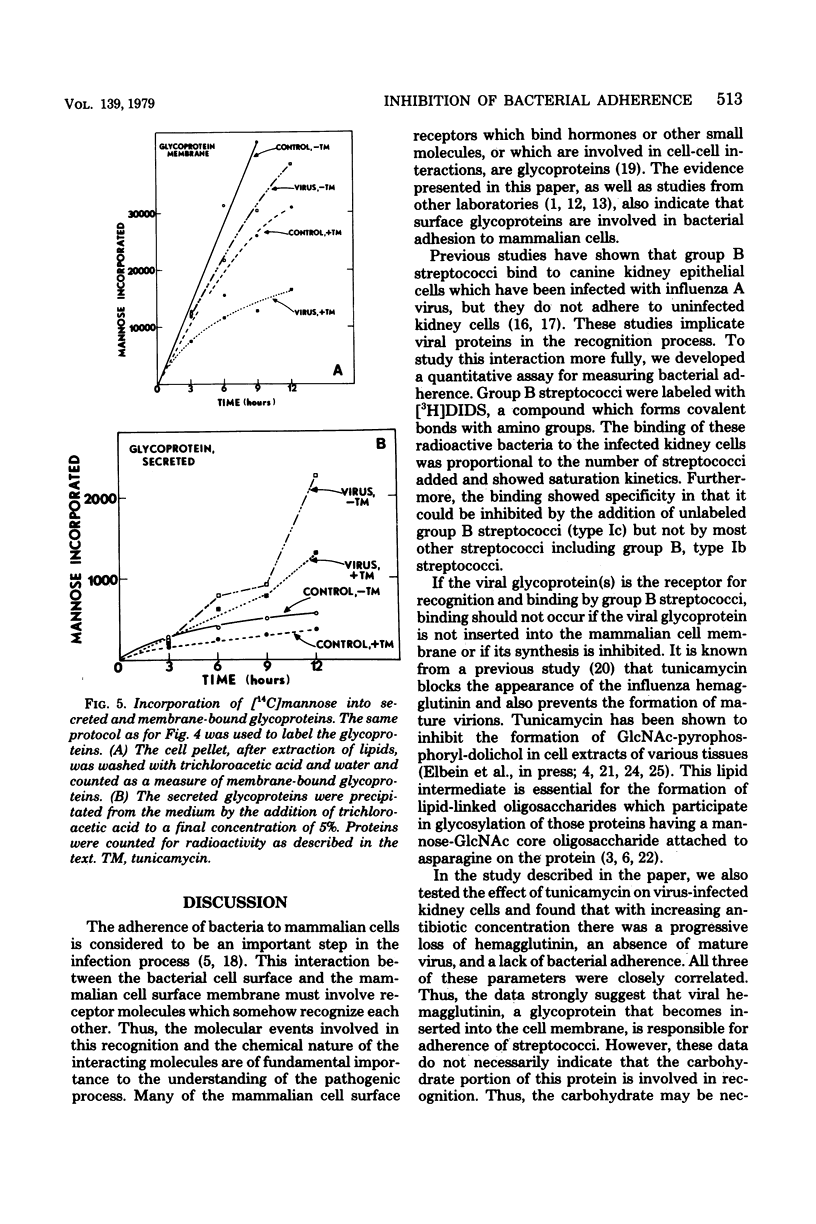
Abstract
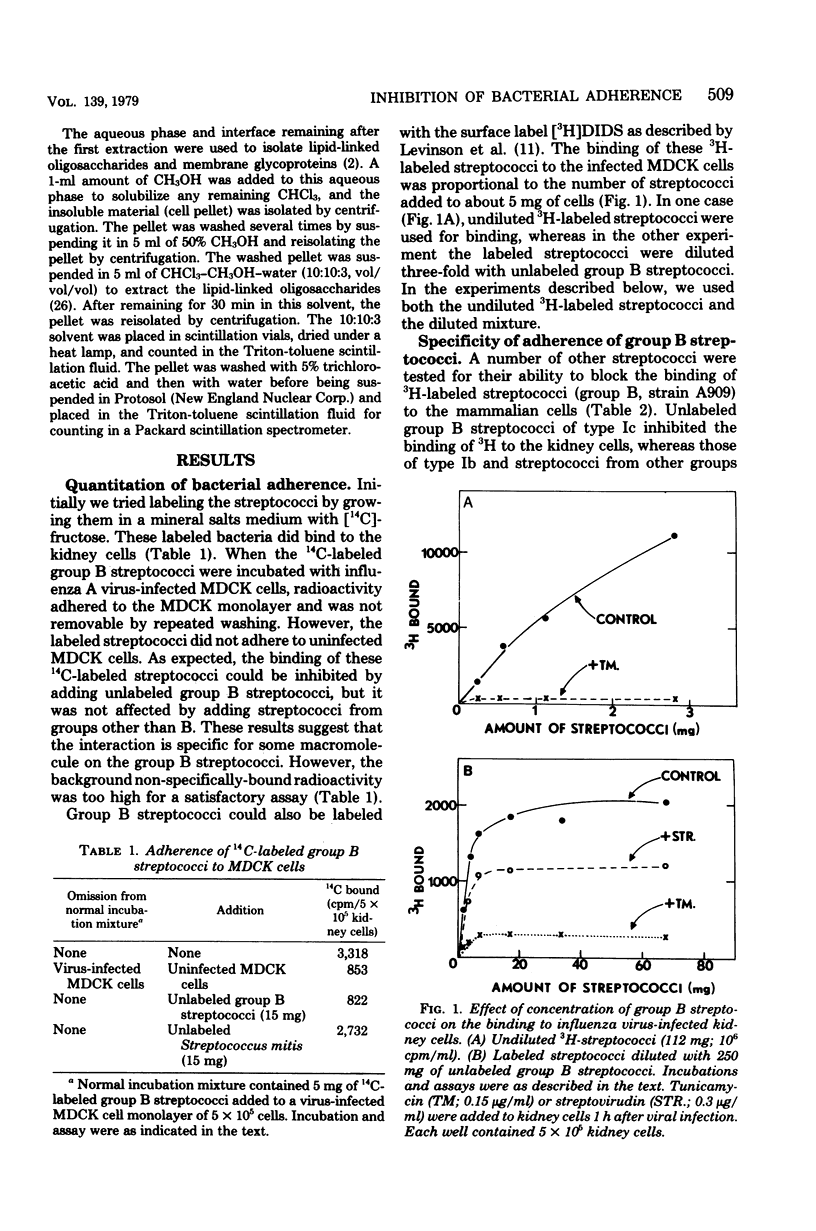
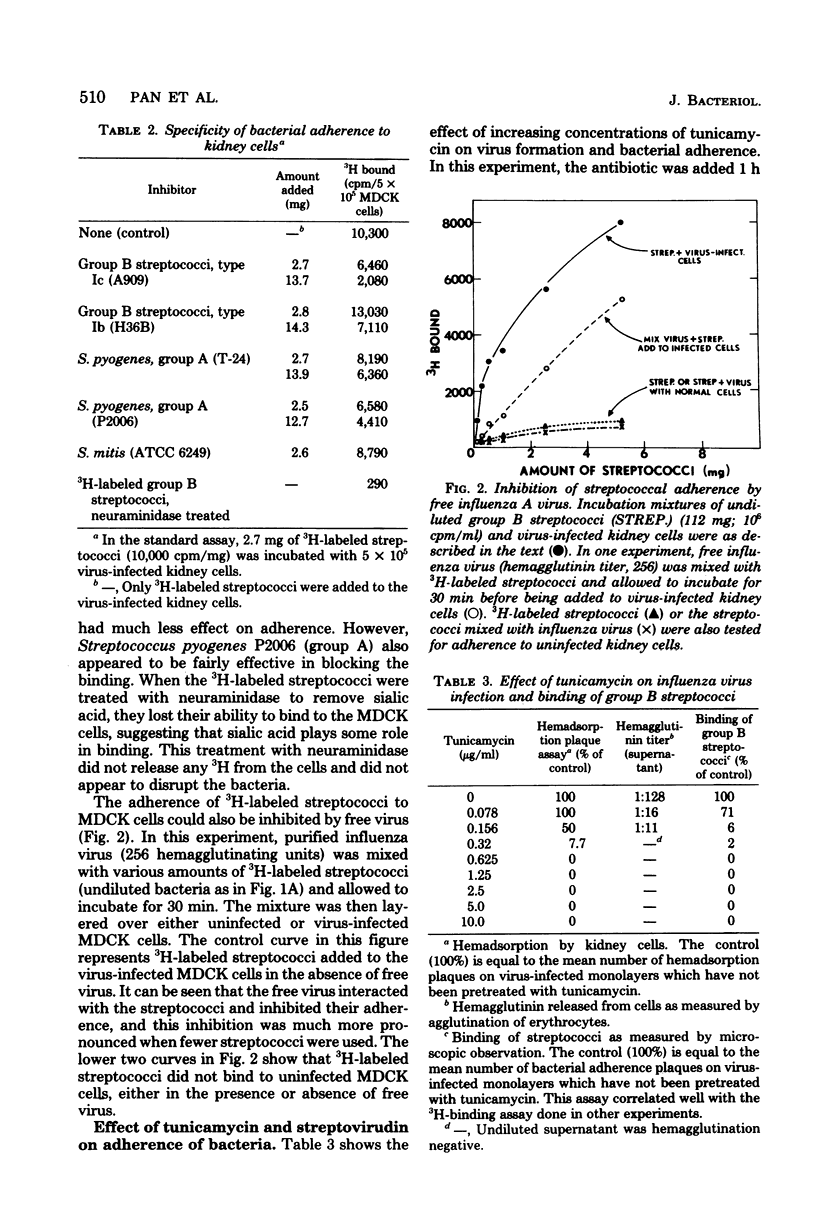
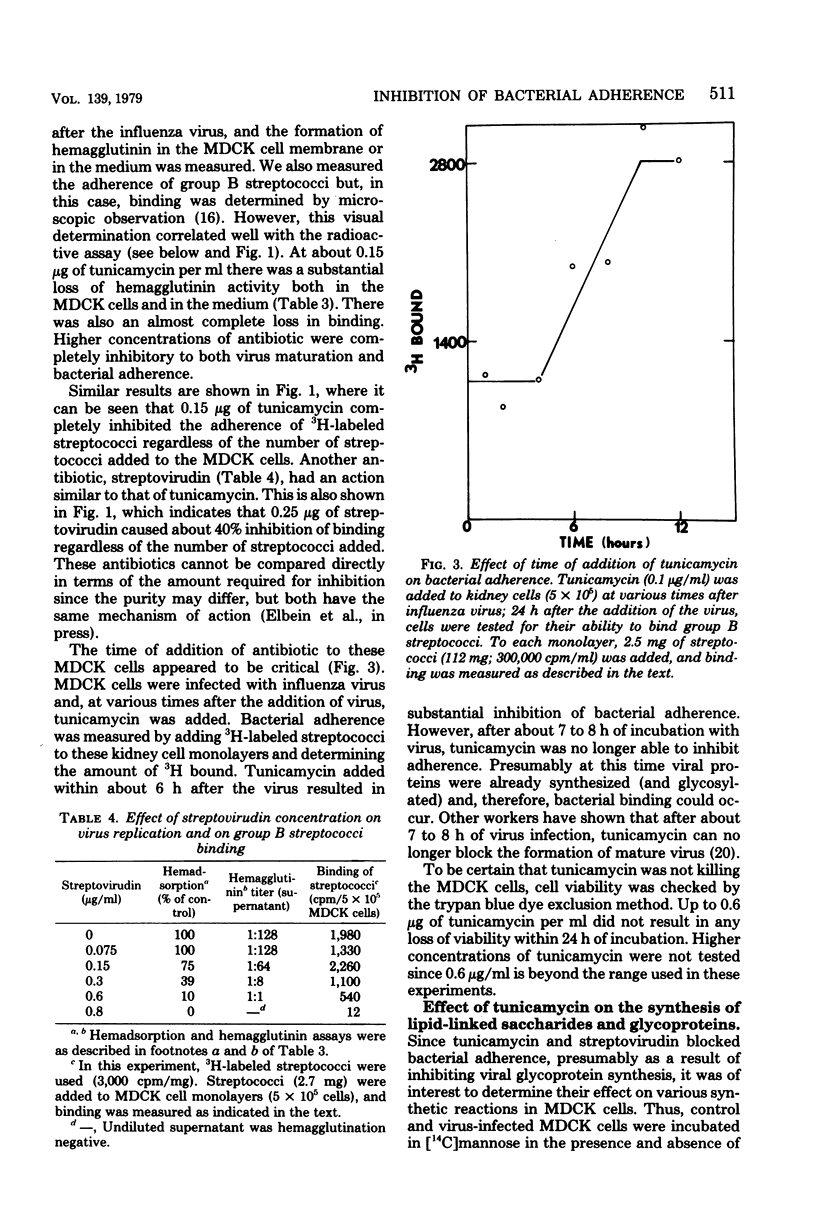
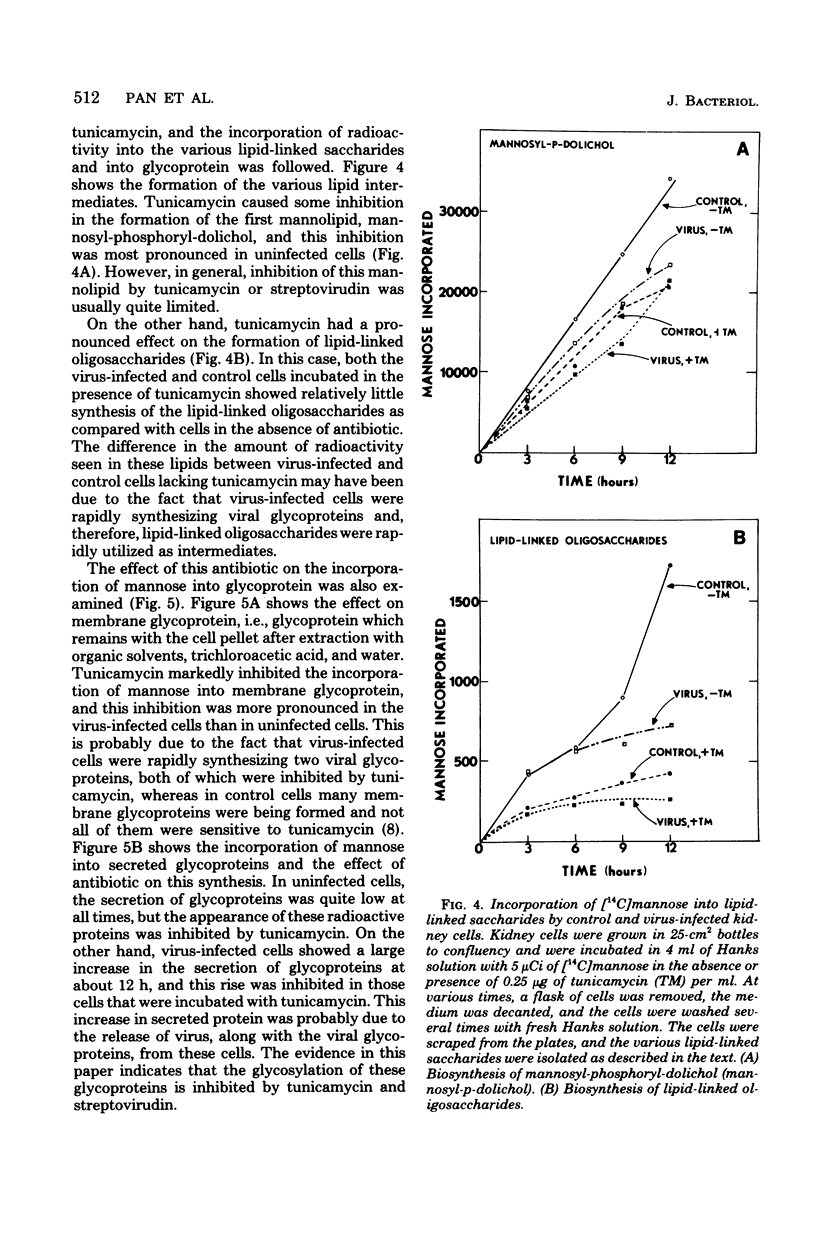
Group B streptococci were labeled either by growing the cells in [14C]fructose or by using the surface label 4,4'-[3H]diisothiocyano-1,2-diphenylethane-2,2'-disulfonic acid, which reacts with amino groups. A quantitative assay was developed by using these labeled bacteria to study the adherence of streptococci to canine kidney epithelial cells. The bacteria adhered to kidney cells that had been infected with influenza A virus, but did not adhere to uninfected cells. The binding of 3H-labeled group B streptococci was proportional to the number of bacteria added and showed saturation kinetics. The binding was blocked by the addition of unlabeled group B streptococci but was not affected by addition of streptococci from other groups. It was also blocked by mixing the 3H-labeled streptococci with influenza A virus before adding the bacteria to the kidney cells. When the kidney cells were infected with influenza virus in the presence of either tunicamycin or streptovirudin, these antibiotics inhibited the appearance of viral hemagglutinin in the kidney cells and also prevented the release of mature virus. In these experiments, the adherence of 3h-labeled streptococci was also inhibited. Tunicamycin was shown to block the incorporation of [14C]mannose into lipid-linked oligosaccharides and glycoprotein in both normal and virus-infected kidney cells. These data give strong support to the notion that adherence of streptococci to mammalian cells involves recognition of viral hemagglutinin, a glycoprotein whose synthesis is blocked by certain antibiotics.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H., Ofek I. Epithelial cell binding of group A streptococci by lipoteichoic acid on fimbriae denuded of M protein. J Exp Med. 1976 Apr 1;143(4):759–771. doi: 10.1084/jem.143.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J., Elbein A. D. Biosynthesis and characterization of lipid-linked sugars and glycoproteins in aorta. J Biol Chem. 1975 Sep 10;250(17):6904–6915. [PubMed] [Google Scholar]
- Duksin D., Bornstein P. Impaired conversion of procollagen to collagen by fibroblasts and bone treated with tunicamycin, an inhibitor of protein glycosylation. J Biol Chem. 1977 Feb 10;252(3):955–962. [PubMed] [Google Scholar]
- Ericson M. C., Gafford J. T., Elbein A. D. Tunicamycin inhibits GlcNAc-lipid formation in plants. J Biol Chem. 1977 Nov 10;252(21):7431–7433. [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- HOTCHIN J. E., DEIBEL R., BENSON L. M. Location of noncytopathic myxovirus plaques by hemadsorption. Virology. 1960 Feb;10:275–280. doi: 10.1016/0042-6822(60)90047-7. [DOI] [PubMed] [Google Scholar]
- Hickman S., Kulczycki A., Jr, Lynch R. G., Kornfeld S. Studies of the mechanism of tunicamycin in hibition of IgA and IgE secretion by plasma cells. J Biol Chem. 1977 Jun 25;252(12):4402–4408. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- Lancefield R. C., McCarty M., Everly W. N. Multiple mouse-protective antibodies directed against group B streptococci. Special reference to antibodies effective against protein antigens. J Exp Med. 1975 Jul 1;142(1):165–179. doi: 10.1084/jem.142.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Tunicamycin inhibits glycosylation and multiplication of Sindbis and vesicular stomatitis viruses. J Virol. 1977 Jan;21(1):375–385. doi: 10.1128/jvi.21.1.375-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson C., Corcoran R. J., Edwards E. H. Interaction of tritium-labeled H2DIDS (4,4'-diisothiocyano-1,2,diphenyl ethane-2,2'disulfonic acid) with the Ehrlich mouse ascites tumor cell. J Membr Biol. 1979 Mar 28;45(1-2):61–79. doi: 10.1007/BF01869295. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Jefferson W., Campbell G. L. Cell membrane-binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975 May 1;141(5):990–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford B. A., Shelokov A., Ramsay M. A. Bacterial adherence to virus-infected cells: a cell culture model of bacterial superinfection. J Infect Dis. 1978 Feb;137(2):176–181. doi: 10.1093/infdis/137.2.176. [DOI] [PubMed] [Google Scholar]
- Sanford B. A., Smith N., Shelokov A., Ramsay M. A. Adherence of group B streptococci and human erythrocytes to influenza A virus-infected MDCK cells. Proc Soc Exp Biol Med. 1979 Feb;160(2):226–232. doi: 10.3181/00379727-160-40424. [DOI] [PubMed] [Google Scholar]
- Schnaar R. L., Weigel P. H., Kuhlenschmidt M. S., Lee Y. C., Roseman S. Adhesion of chicken hepatocytes to polyacrylamide gels derivatized with N-acetylglucosamine. J Biol Chem. 1978 Nov 10;253(21):7940–7951. [PubMed] [Google Scholar]
- Schwarz R. T., Rohrschneider J. M., Schmidt M. F. Suppression of glycoprotein formation of Semliki Forest, influenza, and avian sarcoma virus by tunicamycin. J Virol. 1976 Sep;19(3):782–791. doi: 10.1128/jvi.19.3.782-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck D. K., Lennarz W. J. Evidence for the participation of saccharide-lipids in the synthesis of the oligosaccharide chain of ovalbumin. J Biol Chem. 1977 Feb 10;252(3):1007–1013. [PubMed] [Google Scholar]
- Struck D. K., Siuta P. B., Lane M. D., Lennarz W. J. Effect of tunicamycin on the secretion of serum proteins by primary cultures of rat and chick hepatocytes. Studies on transferrin, very low density lipoprotein, and serum albumin. J Biol Chem. 1978 Aug 10;253(15):5332–5337. [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973 Mar 1;137(3):571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Harford J. B. Evidence for the enzymatic transfer of N-acetylglucosamine from UDP--N-acetylglucosamine into dolichol derivative and glycoproteins by calf brain membranes. Arch Biochem Biophys. 1977 May;181(1):185–198. doi: 10.1016/0003-9861(77)90497-0. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]



