Abstract
Human immunodeficiency virus type 2 (HIV-2) infection leads to a lifelong asymptomatic period in the majority of patients. Even in patients with progressive disease, a slow CD4 count decline characterizes the chronic phase of HIV-2 infection, suggesting that peripheral T-cell homeostasis is controlled better following HIV-2 infection than following HIV-1 infection. Herein we showed that, in contrast to HIV-1-infected patients, HIV-2-infected patients demonstrate enhanced thymic function compared to age-matched healthy individuals. The correlation between higher thymic production and lower CD4 T-cell loss in these patients suggests that efficient thymopoiesis is implicated in the long-lasting maintenance of CD4 T-cell counts in HIV-2 disease.
Human immunodeficiency virus type 2 (HIV-2) infection is associated with a more benign course of disease than HIV-1 infection is. The majority of HIV-2-infected individuals remain asymptomatic for years following infection and their disease behaves like that of HIV-1-infected long-term nonprogressors, while the patients who progress to disease exhibit a much slower rate of CD4 decline than the rate for HIV-1-infected patients (9, 12, 21). The latter group of HIV-2-infected patients is reminiscent of HIV-1 slow progressors, characterized by a slow CD4 T-cell decline despite detectable viremia (3, 13). Furthermore, studies in geographic areas where high HIV-2 prevalence is observed demonstrated that the life expectancy for individuals with HIV-2 infection is often close to that of uninfected individuals living in the same villages (12). The factors determining the delayed disease progression in HIV-2 infection remain largely unknown (14). Although plasma viral load is much lower in HIV-2 infection than in HIV-1 infection (2), the proviral loads of HIV-2-infected patients are similar to those of HIV-1-infected patients at the same disease stage (10, 11), suggesting that the two viruses do not differ significantly in their ability to establish infection and replicate in human cells (1, 17).
On the other hand, similar degrees of T-cell hyperactivation, as well as increased T-cell cycling, were observed for both HIV-1- and HIV-2-infected patients with similar degrees of CD4 depletion (18). This suggests an intimate link between generalized immune activation and associated cell death and CD4 depletion in both infections despite distinct viremias and clinical outcomes.
HIV-1 infection is associated with an impairment of intrathymic precursor T-cell proliferation resulting in a huge reduction of de novo T-cell production thought to participate in the progressive decline of peripheral CD4 T-cell counts, in particular in the recent thymic emigrants (RTEs) and naïve T-cell compartments (4). An imbalance between production and destruction of peripheral CD4 T cells would lead to their progressive decline over time and eventually to AIDS (5, 6). Moreover, in slow progressor HIV-1-infected patients, we recently demonstrated that the maintenance of circulating CD4 T cells is strongly associated with efficient thymopoiesis (3).
We thus hypothesized that the maintenance of de novo T-cell production may counteract the peripheral CD4 loss that is known to occur in HIV-2 infection and represents a major mechanism underlying the slow progression of HIV-2 disease. Here we analyzed the role of thymopoiesis in the long-term maintenance of peripheral CD4+ T-cell numbers in a cohort of untreated chronically HIV-2- and HIV-1-infected subjects currently living in Portugal and attending outpatient clinics in Lisbon, Portugal, compared to age-matched healthy individuals. A summary of the clinical features of the patients studied and healthy controls is shown in Table 1 .
TABLE 1.
Characteristics of the HIV-1- and HIV-2-infected patients and healthy controls studied
| Characteristic | Value for groupa
|
|||||
|---|---|---|---|---|---|---|
| Controls
|
HIV-1-infected patients
|
HIV-2-infected patients
|
||||
| 35 to 45 yr | >45 yr | 35 to 45 yr | >45 yr | 35 to 45 yr | >45 yr | |
| No. of subjects | 11 | 12 | 6 | 9 | 7 | 13 |
| Ethnicityb | 9 C, 2 A | 11 C, 1 A | 5 C, 1 A | 6 C, 3 A | 4 C, 3 A | 7 C, 6 A |
| Age (yr)c | 39 (36-45) | 54 (46-65) | 41 (35-43) | 54 (46-65) | 38 (35-45) | 49 (46-68) |
| Time (mo) after first seropositivityc,d | NA | NA | ND | ND | 19 (7-133) | 18 (2-172) |
| Viral load (log copies/ml)c | NA | NA | 4.06 (2.63-4.98) | 4.55 (3.28-5.41) | <200e | <200e (<200-4,006) |
| Absolute CD4 count (cells/ml)c | 920 (535-1,867) | 882 (347-1,758) | 291** (92-704) | 240** (88-986) | 927 (333-1,184) | 593* (220-830) |
| Absolute CD8 count (cells/ml)c | 336 (105-1,236) | 265 (99-770) | 654* (269-2,007) | 781** (518-2,210) | 845* (611-1,778) | 670** (202-2,180) |
The subjects are grouped by disease status (controls and HIV-1- and HIV-2-infected patients) and age. NA, not applicable; ND, not determined. Statistical differences between the values for HIV-infected patients and age-matched controls (Mann-Whitney test) are indicated as follows: *, P < 0.05; **, P < 0.01.
The number of Caucasians (C) and Africans (A) are shown (all the African subjects had been residing in Europe for several years at the sampling time).
The median value is shown, and the range is shown in parentheses.
All the HIV-2-infected individuals were identified as seropositive at their first visit. (The HIV-1 patients were sampled for another cross-sectional study.)
HIV-2 viremia was quantified by a reverse transcriptase PCR-based test; all but one of the HIV-2 infected patients had a viral load below 200 RNA copies/ml (cutoff value).
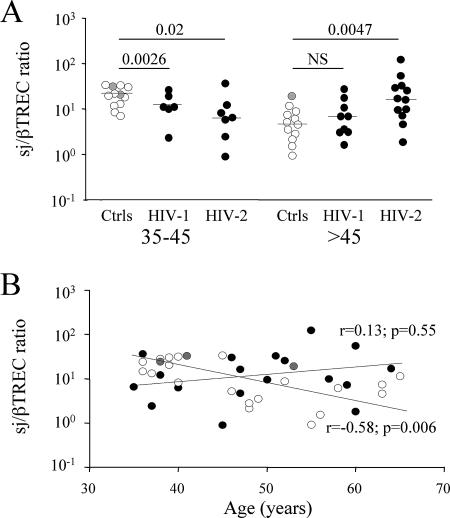
Thymic activity was estimated by measurement of the intrathymic proliferation history of circulating RTEs by quantification of the signal joint/beta T-cell receptor excision circle (sj/βTREC) ratio as described previously (3, 4, 20). This marker, which is independent of peripheral T-cell proliferation and death rate, directly reflects the number of proliferation cycles undergone by precursor T cells during their intrathymic differentiation and thus thymic output (4). Surprisingly, while in both HIV-1 and HIV-2 patients, the younger individuals (35 to 45 years old) demonstrate a low thymic function (median sj/βTREC ratios of 11.3 and 6.5 for HIV-1 and HIV-2 patients, respectively, compared to a ratio of 24.1 in the healthy control group [P = 0.026 and P = 0.02, respectively]; Fig. 1A), a significantly higher sj/βTREC ratio characterizes HIV-2-infected subjects that were >45 years old compared to healthy controls (median sj/βTREC ratio of 17.0 in HIV-2-infected patients compared to 4.9 in control individuals [P = 0.0047]; Fig. 1A). This contrasts with the expected low intrathymic proliferation observed in age-matched HIV-1-infected patients (sj/βTREC ratio of 6.9). In fact, analysis of the sj/βTREC ratio as a function of age shows that the expected age dependence of thymic output is not observed in the HIV-2-infected patients (Fig. 1B). While most patients with chronic HIV-1 infection demonstrate a rapid and persistent defect in thymic output (3, 4), the impairment of thymopoiesis observed in the younger groups of HIV-2-infected patients is not further exacerbated by aging. In contrast, it seems that this function is maintained in this group of patients, so that in aged individuals, the sj/βTREC ratio becomes greater than in healthy individuals, most likely leading to long-term sustained thymic output.
FIG. 1.
HIV-2-infected patients demonstrate high thymic function. (A) The sj/βTREC ratio, a measure of thymic activity, was calculated for healthy individuals (controls [Ctrls]) and HIV-1- and HIV-2-infected patients. The three groups were subdivided into groups by age (35 to 45 years old and >45 years old). Gray symbols represent healthy control individuals of African origin. Statistical differences (P values) between the different groups are shown above the values (Mann-Whitney test). NS, not significant. (B) Correlation between the sj/βTREC ratio and age in healthy controls (white and gray symbols, representing Caucasian and African individuals, respectively, and HIV-2-infected patients (black symbols). Spearman's correlation and associated probability are shown for the control group.
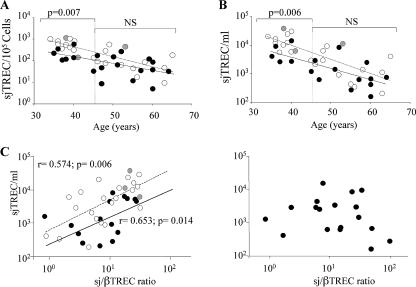
To assess the impact of our observations on the peripheral RTE subset, we evaluated the frequency of sjTREC-containing cells (sjTREC/105 cells) in peripheral blood mononuclear cells from all patients studied. A significant reduction of the sjTREC-positive cell frequency was shown in HIV-2-infected patients compared to the control group (median sjTREC/105 cells of 102 and 297, respectively; P = 0.001 in HIV-2 and controls). However, this reduction was mainly observed in younger (35- to 45-year-old) individuals (median sjTREC/105 cells of 558 and 148, respectively; P = 0.007 in HIV-2 and controls; Fig. 2A). In contrast, despite lower CD4 T-cell counts (Table 1), older individuals (>45 years old) had RTE counts similar to those of age-matched controls (Fig. 2A), supporting our previous assumption that the increased thymic output translates into sustained circulating RTE frequency.
FIG. 2.
Consequences of the increased thymic function on peripheral RTE population. (A) Relationships between the sjTREC frequency and age in HIV-2-infected patients and healthy control individuals. (B) Relationships between sjTREC concentration and age in HIV-2-infected patients and healthy control individuals. In both panels A and B, the white symbols and dashed lines represent healthy controls (gray dots represent healthy controls of African origin), and the black symbols and solid lines represent the HIV-2-infected patients. The P values from the statistical analysis (Mann-Whitney test) for each age group are shown above the values. NS, not significant. (C) Relationships between the sjTREC concentration and thymic function in healthy controls (white and gray symbols, representing Caucasian and African individuals, respectively), HIV-1-infected patients (left graph, black symbols), and HIV-2-infected patients (right graph). When significant, P values from the statistical analysis are shown (Spearman's correlation and associated probability).
Nevertheless, one must consider that the assessment of sjTREC frequency may be influenced by variable degrees of immune activation/proliferation in the groups studied. We thus also determined the sjTREC concentration (sjTREC/ml of blood) which, although influenced by thymic output and RTE survival, does not depend upon the general state of immune activation/proliferation (7, 8).
Interestingly, the analysis of the sjTREC concentration also showed that the patients that were >45 years old behave like age-matched controls do, with a significant reduction characterizing 35- to 45-year-old individuals (median sjTREC/ml of 670 and 1,821 in HIV-2-infected patients and controls, respectively; P = 0.02) (Fig. 2B). The parallelism between sjTREC frequency and concentration (Fig. 2A and B) suggests that the proliferation of TREC-positive cells is not of major importance in the regulation of naïve T-cell counts during HIV-2 infection. However, HIV-2- and HIV-1-infected patients with equivalent degrees of CD4 depletion demonstrate similar enhancement of immune activation levels (18, 19). It is thus most probable, as demonstrated by Sieg et al. in HIV-1 infection that T cells expressing activation/proliferation markers (CD69, HLA-DR, Ki-67…) in HIV-2 infection are also determined to die rapidly in vivo (15, 16). This is further emphasized by analyzing the relationships between the sj/βTREC ratio (thymic production) and the sjTREC concentration (approximate RTE counts). As expected, both parameters correlated nicely in the control group and the HIV-1-infected patients (r = 0.574 and P = 0.006 and r = 0.653 and P = 0.014, respectively [Fig. 2C, left graph]). In contrast, in HIV-2-infected patients, thymic production was not associated with RTE concentration (Fig. 2C, right graph), demonstrating that the sustained thymic function in older HIV-2-infected patients does not lead to the expected enlargement of the TREC-rich T-cell population, likely being concomitantly expanded by increased cell death and/or accelerated maturation. This is further emphasized by the absence of correlation between circulating interleukin 7 levels and sjTREC quantification in HIV-2-infected patients (1).
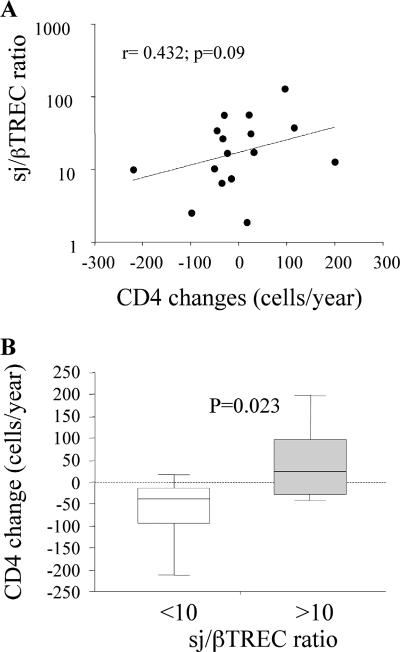
In order to estimate the impact of the observed enhanced thymopoiesis on the relative resistance to disease in the HIV-2-infected patients, we calculated the rate of CD4 count decline in this group of patients over a median period of 2.5 years preceding the analysis of thymic function. In HIV-2-infected patients, the sj/βTREC ratio tends to correlate with the variations in CD4 T-cell counts over time (r = 0.432 and P = 0.09; Fig. 3A). Such a correlation does not exist in the HIV-1-infected patients (r = 0.06 and not statistically significant). Interestingly, when classifying the patients according to their sj/βTREC ratio, a significant difference was observed in their capacity to maintain CD4 T-cell counts over the follow-up period. While patients demonstrating efficient thymopoiesis (i.e., sj/βTREC ratio of >10) preserve their CD4 T-cell counts over the follow-up period (median CD4 change of +25 cells/year [range, −43 to +202]; Fig. 3B), patients with low sj/βTREC ratio (<10) show a slow but definite decline in circulating CD4 T-cell numbers (median loss of −40 cells/year [range, −217 to +19; P = 0.023]). In contrast, in the HIV-1-infected group, the extent of intrathymic precursor T-cell proliferation does not correlate with CD4 T-cell decline (median CD4 changes of −122 and −73 cells/year in patients with sj/βTREC ratios of <10 and >10, respectively; not statistically significant; data not shown).
FIG. 3.
Increased thymic output translates into maintenance of CD4 counts. (A) Relationships between the sj/βTREC ratio and CD4 changes over time in HIV-2-infected patients. The correlation coefficient (Spearman's test) and associated probability are shown. (B) CD4 changes in HIV-2-infected patients with sj/βTREC ratios of <10 and ≥10. CD4 change values were calculated using the slope of CD4 counts measured over a period of >2.5 years before sampling. Statistical differences between the two groups (P values) are shown above the ratios (Mann-Whitney test).
Taken together, these data demonstrate that patients with chronic HIV-2 infections maintain thymic production for prolonged periods of time, even after they reach 45 years of age, when significant thymic involution is observed in healthy individuals. This effect, reminiscent of what was observed in HIV-1-infected slow progressors, suggests that increased CD4 T-cell death can, at least partly, be compensated for by an overproduction of new T cells in HIV-2-induced pathology. The fact that younger HIV-2-infected patients demonstrate a reduced thymic function suggests either that HIV-2 infection can lead to various levels of pathogenesis or that sustained thymic function in aging patients is a mechanism that develops slowly to compensate for increased cell death. Patients who cannot maintain thymic function would exhibit progressively lower CD4 T-cell counts, eventually leading to AIDS, while others whose thymus remains functional maintain CD4 counts and remain asymptomatic for several decades. However, it is possible that efficient thymopoiesis is both a cause and consequence of limited pathogenicity in HIV-2. It is quite possible that low viral loads in HIV-2-infected patients, by inducing limited homeostatic perturbations, leads to maintained thymic potential and that when lymphopenia occurs, this capacity of the thymus to produce new T cells allows the maintenance of CD4 counts and naïve T-cell diversity sufficiently high to limit progression of the disease.
Interestingly, Poulsen et al. observed that in some West African villages, older people (55 to 80 years of age) with HIV-2 infection have the same mortality risk as uninfected individuals do (12). The capacity of these patients to maintain de novo T-cell production through efficient thymopoiesis despite aging may participate to their longevity in the presence of HIV-2 infection. These new data on the role of the thymus in this natural model of attenuated HIV infection bring new arguments to the contribution of ongoing thymopoiesis for HIV pathogenesis and the rate of progression to AIDS and strengthen the importance of the thymus as a target for immune-based therapies.
(This work was carried out by D. Gautier in partial fulfillment of the requirements for a doctoral degree at the Université Paris 7 Denis Diderot, Paris, France, 2007.)
Acknowledgments
This work was supported by the Institut Pasteur and Fundação para a Ciência e Tecnologia (FCT) from Portugal (grant POCI/SAU-MMO/60333 to A.E.S.). D.G. was the recipient of a Ph.D. ANRS scholarship, S.B. was the recipient of a SIDACTION postdoctoral grant, and C.S.C. received a Ph.D. scholarship from FCT.
Footnotes
Published ahead of print on 5 September 2007.
REFERENCES
- 1.Albuquerque, A. S., C. S. Cortesao, R. B. Foxall, R. S. Soares, R. M. Victorino, and A. E. Sousa. 2007. Rate of increase in circulating IL-7 and loss of IL-7Ralpha expression differ in HIV-1 and HIV-2 infections: two lymphopenic diseases with similar hyperimmune activation but distinct outcomes. J. Immunol. 178:3252-3259. [DOI] [PubMed] [Google Scholar]
- 2.Damond, F., M. Gueudin, S. Pueyo, I. Farfara, D. L. Robertson, D. Descamps, G. Chene, S. Matheron, P. Campa, F. Brun-Vezinet, and F. Simon. 2002. Plasma RNA viral load in human immunodeficiency virus type 2 subtype A and subtype B infections. J. Clin. Microbiol. 40:3654-3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dion, M. L., R. Bordi, J. Zeidan, R. Asaad, M. R. Boulassel, J. P. Routy, M. M. Lederman, R. P. Sekaly, and R. Cheynier. 2007. Slow disease progression and robust therapy-mediated CD4+ T-cell recovery are associated with efficient thymopoiesis during HIV-1 infection. Blood 109:2912-2920. [DOI] [PubMed] [Google Scholar]
- 4.Dion, M. L., J. F. Poulin, R. Bordi, M. Sylvestre, R. Corsini, N. Kettaf, A. Dalloul, M. R. Boulassel, P. Debre, J. P. Routy, Z. Grossman, R. P. Sekaly, and R. Cheynier. 2004. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity 21:757-768. [DOI] [PubMed] [Google Scholar]
- 5.Grossman, Z., M. Meier-Schellersheim, W. E. Paul, and L. J. Picker. 2006. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat. Med. 12:289-295. [DOI] [PubMed] [Google Scholar]
- 6.Grossman, Z., M. Meier-Schellersheim, A. E. Sousa, R. M. Victorino, and W. E. Paul. 2002. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat. Med. 8:319-323. [DOI] [PubMed] [Google Scholar]
- 7.Hazenberg, M. D., S. A. Otto, J. W. Cohen Stuart, M. C. Verschuren, J. C. Borleffs, C. A. Boucher, R. A. Coutinho, J. M. Lange, T. F. Rinke de Wit, A. Tsegaye, J. J. van Dongen, D. Hamann, R. J. de Boer, and F. Miedema. 2000. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T-cell population in HIV-1 infection. Nat. Med. 6:1036-1042. [DOI] [PubMed] [Google Scholar]
- 8.Hazenberg, M. D., M. C. Verschuren, D. Hamann, F. Miedema, and J. J. van Dongen. 2001. T cell receptor excision circles as markers for recent thymic emigrants: basic aspects, technical approach, and guidelines for interpretation. J. Mol. Med. 79:631-640. [DOI] [PubMed] [Google Scholar]
- 9.Marlink, R., P. Kanki, I. Thior, K. Travers, G. Eisen, T. Siby, I. Traore, C. C. Hsieh, M. C. Dia, E. H. Gueye, et al. 1994. Reduced rate of disease development after HIV-2 infection as compared to HIV-1. Science 265:1587-1590. [DOI] [PubMed] [Google Scholar]
- 10.Popper, S. J., A. D. Sarr, A. Gueye-Ndiaye, S. Mboup, M. E. Essex, and P. J. Kanki. 2000. Low plasma human immunodeficiency virus type 2 viral load is independent of proviral load: low virus production in vivo. J. Virol. 74:1554-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popper, S. J., A. D. Sarr, K. U. Travers, A. Gueye-Ndiaye, S. Mboup, M. E. Essex, and P. J. Kanki. 1999. Lower human immunodeficiency virus (HIV) type 2 viral load reflects the difference in pathogenicity of HIV-1 and HIV-2. J. Infect. Dis. 180:1116-1121. [DOI] [PubMed] [Google Scholar]
- 12.Poulsen, A. G., P. Aaby, O. Larsen, H. Jensen, A. Naucler, I. M. Lisse, C. B. Christiansen, F. Dias, and M. Melbye. 1997. 9-Year HIV-2-associated mortality in an urban community in Bissau, West Africa. Lancet 349:911-914. [DOI] [PubMed] [Google Scholar]
- 13.Rodes, B., C. Toro, E. Paxinos, E. Poveda, M. Martinez-Padial, J. M. Benito, V. Jimenez, T. Wrin, S. Bassani, and V. Soriano. 2004. Differences in disease progression in a cohort of long-term non-progressors after more than 16 years of HIV-1 infection. AIDS 18:1109-1116. [DOI] [PubMed] [Google Scholar]
- 14.Rowland-Jones, S. L., and H. C. Whittle. 2007. Out of Africa: what can we learn from HIV-2 about protective immunity to HIV-1? Nat. Immunol. 8:329-331. [DOI] [PubMed] [Google Scholar]
- 15.Sieg, S. F., C. V. Harding, and M. M. Lederman. 2001. HIV-1 infection impairs cell cycle progression of CD4+ T cells without affecting early activation responses. J. Clin. Investig. 108:757-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sieg, S. F., J. B. Mitchem, D. A. Bazdar, and M. M. Lederman. 2002. Close link between CD4+ and CD8+ T cell proliferation defects in patients with human immunodeficiency virus disease and relationship to extended periods of CD4+ lymphopenia. J. Infect. Dis. 185:1401-1416. [DOI] [PubMed] [Google Scholar]
- 17.Soares, R., R. Foxall, A. Albuquerque, C. Cortesao, M. Garcia, R. M. Victorino, and A. E. Sousa. 2006. Increased frequency of circulating CCR5+ CD4+ T cells in human immunodeficiency virus type 2 infection. J. Virol. 80:12425-12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sousa, A. E., J. Carneiro, M. Meier-Schellersheim, Z. Grossman, and R. M. Victorino. 2002. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J. Immunol. 169:3400-3406. [DOI] [PubMed] [Google Scholar]
- 19.Sousa, A. E., A. F. Chaves, A. Loureiro, and R. M. Victorino. 2001. Comparison of the frequency of interleukin (IL)-2-, interferon-gamma-, and IL-4-producing T cells in 2 diseases, human immunodeficiency virus types 1 and 2, with distinct clinical outcomes. J. Infect. Dis. 184:552-559. [DOI] [PubMed] [Google Scholar]
- 20.van den Dool, C., and R. J. de Boer. 2006. The effects of age, thymectomy, and HIV infection on alpha and beta TCR excision circles in naive T cells. J. Immunol. 177:4391-4401. [DOI] [PubMed] [Google Scholar]
- 21.Whittle, H., J. Morris, J. Todd, T. Corrah, S. Sabally, J. Bangali, P. T. Ngom, M. Rolfe, and A. Wilkins. 1994. HIV-2-infected patients survive longer than HIV-1-infected patients. AIDS 8:1617-1620. [DOI] [PubMed] [Google Scholar]