Abstract
The interaction between B7 costimulation molecules on antigen-presenting cells and CD28 on antigen-responsive T cells is essential for T-cell activation and maturation of immune responses to herpes simplex virus (HSV) infection. Vaccine-induced immune responses also depend upon adequate upregulation of B7 costimulation molecules, but this signal may be limiting for replication-defective virus vaccines. We investigated whether expression of B7 costimulation molecules by a prototypical replication-defective antiviral vaccine could enhance immune responses to the vaccine and whether B7-1 and B7-2 would be similarly effective. We altered an ICP8− replication-defective strain of HSV type 2 (HSV-2), 5BlacZ, to encode either murine B7-1 or B7-2. B7 molecule expression was detected on the surface of cells infected in vitro and at the RNA level in tissue of immunized mice. Immunization of B7-1/B7-2 knockout mice with B7-encoding virus modestly expanded the number of gamma interferon-producing T cells and significantly augmented class-switched HSV-specific antibody responses compared with the parental virus. Mice immunized with either B7-expressing virus showed less replication of challenge virus in the genital mucosa than mice immunized with 5BlacZ, markedly fewer signs of genital and neurological disease, and little weight loss. Virtually all mice immunized with B7-encoding virus survived challenge with a large dose of HSV-2, whereas most 5BlacZ-immunized mice succumbed to infection. These results indicate that protective immune responses can be enhanced by the inclusion of host B7 costimulation molecules in a prototypical replication-defective HSV vaccine against HSV-2 genital infection and that B7-1 and B7-2 induce immune responses with similar capacities to fight HSV-2 infection.
T-cell activation is the central event in the development of all cell-mediated and most humoral antigen-specific immune responses. Activation of T cells depends on T-cell receptor engagement of an appropriate antigen-major histocompatibility complex (MHC) complex and a second signal mediated by engagement of costimulation molecules (14-16, 20, 34). B7-1 (CD80) and B7-2 (CD86) are two well-characterized costimulation molecules that are up-regulated upon host exposure to a pathogen (2, 23) and are down-regulated in the immunosuppressive environment of tumors (17), suggesting a role for B7-1 and B7-2 in initial T-cell priming. B7-1 and B7-2 engage the positive costimulation regulator on T cells, CD28 and, later, the negative regulator CTLA-4 (CD152) (53). B7-1 and B7-2 expression is limited to professional antigen-presenting cells (APCs). Dendritic cells and macrophages in mice constitutively express B7-2, and all types of professional APCs upregulate B7-2 expression upon activation (12, 32). B7-1, on the other hand, is expressed initially at low levels on dendritic cells, with activation-induced expression occurring on B cells and macrophages (10, 15, 21, 32).
The signal transmitted by interaction of B7-1 or B7-2 with CD28 is central to induction of primary immune responses, promoting proliferation, cytokine production, cytotoxic T-lymphocyte (CTL) activity, and antibody production (19, 29, 50, 52, 54), but the extent to which signals provided by B7-1 and B7-2 are redundant is still controversial. APCs expressing either B7-1 or B7-2 show equivalent capacities to costimulate T-cell proliferation and cytokine production in vitro (29, 33, 44, 50, 50, 51) and to promote CTL activity (29). In mice susceptible to certain allergic or infectious diseases, treatment with anti-B7-2 antibody suppresses production of the Th2 cytokines that accelerate disease progression, but anti-B7-1 antibody has little or no effect (6, 26, 43), suggesting a more central role for B7-2 in induction of immune responses in general and Th2 responses in particular. However, anti-B7-2 antibody blockade may have a more pronounced effect simply because B7-2 is constitutively expressed and more rapidly up-regulated during an immune response than B7-1 (3, 12). Furthermore, B7-1 expressed in the absence of B7-2 can induce the same cytokine patterns as does B7-2, suggesting that neither costimulation molecule inherently biases the Th lineage commitment. In the context of virus infections, the extent of redundancy between B7-1 and B7-2 activities is also in question. Blockade of B7-1 reduces the number of influenza virus-specific CTLs and gamma interferon (IFN-γ) production, whereas CTLA-4Ig administration also reduces antibody titers (35), suggesting incompletely redundant roles for B7-1 and B7-2. Yet, in vesicular stomatitis virus infection, mice deficient in either B7-1 or B7-2 show no differences in antiviral CTL generation or antibody production (38). Thus, although B7 costimulation is central to induction of primary immune responses, there is uncertainty about whether the roles of B7-1 and B7-2 overlap.
Herpes simplex virus (HSV) is a pathogen for which B7 costimulation strongly enhances induction of primary immune responses (11, 45, 58). Mice lacking B7-1 and B7-2 (B7KO) have impaired T-cell responses after primary HSV infection and during memory recall responses in comparison with wild-type mice (59). Fewer IFN-γ-producing T cells are present in the genital lymph nodes of B7KO mice after HSV infection, and T cells isolated from B7KO mice produce less IFN-γ and have attenuated CTL activity. In addition, B7KO mice have a significant deficit in HSV-specific serum immunoglobulin G (IgG) response, and this deficit is likely a consequence of depressed CD40L expression by CD4+ T cells (59). These immune response deficiencies ultimately compromise the capacity of B7KO mice to control HSV-mediated disease and clear the infection (58). Thus, B7 costimulation is a limiting factor in the activation of HSV-specific T cells for cytokine production, CTL activity, and provision of help for class-switched antibody responses.
Given the central role of B7 costimulation in the induction of most immune responses, B7 costimulation molecules have been incorporated into a variety of vaccine formulations. Coinjection of plasmids encoding pathogen-specific epitopes and B7-1 or B7-2 augments immune responses to several pathogens (37, 49, 61, 65), including HSV (13). Oncolytic adenovirus and replication-impaired HSV and vaccinia virus vectors expressing B7 molecules have also been tested as treatments for cancers. The B7-modified viruses enhance antitumor immunity (36, 63) and promote regression following direct intratumoral injection (25, 30, 46, 60). These studies suggest expression of host B7 costimulation molecules by a live attenuated vaccine virus could also potentially be used to induce more vigorous immunity to the homologous viral pathogen. However, the capacity of many viruses, including HSV, to down-regulate B7 molecule expression in infected professional APCs (7, 7, 22, 39, 47, 48) leaves the outcome uncertain.
Although replication-compromised forms of virus are a relatively safe means of inducing antiviral immune responses, the small amount of antigen available to professional APCs for presentation may limit the induction of effective antiviral immunity (66). Vaccination with a replication-defective virus expressing T-cell costimulatory molecules theoretically could provide every virally infected cell with the capacity to activate naïve T cells and thus augment induction of antiviral immunity. We undertook the current study to answer two questions central to this theory: whether virus-encoded B7 molecules would be effective in inducing antiviral immunity in the context of the infected cell and whether B7-1 and B7-2 would have differential effects on the developing antiviral immune responses and the protection they afford. We report here the in vivo immunogenicity and antiviral activity of replication-defective HSV strains genetically altered to encode murine B7-1 or B7-2 costimulation molecules. The B7-expressing vaccine strains were tested in mice lacking B7-1 and B7-2 molecules (4) so that any capacity to augment antiviral immunity could be unambiguously attributed to the virus-encoded B7 molecule expressed in infected cells. Because differences in the roles for B7-1 and B7-2 have been described (26, 28, 31, 43), we developed both B7-1- and B7-2-expressing, replication-defective HSV strains to ensure a more complete understanding of B7-1 versus B7-2 efficacy in induction of the antiviral immune response.
MATERIALS AND METHODS
Cells and viruses.
The replication-defective HSV-2 strain 5BlacZ does not produce the essential viral gene product ICP8 due to a lacZ insertion in the UL29 open reading frame (9). 5BlacZ was propagated in S2 cells, a Vero cell line stably expressing ICP8 (18, 18). HSV-2 strain G-6 (42) was isolated by plaque purification of strain G. Thymidine kinase (TK)-deficient HSV-2, strain 186ΔKpn (24), contains a KpnI-KpnI deletion in the thymidine kinase gene, rendering it replication-competent but nonlethal in mice. HSV-2 strain 333d41 contains a 939-bp excision in UL41 and completely lacks virion host shutoff activity (55). These replication-competent viruses were propagated in Vero cells essentially as previously described (41). Virus titers were determined on S2 or Vero cells by standard plaque assay (27). A20-1.11 murine B lymphoma cells (H-2d) were maintained in Dulbecco's modified Eagle's medium (30% low glucose), supplemented with 10% fetal calf serum, 1% glutamine, 1% nonessential amino acids, and 50 μM 2-mercaptoethanol.
Construction and isolation of recombinant viruses.
The open reading frames of murine B7-1 (CD80) in pCDNAI and B7-2 (CD86) in pCDM8 expression vectors (kindly provided by G. Freeman, Harvard Medical School) were cloned 3′ of the human cytomegalovirus (HCMV) immediate-early (IE) enhancer/promoter in pYBCMV-MP1-lacZ (gift of P. Olivo, Washington University School of Medicine). The HCMVp-B7 expression cassettes were then subcloned into a KpnI-KpnI deletion in the open reading frame of the HSV-2 thymidine kinase gene contained in the plasmid pEH48 (56) (gift of D. Knipe, Harvard Medical School). These plasmids [pEH48(HCMV/B7-1) and pEH48(HCMV/B7-2)] were linearized and cotransfected by calcium phosphate coprecipitation into S2 cells along with full-length 5BlacZ DNA. Potentially recombinant virus plaques (TK−) were selected by growth in the presence of acyclovir (100 μM). Plaque isolates were inoculated onto S2 cell monolayers, and their genotypes were confirmed by Southern blot analysis. Recombinant viruses were plaque purified three times before use in experiments. The B7-1-expressing 5BlacZ virus was named 5B80, and the B7-2-expressing 5BlacZ virus was named 5B86.
Southern blot analysis.
DNAs prepared from 5BlacZ, 5B80, and 5B86 were digested with EcoRI and HindIII, and fragments were separated by agarose gel electrophoresis. Fragments were transferred to a nitrocellulose membrane and hybridized to a random hexamer 32P-labeled probe consisting of an EcoRI-to-SpeI fragment that spanned the 5′ end of the TK gene and the HCMV immediate-early promoter in plasmid pEH48(HCMV/B7-1). Following overnight hybridization, the blot was washed and exposed to film.
Immunofluorescence detection of B7 molecule expression in vitro.
S2 cell monolayers were infected at a low multiplicity (approximately five plaques per well) with 5BlacZ, 5B80, or 5B86 for 48 h. Cells were fixed in 2% paraformaldehyde for 10 min at 4°C and rinsed with phosphate-buffered saline (PBS). Cells were then incubated in the presence of <1 μg biotinylated anti-B7-1 (PharMingen, San Diego, CA) or biotinylated anti-B7-2 (PharMingen) for 30 min at room temperature and washed and stained with streptavidin-fluorescein isothiocyanate (FITC; Immunotech) for 20 min at room temperature protected from light. Washed cells were visualized using a fluorescence microscope, and photographs were captured on a Nikon Diaphot 200 inverted microscope equipped with a Nikon TE-FM epifluorescence attachment and a DVC 1310C charge-coupled-device camera. Images were captured using C-View software (DVC Co., Austin, TX).
Flow cytofluorometric analysis of B7 molecule expression in vitro.
Vero and S2 cell monolayers were infected at a multiplicity of infection (MOI) of 5 with 5BlacZ, 5B80, or 5B86 and incubated at 37°C. Cells were collected by brief incubation in EDTA followed by scraping 15 h after infection. Abdominal paraaortic lymph nodes, hereafter referred to as genital lymph nodes, were harvested from uninfected B7KO mice, and single-cell suspensions were infected at an MOI of 3 for 15 h in DME supplemented to contain 10% fetal calf serum, 2 mM glutamine, 50 U/ml penicillin-streptomycin, and 50 μM 2-mercaptoethanol. Vero, S2, and lymph node cells were incubated in 5% goat serum for 20 min, washed in PBS containing 0.1% bovine serum albumin, and incubated for 30 min on ice with rabbit-anti-HSV polyclonal antibody (Dako) at 1:100 and anti-B7-1-biotin or anti-B7-2-biotin antibodies (PharMingen) at a 1:150 dilution. Washed cells were incubated for 20 min on ice in buffer containing streptavidin-FITC (Immunotech) and goat-anti-rabbit-phycoerythrin (PE; Vector Laboratories), each at a 1:150 dilution. Cells were washed, and staining was visualized by flow cytofluorometric analysis on a FACSCalibur cytometer (Becton Dickinson). Seven thousand to 8,000 gated events for Vero and S2 samples and 50,000 gated events for lymph node samples were collected, and analysis was conducted with CellQuest software (Becton Dickinson). For further analysis of B7-expressing cells, 5B80-infected lymph node cells were stained with anti-B7-1-biotin, anti-CD45-allophycocyanin, anti-CD19-PerCP (peridinin-chlorophyll-protein), anti-CD11b-PE-Cy7, and anti-CD11c-PE (all from PharMingen), followed by streptavidin-FITC.
Immunization of mice.
Female BALB/c mice were purchased from the National Cancer Institute. Female mice genetically deficient in both B7-1 and B7-2 (B7KO) (4), backcrossed to a BALB/c background, were bred at Saint Louis University and housed under specific-pathogen-free conditions in sterile microisolator cages in accordance with institutional and federal guidelines. All mice were used at 6 weeks of age. For immunization, hind flanks of the mice were shaved and injected subcutaneously (s.c.) with 2.5 × 106 PFU of virus suspended in 20 μl of normal saline.
In vivo characterization of B7 expression by RT-PCR.
Mice were immunized with 186ΔKpn or 5B86. Eighteen, 44, or 66 h after immunization, mice were sacrificed and draining genital lymph nodes were removed. Total RNA was prepared from the lymph node cells using TRI reagent (Molecular Research Center, Inc., Cincinnati, OH). Reverse transcription (RT) was performed on 1 μg total RNA using the reverse transcription system (Promega, Madison, WI). Following RT, cDNA preparations were subjected to PCR amplification using primers to B7-2 or glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The sequences of the B7-2 primers were 5′-ATGGTGTGTGGCATATGACC-3′ and 5′-CTTAGAGGCTGTGTTGCTGG-3′, which amplify a 220-bp product absent in the B7KO mouse. The sequences of the B7-1 primers were 5′-TGCAGGATACACCACTCCTCAAGT-3′ and 5′-AAAGGACCAGGCCCAGGATGATAA-3′, which amplify a 311-bp product absent in the B7KO mouse. The sequences of the GAPDH primers were 5′-TCCACCACCCTGTTGCTGTA-3′ and 5′-ACCACAGTCCATGCCATCAC-3′, which amplify a 500-bp product. One-tenth of the reverse transcription reaction was added to PCR mixtures containing high-fidelity Taq buffer with MgCl2 (Roche), 0.2 mM deoxynucleoside triphosphates, 50 pmol each primer, and 1 U Taq polymerase. Each reaction mixture was subjected to a hot start at 94°C for 4 min, followed by denaturation at 94°C for 50 s, annealing at 55°C for 50 s, and extension at 72°C for 60 s. Thirty cycles of amplification were followed by a final extension at 72°C for 5 min. PCR products were visualized on 2% agarose gels stained with ethidium bromide.
Quantitation of serum antibodies.
To determine the titer of HSV-specific serum antibodies induced by vaccination, B7KO mice were unimmunized or immunized with 5BlacZ, 5B80, or 5B86. BALB/c mice immunized with 5BlacZ were used as a positive control. Blood was collected from the tail vein of mice 24 days postimmunization and 14 days after challenge. Serum was prepared by clot retraction and analyzed by enzyme-linked immunosorbent assay (ELISA) as previously described (40). Anti-mouse IgG-biotin (R&D Systems) was used as secondary antibody, and streptavidin-horseradish peroxidase (Pierce) was used as a detection reagent. Plates were developed using OPD reagent (Sigma) according to the manufacturer's recommendations. Plates were read at 490 nm on an EL340 plate reader (Biotek). Antibody titers were determined by comparison to standard curves generated with serum containing known concentrations of IgG captured on plates coated with goat-anti-kappa light chain antibody (Caltag).
Intracellular cytokine staining.
Genital lymph nodes were harvested from control mice or from mice 5 days after immunization with 5BlacZ, 5B80, or 5B86. Single-cell suspensions were prepared and nonspecifically stimulated for 4 h in the presence of phorbol myristate acetate (PMA; 50 ng/ml), calcium ionophore A23187 (1 μg/ml), and GolgiStop (0.67 μl/ml; PharMingen). Cell surface staining was performed using anti-CD3-PerCP-Cy5.5 (1:160), anti-CD4-Pacific Blue (1:300), and anti-CD8-Alexa Fluor 700 (1:80) (all from PharMingen). Intracellular staining was performed using a cytostain kit (PharMingen) according to the manufacturer's instructions with the addition of anti-IFN-γ-PE (PharMingen) at a 1:100 dilution. Staining was visualized by flow cytofluorometric analysis on a FACSCalibur cytometer. At least 100,000 cells in the lymphocyte gate were collected for each sample, and analysis was conducted with CellQuest software (Becton Dickinson). The percentage of CD4+ IFN-γ+ or CD8+ IFN-γ+ cells from unstimulated samples was subtracted from the percentage in PMA-stimulated samples, and the difference was multiplied by the total number of lymph node cells recovered to yield the number of CD4+ IFN-γ+ or CD8+ IFN-γ+ cells. For analysis of memory cells, genital lymph nodes were collected 3 weeks after immunization. Cells were cultured for 4 h in the presence of GolgiStop and A20-1.11 B-lymphoma stimulator cells that had been infected 5 h before with HSV-2 333d41 or left uninfected. Cells were stained with anti-CD4-FITC (PharMingen) and then fixed and stained intracellularly for IFN-γ as described above. The number of CD4+ IFN-γ+ cells in individual mice was determined as described above based on analysis of 40,000 to 100,000 gated events per sample, and the difference between uninfected and infected A20-1.11 stimulator cells was calculated to determine the number of specific responding cells.
In vivo challenge.
At 7 days and 1 day prior to challenge, mice were injected s.c. in the neck ruff with 3 mg Depo-Provera (Pharmacia and Upjohn) suspended in 100 μl normal saline. Mice were anesthetized by intraperitoneal injection of ketamine-xylazine and infected by intravaginal inoculation of 6.5 × 105 PFU virus in a volume of 5 μl. To measure virus replication in the genital mucosa, vaginal vaults were swabbed twice with calcium alginate swabs on days 1 through 5 postinfection. Duplicate swabs for each time point were stored together in 1 ml PBS and frozen until assayed. Virus was quantitated on Vero cell monolayers by standard plaque assay. After challenge, mice were monitored on a daily basis to assess body weight, signs of disease, and survival. Mice were weighed individually, and the mean change from initial body weight was calculated daily for each group. Disease scores were assigned in a blinded fashion based on the following scale: 0, no apparent signs of disease; 1, slight erythema and edema of the genitals; 2, prominent erythema and edema of the genitals; 3, severe erythema and edema with lesions on the genitals. Mean daily disease scores ± standard errors of the mean (SEM) were calculated for each group. Hind limb paralysis was also assessed. Virus replication in neural tissues was analyzed by dissection of brains, brain stems, and spinal cords from a cohort of mice 6 days after challenge. Tissues were stored frozen until use. For virus titer determination, the tissues were thawed and disrupted using a Mini-Bead beater (BioSpec, Inc.) and diluted for a standard plaque assay.
RESULTS
Because replication-defective viruses do not spread in the host, they have limited potential to infect cells naturally capable of expressing the costimulation molecules that are required for activating immune responses. We investigated whether a replication-defective (ICP8−) virus that encodes a host-derived B7 costimulation molecule (ICP8−/B7+) would therefore be more immunogenic than the standard replication-defective vaccine strain. This work was carried out in mice lacking B7-1 and B7-2 costimulation molecules to allow us to ascribe any observed immune stimulatory activity to virally expressed B7 molecules, rather than to B7 induced on APCs, either directly or indirectly, by virus infection. B7-1 and B7-2 are expressed with different kinetics during an immune response and may have nonoverlapping roles in immune induction. We therefore compared individual reconstitution of B7-deficient mice by immunization with virus encoding either B7-1 or B7-2.
Construction and in vitro characterization of replication-defective viruses expressing B7-1 or B7-2.
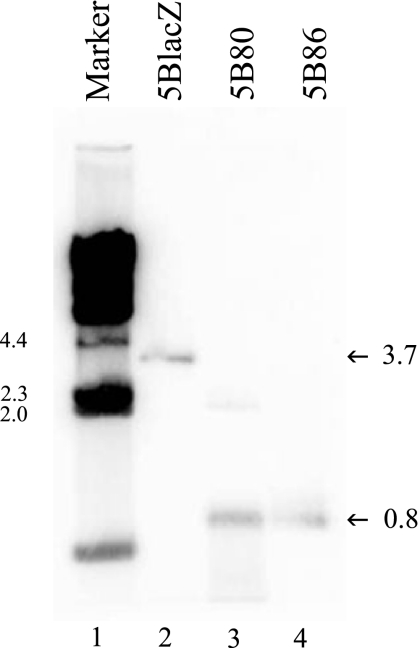
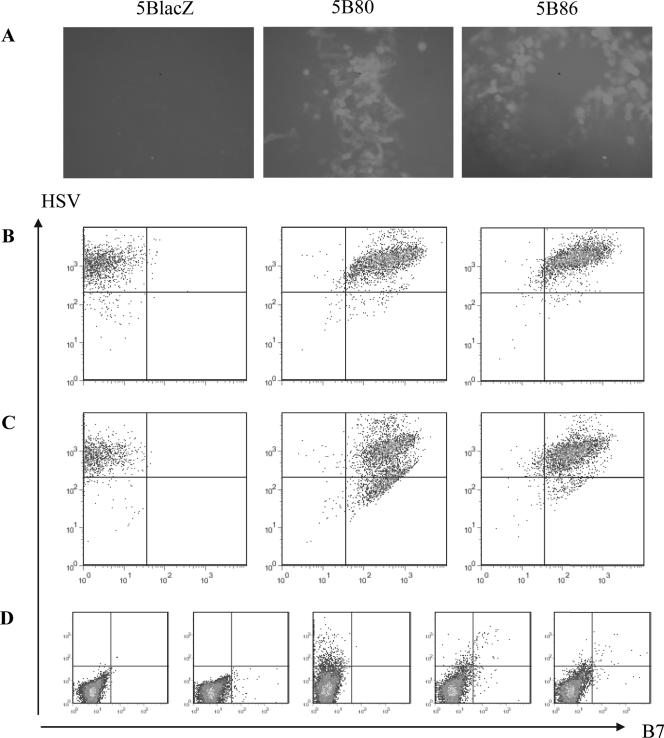
HSV-2 strains encoding either B7-1 or B7-2 were constructed by cloning the open reading frame of the murine B7-1 or B7-2 costimulation molecule downstream of the HCMV IE promoter into the TK gene of HSV-2 in plasmid pEH48 (56). The resultant plasmids were cotransfected with full-length DNA from ICP8− virus (5BlacZ) (40) in ICP8-complementing S2 cells (18). Plaques isolated under acyclovir selection were analyzed by Southern blotting for the presence of a new restriction site in the TK locus (Fig. 1). Using a probe complementary to the amino-terminal portion of TK, replication-defective viruses encoding B7-1 or B7-2 (5B80 and 5B86, respectively) were identified by hybridization to a band of faster mobility (Fig. 1, lanes 3 and 4), indicating the presence of a new restriction site in the adjacent TK sequence. Expression of murine B7-1 or B7-2 from the replication-defective virus genome was confirmed by immunofluorescence detection of B7 on virus plaques in infected S2 cells (Fig. 2A, middle and right panels). B7 coexpression with HSV-2 antigens was also detected on productively infected S2 cells by flow cytometry (Fig. 2B, middle and right panels) and on Vero cells, in which the viruses cannot replicate (Fig. 2C, middle and right panels), indicating that B7 molecules are expressed in the absence of productive infection. Genital lymph node cells of B7KO mice infected in vitro with 5B80 or 5B86 also coexpressed B7 and viral antigen (Fig. 2D, the two right-most panels), in contrast to cells infected with 5BlacZ. Thus, B7-1 and B7-2 encoded in the context of the replication-defective HSV-2 genome are expressed at the surface of infected cells, including primary mouse lymph node cells.
FIG. 1.
Southern blot assay confirmation of genotypes. Potentially recombinant plaque isolates that grew in the presence of acyclovir were screened for the presence of the HCMV IEp-B7 insertion into the TK locus. DNA was prepared from virus-infected cells, digested with EcoRI and HindIII, and subjected to agarose gel electrophoresis. DNA fragments were transferred to a nitrocellulose membrane and hybridized to a random hexamer 32P-labeled probe consisting of an EcoRI-to-SpeI fragment of plasmid pEH48(HCMV/B7-1) spanning the 5′ end of the TK gene and the HCMV IEp. Lane 1, lambda HindIII markers; lanes 2 to 4, viral DNAs as indicated.
FIG. 2.
B7 molecule expression on the surface of cells infected in vitro with 5B80 or 5B86. (A) S2 cell monolayers were mock infected or infected at a very low MOI and stained 48 h later with anti-B7-biotin followed by streptavidin-FITC. Individual plaques were imaged using an inverted immunofluorescence microscope. (B and C) S2 (B) or Vero (C) cell monolayers were mock infected or infected at an MOI of 5 for 15 h, then collected and stained with rabbit anti-HSV-2 and the appropriate anti-B7-biotin antibody followed by goat anti-rabbit-PE and streptavidin-FITC. Cells were analyzed by flow cytometry. (A to C) Results for 5BlacZ-infected cells to which both anti-B7 antibodies were added (left panel) or cells infected with 5B80 (middle panel) or 5B86 (right panel) and stained with the appropriate anti-B7 antibody. (D) Genital lymph node cells from B7KO mice were infected at an MOI of 3 for 15 h and then stained as for panels B and C and analyzed by flow cytometry. Proceeding from the left to the right, the panels show the following: panel 1, 5BlacZ-infected cells stained with secondary reagents only; panel 2, 5B80-infected cells stained with anti-B7-1; panel 3, 5BlacZ-infected cells stained with anti-HSV and anti-B7-1; panels 4 and 5, 5B80- or 5B86-infected cells stained with anti-HSV and the appropriate anti-B7 antibody.
Detection of B7 transcripts in B7KO mice immunized with B7-expressing virus.
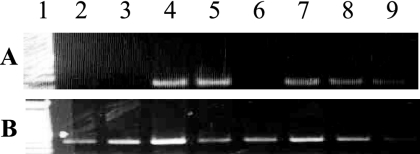
We were unable to detect cells expressing B7 protein in the draining lymph nodes of mice immunized with 5B80 or 5B86 by flow cytometry. This was not unexpected, because few mobile cells are likely to be infected by replication-defective virus. To verify that B7 is expressed in vivo in cells infected with recombinant, replication-defective virus, we immunized B7KO mice with 5B86 s.c. in the hind flank and prepared mRNA from genital lymph nodes collected at various times after immunization for detection of B7-2 transcripts. Wild-type or B7KO mice immunized with replication-competent HSV-2 served as positive and negative controls, respectively. Total RNA was harvested from the genital lymph nodes and reverse transcribed, and PCR was performed using primers to detect murine B7-2 cDNA. B7-2 transcript was detected in immunized wild-type mice but not in samples to which reverse transcriptase was not added (data not shown) or in samples from B7KO mice immunized with replication-competent HSV-2 (Fig. 3A, lanes 2 and 3). B7-2 transcript was also detected in all but one sample of genital lymph node cells from B7KO mice 18 to 66 h after immunization with 5B86 (Fig. 3A, lanes 4 to 9). GAPDH was amplified from all samples (Fig. 3B). Interestingly, B7-1 transcripts could be detected only at 18 h after immunization (data not shown). These results indicate that the B7 open reading frame is transcribed in vivo from the context of the viral genome and suggest that virally encoded B7 molecules are expressed in draining lymph nodes after immunization.
FIG. 3.
Detection of B7 mRNA in immunized B7KO mice. Groups of B7KO mice were immunized with replication-defective viruses, and genital lymph nodes were removed at various times after immunization. Total mRNA was prepared, and reverse transcription was performed on 1 μg per sample. cDNA preparations were subjected to PCR amplification as described in Materials and Methods using primers to B7-2 (A) or GAPDH (B). Lane 1, 100-bp ladder; lanes 2 and 3, B7KO mice immunized with 186ΔKpn (negative control); lanes 4 to 9, B7KO mice 18 h (lanes 4 and 5), 44 h (lanes 6 and 7), or 66 h (lanes 8 and 9) after immunization with 5B86.
IFN-γ-producing cells are stimulated by B7 expression.
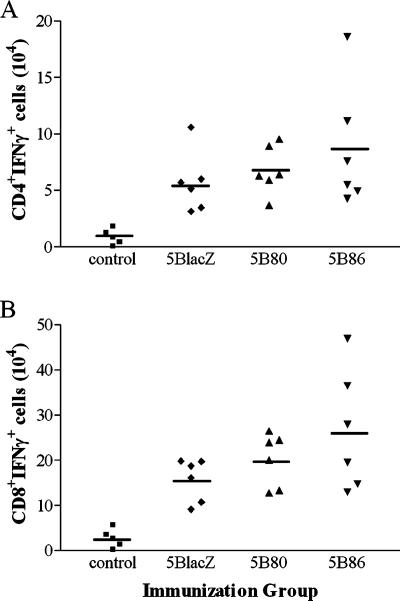
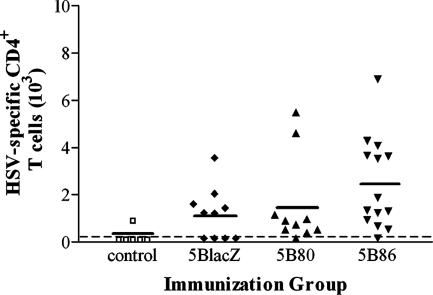
To determine whether immunization of B7KO mice with virus encoding B7 molecules could induce effector T-cell responses, B7KO mice were immunized with 5BlacZ, 5B80, or 5B86, and cells in the draining lymph nodes were examined ex vivo. Genital lymph node cells isolated from mice immunized 5 days previously were stimulated with PMA and calcium ionophore and stained intracellularly for IFN-γ (Fig. 4). Immunization with any of the viruses expanded CD4+ and CD8+ IFN-γ-secreting T cells at least 10-fold compared with control mice (P ≤ 0.0121 and P ≤ 0.0036, respectively). 5B80 and 5B86 stimulated slightly more IFN-γ-secreting cells than did the parental virus, 5BlacZ, but the differences were not statistically significant. Antigen-specific memory T-cell responses in the draining lymph nodes were also analyzed 17 to 21 days after immunization. Cells isolated from genital lymph nodes were restimulated in vitro with HSV-infected A20-1.11 B-lymphoma cells followed by intracellular staining for IFN-γ. Antigen-specific CD4+ IFN-γ+ memory cells were detected in the genital lymph nodes of nearly all mice immunized with 5B80 or 5B86 but in only 60% of mice immunized with 5BlacZ (Fig. 5). Mice immunized with 5B86 had on average the greatest number of antigen-specific CD4+ IFN-γ+ memory cells, 16-fold greater than the background in control mice (P = 0.0152) and more than twice the number found in mice immunized with 5BlacZ, although this difference was not statistically significant. CD8+ IFN-γ+ T-cell responses were not reliably detected by this method of stimulation, possibly due to HSV-mediated inhibition of MHC class I-restricted antigen presentation in the B-lymphoma cells (1). These results indicate that cytokine-secreting T cells are induced by replication-defective virus vaccine and augmented by virally encoded expression of B7 costimulation molecules, particularly B7-2.
FIG. 4.
Acute T-cell responses in draining lymph nodes of immunized B7KO mice. Genital lymph node cells were collected 5 days after immunization of B7KO mice with 5BlacZ, 5B80, or 5B86. Cells were enumerated and stimulated in vitro with PMA and calcium ionophore in the presence of GolgiStop. Cells were stained with FITC-conjugated antibody to CD4 (A) or CD8 (B), fixed and permeabilized, stained with anti-IFN-γ-PE, and analyzed by flow cytometry.
FIG. 5.
Memory CD4+ T-cell responses in the draining lymph nodes of immunized B7KO mice. Genital lymph node cells were collected from control B7KO mice or from mice 3 weeks after immunization with 5BlacZ, 5B80, or 5B86. Cells were enumerated and restimulated in vitro with 5BlacZ-infected A20-1.11 B-lymphoma cells in the presence of GolgiStop, then stained with anti-CD4 and intracellularly for IFN-γ, and analyzed by flow cytometry. P = 0.0152 for 5B86-immunized mice compared with unimmunized mice.
Serum antibody responses to B7-encoding virus.
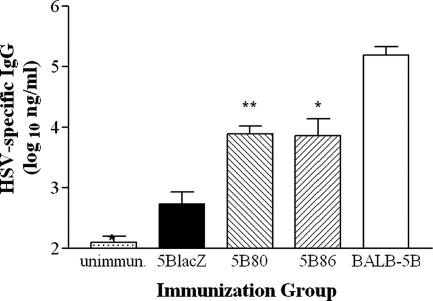
To determine the effect of virally encoded B7 expression on development of the humoral response to HSV, groups of B7KO mice were immunized with 5BlacZ, 5B80, or 5B86. Twenty-four days later, HSV-specific antibody titers in serum were determined by ELISA. Virus-specific IgG reached 13-fold-higher titers in B7KO mice immunized with 5B80 or 5B86 than in B7KO mice immunized with 5BlacZ (Fig. 6), although titers did not reach the concentration found in 5BlacZ-immunized wild-type mice. IgG2a/IgG1 ratios were similar after immunization with either B7-encoding virus or 5BlacZ (data not shown), suggesting that neither B7 molecule skewed the Th phenotype associated with vaccination. Thus, immunization with replication-defective viruses expressing B7 molecules induced stronger virus-specific humoral responses than replication-defective virus alone, but no difference was observed in the magnitude of responses stimulated by B7-1 or B7-2 expression.
FIG. 6.
Titers of HSV-specific antibody in B7KO mice immunized with B7-expressing viruses. Groups of eight to nine B7KO mice were immunized with the indicated viruses, and one group of six BALB/c mice was immunized with 5BlacZ as a positive control. Blood was collected 24 days postimmunization, and HSV-specific serum antibody was quantitated by ELISA. Data represent the geometric mean titer for each group ± the SEM. **, P = 0.0003 for 5B80; *, P = 0.0047 for 5B86 compared with B7KO-5BlacZ, P ≤ 0.0029 for BALB/c-5BlacZ compared with 5B80 or 5B86, and P = 0.0152 for unimmunized controls compared with B7KO-5BlacZ.
B7 expressed by the immunizing virus stimulates protective immunity against HSV infection and genital disease.
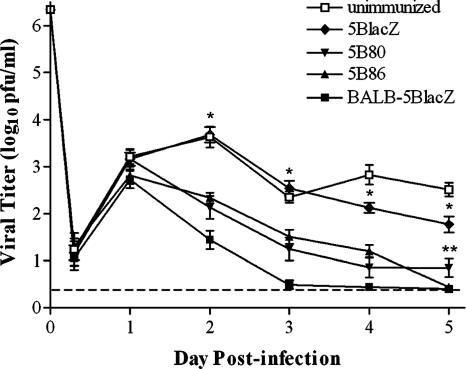
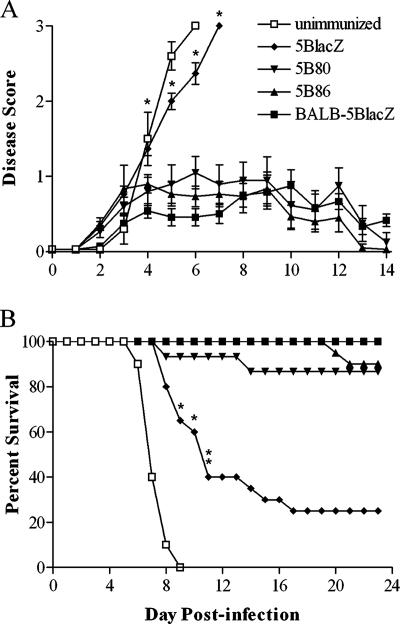
HSV-2 infection of the genital tract produces a burst of virus replication in the genital epithelium, which causes inflammation and vesicular lesions in the genital mucosa. Virus also rapidly ascends along sensory neurons innervating the mucosa to establish latent infection of the nervous system. To assess the protective capacity of vaccination with B7-encoding viruses at several stages of HSV infection, groups of B7KO mice were immunized with 5BlacZ, 5B80, or 5B86 and challenged 1 month later by intravaginal infection with virulent HSV-2 strain G-6. Unimmunized mice were used as a negative control, and wild-type BALB/c mice immunized with 5BlacZ were used as a positive control. HSV-2 G-6 replicated to similar titers in all groups of mice 24 h postchallenge. Thereafter, the level of replication in the genital mucosa of unimmunized or 5BlacZ-immunized B7KO mice remained high, reinforcing previous observations that an anti-HSV immune response is largely dependent on B7 costimulation (11, 58). In contrast, mice immunized with either B7-1- or B7-2-expressing virus prior to challenge showed significantly reduced mucosal replication of HSV-2 from 2 to 5 days postinfection (Fig. 7), although replication was still higher than that seen in wild-type mice immunized with 5BlacZ. Prior immunization with B7-1-expressing virus reduced replication equivalently to B7-2-expressing virus until day 5 postchallenge, when mice immunized with 5B86 but not 5B80 had completely cleared challenge virus (Fig. 7). B7KO mice that were unimmunized or immunized with 5BlacZ developed severe genital disease (Fig. 8A) and rapidly lost weight (P ≤ 0.0056 for days 7 through 9 when comparing 5B80 or 5B86 to 5BlacZ [data not shown]), but genital inflammation and weight loss in B7KO mice immunized with B7-1- or B7-2-expressing virus were reduced nearly to the levels seen in wild-type mice immunized with 5BlacZ (P ≥ 0.1). Thus, immunization with B7-expressing, replication-defective virus provides B7KO mice with enhanced protection from HSV-2 replication in the genital mucosa and development of genital inflammation and lesions compared with virus that does not express host B7 costimulation molecules.
FIG. 7.
Replication of challenge virus in the genital mucosa of immunized mice. Groups of B7KO mice were immunized s.c. with the indicated virus or were left unimmunized, then challenged intravaginally 1 month later with wild-type HSV-2. BALB/c mice immunized with 5BlacZ were a positive control for immunization. Titers from daily swabs of the vagina were determined for the viral titer shed from the mucosa. Data are the geometric means ± SEM for 10 to 15 mice per experimental group and are the combined results of two independent experiments. The dashed line indicates the limit of detection. *, P ≤ 0.0001 for days 2 through 5 comparing 5B80 or 5B86 to B7KO-5BlacZ; **, P = 0.0205 for 5B80 compared with 5B86 on day 5. P ≤ 0.00001 for days 2 through 4 comparing 5B86 to BALB/C-5Blacz.
FIG. 8.
Signs of genital disease and survival. Mice as described in the legend for Fig. 7 were monitored daily for signs of inflammation of the external genitalia and presence of lesions. Data are the arithmetic means + standard deviations. (A) Genital inflammation and lesions. *, P ≤ 0.012 for days 4 through 7 comparing 5B80 or 5B86 to 5BlacZ. (B) Survival of immunized mice after challenge with HSV-2. *, P = 0.0083 to 0.0033 for 5B86 compared with B7KO-5BlacZ; **, P = 0.0049 to <0.0001 for 5B80 or 5B86 compared with B7KO-5BlacZ on days 11 through 23.
Signs of severe disease were apparent in unimmunized B7KO mice, which rapidly lost weight and succumbed to infection (Fig. 8B). Most B7KO mice immunized with 5BlacZ virus also showed hind limb paralysis, and mortality was 75%. 5B80- and 5B86-immunized B7KO mice, however, were strikingly protected from neurologic manifestations and death due to challenge virus infection (Fig. 8B). No mice immunized with either B7-expressing virus showed signs of hind limb paralysis (P ≤ 0.0001 compared with 5BlacZ [data not shown]), and mortality was ≤13% (P ≤ 0.0005), indicating protective efficacy nearly comparable to immunization of wild-type mice with 5BlacZ. The protective effect was dependent on the dose of immunizing virus (data not shown). These data indicate that in mice lacking B7-1 and B7-2 costimulation molecules, expression of B7-1 or B7-2 by an immunizing replication-defective virus establishes significant protection against HSV-2 challenge.
Prophylactic protection of the nervous system by immunization with B7-expressing virus.
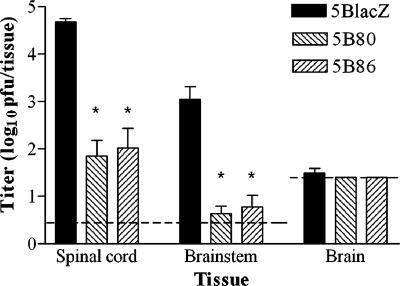
The lack of hind limb paralysis in B7KO mice immunized with B7-expressing virus prior to challenge suggested that less challenge virus was able to reach the spinal cord than in mice immunized with 5BlacZ. To confirm reduced infection of the nervous system and to exclude the possibility that a greater inflammatory infiltrate in 5BlacZ-immunized mice caused the increased incidence of paralysis, groups of immunized B7KO mice were infected intravaginally, and nervous system tissues were dissected 6 days postinfection and assayed for infectious virus. Mice immunized with 5B80 or 5B86 had significantly lower virus titers in all portions of the central nervous system than mice immunized with 5BlacZ prior to challenge (Fig. 9). Unimmunized mice were not included because they succumbed by 6 days postinfection. These results confirm that less challenge virus spread to the nervous system in mice immunized with B7-expressing, replication-defective virus. Thus, expression of B7 costimulation molecules from the HSV genome significantly enhance immune responses and protection over that provided by immunization with replication-defective HSV vaccine.
FIG. 9.
Immunization with B7-expressing virus significantly decreases HSV-2 infection of the nervous system. Groups of 9 to 13 B7KO mice were immunized s.c. with the indicated virus and then challenged intravaginally 1 month later with wild-type HSV-2. Six days postchallenge, virus contained in disrupted spinal cord, brain stem, and brain tissues was quantified by standard plaque assay. Values represent the geometric means ± SEM. The dashed line indicates the limit of detection. *, P < 0.0001 for 5B80 or 5B86 compared with B7KO-5BlacZ.
DISCUSSION
Our approach of equipping a replication-defective virus with host B7 costimulation molecules produced two novel results: first, we demonstrated that reconstitution of B7-deficient mice with HSV-encoded B7 costimulation molecules augments immune responsiveness to HSV-2. Second, the strengthened immune responses induced by replication-defective viruses expressing B7 costimulation molecules provided B7KO mice with better protection from HSV-2 challenge, despite the fact that they lacked endogenous B7-1 and B7-2 and so were otherwise highly susceptible. Challenge virus replication was curtailed in the genital mucosa and nervous system, reducing the pathological consequences of infection. Single reconstitutions of B7-deficient mice showed that B7-2-expressing virus was slightly more effective at inducing T-cell responses and protecting the genital tract than was B7-1-expressing virus. Therefore, virus-mediated expression of B7 costimulation molecules positively influences induction of protective immune responses against the homologous pathogen.
By employing the B7KO model, we were able to definitively ascribe the enhanced in vivo protection observed after vaccination with 5B80 and 5B86 to the virally encoded B7 molecules. B7 expression driven by the HCMV IE promoter was easily detected on cultured S2 cells infected in vitro with 5B80 or 5B86. B7+ cells became actively infected, because B7 expression was not detected on the surface of cells exposed to UV-inactivated 5B86 (data not shown). Similar levels of B7 expression were observed on infected Vero cells even though 5B80 and 5B86 cannot complete a replication cycle, suggesting that B7 could be expressed in vivo after immunization with replication-defective virus. Indeed, a small proportion of B7KO mouse genital lymph node cells infected in vitro also showed strong B7 expression coincident with viral antigen, demonstrating that B7 molecules are expressed in mouse cells in the absence of a complete virus replication cycle. The identities of the lymph node cells infected in vitro have not been precisely determined, but the majority express CD11b and a minority are CD11c+, consistent with cells of the macrophage and dendritic cell lineage that make up a small fraction of the lymph node population. Because we used lymph node cells isolated from a naïve B7KO mouse, they would most likely not have included the subset of dendritic cells that migrate to the lymph node from the submucosa in response to immunization (64). We were unable to detect B7 antigen expression in lymph nodes of B7KO mice after in vivo immunization with 5B80 or 5B86. Reasons for this may include a relatively small number of B7-expressing cells migrating from the flank to the genital nodes, transient B7 expression on or brief survival of infected cells, or B7 expression levels below the limit of detection by flow cytometry. We were, however, able to detect B7 mRNA expression in 5B80- and 5B86-immunized B7KO mice, strongly suggesting that B7 molecules are expressed in vivo. B7-2 mRNA was easier to detect than B7-1, which correlates with the slightly enhanced immunogenicity of 5B86.
The augmented primary immune responses we observed imply that the necessary second signal for immune induction is provided by surface expression of B7 on replication-defective virus-infected cells. It is unlikely that stimulation occurs via B7 protein in the input virus preparation, because there is no evidence to date that host cell B7 molecules are incorporated into HSV virions, and our virus is supernatant derived and thus contains predominantly extracellular virions, not cell components. Rather, detection of B7 transcripts in vivo suggests that the B7 molecules are synthesized de novo within infected cells. Stimulation of HSV-specific T cells may occur through direct antigen presentation by infected cells or by cross-presentation. We favor the former, because it is more straightforward to envision stimulation of T cells via an immunologic synapse containing B7 and MHC/antigen complexes present on the infected cell. Still, some HSV-specific T cells may be stimulated by antigen cross-presented on uninfected dendritic cells after these have engulfed apoptotic, virus-infected cells (5). Interestingly, strong B7-dependent immune stimulation by B7-expressing, replication-defective virus occurred in an otherwise-B7-deficient host, despite the capacity of wild-type HSV to down-regulate B7 costimulation molecules in at least some subsets of dendritic cells (47, 48).
The capacity to reduce HSV-2 replication and disease is the most important consequence of incorporating B7 into replication-defective viruses. This protection was afforded by both B7-1- and B7-2-expressing viruses. The differences detected in T-cell responses of B7KO mice immunized with 5B80 or 5B86 versus 5BlacZ were small. HSV-encoded B7 boosted cellularity of the draining lymph node only about 20% more than parental virus, and the increases in numbers of IFN-γ-producing T cells and IFN-γ production (data not shown) were equivalently modest. Antibody responses, in contrast, were significantly amplified in mice immunized with B7-expressing virus compared with 5BlacZ. Our results are consistent with previous observations that T-cell responses to HSV (59) or lymphocytic choriomeningitis virus (57) in the absence of CD28-B7 signaling are reduced only about twofold, but antibody deficits in the absence of B7 costimulation are profound (38, 59). Thus, T cells induced by the B7-encoding vaccine viruses must have provided additional T-cell help for antibody production, if not through cytokine production then possibly through CD40L expression in a T-cell-dependent induction of class-switched responses (59).
In our current study, B7-1 and B7-2 expressed by replication-defective immunizing virus were quantitatively equivalent in their cell surface abundance after in vitro infection and substantially overlapped in their capacity to augment antiviral immune responses in vivo. In B7KO mice, B7-2-expressing virus showed a slightly greater capacity to augment the number of IFN-γ-secreting CD4+ and CD8+ T cells responding to HSV, but B7-1- and B7-2-expressing viruses were equivalent in terms of titers of HSV-specific IgG achieved and IgG1/IgG2a ratios. Immune responses provoked by vesicular stomatitis virus infection of B7-1−/− or B7-2−/− mice are similarly indistinguishable (38). Thus, in the absence of one or both forms of B7, provision of either B7-1 or B7-2 may drive immune responses roughly equivalently. Alternatively, the strong Th1 stimulus provided by a typical virus infection may mask potentially subtle types of Th bias engendered by B7-1 versus B7-2 expression.
We detected memory CD4+ T cells in local lymph nodes of almost all mice immunized with B7-expressing virus prior to challenge, with a trend toward greater numbers of virus-specific memory cells maintained after immunization with 5B86. HSV-specific antibody responses were higher in mice immunized with either B7-expressing virus than 5BlacZ. These stronger memory immune responses induced by B7-expressing viruses translated into better protection against HSV-2 challenge. Interestingly, antibody titers 14 days after challenge of B7KO mice immunized with 5B80 or 5B86 showed no anamnestic response (data not shown). This result is consistent with the observations of Wu et al. (62) that secondary antibody responses to protein antigen are abrogated if B7 costimulation is blocked at the time of challenge.
B7 expression by the B7-encoding viruses is not completely restorative in a B7-deficient host. Immune responses and protection are still well below the levels seen upon immunization of a B7-sufficient host with the 5BlacZ parental virus, possibly due to B7 expression on fewer cells or different cell types than would be typical in a B7-sufficient host. Nonetheless, the level of protection afforded by the replication-defective virus expressing B7 is impressive considering that a single dose of replication-defective vaccine reduced mucosal replication of challenge virus 10- to 30-fold and turned a predominantly lethal infection into one that could be cleared in mice completely devoid of endogenous B7. By comparison, three immunizations with a mixture of plasmids encoding HSV-2 gD and CD80 were required to reduce HSV replication, disease, and mortality after challenge (13), and pCD86 was ineffective. With the efficacy of vaccine-encoded B7 molecules now established by reconstitution of a B7-deficient host, future studies will determine whether B7-encoding virus immunization enhances the stimulation of anti-HSV immunity in wild-type mice.
Acknowledgments
This work was supported by Public Health Service grant CA75052 from the National Cancer Institute.
We thank David Knipe for the gift of plasmid pEH48 and Arlene Sharpe for the B7KO mice. Rob Reass, Jen Murphy, and Hong Wang provided expert technical assistance. We thank Mike Moxley and John Corbett for assistance with inverted fluorescence microscopy, Sherri Koehm for help with flow cytometry, and Paul Olivo and the Leib, Virgin, and Speck labs for helpful advice and discussion. We are indebted to Paul Allen and Becky Duerst for critical reviews of the manuscript.
Footnotes
Published ahead of print on 5 September 2007.
REFERENCES
- 1.Barcy, S., and L. Corey. 2001. Herpes simplex inhibits the capacity of lymphoblastoid B cell lines to stimulate CD4+ T cells. J. Immunol. 166:6242-6249. [DOI] [PubMed] [Google Scholar]
- 2.Bertram, E. M., W. Dawicki, and T. H. Watts. 2004. Role of T cell costimulation in anti-viral immunity. Semin. Immunol. 16:185-196. [DOI] [PubMed] [Google Scholar]
- 3.Bluestone, J. A. 1995. New perspectives of CD28-B7-mediated T cell costimulation. Immunity 2:555-559. [DOI] [PubMed] [Google Scholar]
- 4.Borriello, F., M. P. Sethna, S. D. Boyd, A. N. Schweitzer, E. A. Tivol, D. Jacoby, T. B. Strom, E. M. Simpson, G. J. Freeman, and A. H. Sharpe. 1997. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity 6:303-313. [DOI] [PubMed] [Google Scholar]
- 5.Bosnjak, L., M. Miranda-Saksena, D. M. Koelle, R. A. Boadel, and C. A. Jones. 2005. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J. Immunol. 174:2220-2227. [DOI] [PubMed] [Google Scholar]
- 6.Brown, J. A., R. G. Titus, N. Nabavi, and L. H. Glimcher. 1996. Blockade of CD86 ameliorates Leishmania major infection by down-regulating the Th2 response. J. Infect. Dis. 174:1303-1308. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhry, A., S. R. Das, A. Hussain, S. Mayor, A. George, V. Bal, S. Jameel, and S. Rath. 1996. The Nef protein of HIV-1 induces loss of cell surface costimulatory molecules CD80 and CD86 in APCs. J. Immunol. 175:4566-4574. [DOI] [PubMed] [Google Scholar]
- 8.Coscoy, L., and D. Ganem. 2001. A viral protein that selectively downregulates ICAM-1 and B7-2 and modulates T cell costimulation. J. Clin. Investig. 107:1599-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Da Costa, X. J., N. Bourne, L. R. Stanberry, and D. M. Knipe. 1997. Construction and characterization of a replication-defective HSV-2. Virology 232:1-12. [DOI] [PubMed] [Google Scholar]
- 10.Ding, L., P. S. Linsley, L. Y. Huang, R. N. Germain, and E. M. Shevach. 1993. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J. Immunol. 151:1224-1234. [PubMed] [Google Scholar]
- 11.Edelmann, K. H., and C. B. Wilson. 2001. Role of CD28/CD80-86 and CD40/CD154 costimulatory interactions in host defense to primary herpes simplex virus infection. J. Virol. 75:612-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elloso, M. M., and P. Scott. 1999. Expression and contribution of B7-1 (CD80) and B7-2 (CD86) in the early immune response to Leishmania major infection. J. Immunol. 162:6708-6715. [PubMed] [Google Scholar]
- 13.Flo, J., S. Tisminetzky, and F. Baralle. 2000. Modulation of the immune response to DNA vaccine by co-delivery of costimulatory molecules. Immunology 100:259-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman, G. J., F. Borriello, R. J. Hodes, H. Reiser, J. G. Gribben, J. W. Ng, J. Kim, J. M. Goldberg, K. Hathcock, and G. Laszlo. 1993. Murine B7-2, an alternative CTLA4 counter-receptor that costimulates T cell proliferation and interleukin 2 production. J. Exp. Med. 178:2185-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman, G. J., S. G. Gray, C. D. Gimmi, D. B. Lombard, L.-J. Zhou, M. White, J. D. Fingeroth, J. G. Gribben, and L. M. Nadler. 1991. Structure, expression, and T cell costimulatory activity of the murine homologue of the human B lymphocyte activation antigen B7. J. Exp. Med. 174:625-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman, G. J., J. G. Gribben, V. A. Boussiotis, J. W. Ng, V. A. Restivo, Jr., L. A. Lombard, G. S. Gray, and L. M. Nadler. 1993. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science 262:909-911. [DOI] [PubMed] [Google Scholar]
- 17.Gajewski, T. F., Y. Meng, and H. Harlin. 2006. Immune suppression in the tumor microenvironment. J. Immunother. 29:233-240. [DOI] [PubMed] [Google Scholar]
- 18.Gao, M., and D. M. Knipe. 1989. Genetic evidence for multiple nuclear functions of the herpes simplex virus ICP8 DNA-binding protein. J. Virol. 63:5258-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harding, F. A., and J. P. Allison. 1993. CD28-B7 interactions allow the induction of CD8+ cytotoxic T lymphocytes in the absence of exogenous help. J. Exp. Med. 177:1791-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hathcock, K. S., G. Laszlo, H. B. Dichler, J. Bradshaw, P. Linsley, and R. J. Hodes. 1993. Identification of an alternative CTLA-γ ligand costimulatory for T cell activation. Science 262:905-907. [DOI] [PubMed] [Google Scholar]
- 21.Hathcock, K. S., G. Laszlo, C. Pucillo, P. Linsley, and R. J. Hodes. 1994. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J. Exp. Med. 180:631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hochreiter, R., C. Ptaschinski, S. L. Kunkel, and R. Rochford. 2007. Murine gammaherpesvirus-68 productively infects immature dendritic cells and blocks maturation. J. Gen. Virol. 88:1896-1905. [DOI] [PubMed] [Google Scholar]
- 23.Janeway, C. A., Jr., and K. Bottomly. 1994. Signals and signs for lymphocyte responses. Cell 76:275-285. [DOI] [PubMed] [Google Scholar]
- 24.Jones, C. A., T. J. Taylor, and D. M. Knipe. 2000. Biological properties of herpes simplex virus 2 replication-defective mutant strains in a murine nasal infection model. Virology 278:137-150. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman, H. L., G. Deraffele, J. Mitcham, D. Moroziewicz, S. M. Cohen, K. S. Hurst-Wicker, K. Cheung, D. S. Lee, J. Divito, M. Voulo, J. Donovan, K. Dolan, K. Manson, D. Panicali, E. Wang, H. Horig, and F. M. Marincola. 2005. Targeting the local tumor microenvironment with vaccinia virus expressing B7.1 for the treatment of melanoma. J. Clin. Investig. 115:1903-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keane-Myers, A. M., W. C. Gause, F. D. Finkelman, X. D. Xhou, and M. Wills-Karp. 1998. Development of murine allergic asthma is dependent upon B7-2 costimulation. J. Immunol. 160:1036-1043. [PubMed] [Google Scholar]
- 27.Knipe, D. M., and A. E. Spang. 1982. Definition of a series of stages in the association of two herpesviral proteins with the cell nucleus. J. Virol. 43:314-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuchroo, V. K., M. P. Das, J. A. Brown, A. M. Ranger, S. S. Zamvil, R. A. Sobel, H. L. Weiner, N. Nabavia, and L. H. Glimcher. 1995. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell 80:707-718. [DOI] [PubMed] [Google Scholar]
- 29.Lanier, L., S. O'Fallon, C. Somoza, J. Phillips, P. Linsley, K. Okumura, D. Ito, and M. Azuma. 1995. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J. Immunol. 154:97-105. [PubMed] [Google Scholar]
- 30.Lee, Y. S., J. H. Kim, K. J. Choi, I. K. Choi, H. Kim, S. Cho, B. C. Cho, and C. O. Yun. 2006. Enhanced antitumor effect of oncolytic adenovirus expressing interleukin-12 and B7-1 in an immunocompetent murine model. Clin. Cancer Res. 12:5859-5868. [DOI] [PubMed] [Google Scholar]
- 31.Lenschow, D. J., S. C. Ho, H. Sattar, L. Rhee, G. Gray, N. Nabavi, K. C. Herold, and J. A. Bluestone. 1995. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J. Exp. Med. 181:1145-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lenschow, D. J., A. I. Sperling, G. J. Cooke, G. J. Freeman, L. Rhee, and D. C. Decker. 1994. Differential up-reguation of the B7-1 and B7-2 costimulatory molecules after Ig receptor engagement by antigen. J. Immunol. 153:1990-1997. [PubMed] [Google Scholar]
- 33.Levine, B. L., Y. Ueda, N. Craighead, M. L. Huang, and C. H. June. 1995. CD28 ligands CD80 (B7-1) and CD86 (B7-2) induce long-term autocrine growth of CD4+ T cells and induce similar patterns of cytokine secretion in vitro. Int. Immunol. 7:891-904. [DOI] [PubMed] [Google Scholar]
- 34.Linsley, P. S., W. Brady, L. Grosmaire, A. Aruffo, N. K. Damle, and J. A. Ledbetter. 1991. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J. Exp. Med. 173:721-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lumsden, J., J. Roberts, N. Harris, R. Peach, and F. Ronchese. 2000. Differential requirement for CD80 and CD80/CD86-dependent costimulation in the lung immune response to an influenza virus infection. J. Immunol. 164:79-85. [DOI] [PubMed] [Google Scholar]
- 36.Marti, W. R., P. Zajac, G. Spagnoli, M. Heberer, and D. Oertli. 1997. Nonreplicating recombinant vaccinia virus encoding human B-7 molecules elicits effective costimulation of naive and memory CD4+ T lymphocytes in vitro. Cell. Immunol. 179:146-152. [DOI] [PubMed] [Google Scholar]
- 37.Maue, A. C., W. R. Waters, M. V. Palmer, D. L. Whipple, F. C. Minion, W. C. Brown, and D. M. Estes. 2004. CD80 and CD86, but not CD154, augment DNA vaccine-induced protection in experimental bovine tuberculosis. Vaccine 23:769-779. [DOI] [PubMed] [Google Scholar]
- 38.McAdam, A. J., E. A. Farkash, B. E. Gewurz, and A. H. Sharpe. 2000. B7 costimulation is critical for antibody class switching and CD8(+) cytotoxic T-Iymphocyte generation in the host response to vesicular stomatitis virus. J. Virol. 74:203-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mintern, J. D., E. J. Klemm, M. Wagner, M. E. Paquet, M. D. Napier, Y. M. Kim, U. H. Koszinowski, and H. L. Ploegh. 2006. Viral interference with B7-1 costimulation: a new role for murine cytomegalovirus Fc receptor-1. J. Immunol. 177:8422-8431. [DOI] [PubMed] [Google Scholar]
- 40.Morrison, L. A., X. J. Da Costa, and D. M. Knipe. 1998. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology 243:178-187. [DOI] [PubMed] [Google Scholar]
- 41.Morrison, L. A., and D. M. Knipe. 1996. Mechanisms of immunization with a replication-defective mutant of herpes simplex virus 1. Virology 320:402-413. [DOI] [PubMed] [Google Scholar]
- 42.Morrison, L. A., L. Zhu, and L. G. Thebeau. 2001. Vaccine-induced serum immunoglobulin contributes to protection from herpes simplex virus type 2 genital infection in the presence of immune T. cells. J. Virol. 75:1195-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy, M. L., C. R. Engwerda, P. M. Gorak, and P. M. Kaye. 1997. B7-2 blockade enhances T cell responses to Leishmania donovani. J. Immunol. 159:4460-4466. [PubMed] [Google Scholar]
- 44.Natesan, M., Z. Razi-Wolf, and H. Reiser. 1996. Costimulation of IL-4 production by murine B7-1 and B7-2 molecules. J. Immunol. 156:2783-2791. [PubMed] [Google Scholar]
- 45.Osorio, Y., S. Cai, and H. Ghiasi. 2005. Treatment of mice with anti-CD86 mAb reduces CD8+ T cell-mediated CTL activity and enhances ocular viral replication in HSV-1-infected mice. Ocul. Immunol. Inflamm. 13:159-167. [DOI] [PubMed] [Google Scholar]
- 46.Petrulio, C. A., and H. L. Kaufman. 2006. Development of the PANVAC-VF vaccine for pancreatic cancer. Expert. Rev. Vaccines 5:9-19. [DOI] [PubMed] [Google Scholar]
- 47.Salio, M., M. Cella, M. Suter, and A. Lanzavecchia. 1999. Inhibition of dendritic cell maturation by herpes simplex virus. Eur. J. Immunol. 29:3245-3253. [DOI] [PubMed] [Google Scholar]
- 48.Samady, L., E. Costigliola, L. MacCormac, Y. McGrath, S. Cleverley, C. E. Lilley, J. Smith, D. S. Latchman, B. Chain, and R. S. Coffin. 2003. Deletion of the virion host shutoff protein (vhs) from herpes simplex virus (HSV) relieves the viral block to dendritic cell activation: potential of vhs-HSV vectors for dendritic cell-mediated immunotherapy. J. Virol. 77:3768-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santra, S., D. H. Barouch, S. S. Jackson, M. J. Kuroda, J. E. Schmitz, M. A. Lifton, A. H. Sharpe, and N. L. Letvin. 2000. Functional equivalency of B7-1 and B7-2 for costimulating plasmid DNA vaccine-elicited CTL responses. J. Immunol. 165:6791-6795. [DOI] [PubMed] [Google Scholar]
- 50.Schweitzer, A., and A. Sharpe. 1998. Studies using antigen-presenting cells lacking expression of both B7-1 (CD80) and B7-2 (CD86) show distinct requirements for B7 molecules during priming versus restimulation of Th2 but not Th1 cytokine production. J. Immunol. 161:2762-2771. [PubMed] [Google Scholar]
- 51.Schweitzer, A. N., F. Borriello, R. C. Wong, A. K. Abbas, and A. H. Sharpe. 1997. Role of costimulators in T cell differentiation: studies using antigen-presenting cells lacking expression of CD80 or CD86. J. Immunol. 158:2713-2722. [PubMed] [Google Scholar]
- 52.Shahinian, A., K. Pfeffer, K. P. Lee, T. M. Kundig, K. Kishihara, A. Wakeham, K. Kawai, P. S. Ohashi, C. B. Thompson, and T. W. Mak. 1993. Differential T cell costimulatory requirements in CD28-deficient mice. Science 261:609-612. [DOI] [PubMed] [Google Scholar]
- 53.Sharpe, A. H., and G. J. Freeman. 2002. The B7-CD28 superfamily. Nat. Rev. Immunol. 2:116-126. [DOI] [PubMed] [Google Scholar]
- 54.Sigal, L. J., H. Reiser, and K. L. Rock. 1998. The role of B7-1 and B7-2 costimulation for the generation of CTL responses in vivo. J. Immunol. 161:2740-2745. [PubMed] [Google Scholar]
- 55.Smith, T. J., L. A. Morrison, and D. A. Leib. 2002. Pathogenesis of herpes simplex virus type 2 virion host shutoff (vhs) mutants. J. Virol. 76:2054-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spang, A. E., P. J. Godowski, and D. M. Knipe. 1983. Characterization of herpes simplex virus 2 temperature-sensitive mutants whose lesions map in or near the coding sequences for the major DNA-binding protein. J. Virol. 45:332-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suresh, M., J. K. Whitmire, L. E. Harrington, C. P. Larsen, T. C. Pearson, J. D. Altman, and R. Ahmed. 2001. Role of CD28-B7 interactions in generation and maintenance of CD8 T cell memory. J. Immunol. 167:5565-5573. [DOI] [PubMed] [Google Scholar]
- 58.Thebeau, L. G., and L. A. Morrison. 2002. B7 costimulation plays an important role in protection from herpes simplex virus type 2-mediated pathology. J. Virol. 76:2563-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thebeau, L. G., and L. A. Morrison. 2003. Mechanism of reduced T-cell effector functions and class-switched antibody responses to herpes simplex virus type 2 in the absence of B7 costimulation. J. Virol. 77:2426-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Todo, T., R. L. Martuza, M. J. Dallman, and S. D. Rabkin. 2001. In situ expression of soluble B7-1 in the context of oncolytic herpes simplex virus induces potent antitumor immunity. Cancer Res. 61:153-161. [PubMed] [Google Scholar]
- 61.Tsuji, T., K. Hamajima, N. Ishii, I. Aoki, J. Fukushima, K. Q. Xin, S. Kawamoto, S. Sasaki, K. Matsunaga, Y. Ishigatsubo, K. Tani, T. Okubo, and K. Okuda. 1997. Immunomodulatory effects of a plasmid expressing B7-2 on human immunodeficiency virus-1-specific cell-mediated immunity induced by a plasmid encoding the viral antigen. Eur. J. Immunol. 27:782-787. [DOI] [PubMed] [Google Scholar]
- 62.Wu, Z. Q., A. Q. Khan, Y. Shen, J. Schartman, R. Peach, A. Lees, J. J. Mond, W. C. Gause, and C. M. Snapper. 2000. B7 requirements for primary and secondary protein- and polysaccharide-specific Ig isotype responses to Streptococcus pneumoniae. J. Immunol. 165:6840-6948. [DOI] [PubMed] [Google Scholar]
- 63.Zajac, P., A. Schutz, D. Oertli, C. Noppen, C. Schaefer, M. Heberer, G. C. Spagnoli, and W. R. Marti. 1998. Enhanced generation of cytotoxic T lymphocytes using recombinant vaccinia virus expressing human tumor-associated antigens and B7 costimulatory molecules. Cancer Res. 58:4567-4571. [PubMed] [Google Scholar]
- 64.Zhao, X., E. Deak, K. Soderberg, M. Linehan, D. Spezzano, J. Zhu, D. M. Knipe, and A. Iwasaki. 2003. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J. Exp. Med. 197:153-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng, J., L. Si, J. Song, X. Sun, J. Yu, and Y. Wang. 2003. Enhanced immune response to DNA-based HPV16L1 vaccination by costimulatory molecule B7-2. Antivir. Res. 59:61-65. [DOI] [PubMed] [Google Scholar]
- 66.Zimmermann, C., P. Seiler, P. Lane, and R. M. Zinkernagel. 1997. Antiviral immune responses in CTLA4 transgenic mice. J. Virol. 71:1802-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]