Abstract
Myxoma virus (MV) is a candidate for oncolytic virotherapy due to its ability to selectively infect and kill tumor cells, yet MV is a species-specific pathogen that causes disease only in European rabbits. To assess the ability of MV to deliver cytokines to tumors, we created an MV (vMyxIL-12) that expresses human interleukin-12 (IL-12). vMyxIL-12 replicates similarly to wild-type MV, and virus-infected cells secrete bioactive IL-12. Yet, vMyxIL-12 does not cause myxomatosis, despite expressing the complete repertoire of MV proteins. Thus, vMyxIL-12 exhibits promise as an oncolytic candidate and is safe in all known vertebrate hosts, including lagomorphs.
Myxoma virus (MV) is a poxvirus and the prototypic member of the Leporipoxvirus genus. MV is the causative agent of myxomatosis, a lethal and deblilitating disease of European rabbits (Oryctolagus cuniculus), characterized by profound systemic immunosuppression (11, 12). MV has been completely sequenced (5), and many virus-encoded immunomodulatory proteins have been identified (2, 20, 32, 41). A significant number of individual MV genes have been genetically removed, and the effect on the ability of the knockout virus to cause mxyomatosis has been evaluated (18, 19). Ablation of some viral genes had minor effects on the ability of the virus to cause disease in rabbits, while others were attenuated to the point that the virus no longer causes significant pathology. These studies, combined with concomitant in vitro analysis of the individual viral gene products, have provided many insights into the mechanism by which MV causes disease in rabbits (20, 32).
Although MV cannot cause disease in any vertebrate species other than lagomorphs, it has been demonstrated that MV can preferentially infect and kill human cancer cells (33). This has led to studies examining MV as a virotherapeutic for human cancer. The ability of MV to infect and clear tumors in vivo has been demonstrated in a model for human glioblastoma, where intratumoral injection of MV effectively “cured” human gliomas in immunocompromised mice (23). The ability of MV to infect human tumor cells has been linked to activity of cellular Akt kinase (36), and drugs that act on this host signaling pathway have been shown to augment MV infection (30).
To stimulate an anticancer immune response within a largely nonresponsive tumor microenvironment, cytokine therapy has been employed. Interleukin-12 (IL-12) is produced by antigen-presenting cells (29) and acts as an important mediator of T-cell responses through promoting T type 1 immunity (15). IL-12 exhibits significant antitumor effects in both animal models and human patients, yet the toxicity induced by systemic administration prevented further use clinically (15, 22, 26, 28, 34, 35, 37). Thus, local dosing of IL-12 within tumors has been explored. IL-12 has been delivered to murine (14) and human (4) tumors using IL-12 encapsulated microspheres (14) or irradiated tumor cells transfected with an IL-12 expression plasmid (13). Viral vectors, including herpesvirus (3, 38-40) and vaccinia virus (8, 9, 25), have also been used to deliver IL-12 into tumors, yet toxicity outside the tumor bed can still be an issue. Here, we have created an MV construct that expresses human IL-12 (hIL-12) with the intention of testing the therapeutic efficacy of this virus in a murine tumor model and assessing whether the oncolytic potential of MV can be enhanced. However, it is important to evaluate the effect of an hIL-2-expressing virus in the only host for which MV is known to be pathogenic.
Expression of hIL-12 from MV does not affect MV replication in vitro.
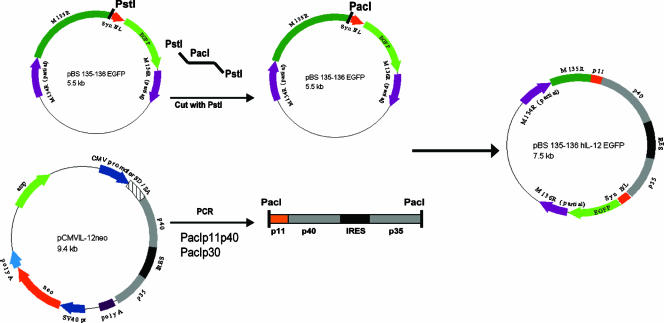
To create a more effective oncolytic virus, we constructed a recombinant MV expressing hIL-12 (Fig. 1). The hIL-12 expression plasmid pCMVIL-12 neo was used as a source of hIL-12 cDNA (13). This plasmid contains the cDNA for the p35 and p40 subunits of IL-12 linked via a viral internal ribosomal entry sequence to allow cotranslation of both subunit chains from a single transcript. Primers for both the p40 and p35 subunits were synthesized to amplify the subunit DNA containing PacI restriction sites at the ends and the p40 primer additionally containing the poxviral P11 minimal late promoter sequence. The PCR product was ligated into the pCR2.1 TOPO TA cloning vector (Invitrogen). A PacI site was inserted into the existing PstI site between open reading frame M135R and the vaccinia virus synthetic early/late promoter driving the expression of the enhanced green fluorescent protein (EGFP) in the cloning plasmid pBS 135-136 EGFP (17). The IL-12 cassette was released from the TOPO vector by PacI digestion, and the recipient plasmid was linearized following PacI digestion. The IL-12 insert was ligated into pBS 135-136 EGFP. This new clone was designated pBS 135-136 hIL-12 EGFP. This plasmid was transfected into MV (strain Lausanne [vMyx])-infected BGMK cells to create, via homologous recombination, the recombinant virus expressing both hIL-12 (under control of the vaccinia virus P11 late promoter) and EGFP (under a synthetic early/late poxvirus promoter). Multiple rounds of single-focus purification were carried out, and PCR analysis was used to determine the purity of the recombinant virus. This procedure is similar to those used to create specific gene-deleted recombinant MV constructs (19).
FIG. 1.
Construction of pBS 135-136 hIL-12 EGFP. The cloning plasmid pBS 135-136 EGFP contains a unique PstI site between M135R and a poxviral synthetic early/late promoter into which a PacI linker was inserted. PacI was also added to the primers used to amplify hIL-12. The IL-12 cassette with both subunits was inserted into pCR2.1 TOPO. In addition, the minimal sequence for the poxviral P11 promoter was inserted before the p40 subunit of hIL-12. Both vectors were digested with PacI, gel purified, and ligated to form the pBS 135-136 hIL-12 EGFP plasmid that was used to create vMyxIL-12 in MV-infected BGMK cells by homologous recombination. CMV, cytomegalovirus; IRES, internal ribosomal entry site.
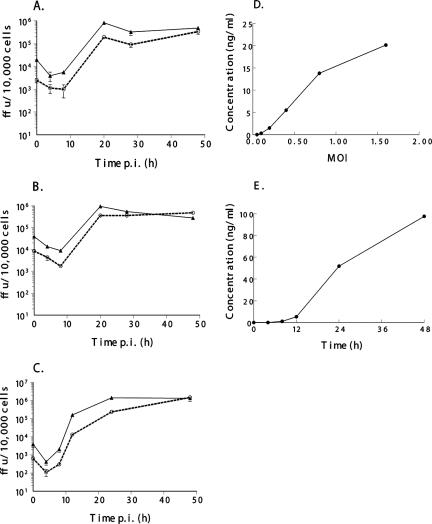
We examined the ability of vMyxIL-12 to infect cells and produce secreted hIL-12 in vitro (Fig. 2). The recombinant MV vMyxgfp, which expresses EGFP, was used as a control (17). This virus has similar growth properties in vitro to wild-type MV (7). To assess viral growth and spread, a single-step growth curve was performed as described previously (33). BGMK cells (control primate cell line), 8484 cells (human lung carcinoma cell line), and RK-13 cells (rabbit fibroblasts [ATCC CCL37]) were used in this study (Fig. 2A to C). In each of these cells, the growth kinetics of infectious vMyxIL-12 mirrored the amplification of vMyxgfp. By 48 hpi, vMyxIL-12 exhibited a slightly greater viral increase over 48 h, indicating that this virus is capable of robust replication in primate, lagomorph, and human cancer cells. In comparison, most recombinant virus gene knockout viruses, unless representing a host range function, are also fully able to infect cells in vitro.
FIG. 2.
Viral growth, replication, and secreted hIL-12 production. Single-step growth analysis of vMyxIL-12 (dashed line and circles) and vMyxgfp (solid line and triangles) was evaluated on BGMK (A), 8484 (B), and RK-13 (C) cells. Cells were infected with virus at an MOI of 3. Cells were harvested at the times indicated, and the titers of infectious virus present at each time point were determined by subsequent infection on BGMK cells. All growth analyses were performed in triplicate. The production of secreted hIL-12 from infected BGMK cells was measured by enzyme-linked immunosorbent assay (D). Cells were infected at the indicated MOI, and the cellular supernatant was collected 48 hpi. RK-13 cells (E) were infected at an MOI of 3, and cell supernatants were collected at the indicated time points.
The ability of vMyxIL-12 to secrete hIL-12 was also examined (Fig. 2D and E). Supernatants from infected cells were collected at the indicated time points, and hIL-12 was measured using a commercially available hIL-12 p70 enzyme-linked immunosorbent assay kit (Ready-SET-Go!; eBioscience). The amount of hIL-12 within the culture supernatant of BGMK cells infected with vMyxIL-12 increased significantly when infected with a larger amount of virus (Fig. 2D). Detectable amounts of hIL-12 (270 pg/ml) could be measured following infection with as little as a multiplicity of infection (MOI) of 0.1 and increased to over 20,000 pg/ml at the highest MOI tested (1.6). In addition, the time course of IL-12 production at a high MOI of infection (MOI of 3) (Fig. 2D) was examined in rabbit RK-13 cells (Fig. 2E). Detectable levels of hIL-12 could be measured in culture supernatant as early as 8 h postinfection (hpi) (1,100 pg/ml) and increased to almost 100,000 pg/ml by 48 hpi. Together, these data indicate that vMyxIL-12 can grow effectively in a variety of cell lines and produce significant quantities of bioactive hIL-12.
Expression of IL-12 counteracts MV virulence in rabbits.
To examine the effect of hIL-12 on the progression of myxomatosis, New Zealand White rabbits (O. cuniculus) were infected with either control viruses (vMyx or vMyxgfp) or vMyxIL-12. Rabbit IL-12 has not been sequenced or isolated. However, other human cytokines, such as granulocyte-macrophage colony-stimulating factor have been shown to be bioactive in rabbits (21). Rabbits were inoculated intradermally with 1,000 focus-forming units (FFU) of virus per site, and injections were performed in both hind flanks. The rabbits were monitored daily for symptoms of myxomatosis, including the size and appearance of the primary lesion and number of satellite lesions. As well, the number and quality of secondary lesions are also important. Other diagnostic features of myxomatosis include orthopnea, mouth breathing, cyanosis, decreased or absent food/water intake, fecal output, dehydration, decreased activity, and unnatural posture (11). Each diagnostic characteristic was evaluated numerically, and clinical scores were used to assist in monitoring the progression of myxomatosis (6). For the pathogenesis study, three rabbits were inoculated with vMyxIL-12, two with vMyx, and two with vMyxgfp. Disease progression was followed for 20 days or until euthanasia was required. Table 1 outlines the gross pathological observations. The vMyxIL-12-injected rabbits made a complete recovery following a delayed and significantly less-pathogenic course of disease, in which they suffered relatively mild clinical signs with reduced size and severity of the primary lesion and restricted spread and establishment of secondary lesions, which were confined to the ears, with little or no development of characteristic lesions on the eyelids and nose and generally healthier constitution. This is also demonstrated in the clinical scores (Fig. 3), determined by well-defined parameters and used to assess clinical progression of myxomatosis (6). Animals infected with vMyxIL-12 exhibited primary lesions which were smaller, did not become protuberant, and healed more quickly (Table 1). In contrast with comparably attenuated MV gene-knockout recombinant viruses, which exhibit a similar pathogenesis during the initial period of infection (10, 24) and then have a significant attenuation after day 7, vMyxIL-12 was attenuated from initial infection. The expression of lesion-localized IL-12 is effective in producing or enhancing a significant antiviral immunity.
TABLE 1.
Pathogenicity of vMyxIL-12 mutant MV in European rabbits
| Day(s) postinfection | Pathogenicity of mutant:
|
||
|---|---|---|---|
| vMyx (2 animals) | vMyxgfp (2 animals) | vMyxIL-12 (3 animals) | |
| 4 | Primary lesion red, raised, 0.5 cm | Primary lesion red, raised 0.5 cm | Primary lesion just visible, small, flat, pink |
| 7 | Large protuberant lesion, 2.5 cm; eyelids red and swelling, lesion beginning; satellites (2-4) around primary lesion; lesions beginning in ears (2-3 each ear) | Primary lesion, 1.5 cm, protuberant; ruffled fur, satellites visible; ear lesions (4-5), ears turning purple; lesions visible on eyelids | Primary lesion dark red, not protuberant, 1.0-2.0 cm; single, small lesions may be appearing in ears |
| 8 | Primary lesion larger, 2.5-3.0 cm, starting to cavitate; head swollen; lesions around nose and muzzle; ears purple, swollen, and heavy, secondary lesion visible | Large protuberant primary lesion; head swollen; multiple ear lesions, purple; 1-2 lesions on nose; multiple lesions on eyelids | Primary lesion flattened, 1.0-2.5 cm, lot of fur overgrowing lesion; 1-2 lesions starting on eyelids; 1 animal with 3-5 lesions in ears |
| 9-11 | Reduced food and water consumption; multiple lesions on nose and muzzle; difficulty breathing; ears purple, drooping, with multiple lesions (10-20 each ear); animals euthanized due to severity of infection (day 10) | Reduced food and water consumption; animals euthanized due to severity of infection (day 11) | Primary lesion large, black but starting to dry out and cavitate; eyes good, slight discharge from 1 eye of 1 animal; multiple (4-10) lesions in each ear |
| 12-19 | Animals healing, primary lesions dried out and scabby, small healed lesions in ears | ||
FIG. 3.
vMyxIL-12 pathogenesis in rabbits. Three rabbits were infected with vMyxIL-12, two rabbits with vMyx, and two rabbits with vMyxgfp. The rabbits were each infected with 2,000 FFU, with 1,000 FFU injected into each hind flank. Rabbits were monitored daily for the clinical symptoms associated with myxomatosis. Scores increase as the disease progresses. Animals are normally sacrificed within 24 to 48 h following a score of 15. Certain symptoms, such as severe orthopnea, mouth breathing, cyanosis, and lack of food and/or water intake for >24 h, required that the rabbits be euthanized for ethical reasons.
Primary and secondary lesions, spleens, and inguinal and popliteal lymph nodes were harvested at time of euthanasia. Tissue was homogenized, and the presence of virus within the lysate was determined by titration on BGMK cells. At the endpoint on day 20, there was no virus present in any of the lesions or organs of the vMyxIL-12-infected rabbits, indicating that by this point, all virus had been cleared from these animals (data not shown). In comparison, at the endpoint on day 11, there was evidence of infectious virus within the primary lesion (6 × 102 FFU/mg), secondary lesion (2.5 × 102 FFU/mg), and spleen (1 × 10−1 FFU/mg) of animals injected with vMyx.
Recombinant technology has been used to insert cytokines into poxviruses (31). When IL-4 has been inserted into MV, vaccinia virus, or ectromelia virus, it has resulted in significantly more severe immunopathology and disease. Ectromelia virus expressing IL-4 was able to break immunity induced by previous infection, a finding not shown in either the MV-IL-4 or vaccinia virus-IL-4 studies. This increased pathogenicity is due to the ability of IL-4 to dampen Th1-type immune processes, particularly IL-12, IL-2, and gamma interferon (1, 16, 27). In contrast, the expression of Th1 cytokines has been hypothesized to result in an attenuated infection, bolstered by a stronger type 1 immune response. However, this is the first report of a fully infectious MV expressing its complete viral genetic complement that is attenuated by the expression of an additional mammalian host immune protein.
Safety is always a concern when constructing recombinant poxviruses expressing a therapeutic cytokine. Therefore, we have tested the ability of infectious MV expressing hIL-12 (vMyxIL-12) to induce myxomatosis in the host to which MV is pathogenic. We have demonstrated that creation of a recombinant MV that could have increased antitumor activity within the tumor microenvironment also exhibited attenuated pathogenicity in its natural host. This would indicate that vMyxIL-12 virus is safer than its wild-type counterpart, and if anything, might be self limiting in vivo. This makes this virus an excellent candidate as a second generation oncolytic virus therapeutic. It is also possible that the IL-12 recombinant virus could produce a more potent and durable protective viral immunity in susceptible rabbit hosts. These issues remain to be determined.
Acknowledgments
This work was supported in part by the National Cancer Institute of Canada and by U.S. Public Health Service grants from the National Cancer Institute CA108970 and CA131407 and the John R. Oshie Foundation. G.M. holds an International Scholarship of the Howard Hughes Medical Institute. M.M.S. is supported by a Postdoctoral Fellowship provided by the Pamela Greenaway Kohlmeier Translational Breast Cancer Research Unit of the London Regional Cancer Program.
Footnotes
Published ahead of print on 29 August 2007.
REFERENCES
- 1.Aung, S., and B. S. Graham. 2000. IL-4 diminishes perforin-mediated and increases Fas ligand-mediated cytotoxicity in vivo. J. Immunol. 164:3487-3493. [DOI] [PubMed] [Google Scholar]
- 2.Barrett, J. W., J. X. Cao, S. Hota-Mitchell, and G. McFadden. 2001. Immunomodulatory proteins of myxoma virus. Semin. Immunol. 13:73-84. [DOI] [PubMed] [Google Scholar]
- 3.Bennett, J. J., S. Malhotra, R. J. Wong, K. Delman, J. Zager, M. St-Louis, P. Johnson, and Y. Fong. 2001. Interleukin 12 secretion enhances antitumor efficacy of oncolytic herpes simplex viral therapy for colorectal cancer. Ann. Surg. 233:819-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broderick, L., S. J. Yokota, J. Reineke, E. Mathiowitz, C. C. Stewart, M. Barcos, R. J. Kelleher, Jr., and R. B. Bankert. 2005. Human CD4+ effector memory T cells persisting in the microenvironment of lung cancer xenografts are activated by local delivery of IL-12 to proliferate, produce IFN-gamma, and eradicate tumor cells. J. Immunol. 174:898-906. [DOI] [PubMed] [Google Scholar]
- 5.Cameron, C., S. Hota-Mitchell, L. Chen, J. Barrett, J. X. Cao, C. Macaulay, D. Willer, D. Evans, and G. McFadden. 1999. The complete DNA sequence of myxoma virus. Virology 264:298-318. [DOI] [PubMed] [Google Scholar]
- 6.Cameron, C. M., J. W. Barrett, L. Liu, A. R. Lucas, and G. McFadden. 2005. Myxoma virus M141R expresses a viral CD200 (vOX-2) that is responsible for down-regulation of macrophage and T-cell activation in vivo. J. Virol. 79:6052-6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron, C. M., J. W. Barrett, M. Mann, A. Lucas, and G. McFadden. 2005. Myxoma virus M128L is expressed as a cell surface CD47-like virulence factor that contributes to the downregulation of macrophage activation in vivo. Virology 337:55-67. [DOI] [PubMed] [Google Scholar]
- 8.Chen, B., T. M. Timiryasova, D. S. Gridley, M. L. Andres, R. Dutta-Roy, and I. Fodor. 2001. Evaluation of cytokine toxicity induced by vaccinia virus-mediated IL-2 and IL-12 antitumour immunotherapy. Cytokine 15:305-314. [DOI] [PubMed] [Google Scholar]
- 9.Chen, B., T. M. Timiryasova, P. Haghighat, M. L. Andres, E. H. Kajioka, R. Dutta-Roy, D. S. Gridley, and I. Fodor. 2001. Low-dose vaccinia virus-mediated cytokine gene therapy of glioma. J. Immunother. 24(1997):46-57. [DOI] [PubMed] [Google Scholar]
- 10.Everett, H., M. Barry, S. F. Lee, X. Sun, K. Graham, J. Stone, R. C. Bleackley, and G. McFadden. 2000. M11L: a novel mitochondria-localized protein of myxoma virus that blocks apoptosis of infected leukocytes. J. Exp. Med. 191:1487-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenner, F., and F. Ratcliffe. 1965. Myxomatosis. University Press, Cambridge, United Kingdom.
- 12.Fenner, F., and G. M. Woodroofe. 1953. The pathogenesis of infectious myxomatosis; the mechanism of infection and the immunological response in the European rabbit (Oryctolagus cuniculus). Br. J. Exp. Pathol. 34:400-411. [PMC free article] [PubMed] [Google Scholar]
- 13.Hess, S. D., N. K. Egilmez, J. Shiroko, and R. B. Bankert. 2001. Antitumor efficacy of a human interleukin-12 expression plasmid demonstrated in a human peripheral blood leukocyte/human lung tumor xenograft SCID mouse model. Cancer Gene Ther. 8:371-377. [DOI] [PubMed] [Google Scholar]
- 14.Hill, H. C., T. F. Conway, Jr., M. S. Sabel, Y. S. Jong, E. Mathiowitz, R. B. Bankert, and N. K. Egilmez. 2002. Cancer immunotherapy with interleukin 12 and granulocyte-macrophage colony-stimulating factor-encapsulated microspheres: coinduction of innate and adaptive antitumor immunity and cure of disseminated disease. Cancer Res. 62:7254-7263. [PubMed] [Google Scholar]
- 15.Hiscox, S., and W. G. Jiang. 1997. Interleukin-12, an emerging anti-tumour cytokine. In Vivo 11:125-132. [PubMed] [Google Scholar]
- 16.Jackson, R. J., A. J. Ramsay, C. D. Christensen, S. Beaton, D. F. Hall, and I. A. Ramshaw. 2001. Expression of mouse interleukin-4 by a recombinant ectromelia virus suppresses cytolytic lymphocyte responses and overcomes genetic resistance to mousepox. J. Virol. 75:1205-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston, J. B., J. W. Barrett, W. Chang, C.-S. Chung, W. Zeng, J. Masters, M. Mann, F. Wang, J. Cao, and G. McFadden. 2003. Role of the serine-threonine kinase PAK-1 in myxoma virus replication. J. Virol. 77:5877-5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston, J. B., and G. McFadden. 2003. Poxvirus immunomodulatory strategies: current perspectives. J. Virol. 77:6093-6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston, J. B., and G. McFadden. 2004. Technical knockout: understanding poxvirus pathogenesis by selectively deleting viral immunomodulatory genes. Cell Microbiol. 6:695-705. [DOI] [PubMed] [Google Scholar]
- 20.Kerr, P., and G. McFadden. 2002. Immune responses to myxoma virus. Viral Immunol. 15:229-246. [DOI] [PubMed] [Google Scholar]
- 21.Kim, J. H., J. Y. Oh, B. H. Park, D. E. Lee, J. S. Kim, H. E. Park, M. S. Roh, J. E. Je, J. H. Yoon, S. H. Thorne, D. Kirn, and T. H. Hwang. 2006. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol. Ther. 14:361-370. [DOI] [PubMed] [Google Scholar]
- 22.Lissoni, P., F. Rovelli, M. R. Rivolta, C. Frigerio, M. Mandala, S. Barni, A. Ardizzoia, F. Malugani, and G. Tancini. 1997. Acute endocrine effects of interleukin-12 in cancer patients. J. Biol. Regul. Homeost. Agents 11:154-156. [PubMed] [Google Scholar]
- 23.Lun, X., W. Yang, T. Alain, Z. Q. Shi, H. Muzik, J. W. Barrett, G. McFadden, J. Bell, M. G. Hamilton, D. L. Senger, and P. A. Forsyth. 2005. Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res. 65:9982-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mossman, K., S. F. Lee, M. Barry, L. Boshkov, and G. McFadden. 1996. Disruption of M-T5, a novel myxoma virus gene member of the poxvirus host range superfamily, results in dramatic attenuation of myxomatosis in infected European rabbits. J. Virol. 70:4394-4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nemeckova, S., V. Sroller, P. Hainz, J. Krystofova, M. Smahel, and L. Kutinova. 2003. Experimental therapy of HPV16 induced tumors with IL12 expressed by recombinant vaccinia virus in mice. Int. J. Mol. Med. 12:789-796. [PubMed] [Google Scholar]
- 26.Robertson, M. J., C. Cameron, M. B. Atkins, M. S. Gordon, M. T. Lotze, M. L. Sherman, and J. Ritz. 1999. Immunological effects of interleukin 12 administered by bolus intravenous injection to patients with cancer. Clin. Cancer Res. 5:9-16. [PubMed] [Google Scholar]
- 27.Rolph, M. S., and I. A. Ramshaw. 2003. Interleukin-4-mediated downregulation of cytotoxic T lymphocyte activity is associated with reduced proliferation of antigen-specific CD8+ T cells. Microbes Infect. 5:923-932. [DOI] [PubMed] [Google Scholar]
- 28.Rook, A. H., G. S. Wood, E. K. Yoo, R. Elenitsas, D. M. Kao, M. L. Sherman, W. K. Witmer, K. A. Rockwell, R. B. Shane, S. R. Lessin, and E. C. Vonderheid. 1999. Interleukin-12 therapy of cutaneous T-cell lymphoma induces lesion regression and cytotoxic T-cell responses. Blood 94:902-908. [PubMed] [Google Scholar]
- 29.Sangro, B., I. Melero, C. Qian, and J. Prieto. 2005. Gene therapy of cancer based on interleukin 12. Curr. Gene Ther. 5:573-581. [DOI] [PubMed] [Google Scholar]
- 30.Stanford, M. M., J. W. Barrett, S. H. Nazarian, S. Werden, and G. McFadden. 2007. Oncolytic virotherapy synergism with signaling inhibitors: rapamycin increases myxoma virus tropism for human tumor cells. J. Virol. 81:1251-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanford, M. M., and G. McFadden. 2005. The ‘supervirus’? Lessons from IL-4-expressing poxviruses. Trends Immunol. 26:339-345. [DOI] [PubMed] [Google Scholar]
- 32.Stanford, M. M., S. J. Werden, and G. McFadden. 2007. Myxoma virus in the European rabbit: interactions between the virus and its susceptible host. Vet. Res. 38:299-318. [DOI] [PubMed] [Google Scholar]
- 33.Sypula, J., F. Wang, Y. Ma, J. Bell, and G. McFadden. 2004. Myxoma virus tropism in human tumour cells. Gene Ther. Mol. Biol. 8:103-114. [Google Scholar]
- 34.Ulchaker, J., J. Panuto, P. Rayman, A. Novick, P. Elson, R. Tubbs, J. H. Finke, and R. M. Bukowski. 1999. Interferon-gamma production by T lymphocytes from renal cell carcinoma patients: evidence of impaired secretion in response to interleukin-12. J. Immunother. 22(1997):71-79. [DOI] [PubMed] [Google Scholar]
- 35.van Herpen, C. M., J. Bussink, A. J. van der Kogel, W. J. Peeters, R. van der Voort, A. van Schijndel, P. C. de Wilde, G. J. Adema, and P. H. de Mulder. 2005. Interleukin-12 has no effect on vascular density, perfusion, hypoxia and proliferation of an implanted human squamous cell carcinoma xenograft tumour despite up-regulation of ICAM-1. Anticancer Res. 25:1015-1021. [PubMed] [Google Scholar]
- 36.Wang, G., J. W. Barrett, M. Stanford, S. J. Werden, J. B. Johnston, X. Gao, M. Sun, J. Q. Cheng, and G. McFadden. 2006. Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc. Natl. Acad. Sci. USA 103:4640-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss, G. R., M. A. O'Donnell, K. Loughlin, K. Zonno, R. J. Laliberte, and M. L. Sherman. 2003. Phase 1 study of the intravesical administration of recombinant human interleukin-12 in patients with recurrent superficial transitional cell carcinoma of the bladder. J. Immunother. 26(1997):343-348. [DOI] [PubMed] [Google Scholar]
- 38.Wong, R. J., M. K. Chan, Z. Yu, R. A. Ghossein, I. Ngai, P. S. Adusumilli, B. M. Stiles, J. P. Shah, B. Singh, and Y. Fong. 2004. Angiogenesis inhibition by an oncolytic herpes virus expressing interleukin 12. Clin. Cancer Res. 10:4509-4516. [DOI] [PubMed] [Google Scholar]
- 39.Wong, R. J., M. K. Chan, Z. Yu, T. H. Kim, A. Bhargava, B. M. Stiles, B. C. Horsburgh, J. P. Shah, R. A. Ghossein, B. Singh, and Y. Fong. 2004. Effective intravenous therapy of murine pulmonary metastases with an oncolytic herpes virus expressing interleukin 12. Clin. Cancer Res. 10:251-259. [DOI] [PubMed] [Google Scholar]
- 40.Wong, R. J., S. G. Patel, S. Kim, R. P. DeMatteo, S. Malhotra, J. J. Bennett, M. St-Louis, J. P. Shah, P. A. Johnson, and Y. Fong. 2001. Cytokine gene transfer enhances herpes oncolytic therapy in murine squamous cell carcinoma. Hum. Gene Ther. 12:253-265. [DOI] [PubMed] [Google Scholar]
- 41.Zuniga, M. C. 2003. Lessons in detente or know thy host: the immunomodulatory gene products of myxoma virus. J. Biosci. 28:273-285. [DOI] [PubMed] [Google Scholar]