Abstract
Modified vaccinia virus Ankara (MVA) is a highly attenuated vaccinia virus strain undergoing clinical evaluation as a replication-deficient vaccine vector against various infections and tumor diseases. To analyze the basis of its high immunogenicity, we investigated the mechanism of how MVA induces type I interferon (IFN) responses. MVA stimulation of bone marrow-derived dendritic cells (DC) showed that plasmacytoid DC were main alpha IFN (IFN-α) producers that were triggered independently of productive infection, viral replication, or intermediate and late viral gene expression. Increased IFN-α levels were induced upon treatment with mildly UV-irradiated MVA, suggesting that a virus-encoded immune modulator(s) interfered with the host cytokine response. Mice devoid of Toll-like receptor 9 (TLR9), the receptor for double-stranded DNA, mounted normal IFN-α responses upon MVA treatment. Furthermore, mice devoid of the adaptors of TLR signaling MyD88 and TRIF and mice deficient in protein kinase R (PKR) showed IFN-α responses that were only slightly reduced compared to those of wild-type mice. MVA-induced IFN-α responses were critically dependent on autocrine/paracrine triggering of the IFN-α/β receptor and were independent of IFN-β, thus involving “one-half” of a positive-feedback loop. In conclusion, MVA-mediated type I IFN secretion was primarily triggered by non-TLR molecules, was independent of virus propagation, and critically involved IFN feedback stimulation. These data provide the basis to further improve MVA as a vaccine vector.
Modified vaccinia virus Ankara (MVA) is a highly attenuated virus strain generated by more than 500 passages in chicken embryo fibroblasts (CEF) of a conventional vaccinia virus (VACV) formerly used as a vaccine against human smallpox in Turkey and Germany. During propagation in tissue culture, about 15% of the parental viral genome, corresponding to approximately 30 kb DNA, got lost (38). Among the genetic information lost or inactivated in the MVA genome, there are host range genes, genes involved in virus-host interaction, and viral immunomodulatory genes (5, 9). Probably as a consequence of genomic deletions, MVA is replication deficient in most cells of mammalian origin (9, 12, 18, 48). The cascade-like pattern of viral early, intermediate, and late gene expression, including DNA replication, is activated in most mammalian cells, whereas viral multiplication is arrested at a late stage after accumulation of immature viral particles (48).
Like many other viruses, the prototype orthopoxvirus VACV evolved strategies to prevent activation of the host immune system or to evade host immune responses. These strategies include expression of viral receptors for certain cytokines, such as soluble type I and type II interferons (IFNs), receptors for tumor necrosis factor, and interleukin-1β (IL-1β), and receptors for CC chemokines (3, 4, 13, 23, 30, 45). In fact, mice deficient for a functional type I IFN system and/or type II IFN system showed an increased sensitivity to lethal VACV infection (50). Moreover, VACV proteins that specifically target Toll-like receptor (TLR) signaling by associating with the Toll/IL-1 receptor domain-containing adaptor molecules MyD88 and TRIF have been identified (46). It has been shown that viral immunomodulatory proteins encoded by VACV contribute to virulence (46), and it has been proposed that the avirulence of MVA, at least in part, is associated with the loss of many of the above-summarized viral escape mechanisms. Although MVA is highly attenuated and replication deficient, it is surprisingly immunogenic compared to conventional VACV (26, 49). Thus, MVA vaccination can induce protective immunity against veterinary orthopoxvirus infections (41) and treatment with MVA enhanced resistance against herpes simplex virus type 1 (HSV-1) in mice 100-fold (21). Considering these properties, recombinant MVA is a promising animal and human vaccine candidate. Since MVA is also regarded as a potential vector for tumor vaccination or vaccination of immunocompromised patients, it is of major interest to gain more insight into the basis of MVA-related immunogenicity. In particular type I IFNs and their main producers, dendritic cells (DC), play a key role in many vaccination strategies. It has been revealed that MVA drives the immune system towards increased production of proinflammatory cytokines such as type I IFNs (9, 11), and human DC were identified as being moderately activated by infection with MVA (19).
Upon many viral infections, plasmacytoid DC (pDC) are important type I IFN producers and are responsible for systemic type I IFN responses in vivo (7; see reference 14 for a review). pDC constitute a subset of DC that is found in humans and mice. Compared to murine myeloid DC (mDC), mouse pDC express CD11c, B220, and CD45RB and show reduced CD11b levels (for a review see reference 14). pDC can be found in many tissues, including secondary lymphoid tissues, liver, and lung at low percentages. By examining different viral model systems it became evident that pDC may be activated directly by both RNA and DNA viruses. In doing so, TLRs turned out to be crucial sensors of virus-associated molecular patterns such as double-stranded DNA (dsDNA), dsRNA, or single-stranded RNA. Among 12 mammalian TLRs discovered so far, several of them are involved in the recognition of viral components. By triggering TLR9, the DNA genomes of HSV-1 (35) and HSV-2 (36) were identified as the pathogen-associated molecular patterns primarily mediating activation of, and cytokine release by, DC in vivo and in vitro. Additionally, in the case of another herpesvirus, DNA-encoded murine cytomegalovirus, TLR9-dependent recognition by pDC has been reported to be the main trigger of type I IFN (35). Which of these mechanisms apply for DNA-encoded MVA has not been investigated yet. In addition, cytosolic non-TLR sensors for dsRNA, such as protein kinase R (PKR), melanoma differentiation-associated gene 5 protein (mda-5), and retinoic acid-inducible gene I protein (RIG-I), that play a role in activation of DC have been identified. PKR is a cytosolic serine/threonine kinase activated by autophosphorylation on binding to dsRNA (52) that has been implicated in the induction of IFN responses to some viruses (6, 17, 45). RIG-I seems to be an essential sensor for infection by RNA viruses of fibroblasts and conventional DC (32), and mda-5 is involved in the induction of antiviral cytokines upon infection with influenza A virus, measles virus, and picornavirus (8, 22, 44). Moreover, novel cytosolic sensors for dsDNA have recently been reported to activate type I IFN responses in a TLR9-independent and IFN regulatory factor 3 (IRF3)-dependent manner (47) or in a TLR- and RIG-I-independent manner (31).
As, so far, little is known of how MVA induces strong immune responses, in this study we addressed MVA-triggered type I IFN responses by DC subsets. We found that pDC were strong producers of type I IFN that were induced primarily independently of TLRs. Moreover, we gained insight into the viral and cellular mechanisms that are critically involved in MVA-mediated activation of DC.
MATERIALS AND METHODS
Mice and viruses.
TLR9-deficient mice (TLR9−/−) and MyD88-deficient mice (MyD88−/−) were provided by Shizuo Akira (1, 25), TRIF-deficient mice (TRIF−/−) were provided by Bruce Beutler (28), PKR-deficient mice (PKR−/−) were provided by Charles Weissmann (53), and IFN-β-deficient mice (IFN-β−/−) were provided by Siegfried Weiß (20). For breeding of MyD88/TRIF double-knockout mice, animals obtained drinking water supplemented with the antibiotics sulfamethoxazole (400 μg/ml) and trimethoprim (80 μg/ml) for 2 weeks of every 4. All mice have been backcrossed at least 10 times on the C57BL/6 background except PKR−/− mice, which were bred on the SV129 background. Type I IFN receptor-deficient mice (IFNAR−/−) (40) have been backcrossed 20 times on the C57BL/6 background. All mice were bred under specific-pathogen-free (SPF) conditions at the Zentrale Tierhaltung of the Paul-Ehrlich-Institut. Unmutated C57BL/6 and SV129 mice were purchased from Charles River. Mouse experimental work was carried out using 8- to 12-week-old mice in compliance with regulations of German animal welfare.
MVA (cloned isolate F6 at the 584th CEF passage) was routinely propagated and titrated on CEF. For analysis of productive infection, bone marrow (BM)-derived Flt3-L or granulocyte-macrophage colony-stimulating factor (GM-CSF) cultures were infected with MVA at a multiplicity of infection (MOI) of 0.1 and cells were harvested after 1 h of viral adhesion (0 h). Other aliquots were washed after 1 h of viral adhesion and incubated with fresh medium. Forty-eight hours later these supernatants plus BM-derived DC (BM-DC) were harvested (48 h). Supernatants plus cells at each time point were freezed-thawed three times, sonicated, and back-titrated in serial dilutions on CEF. MVA-specific plaques were stained using a purified polyclonal anti-VACV immunoglobulin (VIG) prepared from serum of vaccinated humans. As a control, CEF were infected with the original MVA inoculum at an MOI of 0.05 and 0 h and 48 h later virus was determined as described above.
For UV irradiation of virus a UV irradiation chamber (Herolab) was used. In such a device, UV irradiation is adjusted by energy per area (in mJ/cm2). A typical 300-mJ/cm2 irradiation usually took approximately 45 seconds.
Cell isolation and culture.
BM cells were isolated by flushing femur and tibia with RPMI medium supplemented with 10% fetal calf serum. Upon red blood cell lysis, cells were washed and seeded at a density of 1 × 106 cells/ml or 2 × 106 cells/ml in medium supplemented with GM-CSF (100 ng/ml; R&D Systems) or Flt3-L (100 ng/ml; R&D systems), respectively. Flt3-L-supplemented cultures (BM-derived pDC [BM-pDC]) were cultivated for 8 days with one medium change at day 4, whereas medium of GM-CSF-supplemented cultures (BM-derived mDC [BM-mDC]) was changed every 1 to 2 days, depending on the status of cultures, by replacing one-half of the medium with fresh cytokine-supplemented medium.
Flow cytometric analysis and cell enrichment.
Cells were stained with anti-B220-phycoerythrin (PE) or -PECy5.5 monoclonal antibody (MAb), anti-CD11c-allophycocyanin MAb, anti-CD69-PE MAb, or anti-CD86-fluorescein isothiocyanate MAb (all from BD PharMingen). For detection of VACV-specific surface proteins, VIG was used in combination with F(ab)2-PE. For enrichment of CD11c+ B220+ cells from Flt3-L BM cultures, MS or LD columns (Miltenyi Biotech) were used according to the manufacturer's instructions. The purity of pDC (CD11c+ B220+) usually exceeded 80%.
In vitro stimulations and quantification of cytokine production.
For stimulation experiments, ex vivo-isolated BM cells or in vitro-differentiated bulk cultures of BM-mDC and bulk cultures of BM-pDC were seeded at 1 × 106 cells/well in 24-well culture plates in 1 ml medium. Magnetically activated cell sorting (MACS)-sorted cells were seeded at a density of 2 × 105 cells/well in 96-well culture plates in 200 μl medium. CpG 2216 (ggGGGACGATCGTCgggggG [lowercase indicates phosphorothioate-modified nucleotides]; Sigma-ARK) was used at a final concentration of 10 μg/ml. For transfection of 2 μg poly(I:C) (Sigma-Aldrich), the reagent Fugene (Roche) was used according to the manufacturer's instructions. After stimulation, cell-free supernatant was collected and analyzed with a mouse IFN-α or mouse IFN-β enzyme-linked immunosorbent assay (ELISA) kit (PBL Biomedical Laboratories).
RESULTS
MVA but not VACV induces type I IFN responses upon immunization of mice and infection of DC.
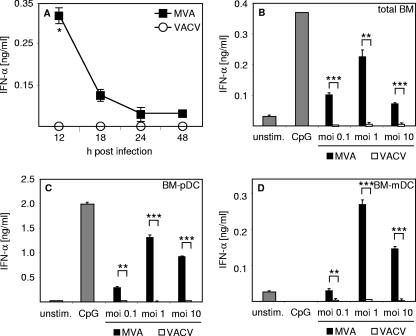
Previously it has been reported that MVA induces Flt3-L-mediated expansion of DC in newborn mice, a process in which IFNs were postulated to play a crucial role (21). However, it is still unclear whether MVA is able to induce systemic type I IFN responses in vivo. Here we show that, 12 h after intravenous (i.v.) inoculation of wild-type (WT) mice with 1 × 107 PFU MVA, serum IFN-α level peaked at about 350 pg/ml, declined within the next 6 h, and reached background levels 24 h after infection (Fig. 1A). In stark contrast, upon inoculation of mice with 1 × 105 PFU VACV, no IFN-α was detected in the serum of mice (Fig. 1A). When 1 × 107 PFU VACV was used for immunization, no IFN-α was measurable either (data not shown). Thus, unlike VACV, MVA is able to induce systemic type I IFN responses in mice.
FIG. 1.
In vivo and in vitro infection with MVA but not VACV induces IFN-α responses. (A) Mice were i.v. inoculated with 1 × 107 PFU MVA or 1 × 105 PFU VACV. Serum was collected at the indicated time points after injection and analyzed for IFN-α by an ELISA method. (B to D) Total BM cells (B), BM-pDC cultures (C), and BM-mDC cultures (D) were infected with MVA or VACV at the indicated MOIs. Control cells were treated with CpG 2216 (10 μg/ml) or were left untreated (unstim.). Twenty-four hours after stimulation or infection, cell-free supernatant was collected and analyzed for IFN-α by an ELISA method. Data shown are representative of two or three independent experiments. *, P < 0.05 ≥ 0.01; **, P < 0.01 ≥ 0.001; ***, P < 0.001 (unpaired two-tailed t test). Error bars indicate standard deviations from triplicate ELISA measurements.
When total BM cells were infected with MVA at different MOIs, IFN-α secretion was elicited, with the highest levels reached at an MOI of 1 (Fig. 1B). To gain more insight into which cell type primarily produced IFN-α, BM-pDC and BM-mDC cultures were generated. Upon MVA infection, BM-pDC secreted high IFN-α levels, whereas BM-mDC produced lower IFN-α quantities (Fig. 1C and D). Reminiscent of the in vivo observations, VACV was not able to induce IFN-α responses in vitro (Fig. 1B, C, and D). As a positive control, the TLR9 ligand double-stranded CpG-containing oligonucleotide 2216 was used to elicit IFN responses by pDC but not by mDC (Fig. 1C and D).
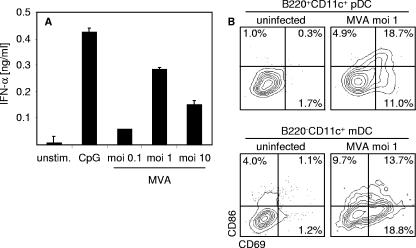
We also analyzed pDC sorted by MACS technique (27, 37) from BM-pDC cultures for type I IFN expression upon MVA infection. These experiments confirmed that pDC were important producers of IFN-α (Fig. 2A). Furthermore, within BM-DC cultures B220+ CD11c+ pDC and B220− CD11+ mDC upregulated activation markers CD69 and CD86 upon MVA infection (Fig. 2B). Thus, we showed that, in contrast to VACV, MVA was able to elicit IFN responses in vivo, and in vitro infection experiments revealed that MVA induced pDC to produce large amounts of IFN-α.
FIG. 2.
DC get activated moderately upon MVA infection. (A) B220+ CD11c+ pDC were MACS sorted from Flt3-L BM-pDC cultures and infected with MVA at the indicated MOIs. Control cells were treated with CpG 2216 (10 μg/ml) or were left untreated (unstim.). Twenty-four hours after stimulation or infection, cell-free supernatant was collected and analyzed for IFN-α by an ELISA method. (B) Flt3-L-derived BM-pDC or GM-CSF-derived BM-mDC cultures were infected with MVA at the MOI of 1. Sixteen hours after infection, upregulation of CD86 and CD69 on B220+ CD11c+ BM-pDC or on B220− CD11c+ mDC documented activation of both DC subsets. Data shown are representative of two independent experiments.
MVA-mediated IFN-α induction in DC is independent of productive infection, viral replication, or intermediate and late viral gene expression.
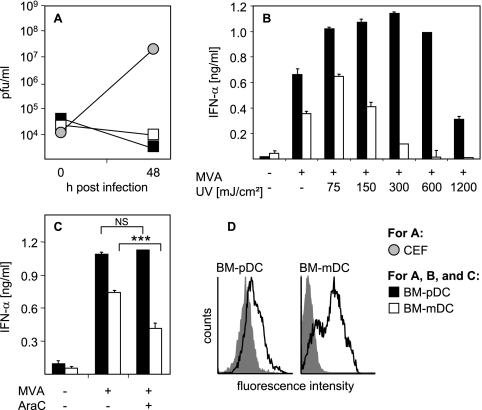
To further explore conditions required for the induction of type I IFN, we investigated whether productive infection took place upon MVA stimulation of BM-DC. To this end, supernatants of MVA-infected BM-DC cultures were back-titrated on CEF. These experiments revealed no evidence for MVA propagation in BM-DC within 48 h after infection (Fig. 3A). Thus, a productive viral infection was not required for the induction of IFN-α in BM-DC. As a positive control CEF were infected with the original MVA inoculum, which indeed showed productive infection of the cells (Fig. 3A). Interestingly, MVA irradiated with low dosages of UV light induced enhanced IFN-α responses by BM-pDC and BM-mDC (Fig. 3B). This further indicated that induction of IFN-α was independent of newly synthesized viral gene products and that MVA might still encode viral inhibitors interfering with the host immune response. To further determine the relevance of the viral life cycle for the induction of IFN-α, DC were infected with MVA in the presence of cytosine β-d-arabinofuranoside (AraC), which inhibits DNA replication and suppresses expression of intermediate and late viral genes. These data showed that AraC had no influence on the secretion of IFN-α by MVA-infected BM-pDC (Fig. 3C). Interestingly, when BM-mDC were MVA infected in the presence of AraC, a slight reduction of IFN-α secretion was observed (Fig. 3C). To test whether viral proteins were synthesized by MVA-infected BM-DC, untreated and MVA-infected cells were stained with a purified polyclonal VIG 18 h after infection (Fig. 3D; for details refer to Materials and Methods). MVA infection led to expression of viral proteins on the surfaces of both BM-DC subsets. Thus, induction of type I IFN upon MVA infection of BM-DC is independent of productive infection and does not involve viral DNA replication and expression of intermediate or late viral gene products.
FIG. 3.
IFN-α induction in BM-DC by MVA does not require productive infection or viral replication. (A) Flt3-L-derived BM-pDC or GM-CSF-derived BM-mDC cultures were infected with MVA at the MOI of 0.1. Zero hours and 48 h after BM-DC infection, supernatants plus cells were harvested and back-titrated on CEF, revealing no evidence for MVA propagation in both BM-DC subsets. As a control CEF were infected with the original MVA inoculum and 0-h and 48-h values were determined as for BM-DC. (B) Flt3-L-derived BM-pDC or GM-CSF-derived BM-mDC cultures were infected at an MOI of 1 with untreated MVA or with MVA that was irradiated with UV light at the indicated doses prior to infection. Control cells were left untreated. Twenty-four hours after infection, cell-free supernatant was collected and analyzed for IFN-α by an ELISA method. Error bars indicate standard deviations from triplicate ELISA measurements. (C) Flt3-L-derived BM-pDC or GM-CSF-derived BM-mDC cultures were infected with MVA at an MOI of 1 in the presence or absence of AraC (40 μg/ml). Control cells were left untreated. Twenty-four hours after stimulation or infection, cell-free supernatant was collected and analyzed for IFN-α by an ELISA method. ***, P < 0.001 by unpaired two-tailed t test; NS, not significant. (D) BM-DC were infected with MVA at an MOI of 1 (black curves) or were left untreated (gray shaded curves). Eighteen hours after infection, cells were stained for virus-specific proteins with VIG in combination with F(ab)2-PE, indicating that viral proteins are expressed on the surfaces of both DC subsets. Data shown are representative of two to four independent experiments.
Sensing MVA is largely independent of TLRs and PKR.
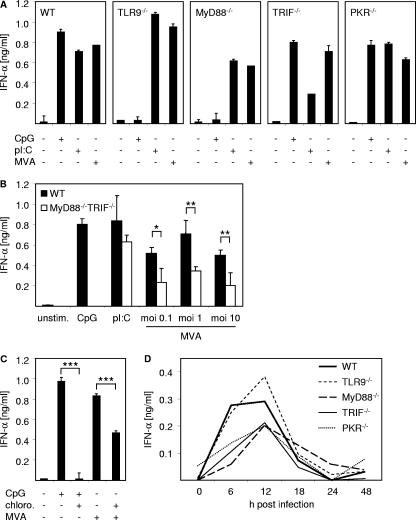
We next wanted to gain insight into cellular mechanisms of sensing MVA infection. Therefore, we generated BM-pDC from WT mice; mice deficient for components of TLR signaling, including TLR9, MyD88, and TRIF; and mice that were deficient for PKR. The total number and the phenotype of BM-pDC derived from the different knockout mice were comparable with those of WT mice (data not shown). BM-pDC were infected with MVA at the MOI of 1, treated with 10 μg/ml CpG or 2 μg transfected poly(I:C), or left untreated as controls. As shown in Fig. 4A, MVA induced IFN-α responses in TLR9-deficient BM-pDC that were as good as those in WT cells, indicating that triggering TLR9 by the DNA genome of MVA was not the relevant mechanism responsible for the induction of IFN-α in BM-pDC. Interestingly, upon MVA stimulation of BM-pDC deficient for MyD88, which is one main adaptor molecule mediating signaling by TLR1, TLR2, and TLR4 to -11, a reduced IFN-α secretion was observed compared to that by WT BM-pDC (Fig. 4A).
FIG. 4.
Sensing of MVA is largely independent of TLRs or PKR. (A) Flt3-L-derived BM-pDC cultures were generated from WT mice and mice deficient for TLR9, MyD88, TRIF, or PKR. Cells were infected with MVA at an MOI of 1, stimulated with CpG 2216 (10 μg/ml) or with poly(I:C) (pI:C) (for transfection of 2 μg poly(I:C), Fugene reagent was used), or left untreated. Twenty-four hours after stimulation or infection, cell-free supernatant was harvested and analyzed for IFN-α by an ELISA method. (B) Flt3-L-derived BM-pDC cultures were generated from WT mice and mice double-deficient for both MyD88 and TRIF. Cells were infected with MVA at the indicated MOI or were stimulated with CpG 2216 (10 μg/ml) or poly(I:C) (for transfection of 2 μg poly(I:C). Fugene reagent was used). Controls were left untreated (unstim.). Twenty-four hours after stimulation, cell-free supernatant was harvested and analyzed for IFN-α by an ELISA method. *, 0.05 > P ≥ 0.01; **, 0.01 > P ≥ 0.001 (unpaired two-tailed t test). (C) WT Flt3-L-derived BM-pDC cultures were infected with MVA at an MOI of 1. Controls were stimulated with CpG 2216 (10 μg/ml) or were left untreated. Where indicated, cells were preincubated with chloroquine (chloro.; 10 μM) for 2 h. Twenty-four hours after stimulation, cell-free supernatant was harvested and analyzed for IFN-α by an ELISA method. ***, P < 0.001 (unpaired two-tailed t test). (D) Indicated mice were i.v. inoculated with 1 × 107 PFU MVA. Serum was collected at the indicated time points after injection and analyzed for IFN-α by an ELISA method. Data shown are representative of two to four independent experiments. Error bars in panels A and C indicate standard deviations from triplicate ELISA measurements. Error bars in panel B indicate standard deviations from three independent experiments. Standard deviation maxima in panel D were ±22.7 pg/ml.
dsRNA is an intermediate of viral replication that has been implicated in type I IFN induction upon viral infections (39, 42). Among TLRs identified so far, TLR3 is involved in sensing dsRNA and in using TRIF as an adaptor for signal transduction (reviewed in reference 2). In line with this, TRIF−/− BM-pDC transfected with poly(I:C) showed reduced IFN-α responses compared to WT controls (Fig. 4A). Nevertheless, MVA infection of TRIF-deficient BM-pDC resulted in IFN-α production similar to that observed upon infection of WT BM-pDC. PKR-deficient BM-pDC infected with MVA showed IFN-α levels comparable to those observed in WT control cells (Fig. 4A), indicating that this pathway did not significantly participate in MVA-mediated IFN-α induction. Since PKR-deficient mice were on the SV129 background, SV129 WT controls were used and showed results comparable to those of C57BL/6 WT controls (data not shown). To gain detailed insight into the role of TLR signaling in the induction of IFN-α responses upon MVA infection, we generated BM-pDC from mice which were deficient for both TLR adaptor molecules MyD88 and TRIF. Infection of MyD88/TRIF double-knockout BM-pDC with different MOIs of MVA resulted in IFN responses that were reduced compared to WT controls (Fig. 4B). Thus, complete loss of TLR signaling in BM-pDC, diminished but did not abrogate IFN-α production upon MVA infection.
It is well accepted that sensing of viruses or artificial stimuli that trigger TLRs located within the endosomes critically involves endosomal acidification (16, 36). Since we observed a reduction of IFN-α secretion when MyD88-deficient or MyD88/TRIF double-knockout BM-pDC were MVA infected (Fig. 4A and B), we aimed to determine whether the sensing of MVA and subsequent IFN-α secretion required endosomal acidification. Therefore, BM-pDC were treated with the virus in the presence or absence of chloroquine, a lysomotropic agent preventing endosomal acidification. Chloroquine completely abrogated CpG oligodeoxynucleotide-induced IFN-α production, which is triggered by TLR9 in the endosomes and critically requires endosomal acidification (Fig. 4A, B, and C) (16, 36). The inhibition was not related to drug toxicity, because chloroquine had no measurable effect on BM-DC viability (data not shown) (16). However, upon MVA infection in the presence of chloroquine, IFN-α responses by BM-pDC were reduced by approximately 40% (Fig. 4C). Taken together these data suggested that MVA-induced IFN-α production by BM-pDC did not involve the sensing of viral replication via the dsRNA binding protein PKR. Furthermore, absence of the TLR adaptor molecule TRIF did not influence IFN-α levels secreted by BM-pDC in vitro. Although DNA sensor TLR9 was not crucially involved in the recognition of MVA resulting in IFN-α secretion by BM-pDC, the TLR adaptor MyD88 seemed to participate in MVA sensing to some extent. Furthermore, MVA recognition by BM-pDC partially involved endosomal acidification.
To investigate the in vivo impact of the data obtained in vitro with BM-pDC, mice deficient for the receptors or signaling components tested above were used. Upon MVA infection of WT control mice, serum IFN-α levels peaked 12 h after challenge (approximately 300 pg/ml), declined within the next 6 h, and reached background levels 24 h after infection (Fig. 4D). In line with the results obtained by infection of BM-pDC in vitro, deficiency of TLR9 had no effect on the serum IFN-α levels observed upon MVA challenge of mice. Furthermore, TRIF-, MyD88-, and PKR-deficient animals mounted IFN-α responses that were only slightly reduced compared to those of WT controls (Fig. 4D). Thus, results obtained by MVA infection of TLR9-, MyD88-, TRIF-, and PKR-deficient mice confirmed in vitro studies performed with BM-pDC.
IFNAR but not IFN-β is critically involved in IFN-α secretion upon MVA infection.
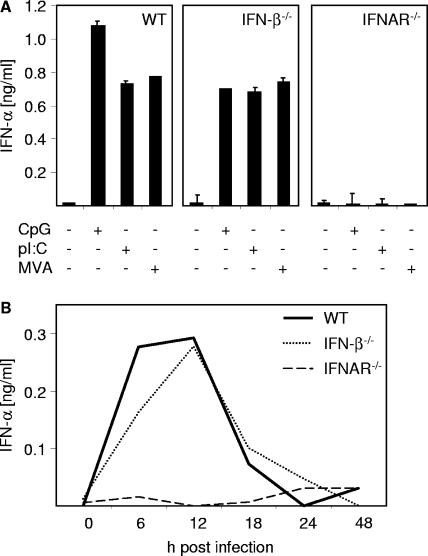
The role of positive feedback in the induction of IFN-α has been discussed controversially. Particularly for pDC a feedback-independent pathway involving TLRs, IRAK4, TRAF6, and IRF7 has been suggested (29, 33). To determine the role of positive feedback, WT, IFN-β-deficient, and type I IFN receptor (IFNAR)-deficient BM-pDC were MVA infected. The number and phenotype of BM-pDC generated from knockout mice were comparable to those of BM-pDC from WT mice (data not shown). As a control, BM-pDC were stimulated with CpG or poly(I:C) or they were left untreated. As shown in Fig. 5A, BM-pDC from IFN-β−/− mice mounted IFN-α levels comparable to that from WT animals. In contrast, IFNAR-deficient cells showed complete abrogation of IFN-α responses upon MVA infection or treatment with CpG or poly(I:C). The inability to produce IFN-α was not associated with an overall impairment of cells, since production of other cytokines such as IL-12p40 was not altered upon CpG stimulation (data not shown). In line with that, IFN-β-deficient mice but not IFNAR-deficient mice challenged with MVA mounted IFN-α responses comparable to those observed in WT animals. A peak of IFN-α production 12 h after infection was detected in WT and IFN-β-deficient mice (Fig. 5B). Thus, secretion of IFN-α upon in vitro MVA infection of BM-pDC and in vivo challenge was independent of IFN-β but critically involved IFNAR triggering.
FIG. 5.
Positive-feedback loop via the IFNAR is critical for MVA-induced IFN-α production. (A) Flt3-L-derived BM-pDC cultures were generated from WT mice and mice deficient for IFN-β or IFNAR. Cells were infected with MVA at an MOI of 1, stimulated with CpG (40 nM) or pI:C (for transfection of 2 μg poly(I:C) (pI:C). Fugene reagent was used), or left untreated. Twenty-four hours after stimulation, cell-free supernatant was harvested and analyzed for IFN-α by an ELISA method. (B) Indicated mice were i.v. inoculated with 1 × 107 PFU MVA. Serum was collected at the indicated time points after injection and analyzed for IFN-α by an ELISA method. Data shown are representative of two to four independent experiments. Error bars in panel A indicate standard deviations from triplicate ELISA measurements. Standard deviation maxima in panel B were ±16.2 pg/ml.
DISCUSSION
MVA was used in large field trials in the 1970s for vaccination against smallpox, with no significant side effects recorded. Considering safety, immunogenicity, and the inability to replicate in most mammalian cells, MVA is an ideal vaccine vector candidate. However, despite the usage of MVA in many vaccination studies, little is known about cellular and viral mechanisms contributing to its high immunogenicity. In this study we provide the first evidence that MVA induces systemic type I IFN responses in vivo that are contributed by pDC (Fig. 1 and 2) largely independently of TLR and PKR signaling (Fig. 4). We showed that MVA-mediated IFN-α induction in DC was independent of productive infection, viral replication, or intermediate and late viral gene expression (Fig. 3). Furthermore, the data presented here indicate that IFNAR, but not IFN-β, is critically required for MVA-mediated IFN-α induction (Fig. 5).
MVA lacks several immunomodulatory proteins encoded by many other orthopoxviruses as well as host range genes and other genes involved in virus-host interaction (5, 9). MVA was found to induce NF-κB activation in human embryonic kidney cells (43) and to activate human DC via an NF-κB-dependent mechanism (19). VACV A52R protein, whose open reading frame was deleted during MVA attenuation, can block activation of NF-κB by multiple TLRs by association with IRAK2 and TRAF6 (10, 24). Furthermore, VACV protein A46R targets TLR adaptors (including MyD88 and TRIF), a process contributing to virulence (46). These findings indicated that targeting and antagonizing TLR pathway components might be important mechanisms to escape from host antiviral defense upon VACV infection. Thus, it was likely that sensing MVA involved components of the TLR pathway. We showed here that, in contrast to other DNA viruses like HSV (35, 36) and murine cytomegalovirus (34), TLR9 was not involved in the induction of type I IFN responses upon MVA infection (Fig. 4A and D). However, upon infection of MyD88-deficient BM-pDC or mice, observed IFN-α levels were slightly reduced compared to WT controls (Fig. 4A and D). Reduced IFN levels induced upon MVA infection of BM-pDC were also detected when MyD88/TRIF double-knockout cells were tested (Fig. 4B). Pretreatment of cells with chloroquine, a lysomotropic agent preventing endosomal acidification and thus proper TLR3, TLR7/8, and TLR9 function, reduced IFN-α production about 40% compared to levels reached in the absence of chloroquine (Fig. 4C). These data suggest that, even though MVA-induced IFN-α secretion was independent of TLR9 triggering, MyD88 was involved in this process at least to some extent. Interestingly, very recent findings indicated that TLR2/MyD88 was required for VACV-induced secretion of proinflammatory cytokines (54). Our results suggest multiple mechanisms involved in sensing MVA infection by TLR-dependent and -independent mechanisms. These mechanisms, most probably, engaged the DNA genome of MVA, early viral RNA, and/or some viral protein(s). Since pDC are the major source of IFN-α upon infection with MVA (Fig. 1 and 2), RIG-I might be of minor importance in detection of MVA infection since this molecule is merely involved in the detection of viral infections by fibroblasts and conventional DC, but not pDC (32). The role of mda-5 and/or the recently discovered yet unidentified cytoplasmic DNA sensor(s) (31, 47) in the recognition of MVA still needs to be elucidated.
As observed by Drillien et al., activation of human DC (upregulation of activation marker CD86) was not impaired when UV-irradiated MVA was used for infection (19). We showed that MVA-induced IFN-α production by (MDC) or pDC cultures was enhanced when mildly UV irradiated virus was used (Fig. 3B). These data suggested that, although MVA is highly attenuated, it probably still has one or more genes interfering with the host's IFN-α responses. Interestingly, when graded MOIs of MVA were used for infection of BM cells or DC subsets, the highest IFN levels were elicited at an MOI of 1 whereas at an MOI of 10 less type I IFN was induced (Fig. 1 and 4). Most probably this was due to cytopathic effects observed in infections with high-dose (MOI, 10) MVA that were not experienced when a 10-fold-lower infection dose was used (data not shown). It will be a matter of future investigations whether further deletions in the MVA genome are able to improve or to decrease MVA-related immunogenicity. The data presented in this study indicate that infection of DC with MVA did not interfere with their capacity to mount IFN responses (Fig. 1). In contrast to other viruses primarily activating pDC (17), MVA activated both pDC and mDC as indicated by upregulation of the activation markers CD69 and CD86 (Fig. 2) and production of IFN-α (Fig. 1). This is in line with the TLR9-independent MVA-induced IFN-α responses (Fig. 4), because only pDC but not mDC can be triggered via TLR9 to produce IFN-α (Fig. 1C and D).
As mentioned above, there is an ongoing debate about the role of the positive feedback via IFNAR in production of IFN-α. Particularly for pDC a feedback-independent pathway involving TLRs, IRAK4, TRAF6, and IRF7 was suggested (29, 33). Our data clearly demonstrate that IFNAR expression is absolutely crucial for IFN-α production in vivo and in vitro. In the absence of this receptor no IFN-α production was observed upon MVA challenge (Fig. 5A and B). In contrast, although MVA induced large amounts of IFN-β upon infection of DC and significant systemic serum levels were found upon in vivo challenge (data not shown), IFN-β expression was no prerequisite for IFN-α secretion, as indicated by MVA-induced IFN-α responses by IFN-β deficient cells and mice. Thus, “one-half” a feedback loop involving IFNAR triggering but not IFN-β was required for production of IFN-α upon MVA infection. Interestingly, the type I IFN system played an essential role in the control of a VACV infection (15, 50, 51). In our experiments both IFN-β- and IFNAR-deficient mice survived MVA infections without developing any signs of disease (Fig. 5 and data not shown), further supporting the safety of MVA compared to the parental VACV.
The study presented here gives important and detailed insight into mechanisms involved in systemic type I IFN production upon in vivo challenge with MVA. We showed that DC of the plasmacytoid lineage played a key role in the secretion of IFN-α. We found that the sensing of MVA infection and subsequent type I IFN secretion constitute a multiple-step process involving TLRs to a minor degree and primarily non-TLR molecules. Furthermore, our data suggest that, although highly attenuated, MVA still encodes one or several immune modulators interfering with the host antiviral defense. Thus, the presented data shed light on mechanisms conferring immunogenicity of MVA and provide the basis for developing new strategies for further improving MVA-related immunogenicity and its capacity as a vaccine vector.
Acknowledgments
We thank Dorothea Kreuz for expert technical assistance, David Nemazee for advice on how to breed MyD88/TRIF double-deficient mice, Bruce Beutler and The Scripps Research Institute for providing TRIF-deficient mice, and Kay-Martin Hanschmann for statistical analyses.
This work was supported in part by the Deutsche Forschungsgemeinschaft (SFB432, B15) and the EU (INVADERS, contract number QLK2-CT-2001-02103; MVACTOR, contract number LSHP-CT-2006-037536).
Footnotes
Published ahead of print on 12 September 2007.
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 3.Alcami, A., and G. L. Smith. 1995. Vaccinia, cowpox, and camelpox viruses encode soluble gamma interferon receptors with novel broad species specificity. J. Virol. 69:4633-4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alcami, A., J. A. Symons, P. D. Collins, T. J. Williams, and G. L. Smith. 1998. Blockade of chemokine activity by a soluble chemokine binding protein from vaccinia virus. J. Immunol. 160:624-633. [PubMed] [Google Scholar]
- 5.Antoine, G., F. Scheiflinger, F. Dorner, and F. G. Falkner. 1998. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology 244:365-396. [DOI] [PubMed] [Google Scholar]
- 6.Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13:129-141. [DOI] [PubMed] [Google Scholar]
- 7.Barchet, W., M. Cella, B. Odermatt, C. Asselin-Paturel, M. Colonna, and U. Kalinke. 2002. Virus-induced interferon alpha production by a dendritic cell subset in the absence of feedback signaling in vivo. J. Exp. Med. 195:507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berghall, H., J. Siren, D. Sarkar, I. Julkunen, P. B. Fisher, R. Vainionpaa, and S. Matikainen. 2006. The interferon-inducible RNA helicase, mda-5, is involved in measles virus-induced expression of antiviral cytokines. Microbes Infect. 8:2138-2144. [DOI] [PubMed] [Google Scholar]
- 9.Blanchard, T. J., A. Alcami, P. Andrea, and G. L. Smith. 1998. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J. Gen. Virol. 79:1159-1167. [DOI] [PubMed] [Google Scholar]
- 10.Bowie, A., E. Kiss-Toth, J. A. Symons, G. L. Smith, S. K. Dower, and L. A. O'Neill. 2000. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 97:10162-10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buttner, M., C. P. Czerny, K. H. Lehner, and K. Wertz. 1995. Interferon induction in peripheral blood mononuclear leukocytes of man and farm animals by poxvirus vector candidates and some poxvirus constructs. Vet. Immunol. Immunopathol. 46:237-250. [DOI] [PubMed] [Google Scholar]
- 12.Carroll, M. W., and B. Moss. 1997. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propagation and generation of recombinant viruses in a nonhuman mammalian cell line. Virology 238:198-211. [DOI] [PubMed] [Google Scholar]
- 13.Colamonici, O. R., P. Domanski, S. M. Sweitzer, A. Larner, and R. M. Buller. 1995. Vaccinia virus B18R gene encodes a type I interferon-binding protein that blocks interferon alpha transmembrane signaling. J. Biol. Chem. 270:15974-15978. [DOI] [PubMed] [Google Scholar]
- 14.Colonna, M., G. Trinchieri, and Y. J. Liu. 2004. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5:1219-1226. [DOI] [PubMed] [Google Scholar]
- 15.Deonarain, R., A. Alcami, M. Alexiou, M. J. Dallman, D. R. Gewert, and A. C. Porter. 2000. Impaired antiviral response and alpha/beta interferon induction in mice lacking beta interferon. J. Virol. 74:3404-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 17.Diebold, S. S., M. Montoya, H. Unger, L. Alexopoulou, P. Roy, L. E. Haswell, A. Al Shamkhani, R. Flavell, P. Borrow, and C. Reis e Sousa. 2003. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 424:324-328. [DOI] [PubMed] [Google Scholar]
- 18.Drexler, I., K. Heller, B. Wahren, V. Erfle, and G. Sutter. 1998. Highly attenuated modified vaccinia virus Ankara replicates in baby hamster kidney cells, a potential host for virus propagation, but not in various human transformed and primary cells. J. Gen. Virol. 79:347-352. [DOI] [PubMed] [Google Scholar]
- 19.Drillien, R., D. Spehner, and D. Hanau. 2004. Modified vaccinia virus Ankara induces moderate activation of human dendritic cells. J. Gen. Virol. 85:2167-2175. [DOI] [PubMed] [Google Scholar]
- 20.Erlandsson, L., R. Blumenthal, M. L. Eloranta, H. Engel, G. Alm, S. Weiss, and T. Leanderson. 1998. Interferon-beta is required for interferon-alpha production in mouse fibroblasts. Curr. Biol. 8:223-226. [DOI] [PubMed] [Google Scholar]
- 21.Franchini, M., H. Hefti, S. Vollstedt, B. Glanzmann, M. Riesen, M. Ackermann, P. Chaplin, K. Shortman, and M. Suter. 2004. Dendritic cells from mice neonatally vaccinated with modified vaccinia virus Ankara transfer resistance against herpes simplex virus type I to naive one-week-old mice. J. Immunol. 172:6304-6312. [DOI] [PubMed] [Google Scholar]
- 22.Gitlin, L., W. Barchet, S. Gilfillan, M. Cella, B. Beutler, R. A. Flavell, M. S. Diamond, and M. Colonna. 2006. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. USA 103:8459-8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham, K. A., A. S. Lalani, J. L. Macen, T. L. Ness, M. Barry, L. Y. Liu, A. Lucas, I. Clark-Lewis, R. W. Moyer, and G. McFadden. 1997. The T1/35kDa family of poxvirus-secreted proteins bind chemokines and modulate leukocyte influx into virus-infected tissues. Virology 229:12-24. [DOI] [PubMed] [Google Scholar]
- 24.Harte, M. T., I. R. Haga, G. Maloney, P. Gray, P. C. Reading, N. W. Bartlett, G. L. Smith, A. Bowie, and L. A. O'Neill. 2003. The poxvirus protein A52R targets Toll-like receptor signaling complexes to suppress host defense. J. Exp. Med. 197:343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 26.Hirsch, V. M., T. R. Fuerst, G. Sutter, M. W. Carroll, L. C. Yang, S. Goldstein, M. Piatak, Jr., W. R. Elkins, W. G. Alvord, D. C. Montefiori, B. Moss, and J. D. Lifson. 1996. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J. Virol. 70:3741-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hochrein, H., B. Schlatter, M. O'Keeffe, C. Wagner, F. Schmitz, M. Schiemann, S. Bauer, M. Suter, and H. Wagner. 2004. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA 101:11416-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoebe, K., X. Du, P. Georgel, E. Janssen, K. Tabeta, S. O. Kim, J. Goode, P. Lin, N. Mann, S. Mudd, K. Crozat, S. Sovath, J. Han, and B. Beutler. 2003. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424:743-748. [DOI] [PubMed] [Google Scholar]
- 29.Honda, K., H. Yanai, T. Mizutani, H. Negishi, N. Shimada, N. Suzuki, Y. Ohba, A. Takaoka, W. C. Yeh, and T. Taniguchi. 2004. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 101:15416-15421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu, F. Q., C. A. Smith, and D. J. Pickup. 1994. Cowpox virus contains two copies of an early gene encoding a soluble secreted form of the type II TNF receptor. Virology 204:343-356. [DOI] [PubMed] [Google Scholar]
- 31.Ishii, K. J., C. Coban, H. Kato, K. Takahashi, Y. Torii, F. Takeshita, H. Ludwig, G. Sutter, K. Suzuki, H. Hemmi, S. Sato, M. Yamamoto, S. Uematsu, T. Kawai, O. Takeuchi, and S. Akira. 2006. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat. Immunol. 7:40-48. [DOI] [PubMed] [Google Scholar]
- 32.Kato, H., S. Sato, M. Yoneyama, M. Yamamoto, S. Uematsu, K. Matsui, T. Tsujimura, K. Takeda, T. Fujita, O. Takeuchi, and S. Akira. 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity 23:19-28. [DOI] [PubMed] [Google Scholar]
- 33.Kawai, T., S. Sato, K. J. Ishii, C. Coban, H. Hemmi, M. Yamamoto, K. Terai, M. Matsuda, J. Inoue, S. Uematsu, O. Takeuchi, and S. Akira. 2004. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 5:1061-1068. [DOI] [PubMed] [Google Scholar]
- 34.Krug, A., A. R. French, W. Barchet, J. A. Fischer, A. Dzionek, J. T. Pingel, M. M. Orihuela, S. Akira, W. M. Yokoyama, and M. Colonna. 2004. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity 21:107-119. [DOI] [PubMed] [Google Scholar]
- 35.Krug, A., G. D. Luker, W. Barchet, D. A. Leib, S. Akira, and M. Colonna. 2004. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood 103:1433-1437. [DOI] [PubMed] [Google Scholar]
- 36.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin, P., G. M. Del Hoyo, F. Anjuere, C. F. Arias, H. H. Vargas, L. Fernandez, V. Parrillas, and C. Ardavin. 2002. Characterization of a new subpopulation of mouse CD8α+ B220+ dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential. Blood 100:383-390. [DOI] [PubMed] [Google Scholar]
- 38.Meyer, H., G. Sutter, and A. Mayr. 1991. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J. Gen. Virol. 72:1031-1038. [DOI] [PubMed] [Google Scholar]
- 39.Mibayashi, M., L. Martinez-Sobrido, Y. M. Loo, W. B. Cárdenas, M. Gale, Jr., and A. García-Sastre. 2007. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J. Virol. 81:514-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 41.Munz, E., S. Linckh, I. C. Renner-Muller, and M. Reimann. 1993. The effectiveness of immunization with vaccinia virus type “MVA” against an infection with cowpox virus type “OPV 85” in rabbits. Zentbl. Veterinarmed. B 40:131-140. [PubMed] [Google Scholar]
- 42.Nakatsu, Y., M. Takeda, S. Ohno, R. Koga, and Y. Yanagi. 2006. Translational inhibition and increased interferon induction in cells infected with a C protein-deficient measles virus. J. Virol. 80:11861-11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oie, K. L., and D. J. Pickup. 2001. Cowpox virus and other members of the orthopoxvirus genus interfere with the regulation of NF-κB activation. Virology 288:175-187. [DOI] [PubMed] [Google Scholar]
- 44.Siren, J., T. Imaizumi, D. Sarkar, T. Pietila, D. L. Noah, R. Lin, J. Hiscott, R. M. Krug, P. B. Fisher, I. Julkunen, and S. Matikainen. 2006. Retinoic acid inducible gene-I and mda-5 are involved in influenza A virus-induced expression of antiviral cytokines. Microbes Infect. 8:2013-2020. [DOI] [PubMed] [Google Scholar]
- 45.Smith, C. A., F. Q. Hu, T. D. Smith, C. L. Richards, P. Smolak, R. G. Goodwin, and D. J. Pickup. 1996. Cowpox virus genome encodes a second soluble homologue of cellular TNF receptors, distinct from CrmB, that binds TNF but not LT alpha. Virology 223:132-147. [DOI] [PubMed] [Google Scholar]
- 46.Stack, J., I. R. Haga, M. Schroder, N. W. Bartlett, G. Maloney, P. C. Reading, K. A. Fitzgerald, G. L. Smith, and A. G. Bowie. 2005. Vaccinia virus protein A46R targets multiple Toll-like-interleukin-1 receptor adaptors and contributes to virulence. J. Exp. Med. 201:1007-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stetson, D. B., and R. Medzhitov. 2006. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity 24:93-103. [DOI] [PubMed] [Google Scholar]
- 48.Sutter, G., and B. Moss. 1992. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc. Natl. Acad. Sci. USA 89:10847-10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sutter, G., L. S. Wyatt, P. L. Foley, J. R. Bennink, and B. Moss. 1994. A recombinant vector derived from the host range-restricted and highly attenuated MVA strain of vaccinia virus stimulates protective immunity in mice to influenza virus. Vaccine 12:1032-1040. [DOI] [PubMed] [Google Scholar]
- 50.van den Broek, M. F., U. Muller, S. Huang, M. Aguet, and R. M. Zinkernagel. 1995. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 69:4792-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van den Broek, M. F., U. Muller, S. Huang, R. M. Zinkernagel, and M. Aguet. 1995. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol. Rev. 148:5-18. [DOI] [PubMed] [Google Scholar]
- 52.Williams, B. R. 2001. Signal integration via PKR. Sci. STKE 2001:RE2. [DOI] [PubMed] [Google Scholar]
- 53.Yang, Y. L., L. F. Reis, J. Pavlovic, A. Aguzzi, R. Schafer, A. Kumar, B. R. Williams, M. Aguet, and C. Weissmann. 1995. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 14:6095-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu, J., J. Martinez, X. Huang, and Y. Yang. 2007. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-β. Blood 109:619-625. [DOI] [PMC free article] [PubMed] [Google Scholar]