Abstract
The attachment and spreading of keratinocyte cells result from interactions between integrins and immobilized extracellular matrix molecules. Human papillomavirus type 16 (HPV-16) E6 augmented the kinetics of cell spreading, while E6 genes from HPV-11 or bovine papillomavirus type 1 did not. The ability of E6 to interact with the E6AP ubiquitin ligase and target p53 degradation was required to augment cell-spreading kinetics; dominant negative p53 alleles also enhanced the kinetics of cell spreading and the level of attachment of cells to hydrophobic surfaces. The targeted degradation of p53 by E6 may contribute to the invasive phenotype exhibited by cervical cells that contain high-risk HPV types.
Human papillomaviruses (HPVs) produce benign epitheliomas, but with a subset of HPV types, infection can eventually lead to invasive cancers (14). HPVs with this property are termed “high-risk” types, and the HPV most frequently isolated from cervical cancers is HPV-16 (23). In cancer cells, the HPV genome is integrated into the host chromosome such that the intact E6 and E7 oncoproteins are expressed. It is as yet unclear whether the expression of E6 and E7 directly contributes to an invasive-cancer phenotype or whether the properties of E6 and E7 (which include the abrogation of normal cell cycle checkpoint controls) contribute to a mutator phenotype, with the subsequent selection for invasive cells.
HPV-16 E6 (16E6) is the prototype high-risk HPV E6 protein. 16E6 directly interacts with a cellular ubiquitin ligase termed E6AP by binding a LXXLL motif of E6AP; the complex of 16E6 together with E6AP binds to the p53 tumor suppressor protein, resulting in ubiquitin-mediated degradation of p53 by the proteasome (7, 8, 22). The in vivo degradation of p53 requires sequences at the amino terminus of E6 that interact with p53 (3). The six amino acids at the carboxy terminus of 16E6 contain a PDZ ligand that interacts with a subset of cellular proteins containing one or more PDZ domains; as with p53, the interaction of E6 with these PDZ-containing cellular proteins may lead to their E6AP-dependent degradation (4, 5, 9, 10, 12, 15). Some evidence supports the involvement of ubiquitin ligases other than E6AP (17, 24).
We and others had previously observed that the E6 oncoprotein of bovine papillomavirus type 1 (BPV-1) E6 binds to paxillin and that paxillin was required for the tyrosine phosphorylation of focal adhesion kinase (FAK), a kinase that plays a regulatory role in cell migration (26-29). These findings prompted us to examine whether expression of E6 proteins might modulate signaling from integrin receptors to the cytoskeleton. We observed that keratinocytes retrovirally transduced with E6 from genital cancer-associated HPV-16 induced a marked increase in early cell spreading after attaching to a matrix-coated surface, while keratinocytes transduced with E6 proteins from low-risk HPV-11 and BPV-1 did not (Fig. 1A). Cultures of normal keratinocyte cell (NIKS cells) (1) were washed twice with phosphate-buffered saline, lightly trypsinized at room temperature, and then rinsed twice to remove feeder fibroblasts, leaving the epithelial cells. A second trypsinization at 37°C detached keratinocytes that were collected by centrifugation, and they were resuspended at 5 × 105 cells/ml in complete media (18) and then incubated with periodic gentle swirling for 20 min at 37°C. A cell suspension mixture (0.2 ml) was added to 24-well, matrix-coated microtiter plates and incubated at 37°C. At various time points, the media were gently removed and the plates were gently rinsed with cold, serum-free media to remove unattached cells. The cells were immediately fixed, stained, and scored as spread if the cytoplasms exceeded twice the diameter of the nuclei (Fig. 1A and 2A and B) or digitally analyzed to determine the areas of the cell surfaces (Fig. 1C). Assays to determine the levels of cell attachment and spreading were performed as previously described (29).
FIG. 1.
HPV-16 E6 enhances early cell spreading after attachment of the cells to matrix-coated surfaces. (A) Cell spreading is altered by cancer-associated HPV E6. Only HPV-16 E6 enhanced early cell spreading after attachment. The values shown are the averages of the results of three assays described in the text. (B) 16E6 enhances early cell spreading. The cells shown were fixed and stained with formalin as described in the text. (C) Quantitation of the enhancement of cell spreading by 16E6. The total cell surface area was quantitated and compared by Image analysis software (NIH Image) 20 min after plating. Student's t test was used to determine the P value for a 300-cell population of each cell type. The error bars indicate the standard errors of the means.
FIG. 2.
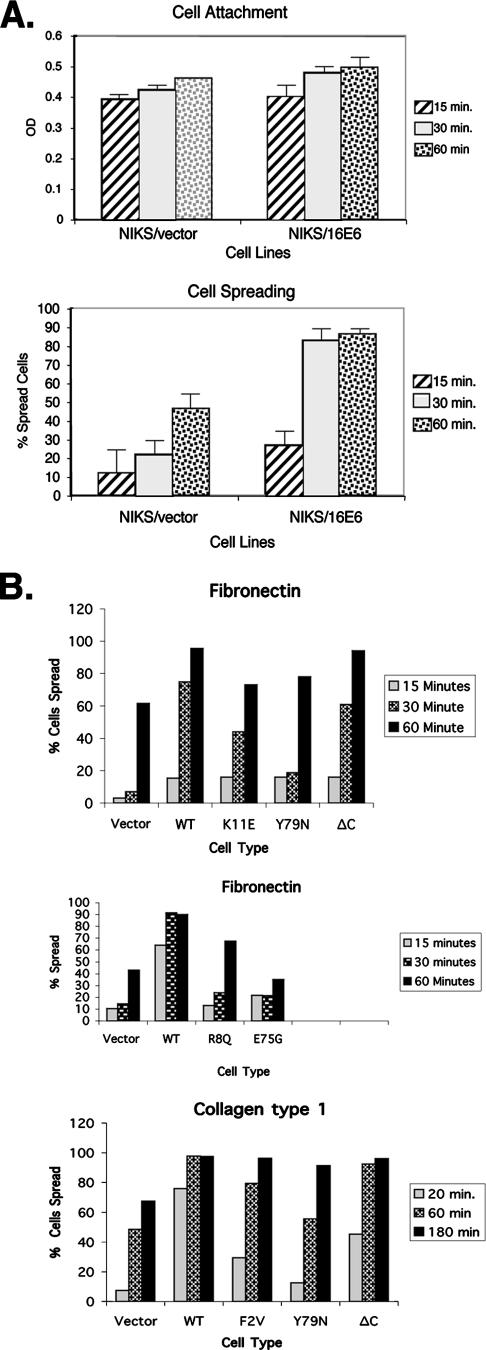
The effects of matrix and E6 mutations on cell spreading. (A) 16E6 augments cell spreading but not cell attachment. NIKS cells transduced with either empty retroviral vectors or 16E6 were assayed to determine the levels of cell attachment (top panel) and cell spreading (bottom panel) 15, 30, or 60 min after being plated on fibronectin-coated plates (10 μg/ml) as previously described (28). OD, optical density. (B) Enhanced spreading is independent of the matrix type and requires amino-terminal sequences of 16E6. NIKS cells transduced with empty retroviral vectors, 16E6 (shown as WT), or the indicated 16E6 mutants were seeded on plastic plates coated with either collagen type 1 or fibronectin for 20, 60, or 180 min. At least 300 cells were counted at each time point in three representative experiments.
Quantitation of early cell spreading showed that 16E6 expression enhanced the early-spread total cell surface area by about 2.5-fold (Fig. 1B and C). The attachment of keratinocytes to the matrix was not altered by 16E6, but subsequent early cell spreading was markedly increased by 16E6 (Fig. 2A) on surfaces coated with fibronectin, laminin, collagen, and vitronectin (Fig. 2B and data not shown). This analysis also demonstrated that three different 16E6 mutants that alter the amino-terminal p53 interaction motif of 16E6 abrogated the enhancement of cell spreading (16E6_K11E, 16E6_R8Q, and 16E6_F2V are defective for degrading p53 [3]), as did mutations that eliminate the ability of 16E6 to interact with the LXXLL motif of E6AP (16E6_Y79N and 16E6_E75G [3]) (Fig. 2B). Surprisingly, deletion of the PDZ ligand of 16E6 (16E6_ΔC) had less effect upon cell spreading (Fig. 2B).
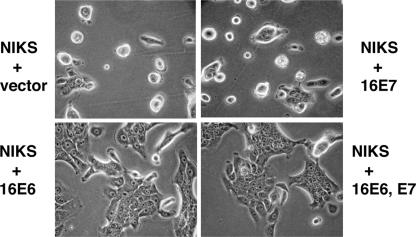
The cell-spreading assay employed measures integrin signaling to the cytoskeleton in the initial minutes after cell attachment (20). To determine whether E6 might also have a prolonged effect upon keratinocyte attachment and spreading, we plated keratinocytes onto a hydrophobic surface, a more-difficult substrate for the cells to attach to. Keratinocytes transduced with 16E6 but not 16E7 had enhanced levels of cell spreading on bacterial plates at 24 and 48 h postplating (Fig. 3). A 16E6 mutant defective for degrading p53 (16E6_F2V) did not augment cell spreading, while 16E6 from cells expressing either a 16E6 mutant with the PDZ ligand deleted or a p53 dominant negative mutant appeared indistinguishable from wild-type 16E6 (Fig. 4A). The expression levels of wild-type and mutant E6 proteins were similar (Fig. 4B) (the 16E6_F2V mutation destroys the 6F4 epitope). These results indicate that interference with wild-type p53 by either targeted degradation or transdominant interference enhances cell spreading.
FIG. 3.
16E6 augments cell spreading on hydrophobic surfaces. (A) Spread of NIKS cells on bacterial plates 24 h after plating. NIKS cells were stably transduced with vectors or the indicated oncogenes, seeded on bacterial plates, and photographed 24 h later.
FIG. 4.

p53 regulates spreading on hydrophobic surfaces. (A) NIKS cells transduced with the indicated 16E6 genes or a dominant negative p53 gene (p53 DD) (25) were photographed 48 h after being plated on bacterial plates. (B) Expression levels of 16E6 and 16E6 mutants in NIKS cells. NIKS cells transduced with retrovirus vectors and lysed in sodium dodecyl sulfate were analyzed by Western blotting, using monoclonal antibodies (MAb) to 16E6 (11). Monoclonal antibody 3F8 recognizes an epitope common to 16E6 and all the mutants, while monoclonal antibody 6F4 recognizes the amino terminus of 16E6; this epitope is disrupted by the 16E6_F2V mutation.
We performed our experiments using a nontransformed but immortalized human keratinocyte cell line (NIKS cells) that supports the full HPV life cycle in organotypic culture but is nontransformed and requires feeder cells, epidermal growth factor, insulin, and serum in the media (1). These cells were chosen so that vector- and 16E6-transduced keratinocytes would retain similar proliferation and differentiation characteristics such that cytoskeleton changes would not be an artifact of the two populations having different degrees of proliferative potential or terminal differentiation. We obtained similar results with transient transfections of NIKS cells with 16E6, 16E6_F2V, and mutant p53 cDNAs (data not shown).
Our experiments examining cell spreading on hydrophobic surfaces were prompted by the intriguing observation that primary keratinocytes that are cultured on bacterial plates have extended proliferative potential (6). Most NIKS keratinocytes fail to attach to a hydrophobic surface, and among those that do attach, most fail to spread well (Fig. 3 and 4). However, the population of cells that do attach and spread can then be passaged upon bacterial plates (6; our unpublished observations). It appears that culturing on hydrophobic plates selects a subpopulation of keratinocytes that both attach to and spread on hydrophobic surfaces and have enhanced proliferative potential. Both 16E6 and dominant negative p53 were able to induce enhanced spreading of NIKS cells on hydrophobic plates (Fig. 4). Dominant negative p53 has recently been shown to cooperate with p16 inhibition to extend the life spans of primary keratinocytes (19).
Mouse fibroblasts null for p53 exhibit enhanced cell spreading and migration, and the mechanisms involved appear complex (21). In studies not shown here, we did not observe a clear and consistent effect of 16E6 upon the tyrosine phosphorylation of FAK or the nucleotide loading of Rac1, Cdc42, or Rho in NIKS cells (data not shown). A previous study of FAK phosphorylation showed enhanced activation of FAK tyrosine phosphorylation in cells expressing HPV-18 E6 and E7 compared to that for vector-transduced primary keratinocytes (13); however, in our experience, there are substantial differences in differentiation and proliferation between these two cell populations which could confound the interpretation. Migration is complex, involving attachment, spreading, polarization, directed cytoskeletal extension, and release of the caudal cell part from the substrate (20). The initial stage of cell spreading that is the subject of this study is a crucial component of migration but is only a part. This report extends the observation of the role of p53 in altering cell spreading to keratinocytes, where the targeted degradation of p53 correlates with HPV types associated with invasive cancers. It is interesting that neither the E6 proteins of HPV-11 nor those of BPV-1 markedly altered early cell spreading, despite the ability of both of these E6 proteins to interact with E6AP and, in the case of HPV-11, to target associated proteins for degradation (2, 16). Neither BPV-1 E6 nor HPV-11 E6 targets the degradation of p53, and upon the mutation of the p53 interaction motif of 16E6, the effect of 16E6 on cell spreading is largely lost. Therefore, it is possible that the degradation of p53 by 16E6 contributes in part to the abnormal invasion and migration that are characteristic of cervical cancer cells.
Acknowledgments
NIH grants CA80931 and CA69292 to S.B.V.P. supported this research.
We thank Martin Schwartz for helpful discussions and Janet Cross for critical reading of the manuscript.
Footnotes
Published ahead of print on 5 September 2007.
REFERENCES
- 1.Allen-Hoffmann, B. L., S. J. Schlosser, C. A. Ivarie, C. A. Sattler, L. F. Meisner, and S. L. O'Connor. 2000. Normal growth and differentiation in a spontaneously immortalized near-diploid human keratinocyte cell line, NIKS. J. Investig. Dermatol. 114:444-455. [DOI] [PubMed] [Google Scholar]
- 2.Brimer, N., C. Lyons, and S. B. Vande Pol. 2007. Association of E6AP (UBE3A) with human papillomavirus type 11 E6 protein. Virology 358:303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper, B., S. Schneider, J. Bohl, Y. Jiang, A. Beaudet, and S. Vande Pol. 2003. Requirement of E6AP and the features of human papillomavirus E6 necessary to support degradation of p53. Virology 306:87-99. [DOI] [PubMed] [Google Scholar]
- 4.Grm, H. S., and L. Banks. 2004. Degradation of hDlg and MAGIs by human papillomavirus E6 is E6-AP-independent. J. Gen. Virol. 85:2815-2819. [DOI] [PubMed] [Google Scholar]
- 5.Handa, K., T. Yugawa, M. Narisawa-Saito, S. Ohno, M. Fujita, and T. Kiyono. 2007. E6AP-dependent degradation of DLG4/PSD95 by high-risk human papillomavirus type 18 E6 protein. J. Virol. 81:1379-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasskarl, J., P. Velupillai, S. O. Piboonniyom, M. Grace, and K. Munger. 2005. Long-term maintenance of human keratinocytes in vitro. J. Investig. Dermatol. 124:475-478. [DOI] [PubMed] [Google Scholar]
- 7.Huibregtse, J. M., M. Scheffner, and P. M. Howley. 1991. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 10:4129-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huibregtse, J. M., M. Scheffner, and P. M. Howley. 1993. Localization of the E6-AP regions that direct human papillomavirus E6 binding, association with p53, and ubiquitination of associated proteins. Mol. Cell. Biol. 13:4918-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jing, M., J. Bohl, N. Brimer, M. Kinter, and S. B. Vande Pol. 2007. Degradation of tyrosine phosphatase PTPN3 (PTPH1) by association with oncogenic human papillomavirus E6 proteins. J. Virol. 81:2231-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuballa, P., K. Matentzoglu, and M. Scheffner. 2007. The role of the ubiquitin ligase E6-AP in human papillomavirus E6-mediated degradation of PDZ domain-containing proteins. J. Biol. Chem. 282:65-71. [DOI] [PubMed] [Google Scholar]
- 11.Lagrange, M., S. Charbonnier, G. Orfanoudakis, P. Robinson, K. Zanier, M. Masson, Y. Lutz, G. Trave, E. Weiss, and F. Deryckere. 2005. Binding of human papillomavirus 16 E6 to p53 and E6AP is impaired by monoclonal antibodies directed against the second zinc-binding domain of E6. J. Gen. Virol. 86:1001-1007. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto, Y., S. Nakagawa, T. Yano, S. Takizawa, K. Nagasaka, K. Nakagawa, T. Minaguchi, O. Wada, H. Ooishi, K. Matsumoto, T. Yasugi, T. Kanda, J. M. Huibregtse, and Y. Taketani. 2006. Involvement of a cellular ubiquitin-protein ligase E6AP in the ubiquitin-mediated degradation of extensive substrates of high-risk human papillomavirus E6. J. Med. Virol. 78:501-507. [DOI] [PubMed] [Google Scholar]
- 13.McCormack, S. J., S. E. Brazinski, J. L. Moore, Jr., B. A. Werness, and D. J. Goldstein. 1997. Activation of the focal adhesion kinase signal transduction pathway in cervical carcinoma cell lines and human genital epithelial cells immortalized with human papillomavirus type 18. Oncogene 15:265-274. [DOI] [PubMed] [Google Scholar]
- 14.Münger, K., A. Baldwin, K. M. Edwards, H. Hayakawa, C. L. Nguyen, M. Owens, M. Grace, and K. Huh. 2004. Mechanisms of human papillomavirus-induced oncogenesis. J. Virol. 78:11451-11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakagawa, S., and J. M. Huibregtse. 2000. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol. Cell. Biol. 20:8244-8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pim, D., M. Thomas, and L. Banks. 2002. Chimaeric HPV E6 proteins allow dissection of the proteolytic pathways regulating different E6 cellular target proteins. Oncogene 21:8140-8148. [DOI] [PubMed] [Google Scholar]
- 17.Pim, D., M. Thomas, R. Javier, D. Gardiol, and L. Banks. 2000. HPV E6 targeted degradation of the discs large protein: evidence for the involvement of a novel ubiquitin ligase. Oncogene 19:719-725. [DOI] [PubMed] [Google Scholar]
- 18.Rheinwald, J. G., and H. Green. 1975. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6:331-343. [DOI] [PubMed] [Google Scholar]
- 19.Rheinwald, J. G., W. C. Hahn, M. R. Ramsey, J. Y. Wu, Z. Guo, H. Tsao, M. De Luca, C. Catricala, and K. M. O'Toole. 2002. A two-stage, p16(INK4A)- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol. Cell. Biol. 22:5157-5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridley, A. J., M. A. Schwartz, K. Burridge, R. A. Firtel, M. H. Ginsberg, G. Borisy, J. T. Parsons, and A. R. Horwitz. 2003. Cell migration: integrating signals from front to back. Science 302:1704-1709. [DOI] [PubMed] [Google Scholar]
- 21.Roger, L., G. Gadea, and P. Roux. 2006. Control of cell migration: a tumour suppressor function for p53? Biol. Cell. 98:141-152. [DOI] [PubMed] [Google Scholar]
- 22.Scheffner, M., J. M. Huibregtse, R. D. Vierstra, and P. M. Howley. 1993. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75:495-505. [DOI] [PubMed] [Google Scholar]
- 23.Schiffman, M. H., H. M. Bauer, R. N. Hoover, A. G. Glass, D. M. Cadell, B. B. Rush, D. R. Scott, M. E. Sherman, R. J. Kurman, S. Wacholder, et al. 1993. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J. Natl. Cancer Inst. 85:958-964. [DOI] [PubMed] [Google Scholar]
- 24.Shai, A., M. L. Nguyen, J. Wagstaff, Y. H. Jiang, and P. F. Lambert. 2007. HPV16 E6 confers p53-dependent and p53-independent phenotypes in the epidermis of mice deficient for E6AP. Oncogene 26:3321-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaulian, E., A. Zauberman, D. Ginsberg, and M. Oren. 1992. Identification of a minimal transforming domain of p53: negative dominance through abrogation of sequence-specific DNA binding. Mol. Cell. Biol. 12:5581-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong, X., and P. M. Howley. 1997. The bovine papillomavirus E6 oncoprotein interacts with paxillin and disrupts the actin cytoskeleton. Proc. Natl. Acad. Sci. USA 94:4412-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vande Pol, S. B., M. C. Brown, and C. E. Turner. 1998. Association of bovine papillomavirus type 1 E6 oncoprotein with the focal adhesion protein paxillin through a conserved protein interaction motif. Oncogene 16:43-52. [DOI] [PubMed] [Google Scholar]
- 28.Wade, R., J. Bohl, and S. Vande Pol. 2002. Paxillin null embryonic stem cells are impaired in cell spreading and tyrosine phosphorylation of focal adhesion kinase. Oncogene 21:96-107. [DOI] [PubMed] [Google Scholar]
- 29.Wade, R., and S. Vande Pol. 2006. Minimal features of paxillin that are required for the tyrosine phosphorylation of focal adhesion kinase. Biochem. J. 393:565-573. [DOI] [PMC free article] [PubMed] [Google Scholar]