Abstract
In the mother cell of sporulating Bacillus subtilis, a regulatory network functions to control gene expression. Four transcription factors act sequentially in the order σE, SpoIIID, σK, GerE. σE and σK direct RNA polymerase to transcribe different regulons. SpoIIID and GerE are DNA-binding proteins that activate or repress transcription of many genes. Several negative regulatory loops add complexity to the network. First, transcriptionally active σK RNA polymerase inhibits early sporulation gene expression, resulting in reduced accumulation of σE and SpoIIID late during sporulation. Second, GerE represses sigK transcription, reducing σK accumulation about twofold. Third, SpoIIID represses cotC, which encodes a spore coat protein, delaying its transcription by σK RNA polymerase. Partially circumventing the first feedback loop, by engineering cells to maintain the SpoIIID level late during sporulation, causes spore defects. Here, the effects of circumventing the second feedback loop, by mutating the GerE binding sites in the sigK promoter region, are reported. Accumulation of pro-σK and σK was increased, but no spore defects were detected. Expression of σK-dependent reporter fusions was altered, increasing the expression of gerE-lacZ and cotC-lacZ and decreasing the expression of cotD-lacZ. Because these effects on gene expression were opposite those observed when the SpoIIID level was maintained late during sporulation, cells were engineered to both maintain the SpoIIID level and have elevated sigK expression late during sporulation. This restored the expression of σK-dependent reporters to wild-type levels, and no spore defects were observed. Hence, circumventing the second feedback loop suppressed the effects of perturbing the first feedback loop. By feeding information back into the network, these two loops appear to optimize target gene expression and increase network robustness. Circumventing the third regulatory loop, by engineering cells to express cotC about 2 h earlier than normal, did not cause a detectable spore defect.
When starved, the gram-positive bacterium Bacillus subtilis initiates a process called sporulation in order to form a dormant spore (for reviews, see references 8 and 23). Sporulation is a complex developmental process in which morphological changes are coupled to temporal and spatial regulation of gene expression. An early morphological change is the formation of an asymmetrically positioned septum that divides the cell into a larger mother cell (MC) compartment and a smaller forespore (FS) compartment. Completion of DNA replication ensures that a copy of the chromosome is available for each cell type. Distinct temporal programs of gene expression occur in the MC and FS, but signaling pathways between the two cell types coordinate the programs and morphogenesis. The MC engulfs the FS, pinching it off as a protoplast within the MC. Cortex, a modified peptidoglycan, is synthesized between the two membranes that surround the FS after engulfment. Proteins assemble on the surface of the FS, producing the coat. Eventually, the mature spore is released by lysis of the MC. The spore is resistant to harsh conditions, such as high temperature and exposure to UV light, lytic enzymes, and chemicals. The spore germinates in the presence of nutrients and grows vegetatively.
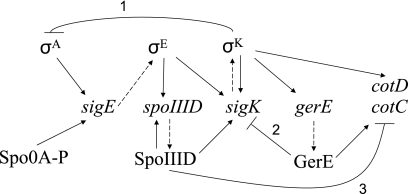
In the MC, a regulatory network controls gene expression during sporulation (for a review, see reference 23). The backbone of the network consists of a cascade of four transcription factors (σE, SpoIIID, σK, and GerE) (43), but several regulatory loops are also present in the network (Fig. 1). Synthesis of σE requires σA RNA polymerase (RNAP), the major form of RNAP in growing cells, and phosphorylated Spo0A (Spo0A-P), a response regulator that governs initiation of sporulation (for a review, see reference 15). The initial product of sigE is inactive pro-σE. It is cleaved to form active σE in response to a signal from the FS (18, 22, 27). σE RNAP transcribes many genes, including spoIIID and sigK (Fig. 1), as well as other genes whose products contribute to engulfment and synthesis of the spore cortex and coat (10, 11, 34). SpoIIID is a DNA-binding protein that activates transcription of sigK by σE RNAP and σK RNAP (13, 24) (Fig. 1). SpoIIID also positively regulates at least seven other transcription units and negatively regulates at least 62 transcription units in the σE regulon (10). In addition, SpoIIID negatively regulates certain genes in the σK regulon, such as cotC and cotD (14, 20, 24) (Fig. 1), which encode spore coat proteins (7). Like SpoIIID, GerR is a transcription factor under σE control, but it is not shown in Fig. 1 since it is not known to affect expression of other transcription factors in the cascade, although it does negatively regulate 10 transcription units in the σE regulon (10). σK, like σE, is first made as an inactive precursor protein that is cleaved in response to a signal from the FS (4, 28). σK RNAP transcribes many genes, including gerE (Fig. 1) and other genes whose products contribute to synthesis of the spore cortex and coat (10, 34). GerE is a DNA-binding protein that activates transcription of certain cot genes, such as cotC and cotD (19, 20, 42), and has an effect opposite that of SpoIIID (Fig. 1). In addition, GerE positively regulates at least 25 other transcription units in the σK regulon and negatively regulates at least 36 transcription units (10), including sigK (19, 42) (Fig. 1).
FIG. 1.
Gene regulatory network in the MC during sporulation of B. subtilis. The dashed arrows indicate gene-protein relationships. The solid arrows and the lines with bars at their ends indicate positive and negative regulation, respectively. The numbers 1, 2, and 3 indicate three negative regulatory loops. See the text for an explanation and references.
Three negative regulatory loops in the MC network are numbered in Fig. 1. Loop 1 involves transcriptionally active σK RNAP inhibition of early gene expression under σA control by an unknown mechanism, resulting in reduced accumulation of σE and SpoIIID late during sporulation (12, 39, 40). Loop 2 involves GerE repression of sigK transcription, lowering the σK level about twofold (19). Loop 3 involves SpoIIID repression of cotC transcription, delaying its expression by at least 1 h compared with other genes under positive control of σK RNAP and GerE (20).
How important are the three negative regulatory loops for sporulation? To begin to address this question, we previously engineered B. subtilis to circumvent the decrease in the level of SpoIIID late during sporulation, which is normally brought about by loop 1 (37). We found that maintaining the SpoIIID level late during sporulation resulted in altered expression of σK-dependent genes, lower numbers of resistant spores, and a structural defect in the coat of most spores that were produced. Here, we report the effect of mutating the GerE binding site in the sigK promoter, which eliminated loop 2 (Fig. 1). As expected, the levels of pro-σK and σK were increased during sporulation. This did not cause detectable spore defects. However, expression of σK-dependent genes was altered, and the effects were opposite those observed when the SpoIIID level was maintained late during sporulation. Interestingly, elimination of GerE negative feedback for sigK transcription suppressed the spore defects caused by maintaining the SpoIIID level late during sporulation. Also, expression of σK-dependent genes in a strain with both perturbations was restored to wild-type levels. We also report that expression of cotC approximately 2 h earlier than normal (circumventing loop 3) did not cause detectable spore defects. Taken together, these results suggest that loops 2 and 3 are less important for sporulation than loop 1 but that loops 1 and 2 exert opposing effects, fine-tuning the expression of target genes in order to optimize spore formation.
MATERIALS AND METHODS
Site-directed mutagenesis and construction of plasmids.
A plasmid bearing the sigK promoter (starting at position −108) and the entire coding region was constructed in several steps. First, pRG2 (32) was digested with BamHI and annealed oligonucleotides (5′-GATCCCACCACCACCACCACCACTAA-3′ and 5′-GATCTTAGTGGTGGTGGTGGTGGTGG-3′) were inserted in the orientation that preserved the BamHI site at the 3′ end of sigK, generating pHP1. Second, a QuikChange site-directed mutagenesis kit (Stratagene) was used with primers 5′-GGCTTTTGCCTACAAGCTTTTGTGGAGGTGACG-3′ and 5′- CGTCACCTCCACAAAAGCTTGTAGGCAAAAGCC-3′ (mutant nucleotides are underlined) and pHP1 as the template to introduce a HindIII site between the sigK promoter and the ribosome binding site, generating pHP12, for which the sigK sequence was verified. Third, site-directed mutagenesis was likewise performed with primers 5′-CCCGAAAAGTGCCACCTGGTGTCTAAGAAACC-3′ and 5′-GGTTTCTTAGACACCAGGTGGCACTTTTCGGG-3′ and with pDG364 as the template to eliminate its AatII site, generating pHP13. Finally, the sigK-containing EcoRI-BamHI fragment from pHP12 was gel purified and ligated into EcoRI-BamHI-digested pHP13, generating pHP14, which due to the BamHI site and vector added four codons (specifying the amino acids GSPA) to the 3′ end of sigK. This sigK allele was flanked by the 5′ and 3′ ends of amyE, and it complemented a sigK mutant when it replaced the normal amyE gene in the B. subtilis chromosome (see below). Plasmid pHP14 was the template for site-directed mutagenesis designed to eliminate the GerE binding site in the sigK promoter region. Four nucleotide changes, GG to CC at positions 4 and 5 and CC to GG at positions 12 and 13, were made using primers 5′-CCGGTCACATACATTTACATATACCCTTTTGGGTACATACTTTTGTGGAGG-3′ and 5′-CCTCCACAAAAGTATGTACCCAAAAGGGTATATGTAAATGTATGTGACCGG-3′. The resulting allele in pJP16 was designated sigKmut and was sequenced to confirm that only the desired mutations were present.
In order to express cotC earlier than normal, we used the gerE promoter to drive its expression. A DNA fragment containing cotC and spanning the region from slightly upstream of its ribosome binding site to slightly downstream of its stop codon was synthesized by performing PCR with primers 5′-CGAAGCTTTAAAGGAGGAGTATATATGGGTTATTAC-3′ and 5′-GCGGATCCACCCGGCAATAGCCGGG-3′, which contained HindIII and BamHI restriction sites (underlined), respectively, and with chromosomal DNA from B. subtilis PY79 as the template. The PCR product was digested with HindIII and BamHI and ligated with HindIII-BamHI-digested pJP1 (37), generating pJP4. The sequence of the entire PgerE-cotC fusion was determined to ensure that no errors occurred during the PCR.
Bacterial strains.
Escherichia coli strain AG115 [araD139 Δ(ara,leu)7697 ΔlacX74 galU galK hsr hsm+ strA (F′ proAB lacIqZ::Tn5)] was obtained from A. Grossman (Massachusetts Institute of Technology). It was used during construction and maintenance of plasmids. Luria-Bertani (LB) medium (33) was used to grow E. coli and B. subtilis and was supplemented with appropriate antibiotics. B. subtilis strains used in this study are listed in Table 1. Plasmid pJP4 was transformed into PY79 with selection on LB agar containing kanamycin sulfate (5 μg/ml) to generate strain BJP3. Plasmids pHP14 and pJP16 bearing wild-type sigK and the sigKmut allele, respectively, were transformed into BK556 with selection on LB agar containing chloramphenicol (5 μg/ml). Strains in which amyE was replaced by sigK (BLW1) or sigKmut (BLW2) were identified as described previously (6). pJP1 (a multicopy plasmid with a PgerE-spoIIID fusion) (37) was transformed into BLW1 and BLW2 with selection on LB agar containing chloramphenicol (5 μg/ml) and kanamycin sulfate (5 μg/ml) to generate strains BLW3 and BLW4, respectively. The chloramphenicol resistance (Cmr) gene of BLW1, BLW2, BLW3, and BLW4 was replaced with the spectinomycin resistance (Spr) gene of pCm::Sp as described previously (35), generating strains BLW5, BLW6, BLW7, and BLW8, respectively. A lysate containing SPβ::cotC-lacZ was obtained by heat induction of strain OR825 as described previously (6). SPβ::cotD-lacZ and SPβ::gerE-lacZ have been described previously (4, 5). Specialized transduction was used to move lacZ fusions into BLW5, BLW6, BLW7, and BLW8 as described previously (16). Transductants were selected on LB agar containing chloramphenicol (5 μg/ml). In each case, at least 10 transductants were transferred onto a DSM agar (16) plate with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (40 μg/ml). Three or more isolates with average blue color were saved for further analysis, excluding occasional isolates with abnormally high or low β-galactosidase activity.
TABLE 1.
B. subtilis strains used
| Strain | Phenotype, genotype, and/or derivation | Reference or source |
|---|---|---|
| PY79 | Spo+ prototroph | 38 |
| OR825 | SPβ::cotC-lacZ Cmr | 3 |
| EUDC9901 | trpC2 pheA1 gerE::kan Kmr | 2 |
| BK556 | spoIVCB23 | 26 |
| BD071 | cotC::cat Cmr | 7 |
| BJP1 | PY79 transformed with pJP1(PgerE-spoIIID), Kmr | 37 |
| BJP2 | PY79 transformed with pJP2(PgerE), Kmr | 37 |
| BJP3 | PY79 transformed with pJP4(PgerE-cotC), Kmr | This study |
| BLW1 | BK556 transformed with pHP6(amyE::sigK), Cmr | This study |
| BLW2 | BK556 transformed with pJP16(amyE::sigKmut), Cmr | This study |
| BLW3 | BLW1 transformed with pJP1, Kmr Cmr | This study |
| BLW4 | BLW2 transformed with pJP1, Kmr Cmr | This study |
| BLW5 | BLW1 transformed with pCm::Sp, Spr | This study |
| BLW6 | BLW2 transformed with pCm::Sp, Spr | This study |
| BLW7 | BLW3 transformed with pCm::Sp, Kmr Spr | This study |
| BLW8 | BLW4 transformed with pCm::Sp, Kmr Spr | This study |
Cell growth and sporulation.
Sporulation was induced by resuspension of cells in SM medium as described previously (16). The time of resuspension was defined as the onset of sporulation (zero time).
Western blot analysis.
Starting at 3 h into sporulation and at hourly intervals thereafter until 9 h into sporulation, 0.5-ml samples were subjected to centrifugation (14,000 × g for 1 min), the supernatants were removed, and the cell pellets were stored at −70°C. Preparation of whole-cell extracts, electrophoresis, and electroblotting were performed as described previously (12, 28). The blots were probed with anti-SpoIIID (12), anti-pro-σK (28), or anti-CotC (21) antibody diluted 1:10,000. Immunodetection of primary antibodies was performed as described previously (25).
Analysis of β-galactosidase activity.
Samples were collected during sporulation as described above. Cell pellets were stored at −70°C prior to the assay. Cells were resuspended and then treated with lysozyme and permeabilized by using toluene as described previously (30). The β-galactosidase specific activity was determined as described previously (30) using o-nitrophenol-β-d-galactopyranoside as the substrate. One unit of the enzyme hydrolyzed 1 μmol of substrate per min per unit of initial culture optical density at 595 nm.
Spore purification and germination and resistance assays.
Spores were harvested at 24 h into sporulation by centrifugation at 7,000 × g for 10 min, washed with 4°C water once, and stored at 4°C overnight. The next day, spores were purified on a step gradient consisting of 20 to 50% RenoCal-76 (Bracco Diagnostics Inc.) as described previously (17). The purity of the spores was verified by microscopy. The germination assay was performed with purified spores using l-alanine (10 mM) as the germinant as described previously (31). Assays to determine resistance to heat, lysozyme, and organic solvents were performed at 24 h into sporulation without spore purification, as described previously (16).
Transmission electron microscopy of spores.
For transmission electron microscopy spores were harvested at 24 h into sporulation, washed with water, and immediately fixed as described previously (29).
RESULTS
Engineering B. subtilis to circumvent GerE repression of sigK.
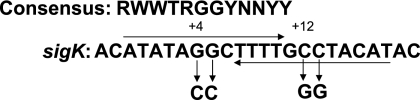
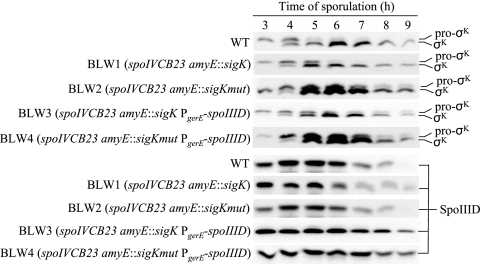
GerE binds to a site overlapping the sigK transcriptional start site and represses transcription about twofold, lowering the level of pro-σK and σK about twofold (19). Within the GerE binding site are two sequences that match the consensus sequence for GerE binding (Fig. 2). To assess the importance of sigK repression by GerE, we mutated two nucleotides in each match to the consensus (Fig. 2). A previous study showed that changing GG to TT near the center of the GerE binding site in the cotH promoter region impaired GerE repression of cotH transcription (1). We changed GG at positions 4 and 5 to CC and CC at positions 12 and 13 to GG in the sigK promoter region (Fig. 2). The resulting mutant allele of sigK, including the promoter and the entire coding region, was integrated at the amyE locus of spoIVCB23 mutant B. subtilis BK556, creating BLW2 (spoIVCB23 amyE::sigKmut). The spoIVCB23 mutation prevents production of pro-σK and σK (28) from the native sigK locus, which is created in the MC during sporulation by site-specific recombination between the spoIVCB and spoIIIC genes (36). As a control, a wild-type sigK allele was integrated at the amyE locus of the spoIVCB23 mutant, creating BLW1 (spoIVCB23 amyE::sigK). BLW1 and BLW2 were grown and induced to sporulate along with B. subtilis wild-type strain PY79 as an additional control. Samples were collected during sporulation, and whole-cell extracts were subjected to Western blot analysis to detect pro-σK and σK. The levels of pro-σK and σK were higher in the spoIVCB23 amyE::sigKmut strain than in either control strain, starting at 5 h into sporulation (Fig. 3). The pro-σK and σK levels were similar in the two control strains. The effect of the sigKmut allele on sigK expression was similar to the effect of a gerE null mutation (19), suggesting that GerE repression of sigK is eliminated in BLW2.
FIG. 2.
Mutation in the GerE binding site in the sigK promoter region. The top line shows the consensus sequence for GerE binding (41) (R = A or G; W = A or T; Y = C or T; N = A, G, C, or T). The bottom line shows the sequence in the sigK promoter region where GerE binds, as defined by DNase I footprinting (19). The numbering is relative to the transcriptional start site. The rightward arrow indicates a perfect match to the consensus. The leftward arrow indicates a match at 9 of 12 positions to the consensus on the strand not shown. The downward arrows show the four changes in the sigKmut allele.
FIG. 3.
Levels of pro-σK and σK and levels of SpoIIID during sporulation. B. subtilis wild-type strain PY79 (WT) and the other strains indicated were induced to sporulate by resuspension in SM medium. Samples were collected at hourly intervals beginning at 3 h after the onset of sporulation. Equal volumes (5 μl) of whole-cell extracts were fractionated on sodium dodecyl sulfate-14% polyacrylamide gels and subjected to Western blot analysis with anti-pro-σK or anti-SpoIIID serum.
Effects of circumventing GerE repression of sigK.
Because σK acts after SpoIIID in the MC gene regulatory network (Fig. 1), we did not expect the SpoIIID level during sporulation to be altered in the spoIVCB23 amyE::sigKmut strain. Indeed, the level was indistinguishable from that in the wild type and the spoIVCB23 amyE::sigK strain (Fig. 3).
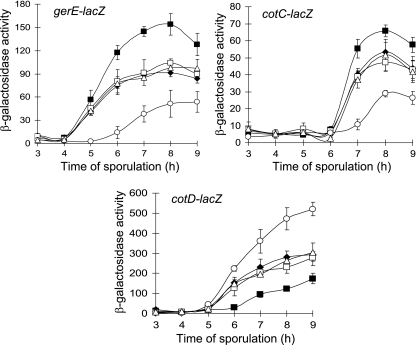
To test whether expression of σK-dependent genes was affected by the elevated level of σK in BLW2, we measured β-galactosidase activity from lacZ fusions to gerE, cotC, and cotD during sporulation. In order to introduce lacZ fusions with associated Cmr markers into strains BLW2 and BLW1, we replaced their Cmr cassettes with Spr cassettes, creating BLW6 and BLW5, respectively. Expression from gerE-lacZ and cotC-lacZ was elevated in BLW6 relative to the BLW5 and PY79 control strains (Fig. 4). Apparently, elevated σK levels in sporulating cells can increase transcription of σK-dependent genes. Interestingly, expression from cotD-lacZ was reduced in BLW6 relative to the control strains (Fig. 4). This might have been due to an elevated level of GerE since a high level of GerE represses cotD transcription by σK RNAP in vitro (19).
FIG. 4.
Effects on gene expression. β-Galactosidase activity during sporulation after resuspension in SM medium was measured for B. subtilis containing the indicated lacZ fusions in wild-type strain PY79 (▵), BLW5 (spoIVCB23 amyE::sigK) (⧫), BLW6 (spoIVCB23 amyE::sigKmut) (▪), BLW7 (spoIVCB23 amyE::sigK PgerE-spoIIID) (○), and BLW8 (spoIVCB23 amyE::sigKmut PgerE-spoIIID) (□). Each symbol indicates the average of three determinations, and the error bars indicate one standard deviation.
Circumventing GerE repression of sigK compensates for persistent spoIIID expression.
The effects on expression of the three σK-dependent reporters (Fig. 4) were the opposite of the effects observed previously when cells were engineered to maintain the SpoIIID level late during sporulation (37). In the previous study, SpoIIID was expressed ectopically from a multicopy plasmid bearing a PgerE-spoIIID fusion (pJP1). Introduction of multicopy PgerE-spoIIID into the spoIVCB23 amyE::sigKmut background (creating BLW4 initially; then the Cmr cassette was replaced with an Spr cassette to create BLW8 for introduction of lacZ fusions [Table 1]) restored expression of gerE-lacZ, cotC-lacZ, and cotD-lacZ to the levels observed in the wild-type PY79 and spoIVCB23 amyE::sigK (BLW5-derived) control strains (Fig. 4). In contrast, introduction of multicopy PgerE-spoIIID into the spoIVCB23 amyE::sigK background (creating BLW3 initially; then the Cmr cassette was replaced with an Spr cassette to create BLW7 for introduction of lacZ fusions [Table 1]) decreased expression of gerE-lacZ and cotC-lacZ and increased expression of cotD-lacZ (Fig. 4), similar to the effects of multicopy PgerE-spoIIID in an otherwise wild-type background (37). As expected, both BLW3 and BLW4 exhibited a higher level of SpoIIID late during sporulation than strains without PgerE-spoIIID (Fig. 3). Also as expected, the levels of pro-σK and σK were higher starting at 5 h into sporulation in BLW4 than in strains without amyE::sigKmut (Fig. 3). An elevated level of σK in BLW4 appears to compensate for an elevated level of SpoIIID, restoring gerE, cotC, and cotD expression to wild-type levels.
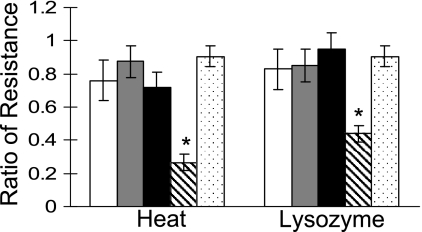
Does circumventing GerE repression of sigK with the sigKmut allele compensate for the other effects of persistent spoIIID expression from PgerE-spoIIID, which include defects in spore resistance properties and coat assembly (37)? Figure 5 shows that BLW4 produced heat- and lysozyme-resistant spores as efficiently as the wild type. These spores germinated normally in response to l-alanine (see Fig. S1 in the supplemental material) and were indistinguishable from wild-type strain PY79 spores when they were examined by thin-section transmission electron microscopy (data not shown). On the basis of the criteria that we tested, elevated levels of σK in BLW4 appeared to fully suppress the sporulation defects caused by maintaining the SpoIIID level during the late stages.
FIG. 5.
Resistance properties of spores. The ratio of the number of CFU after the indicated treatments to the number of CFU before the treatments was determined for B. subtilis wild-type strain PY79 (open bars), BLW1 (spoIVCB23 amyE::sigK) (gray bars), BLW2 (spoIVCB23 amyE::sigKmut) (black bars), BLW3 (spoIVCB23 amyE::sigK PgerE-spoIIID) (striped bars), and BLW4 (spoIVCB23 amyE::sigKmut PgerE-spoIIID) (dotted bars) at 24 h after resuspension in SM medium. The bars indicate the averages of three determinations, and the error bars indicate one standard deviation. An asterisk indicates that the P value is <0.05 for a comparison of BLW3 with PY79 or BLW4.
In contrast to strain BLW4 and as expected from our previous study (37), strain BLW3, in which the SpoIIID level was maintained late during sporulation without an elevated level of σK, produced significantly fewer heat- and lysozyme-resistant spores (Fig. 5). Also, these spores germinated normally (see Fig. S1 in the supplemental material), but about 90% showed a defect in coat assembly when they were examined by electron microscopy (data not shown). The inner and outer coat layers were thinner and less organized than those of wild-type spores, and the coat was not as closely apposed to the cortex and its ridges were less evident, as seen previously for strain BJP1 (containing multicopy PgerE-spoIIID in an otherwise wild-type background) (37).
Despite elevated σK levels (Fig. 3) and altered expression of σK-dependent genes (Fig. 4) in strain BLW2, this strain produced heat- and lysozyme-resistant spores as efficiently as the control strain BLW1 and wild-type strain PY79 (Fig. 5). The spores of all three strains germinated similarly (see Fig. S1 in the supplemental material) and were indistinguishable when they were examined by electron microscopy (data not shown).
We also measured the ability of strains BLW1, BLW2, BLW3, and BLW4 to produce spores resistant to phenol, ethanol, and chloroform. None of the strains differed significantly (i.e., yielded a P value of <0.05 as determined by a Student t test) from wild-type strain PY79 (data not shown).
Effects of circumventing the delay in CotC production.
Expression of cotC-lacZ is delayed by 2 h relative to gerE-lacZ expression during sporulation (Fig. 4). To determine whether this delay is important for sporulation, we fused cotC to the gerE promoter in a plasmid (pJP4) that can be maintained in B. subtilis in multiple copies. The plasmid was transformed into B. subtilis wild-type strain PY79, creating BJP4 with multicopy PgerE-cotC. BJP2 (containing PgerE without cotC in the PY79 background) (37) served as a control. Western blot analysis of whole-cell extracts from sporulating cells showed that in BJP4, CotC accumulates to a low level at 2 to 4 h into sporulation, perhaps due to readthrough transcription from a constitutive promoter on the plasmid, and the level of CotC rises beginning at 5 h into sporulation, presumably due to transcription from PgerE (see Fig. S2 in the supplemental material). As expected, the CotC level began to rise about 2 h later, at 7 h into sporulation, in BJP2 and PY79. Taken together, these results demonstrate that multicopy PgerE-cotC in BJP4 circumvents the 2-h delay in CotC accumulation.
We measured the ability of strains BJP4 and BJP2 to produce spores resistant to heat, lysozyme, phenol, ethanol, and chloroform. Neither strain differed significantly (i.e., yielded a P value of <0.05 as determined by a Student t test) from wild-type strain PY79 (data not shown). Also, the germination kinetics in response to l-alanine were similar for spores produced by strains BJP4 and BJP2 compared to spores produced by PY79 (data not shown). We concluded that accumulating CotC 2 h earlier than normal did not detectably alter spore resistance or germination properties.
DISCUSSION
Our study resulted in two novel findings about loop 2 in the MC gene regulatory network (Fig. 1). First, circumvention of GerE repression of sigK elevated the σK level and probably the GerE level (since expression of a gerE-lacZ translational fusion increased), but this did not detectably alter spore resistance, germination, or structure. This revealed robustness in the network. Second, circumvention of loop 2 suppressed the effects on sporulation of persistent spoIIID expression. This suggests that negative regulatory loops with opposing effects enhance network robustness and presumably optimize target gene expression.
The results of a previous study aided our effort to circumvent GerE repression of sigK. Initially, we deleted nucleotides from position 5 to position 15 relative to the sigK transcriptional start site, but this did not result in elevated pro-σK and σK levels (data not shown), perhaps due to interference with promoter utilization by RNAP. After learning that a mutation in the GG sequence within the GerE binding site in the cotH promoter region increased cotH-lacZ expression (1), we made the sigKmut allele shown in Fig. 2. This allele resulted in levels of pro-σK and σK accumulation similar to those observed previously in a gerE null mutant, which accumulated about twofold more total pro-σK/σK than the wild type (19).
What limits the total pro-σK/σK accumulation to about twofold more than that in the wild type? SpoIIID activates sigK transcription by σE RNAP (13) or σK RNAP (24). Late in sporulation, the SpoIIID and σE levels decrease due to negative feedback by σK RNAP (12, 39, 40). This suggested that SpoIIID might become limiting for sigK transcription by σK RNAP; however, this is not the case because introduction of PgerE-spoIIID into the spoIVCB23 amyE::sigKmut background did not change the pro-σK or σK level (Fig. 3). Perhaps processing of pro-σK to active σK or degradation of σK limits accumulation of SigK products under these conditions. Such posttranslational mechanisms would contribute to network robustness by limiting sigK autoregulation in the event that negative regulatory loops (i.e., GerE repression and loss of the SpoIIID activator) that operate at the transcriptional level fail.
The elevated σK level due to the sigKmut allele did not detectably alter the final product of the MC gene regulatory network, the spores. Elevated σK levels did not significantly alter the number of heat-, lysozyme-, phenol-, ethanol-, or chloroform-resistant spores produced (Fig. 5 and data not shown), nor did they alter the spore germination kinetics in response to l-alanine, as measured by changes in optical density (see Fig. S1 in the supplemental material). Moreover, we detected no difference in spore structure compared to that of the wild type upon examination of thin sections by electron microscopy. On the basis of these criteria, the network is robust in terms of its final output (the spore) when the σK level is elevated. On the other hand, expression of all three σK-dependent genes tested was altered (Fig. 4). Increased expression of gerE and cotC can be understood in terms of the dependence of these genes on σK RNAP and, in the case of cotC, activation by GerE (42). Transcription of cotD also depends on σK RNAP (24), but a high level of GerE represses cotD transcription in vitro (19), so elevated GerE levels in the strain with elevated σK levels might account for the observed decrease in cotD expression (Fig. 4). Based on the findings for these three genes, it seems likely that expression of many of the 108 other genes in the σK regulon (10) is altered by elevated σK levels. In terms of gene expression, the network seems quite susceptible to perturbation of loop 2 (Fig. 1). This provides one rationale for retention of loop 2 during evolution; mutations in loop 2 components may subtly alter MC gene expression, optimizing it for a particular ecological niche.
A second rationale for evolutionary retention of loop 2 is its ability to suppress sporulation defects caused by persistent spoIIID expression. Preliminary results suggest that transposon insertion mutations in several genes elevate expression of an spoIIID-lacZ fusion (L. Wang and L. Kroos, unpublished data). Hence, the MC gene regulatory network appears to be quite susceptible to mutational perturbation leading to elevated SpoIIID levels, which can cause spore defects (37). We show here that mutating the GerE binding site in the sigK promoter elevates the σK level and probably the GerE level (since expression of a gerE-lacZ translational fusion increased), compensating for persistent spoIIID expression by restoring MC gene expression (including expression of gerE-lacZ) and formation of spores with normal resistance properties and coat structure. Undoubtedly, other mutations could elevate the σK level as well, but loop 2 increases network robustness by providing additional targets for mutations that can compensate for changes in the SpoIIID level.
Is GerE repression of sigK (loop 2) likely present in sporeformers related to B. subtilis? A search for orthologs of MC transcription factors revealed that σK is present in Bacillus and Clostridium species, but GerE is absent from Clostridium (10). Among Bacillus species, we searched for a GerE binding site in the sigK promoter region. As shown in Fig. S3A in the supplemental material, Bacillus licheniformis differed from B. subtilis at only one position, which did not affect either match to the GerE consensus binding sequence (Fig. 2). However, most strains of Bacillus cereus, Bacillus weihenstephanensis, all strains of Bacillus anthracis, and one strain of Bacillus thuringiensis have two changes that create mismatches to the TRGGY core of the GerE consensus binding sequence (see Fig. S3B in the supplemental material). One of these changes is a G-to-A transition at a position predicted to interact with Lys41 in B. subtilis GerE (9). This Lys residue is perfectly conserved among GerE homologs of the organisms shown in Fig. S3 in the supplemental material. Moreover, the strains in Fig. S3B in the supplemental material do not exhibit a second match to the GerE consensus binding sequence, as B. subtilis does (Fig. 2). Therefore, GerE is likely to bind more weakly, if at all, to the corresponding position in the sigK promoter region of the strains in Fig. S3B in the supplemental material. For the strains in Fig. S3C in the supplemental material, it seems even less likely that GerE represses sigK transcription. On the other hand, the strains in Fig. S3D in the supplemental material retain more characteristics of the GerE binding site in the B. subtilis sigK promoter region, suggesting that GerE represses sigK transcription in these species. We speculate that about one-half of the distinct species shown in Fig. S3 in the supplemental material have loop 2 in their MC gene regulatory network.
Elevated σK levels in the B. subtilis sigKmut strain did not detectably hasten the decrease in the level of SpoIIID during sporulation (Fig. 3). We infer from this result that σK is not the rate-limiting factor in the regulatory loop (loop 1 in Fig. 1) by which σK RNAP leads to a decrease in the SpoIIID level (12, 39, 40). As depicted in Fig. 1, the evidence suggests that one or more genes transcribed by σK RNAP inhibit the activity of σA RNAP, decreasing transcription of sigE and other early genes, including spoIIID, but the mechanism of inhibition remains a mystery.
Expression of cotC 2 h earlier than normal from the heterologous gerE promoter did not detectably alter spore resistance or germination properties. Why, then, is cotC expression normally delayed? Perhaps repression of cotC by SpoIIID prevents wasteful expression before CotC can assemble into the spore coat. CotH is required for assembly of CotC into the outer coat (21, 31). The cotH gene is expressed by 5 h into sporulation (1) and CotH accumulates by 6 h (44), but earlier times have not been examined. Under our conditions, cotC-lacZ was expressed by 7 h into sporulation (Fig. 4), and CotC began to accumulate at that time (see Fig. S2 in the supplemental material). Earlier expression of cotC from PgerE-cotC on a multicopy plasmid may be inconsequential because CotH availability limits CotC assembly. Interestingly, GerE represses cotH expression, but circumventing this negative regulatory loop by mutating the GerE binding site in the cotH promoter region had no detectable effect on spore structure or function, although it did allow accumulation of CotC in the MC (1). Normally, CotC does not accumulate in the MC, probably because it assembles immediately into the spore coat (21). In cells with GerE-independent expression of cotH, an elevated CotH level appears to stabilize CotC in the MC, suggesting that the spore has a limited capacity to incorporate CotC (1).
In summary, circumvention of GerE repression of sigK elevated the σK level and probably the GerE level, two nodes in the MC gene regulatory network, likely altering expression of many genes in the σK regulon but not detectably changing spore resistance, germination, or structure. However, this perturbation compensated for the effects of persistent spoIIID expression, which also appears to alter two nodes in the network; the SpoIIID level is maintained late during sporulation (when it normally decreases), and the GerE level is probably reduced (since expression of a gerE-lacZ translational fusion is diminished) (37). We infer that the decrease in SpoIIID normally brought about, at least in part, by loop 1 (Fig. 1) promotes gerE expression. In contrast, loop 2 normally inhibits further gerE expression because less σK RNAP is produced. It appears that two negative feedback loops with opposing effects on gerE expression and different effects on other nodes (loop 1 reduces the σE and SpoIIID levels [12, 39, 40], whereas loop 2 reduces the σK level [19] [Fig. 3]) enhance the robustness of the MC network and optimize the expression of target genes.
Supplementary Material
Acknowledgments
We thank H. Prince for constructing pHP14 and R. Losick, A. Grossman, and C. Moran for providing bacterial strains. We also thank E. Ricca for communicating results prior to publication and for providing anti-CotC antibodies.
This work was supported by NIH grant GM43585 and by the Michigan Agricultural Experiment Station.
Footnotes
Published ahead of print on 21 September 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Baccigalupi, L., G. Castaldo, G. Cangiano, R. Isticato, R. Marasco, M. De Felice, and E. Ricca. 2004. GerE-independent expression of cotH leads to CotC accumulation in the mother cell compartment during Bacillus subtilis sporulation. Microbiology 150:3441-3449. [DOI] [PubMed] [Google Scholar]
- 2.Crater, D. L., and C. P. Moran, Jr. 2002. Two regions of GerE required for promoter activation in Bacillus subtilis. J. Bacteriol. 184:241-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crater, D. L., K. H. Wade, O. Resnekov, H. T. Ichikawa, L. Kroos, J. A. Brannigan, and C. P. Moran, Jr. 2002. A mutation in GerE that affects cotC promoter activation in Bacillus subtilis. Biochim. Biophys. Acta 1576:30-38. [DOI] [PubMed] [Google Scholar]
- 4.Cutting, S., V. Oke, A. Driks, R. Losick, S. Lu, and L. Kroos. 1990. A forespore checkpoint for mother-cell gene expression during development in Bacillus subtilis. Cell 62:239-250. [DOI] [PubMed] [Google Scholar]
- 5.Cutting, S., S. Panzer, and R. Losick. 1989. Regulatory studies on the promoter for a gene governing synthesis and assembly of the spore coat in Bacillus subtilis. J. Mol. Biol. 207:393-404. [DOI] [PubMed] [Google Scholar]
- 6.Cutting, S. M., and P. B. V. Horn. 1990. Genetic analysis, p. 27-74. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, New York, NY.
- 7.Donovan, W., L. Zheng, K. Sandman, and R. Losick. 1987. Genes encoding spore coat polypeptides from Bacillus subtilis. J. Mol. Biol. 196:1-10. [DOI] [PubMed] [Google Scholar]
- 8.Driks, A. 2002. Maximum shields: the assembly and function of the bacterial spore coat. Trends Microbiol. 10:251-254. [DOI] [PubMed] [Google Scholar]
- 9.Ducros, V. M., R. J. Lewis, C. S. Verma, E. J. Dodson, G. Leonard, J. P. Turkenburg, G. N. Murshudov, A. J. Wilkinson, and J. A. Brannigan. 2001. Crystal structure of GerE, the ultimate transcriptional regulator of spore formation in Bacillus subtilis. J. Mol. Biol. 306:759-771. [DOI] [PubMed] [Google Scholar]
- 10.Eichenberger, P., M. Fujita, S. T. Jensen, E. M. Conlon, D. Z. Rudner, S. T. Wang, C. Ferguson, K. Haga, T. Sato, J. S. Liu, and R. Losick. 2004. The program of gene transcription for a single differentiating cell type during sporulation in Bacillus subtilis. PLoS Biol. 2:1664-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feucht, A., L. Evans, and J. Errington. 2003. Identification of sporulation genes by genome-wide analysis of the σE regulon of Bacillus subtilis. Microbiology 149:3023-3034. [DOI] [PubMed] [Google Scholar]
- 12.Halberg, R., and L. Kroos. 1992. Fate of the SpoIIID switch protein during Bacillus subtilis sporulation depends on the mother-cell sigma factor, σK. J. Mol. Biol. 228:840-849. [DOI] [PubMed] [Google Scholar]
- 13.Halberg, R., and L. Kroos. 1994. Sporulation regulatory protein SpoIIID from Bacillus subtilis activates and represses transcription by both mother-cell-specific forms of RNA polymerase. J. Mol. Biol. 243:425-436. [DOI] [PubMed] [Google Scholar]
- 14.Halberg, R., V. Oke, and L. Kroos. 1995. Effects of Bacillus subtilis sporulation regulatory protein SpoIIID on transctiption by σK RNA polymerase in vivo and in vitro. J. Bacteriol. 177:1888-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haldenwang, W. G. 1995. The sigma factors of Bacillus subtilis. Microbiol. Rev. 59:1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harwood, C. R., and S. M. Cutting (ed.). 1990. Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, England.
- 17.Henriques, A. O., B. W. Beall, K. Roland, and C. P. Moran. 1995. Characterization of cotJ, a σE-controlled operon affecting the polypeptide composition of the coat of Bacillus subtilis spores. J. Bacteriol. 177:3394-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofmeister, A. E. M., A. Londono-Vallejo, E. Harry, P. Stragier, and R. Losick. 1995. Extracellular signal protein triggering the proteolytic activation of a developmental transcription factor in B. subtilis. Cell 83:219-226. [DOI] [PubMed] [Google Scholar]
- 19.Ichikawa, H., R. Halberg, and L. Kroos. 1999. Negative regulation by the Bacillus subtilis GerE protein. J. Biol. Chem. 274:8322-8327. [DOI] [PubMed] [Google Scholar]
- 20.Ichikawa, H., and L. Kroos. 2000. Combined action of two transcription factors regulates genes encoding spore coat proteins of Bacillus subtilis. J. Biol. Chem. 275:13849-13855. [DOI] [PubMed] [Google Scholar]
- 21.Isticato, R., G. Esposito, R. Zilhao, S. Nolasco, G. Cangiano, M. De Felice, A. O. Henriques, and E. Ricca. 2004. Assembly of multiple CotC forms into the Bacillus subtilis spore coat. J. Bacteriol. 186:1129-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karow, M. L., P. Glaser, and P. J. Piggot. 1995. Identification of a gene, spoIIR, that links the activation of σE to the transcriptional activity of σF during sporulation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 92:2012-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroos, L. The Bacillus and Myxococcus developmental networks and their transcriptional regulators. Annu. Rev. Genet. [Epub ahead of print.] http://arjournals.annualreviews.org/doi/pdf/10.1146/annurev.genet.41.110306.130400. [DOI] [PubMed]
- 24.Kroos, L., B. Kunkel, and R. Losick. 1989. Switch protein alters specificity of RNA polymerase containing a compartment-specific sigma factor. Science 243:526-529. [DOI] [PubMed] [Google Scholar]
- 25.Kroos, L., Y. T. Yu, D. Mills, and S. Ferguson-Miller. 2002. Forespore signaling is necessary for pro-σK processing during Bacillus subtilis sporulation despite the loss of SpoIVFA upon translational arrest. J. Bacteriol. 184:5393-5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunkel, B., L. Kroos, H. Poth, P. Youngman, and R. Losick. 1989. Temporal and spatial control of the mother-cell regulatory gene spoIIID of Bacillus subtilis. Genes Dev. 3:1735-1744. [DOI] [PubMed] [Google Scholar]
- 27.Londono-Vallejo, J. A., and P. Stragier. 1995. Cell-cell signaling pathway activating a developmental transcription factor in Bacillus subtilis. Genes Dev. 9:503-508. [DOI] [PubMed] [Google Scholar]
- 28.Lu, S., R. Halberg, and L. Kroos. 1990. Processing of the mother-cell σ factor, σK, may depend on events occurring in the forespore during Bacillus subtilis development. Proc. Natl. Acad. Sci. USA 87:9722-9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McPherson, D., H. Kim, M. Hahn, R. Wang, P. Grabowski, P. Eichenberger, and A. Driks. 2005. Characterization of the Bacillus subtilis spore morphogenetic coat protein CotO. J. Bacteriol. 187:8278-8290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller, J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 31.Naclerio, G., L. Baccigalupi, R. Zilhao, M. De Felice, and E. Ricca. 1996. Bacillus subtilis spore coat assembly requires cotH gene expression. J. Bacteriol. 178:4375-4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prince, H., R. Zhou, and L. Kroos. 2005. Substrate requirements for regulated intramembrane proteolysis of Bacillus subtilis pro-σK. J. Bacteriol. 187:961-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 34.Steil, L., M. Serrano, A. O. Henriques, and U. Volker. 2005. Genome-wide analysis of temporally regulated and compartment-specific gene expression in sporulating cells of Bacillus subtilis. Microbiology 151:399-420. [DOI] [PubMed] [Google Scholar]
- 35.Steinmetz, M., and R. Richter. 1994. Plasmids designed to alter the antibiotic resistance expression by insertion mutations in Bacillus subtilis, through in vivo recombination. Gene 142:79-83. [DOI] [PubMed] [Google Scholar]
- 36.Stragier, P., B. Kunkel, L. Kroos, and R. Losick. 1989. Chromosomal rearrangement generating a composite gene for a developmental transcription factor. Science 243:507-512. [DOI] [PubMed] [Google Scholar]
- 37.Wang, L., J. Perpich, A. Driks, and L. Kroos. 2007. Maintaining the transcription factor SpoIIID level late during sporulation causes spore defects in Bacillus subtilis. J. Bacteriol. 189:7302-7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Youngman, P., J. B. Perkins, and R. Losick. 1984. Construction of a cloning site near one end of Tn917 into which foreign DNA may be inserted without affecting transposition in Bacillus subtilis or expression of the transposon-borne erm gene. Plasmid 12:1-9. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, B., and L. Kroos. 1997. A feedback loop regulates the switch from one sigma factor to the next in the cascade controlling Bacillus subtilis mother cell gene expression. J. Bacteriol. 179:6138-6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, B., P. Struffi, and L. Kroos. 1999. σK can negatively regulate sigE expression by two different mechanisms during sporulation of Bacillus subtilis. J. Bacteriol. 181:4081-4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang, J., H. Ichikawa, R. Halberg, L. Kroos, and A. I. Aronson. 1994. Regulation of the transcription of a cluster of Bacillus subtilis spore coat genes. J. Mol. Biol. 240:405-415. [DOI] [PubMed] [Google Scholar]
- 42.Zheng, L., R. Halberg, S. Roels, H. Ichikawa, L. Kroos, and R. Losick. 1992. Sporulation regulatory protein GerE from Bacillus subtilis binds to and can activate or repress transcription from promoters for mother-cell-specific genes. J. Mol. Biol. 226:1037-1050. [DOI] [PubMed] [Google Scholar]
- 43.Zheng, L., and R. Losick. 1990. Cascade regulation of spore coat gene expression in Bacillus subtilis. J. Mol. Biol. 212:645-660. [DOI] [PubMed] [Google Scholar]
- 44.Zilhao, R., G. Naclerio, A. O. Henriques, L. Baccigalupi, C. P. Moran, Jr., and E. Ricca. 1999. Assembly requirements and role of CotH during spore coat formation in Bacillus subtilis. J. Bacteriol. 181:2631-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.