Abstract
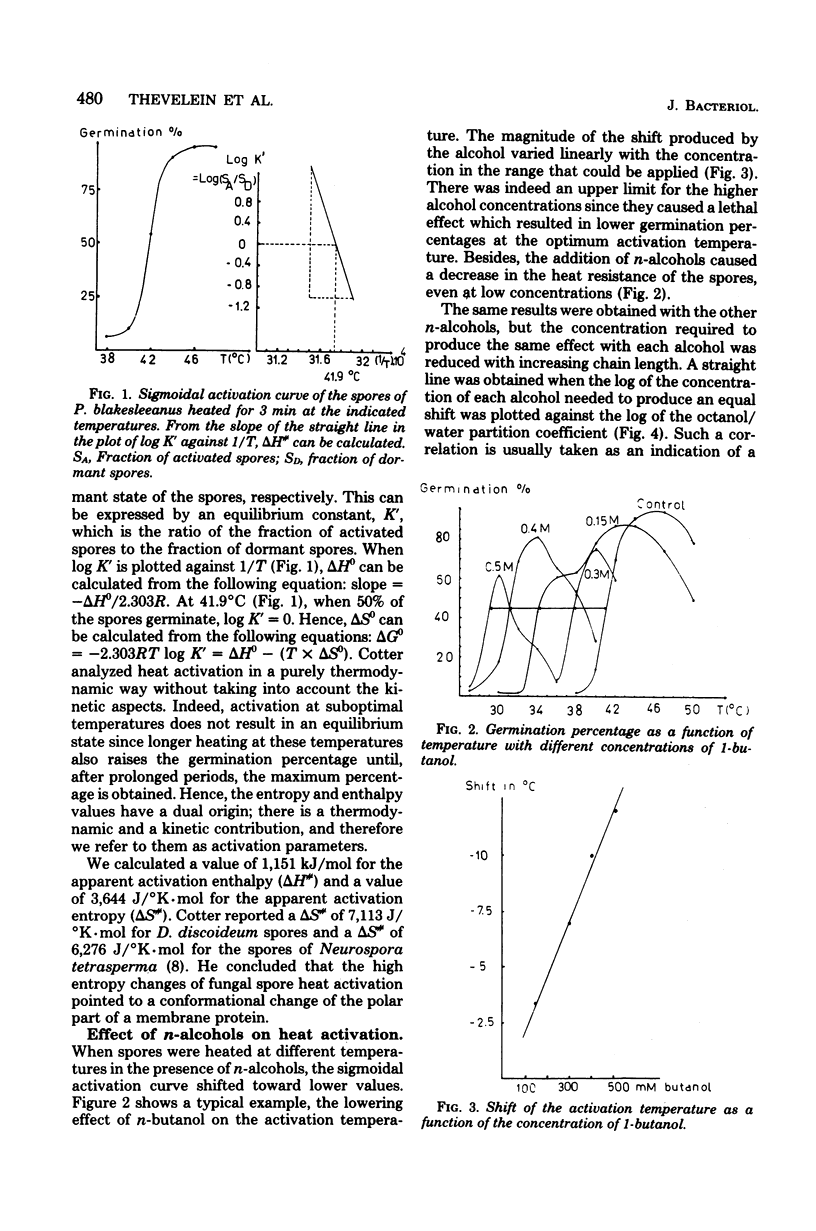
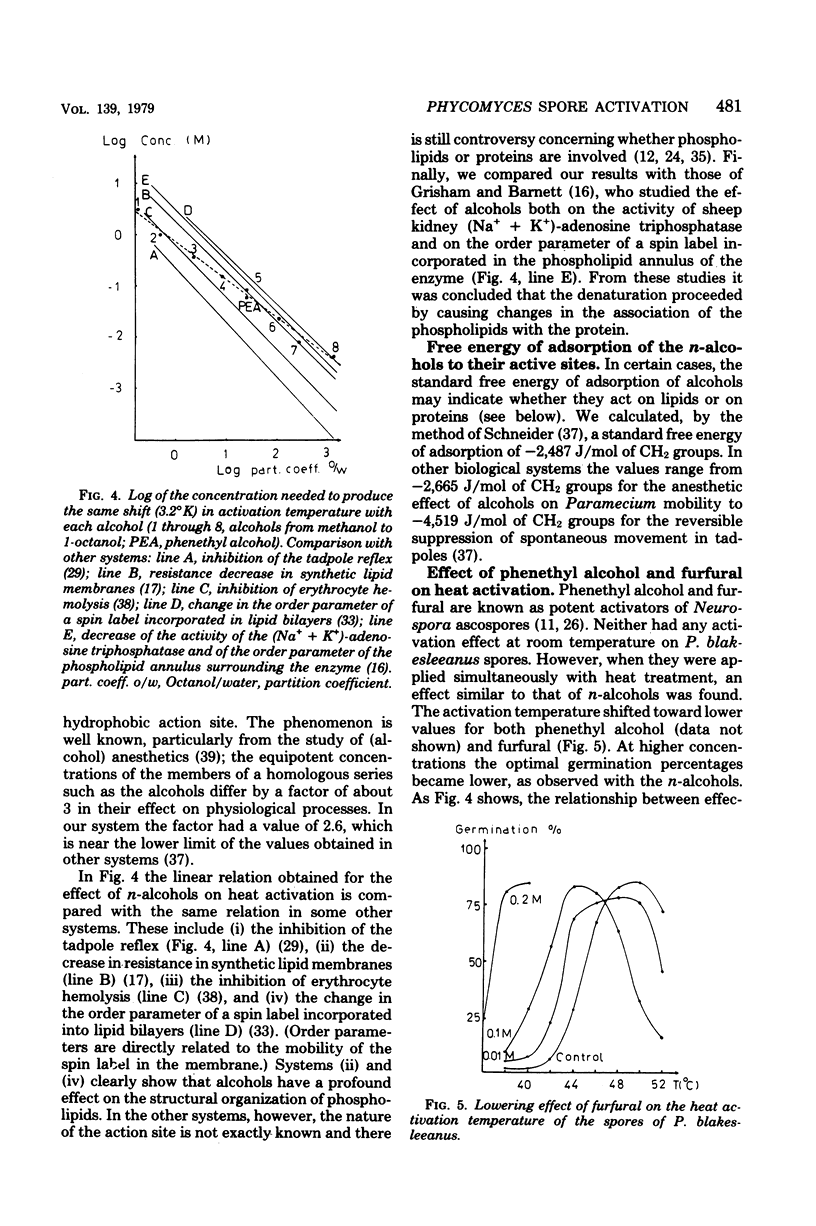
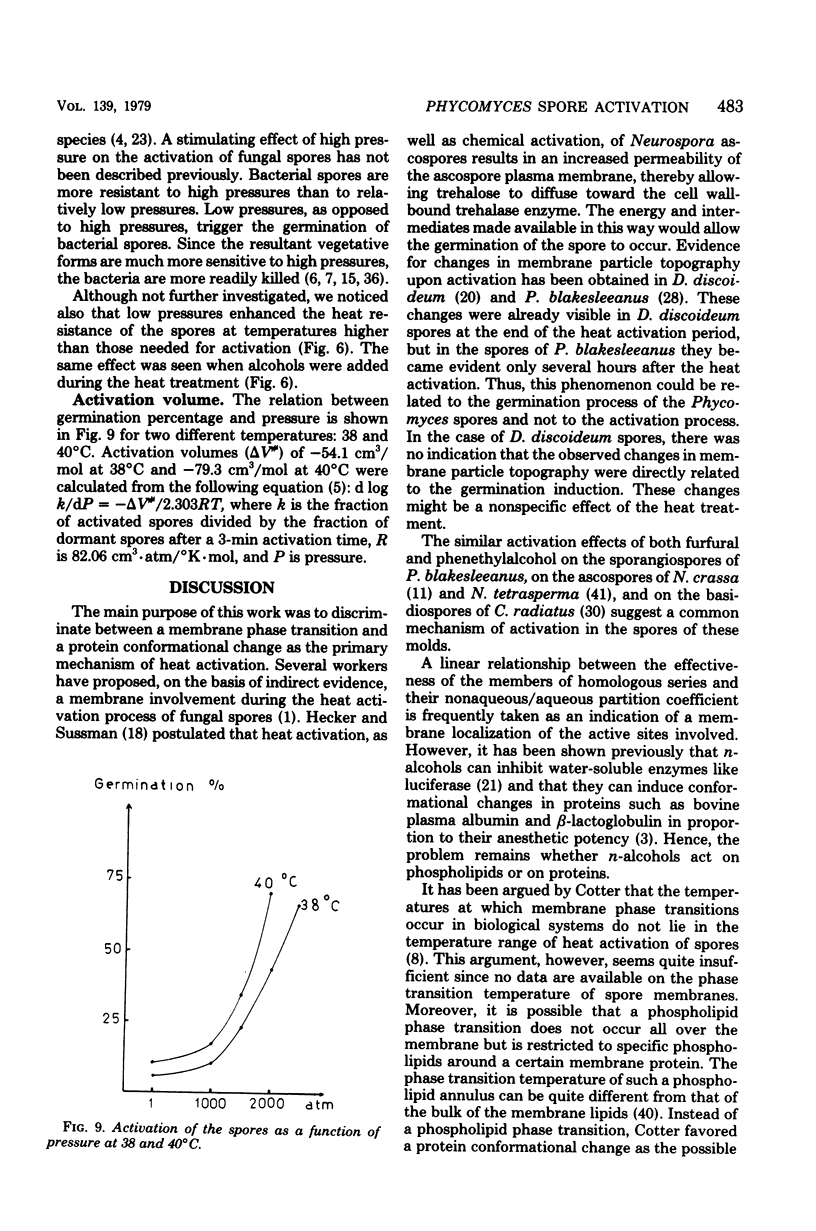
The thermodynamic parameters for the heat activation of the sporangiospores of Phycomyces blakesleeanus were determined. For the apparent activation enthalpy (ΔH#) a value of 1,151 kJ/mol was found, whereas a value of 3,644 J./°K·mol was calculated for the apparent activation entropy (ΔS#). n-Alcohols (from methanol to octanol), phenethyl alcohol, and furfural lowered the activation temperature of P. blakesleeanus spores. The heat resistance of the spores was lowered concomitantly. The effect of the alcohols was a linear function of the concentration in the range that could be applied. When the log of the concentration needed to produce an equal shift of the activation temperature was plotted for each alochol against the log of the octanol/water partition coefficient, a straight line was obtained. The free energy of adsorption of the n-alcohols to their active sites was calculated to be −2,487 J/mol of CH2 groups. Although still inconclusive, this points toward an involvement of protein in the activation process. The effect of phenethyl alcohol was similar to the effect of n-alcohols, but furfural produced a greater shift than would be expected from the value of its partition coefficient. When the heat activation of the spores was performed under high pressure, the activation temperature was raised by 2 to 4°K/1,000 atm. However, with pressures higher than 1,000 atm (1.013 × 105 kPa) the activation temperature was lowered until the pressure became lethal (more than 2,500 atm). It is known that membrane phase transition temperatures are shifted upward by about 20°K/1,000 atm and that protein conformational changes are shifted upward by 2 to 6°K/1,000 atm. Consequently, heat activation of fungal spores seems to be triggered by a protein conformational change and not by a membrane phase transition. Activation volumes of −54.1 cm3/mol at 38°C and −79.3 cm2/mol at 40°C were found for the lowering effect of high pressure on the heat activation temperature.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balasubramanian D., Wetlaufer D. B. Reversible alteration of the structure of globular proteins by anesthetic agents. Proc Natl Acad Sci U S A. 1966 Apr;55(4):762–765. doi: 10.1073/pnas.55.4.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceuterick F., Peeters J., Heremans K., De Smedt H., Olbrechts H. Effect of high pressure, detergents and phospholipase on the break in the Arrhenius plot of Azotobacter nitrogenase. Eur J Biochem. 1978 Jun 15;87(2):401–407. doi: 10.1111/j.1432-1033.1978.tb12389.x. [DOI] [PubMed] [Google Scholar]
- Clouston J. G., Wills P. A. Initiation of germination and inactivation of Bacillus pumilus spores by hydrostatic pressure. J Bacteriol. 1969 Feb;97(2):684–690. doi: 10.1128/jb.97.2.684-690.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouston J. G., Wills P. A. Kinetics of initiation of germination of Bacillus pumilus spores by hydrostatic pressure. J Bacteriol. 1970 Jul;103(1):140–143. doi: 10.1128/jb.103.1.140-143.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D. A. Spore germination in Dictyostelium discoideum. I. The thermodynamics of reversible activation. J Theor Biol. 1973 Sep 14;41(1):41–51. doi: 10.1016/0022-5193(73)90188-4. [DOI] [PubMed] [Google Scholar]
- Emerson M. R. Chemical Activation of Ascospore Germination in Neurospora crassa. J Bacteriol. 1948 Mar;55(3):327–330. doi: 10.1128/jb.55.3.327-330.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Where do general anaesthetics act? Nature. 1978 Jul 27;274(5669):339–342. doi: 10.1038/274339a0. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Sale A. J. Initiation of germination of bacterial spores by hydrostatic pressure. J Gen Microbiol. 1970 Mar;60(3):335–346. doi: 10.1099/00221287-60-3-335. [DOI] [PubMed] [Google Scholar]
- Grisham C. M., Barnett R. E. The effects of long-chain alcohols on membrane lipids and the (Na++K+)-ATPase. Biochim Biophys Acta. 1973 Jul 6;311(3):417–422. doi: 10.1016/0005-2736(73)90322-2. [DOI] [PubMed] [Google Scholar]
- Gutknecht J., Tosteson D. C. Ionic peremability of thin lipid membranes. Effects of n-alkyl alcohols, polyvalent cations, and a secondary amine. J Gen Physiol. 1970 Mar;55(3):359–374. doi: 10.1085/jgp.55.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker L. I., Sussman A. S. Localization of trehalase in the ascospores of Neurospora: relation to ascospore dormancy and germination. J Bacteriol. 1973 Aug;115(2):592–599. doi: 10.1128/jb.115.2.592-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl H. R., Bühlmann M., Wehrli E. Plasma membrane alterations as a result of heat activation in Dictyostelium spores. Arch Microbiol. 1978 Mar;116(3):239–244. doi: 10.1007/BF00417846. [DOI] [PubMed] [Google Scholar]
- Lee A. G. Interactions between anesthetics and lipid mixtures. Normal alcohols. Biochemistry. 1976 Jun 1;15(11):2448–2454. doi: 10.1021/bi00656a031. [DOI] [PubMed] [Google Scholar]
- Lingappa B. T., Lingappa Y., Turian G. Phenethyl alcohol induced germination of ascospores of Neurospora. Arch Mikrobiol. 1970;72(2):97–105. doi: 10.1007/BF00409515. [DOI] [PubMed] [Google Scholar]
- Macdonald A. G. A dilatometric investigation of the effects of general anaesthetics, alcohols and hydrostatic pressure on the phase transition in smectic mesophases of dipalmitoyl phosphatidylcholine. Biochim Biophys Acta. 1978 Feb 2;507(1):26–37. doi: 10.1016/0005-2736(78)90371-1. [DOI] [PubMed] [Google Scholar]
- Malhotra S. K., Tewari J. P. Molecular alterations in the plasma membrane of sporangiospores of Phycomyces related to germination. Proc R Soc Lond B Biol Sci. 1973 Nov 27;184(1075):207–216. doi: 10.1098/rspb.1973.0044. [DOI] [PubMed] [Google Scholar]
- Murphy R. B., Libby W. F. Inhibition of erythrocyte phosphate transport by high pressures. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2767–2769. doi: 10.1073/pnas.73.8.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell W. G., Wills P. A. Initiation of Bacillus spore germination by hydrostatic pressure: effect of temperature. J Bacteriol. 1977 Mar;129(3):1272–1280. doi: 10.1128/jb.129.3.1272-1280.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson S. J., Butler K. W., Huang P., Labelle J., Smith I. C., Schneider H. The effects of alcohols on lipid bilayers: a spin label study. Biochim Biophys Acta. 1972 Jun 20;266(3):597–602. doi: 10.1016/0006-3002(72)90003-0. [DOI] [PubMed] [Google Scholar]
- Péqueux A., Gilles R. Effects of high hydrostatic pressures on the activity of the membrane ATPases of some organs implicated in hydromineral regulation. Comp Biochem Physiol B. 1978;59(3):207–212. doi: 10.1016/0305-0491(78)90248-1. [DOI] [PubMed] [Google Scholar]
- Richards C. D., Martin K., Gregory S., Keightley C. A., Hesketh T. R., Smith G. A., Warren G. B., Metcalfe J. C. Degenerate perturbations of protein structure as the mechanism of anaesthetic action. Nature. 1978 Dec 21;276(5690):775–779. doi: 10.1038/276775a0. [DOI] [PubMed] [Google Scholar]
- Sale A. J., Gould G. W., Hamilton W. A. Inactivation of bacterial spores by hydrostatic pressure. J Gen Microbiol. 1970 Mar;60(3):323–334. doi: 10.1099/00221287-60-3-323. [DOI] [PubMed] [Google Scholar]
- Schneider H. The intramembrane location of alcohol anesthetics. Biochim Biophys Acta. 1968 Dec 10;163(4):451–458. doi: 10.1016/0005-2736(68)90074-6. [DOI] [PubMed] [Google Scholar]
- Seeman P. Erythrocyte membrane stabilization by steroids and alcohols; a possible model for anesthesia. Biochem Pharmacol. 1966 Oct;15(10):1632–1637. doi: 10.1016/0006-2952(66)90214-0. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Stier A., Sackmann E. Spin labels as enzyme substrates. Heterogeneous lipid distribution in liver microsomal membranes. Biochim Biophys Acta. 1973 Jul 6;311(3):400–408. doi: 10.1016/0005-2736(73)90320-9. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Taniguchi Y. Effect of pressure on biopolymers and model systems. Symp Soc Exp Biol. 1972;26:103–124. [PubMed] [Google Scholar]
- Von Hippel P. H., Wong K. Y. On the conformational stability of globular proteins. The effects of various electrolytes and nonelectrolytes on the thermal ribonuclease transition. J Biol Chem. 1965 Oct;240(10):3909–3923. [PubMed] [Google Scholar]
- Wattiaux-De Coninck S., Dubois F., Wattiaux R. Lateral phase separations and structural integrity of the inner membrane of rat-liver mitochondria. Effect of compression. Implications in the centrifugation of these organelles. Biochim Biophys Acta. 1977 Dec 15;471(3):421–435. doi: 10.1016/0005-2736(77)90047-5. [DOI] [PubMed] [Google Scholar]


