Abstract
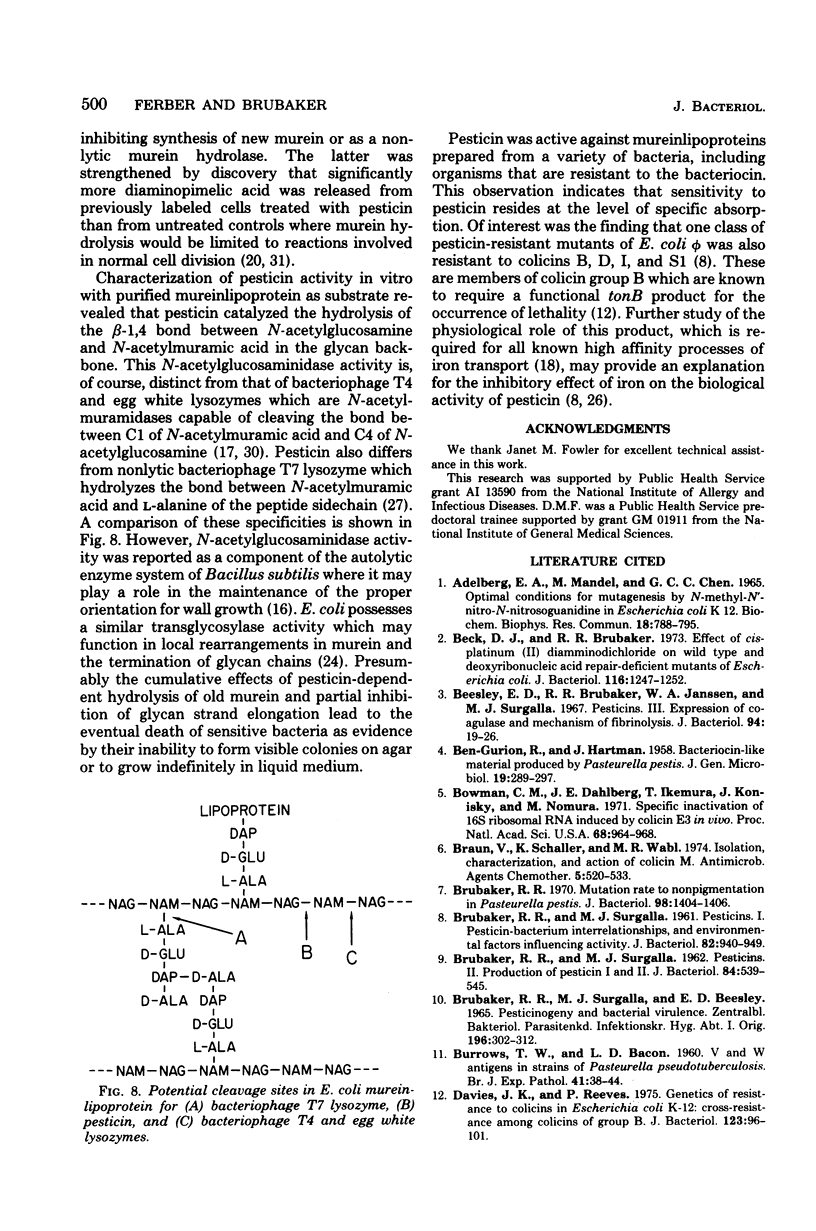
Homogeneous preparations of pesticin, a bacteriocin produced by Yersinia pestis, neither significantly inhibited net synthesis of deoxyribonucleic acid, ribonucleic acid, or protein in Escherichia coli phi nor caused detectable degradation of deoxyribonucleic acid in vivo. Accordingly, its mode of action does not resemble that of colicin E2 as suggested by others. However, incorporation of cell wall-specific label ([14C]diaminopimelic acid) into trichloroacetic acid-insoluble material of growing cells was inhibited by pesticin which also promoted release of such radioactivity from both resting cells and purified mureinlipoprotein. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of reaction mixtures containing appropriately labeled mureinlipoprotein showed that [3H]N-acetylglucosamine comigrated either with [14C]diaminopimelic acid in the murein peptide or with [14C]isoleucine of the Braun lipoprotein. As judged by these findings and pesticin-dependent release of reducing equivalents but not 4-hydroxy-2-acetamido sugars, the bacteriocin possesses N-acetylglucosaminidase activity. Hydrolysis of murein-lipoprotein occurred over a broad pH, with an optimum of 4.7. Mureinlipoproteins from a variety of pesticin-sensitive and -resistant organisms were hydrolyzed by the bacteriocin, indicating that its antibacterial specificity resides at the level of absorption.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEN-GURION R., HERTMAN I. Bacteriocin-like material produced by Pasteurella pestis. J Gen Microbiol. 1958 Oct;19(2):289–297. doi: 10.1099/00221287-19-2-289. [DOI] [PubMed] [Google Scholar]
- BRUBAKER R. R., SURGALLA M. J. Pesticins. I. Pesticinbacterium interrelationships, and environmental factors influencing activity. J Bacteriol. 1961 Dec;82:940–949. doi: 10.1128/jb.82.6.940-949.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUBAKER R. R., SURGALLA M. J. Pesticins. II. Production of pesticin I and II. J Bacteriol. 1962 Sep;84:539–545. doi: 10.1128/jb.84.3.539-545.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W., BACON G. A. V and W antigens in strains of Pasteurella pseudotuberculosis. Br J Exp Pathol. 1960 Feb;41:38–44. [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W., JACKSON S. The pigmentation of Pasteurella pestis on a defined medium containing haemin. Br J Exp Pathol. 1956 Dec;37(6):570–576. [PMC free article] [PubMed] [Google Scholar]
- Beck D. J., Brubaker R. R. Effect of cis-platinum(II)diamminodichloride on wild type and deoxyribonucleic acid repair deficient mutants of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1247–1252. doi: 10.1128/jb.116.3.1247-1252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesley E. D., Brubaker R. R., Janssen W. A., Surgalla M. J. Pesticins. 3. Expression of coagulase and mechanism of fibrinolysis. J Bacteriol. 1967 Jul;94(1):19–26. doi: 10.1128/jb.94.1.19-26.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. M., Dahlberg J. E., Ikemura T., Konisky J., Nomura M. Specific inactivation of 16S ribosomal RNA induced by colicin E3 in vivo. Proc Natl Acad Sci U S A. 1971 May;68(5):964–968. doi: 10.1073/pnas.68.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Schaller K., Wabl M. R. Isolation, characterization, and action of colicin M. Antimicrob Agents Chemother. 1974 May;5(5):520–533. doi: 10.1128/aac.5.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R. Mutation rate to nonpigmentation in Pasteurella pestis. J Bacteriol. 1969 Jun;98(3):1404–1406. doi: 10.1128/jb.98.3.1404-1406.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group A. J Bacteriol. 1975 Jul;123(1):102–117. doi: 10.1128/jb.123.1.102-117.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group B. J Bacteriol. 1975 Jul;123(1):96–101. doi: 10.1128/jb.123.1.96-101.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgat M., Ben-Gurion R. Mode of action of pesticin. J Bacteriol. 1969 May;98(2):359–367. doi: 10.1128/jb.98.2.359-367.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fan D. P., Beckman M. M. New centrifugation technique for isolating enzymes from large cell structures: isolation and characterization of two Bacillus subtilis autolysins. J Bacteriol. 1972 Mar;109(3):1258–1265. doi: 10.1128/jb.109.3.1258-1265.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost G. E., Rosenberg H. Relationship between the tonB locus and iron transport in Escherichia coli. J Bacteriol. 1975 Nov;124(2):704–712. doi: 10.1128/jb.124.2.704-712.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakenbeck R., Messer W. Activity of murein hydrolases in synchronized cultures of Escherichia coli. J Bacteriol. 1977 Mar;129(3):1239–1244. doi: 10.1128/jb.129.3.1239-1244.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P. J., Brubaker R. R. Pesticin-dependent generation of somotically stable spheroplast-like structures. J Bacteriol. 1978 Nov;136(2):786–789. doi: 10.1128/jb.136.2.786-789.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy K. G. Colicinogeny and related phenomena. Bacteriol Rev. 1975 Dec;39(4):464–515. doi: 10.1128/br.39.4.464-515.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschman H. R., Helinski D. R. Purification and characterization of colicin E2 and colicin E3. J Biol Chem. 1967 Nov 25;242(22):5360–5368. [PubMed] [Google Scholar]
- Hu P. C., Brubaker R. R. Characterization of pesticin. Separation of antibacterial activities. J Biol Chem. 1974 Aug 10;249(15):4749–4753. [PubMed] [Google Scholar]
- Hu P. C., Yang G. C., Brubaker R. R. Specificity, induction, and absorption of pesticin. J Bacteriol. 1972 Oct;112(1):212–219. doi: 10.1128/jb.112.1.212-219.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Mirelman D., Sharon N., Schwarz U. Novel type of murein transglycosylase in Escherichia coli. J Bacteriol. 1975 Dec;124(3):1067–1076. doi: 10.1128/jb.124.3.1067-1076.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Arnheim N., Sternglanz R. Bacteriophage T7 lysozyme is an N-acetylmuramyl-L-alanine amidase. J Biol Chem. 1973 Oct 25;248(20):7247–7252. [PubMed] [Google Scholar]
- Kol'tsova E. G., Suchkov Y. G., Lebedeva S. A. Transmission of a bacteriocinogenic factor in Pasteurella pestis. Sov Genet. 1971 Apr;7(4):507–510. [PubMed] [Google Scholar]
- Mirelman D., Kleppe G., Jensen H. B. Studies on the specificity of action of bacteriophage T4 lysozyme. Eur J Biochem. 1975 Jul 1;55(2):369–3-3. doi: 10.1111/j.1432-1033.1975.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Yashouv-Gan Y., Schwarz U. Regulation of murein biosynthesis and septum formation in filamentous cells of Escherichia coli PAT 84. J Bacteriol. 1977 Mar;129(3):1593–1600. doi: 10.1128/jb.129.3.1593-1600.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROODYN D. B., MANDEL H. G. A simple membrane fractionation method for determining the distribution of radioactivity in chemical fractions of Bacillus cereus. Biochim Biophys Acta. 1960 Jun 17;41:80–88. doi: 10.1016/0006-3002(60)90371-1. [DOI] [PubMed] [Google Scholar]
- SMITH D. A., BURROWS T. W. Phage and bacteriocin investigations with Pasteurella pestis and other bacteria. Nature. 1962 Jan 27;193:397–398. doi: 10.1038/193397a0. [DOI] [PubMed] [Google Scholar]
- Schaller K., Nomura M. Colicin E2 is DNA endonuclease. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3989–3993. doi: 10.1073/pnas.73.11.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior B. W., Holland I. B. Effect of colicin E3 upon the 30S ribosomal subunit of Escherichia coli. Proc Natl Acad Sci U S A. 1971 May;68(5):959–963. doi: 10.1073/pnas.68.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surgalla M. J., Beesley E. D. Congo red-agar plating medium for detecting pigmentation in Pasteurella pestis. Appl Microbiol. 1969 Nov;18(5):834–837. doi: 10.1128/am.18.5.834-837.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K., Hedges A. J. The killing of sensitive cells by colicin D. Biochim Biophys Acta. 1972 Mar 14;262(2):200–207. doi: 10.1016/0005-2787(72)90233-x. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Isolation of 4-O-beta-N-acetylmuramyl-N-acetylglucosamine and 4-O-beta-N, 6-O-diacetylmuramyl-N-acetylglucosamine and the structure of the cell wall polysaccharide of Staphylococcus aureus. Biochem Biophys Res Commun. 1966 Jan 4;22(1):48–56. doi: 10.1016/0006-291x(66)90601-2. [DOI] [PubMed] [Google Scholar]



