Abstract
Norovirus, which belongs to the family Caliciviridae, is one of the major causes of nonbacterial acute gastroenteritis in the world. The main human noroviruses are of genogroup I (GI) and genogroup II (GII), which were subdivided further into at least 15 and 18 genotypes (GI/1 to GI/15 and GII/1 to GII/18), respectively. The development of immunological diagnosis for norovirus had been hindered by the antigen specificity of the polyclonal antibody. Therefore, several laboratories have produced broadly reactive monoclonal antibodies, which recognize the linear GI and GII cross-reactive epitopes or the conformational GI-specific epitope. In this study, we characterized the novel monoclonal antibody 14-1 (MAb14-1) for further development of the rapid immunochromatography test. Our results demonstrated that MAb14-1 could recognize 15 recombinant virus-like particles (GI/1, 4, 8, and 11 and GII/1 to 7 and 12 to 15) and showed weak affinity to the virus-like particle of GI/3. This recognition range is the broadest of the existing monoclonal antibodies. The epitope for MAb14-1 was identified by fragment, sequence, structural, and mutational analyses. Both terminal antigenic regions (amino acid positions 418 to 426 and 526 to 534) on the C-terminal P1 domain formed the conformational epitope and were in the proximity of the insertion region (positions 427 to 525). These regions contained six amino acids responsible for antigenicity that were conserved among genogroup(s), genus, and Caliciviridae. This epitope mapping explained the broad reactivity and different titers among GI and GII. To our knowledge, we are the first group to identify the GI and GII cross-reactive monoclonal antibody, which recognizes the novel conformational epitope. From these data, MAb14-1 could be used further to develop immunochromatography.
Norovirus is the major cause of nonbacterial epidemic gastroenteritis (11) and belongs to the family Caliciviridae containing five distinct genera, Vesivirus, Lagovirus, Norovirus, Sapovirus, and Becovirus (33). Norovirus has been identified as the cause of 73% to more than 95% of gastroenteritis outbreaks in the United States and approximately half of those worldwide (1).
Norovirus is classified into five genogroups (genogroup I [GI] to genogroup V [GV]) by genetic diversity: viruses in genogroups I, II, and IV (GI, GII, and GIV, respectively) are associated with diarrhea in humans, with GII also able to infect pigs; genogroups III and V (GIII and GV) are associated with bovines and mice, respectively (19). Moreover, norovirus GI and GII are the main causative agents in humans and subdivided further into at least 15 and 18 genotypes (GI/1 to GI/15 and GII/1 to GII/18), respectively (30).
Because the lack of a cell culture system for norovirus has hindered immunological and structural study, the recombinant virus-like particles (rVLPs), which are morphologically and antigenically similar to native norovirus virions, were expressed by using the baculovirus expression system (12, 16, 37).
Norovirus is composed of 180 molecules (90 dimers) of the single major capsid protein, VP1, which has two principal domains. One is the shell (S) domain, which is highly conserved among animal caliciviruses. The other is the protruding (P) domain, which is divided into three subdomains: N-terminal P1, P2, and C-terminal P1 domains. The P2 domain is the most protruding and diverse domain (37). In addition, the internally located N-terminal domain participates in a network of interactions through domain swapping to assist the assembly of the shell domain into an icosahedral scaffold (6).
Several laboratories have generated polyclonal antibodies by using recombinant VP1 as antigens. The rabbit anti-rVLP polyclonal antibody was highly specific for genotypes used as immunogens (13, 18, 21). This specificity has hindered the development of immunological diagnosis. We previously developed the immunochromatography test for detection of norovirus infection by using the anti-rVLP polyclonal antibody (31); however, this method showed the immunogen's genotype specificity.
Monoclonal antibodies are a useful tool for detecting various kinds of noroviruses, and they are more stable than polyclonal antibodies for use in a rapid immunological assay. The previously reported broadly reactive monoclonal antibodies could be classified into two groups by their epitope properties. The first group recognizes the intergenogroup cross-reactive linear epitopes on the S or P domain, NS14, 1B4, and 1F6 (20, 35, 46, 47). The other group recognizes the intragenogroup cross-reactive conformational epitopes, NV3901 and NV3912 (35, 46). In addition, gaining information about the location of norovirus-specific epitopes is essential for designing diagnostic tools (i.e., enzyme-linked immunosorbent assay [ELISA] and immunochromatography), identifying the neutralizing epitope, and developing antivirals and an effective vaccine.
In this study, we describe characterization of a novel monoclonal antibody, which shows broad reactivity with both GI and GII norovirus rVLPs. These findings could be applied for further development of the rapid immunochromatography test, because immunochromatography using this novel antibody has demonstrated high performance in detecting norovirus infection (28).
MATERIALS AND METHODS
Antigens (rVLPs).
Sixteen rVLPs were previously expressed by the baculovirus expression system and confirmed by electron microscopy (31, 32). The sequences were genetically classified based on the method described by Kageyama et al. (17). Within GI, five genotypes of rVLPs were generated, including genotypes 1 (strain 4656 [sequence accession number EF547392]), 3 (strain 3634 [EF547393]), 4 (strain 2876 [EF547394]), 8 (strain 3006 [EF547395]), and 11 (strain 2258 [EF547396]). For GII, 11 genotypes of rVLPs were generated, including genotypes 1 (strain 3101 [EF547397]), 2 (strain 2840 [EF547398]), 3 (strain 3229 [EF547399]), 4 (strain 1207 [DQ975270]), 5 (strain 3611 [EF5473400]), 6 (strain 3612 [EF5473401]), 7 (strain 419 [EF5473402]), 12 (strain 2087 [EF5473403]), 13 (strain 3385 [EF5473404]), 14 (strain 2468 [EF5473405]), and 15 (strain 3625 [EF5473406]).
Production of monoclonal antibody.
The P363-Ag-U1 myeloma cell line was used as the parent cell. CsCl-purified GII/4 rVLP (r1207) was used as an immunogen for preparing the monoclonal antibody, as previously described (22).
ELISA for titration of the monoclonal antibody.
Plates with 96 wells (Maxisorp; Nunc, Roskilde, Denmark) were coated with 90 ng of rVLP/well in 60 μl of 0.1 M carbonate buffer (pH 9.6) for 1 h at 37°C. To compare the reactivities of ELISAs with different pHs, two coating buffer solutions with different pH conditions were used. Phosphate-buffered saline (PBS) with a pH of 7.4 was used, and carbonate buffer with a pH of 9.6 was used only for GII/3 rVLP r3229 and GII/4 rVLP r1207. The wells were blocked with 1% bovine serum albumin in PBS containing 0.1% Tween 20 (PBS-T). The plates were incubated overnight at 4°C. After the wells were washed three times with PBS-T, for titration of the monoclonal antibody, 60 μl of a twofold serial dilution was added to each well, starting with a 1:100 dilution of the monoclonal antibody in PBS-T containing 1% bovine serum albumin, and the plate was incubated for 1 h at 37°C. After the wells were washed three times with PBS-T, 60 μl of a 1:4,000 dilution of horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) (Biosource International, Camarillo, CA) was allowed to react for 1 h at 37°C as the second antibody. After the wells were washed three times with PBS-T, 60 μl of substrate o-phenylenediamine containing 0.012% H2O2 and 0.2 M citrate-phosphate buffer (pH 5.0) were added to each well and left in the dark for 20 min at room temperature. The reaction was stopped by adding 60 μl of 2 M H2SO4 to each well, and the optical density at 492 nm (OD492) was determined (using OD600 as the reference) with a Labsystems Multiskan MCC microplate reader (Thermo Electron Corporation, Waltham, MA). For this experiment, Tn5 cell lysate was included as a negative control. A sample that which had an OD of ≥0.2 and signal/noise ratio of ≥2.0, was considered positive. Each assay was conducted in duplicate.
Fragment construction.
The pET 100 directional TOPO vector (Invitrogen Corp., Carlsbad, CA) was used to express the capsid fragments with a His tag. The primers used in this study are shown in Table 1. PCR-amplified fragments of r1207 were generated using the primer pairs indicated by the names of the constructs. The template used for the PCR was the previously reported plasmid containing the complete capsid sequence of r1207 (31). PCR fragments were directly cloned into the pET 100 directional TOPO vector. The plasmids were transformed into Escherichia coli One Shot TOP10 (Invitrogen Corp., Carlsbad, CA). Positive transformants were identified by PCR. The plasmids from positive transformants were transformed further into E. coli BL21 Star cells (Invitrogen Corp., Carlsbad, CA). To express the r1207 capsid fragments, overnight cultures of E. coli BL21 cells, transformed with each plasmid, were diluted to a ratio of 1:20 in fresh Luria-Bertani broth supplemented with 100 μg/ml of ampicillin. The cells were grown at 37°C until the culture reached a certain cell density (when the OD600 was 0.5 to 0.7). Expression was induced by adding 1.0 mM of isopropyl-β-d-thiogalactopyranoside (IPTG) (Invitrogen Corp., Carlsbad, CA), and cultures were grown for an additional 3 h. The cells were pelleted by centrifugation for 15 min at 3,000 × g at 4°C. The supernatant was removed, and the cell pellet was suspended in a 1/20 volume of lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, and a protease inhibitor cocktail [complete, Mini, EDTA-free] [1 tablet/10 ml] [Roche Diagnostics GmbH, Roche Applied Science, Mannheim, Germany]) and gently shaken at 4°C for 30 min. Following that treatment, Triton X-100 and lysozyme were added to concentrations of 1% and 0.2 mg/ml, respectively, and gently shaken at 4°C for 20 min. Finally, the cells were centrifuged for 30 min at 12,000 × g at 4°C, after which the protein was found in the insoluble fraction.
TABLE 1.
Capsid fragment primers
| Primera | Sequence (5′ to 3′)b | Position (nucleotide)c | Polarity |
|---|---|---|---|
| 1207cacc-418 | CAC CGC TCC TGC CGT TGC CCC C | 1252-1270 | Sense |
| 1207cacc-427 | CAC CGG TGA GCA ACT TCT TTT C | 1279-1296 | Sense |
| 1207-528 | CTA TGT GTA GAA CTG GTT GAC CC | 1553-1575 | Antisense |
| 1207-534 | CTA CCC CGC TCC ATT TCC CAT | 1582-1602 | Antisense |
| 1207-541 | GGG CCA TTA TAA CGC ACG TC | 1604-1623 | Antisense |
The numbering of the sense primers indicates the nucleotide sequences of the N-terminal (first) norovirus residue contained within a construct generated with a particular primer. The numbering of the antisense primers indicates the nucleotide sequences of the C-terminal (last) norovirus residue contained within a construct generated with a particular primer.
Four bases used for directional cloning are shown underlined.
The 1207 sequence was assigned accession number DQ975270 in GenBank.
This fraction was resuspended in 20 mM of Tris (pH 8), 500 mM of NaCl, and 8 M of urea, filtered, and loaded onto a HisTrap column (GE Healthcare Bio-Science Corp., Piscataway, NJ) equilibrated in 20 mM of Tris (pH 7.4), 500 mM of NaCl, and 8 M of urea. On-column renaturing was performed with 8 to 0 M urea gradient solutions. The elution was performed with a 0 to 1 M imidazole gradient. The peak fractions were pooled, and the solvent displaced PBS (pH 7.4) from the PD10 column (GE Healthcare Bio-Science Corp., Piscataway, NJ).
Fragment analysis.
Analysis of proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis was done by the method of Laemmli et al. (24) with slight modifications. Briefly, 15% polyacrylamide resolving gels and a 5% acrylamide stacking gel were used. Capsid fragments were suspended in electrophoresis sample buffer containing 4% sodium dodecyl sulfate, 10% mercaptoethanol, 125 mM of Tris-HCl (pH 6.8), 0.01% bromophenol blue, and 10% glycerol. Samples were boiled for 5 min. Separated proteins were transferred onto a 0.45 μm polyvinylidene difluoride membrane (Immobilon-P; Millipore, Bedford, MA) in a semidry transfer (CB-09A; ATTO, Tokyo, Japan) at a constant current of 2 mA/cm2 for 30 min. The blotted membrane was washed with PBS-T and blocked with 5% skim milk in PBS-T overnight at 4°C. The membrane was washed with PBS-T and then incubated overnight at 4°C with an antibody against the five-histidine tag (QIAGEN, Hilden, Germany) and antinorovirus monoclonal antibody diluted to 1/10,000 and 1/1,000, respectively, with 0.5% skim milk in PBS-T. The blot was washed with PBS-T and incubated with a 1/10,000 dilution of horseradish peroxidase-conjugated goat anti-mouse IgG (Tago, Burlingame, CA). The blot was then reacted with peroxidase substrate solution (diaminobenzidine; SIGMA, St. Louis, MO) to detect the antigen-antibody complexes on the blot.
Sequence analysis.
The ClustalX multiple-sequence alignment program (version 1.83) was used for multiple alignment of constructed rVLP sequences and other genogroups (40). The capsid subdomains were determined based on previously reported data from Prasad et al. (37).
Mutational analysis.
Specific residues in the capsid fragment, 418 to 534, were altered using the QuikChange XLII site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. Some mutagenesis primers were engineered by the QuikChange Primer Design Program (Stratagene, La Jolla, CA) as shown in Table 2. Generated mutants were purified and analyzed by using the same protocols as those for fragment construction and analysis. Mutant clones were confirmed by sequencing.
TABLE 2.
Site-directed mutagenesis primers
| Primer | Sequence (5′ to 3′)a | Position (nucleotide)b | Polarity |
|---|---|---|---|
| F425G | CTG CCG TTG CCC CCA CTG GCC CGG GTG A | 1256-1283 | Sense |
| F425G antisense | TCA CCC GGG CCA GTG GGG GCA ACG GCA G | 1256-1283 | Antisense |
| P(CCG)426F(GCG) | CGT TGC CCC CAC TTT CGC GGG TGA GCA ACT TCT TTT C | 1260-1296 | Sense |
| P(CGG)426F(CGC) | GAA AAG AAG TTG CTC ACC CGC GAA AGT GGG GGC AAC G | 1260-1296 | Antisense |
| L(CTT)526A(GCT) | GGT CAA CCA GTT CTA CAC AGC TGC CCC CAT GGG AAA TGG | 1557-1595 | Sense |
| L(AAG)526A(AGC) | CCA TTT CCC ATG GGG GCA GCT GTG TAG AAC TGG TTG ACC | 1557-1595 | Antisense |
| A(GCC)527K(AAG) | GGT CAA CCA GTT CTA CAC ACT TAA GCC CAT GGG AAA TGG AGC | 1557-1598 | Sense |
| A(GGC)527K(CTT) | GCT CCA TTT CCC ATG GGC TTA AGT GTG TAG AAC TGG TTG ACC | 1557-1598 | Antisense |
| P(CCC)528A(GCC) | CCA GTT CTA CAC ACT TGC CGC CAT GGG AAA TGG AGC G | 1563-1599 | Sense |
| P(GGG)528A(GGC) | CGC TCC ATT TCC CAT GGC GGC AAG TGT GTA GAA CTG G | 1563-1599 | Antisense |
| G(GGA)530A(GCA) | CAC ACT TGC CCC CAT GGC AAA TGC AGC GGG GTA GAA GG | 1572-1602 | Sense |
| G(TCC)530A(TGC) | CCT TCT ACC CCG CTG CAT TTG CCA TGG GGG CAA GTG TG | 1572-1602 | Antisense |
Mutant nucleotides are shown in boldface type, and vector nucleotides are shown in italic type.
The 1207 sequence was assigned accession number DQ975270 in GenBank.
Structural analysis.
The crystal structure of the prototype Norwalk virus capsid protein (PDB code 1IHM) was used to built homology models for r1207 (37). The initial sequence-to-structure alignments and the refined three-dimensional models of r1207 with minimized side chain conformations were obtained using the T-Coffee and SWISS MODEL (29, 38). The figures were made by using PYMOL (http://pymol.sourceforge.net).
Nucleotide sequence accession numbers.
Newly determined sequences were submitted to GenBank under accession numbers DQ975270 and EF547392 through EF547406.
RESULTS
Cross-reactivity of the novel monoclonal antibody.
The ELISA comparison of the reactivities of the novel monoclonal antibody by using different pH conditions for the coating buffer (pH 7.4 and 9.6) showed that a high pH condition (pH 9.6) could not affect the result of ELISA. The novel monoclonal antibody (MAb14-1) obtained from a mouse immunized with r1207 (GII/4 rVLP) showed broad reactivity against various genotypes of rVLPs by ELISA (Table 3). All the different rVLP norovirus genotypes (GI/1, 3, 4, 8, 11 and GII/1 to 7 and 12 to 15) used in this study were recognized by MAb14-1. However, only a weak affinity to the GI/3 genotype was observed (data not shown). The titers of MAb14-1 were almost the same as those against GII rVLPs, and quite different from those against GI rVLPs (Table 3).
TABLE 3.
Titers of newly developed MAb14-1 with various rVLPs by ELISA
| Monoclonal antibody | Isotype | Titer for rVLP (100)a
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genogroup I genotype
|
Genogroup II genotype
|
||||||||||||||||
| 1 | 3 | 4 | 8 | 11 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 12 | 13 | 14 | 15 | ||
| MAb14-1 | IgG1 | 512 | <1 | 8 | 64 | 16 | 8,192 | 2,048 | 4,096 | 8,192 | 2,048 | 4,096 | 4,096 | 1,024 | 8,192 | 2,048 | 2,048 |
The homologous titer is shown in boldface type.
Minimal binding region on the capsid with monoclonal antibody MAb14-1.
To determine the binding domain of the VP1 capsid region against MAb14-1, five fragments were constructed with the His tag: full VP1 (amino acid positions 1 to 541), full VP1 except for the N-terminal subdomain (positions 46 to 541), P domain (positions 222 to 541), P domain except for the C-terminal P1 domain (positions 222 to 417), and N-terminal P1 domain (positions 222 to 275). Only fragments not containing the C-terminal P1 domain showed nonreactivity for MAb14-1 (Fig. 1B,. top five schematic fragments). This result suggested that the C-terminal P1 domain might contain the specific epitope of MAb14-1.
FIG. 1.
(A) Reactivities of several capsid fragments for MAb14-1 and anti-His5 antibody (a-His) by Western blotting. 20 K, 20,000. (B) Process of mapping the minimal binding region shown in silver on the map of VP1. WB, Western blotting. Symbols; ++, increase in antigenicity; +, same antigenicity as for r1207; +−, decline in antigenicity; −, abolition of antigenicity. (C) Prediction structure of r1207 (a part of the C-terminal P1 domain prediction structure could not be created through lack of structural data from 1IHM [Protein Data Bank identification code for Norwalk virus capsid protein]). The N-terminal domain (amino acid positions 0 to 45) (green), S domain (positions 46 to 221) (yellow), N-terminal and C-terminal P1 domains (positions 222 to 275 and 418 to 541) (red), and P2 domain (positions 276 to 417) (blue) are indicated in panels B and C.
In addition, to determine the minimal binding region of MAb14-1, five capsid fragments were constructed by deletion of both terminal regions of the C-terminal P1 domain. It was found that MAb14-1 showed predictable reactivity for the C-terminal P1 domain, while the N-terminal deletion (amino acid positions 418 to 426 from 418 to 541) induced abolition of reactivity for MAb14-1 and the C-terminal deletion mutants with amino acids 418 to 534, 418 to 528, and 418 to 525 deleted induced rise, decline, and abolition of antigenicity, respectively (Fig. 1A and B, bottom five schematic fragments). These results implied that the minimal binding region is probably from amino acid positions 418 to 534 (Fig. 1) and suggested that nine amino acid residues (positions 418 to 426 [A region]) on the N-terminal domain, and three amino acids (526 to 528 [B region]) and six amino acids (529 to 534 [C region]) on the C-terminal domain were important regions for the antigenicity of MAb14-1.
Epitope for monoclonal antibody 14-1.
Alignment of the minimal binding regions on rVLPs and other genogroups showed that the deleted terminal regions had genus-specific residues (such as A418 and P419) and genogroup-specific residues (such as V421 and F425), but these regions did not have GI/3-specific single point mutations (Fig. 2).
FIG. 2.
Alignment of the amino acid sequence of the minimal binding region on noroviruses for MAb14-1. The MAb14-1-specific residues (•), identical components of the previously reported conformational epitope of NV3901 and NV3912 (▾), and the amino acid positions shared between the epitopes of MAb14-1, NV3901, and NV3912 (▪) are indicated (37). The solid-line and dashed-line boxes represent the N-terminal antigenic region (A region) and C-terminal antigenic region (B and C region), respectively, on the minimal binding region. Dots indicate identical amino acid residues, and dashes indicate gaps. JENAGIII/1 (Bo/Jena/1980/DE [GenBank accession number AJ011099]), BOCHGIII/2 (Bo/CH126/1998/NL [[GenBank accession number AF320625]), ALPH GIV/1 (Hu/Alphatron/1998/NL [GenBank accession number AF195847]), and MUNV GV/I (Mu/Murine norovirus-1/US [GenBank accession number AY228235]).
To confirm the relationship between both terminal regions of highly conserved residues (amino acid positions 418 to 426 [A region] and 526 to 534 [B and C regions]) and antigenicity, genus-specific and GII-specific residues were changed to alanine and GI-specific amino acids, respectively (Fig. 2). The six mutations induced several kinds of changes in antigenicity, whereas other mutations did not affect antigenicity. These effects were classified into four different groups: (i) disappearance of the antigenicity (point mutation of L526A within the B region), (ii) severely attenuated antigenicity (F425G in the A region), (iii) significant reduction in reactivity (P426F in the A region, A527K in the B region, and P528A in the C region), and (iv) moderate reduction (G530A in the C region) (Fig. 3). These results confirmed that three regions affected antigenicity as predicted by alignment analysis.
FIG. 3.

(A) Reactivities of the six point mutations by Western blotting analysis. 20 K, 20,000; a-His, anti-His5 antibody. (B) Position of each point mutation on the r1207 prediction structure. The phenylalanine at position 426 (blue), proline at position 427 (red), leucine at position 526 (yellow), alanine at position 527 (green), proline at position 528 (orange), and glycine at position 530 (magenta) are shown.
To confirm that the relationship between these amino acids affected the antigenicity and structure of r1207, prediction of antigen structure was performed by using the registered Norwalk virus (GI/1) capsid structure (Fig. 1C and 3B). This showed that antigenic residues were contiguous with each other (Fig. 3B). The insert region from amino acid positions 427 to 525 made both the terminal regions proximate; however, they did not have direct interactions via charged residues. The interaction between each terminal region and the insert region (positions 427 to 525) was not observed except for hydrophilic interaction. Moreover, a GI/3-specific single point mutation, close to both terminal regions, was not identified in our study (Fig. 2).
DISCUSSION
In this study, a newly developed monoclonal antibody (MAb14-1) was identified as being a broadly reactive monoclonal antibody, which recognized GI (GI/1, 4, 8, and 11) and GII (GII/1 to 7 and 12 to 15) rVLPs with a weak affinity to GI/3. This recognition range is the broadest of the existing monoclonal antibodies (Table 4) (14, 20, 35, 39, 46, 47). MAb14-1 shows low affinity to GI, but this result was also observed in a previous report by Yoda et al. (46). Therefore, we determined the broad reactivity of MAb14-1 after Yoda's observation.
TABLE 4.
Cross-reactivities of representative previously reported broadly reactive monoclonal antibodies with various norovirus capsids by Western blotting and/or ELISA
| Monoclonal antibody | Isotype | Reactivitya of rVLP or recombinant capsid protein with:
|
Recognition domain | Minimal binding region | Reference(s) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genogroup I genotypeb
|
Genogroup II genotypeb
|
|||||||||||||||||||||||
| 1 | 2 | 3 | 4 | 6 | 8 | 11 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 12 | 13 | 14 | 15 | 17 | |||||
| MAb14-1 | IgG1 | + | + | +/−c | + | + | + | + | + | + | + | + | + | + | + | + | C-terminal P1 | 418-534 | This study | |||||
| NV23 | IgG1 | + | + | + | + | + | + | + | + | 20 | ||||||||||||||
| NS14 | IgG1 | − | − | − | + | + | + | + | + | C-terminal P1 | 473-494d | 20, 34 | ||||||||||||
| NV3901 and NV3912 | IgG1 | + | + | + | − | − | − | − | − | C-terminal P1 | 454-520e | 14, 20, 35 | ||||||||||||
| 1B4 | IgG1 | + | + | + | − | + | + | + | + | + | + | + | + | + | + | S | 31-70f | 46, 47 | ||||||
| 1F6 | IgG2 | +/−g | − | +/−h | − | + | − | + | + | + | + | + | + | + | + | S | 31-70f | 46, 47 | ||||||
| MAb5 | + | + | + | + | + | + | + | + | 39 | |||||||||||||||
Symbols: +, reactive; −, not reactive. Blanks mean the reactivity was unconfirmed. Homologous reactivities are shown in boldface type, especially NS14 against mixture of recombinant norovirus and recombinant Snow Mountain agent (SMA).
Genetic classification based on the method described by Kageyama et al. (17).
MAb14-1 has weak reactivity for GI/3.
Amino acid numbers correspond to the sequence of Norwalk virus (GenBank accession number M87661).
Amino acid numbers correspond to the sequence of Houston virus related to Lordsdale virus (GenBank accession number X86557).
Amino acid numbers correspond to the sequence of the immunogen used to generate the specific antibodies: recombinant norovirus 36 for 1B4 and 1F6 (GenBank accession number AB028244).
In this study, the coating buffer with pH 9.6 was used for the antibody titration. Previous and recent studies repeatedly showed that rVLPs disassemble completely into soluble capsid proteins when a pH value is equal to or higher than 8.9; therefore, rVLPs no longer exist at pH 9.6 (2, 45). From this observation, the same experiments using a coating buffer with pH 7.4 were performed only for r3229 and r1207; however, different conditions showed the same results. Therefore, the data on titer and broad reactivity of the antibody can be compared with not only several laboratory results using rVLPs but also with results using recombinant capsids (or fragments) (Table 4). To explain this reactivity at the molecular level, we demonstrated precise determination of the epitope by using fragment analysis, single point mutants, and structure prediction of antigen.
The results of the fragment analysis for VP1 showed that the epitope for MAb14-1 exists on the C-terminal P1 domain, which is more conserved than the N-terminal P1 and P2 domains based on sequence identity among noroviruses. This location of the epitope may be the reason for the same reactivity under different pH conditions because particle (P domain on the surface of the particle) or a single capsid protein did not relate to the accessibility of the epitope on the P domain for MAb14-1.
The fragment analysis for the C-terminal P1 domain and structural analysis showed that almost the whole C-terminal P1 domain generated the conformation of the minimal binding region. Both terminal antigenic regions (amino acid positions 418 to 426 and 526 to 534) on the minimal binding region approached each other via the insertion region (positions 427 to 525). This motif forms the conformational epitope and may explain the broad reactivity, because MAb14-1 was generated by immunization of GII/4, which is the most sophisticated strain for immune response, implying a potential evolutionary selection.
The components of the epitope for MAb14-1 were determined by mutational analysis. It was found that the components comprise six amino acids and are classified into four major groups, groups 1 to 4, by the following reactivities.
(i) Not only was L526 conserved in all rVLP sequences but also the same conserved residues in other genogroups of norovirus were observed. More interestingly, L526 was even conserved among other caliciviruses, suggesting that this leucine residue might be influence the calicivirus-specific reactivity of MAb14-1 (7).
(ii) There were two interpretations of the role of F425, which is conserved among GII. One of them was the generation of high-titer GII-specific antigenicity for MAb14-1. A previous study by Chakravarty et al. also supported this observation (5). The other interpretation was the generation of genus-specific antigenicity, due to the existence of a GI-specific phenylalanine close to GII-specific phenylalanine. For confirmation of this interpretation, site-directed mutagenesis on the GI capsid needs to be performed in the future.
(iii) Three residues, P426, A527, and P528, gave the same result in inducing a significant reduction in reactivity, but their roles were probably different from each other. P426 possibly constructed the epitope directly. Parker et al. previously reported that K527 (GI) directly interacted with E487, generating the GI-specific structure (35). As a result, K527 (GI) may induce a low titer of MAb14-1 for GI. Our results also supported the previous observations reported by Parker et al. (35) and Chakavraty et al. (5), in that the difference between K and A induced a difference of antigenicity between GI and GII. P528, which is conserved among all noroviruses, except for murine norovirus, is the component of the epitope and induces GI and GII cross-reactivity of MAb14-1.
(iv) G530 is the critical component of the epitope. Functional change of protein was usually ignored in the change from G to A, because there is not much difference in character between G and A (35). Nevertheless, our results showed that the change was important. It is suggested that a slight difference from G to A generates moderate effect on reactivity when glycine is the main component of the epitope.
These mutational analyses elucidated the character of the epitope residues, explaining GI and GII cross-reactivity of the epitope and difference in titer among GI and GII. High conservation of the six amino acids among GII explains high GII-specific titer of MAb14-1. Genus-specific residues generate tolerant reactivity for GI. GI-specific residues induce low reactivity of MAb14-1 for GI. These results imply that the epitope for MAb14-1 is the genus-specific epitope. To investigate this possibility, the reactivity of MAb14-1 for GIII-V rVLPs needs to be elucidated further.
Our results could not explain the low affinity to GI/3 for MAb14-1 because we could not find appropriate GI/3-specific mutations in the minimal binding region. Two possible explanations for this were proposed. First, the epitope on GI/3 may be inhibited by a conformational change derived from the remote amino acid residue(s) in the minimal binding region. Second, other domains, such as N, S, N-terminal P1, and P2 domains, may shield or mask the epitope, as in previous reports about human immunodeficiency virus or picornavirus (4, 8, 23, 26, 36, 44). To confirm these hypotheses, we need to conduct further investigation including crystallography studies.
The fragment, sequence, structural, and mutational analyses identified the epitope formed by the six amino acids and excluded any other amino acids composing the epitope. The structural sequence of these six amino acids generates a linear region; therefore, we can consider this epitope to have potential as a linear epitope with the binding property for the monoclonal antibody. Moreover, in a previous finding on the linear epitope, five amino acid residues were essential for antibody binding, which supports our supposition (10).
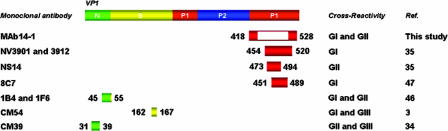
In comparison with the location of a previously reported cross-reactive epitope on VP1 (3, 34, 35, 46, 47), our identified epitope is obviously a novel conformational one (Fig. 4). However, a previously reported GI cross-reactive conformational epitope for monoclonal antibodies, NV3901 and NV3912, and the novel identified epitope in this study shared two amino acid positions, 527 and 528, but the MAb14-1 showed both GI and GII cross-reactivity (Fig. 2) (35). In addition, previous studies reported that broadly reactive monoclonal antibodies, GI and GII cross-reactive antibodies, NS14, 1B4 and 1F6, have linear epitopes (35, 46, 47). Therefore, MAb14-1 had more advantages than previously reported broadly reactive monoclonal antibodies did. In other words, we were the first to identify the GI and GII cross-reactive monoclonal antibody, which recognizes the novel conformational epitope.
FIG. 4.
Location map of the norovirus cross-reactive monoclonal antibody binding sites (being or containing an epitope) on VP1. The blank (amino acids positions 427 to 525) on the binding site for MAb14-1 means that it is not the region for a binding site but is necessary for generating a binding site structurally. Amino acid numbers correspond to the sequences of the immunogens used to generate the specific antibodies: Southampton virus for CM54 (GenBank accession number L07418) Jena virus for CM39 (GenBank accession number AJ011099) Norwalk virus for NV3901 and NV3912 (GenBank accession number M87661), SMA for NS14 (GenBank accession number U70059), recombinant Norwalk virus capsid protein (NV 96-908) for 8C7 (GenBank accession number AB028247), and recombinant genogroup II virus capsid protein (NV 36) for 1B4 and 1F6 (GenBank accession number AB028244). Ref., reference.
With the absence of an appropriate cultivation system, we are not able to use neutralization methods to determine the neutralizing antibody for norovirus. The potentially neutralizing monoclonal antibodies were indirectly determined by Vance's study using histo-blood group antigens assumed to be a receptor for norovirus infection and the putative neutralizing antibodies detecting P2 epitopes (15, 25). Recently, Batten et al. (3) and Oliver et al. (34) reported that anti-human norovirus monoclonal antibody could detect the bovine norovirus, while the opposite was also true. Until now, several neutralizing epitopes have been reported for caliciviruses, except for norovirus (9, 27, 41-43). These previous findings suggest that there are genus-specific neutralizing epitopes on caliciviruses. If this suggestion were true, a broadly reactive monoclonal antibody, such as MAb14-1, which has potential for detecting other caliciviruses, may neutralize calicivirus infection. The use of MAb14-1 may be contribute towards antiviral and vaccine development.
In conclusion, to our knowledge, we were the first group to determine the conformational epitope on the norovirus capsid for GI (GI/1, 4, 8, and 11) and GII (GII/1 to 7 and 12 to 15) cross-reactive novel monoclonal antibody, which showed a weak affinity to GI/3. From these data, MAb14-1 could be applied further for the development of the immunochromatography test.
Acknowledgments
This study was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Sciences and Technology and the Ministry of Health, Labor and Welfare, Japan. It was also supported by the Miyakawa Memorial Research Foundation, Sumitomo Foundation and Mishima-Kaiun Foundation in Japan.
Footnotes
Published ahead of print on 12 September 2007.
REFERENCES
- 1.Atmar, R. L., and M. K. Estes. 2006. The epidemiologic and clinical importance of norovirus infection. Gastroenterol. Clin. N. Am. 35:275-290. [DOI] [PubMed] [Google Scholar]
- 2.Ausar, S. F., T. R. Foubert, M. H. Hudson, T. S. Vedvick, and C. R. Middaugh. 2006. Conformational stability and disassembly of Norwalk virus-like particles. Effect of pH and temperature. J. Biol. Chem. 281:19478-19488. [DOI] [PubMed] [Google Scholar]
- 3.Batten, C. A., I. N. Clarke, S. L. Kempster, S. L. Oliver, J. C. Bridger, and P. R. Lambden. 2006. Characterization of a cross-reactive linear epitope in human genogroup I and bovine genogroup III norovirus capsid proteins. Virology 356:179-187. [DOI] [PubMed] [Google Scholar]
- 4.Cao, J., N. Sullivan, E. Desjardin, C. Parolin, J. Robinson, R. Wyatt, and J. Sodroski. 1997. Replication and neutralization of human immunodeficiency virus type 1 lacking the V1 and V2 variable loops of the gp120 envelope glycoprotein. J. Virol. 71:9808-9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakravarty, S., A. M. Hutson, M. K. Estes, and B. V. Prasad. 2005. Evolutionary trace residues in noroviruses: importance in receptor binding, antigenicity, virion assembly, and strain diversity. J. Virol. 79:554-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, R., J. D. Neill, M. K. Estes, and B. V. Prasad. 2006. X-ray structure of a native calicivirus: structural insights into antigenic diversity and host specificity. Proc. Natl. Acad. Sci. USA 103:8048-8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, R., J. D. Neill, J. S. Noel, A. M. Hutson, R. I. Glass, M. K. Estes, and B. V. Prasad. 2004. Inter- and intragenus structural variations in caliciviruses and their functional implications. J. Virol. 78:6469-6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng-Mayer, C., A. Brown, J. Harouse, P. A. Luciw, and A. J. Mayer. 1999. Selection for neutralization resistance of the simian/human immunodeficiency virus SHIVSF33A variant in vivo by virtue of sequence changes in the extracellular envelope glycoprotein that modify N-linked glycosylation. J. Virol. 73:5294-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geissler, K., K. Schneider, and U. Truyen. 2002. Mapping neutralizing and non-neutralizing epitopes on the capsid protein of feline calicivirus. J. Vet. Med. B Infect. Dis. Vet. Public Health 49:55-60. [DOI] [PubMed] [Google Scholar]
- 10.Geysen, H. M., T. J. Mason, and S. J. Rodda. 1988. Cognitive features of continuous antigenic determinants. J. Mol. Recognit. 1:32-41. [DOI] [PubMed] [Google Scholar]
- 11.Glass, R. I., J. Noel, T. Ando, R. Fankhauser, G. Belliot, A. Mounts, U. D. Parashar, J. S. Bresee, and S. S. Monroe. 2000. The epidemiology of enteric caliciviruses from humans: a reassessment using new diagnostics. J. Infect. Dis. 181(Suppl. 2):S254-S261. [DOI] [PubMed] [Google Scholar]
- 12.Green, K. Y., J. F. Lew, X. Jiang, A. Z. Kapikian, and M. K. Estes. 1993. Comparison of the reactivities of baculovirus-expressed recombinant Norwalk virus capsid antigen with those of the native Norwalk virus antigen in serologic assays and some epidemiologic observations. J. Clin. Microbiol. 31:2185-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hale, A. D., S. E. Crawford, M. Ciarlet, J. Green, C. Gallimore, D. W. Brown, X. Jiang, and M. K. Estes. 1999. Expression and self-assembly of Grimsby virus: antigenic distinction from Norwalk and Mexico viruses. Clin. Diagn. Lab. Immunol. 6:142-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy, M. E., T. N. Tanaka, N. Kitamoto, L. J. White, J. M. Ball, X. Jiang, and M. K. Estes. 1996. Antigenic mapping of the recombinant Norwalk virus capsid protein using monoclonal antibodies. Virology 217:252-261. [DOI] [PubMed] [Google Scholar]
- 15.Hutson, A. M., R. L. Atmar, D. M. Marcus, and M. K. Estes. 2003. Norwalk virus-like particle hemagglutination by binding to H histo-blood group antigens. J. Virol. 77:405-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang, X., M. Wang, D. Y. Graham, and M. K. Estes. 1992. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J. Virol. 66:6527-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kageyama, T., M. Shinohara, K. Uchida, S. Fukushi, F. B. Hoshino, S. Kojima, R. Takai, T. Oka, N. Takeda, and K. Katayama. 2004. Coexistence of multiple genotypes, including newly identified genotypes, in outbreaks of gastroenteritis due to norovirus in Japan. J. Clin. Microbiol. 42:2988-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamata, K., K. Shinozaki, M. Okada, Y. Seto, S. Kobayashi, K. Sakae, M. Oseto, K. Natori, H. Shirato-Horikoshi, K. Katayama, T. Tanaka, N. Takeda, and K. Taniguchi. 2005. Expression and antigenicity of virus-like particles of norovirus and their application for detection of noroviruses in stool samples. J. Med. Virol. 76:129-136. [DOI] [PubMed] [Google Scholar]
- 19.Karst, S. M., C. E. Wobus, M. Lay, J. Davidson, and H. W. Virgin IV. 2003. STAT1-dependent innate immunity to a Norwalk-like virus. Science 299:1575-1578. [DOI] [PubMed] [Google Scholar]
- 20.Kitamoto, N., T. Tanaka, K. Natori, N. Takeda, S. Nakata, X. Jiang, and M. K. Estes. 2002. Cross-reactivity among several recombinant calicivirus virus-like particles (VLPs) with monoclonal antibodies obtained from mice immunized orally with one type of VLP. J. Clin. Microbiol. 40:2459-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi, S., K. Sakae, Y. Suzuki, K. Shinozaki, M. Okada, H. Ishiko, K. Kamata, K. Suzuki, K. Natori, T. Miyamura, and N. Takeda. 2000. Molecular cloning, expression, and antigenicity of Seto virus belonging to genogroup I Norwalk-like viruses. J. Clin. Microbiol. 38:3492-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohler, G., and C. Milstein. 1975. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495-497. [DOI] [PubMed] [Google Scholar]
- 23.Krachmarov, C. P., W. J. Honnen, S. C. Kayman, M. K. Gorny, S. Zolla-Pazner, and A. Pinter. 2006. Factors determining the breadth and potency of neutralization by V3-specific human monoclonal antibodies derived from subjects infected with clade A or clade B strains of human immunodeficiency virus type 1. J. Virol. 80:7127-7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laemmli, U. K., F. Beguin, and G. Gujer-Kellenberger. 1970. A factor preventing the major head protein of bacteriophage T4 from random aggregation. J. Mol. Biol. 47:69-85. [DOI] [PubMed] [Google Scholar]
- 25.Lochridge, V. P., K. L. Jutila, J. W. Graff, and M. E. Hardy. 2005. Epitopes in the P2 domain of norovirus VP1 recognized by monoclonal antibodies that block cell interactions. J. Gen. Virol. 86:2799-2806. [DOI] [PubMed] [Google Scholar]
- 26.Ly, A., and L. Stamatatos. 2000. V2 loop glycosylation of the human immunodeficiency virus type 1 SF162 envelope facilitates interaction of this protein with CD4 and CCR5 receptors and protects the virus from neutralization by anti-V3 loop and anti-CD4 binding site antibodies. J. Virol. 74:6769-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuura, Y., Y. Tohya, M. Mochizuki, K. Takase, and T. Sugimura. 2001. Identification of conformational neutralizing epitopes on the capsid protein of canine calicivirus. J. Gen. Virol. 82:1695-1702. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen, T. A., P. Khamrin, S. Takanashi, L. P. Hoang, D. L. Pham, T. K. Hoang, K. Satou, Y. Masuoka, S. Okitsu, and H. Ushijima. 2007. Evaluation of immunochromatography tests for detection of rotavirus and norovirus among Vietnamese children with acute gastroenteritis and the emergence of a novel norovirus GII.4 variant. J. Trop. Pediatr. -269.53:264. [DOI] [PubMed] [Google Scholar]
- 29.Notredame, C., D. G. Higgins, and J. Heringa. 2000. T-Coffee: a novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 302:205-217. [DOI] [PubMed] [Google Scholar]
- 30.Okada, M., T. Ogawa, I. Kaiho, and K. Shinozaki. 2005. Genetic analysis of noroviruses in Chiba Prefecture, Japan, between 1999 and 2004. J. Clin. Microbiol. 43:4391-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okame, M., H. Yan, S. Akihara, S. Okitsu, H. Tani, Y. Matsuura, and H. Ushijima. 2003. Evaluation of a newly developed immunochromatographic method for detection of norovirus. Kansenshogaku Zasshi 77:637-639. [DOI] [PubMed] [Google Scholar]
- 32.Okitsu-Negishi, S., M. Okame, Y. Shimizu, T. G. Phan, T. Tomaru, S. Kamijo, T. Sato, F. Yagyu, W. E. Muller, and H. Ushijima. 2006. Detection of norovirus antigens from recombinant virus-like particles and stool samples by a commercial norovirus enzyme-linked immunosorbent assay kit. J. Clin. Microbiol. 44:3784-3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliver, S. L., E. Asobayire, A. M. Dastjerdi, and J. C. Bridger. 2006. Genomic characterization of the unclassified bovine enteric virus Newbury agent-1 (Newbury1) endorses a new genus in the family Caliciviridae. Virology 350:240-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliver, S. L., C. A. Batten, Y. Deng, M. Elschner, P. Otto, A. Charpilienne, I. N. Clarke, J. C. Bridger, and P. R. Lambden. 2006. Genotype 1 and genotype 2 bovine noroviruses are antigenically distinct but share a cross-reactive epitope with human noroviruses. J. Clin. Microbiol. 44:992-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker, T. D., N. Kitamoto, T. Tanaka, A. M. Hutson, and M. K. Estes. 2005. Identification of genogroup I and genogroup II broadly reactive epitopes on the norovirus capsid. J. Virol. 79:7402-7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinter, A., W. J. Honnen, Y. He, M. K. Gorny, S. Zolla-Pazner, and S. C. Kayman. 2004. The V1/V2 domain of gp120 is a global regulator of the sensitivity of primary human immunodeficiency virus type 1 isolates to neutralization by antibodies commonly induced upon infection. J. Virol. 78:5205-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prasad, B. V., M. E. Hardy, T. Dokland, J. Bella, M. G. Rossmann, and M. K. Estes. 1999. X-ray crystallographic structure of the Norwalk virus capsid. Science 286:287-290. [DOI] [PubMed] [Google Scholar]
- 38.Schwede, T., J. Kopp, N. Guex, and M. C. Peitsch. 2003. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 31:3381-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka, T., N. Kitamoto, X. Jiang, and M. K. Estes. 2006. High efficiency cross-reactive monoclonal antibody production by oral immunization with recombinant Norwalk virus-like particles. Microbiol. Immunol. 50:883-888. [DOI] [PubMed] [Google Scholar]
- 40.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tohya, Y., K. Masuoka, E. Takahashi, and T. Mikami. 1991. Neutralizing epitopes of feline calicivirus. Arch. Virol. 117:173-181. [DOI] [PubMed] [Google Scholar]
- 42.Tohya, Y., Y. Taniguchi, M. Tsubakimoto, E. Takahashi, and T. Mikami. 1990. Preparation and characterization of neutralizing monoclonal antibodies to feline calicivirus. Nippon Juigaku Zasshi 52:251-256. [DOI] [PubMed] [Google Scholar]
- 43.Tohya, Y., N. Yokoyama, K. Maeda, Y. Kawaguchi, and T. Mikami. 1997. Mapping of antigenic sites involved in neutralization on the capsid protein of feline calicivirus. J. Gen. Virol. 78:303-305. [DOI] [PubMed] [Google Scholar]
- 44.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307-312. [DOI] [PubMed] [Google Scholar]
- 45.White, L. J., M. E. Hardy, and M. K. Estes. 1997. Biochemical characterization of a smaller form of recombinant Norwalk virus capsids assembled in insect cells. J. Virol. 71:8066-8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoda, T., Y. Suzuki, Y. Terano, K. Yamazaki, N. Sakon, T. Kuzuguchi, H. Oda, and T. Tsukamoto. 2003. Precise characterization of norovirus (Norwalk-like virus)-specific monoclonal antibodies with broad reactivity. J. Clin. Microbiol. 41:2367-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoda, T., Y. Terano, Y. Suzuki, K. Yamazaki, I. Oishi, T. Kuzuguchi, H. Kawamoto, E. Utagawa, K. Takino, H. Oda, and T. Shibata. 2001. Characterization of Norwalk virus GI specific monoclonal antibodies generated against Escherichia coli expressed capsid protein and the reactivity of two broadly reactive monoclonal antibodies generated against GII capsid towards GI recombinant fragments. BMC Microbiol. 1:24. [DOI] [PMC free article] [PubMed] [Google Scholar]