Abstract
The expression of retinoblastoma (pRb) and cyclin D3 proteins is highly induced during the process of skeletal myoblast differentiation. We have previously shown that cyclin D3 is nearly totally associated with hypophosphorylated pRb in differentiated myotubes, whereas Rb−/− myocytes fail to accumulate the cyclin D3 protein despite normal induction of cyclin D3 mRNA. Here we report that pRb promotes cyclin D3 protein accumulation in differentiating myoblasts by preventing cyclin D3 degradation. We show that cyclin D3 displays rapid turnover in proliferating myoblasts, which is positively regulated through glycogen synthase kinase 3β (GSK-3β)-mediated phosphorylation of cyclin D3 on Thr-283. We describe a novel interaction between pRb and cyclin D3 that maps to the C terminus of pRb and to a region of cyclin D3 proximal to the Thr-283 residue and provide evidence that the pRb-cyclin D3 complex formation in terminally differentiated myotubes hinders the access of GSK-3β to cyclin D3, thus inhibiting Thr-283 phosphorylation. Interestingly, we observed that the ectopic expression of a stabilized cyclin D3 mutant in C2 myoblasts enhances muscle-specific gene expression; conversely, cyclin D3-null embryonic fibroblasts display impaired MyoD-induced myogenic differentiation. These results indicate that the pRb-dependent accumulation of cyclin D3 is functionally relevant to the process of skeletal muscle cell differentiation.
The differentiation of skeletal muscle cells is characterized by a coordinated sequence of events that include irreversible exit from the cell cycle, the timely, ordered activation of muscle-specific gene expression, and the fusion of postmitotic myoblasts into multinucleated myotubes. This process is regulated by the basic helix-loop-helix family of myogenic regulatory factors (MRFs), which includes MyoD, Myf5, myogenin, and MRF4. The MRFs act in cooperation with members of the myocyte enhancer factor 2 family of transcription factors to synergistically activate muscle-specific gene expression (4, 44).
The transcriptional activity of MRFs is tightly coupled to cell cycle control. It has been well established that mitogenic signaling antagonizes by multiple mechanisms the activity of MRFs, which results in the absence of differentiation-specific gene expression in proliferating myoblasts (51). Conversely, the irreversible withdrawal of myoblasts from the cell cycle promotes their commitment into differentiation. This key step involves the inhibition of cyclin-dependent kinase (cdk) activity, and this is due to the rapid downregulation of most cyclins, as well as to the induction of the cdk inhibitors p21, p27, and p57. In addition, transcription of the retinoblastoma growth suppressor gene (Rb) is highly induced at the onset of differentiation, and the pRb protein, one of the major targets of cdks, accumulates in the hypophosphorylated form, which inhibits cell cycle progression (28, 31, 68).
During the early process of skeletal muscle differentiation, the myogenic factor MyoD not only initiates muscle gene transcription but also cooperates in promoting cell cycle arrest. MyoD-mediated growth arrest is linked to its ability to induce the transcription of critical cell-cycle regulators, such as Rb, p21, and cyclin D3 (6, 20, 27, 30, 37, 54). The induction of Rb gene transcription by MyoD is consistent with the well-described function of pRb as an inhibitor of E2F activity and cell cycle progression (21); the parallel induction of p21 by MyoD contributes to maintaining pRb in the hypophosphorylated (active) state (32). In addition to its role in the establishment of the postmitotic state, pRb mediates progression to terminal differentiation by activating the transcriptional function of myocyte enhancer family 2 in cooperation with MyoD (5, 22, 45, 46). This differentiation-promoting function of pRb is genetically separable from its cell cycle-controlling function and appears to be linked to the pRb's ability to negatively regulate the activity of Ras (29, 62).
The MyoD-mediated induction of cyclin D3 seems contradictory in differentiating myoblasts progressively arresting in G1. Notably, however, cyclin D3 is expressed at high levels not only in differentiated myoblast cell lines but also in skeletal muscle in vivo during the late stages of mouse fetal development and early postnatal life when the muscle cells are already quiescent and cyclin D1 down-regulated (3). In addition to skeletal muscle, cyclin D3 accumulates to high levels in quiescent, differentiated cell types in a wide range of tissues, including liver and adipose tissue. Two recent reports have shed light on the biological function of cyclin D3 in these differentiated cells, showing that cyclin D3 promotes adipogenesis through the activation of PPARγ and supports the growth arrest of quiescent livers and differentiated adipocytes by positively regulating the growth-inhibitory activity of C/EBPα (57, 69).
We have previously shown that cyclin D3 forms catalytically inactive complexes with cdk4, p21, and PCNA in terminally differentiated myotubes. We showed also that in these cells, the bulk of cyclin D3 is pRb-associated, which suggested that cyclin D3 might function as a bridging molecule, allowing pRb to sequester inactive cdk4 complexes, as well as PCNA, into insoluble nuclear structures (6). There is extensive evidence that hypophosphorylated pRb is tethered to the nuclear matrix by interacting with components of the lamina and that the nuclear anchorage of pRb is crucial for its functions (24, 33, 35, 39, 47). Muscle differentiation is associated with both increased nuclear affinity of pRb and a rearrangement in the intranuclear organization of lamin A/C (41, 63), and it is increasingly evident that lamin A/C plays an essential role in myoblast differentiation, as alteration of its activity severely compromises muscle-specific transcription (17, 19, 36). Interestingly, a recent report has shown that cyclin D3 can specifically induce lamin A/C reorganization in myoblasts, but not in other cell types, by a mechanism that requires functional pRb (34).
In previous work, we provided evidence revealing an additional functional link between pRb and cyclin D3 as we observed that the cyclin D3 protein fails to accumulate in Rb-deficient myocytes due to its rapid destruction through the 26S proteasome (6). These earlier observations suggested the hypothesis that pRb promotes cyclin D3 stabilization in differentiating myoblasts. We conducted the present study to (i) determine the pathway that governs the turnover of cyclin D3 in myogenic cells and (ii) elucidate the mechanism by which pRb may prevent cyclin D3 degradation during terminal differentiation. We show that the degradation of cyclin D3 in myogenic cells is regulated through glycogen synthase kinase 3β (GSK-3β)-dependent phosphorylation of cyclin D3 on Thr-283. pRb does not appear to influence the activity of GSK-3β. Instead, we provide evidence that the pRb's interaction with cyclin D3 in terminally differentiated myotubes inhibits the access of GSK-3β to cyclin D3, thus preventing the phosphorylation of Thr-283. We also show that the pRb-dependent cyclin D3 protein stabilization has a functional role during the process of muscle cell differentiation, as we find that the exogenous expression of a stabilized cyclin D3 can enhance muscle-specific gene expression, while cyclin D3 deficiency impairs MyoD-regulated myogenic events.
MATERIALS AND METHODS
Construction of cyclin D3 mutants and expression vectors.
Cyclin D3 mutants were obtained by PCR-mediated mutagenesis using as template murine cyclin D3 cDNA (CYL3) cloned into BlueScript vector (38) and the following oligonucleotide primers flanked by 5′ and 3′ EcoRI sites. Underlining and bold have the meanings described for wild-type cyclin D3 upstream or downstream primers unless otherwise specified.
Wild-type cyclin D3: 5′-CCGGAATTCATGGAGCTGCTGTGTTGCG-3′ (“upstream” oligonucleotide primer representing cyclin D3 sense-strand sequences flanked by a 5′ EcoRI site [underlined] and the adjacent D3 initiation codon [also underlined and bold]) and 5′GGCGAATTCCTACAGGTGAATGGCTGTG-3′ (“downstream” antisense-strand oligonucleotide primer complementary to 3′ cyclin D3 sequences flanked by a 5′ EcoRI site [underlined] and the adjacent stop codon [also underlined and bold]).
Cyclin D3-mLXCXE mutant: 5′-CCGGAATTCATGGAGCTGCTGgGTcaCGAGGGCACC-3′ (upstream sense primer flanked by a 5′ EcoRI site [underlined] and the adjacent initiation codon [also underlined and bold] and containing point mutations [lowercase] in codons 5 and 6 [underlined] that convert the LLCCE motif of cyclin D3 to LLGHE) and 5′-GGCGAATTCCTACAGGTGAATGGCTGTG-3′ (downstream antisense primer).
Cyclin D3(T283A) mutant: 5′-CCGGAATTCATGGAGCTGCTGTGTTGCG-3′ (upstream sense primer) and 5′-GGCGAATTCCTACAGGTGAATGGCTGTGACATCTGTGGGAGcGCTGGTCTG-3′ (downstream antisense primer complementary to 3′ cyclin D3 sequences flanked by a 5′ EcoRI site [underlined] and the adjacent stop codon [also underlined and bold] and containing a mutation in codon 283 [lowercase and underlined] that converts Thr-283 to alanine).
Cyclin D3(1-272) truncation mutant: 5′-CCGGAATTCATGGAGCTGCTGTGTTGCG-3′ (upstream sense primer) and 5′-GGCGAATTCCTAGCCCCGGGGGGCTTTGGG-3′ (downstream antisense primer, complementary to cyclin D3 sequences encoding amino acid residues 267 to 272, flanked by a 5′ EcoRI site [underlined] and the adjacent stop codon [also underlined and bold]).
Cyclin D3(1-249) truncation mutant: 5′-CCGGAATTCATGGAGCTGCTGTGTTGCG-3′ (upstream sense primer) and 5′-GGCGAATTCCTAAGCTTCGATCTGTTCCTGG-3′ (downstream antisense primer, complementary to cyclin D3 sequences encoding amino acid residues 244 to 249, flanked by a 5′ EcoRI site [underlined] and the adjacent stop codon [also underlined and bold]). The resulting PCR products were cloned into the EcoRI site of the pBlueScriptKS(−) cloning vector [pBSKS(−)].
The cyclin D3-dl(250-272) deletion mutant was constructed using a QuikChange XL site-directed mutagenesis kit (Stratagene). The template was murine cyclin D3 cDNA cloned into pBSKS(−), and the oligonucleotide primers were 5′-GCCAGGAACAGATCGAAGCTTCTAGCAGCCAGGGGCCC-3′ and its complementary sequence (spanning nucleotides 728 to 834 and containing a deletion between nucleotides 748 and 816).
The cyclin D3-mLXCXE/dl(250-272) double mutant was generated by ligation of the NcoI-ScaI fragment from pBlueScriptCycD3-mLXCXE and the NcoI-ScaI fragment from pBlueScriptCycD3-dl(250-272).
The cyclin D3(1-225) truncation mutant was generated by exploiting the SacI restriction site located at position 684 in the cyclin D3 coding sequence. The plasmid pBSKS(−) cyclin D3 was digested with SacI, and the fragment encoding cyclin D3 amino acid residues 1 to 225 was ligated to a SacI-Stop-EcoRI oligonucleotide adapter (5′-CTAGAATTCTAGAGCT-3′). The ligation product was digested with EcoRI and then cloned into the EcoRI site of the pBSKS(−) vector.
All constructs were checked by sequencing.
Mutated and wild-type cyclin D3 cDNAs were subcloned into the mammalian expression vector pFlex-1, obtained from the construct pFlex-cyclin D1 (12), provided by C. J. Sherr (St. Jude Children's Research Hospital, Memphis, TN), by EcoRI excision of the cyclin D1 insert and recircularization of the vector by ligation. The polylinker cloning site 3′ from the pFlex-1 promoter is flanked by a 5′ initiator codon followed by sequences encoding the Flag epitope (Met-Asp-Tyr-Lys-Asp4-Lys). Full-length cyclin D3 cDNAs were excised from pBSKS(−) with EcoRI and inserted into the EcoRI cloning site of pFlex-1, thereby creating the Flag epitope fusion to the N-terminal end of cyclin D3. To obtain retroviral expression constructs, the Flag-cyclin D3 coding sequences were excised from pFlex-1 with BamHI and subcloned into the BamHI site of the retroviral vector pBABEpuro (40), provided by B. Amati (IFOM-IEO, Milano, Italy). The retroviral expression construct pBABEpuro-MyoD was generated by subcloning the EcoRI insert from pEMSV-MyoD (70) into the EcoRI site of pBABEpuro.
The expression construct pCMV-Rb, containing the human Rb cDNA subcloned into pCMV-Neo-Bam (52), and constructs expressing the glutathione S-transferase (GST)-RB(379-928), GST-RB(379-792), and GST-RB(792-928) fusion proteins (25) were provided by D. H. Livingston and W. Kaelin (Dana Farber Cancer Institute, Boston). The construct expressing the GST-RB(792-882) fusion protein was constructed by PCR amplification, using the plasmid pCMV-Rb as template, with primers 5′-GGGCGAATTCGGATCCTTTCCTAGTTCACCCTTACGG-3′ (sense primer flanked by a 5′ EcoRI site and 5′ BamHI site [underlined]) and 5′-GCCCGAATTCCTCGAGTCATGATCCTTCAATATCAAAGCG-3′ (antisense primer containing a 5′ EcoRI site, a 5′ XhoI site [underlined], and the adjacent stop codon [also underlined and bold]). The resulting PCR fragment was cloned into the EcoRI cloning site of pGEX-4T-1 in frame with the GST coding sequence. The GST-cyclin D3 construct was constructed by PCR amplification, using as the template murine cyclin D3 cDNA cloned in BlueScript, and the following oligonucleotide primers: 5′-CCGGAATTCATGGAGCTGCTGTGTTGCG-3′ (sense primer flanked by a 5′ EcoRI site [underlined]) and 5′-GGCGAATTCCTACAGGTGAATGGCTGTG-3′ (antisense primer containing a 5′ EcoRI site [underlined]). The resulting PCR fragment was cloned into the EcoRI cloning site of pGEX-4T-1 in frame with the GST coding sequence.
The construct p6HAUbi, encoding ubiquitin fused to six hemagglutinins (HA) (HA-tag) driven by the cytomegalovirus (CMV) promoter (43) was provided by M. Doria (Bambino Gesù Children's Hospital, Roma, Italy).
Cell lines, culture conditions, and transfections.
The C2 line of mouse myoblasts (clone C2/7) was originally obtained by M. Buckingam (Institut Pasteur, Paris, France). CC42 Rb−/− myogenic cells (58) and 3T3 Rb−/− fibroblasts (71) were provided by J. DeCaprio (Dana Farber Cancer Institute, Boston, MA). Primary embryonic fibroblasts isolated from wild-type and cyclin D3−/− mouse embryos were provided by P. Sicinski (Dana Farber Cancer Institute, Boston, MA).
Early passage mouse embryonic fibroblasts (MEFs) were cultured in Dulbecco's modified Eagle's medium (DMEM) plus 10% fetal bovine serum (FBS) by plating at a density of 3 × 105 cells per 6-cm dish and replating at the same density every 3 days (64). C2 and CC42 myoblasts and their derivatives and 3T3 Rb−/− fibroblasts stably expressing MyoD were cultured in DMEM supplemented with 20% FBS. Myogenic differentiation was induced by exposing the cell cultures to DMEM containing 2% horse serum. Insect Sf9 cells were maintained at 27°C in Grace's medium supplemented with 10% FBS. NIH 3T3 fibroblasts, HEK 293 cells, and the Phoenix ecotropic packaging cell line were cultured in DMEM supplemented with 10% FBS.
DNA transfections were carried out with Lipofectamine (Invitrogen) according to the manufacturer's instructions.
Viral infections.
High-titer retrovirus stocks were produced by transient transfection of the Phoenix ecotropic packaging cell line by using calcium phosphate, according to a procedure described previously (49). For transient viral infections, cells were incubated for 5 h with 1:1 to 1:3 mixtures of retrovirus-containing medium from Phoenix cells and culture medium supplemented with Polybrene to a final concentration of 8 μg/ml. The medium was then replaced with fresh growth medium, and cells were harvested 48 h later. For the generation of C2 and CC42 myoblasts stably expressing cyclin D3 mutants or Rb−/− fibroblasts stably expressing MyoD, cells were infected as described above, trypsinized, seeded at low culture densities, and selected for 3 days with 2 μg/ml of puromycin (Sigma). The resulting puromycin-resistant cell populations were then replated for the corresponding assays or frozen for successive experiments.
Adenoviruses expressing a nonphosphorylatable, constitutively active form of pRb (HAΔRb) (Adeno-Rb) or β-galactosidase (Adeno-β-gal) (7) were provided by M. Crescenzi (Istituto Superiore Sanità, Roma, Italy). The adenovirus expressing MyoD (Adeno-MyoD) (42) was provided by G. Falcone (CNR-IBC, Roma, Italy). Viral stocks were amplified and titrated in the permissive HEK 293 cell line using established techniques (48). CC42 myoblasts were infected with Adeno-Rb or Adeno-β-gal at a multiplicity of infection (MOI) of 500 for 90 min. Afterwards, the adenoviral supernatant was replaced with growth medium (GM) or differentiation medium (DM), and the cells were harvested 48 h postinfection. The conversion of wild-type and cyclin D3−/− MEFs into muscle cells was obtained by infecting them with Adeno-MyoD at an MOI of 500 overnight. Twenty-four hours later, the cells were shifted to DM for 48 h.
Baculoviruses expressing cyclin D3 and GSK-3β (11, 26) were provided by C. J. Sherr (St. Jude Children's Research Hospital, Memphis, TN), while the baculovirus expressing Flag-tagged human Rb (50) was provided by V. Sartorelli (NIH, Bethesda, MD). Baculovirus amplification and plaque assays were performed in Sf9 insect cells following standard procedures. For the preparation of insect lysates, 106 Sf9 cells seeded onto a 60-mm-diameter dish were infected with the indicated baculoviruses at an MOI of 10. Infected cells were harvested 40 h postinfection.
Immunoprecipitation, immunoblotting, and kinase assays.
For the preparation of whole-cell extracts, cells were lysed in “NP-40 buffer” containing 50 mM Tris-HCl (pH 7.6), 250 mM NaCl, 5 mM ATP (pH 7.6), 5 mM MgCl2, 1 mM dithiothreitol (DTT) and 1% NP-40 supplemented with 5 mM β-glycerophosphate, 10 mM NaF, 0.1 mM Na-orthovanadate, 1 mM phenylmethylsulfonyl fluoride (PMSF), 5 μg/ml aprotinin, 5 μg/ml leupeptin, 5 μg/ml pepstatin. For the experiments shown in Fig. 9, 10, and 11, cells were lysed in “urea buffer” containing 8 M urea, 100 mM NaH2PO4, 10 mM Tris HCl (pH 8). The lysates were cleared of insoluble materials by centrifugation, and protein concentrations were determined by the Bradford assay.
FIG. 9.
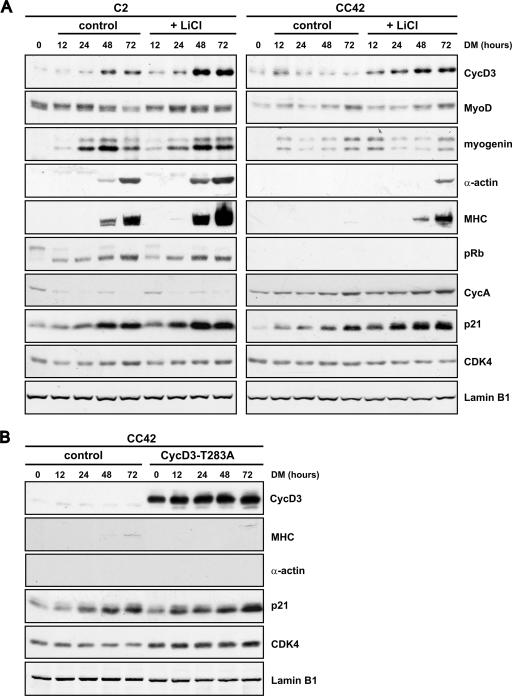
Lithium chloride (LiCl) enhances terminal differentiation of both Rb+/+ and Rb−/− myocytes, but ectopic expression of a stabilized cyclin D3 mutant in Rb−/− myocytes is not sufficient to reproduce the promyogenic effect of LiCl. (A) C2 or CC42 myoblasts, seeded in amounts of 2 × 105 cells into 90-mm dishes, were cultured in proliferation medium for 48 h and then transferred to DM either in the presence or in the absence of LiCl at 10 mM. Cells were harvested at the indicated time points, and equal amounts of protein from whole-cell extracts were analyzed by Western blotting with antibodies to the indicated cell cycle and differentiation markers. (B) CC42 myoblasts were infected with a retrovirus expressing the cyclin D3(T283A) mutant or with the pBABEpuro control virus and selected with puromycin. Cells were seeded as described for panel A and exposed to DM after 48 h. Whole-cell extracts were prepared at the indicated times, and equal amounts of protein were analyzed by Western blotting for expression of the indicated proteins. In the experiments whose results are shown in panels A and B, equal loading was monitored by immunoblotting with anti-lamin B1. Shown are representative results from three independent experiments.
FIG. 10.
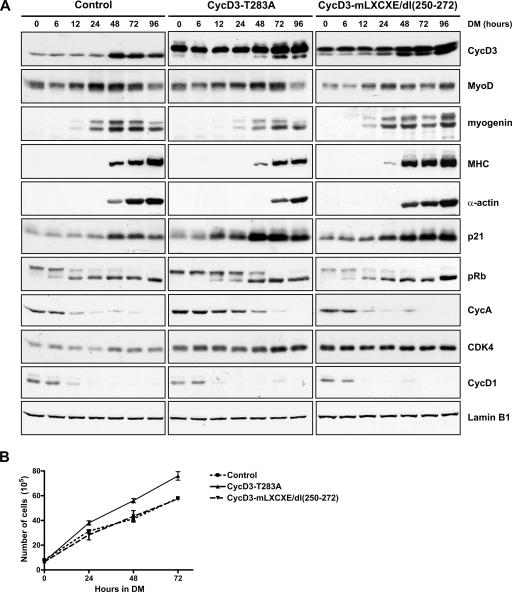
The effect of ectopic expression of stabilized cyclin D3 mutants on differentiation of Rb+/+ myoblasts. (A) C2 myoblasts were infected with pBABEpuro control virus or viruses expressing the cyclin D3(T283A) or the cyclin D3-mLXCXE/dl(250-272) mutant, both Flag-tagged at their N termini. Following selection with puromycin, cells were seeded at 2 × 105 cells into 90-mm dishes in GM and transferred to DM after 48 h, at which time they reached ∼50 to 60% confluence. Whole-cell extracts were prepared at 0, 6, 12, 24, 48, 72, and 96 h after exposure to DM, and equal amounts of protein were subjected to Western blot analysis using antibodies to the indicated proteins. The blots shown are representative results of three independent experiments. (B) Growth curves in DM of C2 cells infected with cyclin D3(T283A), cyclin D3-mLXCXE/dl(250-272), or control retroviruses. Cells were seeded as described for panel A and transferred to DM after 24 h when they reached ∼15% confluence. Counts were made at the time cells were shifted to DM (time, 0) and following 24, 48, and 72 h of incubation in DM. In these culture conditions, cells reach 80 to 100% confluence on day 3, when they start fusing to form multinucleated myotubes. The curves represent the averages of the results of three experiments. Error bars show standard errors of the means.
FIG. 11.
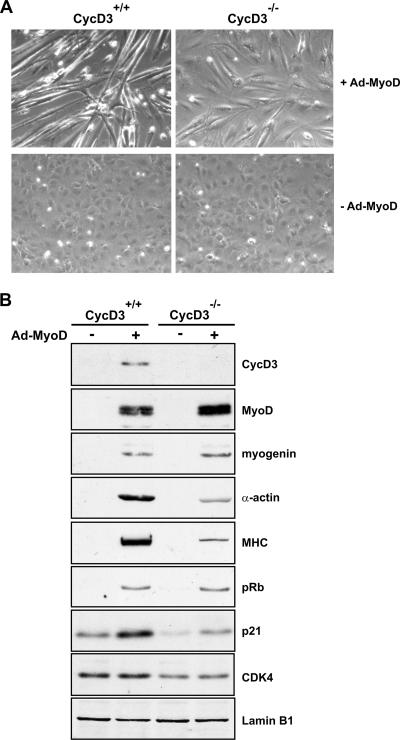
Cyclin D3 deficiency results in defects in MyoD-induced myogenic conversion in MEFs. Wild-type and cyclin D3−/− MEFs were infected with Adeno-MyoD (+Ad-MyoD) at an MOI of 500 or mock infected (−Ad-MyoD). Cells were shifted to DM 24 h postinfection and harvested 48 h later. Phase-contrast microphotographs (A) and Western blot analyses (B) of the indicated proteins in whole-cell lysates normalized for protein amount are shown. Equal loading was verified by immunoblotting with anti-lamin B1. Shown are representative results from four independent experiments. +, present; −, absent.
For immunoprecipitation of ubiquitinated cyclin D3, C2, or CC42, myoblasts expressing wild-type Flag-CycD3 or Flag-CycD3(T283A) were lysed in “NP-40 buffer” containing 5 mM N-ethylmaleimide. The Flag-tagged cyclin D3 derivatives were immunoprecipitated with the M2 monoclonal anti-FLAG antibody covalently linked to agarose beads (M2-agarose; Sigma). To detect interactions between cyclin D3, pRb, and GSK-3β by coimmunoprecipitation, Sf9 insect cells were infected with the indicated baculoviruses and harvested 40 h later. Cells were lysed in “ESB buffer” containing 50 mM Tris-HCl (pH 8), 120 mM NaCl, 1 mM EDTA, and 0.5% NP-40 supplemented with protease and phosphatase inhibitors (0.1 mM PMSF, 5 μg/ml aprotinin, 5 μg/ml leupeptin, 5 μg/ml pepstatin, 10 mM β-glycerophosphate, 0.1 mM NaF, 0.1 mM Na-orthovanadate). Cyclin D3 was immunoprecipitated by incubating the lysates for 2 to 4 h at 4°C with 2 μg of a polyclonal antibody directed against cyclin D3, and the immune complexes were purified using protein A-Sepharose (Amersham). Flag-Rb was immunoprecipitated with the M2 monoclonal anti-FLAG antibody covalently linked to agarose beads (Sigma). Samples were normalized for protein concentration prior to immune precipitation for all determinations.
For immunoblotting analyses, proteins were separated on denaturing polyacrylamide gels, transferred to nitrocellulose membrane (Schleicher & Schuell), and blotted with the indicated primary antibodies. The primary antibodies used include those against pRb (G3-245; Pharmingen); myogenin (hybridoma IF5D); myosin heavy chain (MHC) (hybridoma MF20); HA-tag (hybridoma 12CA5); GSK-3β (610202; BD Transduction Laboratories); Phospho-GSK-3β-Ser9 (9336), Akt (9272), and Phospho-Akt-Ser 473 (9271), all from Cell Signaling Technology; cyclin D3 (C-16), cdk4 (C-22), GSK-3β (0011A), and lamin B (M20), all from Santa Cruz; and α-tubulin (clone DM 1A; Sigma). The sites of antibody binding were visualized by using horseradish peroxidase-conjugated secondary antibodies (Jackson Immunologic) followed by chemiluminescence detection (ECL super signal; Pierce). The immunoblots shown in Fig. 2 and 4C were probed with Alexa 680-conjugated secondary antibodies (Molecular Probes) and detected by using an Odyssey infrared imaging system (Li-Cor).
FIG. 2.
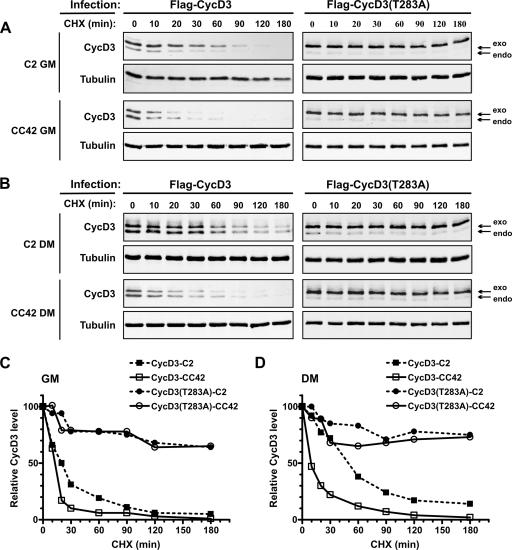
Kinetics of cyclin D3 or cyclin D3(T283A) protein turnover. C2 (Rb+/+) or CC42 (Rb−/−) myoblasts were infected with retroviruses encoding wild-type Flag-CycD3 or Flag-CycD3(T283A). The infected cells were cultured in GM or in DM for 48 h and then treated with CHX (50 μg/ml) over a 180-min time course. Cells were harvested at the indicated time points after the addition of CHX, and whole-cell extracts were prepared. Amounts of 60 μg of protein from cells infected with Flag-CycD3 or 30 μg from those infected with Flag-CycD3(T283A) were analyzed by Western blotting. (A) Immunoblot of cyclin D3 and α-tubulin expression by proliferating C2 or CC42 myoblasts. (B) Immunoblot of cyclin D3 and α-tubulin expression by differentiated C2 or CC42 myocytes. The arrows indicate the endogenous and the exogenous Flag-tagged cyclin D3. The blots shown are representative of the results of three independent experiments. (C, D) The immunoblots in panels A and B were detected by using an Odyssey infrared imaging system (Li-Cor), and the intensities of the protein bands were quantitated. The values for cyclin D3 were normalized to those for α-tubulin and are presented as the percentage of the amount of normalized cyclin D3 in cells not treated with CHX (time, 0 min).
FIG. 4.
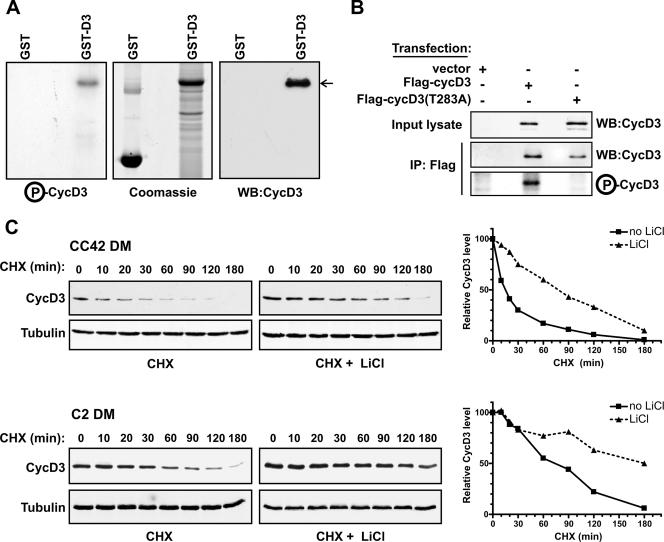
GSK-3β phosphorylates cyclin D3 on Thr-283. (A) Glutathione-Sepharose-bound GST or GST-D3 (1 μg) was incubated with human recombinant GSK-3β in a kinase assay mixture containing [γ-32P]ATP. Proteins were resolved by SDS-PAGE, stained with Coomassie blue, dried, and subjected to autoradiography. Amounts of 250 ng of GST or GST-D3 were resolved on a parallel gel, transferred to a membrane, and immunoblotted with an antibody to cyclin D3. WB, Western blot; P-CycD3, phospho-cyclin D3. (B) NIH 3T3 fibroblasts were transfected with wild-type Flag-CycD3, Flag-CycD3(T283A), or pFlex-1 empty vector. Whole-cell extracts (500 μg) were immunoprecipitated (IP) with anti-FLAG antibody. Cyclin D3 levels were analyzed in input lysates (5%) and in the anti-Flag immunoprecipitates (20%) by immunoblotting; the remainder of the anti-Flag immunoprecipitates (80%) was incubated with human recombinant GSK-3β and [γ-32P]ATP. Proteins were resolved by SDS-PAGE, and phospho-cyclin D3 (P-CycD3) visualized by autoradiography. WB, Western blot; +, present; −, absent. (C) CC42 (Rb−/−) or C2 (Rb+/+) confluent myoblasts were exposed to DM for 48 h and then incubated with or without LiCl (20 mM) for 6 h and treated with CHX (50 μg/ml) for the times indicated. Equal amounts of protein from cell lysates were examined by Western blotting with antibodies against cyclin D3 and α-tubulin. The blots shown are representative of three independent experiments. The graphs on the right show the quantitation of the Western blot data by using an Odyssey infrared imaging system (Li-Cor). The values for cyclin D3 were normalized to those for α-tubulin and are presented as the percentage of the amount of normalized cyclin D3 in cells not treated with CHX (time, 0 min).
For kinase assays, glutathione-Sepharose-bound GST-cyclin D3 or purified immunocomplexes containing cyclin D3 were collected by centrifugation and washed twice with kinase buffer (20 mM Tris-HCl, pH 8.0, 10 mM MgCl2, 5 mM DTT). The beads were then resuspended in 30 μl of kinase buffer containing 0.5 μM ATP, 5 μCi of [γ-32P]ATP (6,000 Ci/mmol; NEN), 0.25 units of recombinant GSK-3β (New England Biolabs) and incubated for 30 min at 30°C. The beads were washed three times with kinase buffer and then denatured in 2× sodium dodecyl sulfate (SDS) sample buffer. Samples were boiled for 10 min and separated by SDS-polyacrylamide gel electrophoresis (PAGE), stained with Coomassie blue, dried, and subjected to autoradiography.
Subcellular fractionation.
C2 and CC42 myoblasts cultured in GM or DM were resuspended in hypotonic buffer (10 mM KCl, 10 mM HEPES-KOH, pH 7.4, 1.5 mM MgCl2, 0.1% Triton X-100, 0.5 mM DTT) at a concentration of 1.5 million cells/ml. After incubation for 20 min on ice, the lysates were centrifuged for 5 min at 1,000 rpm at 4°C, and the supernatant was preserved as the cytosolic fraction. The nuclei were washed once in hypotonic buffer and then lysed in urea buffer. After the protein concentration was determined, 30 μg of both fractions was subjected to SDS-PAGE and Western blot analysis.
Indirect immunofluorescence analysis.
C2 or CC42 cells were grown on coverslips and treated (where indicated) with leptomycin B (40 ng/ml) for 6 h or with LiCl (20 mM) for 3 h. Cells were fixed with freshly prepared 4% paraformaldehyde for 10 min at room temperature and then permeabilized with 0.25% Triton X-100 in phosphate-buffered saline (PBS) for 10 min. Alternatively, cells were fixed with cold methanol-acetone (1:1) for 5 min at −20°C. Coverslips were incubated for 30 min with PBS containing 20% goat serum and then incubated for 2 h with the following antibodies in PBS-5% goat serum: anti-cyclin D3 (C-16; Santa Cruz) used at 2 μg/ml; anti-Rb (G3-245; Pharmingen) used at 10 μg/ml; and anti-GSK-3β (610202; BD Transduction Laboratories) used at 2.5 μg/ml. After three washes in PBS-0.1% NP-40, cells were incubated with fluorescein- or rhodamine-linked anti-rabbit or anti-mouse antibodies (Jackson) diluted 1:150 in PBS-5% goat serum. Cells were washed again, and then the cellular DNA was labeled with 0.1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI).
Preparation of recombinant GST proteins and pull-down assays.
pGEX plasmids encoding GST fusion proteins were transformed into Escherichia coli BL21 in the presence of 2% glucose; protein expression was induced at 30°C for 3 h by the addition of 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) to exponentially growing cultures. The cell pellets were resuspended and sonicated in “NETN buffer” containing 20 mM Tris-HCl (pH 8.0), 100 mM NaCl, 1 mM EDTA, and 0.5% Nonidet P-40 supplemented with 1 mM PMSF and protease inhibitors and clarified by centrifugation. The supernatants were mixed with glutathione-Sepharose 4B (Amersham) for 60 min at room temperature. The beads were washed twice with NETN buffer containing 0.7 M NaCl and three times with NETN buffer. The GST fusion proteins were analyzed and quantified by SDS-PAGE. In vitro-translated 35S-labeled cyclin D proteins were produced by programming reticulocyte lysate (Promega) with in vitro-transcribed RNA from pBSKS(−) plasmids containing murine cyclin D1, cyclin D3, or different cyclin D3 mutants. Reticulocyte lysates containing the translated proteins (10 μl) were mixed with 5 μg of GST-Rb fusion proteins bound to glutathione-Sepharose in 300 μl of NETN buffer for 3 h at 4°C. The beads were washed three times with NETN buffer at room temperature, and bound proteins were eluted with SDS sample buffer. The proteins were then resolved by SDS-PAGE and processed for autoradiography.
RESULTS
pRb is required for cyclin D3 protein accumulation during myogenic differentiation.
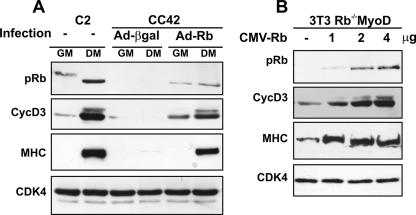
In a previous work we have shown that Rb-null myoblasts that are induced to differentiate fail to accumulate the cyclin D3 protein despite normal induction of cyclin D3 mRNA (6). This was determined in the Rb−/− CC42 myogenic cell line, originally isolated from Rb−/− embryonic stem cells grown as subcutaneous teratomas (58). To rule out the possibility that the reduced levels of cyclin D3 in these cells may be consequent to genetic events other than loss of pRb, we tested whether exogenous pRb expression could restore normal levels of cyclin D3 protein. To this end, CC42 myoblasts were infected with Adeno-Rb (7) or a control virus (Adeno-β-gal). Following incubation in GM or in DM for 48 h, cell extracts were prepared and cyclin D3 protein levels were measured by immunoblotting, along with those of MHC, a late marker of muscle cell differentiation whose expression is known to be inhibited in pRb-negative myocytes (45). As shown in Fig. 1A, the reintroduction of pRb in CC42 myoblasts was sufficient to rescue the expression of both cyclin D3 and MHC to levels comparable to those observed in differentiating Rb+/+ C2 myoblasts.
FIG. 1.
Ectopic expression of pRb restores normal levels of cyclin D3 protein in differentiating Rb−/− myocytes. (A) Proliferating CC42 myoblasts (Rb−/−) were infected with Adeno-Rb or Adeno-β-gal and cultured in GM or DM for 48 h. Equal amounts of protein from cell lysates were analyzed by immunoblotting with antibodies specific to pRb, cyclin D3, or MHC. As positive control, C2 myoblasts (Rb+/+) cultured in GM or in DM were analyzed in parallel. Equal loading of the extracts was monitored by immunoblotting with anti-cdk4. (B) Proliferating 3T3 Rb−/− fibroblasts were infected with the pBABEpuro-MyoD retrovirus and selected for 3 days with puromycin. The puromycin-resistant cell population (3T3 Rb−/−MyoD) was then transfected with increasing amounts of the CMV-Rb expression construct; the CMV-Neo-Bam empty vector was included in transfection mixtures to normalize for DNA amount. Cells were transferred to DM 24 h posttransfection and harvested after 48 h. Equal amounts of protein from cell lysates were analyzed for pRb, cyclin D3, or MHC protein expression by immunoblotting.
The pRb-dependence of cyclin D3 protein accumulation was further examined in Rb−/− fibroblasts induced to transdifferentiate to muscle by the ectopic expression of MyoD. Cultures of Rb−/− fibroblasts were rendered myogenic by infection with a retrovirus encoding MyoD and then transfected with increasing amounts of a pRb expression construct (CMV-Rb). Following incubation in DM for 48 h, the cell lysates were prepared and analyzed for cyclin D3 and MHC protein expression. The results, as seen in Fig. 1B, show that the expression of exogenous pRb in the MyoD-transduced Rb−/− fibroblasts was accompanied by the induction of endogenous cyclin D3 and MHC protein expression. Therefore, pRb appears to play a causal role in the increase of the steady-state levels of cyclin D3 in differentiating myogenic cells. As we have previously shown that cyclin D3 fails to accumulate in pRb-negative myocytes as a result of its rapid degradation through the 26S proteasome (6), the above data strongly suggest that pRb promotes cyclin D3 protein stabilization in terminally differentiating muscle cells.
The threonine 283 residue is involved in the control of cyclin D3 degradation in myogenic cells.
To gain insight into how pRb stabilizes cyclin D3 during the process of myoblast differentiation, we first investigated the mechanism that controls the basal turnover of cyclin D3 in these cells. It has been well established that the rate of cyclin D1 degradation is regulated through the phosphorylation of Thr-286, which induces the ubiquitination and proteasome-dependent degradation of cyclin D1 (12). The alignment of cyclin D1 and cyclin D3 sequences reveals that this residue is conserved in cyclin D3 (Thr-283 in cyclin D3) in a highly homologous amino acid sequence context at the carboxyl terminus of the protein. Therefore, we created a cyclin D3 mutant containing an Ala for Thr-283 substitution (T283A) and analyzed its turnover rate compared to that of wild-type cyclin D3 in Rb+/+ or Rb−/− myogenic cells. C2 (Rb+/+) or CC42 (Rb−/−) myoblasts were infected with retroviruses encoding wild-type cyclin D3 or cyclin D3(T283A) and cultured in GM or DM in the presence of the protein synthesis inhibitor cycloheximide (CHX) in time-course experiments. The levels of cyclin D3 were then examined by immunoblotting, along with those of α-tubulin (Fig. 2A, B). Because the ectopically expressed cyclin D3 proteins were both Flag-tagged at their N termini, they displayed an electrophoretic mobility slower than that of endogenous cyclin D3. Under proliferation conditions, the half-life of the cyclin D3 protein was estimated to be approximately 20 min in C2 myoblasts, compared with a half-life of about 13 min in CC42 myoblasts (Fig. 2C). Following differentiation, the half-life of cyclin D3 extended to 50 min in C2 myotubes while remaining as short as in GM (13 min) in CC42 myocytes (Fig. 2D). As for the cyclin D3(T283A) mutant, it was very stable in both C2 and CC42 cells, either growing or differentiating (Fig. 2C, D). These results indicate that (i) the basal half-life of cyclin D3 is significantly prolonged upon the differentiation of Rb+/+ but not Rb−/− myoblasts and (ii) the threonyl residue 283 regulates the turnover rate of cyclin D3 in both Rb+/+ and Rb−/− myogenic cells.
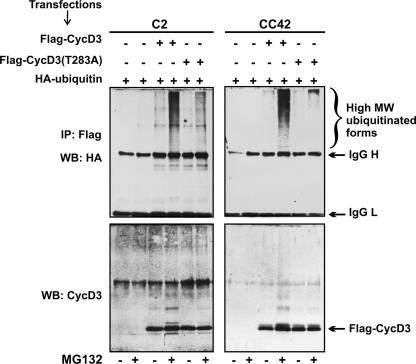
To examine whether Thr-283 is implicated in cyclin D3 ubiquitination, C2 or CC42 myoblasts were transfected with expression constructs for Flag-CycD3 or Flag-CycD3(T283A) together with an HA-tagged ubiquitin expression plasmid. Flag-CycD3 immunocomplexes were then precipitated from cell lysates and subjected to Western blot analysis with anti-HA antibody to detect ubiquitin-conjugated cyclin D3. As shown in Fig. 3, the incubation of cells transfected with Flag-CycD3 in the presence of the proteasome inhibitor MG132 led to the accumulation of high-molecular-weight conjugates immunoprecipitated with the anti-Flag antibody. In contrast, a greatly reduced amount of polyubiquitinated proteins was recovered from MG132-treated cells expressing Flag-CycD3(T283A). The immunoprecipitates were also probed with an anti-cyclin D3 antibody to verify that the wild-type and mutant cyclin D3 proteins were expressed at the same level. In addition to native cyclin D3, slower-migrating discrete proteins were also specifically detected with the antibody to cyclin D3 in immunoprecipitates from MG132-treated cells transfected with wild-type Flag-CycD3 but not in those from cells transfected with the Flag-CycD3(T283A) mutant. Therefore, the integrity of the Thr-283 residue in cyclin D3 appears to be required for the ubiquitination and the subsequent proteasome-mediated degradation of the protein in both Rb+/+ and Rb−/− myoblasts.
FIG. 3.
Thr-283 is required for cyclin D3 ubiquitination. C2 (Rb+/+) or CC42 (Rb−/−) proliferating myoblasts were transfected with expression vectors encoding wild-type Flag-CycD3 or Flag-CycD3(T283A) together with an expression vector for HA-tagged ubiquitin. Transfected cells were treated with the proteasome inhibitor MG132 (10 μM) or with the solvent dimethyl sulfoxide for 4 h before harvesting. Cell extracts were immunoprecipitated with anti-Flag antibody. Immunocomplexes were resolved by SDS-PAGE and analyzed by using either anti-HA (upper panels) or anti-cyclin D3 (lower panels) antibodies. MW, molecular weight; IgG H, immunoglobulin G heavy chains; IgG L, immunoglobulin G light chains; WB, Western blot; IP, immunoprecipitate; +, present; −, absent.
Phosphorylation of Thr-283 by GSK-3β regulates the turnover of cyclin D3 in myogenic cells.
Among several proline-directed kinases tested, GSK-3β was identified for its ability to specifically phosphorylate cyclin D1 on Thr-286 (11). To examine whether GSK-3β phosphorylates cyclin D3, a GST-cyclin D3 fusion protein produced in bacteria (GST-D3) was used as substrate for purified GSK-3β in an in vitro kinase assay. As shown in Fig. 4A, GSK-3β could indeed phosphorylate GST-D3. Next, we determined whether GSK-3β phosphorylates cyclin D3 on Thr-283 (Fig. 4B). To prepare substrates, Flag-CycD3 or Flag-CycD3(T283A) was expressed in NIH 3T3 fibroblasts and recovered by immunoprecipitation with the M2 anti-Flag antibody. Kinase reactions performed in vitro demonstrated that purified recombinant GSK-3β was able to phosphorylate wild-type cyclin D3 but not the cyclin D3(T283A) mutant. The wild-type and mutant cyclin D3 substrate proteins were equally expressed, as determined by immunoblot analysis of the Flag immunoprecipitates with an anti-cyclin D3 antibody. These results indicate that GSK-3β can specifically phosphorylate cyclin D3 on Thr-283.
To verify whether the activity of GSK-3β is required for the degradation of cyclin D3 in myogenic cells, we determined the effect of the GSK-3β-specific inhibitor lithium chloride (LiCl) on cyclin D3 protein stability when protein synthesis was blocked by the addition of CHX (Fig. 4C). Treatment of Rb−/− myocytes with LiCl significantly prolonged cyclin D3 turnover (13 min versus 80 min). Likewise, the inhibitor further extended the half-life of cyclin D3 in Rb+/+ differentiated myocytes (60 min versus ≥180 min), indicating that direct inhibition of GSK-3β had an effect that was additive to that of pRb on the stimulation of cyclin D3 stability in these cells. Altogether, the above results indicate that GSK-3β-mediated phosphorylation of cyclin D3 at Thr-283 regulates the rate of cyclin D3 degradation in both Rb−/− and Rb+/+ myogenic cells and suggest that pRb may impinge on cyclin D3 stability by modulating the activity of GSK-3β.
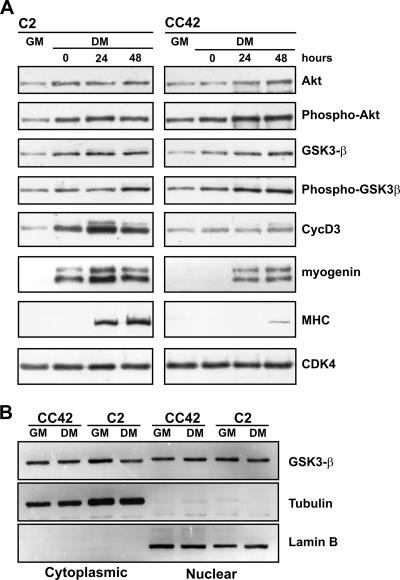
The kinase activity of GSK-3β is known to be down-regulated through the specific phosphorylation of Ser-9 by the serine threonine kinase Akt, which, in turn, is activated through the phosphorylation of Ser-473 by signals originating with phosphatidylinositol 3-kinase (1, 10). To explore the possibility that pRb may influence this pathway, we analyzed the GSK-3β and Akt expression profiles and the phosphorylation state during the differentiation of Rb−/− myoblasts compared to that of Rb+/+ myoblasts. As shown in Fig. 5A and as expected, the differentiation of CC42 myoblasts was characterized by normal induction of early differentiation markers, such as myogenin, but greatly reduced expression of MHC (a late differentiation marker) and cyclin D3. The phosphorylated forms of Akt and GSK-3β were both up-regulated as early as myogenin during the differentiation of C2 myoblasts, and this increase corresponded to an augmentation of the total Akt and GSK-3β levels. A similar pattern of expression was observed in differentiating CC42 myoblasts. Because GSK-3β has been shown to shuttle between nuclear and cytoplasmic compartments in a cell cycle-dependent manner (11), we also examined whether pRb may affect the subcellular localization of GSK-3β in myogenic cells. To this end, C2 (Rb+/+) and CC42 (Rb−/−) myoblasts, cultured in GM or DM, were separated into nuclear and cytoplasmic fractions, which were blotted for GSK-3β. The purities of the cytoplasmic and nuclear fractions were verified by immunoblotting with anti-α-tubulin and anti-lamin B1, respectively. As shown in Fig. 5B, GSK-3β was detected in both the cytosolic and nuclear fractions of proliferating or differentiating C2 myoblasts, and its subcellular distribution remained unchanged in myocytes lacking pRb. Altogether, these data indicate that differentiating Rb−/− myoblasts display normal regulation of GSK-3β in terms of expression, phosphorylation state, and subcellular localization.
FIG. 5.
Analysis of expression, phosphorylation state, and subcellular localization of GSK-3β in Rb+/+ and Rb−/− differentiating myoblasts. (A) C2 (Rb+/+) or CC42 (Rb−/−) myoblasts were grown to 90% confluence and then shifted to DM. Whole-cell extracts were prepared from proliferating myoblasts (GM) or from cultures exposed to DM for the indicated periods of time. Equal amounts of protein were separated by SDS-PAGE and subjected to immunoblot analysis using antibodies against Akt, GSK-3β, cyclin D3, myogenin, MHC, and cdk4 (as loading control). The phosphorylation of Akt or GSK-3β was analyzed with phospho-specific antibodies that recognize the phosphorylated Ser-473 residue of Akt or Ser-9 residue of GSK-3β. (B) C2 and CC42 myoblasts were cultured in GM or exposed to DM for 48 h. Equal amounts of protein (30 μg) from nuclear and cytoplasmic fractions were separated on denaturing gels and subjected to Western blot analysis with antibodies against GSK-3β, α-tubulin, or lamin B1.
Characterization of the pRb-cyclin D3 interaction.
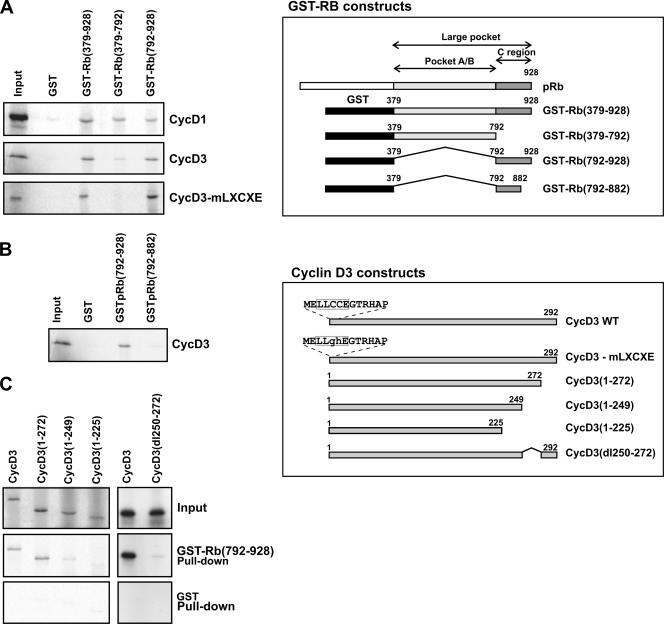
We have previously shown that cyclin D3 is nearly totally complexed to hypophosphorylated pRb in differentiated C2 myotubes (6), raising the possibility that pRb's binding may hinder the access of GSK-3β to cyclin D3. The in vitro association between D-type cyclins and pRb has been previously mapped to a pRb domain that includes the T/E1A/E7-binding region (termed A/B pocket) and additional carboxy-terminal sequences and to an amino terminal LXCXE motif found both in D-type cyclins and pRb-binding oncoproteins. It has also been observed that cyclins D2 and D3 can bind more efficiently than cyclin D1 to pRb (14, 15, 26). To define in more detail the protein domains required for the association between pRb and cyclin D3, we performed binding assays using in vitro-translated cyclin D3 and bacterially produced GST-Rb fusion proteins (schematized in Fig. 6, upper inset) which contained the A/B pocket (residues 379-792), the C-terminal region (residues 792-928), or the A/B pocket plus the C-terminal region (residues 379-928; termed large pocket). As a control, we also measured the ability of these GST-Rb constructs to bind cyclin D1. As shown in Fig. 6A, cyclin D3 bound with similar efficiencies to the large pocket and to the C terminus of pRb, but only very weakly to the A/B pocket. By comparison, cyclin D1 bound weakly to all Rb fusion proteins tested. These results not only confirm that cyclin D3 binding requires the C terminus of pRb in addition to the A/B pocket but also reveal that, in fact, the C-terminal region of pRb contains a strong cyclin D3 binding site which can act independently of the adjacent A/B pocket unit.
FIG. 6.
Binding of cyclin D3 to pRb in vitro. The schematic in the upper inset shows the structure of the GST-Rb fusion proteins used and illustrates the A/B pocket region and the carboxy-terminal region of Rb. The lower inset shows schematic representations of wild-type (WT) cyclin D3 and cyclin D3 mutants carrying substitutions in the N-terminal LXCXE motif or deletions in the carboxy-terminal region. (A and B) The indicated GST-Rb fusion proteins or GST alone were bound to glutathione-Sepharose beads and incubated with in vitro-translated, 35S-labeled cyclin D1, cyclin D3, or the cyclin D3-mLXCXE mutant. Bound proteins were resolved by SDS-PAGE and processed for autoradiography. (C) The GST-Rb(792-928) fusion protein was incubated with the indicated cyclin D3 proteins translated and radiolabeled in vitro. Bound proteins were resolved by SDS-PAGE and processed for autoradiography.
It has been previously shown that cyclin D1 and cyclin D2 mutants carrying substitutions that destroy their LXCXE sequence fail to associate with the pRb pocket region (14, 15); thus, we introduced analogous substitutions into cyclin D3 to create the mutant designated CycD3-mLXCXE (schematized in Fig. 6, lower inset). As shown in Fig. 6A (lower panel), this cyclin D3 mutant, though unable to bind to the A/B pocket, could still bind efficiently to the large pocket, as well as to the C terminus of pRb. Therefore, it can be concluded that stable interaction between cyclin D3 and pRb requires a domain in cyclin D3 distinct from the LXCXE motif, as well as sequences located in the C terminus of pRb. Furthermore, we have been able to narrow down the cyclin D3 binding site to the most C-terminal 46 amino acids of pRb, since a GST-Rb fusion protein containing a C terminus truncated at amino acid residue 882 [GST-Rb(792-882)] resulted in the inability to bind cyclin D3 (Fig. 6B). Finally, we sought to identify the domain through which cyclin D3 binds the C terminus of pRb. To this end, we created a series of C-terminally truncated cyclin D3 mutants, whose structures are schematized in the lower inset of Fig. 6. GST pull-down assays were then performed using in vitro-translated wild-type cyclin D3 and mutant proteins and the bacterially produced GST-Rb(792-928) fusion protein. The results, as shown in Fig. 6C, indicate that a cyclin D3 protein truncated at residue 272 could still bind the C-terminal region of pRb, whereas cyclin D3 proteins truncated at residues 225 or 249 have lost such capability. Then, we created a cyclin D3 mutant carrying an internal deletion that eliminates the residues between amino acids 250 and 272; as shown in Fig. 6C and as expected, we found that this mutant was unable to bind GST-Rb(792-928). Collectively, the mapping analyses described above identify a novel domain in cyclin D3 between residues 250 and 272, required for stable binding of cyclin D3 to the pRb C-terminal 46 residues.
pRb protects cyclin D3 from GSK-3β-mediated phosphorylation.
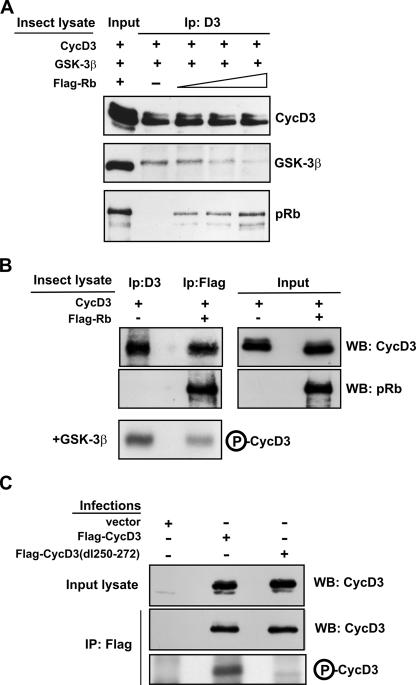
The above data reveal that cyclin D3 interacts with pRb through a region lying in close proximity to the Thr-283 residue, which is consistent with the hypothesis that the formation of the pRb-cyclin D3 complex may prevent association between cyclin D3 and GSK-3β. To examine this possibility, we performed in vitro binding assays using the relevant proteins expressed from baculovirus vectors. Sf9 insect cells were infected separately with baculoviruses expressing cyclin D3, GSK-3β, or pRb, and cell lysates containing high levels of these proteins were prepared 40 h later. Extracts expressing cyclin D3 were incubated with those expressing GSK-3β in the presence or absence of increasing amounts of extracts containing pRb. Cyclin D3 was then immunoprecipitated, and the quantities of GSK-3β and pRb recovered in the anti-cyclin D3 immunocomplexes were determined by Western blotting. As shown in Fig. 7A, GSK-3β precipitated with cyclin D3, and this interaction was inhibited by pRb in a dose-dependent manner. Next, we sought to determine whether cyclin D3 is refractory to GSK-3β-mediated phosphorylation when complexed to pRb. Hence, Sf9 cells were infected with baculoviruses expressing cyclin D3 alone or cyclin D3 together with Flag-Rb. Free cyclin D3 was directly immunoprecipitated with an antibody against cyclin D3, while cyclin D3 bound to pRb was isolated by immunoprecipitation with the M2 anti-Flag antibody. The anti-Flag and anti-cyclin D3 immunoprecipitates were normalized by immunoblotting to yield roughly equivalent levels of cyclin D3 and then used as substrates for purified GSK-3β in an in vitro kinase assay. Consistent with our hypothesis, it was found that pRb-bound cyclin D3 was phosphorylated by GSK-3 much less efficiently than free cyclin D3 (Fig. 7B). Finally, we wished to verify whether the region required for cyclin D3 binding to pRb (amino acids 250 to 272) (Fig. 6) is also necessary for cyclin D3 to be recognized and, thus, phosphorylated by GSK-3β. Therefore, Flag-CycD3 or Flag-CycD3-dl(250-272) was expressed in NIH 3T3 fibroblasts and immunoprecipitated with the M2 anti-Flag antibody, and kinase reactions were performed using purified recombinant GSK-3β. As shown in Fig. 7C, GSK-3β was able to phosphorylate wild-type cyclin D3 but not the dl(250-272) cyclin D3 mutant. The wild-type and mutant cyclin D3 substrate proteins were expressed equally, as determined by immunoblot analysis of the Flag immunoprecipitates with an anti-cyclin D3 antibody. Taken together, the above results indicate that the association between pRb and cyclin D3 can prevent GSK-3β-mediated phosphorylation of cyclin D3 on Thr-283.
FIG. 7.
pRb inhibits the association between cyclin D3 and GSK-3β, thus preventing cyclin D3 phosphorylation by GSK-3β. (A) Insect Sf9 cells were infected with baculoviruses encoding cyclin D3, GSK-3β, or Flag-Rb. Sf9 cell extract containing cyclin D3 (30 μg protein) was incubated with extract containing GSK-3β (50 μg) in the presence or absence of increasing amounts of extract containing Flag-Rb (50 μg, 150 μg, or 300 μg). Lysate from uninfected Sf9 cells was added to the reaction mixtures to adjust for total protein amount. Cyclin D3-containing complexes were then immunoprecipitated (Ip:D3). Of each immunoprecipitate, 10% was analyzed by immunoblotting with anti-cyclin D3 and 90% by immunoblotting with anti-GSK-3β or anti-Rb. The input lane contains 3 μg of protein of cyclin D3 lysate, 5 μg of GSK-3β lysate, and 5 μg of Flag-Rb lysate. The blot shown is representative of three independent experiments. (B) Insect Sf9 cells were infected with a baculovirus encoding cyclin D3 or coinfected with baculoviruses encoding cyclin D3 or Flag-Rb. The Sf9 lysate containing cyclin D3 (100 μg) was immunoprecipitated with an antibody to cyclin D3, whereas the Sf9 lysate containing cyclin D3 and Flag-Rb (100 μg) was subjected to precipitation with anti-Flag antibody. The expression of cyclin D3 and pRb was analyzed in the input lysates (5%) and in the immunoprecipitates (25%) by Western blotting. The anti-cyclin D3 (25%) and the anti-Flag (50%) immunoprecipitates were incubated in kinase reaction mixtures with human recombinant GSK-3β and [γ-32P]ATP. Samples were then subjected to SDS-PAGE and autoradiography. The results of one experiment representative of three are shown. (C) NIH 3T3 fibroblasts were infected with retroviruses expressing Flag-CycD3, Flag-CycD3-dl(250-272), or empty vector and harvested 48 h postinfection. Cell extracts (500 μg) were immunoprecipitated with anti-Flag antibody, and afterwards, 20% of each immunoprecipitate was analyzed by immunoblotting with an antibody to cyclin D3 while the remainder was incubated with human recombinant GSK-3β and [γ-32P]ATP in kinase reaction mixtures. Samples were then subjected to SDS-PAGE and autoradiography. Input lysates (5%) were also analyzed. WB, Western blot; IP, immunoprecipitate; +, present; −, absent; P-CycD3, phospho-cyclin D3.
Functional significance of cyclin D3 protein accumulation during muscle cell differentiation.
The above-described mechanism of cyclin D3 stabilization by pRb led us to question the biological significance of cyclin D3 accumulation during the process of muscle cell differentiation. To address this issue, we undertook a series of cellular and biochemical studies.
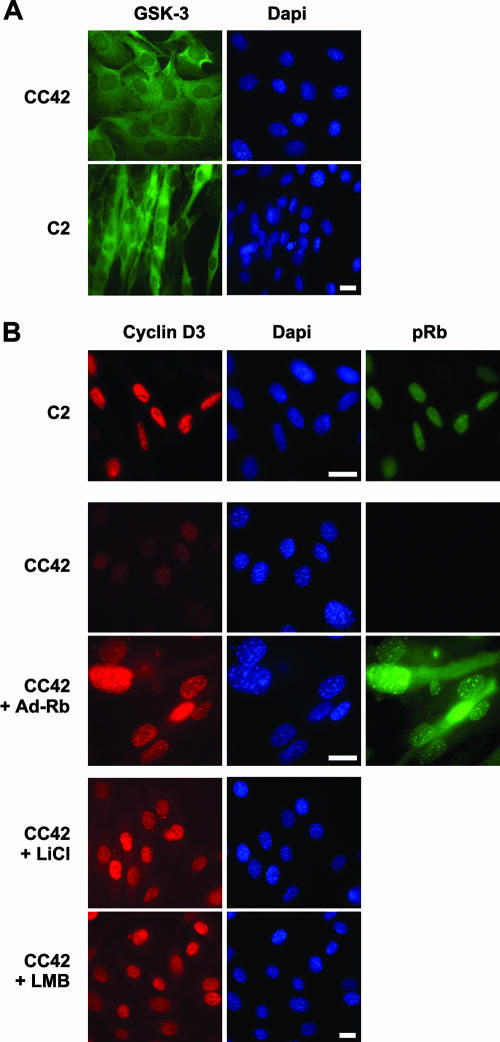
As shown in Fig. 8A, immunofluorescence staining detected GSK-3β both in the cytoplasm and nuclei of C2 and CC42 differentiated myocytes, indicating that cyclin D3 may be subjected to GSK-3β-mediated phosphorylation in both cellular compartments. Cyclin D3 was found to accumulate and colocalize with pRb in the nuclei of differentiated C2 (Rb+/+) myotubes but was hardly detectable in CC42 (Rb−/−) differentiated myocytes. Interestingly, the ectopic expression of a nonphosphorylatable, constitutively active pRb mutant in CC42 myocytes resulted in the nuclear accumulation of cyclin D3 (Fig. 8B), which occurs concomitantly with the induction of late differentiation markers (Fig. 1A). Previous studies have shown that GSK-3β-dependent phosphorylation of cyclin D1 triggers nuclear export of cyclin D1 to the cytoplasm where it is degraded by the proteasome (2). To examine whether cyclin D3 is similarly subjected to active export from the nuclei of pRb-negative myoblasts, CC42 myocytes were treated with leptomycin B, an inhibitor of nuclear export, or with the GSK-3β-specific inhibitor LiCl. As shown in Fig. 8B, leptomycin B could indeed restore the accumulation of cyclin D3 in the nuclei of a majority of CC42 myocytes, and a similar effect was observed following the inactivation of GSK-3β by LiCl, which indicates that, in the absence of pRb, GSK-3β-phosphorylated cyclin D3 is rapidly exported to the cytoplasm to be degraded through the cytoplasmic proteasome. Taken together, these results are consistent with the idea that pRb promotes the nuclear accumulation of cyclin D3 by preventing its phosphorylation by GSK-3β.
FIG. 8.
The nuclear accumulation of cyclin D3 is restored in Rb−/− myocytes by expression of exogenous pRb, inhibition of GSK-3β activity, or inhibition of nuclear export. (A) C2 or CC42 myoblasts plated on glass coverslips were exposed to DM for 48 h and then stained with a GSK-3β-specific antibody and DAPI dye. (B) C2 myoblasts exposed to DM for 48 h were stained with antibodies to cyclin D3 or pRb and DAPI dye. CC42 myoblasts plated on glass coverslips were either mock-infected or infected with Adeno-Rb and then exposed to DM for 48 h. Cells were fixed and processed for immunofluorescence with antibodies specific to cyclin D3 or pRb and DAPI dye. Where indicated, CC42 myocytes were treated with LiCl (20 mM) or leptomycin B (LMB, 40 ng/ml) for 3 h or 6 h, respectively, prior to fixation. Bar, 10 μm.
Having established that GSK-3β inhibition promotes cyclin D3 stabilization and nuclear accumulation in pRb-negative myocytes (Fig. 4C and 8B), we next examined the consequences for myogenic differentiation of blocking GSK-3β activity in either the absence or presence of pRb. Therefore, CC42 or C2 myoblasts were cultured in DM alone or complemented with LiCl and the relative expression levels of a number of muscle differentiation markers were determined by Western blotting. The results, as seen in Fig. 9A, show that, following treatment with LiCl, both CC42 and C2 differentiating myoblasts accumulated increased levels of cyclin D3. Concurrently, LiCl-treated cells exhibited enhanced expression of the late differentiation markers MHC and sarcomeric α-actin and the cdk inhibitor p21, while MyoD and myogenin levels were not grossly affected. Previous studies have demonstrated that, besides expressing extremely low levels of late differentiation markers, differentiated myocytes lacking pRb fail to undergo G0 arrest and accumulate in the S and G2 phases of the cell cycle (45, 58). We observed that, in contrast to the induction of MHC and sarcomeric α-actin, an inhibition of GSK-3β could not restore terminal G0 arrest to differentiating Rb−/− myocytes, as indicated by the persistence of high levels of cyclin A in the presence of LiCl (Fig. 9A). The above findings indicate that GSK-3β inactivation can promote myogenic differentiation concomitantly with enhanced expression of cyclin D3 not only in the presence but also in the absence of pRb, suggesting that cyclin D3 may be implicated in the control of muscle gene expression.
To assess the effect of cyclin D3 overexpression on the differentiation of Rb−/− myoblasts in the absence of other physiological perturbations elicited by GSK-3β inactivation, CC42 myoblasts were infected with a retrovirus expressing the cyclin D3(T283A) mutant, which is refractory to GSK-3β-mediated phosphorylation and, hence, very stable. After puromycin selection, cells were induced to differentiate and muscle-specific gene expression was examined by Western blotting. As shown in Fig. 9B, ectopic cyclin D3(T283A) expression led to enhanced expression of p21 and cdk4 compared with the levels in cells infected with control virus (twofold increase relative to the constitutive levels of lamin B1) but was not sufficient by itself to induce the expression of MHC and sarcomeric α-actin. This indicates that, in the absence of pRb, a stabilized cyclin D3 cannot resume the differentiation-promoting action of GSK-3β inhibition.
We next determined whether the ectopic expression of cyclin D3(T283A) affected the differentiation of Rb+/+ myoblasts (Fig. 10A). The expression of retrovirally transduced Flag-tagged cyclin D3(T283A) in differentiating C2 myoblasts appeared to inhibit the accumulation of endogenous cyclin D3, which likely reflects a competition for pRb binding, with a consequent destabilization of endogenous cyclin D3. The differentiation of these cells was characterized by normal expression of MyoD but delayed expression of MyoD transcriptional targets, including myogenin, MHC, and sarcomeric α-actin. However, within 3 to 5 days of differentiation, they fully differentiated at the biochemical level and formed myotubes grossly similar to those of C2 myoblasts infected with control virus. The delayed kinetics of muscle gene induction observed in C2 myoblasts expressing cyclin D3(T283A) correlated with the delayed kinetics of pRb dephosphorylation; in fact, pRb remained in its hyperphosphorylated form, and cyclin A was highly expressed, for about 2 days after the initiation of differentiation, while cyclin D1 was down-regulated normally within the first 12 h of differentiation. pRb was afterwards dephosphorylated, and cyclin A repressed, at the time of induction of high levels of p21. Notably, as observed also in pRb-negative myocytes, ectopic cyclin D3(T283A) expression in C2 myoblasts was associated with an ∼twofold increase in p21 and cdk4 protein levels.
The sustained levels of pRb phosphorylation and cyclin A expression observed in early differentiating myoblasts expressing cyclin D3(T283A) suggest an increased proliferation rate for these cells in DM conditions. To verify this prediction, sparsely seeded cells were exposed to DM and counted daily for 3 days (Fig. 10B). After 24 h in DM, the population-doubling time of myoblasts expressing cyclin D3(T283A) was ∼20% shorter than that of control C2 myoblasts (9.5 h versus 12 h). Furthermore, in one representative experiment, flow cytometric analysis performed on cells at this time point showed that C2 expressing cyclin D3(T283A) had an increased percentage of cells in the S phase (22.45% versus 9.33% for control C2 cells), which was associated with a decrease in the percentage of cells in G1 (53.79% versus 70% for control cells).
To evaluate whether the overexpression of cyclin D3 may positively influence the differentiation of postmitotic myoblasts, we sought to exploit a stabilized cyclin D3 mutant that had lost the ability to bind pRb, reasoning that this mutant should be able to accumulate in differentiating myoblasts without promoting pRb phosphorylation and the consequent delay of their terminal differentiation. Therefore, we generated a cyclin D3 double mutant carrying a mutated LXCXE motif and a deletion of amino acids 250 to 272; as shown in Fig. 6 and 7, such mutations render cyclin D3 unable to bind pRb and refractory to GSK-3β-mediated phosphorylation. When retrovirally transduced into C2 myoblasts, the Flag-tagged cyclin D3-mLXCXE/dl(250-272) double mutant accumulated to high levels without inhibiting the accumulation of endogenous cyclin D3, which was expected given its inability to bind pRb (Fig. 10A). The differentiation of these cells was characterized by normal timing of pRb dephosphorylation and G0 arrest and substantially enhanced expression of the muscle-specific genes for MHC and α-actin, as well as those for p21 and cdk4. Furthermore, the proliferation rate in DM of C2 myoblasts expressing the cyclin D3-mLXCXE/dl(250-272) double mutant was very similar to that of C2 control myoblasts (Fig. 10B).
We finally investigated whether cyclin D3 is required for myogenesis by examining the MyoD-stimulated myogenic conversion of cyclin D3-deficient MEFs. MEFs from cyclin D3−/− and wild-type littermate embryos were infected with Adeno-MyoD, incubated in DM for 2 days, and then examined for their abilities to form myotubes and to express muscle-specific genes (Fig. 11). We observed that while the vast majority of wild-type MEFs infected with MyoD formed long, multinucleated myotubes, the MyoD-transduced cyclin D3−/− MEFs showed a greatly decreased degree of myogenic conversion. Consistently, the expression of late, muscle-specific genes, such as the MHC and sarcomeric actin genes, was significantly reduced in MyoD-transduced cyclin D3-null MEFs compared with their expression in wild-type MEFs. By contrast, cyclin D3 deficiency did not affect MyoD-mediated induction of myogenin or pRb.
Interestingly, the p21 and cdk4 protein levels appeared to decline in cells lacking cyclin D3, in both the presence and absence of MyoD. The decreased expression of p21 and cdk4 in cyclin D3−/− MEFs is consistent with the previously observed increase of p21 and cdk4 in response to ectopic cyclin D3 expression in either Rb−/− or Rb+/+ myoblasts (Fig. 9B and 10A). These observations reveal that the regulation of cyclin D3 expression is linked to that of the cdk4 and p21 partners.
DISCUSSION
In an earlier study we have shown that, while the expression of cyclin D3 mRNA is highly induced by MyoD at the onset of myoblast differentiation, the cyclin D3 protein becomes stabilized during the process of terminal differentiation by a mechanism that appears to require functional pRb. We observed, indeed, that cyclin D3 mRNA is induced normally in Rb−/− myocytes but the cyclin D3 protein fails to accumulate as a result of its rapid degradation through the 26S proteasome (6). Here we demonstrate that the reintroduction of pRb expression in pRb-negative myoblasts rescues high levels of cyclin D3 protein, in parallel with the levels of late markers of muscle cell differentiation. Furthermore, we identify the mechanism by which pRb stabilizes the cyclin D3 protein in differentiating myoblasts and provide evidence that the pRb-dependent accumulation of cyclin D3 is functionally relevant to the process of muscle cell differentiation.
GSK-3β regulates the turnover of cyclin D3 in myogenic cells.
Our half-life determination experiments indicate that the basal turnover of cyclin D3 is rapid in proliferating C2 myoblasts but is significantly prolonged upon their terminal differentiation into myotubes, which are known to accumulate high levels of hypophosphorylated pRb. By contrast, myoblasts lacking pRb display basal turnover rates of cyclin D3 in both proliferation and differentiation conditions. Previous studies have demonstrated that the ubiquitin-mediated proteasomal degradation of cyclin D1 is triggered by its phosphorylation on Thr-286 by GSK-3β (11). We provide evidence that a similar mechanism regulates the turnover of cyclin D3 in myogenic cells. We found that purified GSK-3β could phosphorylate cyclin D3 on a specific threonine residue (Thr-283). Mutation of Thr-283 to alanine (T283A) markedly stabilized cyclin D3 by inhibiting its polyubiquitination and subsequent degradation. Finally, the treatment of Rb−/− myocytes with LiCl, a specific inhibitor of GSK-3β activity, resulted in the stabilization of cyclin D3. In addition, we observed that LiCl could further prolong the turnover of cyclin D3 in Rb+/+ myotubes, indicating additive effects of pRb and GSK-3β inactivation on the stimulation of cyclin D3 stability in these cells.
The multifunctional GSK-3β serine/threonine kinase is normally active in cells, being primarily regulated through inhibition of its activity in response to multiple signals (13), including insulin and insulin-like growth factors. Insulin-like growth factors potently stimulate skeletal myogenesis and muscle hypertrophy through the activation of the phosphatidylinositol 3-kinase/Akt signaling pathway (8, 23, 56, 65, 66). Once activated, the serine-threonine protein kinase Akt phosphorylates an array of substrates that include GSK-3β. Phosphorylation by Akt results in the inactivation of GSK-3β (10). Given that pRb controls many aspects of muscle cell differentiation, we considered the possibility that pRb may positively regulate the expression and/or the activity of Akt, with a consequent downregulation of GSK-3β and stabilization of cyclin D3. We observed, however, that the differentiation of Rb+/+ or Rb−/− myoblasts is associated with a similar increase in Akt activity, mirroring protein levels, and a parallel induction of both total GSK-3β and the GSK-3β isoform phosphorylated by Akt. Furthermore, GSK-3β was found to localize in both the cytoplasm and nuclei of myogenic cells in either the presence or absence of pRb. Therefore, pRb does not appear to regulate GSK-3β activity and subcellular distribution in myogenic cells. Instead, the evidence discussed below indicates that pRb affects cyclin D3 stability by specifically preventing its phosphorylation by GSK-3β.
pRb counteracts GSK-3β-mediated phosphorylation of cyclin D3 by directly binding cyclin D3.
Earlier studies have shown that pRb and cyclin D3 can directly interact and that such interaction requires the large pocket region of pRb, comprising the A/B pocket and the adjacent C-terminal domain, and the LXCXE motif located at the N terminus of cyclin D3 (14, 15). Our in vitro binding assays extend the understanding of the interaction between pRb and cyclin D3 by showing that pRb contains two distinct binding sites for cyclin D3. One binding site resides in the A/B pocket unit and contacts the LXCXE motif of cyclin D3, while the other is located within the C-terminal 46 amino acids of pRb and is used to bind a region of cyclin D3 between amino acids 250 and 272. This region lies at the C terminus of cyclin D3 in close proximity to the Thr-283 residue targeted by GSK-3β. Interestingly, a cyclin D3 deletion mutant lacking the sequence between amino acids 250 and 272 was both unable to bind pRb and refractory to GSK-3β-mediated phosphorylation, which indicates that the C terminus of pRb contacts cyclin D3 protein determinants required for the interaction of cyclin D3 with GSK-3β and/or for the adoption of a conformational structure that renders the Thr-283 residue available for GSK-3β-mediated phosphorylation. These observations strongly suggest that pRb's binding to cyclin D3 prevents the phosphorylation of Thr-283 by GSK-3β. In support of this notion, in vitro binding and kinase assays showed that pRb could displace GSK-3β from cyclin D3 and that pRb-bound cyclin D3 was refractory to GSK-3β-mediated phosphorylation.
While cyclin D3 and pRb accumulate in the nuclei of terminally differentiated Rb+/+ myotubes, we found that cyclin D3 is almost undetectable in Rb−/− myocytes, which reflects the high turnover rate of cyclin D3 in these cells. However, the transduction of constitutively active pRb into Rb−/− myocytes resulted in the nuclear accumulation of cyclin D3, which was also observed following the treatment of pRb-negative myocytes with an inhibitor of nuclear export or an inhibitor of GSK-3β. These results strongly suggest that, in the absence of pRb, GSK-3β-phosphorylated cyclin D3 is actively exported from the nucleus. Altogether, the above observations are consistent with the idea that pRb specifically prevents the GSK-3β-mediated phosphorylation of cyclin D3 through direct binding, thereby inhibiting cyclin D3's nuclear export and subsequent cytoplasmic degradation.
Stabilization of cyclin D3 by pRb has a functional role in myogenesis.
GSK-3β inhibition has recently been shown to play an integral role in the processes of myoblast differentiation and myotube hypertrophy (55, 56, 67). We observed that, in addition to cyclin D3 protein stabilization and nuclear accumulation, LiCl-treated Rb−/− myocytes exhibited induced expression of muscle genes specific to the late phase of differentiation, although their G0 arrest remained compromised. These results reveal that the inhibition of GSK-3β can rescue some differentiation defects due to the absence of pRb. However, cyclin D3 does not appear to mediate the promyogenic action of GSK-3β inhibition, as ectopic expression of the stabilized cyclin D3(T283A) mutant was not sufficient by itself to induce late muscle gene expression in pRb-negative myocytes. This was not unexpected since the inactivation of GSK-3β likely targets different substrates that cooperate to enable differentiation in the absence of pRb. The above observations also suggest that any potential positive effect of cyclin D3 on muscle gene expression requires pRb.
When ectopically expressed in pRb-positive myoblasts, the stabilized cyclin D3(T283A) mutant stimulated myoblast proliferation in DM and delayed the onset of terminal differentiation. We observed that this effect of cyclin D3(T283A) correlates with its ability to prolong pRb hyperphosphorylation, which temporarily inhibits the expression of MyoD transcriptional targets, until p21 has accumulated above a threshold; the pRb kinase activity of cyclin D3/cdk4 is then repressed, which permits permanent G0 arrest and the ensuing terminal differentiation. In this regard, we noticed that differentiating C2 myoblasts expressing ectopic cyclin D3(T283A) display higher levels of p21 and cdk4 than control myoblasts. As expected, the ectopic expression of a stabilized cyclin D3 double mutant without the ability to bind pRb did not alter the normal timing of pRb dephosphorylation and G0 arrest during C2 myoblast differentiation. This mutant revealed a differentiation-promoting effect of cyclin D3, as it could significantly enhance late, muscle-specific gene expression in postmitotic myocytes. Altogether, the above observations indicate that an overexpression of cyclin D3 in predifferentiation myoblasts increases the number of cells progressing toward terminal differentiation by temporarily delaying muscle-specific expression, whereas an enhanced accumulation of cyclin D3 in postmitotic myocytes appears to stimulate their terminal differentiation.
In striking contrast to cyclin D3, an overexpression of cyclin D1 has been demonstrated to inhibit muscle gene expression through both pRb-dependent and pRb-independent mechanisms (53, 54, 61). This underscores important functional differences between the two proteins.
In the present study, we also provide evidence that cyclin D3 is required for myogenesis, as we show that MyoD-transduced MEFs from cyclin D3-null mice fail to undergo terminal differentiation. Indeed, cyclin D3 deficiency does not appear to affect the expression of early differentiation markers such as myogenin but strongly inhibits the high-level expression of late differentiation markers and myotube formation. In addition, and in agreement with the increase of p21 and cdk4 levels noticed in myoblasts expressing cyclin D3(T283A), cyclin D3-deficient MEFs express reduced levels of p21 and cdk4 compared with the levels in wild-type MEFs; such decreases, however, do not appear to be specific to muscle differentiation, as they were observed both in the absence and presence of MyoD. Furthermore, the lower levels of p21 cannot account for the aberrant differentiation of cyclin D3-null cells, as p21−/− fibroblasts have been shown to undergo normal MyoD-induced myogenesis (18). In any case, these observations reveal the existence of a mechanism linking the expression of cyclin D3 to that of the cdk4 and p21 partners.
Several pieces of evidence accumulated by us and by others concur to indicate that cyclin D3 is implicated in the establishment of muscle cell terminal differentiation. Cyclin D3 is induced by MyoD at the onset of myoblast differentiation (6) and is highly expressed during the late stages of mouse muscle development and early postnatal life (3). Cyclin D3 promoter activity is stimulated during insulin-dependent myoblast differentiation through phospholipase C signaling (16). In terminally differentiated myotubes, cyclin D3 is recruited into insoluble nuclear structures by forming kinase-inactive complexes with cdk4, p21, and PCNA and mediating their interaction with unphosphorylated pRb (6). Further, cyclin D3 brings about changes in intranuclear lamin A/C organization in differentiating myogenic cells with the requirement of pRb (34). In this paper, we identify cyclin D3 as a downstream target of pRb regulation, as we demonstrate that pRb stabilizes cyclin D3 protein during terminal muscle cell differentiation. Furthermore, we demonstrate for the first time that cyclin D3 is required for proper myogenic differentiation. Importantly, the aberrant myogenic phenotype observed in MyoD-converted, cyclin D3-null MEFs, i.e., greatly reduced expression of late, muscle-specific genes, strikingly overlaps that of pRb-deficient myoblasts, which strongly suggests that cyclin D3 takes part in the differentiation-promoting function of pRb.
Cyclin D3 knockout mice are viable and display focal defects in the maturation of specific myelolymphocyte precursors (9, 59, 60). No overt abnormalities were reported in skeletal muscle; our observations, however, raise the possibility that cyclin D3 may play an essential role in the developmental program of adult muscle cell precursors.
Acknowledgments
This work was supported by Telethon (grant 1247), MIUR-FIRB (progetto RBAU01PCRL), MIUR-CNR (progetto Oncologia), and in part by Filas-Regione Lazio.
We thank P. Sicinski, C. J. Sherr, J. DeCaprio, D. H. Livingston, W. Kaelin, V. Sartorelli, B. Amati, G. Falcone, M. Crescenzi, and M. Doria for the generous gift of plasmids, viruses, and cell lines.
Footnotes
Published ahead of print on 20 August 2007.
We dedicate this work to the memory of Cesare Vesco.
REFERENCES
- 1.Alessi, D. R., M. Andjelkovic, B. Caudwell, P. Cron, N. Morrice, P. Cohen, and B. A. Hemmings. 1996. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15:6541-6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Alt, J. R., J. L. Cleveland, M. Hannink, and J. A. Diehl. 2000. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 14:3102-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartkova, J., J. Lukas, M. Strauss, and J. Bartek. 1998. Cyclin D3: requirement for G1/S transition and high abundance in quiescent tissues suggest a dual role in proliferation and differentiation. Oncogene 17:1027-1037. [DOI] [PubMed] [Google Scholar]
- 4.Berkes, C. A., and S. J. Tapscott. 2005. MyoD and the transcriptional control of myogenesis. Semin. Cell Dev. Biol. 16:585-595. [DOI] [PubMed] [Google Scholar]
- 5.Camarda, G., F. Siepi, D. Pajalunga, C. Bernardini, R. Rossi, A. Montecucco, E. Meccia, and M. Crescenzi. 2004. A pRb-independent mechanism preserves the postmitotic state in terminally differentiated skeletal muscle cells. J. Cell Biol. 167:417-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cenciarelli, C., F. De Santa, P. L. Puri, E. Mattei, L. Ricci, F. Bucci, A. Felsani, and M. Caruso. 1999. Critical role played by cyclin D3 in the MyoD-mediated arrest of cell cycle during myoblast differentiation. Mol. Cell. Biol. 19:5203-5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, M. W., E. Barr, J. Seltzer, Y. Q. Jiang, G. J. Nabel, E. G. Nabel, M. S. Parmacek, and J. M. Leiden. 1995. Cytostatic gene therapy for vascular proliferative disorders with a constitutively active form of the retinoblastoma gene product. Science 267:518-522. [DOI] [PubMed] [Google Scholar]
- 8.Coolican, S. A., D. S. Samuel, D. Z. Ewton, F. J. McWade, and J. R. Florini. 1997. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J. Biol. Chem. 272:6653-6662. [DOI] [PubMed] [Google Scholar]
- 9.Cooper, A. B., C. M. Sawai, E. Sicinska, S. E. Powers, P. Sicinski, M. R. Clark, and I. Aifantis. 2006. A unique function for cyclin D3 in early B cell development. Nat. Immunol. 7:489-497. [DOI] [PubMed] [Google Scholar]
- 10.Cross, D. A., D. R. Alessi, P. Cohen, M. Andjelkovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785-789. [DOI] [PubMed] [Google Scholar]
- 11.Diehl, J. A., M. Cheng, M. F. Roussel, and C. J. Sherr. 1998. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 12:3499-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diehl, J. A., F. Zindy, and C. J. Sherr. 1997. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 11:957-972. [DOI] [PubMed] [Google Scholar]
- 13.Doble, B. W., and J. R. Woodgett. 2003. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116:1175-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowdy, S. F., P. W. Hinds, K. Louie, S. I. Reed, A. Arnold, and R. A. Weinberg. 1993. Physical interaction of the retinoblastoma protein with human D cyclins. Cell 73:499-511. [DOI] [PubMed] [Google Scholar]
- 15.Ewen, M. E., H. K. Sluss, C. J. Sherr, H. Matsushime, J. Kato, and D. M. Livingston. 1993. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell 73:487-497. [DOI] [PubMed] [Google Scholar]
- 16.Faenza, I., G. Ramazzotti, A. Bavelloni, R. Fiume, G. C. Gaboardi, M. Y. Follo, R. S. Gilmour, A. M. Martelli, K. Ravid, and L. Cocco. 2007. Inositide-dependent phospholipase C signaling mimics insulin in skeletal muscle differentiation by affecting specific regions of the cyclin D3 promoter. Endocrinology 148:1108-1117. [DOI] [PubMed] [Google Scholar]
- 17.Favreau, C., D. Higuet, J. C. Courvalin, and B. Buendia. 2004. Expression of a mutant lamin A that causes Emery-Dreifuss muscular dystrophy inhibits in vitro differentiation of C2C12 myoblasts. Mol. Cell. Biol. 24:1481-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Figliola, R., and R. Maione. 2004. MyoD induces the expression of p57Kip2 in cells lacking p21Cip1/Waf1: overlapping and distinct functions of the two cdk inhibitors. J. Cell Physiol. 200:468-475. [DOI] [PubMed] [Google Scholar]
- 19.Frock, R. L., B. A. Kudlow, A. M. Evans, S. A. Jameson, S. D. Hauschka, and B. K. Kennedy. 2006. Lamin A/C and emerin are critical for skeletal muscle satellite cell differentiation. Genes Dev. 20:486-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halevy, O., B. G. Novitch, D. B. Spicer, S. X. Skapek, J. Rhee, G. J. Hannon, D. Beach, and A. B. Lassar. 1995. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science 267:1018-1021. [DOI] [PubMed] [Google Scholar]
- 21.Harbour, J. W., and D. C. Dean. 2000. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 14:2393-2409. [DOI] [PubMed] [Google Scholar]
- 22.Huh, M. S., M. H. Parker, A. Scime, R. Parks, and M. A. Rudnicki. 2004. Rb is required for progression through myogenic differentiation but not maintenance of terminal differentiation. J. Cell Biol. 166:865-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang, B. H., M. Aoki, J. Z. Zheng, J. Li, and P. K. Vogt. 1999. Myogenic signaling of phosphatidylinositol 3-kinase requires the serine-threonine kinase Akt/protein kinase B. Proc. Natl. Acad. Sci. USA 96:2077-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson, B. R., R. T. Nitta, R. L. Frock, L. Mounkes, D. A. Barbie, C. L. Stewart, E. Harlow, and B. K. Kennedy. 2004. A-type lamins regulate retinoblastoma protein function by promoting subnuclear localization and preventing proteasomal degradation. Proc. Natl. Acad. Sci. USA 101:9677-9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaelin, W. G. J., D. C. Pallas, J. A. DeCaprio, F. J. Kaye, and D. M. Livingston. 1991. Identification of cellular proteins that can interact specifically with the T/E1A-binding region of the retinoblastoma gene product. Cell 64:521-532. [DOI] [PubMed] [Google Scholar]
- 26.Kato, J., H. Matsushime, S. W. Hiebert, M. E. Ewen, and C. J. Sherr. 1993. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 7:331-342. [DOI] [PubMed] [Google Scholar]
- 27.Kiess, M., R. Montgomery Gill, and P. A. Hamel. 1995. Expression of the positive regulator of cell cycle progression, cyclin D3, is induced during differentiation of myoblasts into quiescent myotubes. Oncogene 10:159-166. [PubMed] [Google Scholar]
- 28.Kitzmann, M., and A. Fernandez. 2001. Crosstalk between cell cycle regulators and the myogenic factor MyoD in skeletal myoblasts. Cell. Mol. Life Sci. 58:571-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, K. Y., M. H. Ladha, C. McMahon, and M. E. Ewen. 1999. The retinoblastoma protein is linked to the activation of Ras. Mol. Cell. Biol. 19:7724-7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magenta, A., C. Cenciarelli, F. De Santa, P. Fuschi, F. Martelli, M. Caruso, and A. Felsani. 2003. MyoD stimulates RB promoter activity via the CREB/p300 nuclear transduction pathway. Mol. Cell. Biol. 23:2893-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maione, R., and P. Amati. 1997. Interdependence between muscle differentiation and cell-cycle control. Biochim. Biophys. Acta Rev. Cancer 1332:M19-M30. [DOI] [PubMed] [Google Scholar]
- 32.Mal, A., D. Chattopadhyay, M. K. Ghosh, R. Y. Poon, T. Hunter, and M. L. Harter. 2000. p21 and retinoblastoma protein control the absence of DNA replication in terminally differentiated muscle cells. J. Cell Biol. 149:281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mancini, M. A., B. Shan, J. A. Nickerson, S. Penman, and W. H. Lee. 1994. The retinoblastoma gene product is a cell cycle-dependent, nuclear matrix-associated protein. Proc. Natl. Acad. Sci. USA 91:418-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mariappan, I., and V. K. Parnaik. 2005. Sequestration of pRb by cyclin D3 causes intranuclear reorganization of lamin A/C during muscle cell differentiation. Mol. Biol. Cell 16:1948-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markiewicz, E., T. Dechat, R. Foisner, R. A. Quinlan, and C. J. Hutchison. 2002. Lamin A/C binding protein LAP2alpha is required for nuclear anchorage of retinoblastoma protein. Mol. Biol. Cell 13:4401-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Markiewicz, E., M. Ledran, and C. J. Hutchison. 2005. Remodelling of the nuclear lamina and nucleoskeleton is required for skeletal muscle differentiation in vitro. J. Cell Sci. 118:409-420. [DOI] [PubMed] [Google Scholar]
- 37.Martelli, F., C. Cenciarelli, G. Santarelli, B. Polikar, A. Felsani, and M. Caruso. 1994. MyoD induces retinoblastoma gene expression during myogenic differentiation. Oncogene 9:3579-3590. [PubMed] [Google Scholar]
- 38.Matsushime, H., M. F. Roussel, R. A. Ashmun, and C. J. Sherr. 1991. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell 65:701-713. [DOI] [PubMed] [Google Scholar]
- 39.Mittnacht, S., and R. A. Weinberg. 1991. G1/S phosphorylation of the retinoblastoma protein is associated with an altered affinity for the nuclear compartment. Cell 65:381-393. [DOI] [PubMed] [Google Scholar]
- 40.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muralikrishna, B., J. Dhawan, N. Rangaraj, and V. K. Parnaik. 2001. Distinct changes in intranuclear lamin A/C organization during myoblast differentiation. J. Cell Sci. 114:4001-4011. [DOI] [PubMed] [Google Scholar]
- 42.Murry, C. E., M. A. Kay, T. Bartosek, S. D. Hauschka, and S. M. Schwartz. 1996. Muscle differentiation during repair of myocardial necrosis in rats via gene transfer with MyoD. J. Clin. Investig. 98:2209-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naviglio, S., C. Mattecucci, B. Matoskova, T. Nagase, N. Nomura, P. P. Di Fiore, and G. F. Draetta. 1998. UBPY: a growth-regulated human ubiquitin isopeptidase. EMBO J. 17:3241-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naya, F. J., and E. Olson. 1999. MEF2: a transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation. Curr. Opin. Cell Biol. 11:683-688. [DOI] [PubMed] [Google Scholar]
- 45.Novitch, B. G., G. J. Mulligan, T. Jacks, and A. B. Lassar. 1996. Skeletal muscle cells lacking the retinoblastoma protein display defects in muscle gene expression and accumulate in S and G2 phases of the cell cycle. J. Cell Biol. 135:441-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Novitch, B. G., D. B. Spicer, P. S. Kim, W. L. Cheung, and A. B. Lassar. 1999. pRb is required for MEF2-dependent gene expression as well as cell-cycle arrest during skeletal muscle differentiation. Curr. Biol. 9:449-459. [DOI] [PubMed] [Google Scholar]
- 47.Ozaki, T., M. Saijo, K. Murakami, H. Enomoto, Y. Taya, and S. Sakiyama. 1994. Complex formation between lamin A and the retinoblastoma gene product: identification of the domain on lamin A required for its interaction. Oncogene 9:2649-2653. [PubMed] [Google Scholar]
- 48.Parks, R. J., J. L. Bramson, Y. Wan, C. L. Addison, and F. L. Graham. 1999. Effects of stuffer DNA on transgene expression from helper-dependent adenovirus vectors. J. Virol. 73:8027-8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pear, W. S., G. P. Nolan, M. L. Scott, and D. Baltimore. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90:8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puri, P. L., S. Iezzi, P. Stiegler, T. T. Chen, R. L. Schiltz, G. E. Muscat, A. Giordano, L. Kedes, J. Y. Wang, and V. Sartorelli. 2001. Class I histone deacetylases sequentially interact with MyoD and pRb during skeletal myogenesis. Mol. Cell 8:885-897. [DOI] [PubMed] [Google Scholar]
- 51.Puri, P. L., and V. Sartorelli. 2000. Regulation of muscle regulatory factors by DNA-binding, interacting proteins, and post-transcriptional modifications. J. Cell. Physiol. 185:155-173. [DOI] [PubMed] [Google Scholar]
- 52.Qin, X. Q., T. Chittenden, D. M. Livingston, and W. G. J. Kaelin. 1992. Identification of a growth suppression domain within the retinoblastoma gene product. Genes Dev. 6:953-964. [DOI] [PubMed] [Google Scholar]
- 53.Rao, S. S., C. Chu, and D. S. Kohtz. 1994. Ectopic expression of cyclin D1 prevents activation of gene transcription by myogenic basic helix-loop-helix regulators. Mol. Cell. Biol. 14:5259-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rao, S. S., and D. S. Kohtz. 1995. Positive and negative regulation of D-type cyclin expression in skeletal myoblasts by basic fibroblast growth factor and transforming growth factor beta. A role for cyclin D1 in control of myoblast differentiation. J. Biol. Chem. 270:4093-4100. [DOI] [PubMed] [Google Scholar]
- 55.Rochat, A., A. Fernandez, M. Vandromme, J. P. Moles, T. Bouschet, G. Carnac, and N. J. Lamb. 2004. Insulin and wnt1 pathways cooperate to induce reserve cell activation in differentiation and myotube hypertrophy. Mol. Biol. Cell 15:4544-4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rommel, C., S. C. Bodine, B. A. Clarke, R. Rossman, L. Nunez, T. N. Stitt, G. D. Yancopoulos, and D. J. Glass. 2001. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 3:1009-1013. [DOI] [PubMed] [Google Scholar]
- 57.Sarruf, D. A., I. Iankova, A. Abella, S. Assou, S. Miard, and L. Fajas. 2005. Cyclin D3 promotes adipogenesis through activation of peroxisome proliferator-activated receptor gamma. Mol. Cell. Biol. 25:9985-9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schneider, J. W., W. Gu, L. Zhu, V. Mahdavi, and B. Nadal-Ginard. 1994. Reversal of terminal differentiation mediated by p107 in Rb−/− muscle cells. Science 264:1467-1471. [DOI] [PubMed] [Google Scholar]
- 59.Sicinska, E., I. Aifantis, L. Le Cam, W. Swat, C. Borowski, Q. Yu, A. A. Ferrando, S. D. Levin, Y. Geng, H. von Boehmer, and P. Sicinski. 2003. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell 4:451-461. [DOI] [PubMed] [Google Scholar]
- 60.Sicinska, E., Y. M. Lee, J. Gits, H. Shigematsu, Q. Yu, V. I. Rebel, Y. Geng, C. J. Marshall, K. Akashi, D. M. Dorfman, I. P. Touw, and P. Sicinski. 2006. Essential role for cyclin D3 in granulocyte colony-stimulating factor-driven expansion of neutrophil granulocytes. Mol. Cell. Biol. 26:8052-8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skapek, S. X., J. Rhee, P. S. Kim, B. G. Novitch, and A. B. Lassar. 1996. Cyclin-mediated inhibition of muscle gene expression via a mechanism that is independent of pRB hyperphosphorylation. Mol. Cell. Biol. 16:7043-7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takahashi, C., R. T. Bronson, M. Socolovsky, B. Contreras, K. Y. Lee, T. Jacks, M. Noda, R. Kucherlapati, and M. E. Ewen. 2003. Rb and N-ras function together to control differentiation in the mouse. Mol. Cell. Biol. 23:5256-5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thorburn, A. M., P. A. Walton, and J. R. Feramisco. 1993. MyoD induced cell cycle arrest is associated with increased nuclear affinity of the Rb protein. Mol. Biol. Cell 4:705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Todaro, G. J., and H. Green. 1963. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 17:299-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tureckova, J., E. M. Wilson, J. L. Cappalonga, and P. Rotwein. 2001. Insulin-like growth factor-mediated muscle differentiation: collaboration between phosphatidylinositol 3-kinase-Akt-signaling pathways and myogenin. J. Biol. Chem. 276:39264-39270. [DOI] [PubMed] [Google Scholar]
- 66.Vandromme, M., A. Rochat, R. Meier, G. Carnac, D. Besser, B. A. Hemmings, A. Fernandez, and N. J. Lamb. 2001. Protein kinase B beta/Akt2 plays a specific role in muscle differentiation. J. Biol. Chem. 276:8173-8179. [DOI] [PubMed] [Google Scholar]
- 67.Vyas, D. R., E. E. Spangenburg, T. W. Abraha, T. E. Childs, and F. W. Booth. 2002. GSK-3beta negatively regulates skeletal myotube hypertrophy. Am. J. Physiol. Cell Physiol. 283:C545-C551. [DOI] [PubMed] [Google Scholar]
- 68.Walsh, K., and H. Perlman. 1997. Cell cycle exit upon myogenic differentiation. Curr. Opin. Genet. Dev. 7:597-602. [DOI] [PubMed] [Google Scholar]
- 69.Wang, G. L., X. Shi, E. Salisbury, Y. Sun, J. H. Albrecht, R. G. Smith, and N. A. Timchenko. 2006. Cyclin D3 maintains growth-inhibitory activity of C/EBPα by stabilizing C/EBPα-cdk2 and C/EBPα-Brm complexes. Mol. Cell. Biol. 26:2570-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weintraub, H., S. J. Tapscott, R. L. Davis, M. J. Thayer, M. A. Adam, A. B. Lassar, and A. D. Miller. 1989. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc. Natl. Acad. Sci. USA 86:5434-5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zalvide, J., and J. A. DeCaprio. 1995. Role of pRb-related proteins in simian virus 40 large-T-antigen-mediated transformation. Mol. Cell. Biol. 15:5800-5810. [DOI] [PMC free article] [PubMed] [Google Scholar]