Abstract
Populations of Bacillus subtilis spores in which 90 to 99.9% of the spores had been killed by moist heat gave only two fractions on equilibrium density gradient centrifugation: a fraction comprised of less dense spores that had lost their dipicolinic acid (DPA), undergone significant protein denaturation, and were all dead and a fraction with the same higher density as that of unheated spores. The latter fraction from heat-killed spore populations retained all of its DPA, but ≥98% of the spores could be dead. The dead spores that retained DPA germinated relatively normally with nutrient and nonnutrient germinants, but the outgrowth of these germinated spores was significantly compromised, perhaps because they had suffered damage to some proteins such that metabolic activity during outgrowth was greatly decreased. These results indicate that DPA release takes place well after spore killing by moist heat and that DPA release during moist-heat treatment is an all-or-nothing phenomenon; these findings also suggest that damage to one or more key spore proteins causes spore killing by moist heat.
Spores of Bacillus and Clostridium species formed in sporulation are metabolically dormant and extremely resistant to a variety of stress factors, including moist heat, dry heat, UV and gamma radiation, desiccation, and many toxic chemicals (18, 32). Since spores of many of these species are commonly present in foodstuffs and since cells of some species can cause food spoilage or food-borne disease, much effort is expended in eliminating spores from foods. Moist heat is used routinely for inactivation of spores, generally at temperatures of ≥100°C for short to moderate periods of time. This method has been used for many, many years and is the gold standard for inactivation of spores in a food product.
In general, spores are resistant to moist-heat temperatures that are ∼45°C higher than those that inactivate growing cells of the same organism (36). A number of factors are responsible for spore moist-heat resistance, including the following: (i) the optimum growth temperature of the bacterial strain and the sporulation temperature (higher optimum growth and sporulation temperatures result in more resistant spores), (ii) the spore core's high level of dipicolinic acid (DPA) and its associated divalent cations, (iii) the type of divalent cations associated with DPA, (iv) the protection of spore DNA by its saturation with a group of α/β-type small, acid-soluble spore proteins, and (v) the low water content in the spore core, which may contain as little as 25% of its wet weight as water in the most resistant spores (9, 18, 32).
Even though the mechanisms of spore resistance to moist heat are fairly well understood, there is only a rudimentary understanding of the mechanism whereby spores are killed by this treatment, although this is not by DNA damage, since spore DNA is well protected by its saturation with α/β-type small, acid-soluble spore proteins (18, 32). Moist-heat-treated spores often appear injured, and although they can be recovered on rich medium plates, in contrast to unheated spores they are only poorly recovered on plates with high salt or with low nutrient levels (5, 8, 13). This suggests that some spore protein or proteins can be damaged by moist-heat treatment, although this damage may be only conditionally lethal. Moist-heat-killed spores often, but not always, have also lost DPA and may have a few core enzymes inactivated and/or denatured (5, 15, 37). However, it is not known if any or all of these events are the cause of spore killing or take place only after some other initial lethal event. Identification of the initial event that results in spore killing by moist heat might have significant practical import, since such knowledge could allow rational design of more efficient and less costly regimens for spore inactivation. Consequently, in this communication we report results of studies aimed at elucidating the initial event or events in the killing of spores of Bacillus subtilis by moist heat. While B. subtilis is not a major agent of food spoilage or food-borne disease, this organism is a model spore former and one that is genetically tractable, with many strains available with mutations and reporter genes that can facilitate analysis of spore properties, including spore resistance. Indeed, there is more known about the spores of B. subtilis than any other spore former.
MATERIALS AND METHODS
B. subtilis strains used and spore preparation and purification.
All B. subtilis strains used are isogenic and derived from strain PS832, a prototrophic derivative of strain 168. Strain PS533 (29) carries plasmid pUB110 that encodes resistance to kanamycin (Kmr; 10 μg/ml). Strain PS3518 (7, 38) carries the gfp gene encoding green fluorescent protein (GFP) under the control of the strong forespore-specific promoter of the B. subtilis sspE gene, and the strain is also Kmr. This strain accumulates high levels of GFP in the spore core (7). Strain PS3379 (10, 28) contains the luxAB genes from Vibrio harveyi under the control of the strong forespore-specific promoter of the B. subtilis sspB gene (34) and accumulates high levels of LuxA and LuxB in the spore core; this strain is also resistant to erythromycin (Emr; 1 μg/ml). When PS3379 spores germinate and initiate outgrowth and metabolism resumes, LuxA and LuxB generate light if a long chain aldehyde such as dodecanal is added to the medium (10, 28). Strain PS4006 (35) carries genes encoding resistance to both chloramphenicol (Cmr; 5 μg/ml) and tetracycline (Tcr; 10 μg/ml) and has a gene encoding a minor mechanosensitive channel under the control of the promoter of the xylose operon inserted at the amyE locus. The sporulation of this strain in the presence or absence of xylose and the properties of the spores produced appear identical to those of wild-type spores (35). Strain PS2583 is spectinomycin resistant (Spr; 100 μg/ml) and contains a deletion replacement of the sigX gene. This strain was constructed by transformation of strain PS832 with chromosomal DNA from strain AB406 (12) and selection for Spr. Strain PS2586 is Emr and contains a plasmid insertion that prevents expression of the ypuN gene (12). This strain was constructed by transformation of strain PS832 with chromosomal DNA from strain A29.1 (12) with selection for Emr.
Spores of all strains were prepared at 37°C on 2× SG medium agar plates without antibiotics as described previously (19, 21). After incubation for 2 to 3 days at 37°C, plates were incubated for several days at 23°C to allow completion of lysis of sporulating cells. The spores were scraped from plates, suspended in water, purified by repeated centrifugation and washing with water as well as several sonication treatments, and stored protected from light in water at 4°C (19). All spore preparations used in this work were free (>98%) from germinated spores, growing or sporulating cells, and cell debris as determined by observation in a phase-contrast microscope.
Spore treatment with moist heat and assessment of spore killing.
Spores were incubated in water at an optical density at 600 nm (OD600) of 1 (∼1.5 × 108 spores/ml) or 10 with a measured pH of ∼7.5 at 87 to 90°C. At various times aliquots (10 μl) were diluted 1/100 in 23°C water. Following serial dilution in water, aliquots were routinely spotted on LB medium plates (22) without antibiotics, the plates were incubated for 18 to 36 h at 37°C, and colonies were counted. Incubation for longer times gave no increase in numbers of colonies. In a few experiments, dilutions of spores of strains carrying genes encoding Cmr, Emr, Kmr, Spr, or Tcr were also spotted on LB medium plates containing appropriate antibiotics.
Several additional treatments were also tested for their ability to recover heat-treated spores, including addition of lysozyme (3 μg/ml) in recovery plates without antibiotics (39); preincubation for 1 h in a nonnutrient germinant, a 1:1 mixture of 40 mM Ca2+ and DPA (20); and lysozyme treatment in hypertonic medium after spore coats had been permeabilized (23).
Fractionation of heat-treated spores.
For equilibrium density gradient centrifugation of heat-treated spores, spores (200 μl) treated at an OD600 of 10 were chilled on ice and centrifuged, and the pellet was suspended in 100 μl of 20% 5-(N-2,3-dihydroxypropylacetamido)-2,4,6-triiodo-N,N′-bis(2,3-dihydroxypropyl)- isophthalamide (Nycodenz) (24) and layered on a 2-ml density gradient of 75 to 37% Nycodenz in steps of 2%. The tubes were centrifuged at 23°C for 45 min at 14,000 rpm in a TLS55 rotor in a Beckman TL-100 ultracentrifuge. Following centrifugation, bands were removed, diluted with water, and washed five to six times by centrifugation with water to remove the Nycodenz.
For preparation of large amounts of fractionated heat-treated spores, spores (100 μl at an OD600 of 100 to 200 in 20% Nycodenz) were layered on 50% Nycodenz in a 2-ml ultracentrifuge tube and centrifuged as described above. Under these conditions spores that retained their DPA pelleted, and spores that had lost their DPA banded close to the top of the tube. The Nycodenz was removed from the fractionated spores as described above, and the spores were suspended in cold water and stored at 4°C.
Spore germination.
Unless noted otherwise, spores were germinated at an OD600 of 1 at 37°C in LB medium (22) plus 10 mM l-alanine, and the extent of spore germination was assessed either by phase-contrast microscopy or by flow cytometry after the spores were stained with the dye Syto 9 (Molecular Probes, Eugene, OR) as described previously (3). In some cases 2-ml aliquots of spores incubated in this medium were centrifuged in a microcentrifuge, suspended in 300 μl of 4% formaldehyde, and incubated for 20 min at 23°C; they were then washed three times with phosphate-buffered saline (PBS) (25 mM KPO4 buffer [pH 7.4]-0.15 M NaCl), suspended in 200 μl of PBS, and examined by differential interference contrast (DIC) microscopy on an LSM510 confocal microscope (Carl Zeiss Inc., Thornwood, NY) using a 63× planapochromat lens (numerical aperture, 1.4) with the confocal aperture at its widest setting. Spores at an OD600 of 2 were also germinated at 37°C in 10 mM l-alanine-10 mM Tris-HCl buffer (pH 7.4) or in 25 mM Tris-HCl buffer (pH 7.4) with either 0.2 mM dodecylamine at 45°C or 1 mM dodecylamine at 50°C (26). In these three cases, spore germination was assessed by measuring the release of spore DPA by monitoring the OD270 of the supernatant fluid from 1 ml of culture, since ≥90% of the OD270 released by germinating spores is due to DPA (4). The total DPA content of the spores was determined from the OD270 of the supernatant fluid from a 1-ml aliquot of culture that was boiled for 20 min (4, 26).
Analytical procedures.
DPA was analyzed chemically (25) in supernatant fluid from spores boiled 30 min as described previously (19). Untreated and moist-heat-treated and fractionated PS3518 spores were examined by DIC and fluorescence microscopy on an LSM510 confocal microscope (Carl Zeiss Inc., Thornwood, NY) using a 63× planapochromat lens (numerical aperture, 1.4) with the confocal aperture at its widest setting; alternatively, spores were examined directly by flow cytometry. In some experiments spores were stained with the BacLight viability stain (Molecular Probes, Eugene, OR), and the stained spores were examined by fluorescence microscopy as described previously (16, 17).
Light production during germination and outgrowth of spores of strain PS3379 germinated in LB medium plus l-alanine as described above was measured in a luminometer (28); 0.5-ml aliquots of culture were taken at various times, 0.5 ml of fresh LB medium plus dodecanal (10 μg) was added, and light production was measured at 23°C. The Raman spectra of individual fractionated and untreated spores were obtained and averaged as described previously (11). For analysis of ATP accumulated during spore outgrowth, spores were germinated in LB medium plus l-alanine as described above, but at an OD600 of 5, and at various times 1-ml aliquots were added to 4 ml of boiling 1-propanol; the samples were processed, and ATP was measured by the firefly luciferase assay as described previously (33).
RESULTS
Fractionation of heat-treated spores by equilibrium density gradient centrifugation.
Spores of Bacillus species have a relatively high wet density because of the high solid and low water content in the spore core (23). Since much of the solid content in the spore core is DPA and its associated cations, DPA release should result in a significant decrease in spore wet density, a change that has been well documented during spore germination (23, 28). Consequently, spores of B. subtilis PS533 (wild type) in water were treated at 87 to 90°C for various times, spore viability was determined, and aliquots of untreated and treated spores were run on equilibrium density gradients (Fig. 1). As expected, untreated spores gave only a single band at a rather high density (∼1.36 g/ml), while with increasing exposure to heat more and more spores banded at a significantly lower density (∼1.27 g/ml). There were two particularly notable findings in this experiment. The first was that no bands of intermediate density were detected (Fig. 1). The second was that the rate of generation of the less dense upper band was clearly much slower than the rate of spore killing (Fig. 1; the ratios of the amounts of spores in the upper and lower bands are given in the figure legend). Consequently, while the spores in the upper band were not viable, some of the spores in the lower band obtained from heat-treated spores were viable, but this was a much lower percentage than for untreated spores (Fig. 1; also data not shown). Similar results in this experiment were also obtained with a number of other B. subtilis strains including PS832, PS3379, and PS3518 (data not shown).
FIG. 1.
Equilibrium density gradient centrifugation of spore populations with various percentages of spores killed by moist heat. Spores of strain PS533 (wild type) were either untreated (tube 1) or treated for increasing times at ∼89°C (40, 80, and 120 min for tubes 2 to 4, respectively), and viability was determined; spores were run on Nycodenz step gradients, and the spores in the upper (U) or lower (L) bands were isolated. The viability of the spores was determined as the number of CFU/0.5 ml of spores at an OD600 of 1, as described in Materials Methods. The values for the viability of untreated spores before centrifugation or from the lower band in tube 1 were each set at 100%, and the absolute viabilities for these two samples were almost identical. Values for the viability of spores from upper and lower bands in tubes 2 to 4 are expressed relative to the value for the lower band from untreated spores in tube 1. Based on the OD600 of spores recovered from the upper and lower bands, the upper band comprised 4%, 20%, and 60% of total spores in tubes 2, 3, and 4, respectively. Since the upper band had lost DPA and this decreases the OD600 of spore suspensions ∼25% (28), the ratios of the amounts of spores in the upper to the lower bands are 0.05, 0.3, and 3 in tubes 2 to 4, respectively. This experiment was repeated three times with essentially identical findings.
There are a number of examples of spores that appear to be killed by various agents when the spores are directly applied to nutrient-containing plates, yet these spores are recovered if they are treated to enhance their recovery and/or germination (6, 23, 28, 27, 39). Consequently, we tested several of these treatments that have sometimes enhanced spore recovery, including the addition of low levels of lysozyme to plating medium, pretreatment of spores with a 1:1 chelate of Ca-DPA, and decoating of spores followed by lysozyme treatment in hypertonic medium (20, 21, 23, 27, 39). Lysozyme treatment can recover spores that only appear dead due to inactivation of the cortex lytic enzymes that are essential for the degradation of the peptidoglycan cortex during spore germination, while Ca-DPA pretreatment can recover spores that lack only nutrient germinant receptors (22, 23, 27, 31, 39). However, in a spore population in which 90 to 99% of the spores had been killed by heat treatment, none of these treatments significantly (≤25%) increased the viability of the spores in the lower or upper bands obtained from equilibrium density gradient centrifugation (data not shown), although the lysozyme treatment in hypertonic medium did germinate the heat-killed spores (data not shown). These results indicate that moist-heat killing of B. subtilis spores is not through inactivation of spore nutrient germinant receptors or cortex lytic enzymes.
DPA content of spores in fractions from heat-treated spores.
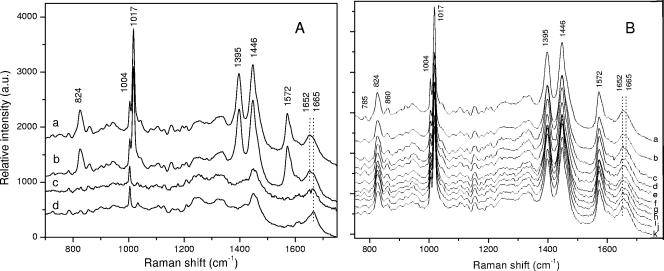
In addition to being dead, spores in the upper band from heat-treated spores contained no detectable DPA. This was determined both by chemical assays of DPA from these spores (data not shown) and by Raman spectroscopy of ∼100 individual spores (Fig. 2A). Notably, the multiple Raman spectral bands due to Ca-DPA in untreated spores (824, 1017, 1395, 1446, and 1572 cm−1) (previously reported in reference 11) were absent in spores from the upper band from heat-treated spore preparations (Fig. 2A, compare curves a and d).
FIG. 2.
Mean Raman spectra of individual untreated spores and fractionated moist-heat-treated spores and of individual spores from moist-heat-killed spore populations that retained DPA. (A) The Raman spectra of 20 strain PS533 (wild type) spores of various types were collected, and the mean Raman spectra were determined as described in Materials and Methods. The individual spectra are from untreated spores (a) or spores from a population in which 97% were killed by moist heat at 87 to 90°C, as follows: spores that retained DPA isolated from the lower band on an equilibrium density gradient (b), spores that had lost DPA isolated from the lower band on an equilibrium density gradient (c), and spores from the upper band on an equilibrium density gradient (d). (B) Ninety-seven percent of strain PS533 spores (wild type) were killed by moist heat at 87 to 90°C; the spores were separated by equilibrium density gradient centrifugation, and Raman spectra of nine individual spores (curves c to k) that retained DPA from the lower, denser band as well as the mean Raman spectrum (curve b) for these nine spores were determined as described in Materials and Methods. The different individual spectra are offset vertically for clarity. The mean Raman spectrum of 10 individual untreated spores (curve a) is also shown for comparative purposes.
In contrast to the absence of DPA in heat-treated spores from the upper band on equilibrium density gradients, the great majority (≥94%) of the spores in the lower band from a heat-treated spore population in which ∼99% of the spores were killed retained their DPA, as determined by chemical assays (data not shown) and Raman spectroscopy of individual spores (Fig. 2A; compare curves a and b) (note that ∼98% of the spores in this fraction were dead). Of 100 individual spores in the lower band from a spore population in which 99% of the spores had been killed, only 6% had lost DPA. It is possible either that these spores lost DPA in the ∼36 h following equilibrium density gradient centrifugation that it took for shipment of the spores from the site of fractionation to the site where Raman spectroscopy was performed or that they were contaminants from the upper band. More importantly, ≥90% of the spores in the lower band retained DPA, and these spores contained the same amount of DPA (± 15%) as untreated spores (Fig. 2A, compare curves a and b, and B, compare heights of major Ca-DPA peaks at 1,017, 1,446, and 1,572 cm−1 in curve a with those in curves b to k).
Status of proteins in fractions from heat-treated spores.
In addition to information about spore DPA content, the Raman spectra of individual spores can also provide information on the status of spore proteins, since the band at 1,652 cm−1 in untreated spores (Fig. 2A, curve a) is due to the amide stretch motion of proteins with a largely α-helical structure (11, 14, 40). However, in the less dense heat-treated spores that had lost DPA, the position of this Raman band had shifted to 1,665 cm−1 (Fig. 2A, curve d), a position characteristic of denatured proteins (11, 14, 40). The situation with the denser spores from heat-treated spore preparations was more complicated. With spores from this fraction that had lost DPA, the amide band had largely shifted to 1,665 cm−1 (Fig. 2A, compare curves a and c), although as noted above, the source of these spores in the lower band that had lost DPA is not clear. Importantly, analysis of ∼100 spores from the lower band that retained DPA gave a mean spectrum in which the protein peak was at 1,652 cm−1, the position of the native protein band, but also showed a distinct shoulder at 1,665 cm−1, the position of the denatured protein band (Fig. 2A, compare curves a and b). Even more notable was the significant variability in the relative heights of the 1,665 and 1,652 cm−1 bands in this group of spores, with some even showing two distinct peaks at these wave numbers (Fig. 2B, compare curve a with curves c to k).
As an additional test of the state of a protein in heat-treated spores, we used spores of strain PS3518 that contain a large amount of GFP in the spore core. When spores of this strain were subjected to heat treatment, ∼99% were killed; when these spores were then fractionated by equilibrium density gradient centrifugation, we again obtained two bands, and as with wild-type spores, >99.999% of the spores in the upper band were dead while only ∼98% of spores in the lower band were dead (data not shown). Examination by fluorescence microscopy of spores from both bands as well as untreated spores revealed that spores from the upper band exhibited little or no detectable GFP fluorescence, while untreated spores and spores from the lower band exhibited similar levels of GFP fluorescence (Fig. 3). Western blot analysis using anti-GFP antiserum also showed that the spores from the upper band from heat-treated PS3518 spores retained the GFP protein (data not shown) even though these spores exhibited no GFP fluorescence.
FIG. 3.
GFP fluorescence of untreated spores and fractions from moist-heat-treated spore populations. Spores of strain PS3518 that accumulate significant amounts of GFP in the spore core were treated with moist heat at 87 to 90°C so that 99% of the spores were killed; the treated spores were run on an equilibrium density gradient, and the spores in the lower and upper bands were isolated and examined by fluorescence and DIC microscopy as described in Materials and Methods. The DIC images (a, c, and e) and fluorescence images (b, d, and f) in the various frames are all at the same magnification and are as follows:: untreated spores (a and b), spores from the lower band (c and d), and spores from the upper band (e and f). Scale bar, 20 μm. Images from unfixed spores looked identical to those shown in this figure (data not shown).
As a more direct assessment of protein function in heat-treated spores, we used spores of strains carrying resistance to various antibiotics and measured spore survival during heat treatment on plates with and without the appropriate antibiotic. With spores of strains that were Cmr, Kmr, Spr, or Tcr, there was no difference (±15%) in spore resistance to moist heat at 87 to 90°C assessed on plates with or without antibiotics (Fig. 4A and data not shown). However, spores of two different Emr strains appeared to die more rapidly on plates with erythromycin than on drug-free plates (Fig. 4B), suggesting that a protein involved in Emr is slightly more heat sensitive than is spore viability.
FIG. 4.
Rates of moist-heat killing of spores of various strains determined by survival on plates with or without antibiotics. Spores of various strains in water were heated at 87 to 90°C, and at various times aliquots were taken and cooled, and spore survival on plates with or without antibiotics was determined as described in Materials and Methods. (A) Survival of spores of strain PS4006 (Tcr) measured on plates without (○) or with (•) tetracycline. (B) Survival of spores of strains PS2586 (Emr; circles) or PS3379 (Emr; triangles) measured on plates without (open symbols) or with (filled symbols) the appropriate antibiotic. Values shown are averages from duplicate determinations on at least two different serial dilutions and were ±25%.
Germination of spores in fractions from heat-treated spores.
The results noted above are consistent with the idea that damage to one or more spore proteins is a major factor in spore killing by moist heat. One group of proteins whose inactivation would result in apparently dead spores includes proteins that are essential for spore germination since a spore that cannot germinate will appear dead. This seemed unlikely, since several artificial germination treatments did not recover heat-killed spores, as noted above. However, we directly measured the ability of heat-killed spores to germinate by measuring the response of spores in fractions from equilibrium density gradients of heat-treated spores to either the nutrient germinant l-alanine or a nonnutrient germinant, dodecylamine (26, 31). As expected, there was no change in the appearance of the less dense spores in the phase-contrast microscope after 90 min of incubation with l-alanine under germination conditions (data not shown). However, the denser spores from equilibrium density gradient fractionation germinated almost as rapidly as untreated spores with l-alanine when spore germination was measured by monitoring DPA release; similar results were obtained when germination was with dodecylamine (Fig. 5). The denser spores from a spore population in which 97% of the spores were killed by moist heat also germinated almost as well as wild-type spores in LB medium plus l-alanine, as determined by flow cytometry (data not shown). In LB medium plus l-alanine, the majority of the untreated spores not only germinated but also elongated and began vegetative growth after 75 min (Fig. 6a to c). In contrast, while >93% of the spores from the lower band (viability of ∼3% that of untreated spores) from heat-treated spores germinated in this medium, <5% of these spores elongated and began vegetative growth even after 5 h (Fig. 6d; also data not shown).
FIG. 5.
l-Alanine and dodecylamine germination of spores from the lower band on equilibrium density gradients from heat-treated spores. Ninety-seven percent of strain PS533 spores (wild type) were killed by moist heat at 87 to 90°C, and these spores were fractionated by equilibrium density gradient centrifugation. The spores in the lower band and untreated spores were germinated with l-alanine or dodecylamine, and spore germination was quantitated by measuring DPA release as described in Materials and Methods. (A) l-Alanine germination of untreated spores (○) or spores from the lower band (•). (B) Dodecylamine germination with either 0.25 mM dodecylamine at 45°C (circles) or 1 mM dodecylamine at 50°C (triangles) of untreated spores (open symbols) or spores from the lower band (filled symbols).
FIG. 6.
Outgrowth of untreated and heat-treated spores. Ninety-nine percent of strain PS533 spores (wild type) were killed by moist-heat treatment at 87 to 90°C. The spores were separated by equilibrium density gradient centrifugation; the spores in the lower, denser band were isolated, and untreated spores and spores from the lower band were germinated or outgrown in LB medium plus l-alanine as described in Materials and Methods. After incubation in LB medium plus l-alanine for either 75 min (untreated spores) or 5 h (spores from the lower band), aliquots were harvested, fixed with formaldehyde, suspended in 200 μl of PBS, and examined by DIC microscopy as described in Materials and Methods. The images of untreated spores (a to c) and spores from the lower band (d) are all at the same magnification. Scale bar, 20 μm.
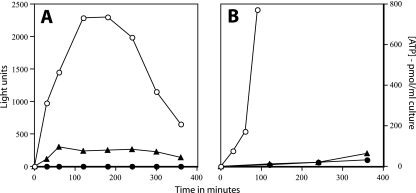
As a further assessment of the ability of fractions from heat-killed spores to undergo outgrowth, we used spores of strain PS3379 that contained high levels of the V. harveyi LuxA and LuxB proteins in spores. These proteins can generate light if provided with FMNH2 and a long chain alkyl aldehyde such as dodecanal (10). Although reduced coenzymes are not present in dormant spores, FMNH2 is generated early in spore outgrowth when metabolism resumes (6, 10, 30, 31, 33). Consequently, PS3379 spores generate light early in outgrowth, provided an appropriate aldehyde is added, and this continues until eventually the LuxA and/or LuxB proteins are lost (6, 10, 28). As expected, untreated PS3379 spores generated high levels of light when germination was initiated in LB medium plus l-alanine and dodecanal was provided (Fig. 7A). However, in a spore population in which 99% were killed by moist heat, the less dense and more dense spores from equilibrium density gradient fractionation generated ≤1% of the light generated by untreated spores even after 6 h of incubation in LB medium plus l-alanine; spores from a population in which ∼80% were killed by heat treatment generated only 10 to 15% of the maximum light production of untreated spores (Fig. 7A).
FIG. 7.
Light production and ATP accumulation during outgrowth of untreated and heat-killed spores. (A) In populations of PS3379 (luxAB) spores that contained luciferase, 80 or 99% of spores were killed by incubation at 87 to 90°C in water. For the spore population killed at the 99% level, the lower band containing spores that retained DPA but were dead was isolated by equilibrium density gradient centrifugation. For the spore population killed at the 80% level, essentially all spores retained DPA, so these were not fractionated by density gradient centrifugation. The killed spores that retained DPA were germinated in LB medium plus l-alanine, and light production was measured at various times as described in Materials and Methods. ○, untreated spores; •, spores from the lower band in which 97% of spores were dead; ▴, spore population in which 80% of spores were dead. (B) ATP accumulation during outgrowth of untreated and heat-killed spores. In a population of PS533 (wild type) spores, 94 to 99% of spores were killed by incubation at 87 to 90°C in water. For the heat-treated spores, the lower band containing spores that retained DPA but were dead was isolated by equilibrium density gradient centrifugation. The untreated spores and the heat-killed spores that retained DPA were germinated at an OD600 of 5 in LB medium plus l-alanine, and samples were extracted, processed, and assayed for ATP as described in Materials and Methods. ○, untreated spores; •, spore population in which ∼94% of spores were dead; ▴, spore population in which ∼99% of spores were dead. Examination of cultures by phase-contrast microscopy indicated that ∼80% of untreated spores had germinated and many were elongating after 60 min of incubation and that ∼80% of heat-treated spores had germinated after 2 h but none were elongating.
As another assessment of the capacity of heat-killed spores to carry out metabolism during outgrowth, we measured ATP production during the germination and outgrowth of untreated and heat-killed spores in a complete nutrient medium (Fig. 7B). As expected (28, 31, 33), untreated spores accumulated ATP rapidly during incubation in this medium. However, in a population in which 94 to 99% of the spores were killed by heat treatment, ATP accumulation was much less, even though these spores had largely germinated after 2 h of incubation.
Permeability properties of untreated spores and fractionated heat-killed spores.
The fact that the less dense spores isolated by equilibrium density gradient fractionation of heat-treated spore populations had lost their DPA suggested that the permeability barriers that block movement of small molecules in and out of the spore core had been breached in these spores. Normally, the nucleic acids in the spore core are not readily stained by dyes such as Syto9 and propidium iodide, the two dye components of a commonly used bacterial viability strain, BacLight. However, it was possible that these stains could penetrate into the core of less dense spores from heat-treated spore populations. To test this possibility, from populations in which 97% of spores were killed by wet heat, untreated spores and less dense and more dense spores were stained with the BacLight reagent with or without prior incubation in germination medium with l-alanine for 90 min; from 200 to 2,000 spores of each type were scored for the amount and color (green, perhaps alive; red, almost certainly dead) of staining of the core region (Table 1). As expected, untreated dormant spores stained very poorly, with only ∼5% of the spores staining and almost all of these staining red. The denser dormant spores from heat-treated populations in which ∼95% of spores were determined to be dead by plate assays gave results similar to those for untreated spores. However, when these denser spores were germinated and then stained, the percentage staining red (i.e., dead) was higher than for untreated spores. The most dramatic result was obtained with the less dense spores from populations in which 97% of the spores were killed by heat treatment as these spores all stained with BacLight, and >90% stained red. As expected, incubation in germination medium did not affect the staining of these spores with BacLight. Similar results with BacLight staining were also obtained with fractions from spore populations in which only 90% were killed by moist heat (data not shown).
TABLE 1.
Permeability and viability of untreated and fractionated heat-treated spores as assessed by BacLight staininga
| Spore type analyzed | No. of spores examined | % Stained (total) | % Staining red | % Staining green |
|---|---|---|---|---|
| Untreated, dormant | ∼1,000 | 5 | 4 | 1 |
| Untreated, germinated | ∼2,000 | 94 | 6 | 88 |
| Denser band, dormant | ∼1,000 | 9 | 7.5 | 1.5 |
| Denser band, germinated | ∼1,000 | 93 | 23 | 70 |
| Lighter band, dormant | 200 | 100 | 93 | 7 |
| Lighter band, germinated | 200 | 100 | 91 | 9 |
PS533 (wild type) spores from a population in which 97% were killed by moist heat were separated by equilibrium density gradient centrifugation. Untreated spores or spores from the upper, less dense and lower, denser bands were then treated with the BacLight viability stain with or without prior germination with l-alanine for 90 min. Spores were examined by fluorescence microscopy as described in Materials and Methods, and the stained spores were counted. Only spores whose central region or core was stained were scored as staining, since even untreated dormant spores give weak peripheral staining with the BacLight reagent (27).
DISCUSSION
The work in this report leads to a number of significant conclusions about the events in and the mechanism of the killing of spores of B. subtilis by moist heat. These conclusions include the following: (i) DPA release during moist-heat treatment is accompanied by a large amount of protein damage, most likely heat denaturation due to the increased core water content of spores from which DPA has been released (21, 28); (ii) DPA release is an all-or-nothing phenomenon and does not take place in discrete steps; (iii) while DPA release is a major event during spore treatment with moist heat, this release takes place largely if not exclusively after spore death; (iv) spores that retain DPA after moist-heat treatment can still initiate germination with both nutrient and nonnutrient germinants but cannot progress into outgrowth; and (v) spores that retain DPA after moist-heat treatment but are dead have suffered some protein damage, probably denaturation; this is the most likely cause of spore death caused by moist heat, although the identity of the key protein or proteins whose denaturation results in spore death is not yet clear. The spore's inner membrane as an alternative target of moist-heat treatment seems much less likely, since the changes in the permeability properties of the inner membrane upon moist-heat treatment take place at a much slower rate than spore killing.
While the conclusions listed above can be drawn only for wild-type B. subtilis spores treated with moist heat, there are previous data from work with spores of several other Bacillus species that are consistent with these conclusions. Thus, DPA release was also shown to take place more slowly than killing of Bacillus cereus and Bacillus megaterium spores by moist heat, while inactivation of some spore core enzymes was actually faster than loss in spore viability (2, 37). That there is significant protein damage during and, in some cases, preceding spore inactivation by moist heat has also been shown by analysis of moist-heat killing of B. megaterium spores using differential scanning calorimetry (2) and of B. subtilis spores treated with moist heat at acidic pH values (6). In the latter case, as well as in some other cases where spores were killed by moist heat, spore killing was due to the inactivation of some essential component of the germination apparatus (1, 6, 27, 39). However, this is clearly not the case in the current work, since moist-heat-inactivated spores could not be recovered by artificial germination treatments, in particular by lysozyme treatment in hypertonic medium of spores whose coats were made permeable, as this treatment germinated heat-killed spores well but did not increase their viability.
Based on our observations, we propose the following model for the killing of B. subtilis spores by moist heat. As moist-heat treatment continues, spores suffer increasing amounts of damage to a number of proteins, although different proteins are inactivated at different rates. When loss of some crucial protein becomes too great, the spores are dead. However, these dead spores retain DPA and the capacity to initiate germination but cannot progress in outgrowth due to the inactivation of some crucial protein or proteins. As moist-heat treatment continues, there is eventually sufficient damage to one or more proteins in the spore's inner membrane such that this membrane ruptures, leading to the rapid release of the spore's DPA and perhaps other small molecules, although the latter has not yet been tested. With the loss of spore DPA and its likely replacement by water as in spore germination (31), the spore's core water content almost immediately rises significantly, from ∼35% of wet weight to ∼45% based on the increase in core wet density (28). This will undoubtedly result in much more rapid heat inactivation of spore core proteins, as has been seen previously with B. subtilis dormant spores that have increased levels of core water (21, 28), and lead to more rapid denaturation of spore core proteins.
The major question raised by this model and the work in this report is the identity of the protein or proteins whose inactivation or denaturation results in spore killing. In the case of the sensitization of spores to erythromycin by moist-heat treatment, it is likely that a protein essential for Emr is more sensitive to moist heat than is spore viability. In the Emr B. subtilis strains used in this work, the Emr phenotype is the result of the erm gene that encodes a methylase that modifies rRNA. Perhaps it is this methylase that is heat sensitive such that rRNA synthesized during outgrowth is not modified, and modified rRNA carried over in the dormant spore is not sufficient for progression into vegetative growth.
In experiments where there was no antibiotic selection, heat-killed spores that retained DPA germinated but did not go through outgrowth. The lack of outgrowth of heat-killed spores that retain DPA suggests that the lesion in these spores is in either macromolecular synthesis or metabolism. Indeed, with B. cereus spores at least one enzyme of metabolism, glucose-6-phosphate dehydrogenase, is inactivated with or before loss of spore viability (37), although it is not clear why loss of this enzyme alone would block spore outgrowth. The lack of light production during outgrowth of heat-killed B. subtilis spores that retained DPA and contained the LuxAB proteins is certainly consistent with there being a block in metabolism of these heat-killed spores, although it is possible that it is LuxA or LuxB that has been inactivated by the moist heat. However, the very poor ATP accumulation following germination of heat-killed spores that retain DPA in a complete nutrient medium strongly suggests that inactivation of some enzyme involved in metabolism causes the death of these spores. Certainly, the work in the present study and the model presented above focus attention on one or more proteins, most likely enzymes involved in metabolism, as the crucial targets for moist-heat killing of bacterial spores.
Acknowledgments
This work was supported by a grant from the Army Research Office (P.S.).
Footnotes
Published ahead of print on 21 September 2007.
REFERENCES
- 1.Atrih, A., and S. J. Foster. 2001. In vivo roles of the germination-specific lytic enzymes of Bacillus subtilis 168. Microbiology 147:2925-2932. [DOI] [PubMed] [Google Scholar]
- 2.Belleveau, B. H., T. C. Beaman, H. S. Pankratz, and P. Gerhardt. 1992. Heat killing of bacterial spores analyzed by differential scanning calorimetry. J. Bacteriol. 174:4463-4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black, E. P., K. Koziol-Dube, D. Guan, J. Wei, B. Setlow, D. E. Cortezzo, D. G. Hoover, and P. Setlow. 2005. Factors influencing the germination of Bacillus subtilis spores via the activation of nutrient receptors by high pressure. Appl. Environ. Microbiol. 71:5879-5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabrera-Martinez, R. M., F. Tovar-Rojo, V. R. Vepachedu, and P. Setlow. 2003. Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis. J. Bacteriol. 185:2457-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cazemier, A. E., S. F. Wagenaars, and P. F. ter Steeg. 2001. Effect of sporulation and recovery medium on the heat resistance and amount of injury of spores from spoilage bacilli. J. Appl. Microbiol. 90:761-770. [DOI] [PubMed] [Google Scholar]
- 6.Ciarciaglini, G., P. J. Hill, K. Davies, P. J. McClure, D. Kilsby, M. H. Brown, and P. J. Coote. 2000. Germination-induced bioluminescence, a route to determine the inhibitory effect of a combination preservation treatment on bacterial spores. Appl. Environ. Microbiol. 66:3735-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowan, A. E., D. E. Koppel, B. Setlow, and P. Setlow. 2003. A soluble protein is immobile in dormant spores of Bacillus subtilis but is mobile in germinated spores: implications for spore dormancy. Proc. Natl. Acad. Sci. USA 100:4209-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feeherry, F. E., D. T Munsey, and D. B. Rowley. 1987. Thermal inactivation and injury of Bacillus stearothermophilus spores. Appl. Environ. Microbiol. 53:365-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerhardt, P., and R. E. Marquis. 1989. Spore thermoresistance mechanisms, p. 43-63. In I. Smith, R. A. Slepecky and P. Setlow (ed.), Regulation of prokaryotic development. American Society for Microbiology, Washington, DC.
- 10.Hill, P. J., L. Hall, D. A. Vinicombe, C. J. Soper, P. Setlow, W. M. Waites, S. Denyer, and G. S. Stewart. 1994. Bioluminescence and spores as biological indicators of inimical processes. Soc. Appl. Bacteriol. Symp. Ser. 23:129S-134S. [DOI] [PubMed] [Google Scholar]
- 11.Huang, S.-S., D. Chen, P. L. Pelczar, V. R. Vepachedu, P. Setlow, and Y.-Q. Li. 2007. Levels of Ca2+-dipicolinic acid in individual Bacillus spores determined using microfluidic Raman tweezers. J. Bacteriol. 189:4681-4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang, X., A. Decatur, A. Sorokin, and J. D. Helmann. 1997. The Bacillus subtilis σX protein is an extracytoplasmic function σ factor contributing to survival at high temperature. J. Bacteriol. 179:2915-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurst, A. 1983. Injury, p. 255-274. In A. Hurst and G. W. Gould (ed.), The bacterial spore, vol. 2. Academic Press, London, England. [Google Scholar]
- 14.Lin, V. J. C., and J. L. Koenig. 1976. Raman studies of bovine serum albumin. Biopolymers 15:203-218. [DOI] [PubMed] [Google Scholar]
- 15.Mallidis, C. G., and J. S. Scholefield. 1985. The release of dipicolinic acid during heating and its relation to the heat destruction of Bacillus stearothermophilus spores. J. Appl. Bacteriol. 59:479-486. [DOI] [PubMed] [Google Scholar]
- 16.Melly, E., B. Setlow, and P. Setlow. 2002. Studies on the mechanism of killing of Bacillus subtilis spores by hydrogen peroxide. J. Appl. Microbiol. 93:316-325. [DOI] [PubMed] [Google Scholar]
- 17.Murray, T., D. L. Popham, C. B. Pearson, A. R. Hand, and P. Setlow. 1998. Analysis of the outgrowth of Bacillus subtilis spores lacking penicillin-binding protein 2a. J. Bacteriol. 180:6493-6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicholson, W. L., N. Munakata, G. Horneck, H. J. Melosh, and P. Setlow. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64:548-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, England.
- 20.Paidhungat, M., K. Ragkousi, and P. Setlow. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J. Bacteriol. 183:4886-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paidhungat, M., B. Setlow, A. Driks, and P. Setlow. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182:5505-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paidhungat, M., and P. Setlow. 2000. Role of Ger-proteins in nutrient and non-nutrient triggering of spore germination in Bacillus subtilis. J. Bacteriol. 182:2513-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popham, D. L., J. Helin, C. E. Costello, and P. Setlow. 1996. Muramic lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not for spore dehydration or heat resistance. Proc. Natl. Acad. Sci. USA 93:15405-15410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rickwood, D., T. Ford, and J. Graham. 1982. Nycodenz: a new nonionic iodinated gradient medium. Anal. Biochem. 123:23-31. [DOI] [PubMed] [Google Scholar]
- 25.Rotman, Y., and M. L. Fields. 1968. A modified reagent for dipicolinic acid analyses. Anal. Biochem. 22:168. [DOI] [PubMed] [Google Scholar]
- 26.Setlow, B., A. E. Cowan, and P. Setlow. 2003. Germination of spores of Bacillus subtilis with dodecylamine. J. Appl. Microbiol. 95:637-648. [DOI] [PubMed] [Google Scholar]
- 27.Setlow, B., C. A. Loshon, P. C. Genest, A. E. Cowan, C. Setlow, and P. Setlow. 2002. Mechanisms of killing of spores of Bacillus subtilis by acid, alkali and ethanol. J. Appl. Microbiol. 92:362-375. [DOI] [PubMed] [Google Scholar]
- 28.Setlow, B., E. Melly, and P. Setlow. 2001. Properties of spores of Bacillus subtilis blocked at an intermediate stage of spore germination. J. Bacteriol. 183:4894-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Setlow, B., and P. Setlow. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 178:3486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Setlow, P. 1994. Mechanisms that contribute to the long-term survival of spores of Bacillus species. J. Appl. Bacteriol. 76:49S-60S. [DOI] [PubMed] [Google Scholar]
- 31.Setlow, P. 2003. Spore germination. Curr. Opinion Microbiol. 6:550-556. [DOI] [PubMed] [Google Scholar]
- 32.Setlow, P. 2006. Spores of Bacillus subtilis: their resistance to radiation, heat and chemicals. J. Bacteriol. 101:514-525. [DOI] [PubMed] [Google Scholar]
- 33.Setlow, P., and A. Kornberg. 1970. Biochemical studies of bacterial sporulation and germination. XXII. Energy metabolism in early stages of germination of Bacillus megaterium spores. J. Biol. Chem. 245:3637-3644. [PubMed] [Google Scholar]
- 34.Sun, D., P. Stragier, and P. Setlow. 1989. Identification of a new σ-factor involved in compartmentalized gene expression during sporulation of Bacillus subtilis. Genes Dev. 3:141-149. [DOI] [PubMed] [Google Scholar]
- 35.Wahome, P., and P. Setlow. 31 July 2007, posting date. Growth, osmotic downshock resistance and differentiation of Bacillus subtilis strains lacking mechanosensitive channels. Arch. Microbiol. doi: 10.1007/s00203-007-0292-z. [DOI] [PubMed]
- 36.Warth, A. D. 1978. Relationship between the heat resistance of spores and the optimum temperature and maximum growth temperatures of Bacillus species. J. Bacteriol. 131:699-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warth, A. D. 1980. Heat stability of Bacillus cereus enzymes within spores and in extracts. J. Bacteriol. 143:27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webb, C. D., A. Decatur, A. Teleman, and R. Losick. 1995. Use of green fluorescent protein for visualization of cell-specific gene expression and subcellular protein localization during sporulation in Bacillus subtilis. J. Bacteriol. 177:5906-5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams, N. D., and A. D. Russell. 1993. Revival of Bacillus subtilis spores from biocide-induced injury in the germination process. J. Appl. Bacteriol. 75:76-81. [DOI] [PubMed] [Google Scholar]
- 40.Xie, C. A., Y.-Q. Li, W. Tang, and R. J. Newton. 2003. Study of dynamical process of heat denaturation in optically trapped single microorganisms by near-infrared Raman spectroscopy. J. Appl. Phys. 94:6138-6142. [Google Scholar]