Abstract
Enterococcus faecalis plasmid pAD1 is a 60-kb conjugative, low-copy-number plasmid that encodes a mating response to the peptide sex pheromone cAD1 and a cytolytic exotoxin that contributes to virulence. Although aspects of conjugation have been studied extensively, relatively little is known about the control of pAD1 maintenance. Previous work on pAD1 identified a 5-kb region of DNA sufficient to support replication, copy control, and stable inheritance (K. E. Weaver, D. B. Clewell, and F. An, J. Bacteriol. 175:1900-1909, 1993), and recently, the pAD1 replication initiator (RepA) and the origin of vegetative replication (oriV) were characterized (M. V. Francia, S. Fujimoto, P. Tille, K. E. Weaver, and D. B. Clewell, J. Bacteriol. 186:5003-5016, 2004). The present study focuses on the adjacent determinants repB and repC, as well as a group of 25 8-bp direct repeats (iterons with the consensus sequence TAGTARRR) located between the divergently transcribed repA and repB. Through mutagenesis and trans-complementation experiments, RepB (a 33-kDa protein, a member of the ParA superfamily of ATPases) and RepC (a protein of 14.4 kDa) were shown to be required for maximal stabilization. Both were active in trans. The iteron region was shown to act as the pAD1 centromere-like site. Purified RepC was shown by DNA mobility shift and DNase I footprinting analyses to interact in a sequence-specific manner with the iteron repeats upstream of the repBC locus. The binding of RepC to the iteron region was shown to be modified by RepB in the presence of ATP via a possible interaction with the RepC-iteron complex. RepB did not bind to the iteron region in the absence of RepC.
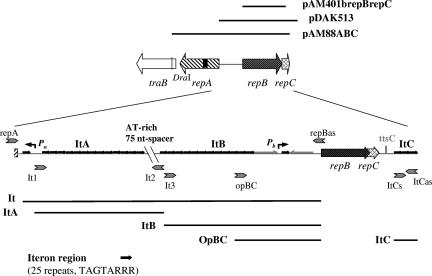
pAD1 is representative of a large and globally disseminated family of conjugative plasmids in Enterococcus faecalis. It is a 60-kb, low-copy-number (one to four copies per chromosome) element that encodes a mating response to the peptide sex pheromone cAD1. It also determines a virulence-related cytolytic (hemolysin/bacteriocin) exotoxin, as well as resistance to UV light. (For recent reviews of pheromone-responding plasmids, see references 6, 7, and 8.) Although aspects of conjugation have been studied extensively, much less is known about the control of pAD1 replication and maintenance. A previous investigation of pAD1 identified a 5-kb region of DNA able to support replication, copy control, and stable inheritance, and it was found that three determinants, designated repA, repB, and repC, were necessary for normal maintenance (46). Data suggested that RepA (336 amino acids) was involved in replication initiation whereas RepB (281 amino acids) and RepC (123 amino acids) were involved in maintaining copy number and stability. As illustrated in Fig. 1, the repA and repB determinants diverge and are separated by a series of iterons, whereas repC is immediately downstream of, and probably transcribed together with, repB. The iterons correspond to two groups of octanucleotide repeats with the sequence TAGTARRR: 13 iterons and 12 iterons are separated by a 75-bp AT-rich (80%) “spacer.” There are also three tandemly arranged iterons located downstream of repC.
FIG. 1.
pAD1 genetic map (not to scale) showing the replication and maintenance region. Putative promoters are indicated by the letter P above the map, and the adjacent arrows indicate the directions of transcription. Transcriptional terminators are represented by tts. Iteron repeats are represented by short black arrows. Thick gray arrows (above and below) represent the positions and orientations of the various primers (specified in Table 2) used in generating PCR products. Different DNA fragments specifically analyzed are indicated by various lines labeled accordingly.
Recently, the pAD1 replication initiator (RepA) and the origin of vegetative replication (oriV) were identified and characterized (15). It was found that repA alone, under an artificial promoter, was sufficient for replication when cloned on a plasmid devoid of replication ability. The chimera maintained itself at a high copy number (20 to 30 copies per chromosome), in contrast to the low copy number of intact pAD1. In addition, the replication origin was found to be located within a 170-bp segment of repA, which alone could facilitate replication in cis as long as RepA was provided in trans. RepA was also found to bind specifically to several sites containing inverted-repeat sequences within oriV and bound nonspecifically to single-stranded DNA (15).
Whereas RepA alone was able to support replication, the high-copy-number repA chimera was not highly stable in that after 30 generations of unselected growth in broth about 60% of the cells were observed to have lost the plasmid (15); an efficient partitioning system was clearly missing. Good candidates for components of a partition system in the intact pAD1 are the iterons adjacent to repA, together with the products of repB and repC. A BLAST search indicated that RepB has significant homology with members of the “ParA” family of partition proteins, including the essential Walker motifs associated with ATPase activity (38), and although a RepC search revealed no significant group of homologues, it did show homology with a similarly small replication-associated protein encoded by pTE15 (32% identity and 59% similarity; accession number AAC02984). repC would not be inconsistent with a ParB-like component, as such elements frequently do not exhibit homology (20). Since partitioning systems frequently include a cis-acting site consisting of a series of repeat sequences, it appears reasonable that the iterons adjacent to repB may be part of such an apparatus. The present study provides data indicating that repB, repC, and the iterons of pAD1 indeed represent an active partitioning system.
(Much of the information reported here was part of a communication presented at the 2nd International ASM-FEMS Conference on Enterococci, Helsingor, Denmark, 2005, by M. V. Francia.)
MATERIALS AND METHODS
Bacterial strains, plasmids, oligonucleotides, media, and culture conditions.
The Escherichia coli K-12 and E. faecalis strains, plasmids, and synthetic oligonucleotides used in this study are listed in Tables 1 and 2. E. faecalis strains were grown in Todd-Hewitt broth (THB) (Difco Laboratories, Detroit, MI) at 37°C. E. coli strains were grown in Luria-Bertani (LB) broth (42). Plating was on THB or LB agar, respectively. The following antibiotics were used at the indicated concentrations for E. faecalis: erythromycin, 20 μg/ml; chloramphenicol, 20 μg/ml; rifampin, 25 μg/ml; and fusidic acid, 25 μg/ml. For E. coli, the concentrations were as follows: ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; chloramphenicol, 25 μg/ml; erythromycin, 200 μg/ml; and nalidixic acid, 20 μg/ml. All antibiotics were obtained from Sigma Chemical Co. X-Gal (5-bromo-4-chloro-3-indolyl-d-galactopyranoside) and IPTG (isopropyl-β-d-thiogalactoside) were from Invitrogen and were used at concentrations of 40 μg/ml and 1 mM, respectively.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant features | Reference |
|---|---|---|
| Strains | ||
| E. faecalis | ||
| JH2-2 | rif fus | 26 |
| UV202 | rif fus recA | 50 |
| E. coli | ||
| DH5α | λ− φ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK−) supE44 thi-1 gyrA relA1 | BRL |
| BL21(DE3) C41 | F−ompT hsdSB(rB− mB−) gal dcm (DE3) | 34 |
| Plasmids | ||
| pAM714 | pAD1::Tn917 erm Agg Tra | 25 |
| pAM714SW2 | DryC phase variant form of pAM714 | 24 |
| pAM88 | Suicide vector; cm p15A cm(+) | 14 |
| pAM88ABC | pAD1 rep region cloned in pAM88 | 15 |
| pAM88ABC17 | pAM714SW2 rep region cloned in pAM88 | This study |
| pAM401 | E. coli-E. faecalis shuttle vector; cm(+) | 49 |
| pDAK513 | pAD1 iteron-repB-repC region cloned in pAM401 | This study |
| pTAd | E. coli cloning vector; ap km | Clontech |
| pTAdIt | It region in pTAd | 15 |
| pTAdItA | 5′ Iteron repeats (Fig. 1) in pTAd | 15 |
| pTAdItB | 3′ Iteron repeats (Fig. 1) in pTAd | 15 |
| pTAdItC | ItC repeats (Fig. 1) in pTAd | 15 |
| pAM434brepA(pA) | bac + repA cloned in pAM434 | 15 |
| pAIt | pAD1 iteron region cloned in pA | This study |
| pAItA | pAD1 ItA repeats cloned in pA | This study |
| pAItB | pAD1 ItB repeats cloned in pA | This study |
| pAItC | pAD1 ItC repeats cloned in pA | This study |
| pAM401It | pAD1 iteron region cloned in pAM401 | This study |
| pSU18bac* | Modified bac promoter cloned in pSU18 | 15 |
| pAM401b | Modified bac promoter cloned in pAM401 | This study |
| pAM401brepBrepC | bac + repB and repC genes cloned in pAM401 | This study |
| pAM401brepB(-)repC | repB frameshift mutant in NdeI | This study |
| pAM401brepBrepC(-) | repC frameshift mutant in BamHI | This study |
| pET30c | Expression vector | Novagen |
| pASK60 | Expression vector | Biometra |
| pET30RepC | repC gene cloned in pET30b | This study |
| pASK60RepB | repB gene cloned in pASK60 | 15 |
| pBlueScriptoriV | oriV region (Fig. 1) in pBluescript | 15 |
| pTAdopBC | 3 It repeats upstream of repB (Fig. 1) in pTAd | This study |
TABLE 2.
Oligonucleotides used in this study
| Primer | Sequence (5′-3′) | Plasmid(s) generated |
|---|---|---|
| RepA | TTCATTGTAAATACGGTT | pTAdIt, pAIt, pAM401It |
| RepBas | CTTCCCAACGCCGCC | pTAdIt, pAIt, pAM401It, pAItB, pTAdItB, pTAdopBC |
| ASK60repB.1 | TTTAACGGCCGGCATGGTTAAAAAAATTGTATT | pAM401brepBrepC |
| ASK60repC.2 | ATTTTTCGCGATTTTTGTTTCTTTTTGTCTCGTGA | pAM401brepBrepC |
| ETRepC.1 | CCCCATATGAGTAAGTATACATTTCATAAGGA | pET30RepC |
| ETRepC.2 | CCCCTCGAGTTTTTGTTTCTTTTTGTCTCGTGA | pET30RepC |
| It1 | TTAAGAATACCAAAACATTATT | pTAdItA, pAItA |
| It2 | CCTTTCTACAAAAGGATT | pTAdItA, pAItA |
| It3 | CCTTTTGTAGAAAGGTT | pTAdItB, pAItB |
| ItCs | TTTTTTACTATCTTACTATTTTACTAC | pTAdItC, pAItC |
| ItCas | ATTTTTTGTAGTAAAATAGTAAGATAG | pTAdItC, pAItC |
| OpBC | GATAGTAAAATAGTAAATTTTATAAATATTATTTGTAG | pTAdopBC |
| Itseq1 | AGCTTGGTACCGAGCTCGGA | DNase I footprint |
| Itseq2 | CTAGATGCATGCTCGAGCGG | DNase I footprint |
Standard molecular techniques.
Recombinant plasmids were generated in E. coli DH5α. Introduction of plasmid DNA into bacterial cells was by transformation, as previously described (9, 22). Electrotransformation of E. faecalis was as described by Flannagan and Clewell (13). Plasmid DNA was purified from E. coli using established techniques described elsewhere (42). Isolation of plasmid DNA from E. faecalis was also as previously described (45). When necessary, DNA fragments were purified with silica gel, as described by Boyle and Lew (5). Recombinant DNA methodology, as well as analyses of plasmid DNA using restriction enzymes and agarose gel electrophoresis, involved procedures described by Sambrook et al. (42). Restriction enzymes were purchased from Roche, and reactions were carried out under the conditions recommended by the manufacturers. PCR was performed with a Perkin-Elmer Cetus apparatus under conditions recommended by the manufacturer. Specific primers were purchased from Invitrogen and Taq DNA polymerase from Bioline. PCR-generated fragments were purified by using QIAquick spin columns (Qiagen). Ligations made use of T4 DNA ligase from New England Biolabs. Nucleotide sequence analyses were carried out at either the University of Michigan or the Hospital Universitario Marqués de Valdecilla sequencing core facility or using the “fmol DNA cycle-sequencing system” as specified by the manufacturer (Promega).
Plasmid constructions.
Different segments of pAD1 included in the replication-maintenance region were amplified by PCR from template pAM714 using the oligonucleotides indicated in Table 2 and cloned into pTAd (Clontech) via TA cloning. From there, XbaI/HindIII fragments representing either the complete pAD1 repA-repB intergenic (iteron-containing) region (It), the repA upstream iteron region (ItA), the repB upstream iteron region (ItB), or the repC downstream iteron region (ItC) were subcloned into the E. faecalis-E. coli shuttle vector pAM434brepA (abbreviated here as pA) (15), generating the plasmid pAIt or pAItC, respectively (Fig. 1). An XbaI/BamHI It DNA fragment was subcloned into pAM401 (49), generating plasmid pAM401It. The repB and repC coding sequences in pAM401 were cloned in several steps. The fragment repB repC contained in pTAd (Clontech) was generated by digestion using the restriction enzymes EagI/NruI, purified, and cloned in the BsaI and Eco47III sites of pSU18b* (15). Next, the SalI/XbaI fragment, which now contained the genes behind the modified bac promoter, was subcloned into pAM401, generating the plasmid pAM401brepBrepC. pAM401b was obtained by subcloning instead the SalI/XbaI fragment, which contained only the modified bac promoter derived from pSU18b*. The corresponding plasmid derivatives containing repB and repC mutants, pAM401brepB(−)repC and pAM401brepBrepC(−), were obtained by NdeI or BamHI digestion, respectively; filling with Klenow; and religation. A fragment of DNA containing the repC sequence was amplified by PCR from pAM714 using the primers ETrepC.1 and ETrepC.2, digested with NdeI and XhoI, and cloned into the same sites of pET30c to construct the plasmid pET30RepC. In the expression vector pET30, RepC is under the control of the T7 promoter. All the constructions were confirmed by DNA sequencing.
Stability assays.
Stability assays were performed in E. faecalis JH2-2. Test plasmids were transformed into the bacterial hosts and spread onto THB agar plates containing the appropriate antibiotics. A fresh transformant was used to inoculate a flask of 10 ml of THB containing antibiotics, and the culture was grown to late log phase. Ten microliters of the culture was then inoculated into 10 ml of prewarmed THB lacking the corresponding antibiotics and grown for 30 generations, at which time a sample of the culture was spread onto nonselective medium. About 300 colonies were picked and tested for plasmid retention by stabbing them into selective media. The data shown represent the average of at least two independent experiments.
Protein purification and analysis.
The His-tagged RepC fusion protein was purified from the derivative C41 of E. coli BL21(DE3) (34) induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) by a Ni-agarose column as described in the manufacturer's instructions (QIAGEN GmbH). The Strep-tagged fusion protein RepB was purified from recombinant E. coli JM83 induced with 1 mM IPTG by a streptavidin-immobilized column as described in the manufacturer's instructions (Boehringer Mannheim). All protein preparations used in DNA-binding studies were at least 90% pure based on sodium dodecyl sulfate-polyacrylamide gel electrophoresis estimates, according to the method of Laemmli (31). Gels were stained with Coomassie brilliant blue R-250.
DNA-binding experiments.
Double-stranded DNA containing iteron repeat fragments for binding assays was generated as previously described (15). Gel mobility shift assays were performed basically as described previously (15). Briefly, labeled DNA fragments (1 pmol) were incubated with various amounts of either RepC, RepB, or both protein preparations, as indicated, for 15 min at 30°C in 20-μl volumes containing 50 mM Tris, pH 7.5, 100 mM NaCl, 0.2 mM EDTA, 5% glycerol, 1 mM dithiothreitol, 1.5 μg poly(dI-dC) DNA, and 0.7 μg of bovine serum albumin. Unlabeled competitor DNA fragments and/or specific antibodies or 1 mM of ATP or ATPγS was also used as indicated. After the incubation period, the binding reaction mixtures were placed on ice, loaded onto a 5% prerun polyacrylamide gel, and electrophoresed at room temperature in 0.5× Tris-borate-EDTA buffer. After electrophoresis, the gel was dried on Whatman 3MM paper and exposed to X-ray film at −70°C using an intensifying screen.
DNase I footprinting assays were carried out as described elsewhere (16). The same DNA fragments containing the iteron repeats that were used in the mobility assays were also used in DNase I protection assays; however, the fragments were labeled at only one end. Two picomoles of labeled DNA aliquots was incubated with various amounts of either purified RepC, RepB, or both, as indicated, in a buffer containing 50 mM Tris (pH 7.5), 100 mM NaCl, 5 mM MgCl2, 1 mM CaCl2, 5% glycerol, 1 mM dithiothreitol, 1 mM ATP, 0.2 mM EDTA, 1.5 μg of poly(dI-dC) DNA, and 0.7 μg of bovine serum albumin in a 20-μl final volume for 15 to 20 min at 30°C. Then, 10−3 DNase I Kunitz units were added to each binding reaction mixture and incubated for 15 min at 30°C. The resulting products were separated on 6% polyacrylamide sequencing gels and visualized by autoradiography.
RESULTS
RepB and RepC operate in trans on cis-acting iterons.
To analyze the components of the hypothesized pAD1 partitioning system, we constructed a series of plasmids carrying parts of the related region and examined the effects on plasmid stability. The vector used to test stability was pA (Fig. 2). pA is the previously reported (15) high-copy-number plasmid chimera (4.6 kb) containing repA under an artificial promoter (a variant form of a bacteriocin promoter in which the −10 box contained CATAAT rather than TATAAT). pA contains a multiple cloning site, and it is able to replicate efficiently and express resistance to erythromycin in both E. coli and E. faecalis. Insertion of an XbaI/HindIII fragment containing various iteron representations (created by PCR; see Materials and Methods) into the multiple cloning site generated the plasmids pAIt, pAItA, pAItB, and pAItC (Fig. 1 and 2). DNA containing the repB and repC determinants or segments containing a mutation in one determinant or the other were cloned in the compatible plasmid pAM401b downstream of the engineered bac promoter (Fig. 2). After the two types of plasmids were combined, selection for the pAM401b derivatives was maintained using chloramphenicol to determine the stability of the pA derivatives. As seen in Table 3, the insertion of the XbaI/HindIII fragments containing any of the four iteron representations resulted in a major destabilization of the plasmid in the absence of repB and repC. After 30 generations in broth without selection, the pA derivatives were lost from 99% or more of the population. Without the iterons present, the plasmid (i.e., pA) was lost from only 60% of the cells. The presence of both RepB and RepC in trans (plasmid pAM401brepBrepC) led to a major restabilization of the plasmids containing the iterons, except for pAItC, which contained only three iteron repeats. Indeed, stability was significantly greater than even that of the pA plasmid devoid of iterons, i.e., loss by only 1 to 18% of the cells (Table 3). The stability of iteron-free pA was not affected when both RepB and RepC were provided in trans. Although the destabilization of the pA derivatives was surprising, it is clear that regions of DNA containing 12 tandem iterons or more are sufficient to ensure plasmid stabilization by the RepB and RepC proteins and appear to constitute centromere-like sites. In contrast, the pAItC chimera, which contained only three iteron sequences, was not stabilized by these proteins.
FIG. 2.
Schematic representation of plasmid constructions used in stability studies. The restriction sites used are indicated. The two clusters of iteron repeats are indicated by arrows labeled ItA and ItB. The shorter ItC cluster represents three iterons located downstream of repC. The bac promoter is represented by a triangular arrowhead, while the pAD1 replication initiator gene repA is represented by a hatched gray arrow. The repB and repC determinants are indicated on the right, along with designations noting the corresponding frameshift mutations, labeled as (-) in each case. The plasmid pA has a determinant for erythromycin resistance (Err), and pAM401b has a chloramphenicol resistance (Cmr) marker.
TABLE 3.
Plasmid segregational stability mediated by wild-type and mutated pAD1 par open reading frames
| Coresident plasmid | % pAIta | % pAa | % pAItAb | % pAItBb | % pAItCb |
|---|---|---|---|---|---|
| pAM401b | 0 | 40 | 0 | 0 | 1 |
| pAM401brepBrepC | 95 | 35 | 82 | 99 | 4 |
| pAM401brepB(-)repCc | 35 | NDe | ND | ND | ND |
| pAM401brepBrepC(-)d | 0 | ND | ND | ND | ND |
Measured as the percentage of plasmid retention after 30 generations of nonselected growth, it represents the average of three independent experiments. At least 300 colonies were tested in each case.
Measured as the percentage of plasmid retention after 30 generations of nonselected growth, it represents the average of two independent experiments. At least 300 colonies were tested in each case.
The repB open reading frame was disrupted by a frameshift in the NdeI restriction site.
The repC open reading frame was disrupted by a frameshift in the BamHI restriction site.
ND, not determined.
A frameshift mutation in repC (see Material and Methods) resulted in loss of pAIt stability (>99% loss), whereas a frameshift mutation in repB resulted in a 35% retention rate for pAIt (Table 3), indicating that both protein products were needed for maximal stabilization. It is interesting that when RepB was absent but RepC was present, segregation of pAIt was similar to that of pA, suggesting that an interaction of RepC alone with iterons is enough to neutralize the iteron-promoted destabilization but not enough to further stabilize the plasmid.
It is not clear why the presence of iterons resulted in a major destabilization; conceivably it derived from interaction with a host- or plasmid-encoded product or even a structural effect caused by the repeats themselves. To further examine this question, the It fragment (Fig. 1) was cloned in pAM401, a commonly used E. coli-E. faecalis shuttle vector (49), giving rise to pAM401It, and its stability was tested. No significant differences were observed compared to the iteron-free pAM401 (15% versus 18% plasmid retention, respectively), implying that neither the repeats themselves nor their interaction with a host factor is responsible for plasmid destabilization. A likely candidate for a protein interacting with iterons and destabilizing the plasmid is therefore the RepA product encoded by the pA derivatives. In this respect, the ability of RepC alone to stabilize pAIt to the level of pA would be due to interference with RepA binding to the iterons, not a legitimate partition effect. Both, RepB and RepC are needed for stabilization beyond the basal level of vector stability, and therefore, both are required for active partitioning of pAD1. Alternatively, the differences in the effects of the iterons on pA and pAM401 may be due to different mechanisms of replicon function or differences in the distances of the inserted iterons from the replicon.
To determine if the apparent partition system was functionally independent of a repA-controlled plasmid, a fragment containing the iterons, repB, and repC was cloned in the unrelated replicon pAM401 (49) to generate pDAK513 (Fig. 1). In this case, stability studies showed that after 30 generations pDAK513 was maintained in approximately 90% of the population, in contrast to less than 20% in a control culture carrying “empty” pAM401 grown without selection, suggesting active partitioning.
RepC binds directly to the pAD1 iterons.
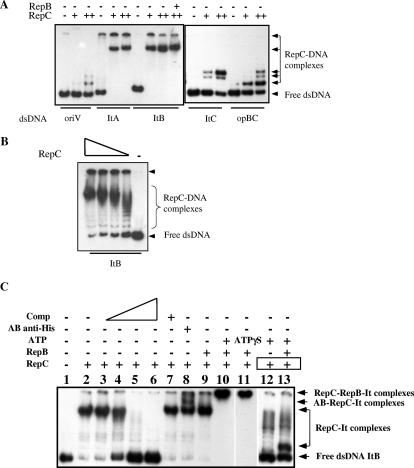
It has been shown that ParB-like components from gram-negative systems recognize and bind to their corresponding centromeres. To determine if this is also the case for pAD1, RepC bearing a His tag at the C terminus was purified to more than 95% purity as described in Materials and Methods and examined using a gel band retardation technique for its ability to bind to iteron DNA. The protein was incubated with various 32P-labeled linear DNA fragments, and the mixtures were subjected to polyacrylamide gel electrophoresis. Under these conditions, protein-DNA complexes migrate more slowly through the gel than free DNA fragments. The results, shown in Fig. 3A, indicate that a binding activity in the RepC preparation specifically recognized DNA containing the iteron repeats, forming a distinct retarded complex, independently of the iteron-containing fragment tested. In contrast, DNA not containing iterons but rather containing oriV was not significantly bound unless an elevated concentration of protein was used (in this case, a small amount of binding was evident and involved much less retardation). Although not shown, similarly prepared RepB, under the same conditions, did not bind any of the fragments tested. As can be seen in Fig. 3B, when the RepC concentration was decreased, a series of bands was resolved, suggesting there is cooperative binding to the iteron sequences on the DNA fragment. Although only shown for ItB, similar results were obtained for ItA and ItC fragments. The addition of unlabeled iteron-containing DNA exhibited competition resulting in reduced RepC binding to the related labeled DNA (Fig. 3C, lanes 3 to 6), whereas addition of unlabeled oriV-containing DNA showed no effect (lane 7). Supershift experiments using anti-His tag antibody showed that in fact the RepC protein participates in the DNA-protein complexes detected (Fig. 3C, lane 8).
FIG. 3.
Gel mobility shift assays showing RepC-DNA binding. PCR or end-labeled double-stranded DNA (dsDNA) fragments containing oriV or iteron repeats were incubated with purified RepC with or without purified RepB in the absence or presence of increasing concentrations of unlabeled competitor DNA fragments or specific antibodies. (A) Assays showing RepC-specific binding to dsDNA. The substrate DNA used is indicated at the bottom of the figure as follows: lanes 1 to 3, dsDNA corresponding to the repA MfeI/RsaI internal sequence (oriV); lanes 4 to 6, dsDNA (XbaI/HindIII restriction fragment) corresponding to the repA-proximal cluster of iterons (ItA); lanes 7 to 10, dsDNA (XbaI/HindIII restriction fragment) corresponding to the repB-proximal iteron repeats (ItB); lanes 11 to 13, dsDNA (XbaI/HindIII restriction fragment) corresponding to the repC-proximal iteron repeats (ItC); lanes 14 to 16, dsDNA (XbaI/HindIII restriction fragment) containing the last three repB-proximal iteron repeats (OpBC). Purified protein added in each lane is indicated at the top of the figure: 0.1 (+) or 1.0 (++) μg of RepC and 1.0 μg of RepB were used. Free DNA forms and RepC-DNA complexes are indicated. (B) Assays showing RepC cooperative binding to the iteron repeats. Decreasing amounts of RepC (from left to right, 50, 10, 5, 1, and 0 ng, respectively) were incubated with the XbaI/HindIII dsDNA fragment corresponding to the ItB region. Free DNA forms and RepC-DNA complexes are indicated. (C) Competition and supershift experiments showing RepC-specific binding to the iteron repeats (ItB). Increasing amounts of specific unlabeled DNA fragments (1-, 10-, 50-, and 100-fold excess) were incubated with a fixed amount of purified protein as indicated at the top of the figure (lanes 3 to 6). A 100-fold excess of unrelated unlabeled DNA (oriV) was incubated instead in lane 7. Supershift experiments using specific anti-His antibody incubation after the binding reaction are shown in lane 8. The effects of RepB in RepC recognition and binding in the absence or presence of 1 mM ATP or ATPγS are shown in lanes 9, 10, and 11, respectively. The protein levels used were 50 ng of RepC with/without 1 μg of RepB, as indicated at the top of the figure (lanes 2 to 11). Five nanograms of RepC and 12.5 ng of RepB were used for the last two lanes, as also shown at the top. Free DNA forms and RepC-DNA or RepB-RepC-DNA complexes are indicated. Also, specific anti-His antibody-RepC-DNA complexes are shown.
RepB does not bind to iterons directly but binds to the RepC-iteron complex.
Although RepB did not bind directly to iteron-containing DNA fragments (see above), it did bind to RepC-iteron complexes in the presence of ATP (compare lanes 9 and 10 in Fig. 3C). A similar complex was obtained in the presence of the nonhydrolysable ATPγS (lane 11), implying that hydrolysis of ATP is not needed to maintain the complex. Again, although results are shown only for ItB, similar data were obtained using ItA and ItC.
Next, to examine the influence RepB might have on the cooperativity of RepC binding to the iterons, equimolar concentrations of RepB and RepC in the binding mixture were used. The formation of a smaller complex was now observed, presumably reflecting a RepB enhancement of the binding of one RepC molecule to one iteron repeat sequence (Fig. 3C, compare lanes 12 and 13), suggestive of loss of the RepC cooperativity described above. Thus, the relative RepB concentration appears to play an influential role in the binding of RepC to iterons.
Further evidence that RepC binds specifically to the iterons.
Figure 4A shows the results of DNase I protection experiments conducted at two concentrations of protein (50 ng and 0.5 μg). At the lower concentration of protein, no protection was observed; however, at the higher concentration, RepC was easily seen to have protected a region of DNA corresponding to the iteron repeats. In contrast, RepB exhibited no protection, which is consistent with the mobility shift results showing no direct interaction between RepB and the iterons. Due to RepC cooperative binding and the high number of repeats present in the DNA fragment used, we were unable to see if RepC showed preferential binding to any of the iteron repeats. As was shown in Fig. 3A, RepC still recognized and bound readily to DNA fragments containing just three iteron repeats (as ItC and opBC). When a shorter fragment of DNA containing only the last three repB-proximal iterons (opBC) (Fig. 1) was used in the DNase I footprinting assays, protection by RepC was still observed, and in the presence of both RepB and RepC, an extension in the region of protection corresponding to the inverted repeats upstream of repB was evident (Fig. 4B). This extended protection was more clearly seen in the “lower strand” and is conceivably related to influence that RepB may have on the binding of RepC to the repB-proximal iteron, perhaps via a simultaneous interaction or effect on the inverted repeat.
FIG. 4.
DNase footprinting analysis of the RepC-iteron repeat interaction. One-end-labeled double-stranded DNA fragments containing the iteron repeats were incubated with purified RepC with or without purified RepB, and the binding reaction mixtures were then subjected to partial DNase I digestion and separated on a denaturing sequencing gel. (A) Interaction of RepC on the coding strand of the iteron DNA fragment ItA. Sequencing ladders T, C, G, and A generated using the same DNA fragment as a template and the fmol DNA cycle-sequencing system (Promega) are included (left lanes). Two picomoles of labeled DNA fragments was incubated alone or with various amounts of proteins as indicated at the top of the figure: 50 ng of purified RepC or 0.5 μg of RepB (central lanes) and 0.5 μg of RepC or 5 μg of RepB, (right lanes) prior to DNase I digestion. DNase I protection was shown over the 13 iteron repeats contained in the DNA fragment tested, as indicated by the arrows on the left. (B) Interaction of RepC on the coding (left; “upper”) and noncoding (right; “lower”) strand of the iteron DNA fragment OpBC (Fig. 1). Again, 2 picomoles of labeled DNA fragments was incubated alone or with increasing concentrations of purified RepC (5, 10, and 20 ng) and RepB (12.5 and 25 ng), as indicated at the top of the figure. Iteron repeats on the tested DNA fragment are represented by black arrows on the left of each gel. Other inverted-repeat sequences are shown by gray arrows. The hatched bars represent the DNase I protection regions when RepC and then RepB were added. The gray arrowheads on the left of each gel indicate the extension in the region of protection in the presence of both RepB and RepC.
Effect of change in the iteron number.
pAD1 has been shown to undergo a reversible phase variation that results in a constitutive expression of conjugation functions (24, 41). Variants are distinguishable by their colony morphologies, whereby cells that exhibit a “switched-on” conjugation system give rise to “dry” colonies (Dryc) compared to the more “watery” colonies (Dry+) characteristic of the “switched-off” state. (The possible basis of this phenomenon is noted in Discussion below.) The spontaneous event occurs at a frequency of 10−4 to 10−3 and involves a change in the number of iterons located between repA and repB. For example, in the case of cells in the Dryc state, the cluster of 13 iterons may instead have 17 iterons (24). In the present context, it was of interest to determine if the change in the number of iterons could affect stability, and the following experiment was conducted in an attempt to answer this question.
pAM88 is an E. coli vector unable to replicate in E. faecalis, but it carries a chloramphenicol resistance gene able to be expressed in gram-positive bacteria (15). A 3-kb fragment, which contained pAD1 iterons, repB, and repC, as well as repA, was cloned into the multiple cloning site of pAM88, generating pAM88ABC (Table 1), and introduced into JH2-2. A similar construct was generated using a fragment from a Dryc phase variant containing 17 iterons in place of the 13-iteron set (pAM88ABC17). When the stabilities of the two constructs were compared, the difference was only slight and thus of questionable significance. After 30 generations of unselected growth, there was 96% maintenance of pAM88ABC compared to 85% in the case of pAM88ABC17. A significant effect on stability would therefore not appear to be a consequence of phase variation, at least under the conditions tested.
DISCUSSION
Partitioning systems are ubiquitous among plasmids and frequently consist of two plasmid-encoded proteins, generically called ParA and ParB, that act in trans on a cis-acting centromere-like DNA site, sometimes called parS, to facilitate efficient partitioning (3, 10, 19, 20, 23). ParB generally binds to the parS site, which frequently contains a number of repeat sequences, whereas ParA, an ATPase, interacts with the ParB-centromere complex. Significant homology is typical among the ParA proteins of different plasmid systems, but usually there is little, if any, similarity among ParB proteins. Most of the reported characterized systems have involved plasmids of gram-negative bacteria; however, similar functional systems among gram-positive bacteria have recently been identified (28, 48, 51). Clearly, the results from the present study are consistent with RepB and RepC corresponding to pAD1 ParA and ParB, respectively, and the iterons correspond to the “centromere” (parS). Functional analyses showed that RepB and RepC acted in trans to efficiently stabilize a plasmid chimera containing iteron sequences. RepC was found to be absolutely necessary to stabilize a plasmid containing the pAD1 centromere. The very efficient partitioning that occurs with the additional presence of RepB is consistent with the latter protein serving to “connect” the DNA-RepC complex with a host segregation system, as suggested for other partition systems (19). The observations were consistent with the in vitro data showing that RepC was able to bind to the iterons even in the absence of RepB, representing one of the initial steps in the formation of the pAD1 partition complex. Then, RepB (which was not itself able to bind to iterons) would bind to RepC-iteron complexes in the presence of ATP. Based on the general organization of pAD1 having a functional centromere upstream of the partitioning determinants and the characteristics of RepB (a ParA-like protein lacking an N-terminal HTH motif for DNA binding) and RepC (a relatively short ParB DNA-binding protein with a sequence unrelated to other known ParB-like components), the partitioning system fits most closely with the Type Ib category as defined by Gerdes et al. (20). However, unlike the in typical type Ib systems, a set of three downstream iterons might also play a role in partitioning.
RepB-RepC-iteron complex formation was found to depend on the relative RepB concentration and the presence of ATP. RepB did not bind to the RepC-It complex in the absence of ATP, in contrast to what has been reported for TP228 partitioning, the paradigm of the Type Ib systems (1, 2). Our data implying the importance of maintaining an appropriate RepC/RepB ratio for partition are consistent with what has been reported previously for the corresponding components encoded by the well-studied F, P1, and TP228 systems (1, 4, 18, 32, 36, 37). At a high RepC/RepB ratio, recruitment of RepB to the RepC-It complex occurred, and cooperative binding of RepC to the iteron repeats located on the same DNA fragment was maintained, as shown by the high-molecular-weight complexes obtained in the corresponding mobility shift assays. However, when this ratio was reduced to equimolar concentrations of both proteins, cooperativity was lost and RepC binding to just one iteron repeat appeared to be favored. RepB might therefore influence an intrinsic pairing activity of RepC, similar to what has been reported for the R1 par system (27), allowing a step involving the “pairing” of plasmids prior to pAD1 segregation.
DNase I footprinting assays done using only the three iteron sequences closest to repB indicated that while RepC showed binding, the addition of RepB resulted in extended protection over inverted repeats that flanked the isolated repB-proximal iteron. Since this region includes the repBC promoter, it is conceivable that the interactions reflect a self-regulatory aspect of expression of the partitioning proteins. Indeed, a number of partitioning proteins are known to negatively regulate their own genes (19). In this regard, preliminary experiments have shown that indeed, RepC represses the repBC promoter (fourfold) (M. V. Francia, unpublished data). Alternatively, it could represent a key process in the pAD1 partition mechanism. However, efficient partitioning by ItA alone seems to rule out this possibility, as similar inverted repeats are absent in ItA. As no obvious effects of ParA homologues on the mobility shift and/or the DNase I protection patterns were observed in Type Ib par systems (with the exception of TP228 mentioned above), some mechanistic differences among them are possible (1, 30, 51).
pAD1 is believed to replicate by a theta-type mechanism based on its low copy number and the fact that RepA exhibits strong homology with initiator proteins associated with other theta replication plasmids (15). Many low-copy-number plasmids have iteron sites located close to the initiator determinant, and in many cases, there is evidence that these sequences, along with an adjacent AT-rich segment of DNA, are involved in assembly of the replisome and represent the replication origin (29). In the case of pAD1, however, as well as some other plasmids with RepA homologues, the origin of replication, including specific repeat sequences, is located within the repA determinant (12, 15, 21, 43, 44). The rather extensive iteron system of pAD1 with its two clusters separated by an AT-rich region (Fig. 1) was at first suggestive of a replication origin (46); subsequent analyses, however, demonstrated that this was not the case (15). In this respect, the basis of a significant instability resulting from the presence of iterons in pA, in the absence of RepC, was not clear, but the fact that the addition of iterons to the unrelated pAM401 did not result in destabilizing the plasmid implied that it was not due to a host factor or a structural effect of the iterons. We are currently investigating the possibility that RepA might actually be involved in this phenomenon. If the iteron-enhanced instability of the pA iteron derivatives turns out to be related to RepA interaction with the iterons, such interaction might be related in some way to an autoregulation of repA expression. Other examples showing iterons involved in replication initiator expression control have been well documented in the literature (11). In this sense, a possible coregulation of pAD1 replication and partitioning might be needed, as has been reported for the gram-negative R1 plasmid and the RepABC plasmids of Rhizobiaceae, to ensure plasmid segregation (33, 35, 39).
In addition to their roles in partitioning, the pAD1 iteron arrays are also known to be connected with a phase variation event related to a reversible switching on of the expression of the pAD1 conjugation system at a frequency of about 10−4 to 10−3 (24). The phenomenon was found, surprisingly, to be associated with an increase in the number of iterons. Variants were noted in three independent cases to involve a 32-bp insertion corresponding to an increase from 13 to 17 of the octanucleotide iterons in the repA-proximal array. A fourth variant involved an insertion of a 31-bp duplication of the region that includes the isolated iteron in the repB promoter (24). The basis of the connection between the iteron number and the expression of conjugation functions is not certain, although it was speculated that changes in the expression of one or more of the diverging flanking determinants may lead to binding of one of the protein products to iteron-like sequences located within traA, a key negative regulator of the conjugation system (17, 40). From an evolutionary perspective, a mechanism by which changes in the iteron structure/number affects the conjugation potential could be advantageous. For example, such changes might have a negative effect on partitioning and reduce the stability of the plasmid, which could be compensated for by enhancement of plasmid survival via conjugation. In this regard, the stability of the repA-iteron-repB-repC region from a phase variant when cloned in pAM88 was only slightly, if at all, reduced (85% versus 96% loss); however, it should be kept in mind that the test system involved an “artificial” high-copy-number plasmid. It is conceivable that the stability of the wild-type low-copy-number pAD1 could be more sensitive to differences in the DNA structure.
Other pheromone-responding plasmids, such as pCF10, pPD1, and pAM373, are similar to pAD1 in that they have repA and repB homologues adjacent to a key negative regulator of conjugation equivalent to traA, and the presence of similar partitioning systems appears likely (47). However, there is little if any similarity among their repC equivalents, a feature consistent with the fact that while iterons are present, they are very different in sequence and their numbers are much lower. The sequence differences among the ParB-like analogs of pheromone-responding plasmids and their respective iterons may have evolved as a strategy to ensure that coresident plasmids harboring these diverse partitioning systems are maintained and distributed accurately. (E. faecalis clinical isolates carrying more than two pheromone-responding plasmids have been described [6].)
Plasmid partitioning systems play an important role in the survival of plasmids. The results reported here for pAD1, as well as two other recent reports (28, 51), show that systems closely resembling those found in a number of gram-negative bacteria are also functional in at least some gram-positive organisms. The system of pAD1 characterized here represents the first among the widely disseminated enterococcal pheromone-responding plasmids.
Acknowledgments
This study was supported by grants FIS 02/3029 and FIS PI04/0802 from the Spanish Fondo de Investigación Sanitaria, Instituto de Salud Carlos III, and API 04/12 from the Fundación Marqués de Valdecilla-IFIMAV to M.V.F.; National Institute of Health grants GM33956 to D.B.C. and GM55544 to K.E.W; and American Heart Association grant 9804088X to P.T. This work was also partially supported by grant LSHE-CT-2007-037410 from the European Union and grant REIPI RD06/0008 from the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III-FEDER, Spanish Network for Research in Infectious Diseases. P.G. was supported by a grant from IFIMAV.
We thank all members of our laboratories for helpful discussions.
Footnotes
Published ahead of print on 28 September 2007.
REFERENCES
- 1.Barilla, D., and F. Hayes. 2003. Architecture of the ParF*ParG protein complex involved in prokaryotic DNA segregation. Mol. Microbiol. 49:487-499. [DOI] [PubMed] [Google Scholar]
- 2.Barilla, D., M. F. Rosenberg, U. Nobbmann, and F. Hayes. 2005. Bacterial DNA segregation dynamics mediated by the polymerizing protein ParF. EMBO J. 24:1453-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bignell, C., and C. M. Thomas. 2001. The bacterial ParA-ParB partitioning proteins. J. Biotechnol. 91:1-34. [DOI] [PubMed] [Google Scholar]
- 4.Bouet, J. Y., and B. E. Funnell. 1999. P1 ParA interacts with the P1 partition complex at parS and an ATP-ADP switch controls ParA activities. EMBO J. 18:1415-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle, J. S., and A. M. Lew. 1995. An inexpensive alternative to glassmilk for DNA purification. Trends Genet. 11:8. [DOI] [PubMed] [Google Scholar]
- 6.Clewell, D. B. 1999. Sex pheromone systems in enterococci, p. 47-65. In G. M. Dunny and S. C. Winans (ed.), Cell-cell signaling in bacteria. ASM Press, Washington, DC.
- 7.Clewell, D. B., and M. V. Francia. 2004. Conjugation in gram-positive bacteria, p. 227-256. In B. E. Funnell and G. Phillips (ed.), Plasmid biology. ASM Press, Washington, DC.
- 8.Clewell, D. B., and G. M. Dunny. 2002. Conjugation and genetic exchange in enterococci, p. 265-300. In S. Gilmore (ed.), The enterococci: pathogenesis, molecular biology, and antibiotic resistance. ASM Press, Washington, DC.
- 9.Dower, W. J., J. F. Miller, and C. W. Ragsdale. 1988. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 16:6127-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebersbach, G., D. J. Sherratt, and K. Gerdes. 2005. Partition-associated incompatibility caused by random assortment of pure plasmid clusters. Mol. Microbiol. 56:1430-1440. [DOI] [PubMed] [Google Scholar]
- 11.Espinosa, M., S. Cohen, M. Couturier, G. del Solar, R. Diaz-Orejas, R. Giraldo, L. Jánniere, C. Miller, M. Osborn, and C. M. Thomas. 2000. Plasmid replication and copy number control, p. 1-47. In C. M. Thomas (ed.), The horizontal gene pool: bacterial plasmids and gene spread. Harwood Academic Publishers, Amsterdam, The Netherlands.
- 12.Firth, N., S. Apisiridej, T. Berg, B. A. O'Rourke, S. Curnock, K. G. Dyke, and R. A. Skurray. 2000. Replication of staphylococcal multiresistance plasmids. J. Bacteriol. 182:2170-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flannagan, S. E., and D. B. Clewell. 1991. Conjugative transfer of Tn916 in Enterococcus faecalis: trans activation of homologous transposons. J. Bacteriol. 173:7136-7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francia, M. V., and D. B. Clewell. 2002. Transfer origins in the conjugative Enterococcus faecalis plasmids pAD1 and pAM373: identification of the pAD1 nic site, a specific relaxase and a possible TraG-like protein. Mol. Microbiol. 45:375-395. [DOI] [PubMed] [Google Scholar]
- 15.Francia, M. V., S. Fujimoto, P. Tille, K. E. Weaver, and D. B. Clewell. 2004. Replication of Enterococcus faecalis pheromone-responding plasmid pAD1: location of the minimal replicon and oriV site and RepA involvement in initiation of replication. J. Bacteriol. 186:5003-5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francia, M. V., J. C. Zabala, F. de la Cruz, and J. M. Garcia Lobo. 1999. The IntI1 integron integrase preferentially binds single-stranded DNA of the attC site. J. Bacteriol. 181:6844-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujimoto, S., and D. B. Clewell. 1998. Regulation of the pAD1 sex pheromone response of Enterococcus faecalis by direct interaction between the cAD1 peptide mating signal and the negatively regulating, DNA-binding TraA protein. Proc. Natl. Acad. Sci. USA 95:6430-6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Funnell, B. E. 1988. Mini-P1 plasmid partitioning: excess ParB protein destabilizes plasmids containing the centromere parS. J. Bacteriol. 170:954-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funnell, B. E., and R. A. Slavcev. 2004. Partition systems of bacterial plasmids, p. 81-103. In B. E. Funnell and G. Phillips (ed.), Plasmid biology. ASM Press, Washington, DC.
- 20.Gerdes, K., J. Moller-Jensen, and R. Bugge Jensen. 2000. Plasmid and chromosome partitioning: surprises from phylogeny. Mol. Microbiol. 37:455-466. [DOI] [PubMed] [Google Scholar]
- 21.Gering, M., F. Gotz, and R. Bruckner. 1996. Sequence and analysis of the replication region of the Staphylococcus xylosus plasmid pSX267. Gene 182:117-122. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 23.Hayes, F., and D. Barilla. 2006. The bacterial segrosome: a dynamic nucleoprotein machine for DNA trafficking and segregation. Nat. Rev. Microbiol. 4:133-143. [DOI] [PubMed] [Google Scholar]
- 24.Heath, D. G., F. Y. An, K. E. Weaver, and D. B. Clewell. 1995. Phase variation of Enterococcus faecalis pAD1 conjugation functions relates to changes in iteron sequence region. J. Bacteriol. 177:5453-5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ike, Y., and D. B. Clewell. 1984. Genetic analysis of the pAD1 pheromone response in Streptococcus faecalis, using transposon Tn917 as an insertional mutagen. J. Bacteriol. 158:777-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacob, A. E., and S. J. Hobbs. 1974. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 117:360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen, R. B., R. Lurz, and K. Gerdes. 1998. Mechanism of DNA segregation in prokaryotes: replicon pairing by parC of plasmid R1. Proc. Natl. Acad. Sci. USA 95:8550-8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kearney, K., G. F. Fitzgerald, and J. F. Seegers. 2000. Identification and characterization of an active plasmid partition mechanism for the novel Lactococcus lactis plasmid pCI2000. J. Bacteriol. 182:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krüger, R., S. A. Rakowski, and M. Filutowicz. 2004. Participating elements in the replication of iteron-containing plasmids (ICPs), p. 25-45. In B. E. Funnell and G. Phillips (ed.), Plasmid biology. ASM Press, Washington, DC.
- 30.Kwong, S. M., C. C. Yeo, and C. L. Poh. 2001. Molecular analysis of the pRA2 partitioning region: ParB autoregulates parAB transcription and forms a nucleoprotein complex with the plasmid partition site, parS. Mol. Microbiol. 40:621-633. [DOI] [PubMed] [Google Scholar]
- 31.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 32.Lemonnier, M., J. Y. Bouet, V. Libante, and D. Lane. 2000. Disruption of the F plasmid partition complex in vivo by partition protein SopA. Mol. Microbiol. 38:493-505. [DOI] [PubMed] [Google Scholar]
- 33.Li, P. L., and S. K. Farrand. 2000. The replicator of the nopaline-type Ti plasmid pTiC58 is a member of the repABC family and is influenced by the TraR-dependent quorum-sensing regulatory system. J. Bacteriol. 182:179-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miroux, B., and J. E. Walker. 1996. Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J. Mol. Biol. 260:289-298. [DOI] [PubMed] [Google Scholar]
- 35.Moller-Jensen, J., J. Borch, M. Dam, R. B. Jensen, P. Roepstorff, and K. Gerdes. 2003. Bacterial mitosis: ParM of plasmid R1 moves plasmid DNA by an actin-like insertional polymerization mechanism. Mol. Cell 12:1477-1487. [DOI] [PubMed] [Google Scholar]
- 36.Mori, H., A. Kondo, A. Ohshima, T. Ogura, and S. Hiraga. 1986. Structure and function of the F plasmid genes essential for partitioning. J. Mol. Biol. 192:1-15. [DOI] [PubMed] [Google Scholar]
- 37.Mori, H., Y. Mori, C. Ichinose, H. Niki, T. Ogura, A. Kato, and S. Hiraga. 1989. Purification and characterization of SopA and SopB proteins essential for F plasmid partitioning. J. Biol. Chem. 264:15535-15541. [PubMed] [Google Scholar]
- 38.Motallebi-Veshareh, M., D. A. Rouch, and C. M. Thomas. 1990. A family of ATPases involved in active partitioning of diverse bacterial plasmids. Mol. Microbiol. 4:1455-1463. [DOI] [PubMed] [Google Scholar]
- 39.Pappas, K. M., and S. C. Winans. 2003. The RepA and RepB autorepressors and TraR play opposing roles in the regulation of a Ti plasmid repABC operon. Mol. Microbiol. 49:441-455. [DOI] [PubMed] [Google Scholar]
- 40.Pontius, L. T., and D. B. Clewell. 1992. Conjugative transfer of Enterococcus faecalis plasmid pAD1: nucleotide sequence and transcriptional fusion analysis of a region involved in positive regulation. J. Bacteriol. 174:3152-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pontius, L. T., and D. B. Clewell. 1991. A phase variation event that activates conjugation functions encoded by the Enterococcus faecalis plasmid pAD1. Plasmid 26:172-185. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 43.Tanaka, T., H. Ishida, and T. Maehara. 2005. Characterization of the replication region of plasmid pLS32 from the Natto strain of Bacillus subtilis. J. Bacteriol. 187:4315-4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka, T., and M. Ogura. 1998. A novel Bacillus natto plasmid pLS32 capable of replication in Bacillus subtilis. FEBS Lett. 422:243-246. [DOI] [PubMed] [Google Scholar]
- 45.Weaver, K. E., and D. B. Clewell. 1988. Regulation of the pAD1 sex pheromone response in Enterococcus faecalis: construction and characterization of lacZ transcriptional fusions in a key control region of the plasmid. J. Bacteriol. 170:4343-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weaver, K. E., D. B. Clewell, and F. An. 1993. Identification, characterization, and nucleotide sequence of a region of Enterococcus faecalis pheromone-responsive plasmid pAD1 capable of autonomous replication. J. Bacteriol. 175:1900-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weaver, K. E., L. R. Rice, and G. Churchward. 2002. Plasmids and transposons, p. 219-263. In S. Gilmore (ed.), The enterococci: pathogenesis, molecular biology, and antibiotic resistance. ASM Press, Washington, DC.
- 48.Weihofen, W. A., A. Cicek, F. Pratto, J. C. Alonso, and W. Saenger. 2006. Structures of omega repressors bound to direct and inverted DNA repeats explain modulation of transcription. Nucleic Acids Res. 34:1450-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wirth, R., F. Y. An, and D. B. Clewell. 1986. Highly efficient protoplast transformation system for Streptococcus faecalis and a new Escherichia coli-S. faecalis shuttle vector. J. Bacteriol. 165:831-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yagi, Y., and D. B. Clewell. 1980. Recombination-deficient mutant of Streptococcus faecalis. J. Bacteriol. 143:966-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yin, P., T. Y. Li, M. H. Xie, L. Jiang, and Y. Zhang. 2006. A Type Ib ParB protein involved in plasmid partitioning in a gram-positive bacterium. J. Bacteriol. 188:8103-8108. [DOI] [PMC free article] [PubMed] [Google Scholar]