified cyclin D3, and the AR TAD were employed as elution peptides for mouse monoclonal anti-HA, anti-CCND3 (D-7), and anti-AR (441) antibodies, respectively. For example, the transfected cells were lysed in CoIP buffer. Anti-CCND3 antibody was used to immunoprecipitate cyclin D3. The primary immunoprecipitates were eluted with 250 μl CoIP buffer containing 200 μg/ml recombinant glutathione S-transferase (GST)-CCND3 peptides. The eluates were then immunoprecipitated with anti-HA antibody. The secondary immunoprecipitates were extensively washed and probed with mouse monoclonal anti-CCND3 and anti-AR and rabbit polyclonal anti-PITSLRE antibodies.
In vitro binding assays.
GST control, GST-CDK11p58, or GST-CDK11p110 was in vitro translated, [35S]methionine labeled, preimmobilized onto glutathione-Sepharose 4B beads, and incubated with in vitro [35S]methionine-labeled AR in binding buffer (20 mM Tris [pH 7.5], 50 mM NaCl, 10% glycerol, 10 mM NaF, 1% Nonidet P-40, 1 mM NaVO4, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM PMSF) for 4 h at 4°C with gentle rotation. Bound proteins were eluted with sodium dodecyl sulfate (SDS) protein sample buffer, denatured, subjected to SDS-polyacrylamide gel electrophoresis (PAGE), and visualized using phosphorimaging. Cyclin D3 was expressed in Escherichia coli cells as a GST fusion protein, purified, and preimmobilized on glutathione-Sepharose 4B beads. GST protein was used as a negative control. In vitro [35S]methionine-labeled AR was preimmobilized GST or GST-CCND3. GST pull-down assays were performed as described above.
In vitro and in vivo phosphorylation assays.
The cyclin D3/CDK11p58 protein kinase activity assay was carried out as described previously, with minor modifications (27). After transfection with 4 μg of cyclin D3 and 4 μg of CDK11p58 plasmids, 500 μg of COS-1 cell lysates was immunoprecipitated with anti-HA antibody. The protein kinase activity of the immunoprecipitates was assayed using 30 μl of kinase buffer (50 mM Tris-HCl [pH 7.5], 15 mM MgCl2, 1 mM dithiothreitol, 50 μM ATP, 5 μCi [γ-32P]ATP) containing 1 μg of histone H1 or purified AR deletions. After incubation at 30°C for 30 min, reactions were terminated by the addition of SDS sample buffer to the reaction mixture. The samples were centrifuged after denaturation at 95°C for 5 min. The supernatants were subjected to 10% SDS-PAGE. The gel was fixed, dried, and analyzed by use of the FLA-5100 phosphorimaging system (Fujifilm).
To test AR phosphorylation in cells, LNCaP cells were transfected with CDK11p58, cyclin D3, or siCCND3 as indicated. At 24 h posttransfection, cells were washed with phosphate-free DMEM (Invitrogen) and then incubated with 0.5 mCi/ml [32P]orthophosphate in the presence of 10 nM DHT for 6 h at 37°C (45). The cell lysates were prepared with CoIP buffer, immunoprecipitated with anti-AR antibody, and subjected to 10% SDS-PAGE followed by phosphorimaging.
Immunohistochemistry and immunofluorescence.
Prostate samples were prepared from 8-week-old male C-57 black mice. Three-micrometer-thick sections of formalin-fixed, paraffin-embedded prostate tissue were dewaxed in xylene and rehydrated in decreasing concentrations of ethanol, and endogenous peroxidase activity was blocked using 0.3% (vol/vol) H2O2-methanol. Immunohistochemistry assays were performed as described previously (3). Rabbit polyclonal anti-AR (AR441, 1:100 dilution), anti-PITSLRE (1:50 dilution), and anti-CCND3 (1:100 dilution) antibodies were used as primary antibodies. After being washed, sections were incubated with HRP-conjugated goat anti-rabbit IgG for 60 min. The sections were washed three times, and the staining of these sections was then obtained by treating the sections with 50 mM Tris-HCl-buffered saline containing 0.01% H2O2 and 25 mg diaminobenzidine for 2 to 10 min.
For immunofluorescence, LNCaP cells were cultured in six-well dishes and were transfected with 2 μg of CDK11p58, pSi-CCND3, or both along with 0.5 μg of green fluorescent protein (GFP) plasmids using Lipofectamine 2000. At 24 h posttransfection, cells were washed and cultured in F12 medium containing 5% FCS, 10 nM DHT, and 10 μM BrdU (Sigma) for 24 h. Cells were then fixed in 4% paraformaldehyde. BrdU-labeled cells were evaluated under a fluorescent microscope as described previously (25). The percentages of BrdU-labeled cells were calculated based on 100 GFP-positive cells from each sample.
PSA detection.
LNCaP cells seeded on six-well plates were transfected with 2 μg of cyclin D3, CDK11p58, CDK11p110, D224N, or pSi-CCND3 alone or in combination. At 16 h after transfection, LNCaP cells were treated with 10 nM DHT for another 24 h. Cells were than collected in TRIzol reagent (Invitrogen) and subjected to RNA extraction according to the manufacturer's instructions. One microgram of total RNA extracted from the transfected cells was subjected to reverse transcription (RT). The primers used for real-time RT-PCR were 5′-CAC CTG CTC GGG TGA TTC TG-3′ and 5′-CCA CTT CCG GTA ATG CAC CA-3′. Real-time RT-PCR was performed using an iCycler iQ multicolor real-time PCR detection system (Bio-Rad) with the following cycling conditions: (i) 15 s at 94°C and (ii) 45 cycles, with 1 cycle consisting of 15 s at 94°C, 20 s at 55°C, and 15 s at 72°C. Each sample was run in triplicate. β-Actin was employed as an internal reference under the same experimental conditions. Data were analyzed by using iCycler iQ software (Bio-Rad). The values were obtained through normalizing PSA copies to β-actin copies. The secreted PSA in conditioned medium was analyzed by use of an enzyme-linked immunosorbent assay method.
RESULTS
Cyclin D3 and CDK11p58 repress AR-dependent transcription.
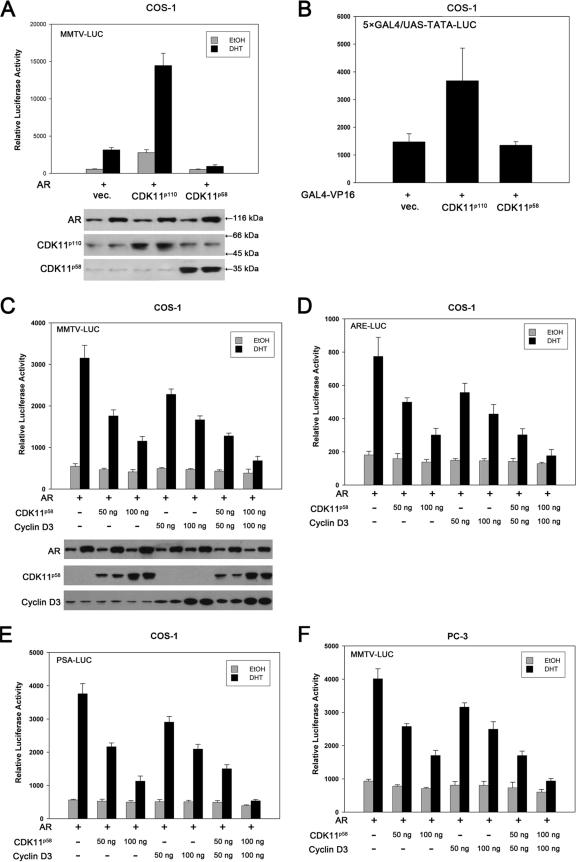
We took advantage of a dual luciferase assay system using the MMTV-LUC reporter, which contains multiple androgen-responsive elements from the long terminal repeat promoter of MMTV, and the internal control plasmid pRL, which encodes cytomegalovirus promoter-regulated Renilla luciferase, together to investigate whether CDK11 proteins are involved in the transcription activity of AR. Figure 1A demonstrated that CDK11p110 potentiated AR-mediated transcription by five times in both EtOH- and DHT-treated cells. Interestingly, AR transcriptional activity was slightly decreased by CDK11p58 in quiescent cells but significantly repressed in DHT-treated COS-1 cells. GAL4-VP16 and its reporter plasmid, 5×GAL4/UAS-TATA-LUC, which contains a synthetic promoter with five tandem repeats of the yeast GAL4 binding sites that control the expression of the Photinus pyralis luciferase gene, were employed to determine whether the transcriptional regulatory roles of CDK11 proteins were specific. GAL4-VP16 activity was also promoted by CDK11p110, suggesting its nonspecific role in eukaryotic transcription (Fig. 1B). Thus, we propose that the promotion of AR activity in both the presence and absence of androgens by CDK11p110 may be due to the fact that CDK11p110 associates with RNA transcription and splicing complexes, such as cyclin L, TFIIF, TFIIS, and ELL, and enhances their activities in a less specific manner (49). In contrast, the overexpression of CDK11p58 did not affect GAL4-VP16-mediated transactivation, implying that AR repression by CDK11p58 is specific. Cyclin D3 is reported to be an AR repressor, but its mode of repressive signaling still remains unclear (26). The kinase activity of CDK11p58 is cyclin D3 dependent (58). We then investigated whether CDK11p58 was involved in AR regulation in a cyclin D3-dependent manner. As shown in Fig. 1C, DHT-induced AR transactivation was inhibited by 44% when 50 ng of CDK11p58 was transfected. In the presence of 100 ng of CDK11p58 expression plasmids, AR activity was downregulated by 63%. Similar to data reported previously (26), 50 ng of cyclin D3 AR transcriptional activity was repressed by 27% when 50 ng of cyclin D3 was transfected and by 50% when 100 ng of cyclin D3 was transfected. In the presence of 50 ng of CDK11p58 and 50 ng of cyclin D3, AR activity was repressed by 60%, and the combination of 100 ng of CDK11p58 and 100 ng of cyclin D3 resulted in the decrease of AR-dependent transcription by over 80%. These data implied that the repression of AR by CDK11p58 is dependent on cyclin D3 and vice versa.
FIG. 1.
Cyclin D3 and CDK11p58 repress AR-dependent transcriptional activation in multiple human cell lines. (A) Twenty nanograms of AR and 200 ng of MMTV-LUC were transfected with 50 ng of CDK11p110 or CDK11p58 or control vector (vec.). The cells were treated with EtOH or 10 nM DHT at 24 h after transfection and were harvested at 24 h after androgen treatment. Luciferase activity was measured and normalized to Renilla luciferase activity. Expression of AR, CDK11p110, and CDK11p58 was monitored by Western blotting using rabbit polyclonal anti-AR (N-20) targeting the AR N terminus and rabbit polyclonal anti-PITSLRE, recognizing the C-terminal region of CDK11p110/p58. (B) Twenty nanograms of GAL4-VP16 and 200 ng of 5×GAL4/UAS-TATA-LUC were transfected with 50 ng of CDK11p110 or CDK11p58 or control vector. At 48 h posttransfection, luciferase activity was assayed as described above. (C) Twenty nanograms of AR and 200 ng of MMTV-LUC were transfected with indicated amounts of CDK11p58, cyclin D3, or both into COS-1 cells. Expression of AR, cyclin D3, and CDK11p58 was probed with anti-AR (N-20), anti-PITSLRE, and mouse monoclonal anti-CCND3, which does not cross-link with other D-type cyclins. (D) COS-1 cells were transfected and treated as described above except that ARE-LUC was used as the reporter plasmid. (E) PSA-LUC was used as the reporter plasmid in the transfected COS-1 cells. (F) AR and MMTV-LUC were transfected with the indicated amounts of CDK11p58, cyclin D3, or both into AR-independent PC-3 PCa cells. Luciferase activity was assayed as described above. (G) MMTV-LUC was transfected with the indicated amounts of CDK11p58, cyclin D3, or both into androgen-dependent LNCaP PCa cells. Luciferase activity was then assayed. (H) Two hundred nanograms of MMTV-LUC was cotransfected with 200 ng pSi-CCND3 alone or in combination with 50 ng of CDK11p58 into LNCaP cells. The pSilencer vector was used as a control. (I) MMTV-LUC was cotransfected with 20 nM siCDK11 alone or with CDK11p110, CDK11p58, or cyclin D3 or in combinations, as indicated, into LNCaP cells. Scrambled siRNA was used as a control. Data shown are the means and standard errors for three independent experiments. Expression of AR, CDK11p58/p110, and cyclin D3 was monitored by Western blotting.
Two other reporter plasmids for AR, ARE-LUC, containing two tandem copies of the ARE, and PSA-LUC, involving both the distal and proximal ARE-containing enhancer regions of the PSA promoter, were tested to confirm the repressive effect of CDK11p58 on AR transcriptional activity. Both of the reporters displayed similar patterns of AR repression by cyclin D3 and CDK11p58 in COS-1 cells (Fig. 1D and E). Consistent results were obtained using AR-transfected PC-3 cells, an AR-independent human PCa cell line that does not express AR (Fig. 1F). To further investigate whether endogenous AR was regulated by cyclin D3 and CDK11p58, AR-dependent LNCaP human PCa cells, which express AR and secrete PSA (20), were transfected with MMTV-LUC, cyclin D3, and CDK11p58 expression plasmids. The dual luciferase reporter assay showed that androgen-dependent endogenous AR-mediated transactivation was repressed by cyclin D3 and CDK11p58 as well (Fig. 1G). These observations were not due to the changes in AR expression, as both the exogenous and the endogenous AR levels remained constant in the presence of ectopic cyclin D3 and CDK11p58 (Fig. 1A, C, and G, bottom).
To further determine whether cyclin D3 and CDK11p58 cooperate in AR regulation, pSilencer-CCND3 and siCDK11 were used to knock down the expression of cyclin D3 or CDK11p58. Transfection of pSilencer-CCND3 (200 ng per well in 24-well plates) led to an increase in AR-dependent transactivation of 40%. Cotransfection of pSilencer-CCND3 with CDK11p58 almost totally abolished CDK11p58-mediated AR repression (Fig. 1H). siCDK11 was generated from the CDK11 cDNA sequence from bp 1664 to 1688. The expression of CDK11 was downregulated by 72% in the presence of 20 nM siCDK11, while AR-mediated transactivation was enhanced by 60%. The overexpression of CDK11p110 in siCDK11-transfected cells led to a further enhancement of AR activity. However, ectopic CDK11p58 antagonized the transcription that was increased by siCDK11 (Fig. 1I). These data suggest that among the CDK11 isoforms, CDK11p58 might play a specific and dominant role in AR regulation. The overexpression of cyclin D3 caused only a minor decrease in AR activity in siCDK11-transfected cells, implying that cyclin D3 cooperates with CDK11p58 in AR repression.
PSA expression in PCa cells was inhibited by cyclin D3/CDK11p58.
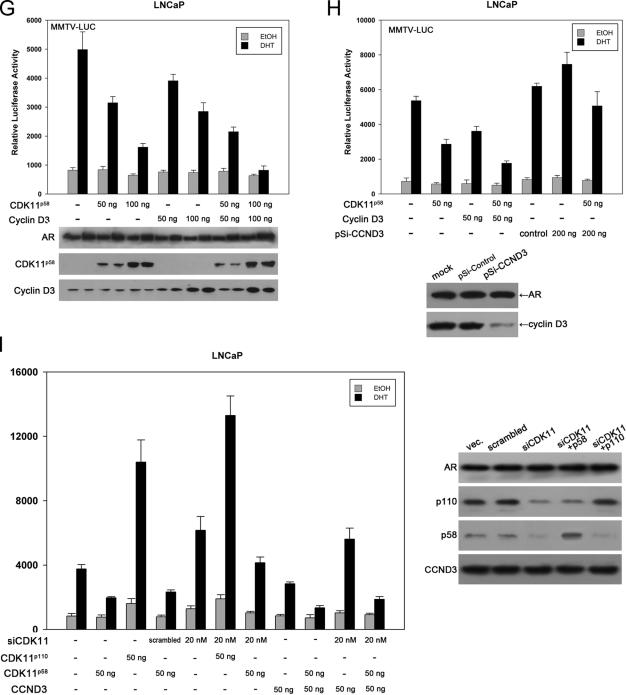
To support the regulatory role of cyclin D3/CDK11p58 in AR function, PSA expression in LNCaP cells was analyzed. Total RNA was extracted from transfected cells. PSA mRNA expression was quantitated by use of real-time RT-PCR. PSA transcripts were normalized to β-actin mRNA, and data are presented as increases (n-fold) compared to control vector-transfected cells. As shown in Fig. 2A, transfection with 2 μg of CDK11p110 per well in a six-well plate led to an increase in PSA mRNA of 56%. The overexpression of CDK11p58 inhibited the PSA mRNA level by 32%. The overexpression of cyclin D3 repressed PSA mRNA levels by 12%. In cyclin D3- and CDK11p58-cotransfected cells, PSA mRNA was downregulated by 52%. siCDK11 increased PSA transcripts by 23% and recovered cyclin D3-induced inhibition of PSA mRNA. The downregulation of the endogenous cyclin D3 expression using pSi-CCND3 resulted in a 15% increase in PSA mRNA expression. When pSi-CCND3 was cotransfected with CDK11p58, the inhibitive effect of CDK11p58 was obliterated. A concordant change of secreted PSA protein in conditioned medium was observed to a lesser extent (Fig. 2B).
FIG. 2.
PSA expression in LNCaP cells is inhibited by cyclin D3/CDK11p58. (A) LNCaP cells were transfected with the plasmids indicated. Twenty-four hours after transfection, LNCaP cells were treated with 10 nM DHT for another 24 h. Quantitative real-time RT-PCR was performed using 1 μg of total RNA extracted from the transfected cells. Real-time RT-PCR for β-actin was also performed as an internal reference to normalize PSA mRNA amounts. The data are presented as increases (n-fold) compared to the control vector (vec.)-transfected group. (B) LNCaP cells were transfected and treated as described above. The secreted PSA in conditioned medium was analyzed by enzyme-linked immunosorbent assays. The values were obtained as increases (n-fold) compared to the vector control group. Data shown are the means and standard errors for three independent experiments.
CDK11p58 interacts with AR in vitro and in mammalian cells.
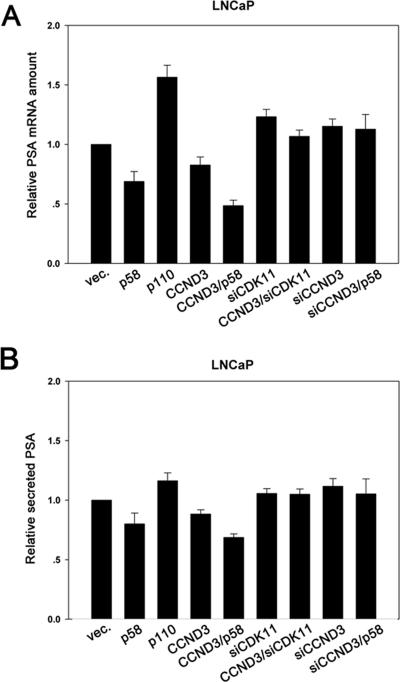
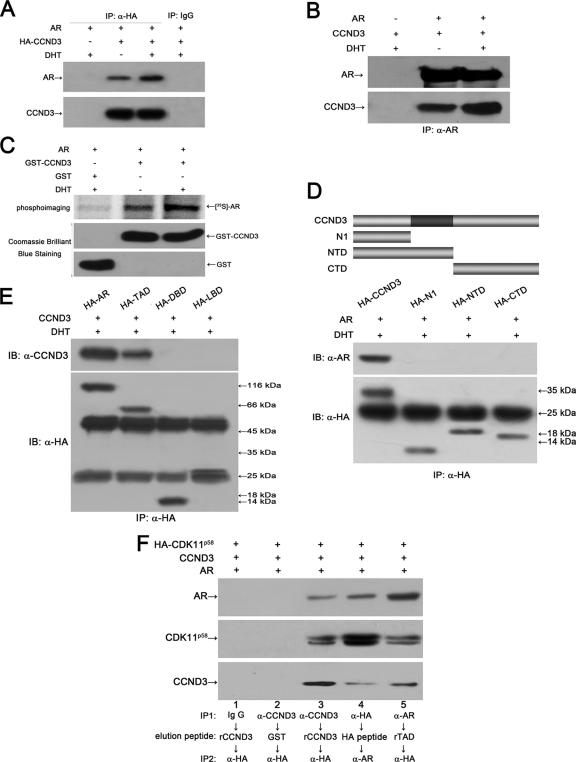
To examine the association between CDK11p58 and AR, GST pull-down and CoIP assays were performed. GST, GST-CDK11p58, GST-CDK11p110, and AR were translated and [35S]methionine labeled in vitro. AR was incubated with either GST, GST-CDK11p110, or GST-CDK11p58 with glutathione-Sepharose 4B beads. AR interacted with CDK11p58 in the absence of DHT. The inclusion of ligand further intensified the binding. Interactions between CDK11p110 and AR were not observed in the assays. Anti-HA was used to immunoprecipitate HA-CDK11p110 or HA-CDK11p58 from transfected cells. The coimmunoprecipitates were probed with anti-AR antibody and reprobed with anti-PITSLRE antibody, which recognizes the C-terminal region of CDK11p110/p58 and can thus detect both the p110 and p58 isoforms of CDK11. As a result, AR was not coimmunoprecipitated with HA-CDK11p110, while the binding of AR to CDK11p58 was detected and potentiated by DHT stimulation (Fig. 3B). Consistently, CDK11p58 was coimmunoprecipitated with AR specifically by the anti-AR antibody in transfected COS-1 cells (Fig. 3C). To gain further insight into the interaction, CDK11p58 and AR were fragmented according to their functional domains. As a result, the Ser/Thr PKD of CDK11p58 and AR TAU1 were the key regions mediating the interaction between CDK11p58 and AR (Fig. 3D and E). A conserved leucine-rich motif (the LXXLL or LXXXLL motif) is considered to be an important region for many protein factors to interact with steroid receptors (42). The CDK11p58 PKD contains an 186LMIQLL191 motif. Leu-186, Leu-190, and Leu-191 were mutated separately or in combination. CoIP assays showed that all the CDK11p58 mutants were capable of binding and inhibiting AR (Fig. 3F and G), suggesting that the CDK11p58-AR interaction is LXXXLL motif independent.
FIG. 3.
AR interacts with CDK11p58. (A) GST control, GST-CDK11p58, or GST-CDK11p110 was in vitro translated, [35S]methionine labeled, preimmobilized onto glutathione-Sepharose 4B beads, and incubated with in vitro [35S]methionine-labeled AR in binding buffer. Bound proteins were subjected to SDS-PAGE and visualized by phosphorimaging. (B) COS-1 cells were transfected with AR alone or in combination with HA-CDK11p110 or HA-CDK11p58. At 16 h after transfection, cells were treated with 10 nM DHT or EtOH for 24 h. Lysates from the transfected cells were immunoprecipitated (IP) with mouse monoclonal anti-HA antibody (α-HA). The immunoprecipitates were blotted with anti-AR (N-20) antibody and then reprobed with anti-PITSLRE antibody. vec., vector control. (C) COS-1 cells were transfected with HA-CDK11p58 alone or in combination with AR. Immunoprecipitation was carried out using mouse monoclonal anti-AR (441) antibody or control IgG. The immunoprecipitates were blotted with anti-AR and anti-PITSLRE antibodies. (D) Expression plasmids encoding HA-tagged CDK11p58 deletions were constructed as described in Materials and Methods and were cotransfected with AR into COS-1 cells. Immunoprecipitation was performed using anti-HA antibody. The immunoprecipitates were blotted with rabbit polyclonal anti-AR (N-20) antibody. The membrane was then stripped and reprobed with anti-HA antibody. IB, immunoblot. (E) Expression plasmids encoding HA-tagged AR deletions were constructed as described in Materials and Methods and were cotransfected with CDK11p58 into COS-1 cells. Immunoprecipitation was performed using anti-HA antibody. The immunoprecipitates were blotted with anti-PITSLRE. The membrane was then stripped and reprobed with anti-HA antibody. (F) AR was cotransfected with HA-tagged CDK11p58 or its point mutants, including L186A, L190A, L191A, L186A/L190A, and L186A/L190A/L191A, into COS-1 cells. Immunoprecipitation was carried out using anti-HA antibody. The immunoprecipitates were blotted with anti-AR and anti-PITSLRE antibodies. (G) AR and MMTV-LUC were cotransfected with CDK11p58 or its point mutants. MMTV-LUC activity was assayed and normalized to Renilla luciferase activity.
AR forms a ternary complex with cyclin D3/CDK11p58.
In vivo and in vitro binding assays were also performed to test the interaction between cyclin D3 and AR. AR was coimmunoprecipitated with HA-tagged cyclin D3 using an anti-HA antibody. The anti-HA antibody did not pull down AR in AR- and control vector-cotransfected cells. Control IgG also did not immunoprecipitate AR in HA-CCND3- and AR-transfected COS-1 cells (Fig. 4A). Cyclin D3 was also coimmunoprecipitated by anti-AR antibody in AR- and cyclin D3-overexpressing COS-1 cells (Fig. 4B). For in vitro binding assays, cyclin D3 was expressed and purified in E. coli cells as a GST fusion protein. GST pull-down assays demonstrated that the in vitro-translated 35S-labeled AR bound significantly to recombinant cyclin D3 (compared to the GST control). In the presence of DHT, the binding of AR and cyclin D3 was enhanced (Fig. 4C). These findings proved a specific interaction between cyclin D3 and AR.
FIG. 4.
AR associates with cyclin D3 and forms a ternary complex with cyclin D3/CDK11p58. (A) AR was cotransfected with HA-CCND3 or control vectors. Immunoprecipitation (IP) was performed using anti-HA antibody (α-HA) or normal mouse IgG. The immunoprecipitates were blotted with anti-AR and anti-CCND3 antibodies. (B) Cyclin D3 was cotransfected with AR or control vectors. Immunoprecipitation was performed using anti-AR (441) antibody. The immunoprecipitates were blotted with anti-AR (441) and anti-CCND3 antibodies. (C) GST-fused cyclin D3 was bacterially expressed, purified, immobilized onto glutathione-Sepharose 4B beads, and incubated with [35S]methionine-labeled AR. Bound proteins were analyzed by SDS-PAGE followed by phosphorimaging. (D) AR was cotransfected with HA-tagged expression plasmids encoding different regions of cyclin D3 into COS-1 cells. Lysates from transfected cells were immunoprecipitated using anti-HA antibody. The immunoprecipitates were blotted using anti-AR (N-20) antibody and reprobed with anti-HA antibody. IB, immunoblot. (E) Cyclin D3 was cotransfected with HA-tagged AR deletions. The immunoprecipitates were obtained using anti-HA antibody, blotted with anti-CCND3 antibody, and reprobed with anti-HA antibody. (F) HA-CDK11p58, cyclin D, and AR were cotransfected into COS-1 cells. Lysates prepared from the transfected cells were subjected to sequential immunoprecipitations according to the experimental strategies indicated below. HA peptide and bacterially expressed and purified GST fusion proteins of cyclin D3 (rCCND3) and AR TAD (rTAD) were employed as elution peptides for the corresponding antibodies after the primary immunoprecipitation. Normal mouse IgG and purified GST protein were used as negative controls.
Cyclin D3 and AR were then fragmented to search for the key regions mediating their interaction. As shown in Fig. 4D and E, the full length was indispensable for cyclin D3 to bind to AR, while the AR TAD was the essential domain to mediate the interaction between AR and cyclin D3.
In view of the interactions of AR-CDK11p58 and AR-cyclin D3, we asked whether the three proteins formed a ternary complex in cells. COS-1 cells were cotransfected with HA-CDK11p58, cyclin D3, and AR expression plasmids. Sequential CoIP using whole-cell lysates from the transfected cells was performed with the strategies indicated in the legend of Fig. 4F. HA peptide, bacterially expressed cyclin D3, and AR TAD were used as the elution peptides for mouse monoclonal anti-HA, anti-CCND3, and anti-AR antibodies, respectively. The final immunoprecipitates were blotted with mouse monoclonal anti-CCND3 and anti-AR and rabbit polyclonal anti-PITSLRE antibodies. As shown in Fig. 4F, CDK11p58, cyclin D3, and AR were present in the secondary immunoprecipitates (lanes 3, 4, and 5). As negative controls, when the immunoprecipitates were eluted with nonspecific peptides (GST) or the immunoprecipitation was performed using normal IgG, the three proteins were not detected in the secondary immunoprecipitates (Fig. 4F, lanes 1 and 2). This provides the evidence that AR forms a ternary complex with CDK11p58/cyclin D3 in mammalian cells.
AR associates with cyclin D3 and CDK11p58 in male reproductive tissues.
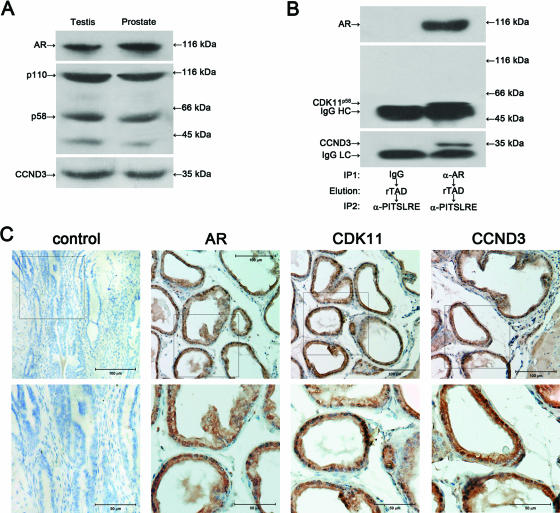
To verify the above-described findings, reproductive tissues including prostate and testis were obtained from 8-week-old adult male C-57 mice and subjected to CoIP and immunohistochemistry assays. Although CDK11p58 is expressed at a low level in cells due to its unique internal ribosome entry site-dependent translation in the G2/M phase of the cell cycle, it exhibited a relatively high level of expression in mice prostate and testis (Fig. 5A). Compared to CDK11p58, the expression of CDK11p110 is about 0.8-fold higher in mouse testis and 1.2-fold higher in mouse prostate. Cyclin D3 and AR displayed high expression levels in the mouse reproductive tissues examined. The ternary complex of cyclin D3-CDK11p58-AR in mouse prostates was also found by sequential CoIP assay, while the CDK11p110 isoform was not present in the immunoprecipitates (Fig. 5B). The immunohistochemistry assay demonstrated that CDK11, cyclin D3, and AR were all highly expressed in the luminal epithelial layer of murine prostate and were located mainly in the nuclear region of prostate epithelial cells (Fig. 4C). Expression of CDK11, cyclin D3, and AR in mouse testis was also visualized through immunohistochemistry, and colocalization of the three proteins was observed (data not shown). All these data confirmed the association between AR and cyclin D3/CDK11p58.
FIG. 5.
AR associates with cyclin D3/CDK11p58 in mouse testis and prostate. (A) Fifty micrograms of total protein extracted from testis and prostate of an 8-week-old male C-57 black mouse was subjected to SDS-PAGE and immunoblotted with anti-AR (N-20), anti-CCND3, and anti-PITSLRE antibodies. (B) One milligram of total protein extracted from testis and prostate of a male C-57 black mouse was immunoprecipitated (IP) with mouse monoclonal anti-AR (441) antibody, eluted with recombinant AR TAD (rTAD), and immunoprecipitated with rabbit polyclonal anti-PITSLRE antibody (α-PITSLRE). Normal mouse IgG was used as a negative control. The immunoprecipitates were blotted with mouse monoclonal anti-AR and anti-CCND3 antibodies. The membrane was then stripped and reprobed with rabbit polyclonal anti-PITSLRE antibody. (C) Sections of prostate samples prepared from the 8-week-old male C-57 black mouse were stained with anti-AR (N-20), anti-CCND3, and anti-PITSLRE antibodies or control IgG followed by diaminobenzidine staining. Magnifications, ×20 (top panels) and ×40 (bottom panels).
AR was phosphorylated by the cyclin D3/CDK11p58 complex in vitro and in vivo.
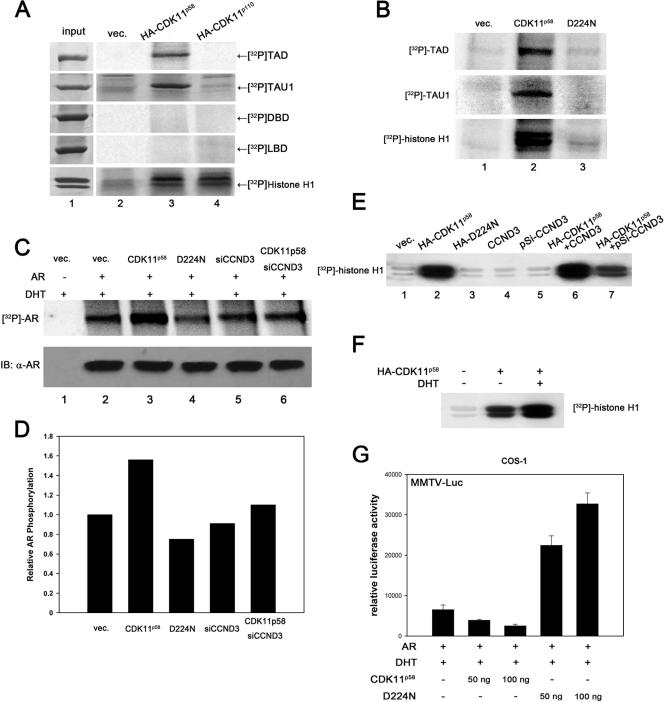
Considering the association between cyclin D3/CDK11p58 and AR, we explored whether AR was phosphorylated by cyclin D3/CDK11p58. HA-tagged CDK11p110 or CDK11p58 was transfected into COS-1 cells and immunoprecipitated with anti-HA antibody. The AR TAD, DBD, and LBD were bacterially expressed as GST fusion proteins and were used as substrates in the in vitro CDK11 kinase assay. As shown in Fig. 6A, The AR TAD, but not the DBD and LBD, was phosphorylated in vitro by CDK11p58. Furthermore, AR TAU1, which is located within the N terminus of the TAD and is the minimal domain found to interact with CDK11p58, was phosphorylated by CDK11p58. None of the AR domains tested were phosphorylated by CDK11p110 in vitro. D224N, the kinase-dead mutant of CDK11p58, did not phosphorylate the TAD, TAU1, and histone H1 in the in vitro kinase assay (Fig. 6B).
FIG. 6.
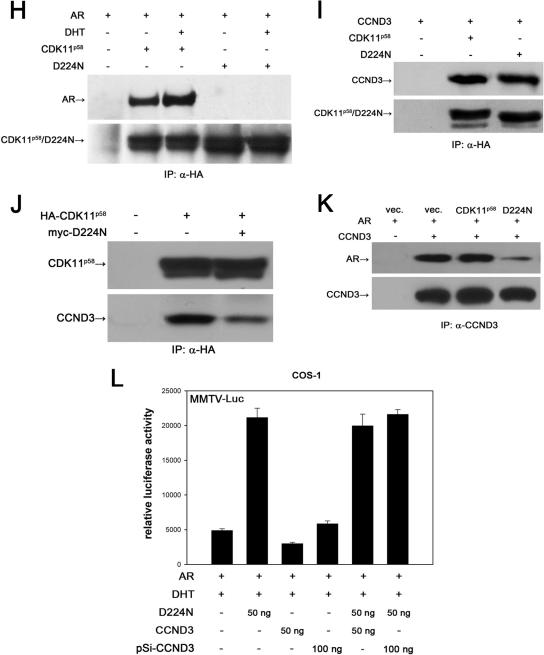
AR is phosphorylated by cyclin D3/CDK11p58 in vitro and in vivo. (A) HA-CDK11p110 or HA-CDK11p58 was transfected into COS-1 cells. At 48 h posttransfection, lysates from 107 cells were immunoprecipitated with anti-HA antibody. Bacterially expressed and purified AR TAD, DBD, and LBD were used as substrates for immunoprecipitated HA-CDK11p110 or HA-CDK11p58. The supernatants were subjected to SDS-PAGE and phosphorimaging. Histone H1 was employed as a positive control. vec., vector. (B) HA-CDK11p58 or HA-D224N was immunoprecipitated from transfected COS-1 cells. The in vitro kinase assay was performed as described above. (C) AR was cotransfected with HA-CDK11p58, HA-D224N, or siCCND3 expression plasmids as indicated. At 24 h after transfection, cells were washed with phosphate-free DMEM and then incubated with 0.5 mCi/ml [32P]orthophosphate in the presence of 10 nM DHT for 6 h at 37°C. Immunoprecipitated AR from the transfected cells was subjected to SDS-PAGE and phosphorimaging. AR input was monitored with Western blotting (IB) of 5% whole-cell lysates. (D) Semiquantitation of AR phosphorylation was obtained through grayscale scanning of the image obtained in C. (E) Anti-HA antibody was used to immunoprecipitate HA-CDK11p58 or HA-D224N from the LNCaP cells transfected with the indicated plasmids. In vitro kinase assays for CDK11p58 or D224N were monitored using histone H1. (F) HA-CDK11p58-transfected LNCaP cells were treated with EtOH or 10 nM DHT. CDK11p58 was immunoprecipitated using anti-HA antibody and subjected to in vitro histone H1 kinase assays. (G) AR and MMTV-LUC plasmids were cotransfected with wild-type CDK11p58 or the kinase mutant D224N. After treatment with 10 nM DHT for 24 h, the transfected cells were assayed for luciferase activity. (H) AR was cotransfected with HA-CDK11p58 or HA-D224N. The immunoprecipitates (IP) were obtained using anti-HA antibody (α-HA) and were probed with anti-AR (N-20) and anti-PITSLRE antibodies. (I) Cyclin D3 was cotransfected with HA-CDK11p58 or HA-D224N. The immunoprecipitates were obtained using anti-HA antibody and were probed with anti-CCND3 and anti-PITSLRE antibodies. (J) Cyclin D3 and HA-CDK11p58 were cotransfected with control vectors or Myc-D224N. Immunoprecipitation was performed using anti-HA antibody. The immunoprecipitates were detected using anti-CCND3 and anti-PITSLRE antibodies. (K) AR, cyclin D3, and HA-CDK11p58 were cotransfected with control vector or D224N. The immunoprecipitation was performed using anti-CCND3 antibody. The immunoprecipitates were detected using anti-AR, anti-CCND3, and anti-PITSLRE antibodies. (L) AR and MMTV-LUC were cotransfected with D224N, pSi-CCND3, or both. After treatment with 10 nM DHT for 24 h, the transfected cells were assayed for luciferase activity.
To determine whether CDK11p58 is associated with AR phosphorylation in vivo, LNCaP cells were transfected with CDK11p58 or D224N and were metabolically labeled with [32P]orthophosphate for 6 h in the presence of DHT. AR was immunoprecipitated from the cell lysates and subjected to SDS-PAGE and phosphorimaging. The kinase activity of cyclin D3/CDK11p58 in parallel treated cells was monitored by the in vitro phosphorylation of histone H1. As shown is Fig. 6C, AR phosphorylation was increased by the overexpression of CDK11p58 (compare lane 3 to lane 2), while it exhibited a decrease in D224N-overexpressing cells (compare lane 4 to lane 2). When pSi-CCND3 was used to knock down endogenous cyclin D3, the overexpression of CDK11p58 failed to enhance the phosphorylation of AR in vivo (Fig. 6C, compare lane 6 to lane 2). The grayscale scan showed that the overexpression of CDK11p58 enhanced AR phosphorylation by 56%, while D224N inhibited AR phosphorylation by 25%. pSi-CCND3 downregulated AR phosphorylation by 10%. When endogenous cyclin D3 expression was knocked down, the overexpression of CDK11p58 resulted in only a 10% increase in AR phosphorylation (Fig. 6D). It was confirmed in the histone H1 kinase assays that cyclin D3 was vital for the histone H1 kinase activity of CDK11p58 (Fig. 6E, compare lanes 6 and 7 to lane 2). Interestingly, we also found that the kinase activity of CDK11p58 was stimulated by DHT in LNCaP cells (Fig. 6F, compare lane 3 to lane 2). The effect of the CDK11p58 kinase-dead mutant on AR-dependent transcription was then assessed. Surprisingly, D224N facilitated AR-dependent transcription significantly (Fig. 6G). In search of the mechanism by which D224N enhances transcription, we found that D224N failed to bind AR in vivo (Fig. 6H) but retained the ability to interact with cyclin D3 (Fig. 6I). Thus, we questioned whether D224N potentiates AR-dependent transcription through blocking the association between AR and cyclin D3/CDK11p58. As shown in Fig. 6J, the overexpression of D224N interfered with the interaction between cyclin D3 and CDK11p58. Figure 6K shows that the association between AR and cyclin D3/CDK11p58 was inhibited by ectopic D224N. The repressive effect of cyclin D3 was hence interrupted in D224N-overexpressing LNCaP cells (Fig. 6L).
Cyclin D3/CDK11p58 represses AR through phosphorylating TAU1 at Ser-308.
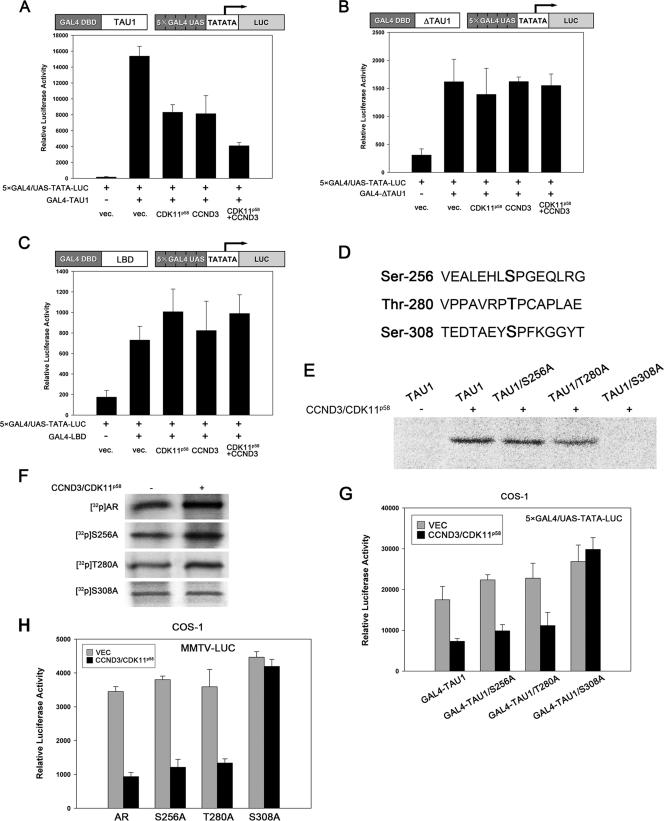
In our endeavor to elucidate how cyclin D3/CDK11p58 exerts the repressive effect on AR, we found that neither the AR nuclear translocation, the AR N/C functional interaction, nor coregulator binding as measured by AR interactions with p300 or p/CAF or HDAC1 was influenced by CDK11p58 overexpression. Chromatin immunoprecipitation assays indicated that CDK11p58 was not present at PSA promoters (data not shown). Given the fact that AR TAU1 was phosphorylated by cyclin D3/CDK11p58 in vitro, we tested whether TAU1 played a central role in AR repression by cyclin D3/CDK11p58. TAU1, ΔTAU1 lacking the TAU1 domain, and AF-2 located in the AR LBD were fused in frame downstream of the GAL4 DBD. Plasmid 5×GAL4/UAS-TATA-LUC was employed as the reporter plasmid for GAL4 DBD-fused AR domains. In the three AR fragments examined, only AR TAU1-mediated transactivation was repressed in response to cyclin D3/CDK11p58 overexpression (Fig. 7A to C). Thus, cyclin D3/CDK11p58 might directly target the AR TAU1 domain for phosphorylation and transcriptional repression.
FIG. 7.
Cyclin D3/CDK11p58 represses AR TAU1 through phosphorylation of Ser-308. (A) 5×GAL4/UAS-TATA-LUC and GAL4-TAU1 were cotransfected with cyclin D3, CDK11p58, or both. At 48 h posttransfection, luciferase activity was determined. (B) 5×GAL4/UAS-TATA-LUC and GAL4-ΔTAU1 containing the TAU1-deleted AR fragment were cotransfected with cyclin D3, CDK11p58, or both. At 48 h posttransfection, luciferase activity was determined. (C) 5×GAL4/UAS-TATA-LUC and GAL4-LBD were cotransfected with cyclin D3, CDK11p58, or both. At 48 h posttransfection, luciferase activity was determined. (D) Three putative phosphosites within AR TAU1. (E) Wild-type TAU1 and TAU1 mutants from AR S256A, T280A, S308A were bacterially expressed and purified. Cyclin D3/HA-CDK11p58 was immunoprecipitated from cyclin D3- and HA-CDK11p58-cotransfected COS-1 cells. In vitro kinase assays were performed as described above. (F) AR S256A, T280A, and S308A were transfected into COS-1 cells with vector control (vec.) or cyclin D3/CDK11p58. In vivo AR phosphorylation was assayed as described above. (G) 5×GAL4/UAS-TATA-LUC- and GAL4-fused TAU1 point mutants were transfected with vector control or cyclin D3/CDK11p58. Luciferase activity was assayed. (H) MMTV-LUC was transfected with AR S256A, T280A, or S308A together with vector control (VEC) or cyclin D3/CDK11p58. MMTV-LUC activity was assayed as described above.
Ser-256, Thr-280, and Ser-308 were found to be potential proline-directed Ser/Thr phosphosites in TAU1 (Fig. 7D). TAU1 was mutated at these three sites from Ser/Thr to Ala individually and tested for phosphorylation by cyclin D3/CDK11p58 in vitro. Cyclin D3/CDK11p58 was capable of phosphorylating TAU1/S256A and TAU1/T280A but failed to phosphorylate TAU1/S308A (Fig. 7E). Wild-type AR, S256A, T280A, and S308A were then transfected into COS-1 cells and assayed for in vivo phosphorylation. The overexpression of cyclin D3/CDK11p58 increased the in vivo phosphorylation levels of wild-type AR, S256A, and T280A but had no influence on the phosphorylation of S308A (Fig. 7F). Reporter assays showed that cyclin D3/CDK11p58 could not repress the transcription mediated by the TAU1/S308A mutant (Fig. 7G). Compared with the other two AR mutants, AR S308A possessed a transcriptional activity that was 30% higher than that of wild-type AR and was not altered by the overexpression of cyclin D3/CDK11p58 (Fig. 7H). All these data suggest that the cyclin D3/CDK11p58 complex represses AR function through phosphorylating Ser-308 and repressing the activity of TAU1.
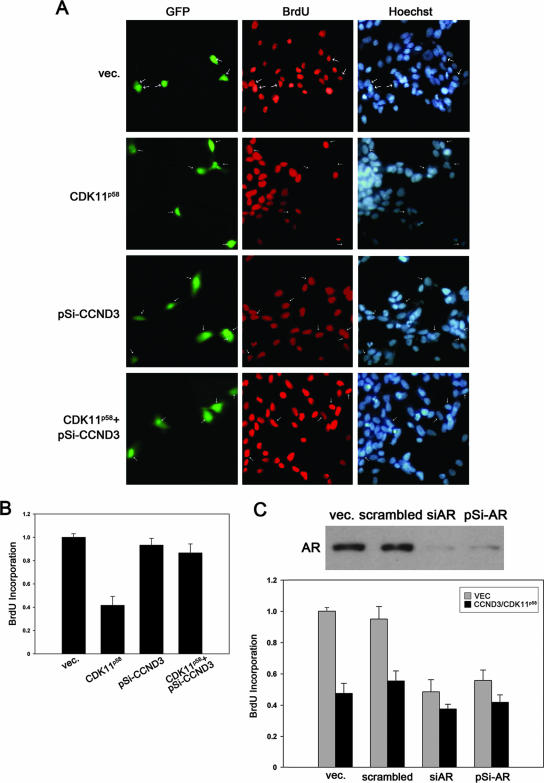
Cyclin D3/CDK11p58 inhibits androgen-dependent proliferation of PCa cells.
AR activity is required for the proliferation of prostate cells and androgen-sensitive PCa cells. We herein investigated the influence of cyclin D3/CDK11p58 on the growth of AR-dependent PCa cells. LNCaP cells were transfected with CDK11p58 expression plasmids alone or in combination with pSi-CCND3 and tested for cell proliferation through immunofluorescence for incorporated BrdU. Expression plasmids encoding GFP were also cotransfected in each sample. Cells transfected with CDK11p58 displayed decreased BrdU incorporation compared with that of vector-transfected cells (Fig. 8A). However, when endogenous cyclin D3 was knocked down by pSi-CCND3, the proliferation of PCa cells was not affected even in the presence of ectopic CDK11p58. GFP-positive cells were then scored for percent BrdU incorporation. Cell proliferation was inhibited by 56% when CDK11p58 was overexpressed. In pSi-CCND3- and CDK11p58-cotransfected LNCaP cells, cell proliferation was inhibited by only 13%, suggesting that the kinase activity of cyclin D3/CDK11p58 is involved in the regulation of PCa cell growth (Fig. 8B). To confirm that the inhibited proliferation of PCa cells by cyclin D3/CDK11p58 is due to AR regulation, siAR and pSi-AR expression plasmids were designed to knock down AR expression. Cyclin D3 and CDK11p58 were cotransfected with control vector, scrambled siRNA, siAR, or pSi-AR in LNCaP cells. Cell proliferation was monitored by BrdU incorporation. The overexpression of cyclin D3/CDK11p58 inhibited LNCaP cell proliferation by 52% in control vector-transfected cells or 45% in scrambled siRNA-transfected cells. siAR led to a 48% decrease in LNCaP cell proliferation, and pSi-AR downregulated cell proliferation by 43%. In both groups, we failed to observe significant alterations of cell proliferation by cyclin D3/CDK11p58 (Fig. 8C). These data suggest that the cyclin D3/CDK11p58 complex is a negative regulator of androgen-sensitive PCa cells through AR repression.
FIG. 8.
Androgen-dependent proliferation of PCa cells was inhibited by cyclin D3/CDK11p58. GFP was cotransfected into LNCaP cells with CDK11p58, pSi-CCND3, or both. At 24 h posttransfection, cells were labeled with 10 μM BrdU for another 24 h. BrdU incorporation was then visualized via indirect immunofluorescence (A). Transfected (GFP-positive) cells were scored for percent BrdU incorporation (B). (C) LNCaP cells were transfected with vector control (vec.) or scrambled siRNA, siAR, or pSi-AR together with vector control or cyclin D3/CDK11p58. GFP-positive cells were scored for percent BrdU incorporation. Experiments were performed at least in duplicate. The expression of AR in the presence of siAR or pSi-AR in LNCaP cells was monitored by Western blotting.
DISCUSSION
Androgens and AR are indispensable for the development, regulation, and maintenance of male phenotype and reproductive physiology (9). The AR signaling pathways play critical roles in the development and progression of PCa, a leading cause of cancer death second to lung cancer in men in the United States (15, 22). Binding of AR to androgens results in changes in receptor conformation, dissociation from heat shock proteins, nuclear localization, dimerization, interaction between functional domains, DNA binding, recruitment of coactivators, and initiation of transcription (19). In addition to the direct association with the general transcriptional machinery at the target gene promoters upon binding to the ligands, AR is also regulated by a large variety of corepressors in a sophisticated and complex manner (41). Many protein factors have been identified as being AR corepressors, including cyclin D1, HBO1, HDAC1, Akt, PTEN, etc. (50).
It is well known that cyclin D1 represses AR function through multiple mechanisms (8, 37, 42). On the contrary, CDK6, which binds to and is activated by cyclin D1 and thereby enhances the transition of cells through the G1 phase of the cell cycle, stimulates AR transcriptional activity in a cyclin D1- or CDK-independent manner (29). CDK4, another cyclin D1-dependent kinase, has little effect on AR (29). Thus, the regulatory roles of cyclin D1 and its CDKs are irrelevant to their kinase activities. Cyclin D3 is also reported to repress AR-mediated transactivation with an unknown mechanism (26). Our data demonstrate a mechanism of cyclin D3 that is distinct from that of cyclin D1 in the regulation of AR-dependent transcription. Ectopic expression of cyclin D3 and CDK11p58 led to a significant inhibition of AR-dependent transcription in a wide spectrum of cell lines including COS-1, 293T, PC-3, and LNCaP cells. Cyclin D3 fails to repress AR in the absence of CDK11p58 and vice versa. Thus, it provides the clue that cyclin D3 and CDK11p58 cooperate in the regulation of AR function.
In elucidating the repressive mechanism of cyclin D3/CDK11p58 on AR, we found that neither the AR nuclear translocation, the functional interaction between AR N- and C-terminal domains, nor coregulator binding as measured by the interaction between AR and HDAC1 or p300 or p/CAF was influenced by CDK11p58 overexpression, although other coregulators remain to be examined. Unlike CDK6, CDK11p58 was not detected at PSA promoters (data not shown). The phosphorylation of steroid hormone receptors is thought to be involved in the regulation of steroid hormone action. AR is phosphorylated at multiple sites. Some of these sites are constitutively phosphorylated, and some are transiently phosphorylated upon androgen stimulation (51). Several kinases have been shown to phosphorylate AR in vitro, including Akt, GSK3β, p38, JNK1, ANPK, etc. (17, 30, 33, 45). It was shown in the in vitro kinase assays that cyclin D3/CDK11p58 phosphorylates and represses amino acids 101 to 372 of AR, the TAU1 domain that is important for AR transcriptional activation. Ser/Thr-Pro motifs are found to be phosphorylated in many steroid receptors, implying the potential participation of proline-directed Ser/Thr protein kinases including CDKs (5). Most recently, AR Ser-81 was reported to be phosphorylated by CDK1 (11). Two Ser-Pro motifs and one Thr-Pro motif are also found in AR TAU1. By mutagenesis, we found that AR was phosphorylated at Ser-308 by cyclin D3/CDK11p58 in vitro and in vivo. Ser-308 was the first phosphorylation site identified in baculovirus-overexpressed AR using mass spectrometry and was confirmed later on in mammalian cells (18, 61). It should be noted that AR phosphorylation at different sites possesses different functions. AR phosphorylation at Ser-81 by CDK1 is associated with AR stability, and phosphorylation at Ser-650 by stress kinases is closely related to the nuclear export of AR (11, 17). S308A was reported to be the only mutant that displayed increased transcriptional activity of AR (18), which is consistent with our findings. The enhanced phosphorylations and inhibited transcriptional activities of AR S256A and T280A were still observed in cyclin D3/CDK11p58-overexpressing cells. However, the phosphorylation and activity of S308A remained unchanged by this kinase complex, implicating the key role of this phosphosite in cyclin D3/CDK11p58 function. It is to our great interest that although D224N, the kinase-dead mutant of CDK11p58, does not interact with AR in vitro and in vivo, it sabotages the association between cyclin D3/CDK11p58 and AR and subsequently enhances AR-mediated transactivation. Given the above-described data, our results provide evidence of cross talk between cell cycle and AR signaling. It is possible that some other protein factors that are present in CDK11p58 coimmunoprecipitates might also be involved in the regulation of CDK11p58 kinase as well as cyclin D3. Interestingly, in CDK11p110-transfected COS-1 and PC-3 cells, the transcriptional activity of AR was enhanced by more than fivefold. However, we failed to detect a direct association between CDK11p110 and AR. Thus, CDK11p110 may activate eukaryotic transcription through its association with the general transcription machinery (49). It was suggested previously that the regulation of CDK11p58 kinase activity differs from that of CDK11p110 kinase (28) and may also be involved in the control of spindle bipolarity (38). In combination, these data suggest that although CDK11p58 is structurally located within the C-terminal region of CDK11p110, their functions diverge distinctly due to different crystal conformations. CDK11p58 regulates cell cycle progression and cell apoptosis through mediating the specific repression of certain transcription factors, changes in the cell skeleton, and the formation of centrosome composition. Androgens such as DHT have a biphasic stimulatory effect on LNCaP cell proliferation (16). Enhanced CDK11p58 kinase activity was found in the presence of 10 nM DHT, an inhibitory dose for cell growth, suggesting that the antiproliferative effect of high levels of androgens might be due to the hyperphosphorylation of AR at inhibitory sites such as Ser-308.
Although cyclin D1 and D2 are regarded as proto-oncogenes based on genetic aberrations leading to their overabundance in human and animal malignancies, the role of cyclin D3 in carcinogenesis is still a paradox (52). Deletions and abnormalities of the CDK11 gene have been found in many tumors including childhood neuroblastoma, childhood endodermal sinus tumors, non-Hodgkin's lymphoma, malignant melanoma, etc. (13, 27, 35, 36, 39). Combined with the repressive role of CDK11p58 in the cell cycle, these findings imply that CDK11p58 might be a potential repressor of tumorigenesis. Current therapies for PCa target the AR LBD to block the ligand-dependent activation of AR. However, ligand-independent AR activity is mediated by the TAD and resistant to antiandrogens in androgen-refractory PCa cells (14). Novel therapeutic strategies targeting the AR TAD have been developed to antagonize AR in androgen-refractory PCas (40). It needs to be clarified in the future whether AR hypersensitivity to androgen and androgen-refractory PCa are associated with the alterations of repressive signaling such as cyclin D3/CDK11p58.
Taken together, we demonstrate a new role for the cyclin D3/CDK11p58 complex as a negative regulator of AR via its kinase activity. The cyclin D3/CDK11p58 signaling pathway might participate in the sophisticated regulation of AR-dependent physiological and pathological activities in male reproductive systems.
Acknowledgments
This work was supported by the National Natural Scientific Foundation of China (30470442 and 30330320) and CNHLPP (2004BA711A19).
We thank DZijie Sun and Roland Schüle for the AR expression and reporter plasmids described in Materials and Methods.
Footnotes
Published ahead of print on 13 August 2007.
REFERENCES
- 1.Alen, P., F. Claessens, G. Verhoeven, W. Rombauts, and B. Peeters. 1999. The androgen receptor amino-terminal domain plays a key role in p160 coactivator-stimulated gene transcription. Mol. Cell. Biol. 19:6085-6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartkova, J., J. Lukas, M. Strauss, and J. Bartek. 1998. Cyclin D3: requirement for G1/S transition and high abundance in quiescent tissues suggest a dual role in proliferation and differentiation. Oncogene 17:1027-1037. [DOI] [PubMed] [Google Scholar]
- 3.Belandia, B., S. M. Powell, J. M. Garcia-Pedrero, M. M. Walker, C. L. Bevan, and M. G. Parker. 2005. Hey1, a mediator of Notch signaling, is an androgen receptor corepressor. Mol. Cell. Biol. 25:1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blok, L. J., P. E. de Ruiter, and A. O. Brinkmann. 1996. Androgen receptor phosphorylation. Endocr. Res. 22:197-219. [DOI] [PubMed] [Google Scholar]
- 5.Bodwell, J. E., E. Orti, J. M. Coull, D. J. Pappin, L. I. Smith, and F. Swift. 1991. Identification of phosphorylated sites in the mouse glucocorticoid receptor. J. Biol. Chem. 266:7549-7555. [PubMed] [Google Scholar]
- 6.Bodwell, J. E., J. C. Webster, C. M. Jewell, J. A. Cidlowski, J. M. Hu, and A. Munck. 1998. Glucocorticoid receptor phosphorylation: overview, function and cell cycle-dependence. J. Steroid Biochem. Mol. Biol. 65:91-99. [DOI] [PubMed] [Google Scholar]
- 7.Bunnell, B. A., L. S. Heath, D. E. Adams, J. M. Lahti, and V. J. Kidd. 1990. Increased expression of a 58-kDa protein kinase leads to changes in the CHO cell cycle. Proc. Natl. Acad. Sci. USA 87:7467-7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burd, C. J., C. E. Petre, H. Moghadam, E. M. Wilson, and K. E. Knudsen. 2005. Cyclin D1 binding to the androgen receptor (AR) NH2-terminal domain inhibits activation function 2 association and reveals dual roles for AR corepression. Mol. Endocrinol. 19:607-620. [DOI] [PubMed] [Google Scholar]
- 9.Chang, C., A. Saltzman, S. Yeh, W. Young, E. Keller, H. J. Lee, C. Wang, and A. Mizokami. 1995. Androgen receptor: an overview. Crit. Rev. Eukaryot. Gene Expr. 5:97-125. [DOI] [PubMed] [Google Scholar]
- 10.Chang, C. S., J. Kokontis, and S. T. Liao. 1988. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science 240:324-326. [DOI] [PubMed] [Google Scholar]
- 11.Chen, S., Y. Xu, X. Yuan, G. J. Bubley, and S. P. Balk. 2006. Androgen receptor phosphorylation and stabilization in prostate cancer by cyclin-dependent kinase 1. Proc. Natl. Acad. Sci. USA 103:15969-15974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornelis, S., Y. Bruynooghe, G. Denecker, S. Van Huffel, S. Tinton, and R. Beyaert. 2000. Identification and characterization of a novel cell cycle-regulated internal ribosome entry site. Mol. Cell 5:597-605. [DOI] [PubMed] [Google Scholar]
- 13.Dave, B. J., D. L. Pickering, M. M. Hess, D. D. Weisenburger, J. O. Armitage, and W. G. Sanger. 1999. Deletion of cell division cycle 2-like 1 gene locus on 1p36 in non-Hodgkin lymphoma. Cancer Genet. Cytogenet. 108:120-126. [DOI] [PubMed] [Google Scholar]
- 14.Dehm, S. M., and D. J. Tindall. 2006. Ligand-independent androgen receptor activity is activation function-2-independent and resistant to antiandrogens in androgen refractory prostate cancer cells. J. Biol. Chem. 281:27882-27893. [DOI] [PubMed] [Google Scholar]
- 15.Dehm, S. M., and D. J. Tindall. 2005. Regulation of androgen receptor signaling in prostate cancer. Expert Rev. Anticancer Ther. 5:63-74. [DOI] [PubMed] [Google Scholar]
- 16.de Launoit, Y., R. Veilleux, M. Dufour, J. Simard, and F. Labrie. 1991. Characteristics of the biphasic action of androgens and of the potent antiproliferative effects of the new pure antiestrogen EM-139 on cell cycle kinetic parameters in LNCaP human prostatic cancer cells. Cancer Res. 51:5165-5170. [PubMed] [Google Scholar]
- 17.Gioeli, D., B. E. Black, V. Gordon, A. Spencer, C. T. Kesler, S. T. Eblen, B. M. Paschal, and M. J. Weber. 2006. Stress kinase signaling regulates androgen receptor phosphorylation, transcription, and localization. Mol. Endocrinol. 20:503-515. [DOI] [PubMed] [Google Scholar]
- 18.Gioeli, D., S. B. Ficarro, J. J. Kwiek, D. Aaronson, M. Hancock, A. D. Catling, F. M. White, R. E. Christian, R. E. Settlage, J. Shabanowitz, D. F. Hunt, and M. J. Weber. 2002. Androgen receptor phosphorylation. Regulation and identification of the phosphorylation sites. J. Biol. Chem. 277:29304-29314. [DOI] [PubMed] [Google Scholar]
- 19.Heinlein, C. A., and C. Chang. 2004. Androgen receptor in prostate cancer. Endocr. Rev. 25:276-308. [DOI] [PubMed] [Google Scholar]
- 20.Horoszewicz, J. S., S. S. Leong, E. Kawinski, J. P. Karr, H. Rosenthal, T. M. Chu, E. A. Mirand, and G. P. Murphy. 1983. LNCaP model of human prostatic carcinoma. Cancer Res. 43:1809-1818. [PubMed] [Google Scholar]
- 21.Hu, D., A. Mayeda, J. H. Trembley, J. M. Lahti, and V. J. Kidd. 2003. CDK11 complexes promote pre-mRNA splicing. J. Biol. Chem. 278:8623-8629. [DOI] [PubMed] [Google Scholar]
- 22.Jemal, A., R. Siegel, E. Ward, T. Murray, J. Xu, C. Smigal, and M. J. Thun. 2006. Cancer statistics, 2006. CA Cancer J. Clin. 56:106-130. [DOI] [PubMed] [Google Scholar]
- 23.Jenster, G., H. A. van der Korput, C. van Vroonhoven, T. H. van der Kwast, J. Trapman, and A. O. Brinkmann. 1991. Domains of the human androgen receptor involved in steroid binding, transcriptional activation, and subcellular localization. Mol. Endocrinol. 5:1396-1404. [DOI] [PubMed] [Google Scholar]
- 24.Knotts, T. A., R. S. Orkiszewski, R. G. Cook, D. P. Edwards, and N. L. Weigel. 2001. Identification of a phosphorylation site in the hinge region of the human progesterone receptor and additional amino-terminal phosphorylation sites. J. Biol. Chem. 276:8475-8483. [DOI] [PubMed] [Google Scholar]
- 25.Knudsen, K. E., K. C. Arden, and W. K. Cavenee. 1998. Multiple G1 regulatory elements control the androgen-dependent proliferation of prostatic carcinoma cells. J. Biol. Chem. 273:20213-20222. [DOI] [PubMed] [Google Scholar]
- 26.Knudsen, K. E., W. K. Cavenee, and K. C. Arden. 1999. D-type cyclins complex with the androgen receptor and inhibit its transcriptional transactivation ability. Cancer Res. 59:2297-2301. [PubMed] [Google Scholar]
- 27.Lahti, J. M., M. Valentine, J. Xiang, B. Jones, J. Amann, J. Grenet, G. Richmond, A. T. Look, and V. J. Kidd. 1994. Alterations in the PITSLRE protein kinase gene complex on chromosome 1p36 in childhood neuroblastoma. Nat. Genet. 7:370-375. [DOI] [PubMed] [Google Scholar]
- 28.Lahti, J. M., J. Xiang, L. S. Heath, D. Campana, and V. J. Kidd. 1995. PITSLRE protein kinase activity is associated with apoptosis. Mol. Cell. Biol. 15:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim, J. T., M. Mansukhani, and I. B. Weinstein. 2005. Cyclin-dependent kinase 6 associates with the androgen receptor and enhances its transcriptional activity in prostate cancer cells. Proc. Natl. Acad. Sci. USA 102:5156-5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin, H. K., S. Yeh, H. Y. Kang, and C. Chang. 2001. Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor. Proc. Natl. Acad. Sci. USA 98:7200-7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacLean, H. E., G. L. Warne, and J. D. Zajac. 1997. Localization of functional domains in the androgen receptor. J. Steroid Biochem. Mol. Biol. 62:233-242. [DOI] [PubMed] [Google Scholar]
- 32.Metzger, E., M. Wissmann, N. Yin, J. M. Muller, R. Schneider, A. H. Peters, T. Gunther, R. Buettner, and R. Schule. 2005. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature 437:436-439. [DOI] [PubMed] [Google Scholar]
- 33.Moilanen, A. M., U. Karvonen, H. Poukka, O. A. Janne, and J. J. Palvimo. 1998. Activation of androgen receptor function by a novel nuclear protein kinase. Mol. Biol. Cell 9:2527-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narayanan, R., A. A. Adigun, D. P. Edwards, and N. L. Weigel. 2005. Cyclin-dependent kinase activity is required for progesterone receptor function: novel role for cyclin A/Cdk2 as a progesterone receptor coactivator. Mol. Cell. Biol. 25:264-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson, M. A., M. E. Ariza, J. M. Yang, F. H. Thompson, R. Taetle, J. M. Trent, J. Wymer, K. Massey-Brown, M. Broome-Powell, J. Easton, J. M. Lahti, and V. J. Kidd. 1999. Abnormalities in the p34cdc2-related PITSLRE protein kinase gene complex (CDC2L) on chromosome band 1p36 in melanoma. Cancer Genet. Cytogenet. 108:91-99. [DOI] [PubMed] [Google Scholar]
- 36.Perlman, E. J., M. B. Valentine, C. A. Griffin, and A. T. Look. 1996. Deletion of 1p36 in childhood endodermal sinus tumors by two-color fluorescence in situ hybridization: a pediatric oncology group study. Genes Chromosomes Cancer 16:15-20. [DOI] [PubMed] [Google Scholar]
- 37.Petre, C. E., Y. B. Wetherill, M. Danielsen, and K. E. Knudsen. 2002. Cyclin D1: mechanism and consequence of androgen receptor co-repressor activity. J. Biol. Chem. 277:2207-2215. [DOI] [PubMed] [Google Scholar]
- 38.Petretti, C., M. Savoian, E. Montembault, D. M. Glover, C. Prigent, and R. Giet. 2006. The PITSLRE/CDK11p58 protein kinase promotes centrosome maturation and bipolar spindle formation. EMBO Rep. 7:418-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poetsch, M., T. Dittberner, and C. Woenckhaus. 2001. Does the PITSLRE gene complex contribute to the pathogenesis of malignant melanoma of the skin? A study of patient-derived tumor samples. Cancer Genet. Cytogenet. 128:181-182. [DOI] [PubMed] [Google Scholar]
- 40.Quayle, S. N., and M. D. Sadar. 2007. 14-3-3 sigma increases the transcriptional activity of the androgen receptor in the absence of androgens. Cancer Lett. 254:137-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reid, J., R. Betney, K. Watt, and I. J. McEwan. 2003. The androgen receptor transactivation domain: the interplay between protein conformation and protein-protein interactions. Biochem. Soc. Trans. 31:1042-1046. [DOI] [PubMed] [Google Scholar]
- 42.Reutens, A. T., M. Fu, C. Wang, C. Albanese, M. J. McPhaul, Z. Sun, S. P. Balk, O. A. Janne, J. J. Palvimo, and R. G. Pestell. 2001. Cyclin D1 binds the androgen receptor and regulates hormone-dependent signaling in a p300/CBP-associated factor (P/CAF)-dependent manner. Mol. Endocrinol. 15:797-811. [DOI] [PubMed] [Google Scholar]
- 43.Rogatsky, I., J. M. Trowbridge, and M. J. Garabedian. 1999. Potentiation of human estrogen receptor alpha transcriptional activation through phosphorylation of serines 104 and 106 by the cyclin A-CDK2 complex. J. Biol. Chem. 274:22296-22302. [DOI] [PubMed] [Google Scholar]
- 44.Sachs, A. B. 2000. Cell cycle-dependent translation initiation: IRES elements prevail. Cell 101:243-245. [DOI] [PubMed] [Google Scholar]
- 45.Salas, T. R., J. Kim, F. Vakar-Lopez, A. L. Sabichi, P. Troncoso, G. Jenster, A. Kikuchi, S. Y. Chen, L. Shemshedini, M. Suraokar, C. J. Logothetis, J. DiGiovanni, S. M. Lippman, and D. G. Menter. 2004. Glycogen synthase kinase-3 beta is involved in the phosphorylation and suppression of androgen receptor activity. J. Biol. Chem. 279:19191-19200. [DOI] [PubMed] [Google Scholar]
- 46.Sharma, M., M. Zarnegar, X. Li, B. Lim, and Z. Sun. 2000. Androgen receptor interacts with a novel MYST protein, HBO1. J. Biol. Chem. 275:35200-35208. [DOI] [PubMed] [Google Scholar]
- 47.Shen, X., Y. Yang, W. Liu, M. Sun, J. Jiang, H. Zong, and J. Gu. 2004. Identification of the p28 subunit of eukaryotic initiation factor 3(eIF3k) as a new interaction partner of cyclin D3. FEBS Lett. 573:139-146. [DOI] [PubMed] [Google Scholar]
- 48.Slagsvold, T., I. Kraus, T. Bentzen, J. Palvimo, and F. Saatcioglu. 2000. Mutational analysis of the androgen receptor AF-2 (activation function 2) core domain reveals functional and mechanistic differences of conserved residues compared with other nuclear receptors. Mol. Endocrinol. 14:1603-1617. [DOI] [PubMed] [Google Scholar]
- 49.Trembley, J. H., D. Hu, L. C. Hsu, C. Y. Yeung, C. Slaughter, J. M. Lahti, and V. J. Kidd. 2002. PITSLRE p110 protein kinases associate with transcription complexes and affect their activity. J. Biol. Chem. 277:2589-2596. [DOI] [PubMed] [Google Scholar]
- 50.Wang, L., C. L. Hsu, and C. Chang. 2005. Androgen receptor corepressors: an overview. Prostate 63:117-130. [DOI] [PubMed] [Google Scholar]
- 51.Weigel, N. L. 1996. Steroid hormone receptors and their regulation by phosphorylation. Biochem. J. 319:657-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu, W., B. M. Slomovitz, P. T. Soliman, K. M. Schmeler, J. Celestino, M. R. Milam, and K. H. Lu. 2006. Correlation of cyclin D1 and cyclin D3 overexpression with the loss of PTEN expression in endometrial carcinoma. Int. J. Gynecol. Cancer 16:1668-1672. [DOI] [PubMed] [Google Scholar]
- 53.Xiang, J., J. M. Lahti, J. Grenet, J. Easton, and V. J. Kidd. 1994. Molecular cloning and expression of alternatively spliced PITSLRE protein kinase isoforms. J. Biol. Chem. 269:15786-15794. [PubMed] [Google Scholar]
- 54.Yamamoto, A., Y. Hashimoto, K. Kohri, E. Ogata, S. Kato, K. Ikeda, and M. Nakanishi. 2000. Cyclin E as a coactivator of the androgen receptor. J. Cell Biol. 150:873-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang, Q., K. M. Fung, W. V. Day, B. P. Kropp, and H. K. Lin. 2005. Androgen receptor signaling is required for androgen-sensitive human prostate cancer cell proliferation and survival. Cancer Cell Int. 5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ye, J. Z., and T. de Lange. 2004. TIN2 is a tankyrase 1 PARP modulator in the TRF1 telomere length control complex. Nat. Genet. 36:618-623. [DOI] [PubMed] [Google Scholar]
- 57.Yeh, S., H. K. Lin, H. Y. Kang, T. H. Thin, M. F. Lin, and C. Chang. 1999. From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc. Natl. Acad. Sci. USA 96:5458-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang, S., M. Cai, S. Zhang, S. Xu, S. Chen, X. Chen, C. Chen, and J. Gu. 2002. Interaction of p58(PITSLRE), a G2/M-specific protein kinase, with cyclin D3. J. Biol. Chem. 277:35314-35322. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, Y., C. A. Beck, A. Poletti, J. P. Clement IV, P. Prendergast, T.-T. Yip, T. W. Hutchens, D. P. Edwards, and N. L. Weigel. 1997. Phosphorylation of human progesterone receptor by cyclin-dependent kinase 2 on three sites that are authentic basal phosphorylation sites in vivo. Mol. Endocrinol. 11:823-832. [DOI] [PubMed] [Google Scholar]
- 60.Zhou, Z. X., J. A. Kemppainen, and E. M. Wilson. 1995. Identification of three proline-directed phosphorylation sites in the human androgen receptor. Mol. Endocrinol. 9:605-615. [DOI] [PubMed] [Google Scholar]
- 61.Zhu, Z., R. R. Becklin, D. M. Desiderio, and J. T. Dalton. 2001. Identification of a novel phosphorylation site in human androgen receptor by mass spectrometry. Biochem. Biophys. Res. Commun. 284:836-844. [DOI] [PubMed] [Google Scholar]
- 62.Zong, H., Z. Li, L. Liu, Y. Hong, X. Yun, J. Jiang, Y. Chi, H. Wang, X. Shen, Y. Hu, Z. Niu, and J. Gu. 2005. Cyclin-dependent kinase 11(p58) interacts with HBO1 and enhances its histone acetyltransferase activity. FEBS Lett. 579:3579-3588. [DOI] [PubMed] [Google Scholar]