Abstract
The E6 protein of the oncogenic human papillomaviruses (HPVs), in combination with the E7 protein, is essential for the efficient immortalization of human foreskin keratinocytes (HFKs). Since we recently demonstrated that E6 activates the human telomerase reverse transcriptase (hTERT) promoter via a Myc-dependent mechanism, we speculated that overexpressed Myc might be able to substitute for E6 in cell immortalization. Myc (similar to E6) was unable to immortalize HFKs when transduced alone, despite inducing high levels of telomerase activity. However, when transduced with E7, Myc immortalized HFKs following a brief but detectable crisis period. In contrast to E6 + E7-immortalized cells, the Myc + E7-immortalized cells expressed high levels of p53 protein as well as two p53-regulated proteins, p21 and hdm-2. The increase in p21 and hdm-2 proteins correlated directly with their mRNA levels, suggesting transcriptional activation of the respective genes by the overexpressed p53 protein. Interestingly, a significant proportion of the p53 protein in the Myc + E7-immortalized cells was localized to the cytoplasm, potentially due to interactions with the overexpressed hdm-2 protein. Regardless, cell immortalization by the Myc + E7 genes occurred independently of p53 degradation. Since we have already observed high-efficiency cell immortalization with the hTERT + E7 or E6 mutant (p53 degradation-defective) + E7 genes (i.e., no crisis period) that proceeds in the presence of high levels of p53, we hypothesize that the crisis period in the Myc + E7 cells is due not to the levels of the p53 protein but rather to unique properties of the Myc protein. The common factor in cell immortalization by the three gene sets (E6 + E7, Myc + E7, and hTERT + E7 genes) is the induction of telomerase activity.
High-risk human papillomaviruses (HPVs), such as types 16 and 18, are etiological agents of nearly all cervical cancers (49-51). The major transforming genes of HPV-16 are the E6 and E7 genes, both of which are necessary and sufficient for the efficient immortalization of primary human ectocervical keratinocytes and human foreskin keratinocytes (HFKs) (1, 5, 15, 30). The E7 oncoprotein has been shown to bind and degrade the retinoblastoma protein, pRb, thereby releasing repression of the E2F transcription factor and allowing cells to progress into S phase (9, 31). However, in the absence of E6, the E7 protein has also been shown to induce apoptosis (38, 41) and to sensitize cells to tumor necrosis factor-induced apoptosis (4). These apoptotic responses may be the consequence of E7's ability to stabilize the p53 protein (7, 18, 38, 41), a proposal which is supported by the ability of E6 to abrogate such responses via the degradation of p53 (36, 37, 46).
Although p53 binding and degradation were its first described activities, the E6 protein also displays biological activities that are p53 independent (11, 21, 23, 24) and interacts with many other cell regulatory proteins, some of which contain PDZ domains (13, 22, 26). E6 transactivates the promoter of the catalytic subunit of telomerase, human telomerase reverse transcriptase (hTERT) (11, 21, 34, 43, 44), but E6 mutants that cannot degrade p53 are still able to induce telomerase activity (11, 21, 23, 27) and immortalize HFKs in cooperation with E7 (21). This suggests that the role of E6 in cell immortalization can at least be partially separated from p53 degradation. In agreement with this hypothesis, E6 mutants incapable of degrading p53 can still inhibit serum- and calcium-induced differentiation of HFK cells (16, 40) and a dominant-negative mutant of p53 cannot inhibit HFK differentiation (40), transform NIH 3T3 cells, or transactivate the adenovirus E2 promoter like E6 can (39). Thus, it appears that there are critical, p53-independent functions of E6 that are required for the induction of telomerase activity and cell immortalization.
Myc is able to substitute for E6 in the immortalization of primary HFKs when expressed in combination with E7.
E6 transactivation of the hTERT promoter requires Myc as well as Myc binding sites (E boxes) on the hTERT promoter (11, 34, 43), and both Myc and E6 are present on the activated promoter (44). Since Myc has already been shown to transactivate the hTERT promoter independently, these findings suggest that E6 may be enhancing Myc-induced gene transactivation. To evaluate this possibility, we examined whether the overexpression of Myc could substitute for E6 in the immortalization of primary keratinocytes. Primary keratinocytes were transduced, as previously described (8), at passage 2 with LXSN-based retroviruses containing no insert (LXSN), Myc, or HPV-16 E6 + E7. Twenty-four hours later, the cells were selected in G418 and passed in culture twice. The LXSN and Myc lines were subsequently transduced with pBABEpuro-based retroviruses, either control or expressing HPV-16 E7. The cells were then selected in puromycin and G418 for an additional 10 days. Cells were then passed serially in vitro to assay for immortalization. When cells reached 80% confluence, they were split at a 1:4 ratio. Therefore, one split corresponds to two cell population doublings.
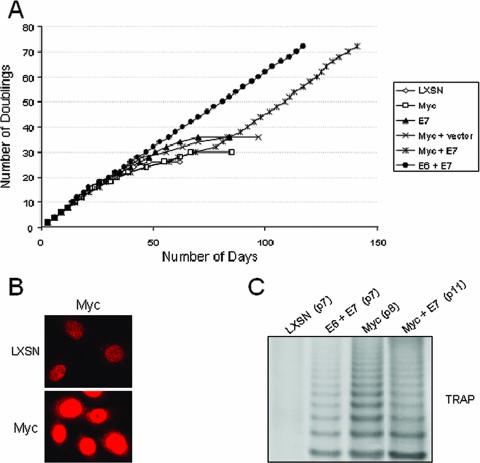
As shown in Fig. 1A, neither Myc nor E7 could independently immortalize keratinocytes, and these cells became senescent at approximately 25 to 30 population doublings. However, the combined activity of Myc and E7 allowed the keratinocytes to bypass senescence and become established as a cell line. As anticipated, the combined activity of E6 and E7 also generated a cell line but with no detectable crisis period.
FIG. 1.
Keratinocyte immortalization and telomerase activity. (A) Transduction of E7 + Myc genes immortalizes human keratinoctyes. Primary HFKs were transduced with the indicated retroviruses and selected as previously described (8). Cultures were passaged continuously in vitro as described in the text, and the number of cell doublings was calculated and plotted versus time in culture. Cultures that did not proliferate and expand in 14 days were considered senescent and were terminated at the indicated times. This experiment was repeated a second time with similar results. (B) Myc protein is overexpressed in cells transduced with the Myc retrovirus. IF microscopy was performed on early passage keratinocytes infected with LXSN control cells or Myc retroviruses (8). The anti-c-myc rabbit polyclonal antibody from Santa Cruz (N-262) was used at a 1:200 dilution, and the secondary antibody was goat anti-rabbit antibody conjugated with Texas Red (Jackson ImmunoResearch). (C) Myc induces telomerase activity in early passage cells. Telomerase activity was highly induced in HFK cells transduced with Myc or the combination of Myc + E7 utilizing the TRAP assay as previously described (43). HPV-16 E6 + E7-transduced HFKs were included as a positive control and LXSN-transduced HFKs as a negative control.
Despite undergoing senescence, cells expressing Myc alone overexpressed the transduced gene (Fig. 1B) and exhibited high levels of telomerase activities (Fig. 1C). Thus, similar to E6, Myc can induce very significant levels of telomerase at early times but cannot mediate cell immortalization. Interestingly, in contrast to the LXSN- or E7-transduced cells, the proliferation of Myc cells was accompanied by significant cell death during passaging, as evidenced by detached cells (data not shown). This was not observed with cells coexpressing Myc and E7, indicating that E7 can apparently dampen apoptotic signaling by Myc. Despite this apparent reduction in apoptosis, the coexpression of Myc and E7 was still characterized by a short but detectable crisis period that was not observed in E6 + E7 cells (Fig. 1A), hTERT + E7 cells, or E6 mutant (p53 degradation-defective) + E7 cells (data not shown).
The inability of Myc to immortalize primary HFK cells is in contrast to the findings of two recent reports that demonstrated that Myc can immortalize primary prostate and mammary epithelial cells (12, 45). However, the efficiency of immortalization was not evaluated in these studies and, at least in the case of mammary epithelial cells, there are very significant differences from HFKs in the gene requirements for immortalization. For example, the HPV E6 gene is sufficient to immortalize mammary cells but cannot immortalize HFKs (2, 3).
Analysis of immortalized cell lines.
The immortalized Myc + E7 and E6 + E7 cell lines were analyzed for expression of the appropriate transduced genes (Fig. 2). For reverse transcription-PCR (RT-PCR) analysis of mRNA expression, RNA was harvested as previously described (23). The primers used for RT-PCR were as follows: 5′-ATGTTTCAGGACCCACAGGA-3′ (forward) and 5′-CAGCTGGGTTTCTCTACGTGTT-3′ (reverse) for HPV-16 E6; 5′-ATGCATGGAGATACACCTAC-3′ (forward) and 5′-CATTAACAGGTCTTCCAAAG-3′ (reverse) for HPV-16 E7; 5′-ATGCCCCTCAACGTTAGCTTC-3′ (forward) and 5′-CTGAGACGAGGATGTTTTTGATGAAGG-3′ (reverse) for c-myc; and 5′-CTCAGACACCATGGGGAAGGTGA-3′ (forward) and 5′-ATGATCTTGAGGCTGTTGTCATA-3′ (reverse) for GAPDH (glyceraldehyde-3-phosphate dehydrogenase). Immunoprecipitations (IP) and immunoblottings (IB) were performed as described previously (8).
FIG. 2.
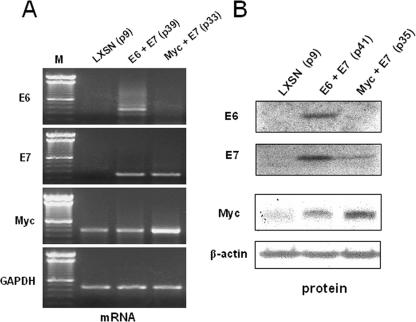
Expression of transduced genes. (A) mRNA expression of transduced genes. Immortalized cells were analyzed to verify expression of E6, E7, and Myc by RT-PCR. Total cellular RNA was isolated using TRIzol reagent, and DNA was removed using the DNA-free kit (Ambion) according to the manufacturer's protocol. RT and PCR were performed under conditions described previously (23). Lane M, molecular weight ladder. (B) Protein expression of transduced genes. The E6 protein was quantified by IP/IB of cellular lysates using the AU1 antibody (Covance) to detect the AU1-tagged E6 protein (4 μl and 1:1,000 dilution, respectively). The E7 protein was detected by IP/IB using an anti-E7 antibody from Santa Cruz (15 μl and 1:1,000 dilution, respectively). Myc protein was also quantified by direct IB, using the N-262 antibody from Santa Cruz (1:1,000 dilution). β-Actin was detected on a stripped direct IB using the anti-β-actin antibody from Sigma at a 1:10,000 dilution.
Compared to Myc expression in control LXSN cells, Myc expression in the Myc + E7 cells was clearly increased at both the mRNA level (Fig. 2A) and the protein level (Fig. 2B). In the E6 + E7 cells, there was no increase in Myc mRNA expression (Fig. 2A) and only a slight increase in Myc protein (Fig. 2B). As anticipated, only the E6 + E7 cells expressed E6 mRNA (Fig. 2A) and protein (Fig. 2B), and both the E6 + E7 and Myc + E7 cells expressed E7 mRNA (Fig. 2A) and protein (Fig. 2B). Thus, all the immortalized cells lines stably expressed the appropriate transgenes.
Analysis of hTERT expression and telomerase activity in the immortalized cell lines.
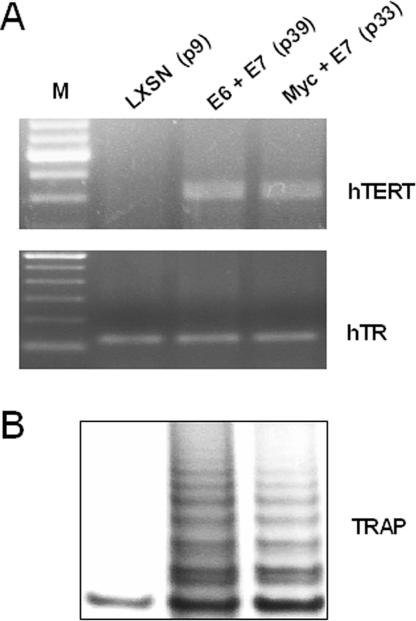
The mRNA levels of the catalytic and template subunits of telomerase were assayed by RT-PCR as previously described (23). The primer sets were as follows: 5′-GCCTGAGCTGTACTTTGTCAA-3′ (forward) and 5′-CGCAAACAGCTTGTTCTCCATGTC-3′ (reverse) for hTERT and 5′-TCTAACCCTAACTGAGAAGGGCGTAG-3′ (forward) and 5′-GTTTGCTCTAGAATGAACGGTGGAAG-3′ (reverse) for hTR. Telomerase activity was measured by a telomerase repeat amplification protocol (TRAP) assay as previously described (23). The increased telomerase activity that was observed in the cell lines at early passages (Fig. 1C) was also observed at late passages (Fig. 3B) and correlated with overexpression of cellular hTERT mRNA (Fig. 3A). There was no observable increase in the template subunit of telomerase, hTR (Fig. 3A), which is consistent with several previous studies indicating that hTERT is the rate-limiting factor for telomerase activity (19, 29, 32, 35, 42). The ability of the HPV-16 E6 protein to induce the hTERT promoter is postulated to be the result of direct E6-Myc interactions at the hTERT promoter (44). In addition, the minimal hTERT promoter exhibits an absolute requirement for the proximal E box (Myc binding site) (11, 34, 43). These findings indicate a direct requirement for Myc in the E6 induction of hTERT, even though E6 does not increase the levels of Myc itself (11, 34, 43). In addition, our data and previous data show that overexpression of Myc is sufficient to induce endogenous telomerase activity in primary HFKs (11). However, the telomerase activity (measured with a TRAP assay) of the immortalized cell lines was not proportional to the level of Myc protein. Myc + E7 cells have a higher level of Myc protein but a lower level of telomerase activity than the E6 + E7 cell line. This finding is similar to those of previous studies in which the level of Myc protein did not correlate with the level of telomerase activity (11). There are several potential explanations for the dissociation of Myc levels from telomerase activity. First of all, the TRAP and RT-PCR assays are not quantitative and are not necessarily expected to generate precise correlations. Second, there may be rate-limiting accessory proteins required for telomerase activity that are induced only in the E6 + E7 cells, not in the Myc + E7 cells. This scenario may be plausible since the levels of hTERT mRNA in the Myc + E7 and E6 + E7 cells are similar despite differences in TRAP activity. Finally, until antibodies that can reliably and quantitatively detect endogenous hTERT protein are available, we cannot be certain that the levels of hTERT mRNA correlate directly with those of hTERT protein. Despite these qualifications, it is clear that the E6, Myc, and hTERT genes are sufficient to induce telomerase activity and, in cooperation with E7, to facilitate cell immortalization.
FIG. 3.
hTERT expression in immortalized cell lines. (A) Total cellular RNA was isolated from the two immortalized cell lines, E6 + E7 and Myc + E7, as previously described (23), and the levels of hTERT and hTR mRNA were assayed by RT-PCR and compared to the LXSN control cells. Lane M, molecular weight ladder. (B) The telomerase activities of the same cells were measured using the TRAP assay as described in the legend to Fig. 1C.
Analysis of p53 protein and function in Myc + E7 and E6 + E7 cells.
While we noted previously that Myc cells exhibited significant cell death during passaging, it was also clear that Myc + E7 and E6 + E7 keratinocytes did not. The ability of E6 + E7 cells to bypass p53-dependent apoptotic pathways was explicable by the ability of E6 to bind and mediate the ubiquitin-dependent degradation of p53 (Fig. 4B). However, it was unclear how the Myc + E7 cells bypassed this cell death pathway. We therefore examined the Myc + E7 keratinocytes to determine if p53 protein might be reduced via alternative mechanisms. Surprisingly, rather than being reduced in abundance, the p53 protein was found to be significantly overexpressed in the Myc + E7 cells compared to the control LXSN cells (Fig. 4A). There was no p53 protein detectable in the E6 + E7 cells by IB as there was for the control and Myc + E7 cells at the same exposure time. It is possible that this increase is due to the known ability of E7 to stabilize the p53 protein, as mentioned earlier. In addition, Myc has been shown to increase cellular levels of p53 by the induction of Arf and p53 itself (reviewed in references 14 and 33). Most recently, we also observed that the overexpression of Myc in either HFKs or human mammary epithelial cells was able to induce p53 mRNA and protein (data not shown). It appears that the p53 protein does not need to be degraded in order to permit cell proliferation, immortalization, induction of the hTERT gene, and a consequent increase in telomerase activity.
FIG. 4.
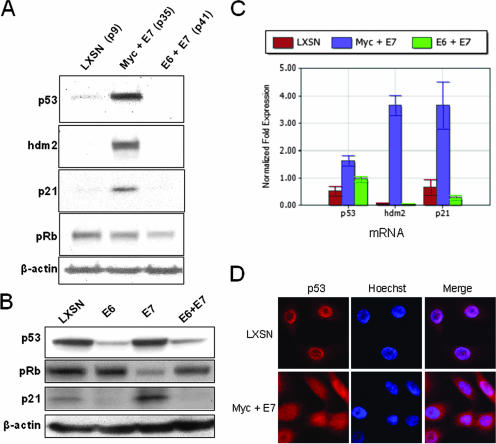
Proteins of the p53/pRb tumor suppressor pathways show differential expression in the Myc + E7 and E6 + E7 cells. (A) The Myc + E7 cells overexpress p53, hdm-2, and p21 proteins. IB analysis was used to quantify the amount of p53, hdm-2, pRb, and p21 in the immortalized keratinocytes. Primary cells transduced with the control LXSN retrovirus were included as a reference. IB for β-actin was used to standardize sample loading. Antibodies to the following proteins were used to detect the corresponding proteins: p53 (Cell Signal; 1:1,000), hdm-2 (Santa Cruz; 1:1,000), p21 (Santa Cruz; 1:1,000), pRb (Cell Signal; 1:1,000), and β-actin (Sigma; 1:10,000). Secondary antibodies were goat anti-rabbit antibody conjugated to alkaline phosphatase for p53 and pRb or goat anti-mouse antibody for hdm-2, p21, and β-actin. (B) Overexpression of p53 requires both Myc and E7. IB was used to quantify the amount of p53, pRb, and p21 proteins in HFKs stably expressing LXSN, E6, E7, or E6 + E7. β-Actin was used for normalization of the loading control. In contrast to Myc + E7 cells that markedly overexpress p53 (Fig. 4A), cells expressing only E7 contained the same amount of p53 as control LXSN cells. Again, E6 + E7 cells showed reduced p53 and p21 protein levels. (C) p53, hdm-2, and p21 mRNA levels are increased in Myc + E7 cells. These RNA levels were subjected to SYBR Green-based real-time RT-PCR on a Bio-Rad iQ5 system according to the manufacturer's instructions. GAPDH was used as an internal control, and data were analyzed using the normalized expression (ΔΔCT) method according to the manufacturer's guidelines (Bio-Rad). (D) The p53 protein is aberrantly localized in the cytoplasm of Myc + E7 cells. p53 protein was localized in the Myc + E7 and LXSN cell lines by IF microscopy. Cells were fixed and stained as described previously (8) using the p53 antibody from Cell Signal (at a 1:200 dilution). The secondary antibody was a goat anti-rabbit antibody conjugated to Texas Red (Jackson ImmunoResearch). DNA was counterstained with Hoechst dye.
Although the immortalized Myc + E7 cells contained high levels of p53 protein, there are compensatory cellular mechanisms that can functionally inactivate p53 transcriptional activity and permit continued cell proliferation. We therefore examined whether the overexpressed p53 protein was able to transactivate endogenous p53-responsive genes, such as the p21 and hdm-2 genes (reviewed in reference 14). Figure 4A shows that both the p21 and hdm-2 proteins are increased in the Myc + E7 cell line compared to both the E6 + E7 cell line and the LXSN control. In the E6 + E7 cells, there was no or very little p53 protein present; therefore, p21 and hdm-2 proteins were undetectable (Fig. 4A and B). These studies suggested that the overexpressed p53 protein was transcriptionally active, at least in part. However, to confirm that the p21 and hdm-2 protein overexpression reflected increased mRNA expression, we measured the p53, hdm-2, and p21 mRNA levels using real-time RT-PCR with SYBR Green supermix (Bio-Rad) on a Bio-Rad iQ5 system. The primer sets used were as follows: 5′-GGAGCCGCAGTCAGATCCTA-3′ and 5′-GGGGACAGAACGTTGTTTTC-3′ for p53; 5′-CTAGGAGATTTGTTTGGCGTGCC-3′ and 5′-GTCCTTTTGATCACTCCCACCTTC-3′ for hdm-2; 5′-ATGTCAGAACCGGCTGGGGA-3′ and 5′-GCCGTTTTCGACCCTGAGAG-3′ for p21; and 5′-TCTCCTCTGACTTCAACAGC-3′ and 5′-GAAATGAGCTTGACAAAGTG-3′ for GAPDH. The quantitative data were normalized by GAPDH using the expression (ΔΔCT) method of the Bio-Rad iQ5 system. Correlating with p53 protein levels, the p53 mRNA level was slightly increased in the Myc + E7 cells compared to that in the control cells (Fig. 4C). p53 mRNA was also increased in E6 + E7 cells (where p53 is degraded by E6) by a feedback mechanism in those cells. Thus, the elevated level of p53 protein in Myc + E7 cells is most likely due to a combination of increased p53 gene transcription as well as direct protein stabilization by E7. More importantly, our data demonstrate that hdm-2 and p21 mRNA levels were dramatically increased in Myc + E7 cells (Fig. 4C), suggesting that p53 is transcriptionally active in these cells. However, despite the gross overexpression of p53 in Myc + E7 cells and the induction of endogenous p53 target genes, we noted that there was a twofold reduction in p53 transcriptional activity when measured with an exogenous reporter construct, compared to the control cells (data not shown). While measurement of transcriptional activity using exogenous constructs can sometimes be misleading, we speculated that it might be possible that the cell was utilizing alternative methods to downregulate p53 function.
One method to inactivate p53 is by the overexpression of the hdm-2 protein (Fig. 4A), which is known to bind and sequester p53 in the cytoplasm (reviewed in references 6 and 28). To determine if the p53 protein might be mislocalized in the Myc + E7 cell line, we used immunofluorescence (IF) microscopy (Fig. 4D). The fluorescent signal with p53 antibodies was clearly increased in the Myc + E7 cells compared to the LXSN cells, corroborating the results with IB (Fig. 4A). More importantly, there was also a significant difference in intracellular localization. Myc + E7 cells exhibited strong cytoplasmic fluorescence as well as nuclear fluorescence in contrast to the typical nuclear localization observed in LXSN cells. The cytoplasmic localization of p53 is best observed with merged images of Myc + E7 cells stained for p53 and DNA.
We also examined the level of pRb in the cell lines and, as shown in Fig. 4A, the level of pRb was decreased (but only moderately) in both cell lines containing E7 (Fig. 4A). In these two cell lines, the level of pRb corresponds with the level of E7 (Fig. 2B) due to the known ability of E7 to facilitate the degradation of pRb (9). From a theoretical viewpoint, it is somewhat perplexing why E7 is required to be expressed simultaneously with Myc for cell immortalization. For example, it has been shown that hdm-2 (which is overexpressed in the Myc + E7 cells) can bind and disrupt the function of pRb (48) as well as bind to and activate the E2f1/DP1 transcription factor complex (25), making the need for E7 appear somewhat redundant. However, it is very possible that the pRb-independent ability of E7 to abolish p21-mediated cell arrest via a direct interaction or mislocalization of the p21 protein (10, 17, 47) is critical for cell immortalization. Further analysis of the Myc + E7 and E6 + E7 cells may uncover additional functions of E7 which are pRb independent.
In summary, it is apparent that immortalization of HFK cells by E6 or Myc requires the coexpression of E7. In the case of Myc + E7 cells, immortalization occurs in the presence of high levels of p53, which is functionally active, at least in part. This finding (immortalization in the presence of p53) has been strengthened by two independent experiments with hTERT + E7 and E6 mutant + E7 genes in primary HFKs (data not shown). This phenomenon has also been reported for E6 mutants which are defective for p53 degradation in epithelial cells (20, 21), although these investigations did not pursue secondary changes in p53 function. Regardless, it appears that a critical activity of E6 is not only to degrade p53 but also to transactivate Myc-responsive genes such as the hTERT gene.
Acknowledgments
This work was supported by NIH grants R01CA106440 and R01CA53371 to R.S.
Footnotes
Published ahead of print on 5 September 2007.
REFERENCES
- 1.Baege, A. C., A. Berger, R. Schlegel, T. Veldman, and R. Schlegel. 2002. Cervical epithelial cells transduced with the papillomavirus E6/E7 oncogenes maintain stable levels of oncoprotein expression but exhibit progressive, major increases in hTERT gene expression and telomerase activity. Am. J. Pathol. 160:1251-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Band, V., S. Dalal, L. Delmolino, and E. J. Androphy. 1993. Enhanced degradation of p53 protein in HPV-6 and BPV-1 E6-immortalized human mammary epithelial cells. EMBO J. 12:1847-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Band, V., J. A. De Caprio, L. Delmolino, V. Kulesa, and R. Sager. 1991. Loss of p53 protein in human papillomavirus type 16 E6-immortalized human mammary epithelial cells. J. Virol. 65:6671-6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basile, J. R., V. Zacny, and K. Munger. 2001. The cytokines tumor necrosis factor-α (TNF-α) and TNF-related apoptosis-inducing ligand differentially modulate proliferation and apoptotic pathways in human keratinocytes expressing the human papillomavirus-16 E7 oncoprotein. J. Biol. Chem. 276:22522-22528. [DOI] [PubMed] [Google Scholar]
- 5.Berger, A. J., A. Baege, T. Guillemette, J. Deeds, R. Meyer, G. Disbrow, R. Schlegel, and R. Schlegel. 2002. Insulin-like growth factor-binding protein 3 expression increases during immortalization of cervical keratinocytes by human papillomavirus type 16 E6 and E7 proteins. Am. J. Pathol. 161:603-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deb, S. P. 2003. Cell cycle regulatory functions of the human oncoprotein HDM-2. Mol. Cancer Res. 1:1009-1016. [PubMed] [Google Scholar]
- 7.Demers, G. W., C. L. Halbert, and D. A. Galloway. 1994. Elevated wild-type p53 protein levels in human epithelial cell lines immortalized by the human papillomavirus type 16 E7 gene. Virology 198:169-174. [DOI] [PubMed] [Google Scholar]
- 8.Disbrow, G. L., I. Sunitha, C. C. Baker, J. Hanover, and R. Schlegel. 2003. Codon optimization of the HPV-16 E5 gene enhances protein expression. Virology 311:105-114. [DOI] [PubMed] [Google Scholar]
- 9.Dyson, N., P. M. Howley, K. Munger, and E. Harlow. 1989. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243:934-937. [DOI] [PubMed] [Google Scholar]
- 10.Funk, J. O., S. Waga, J. B. Harry, E. Espling, B. Stillman, and D. A. Galloway. 1997. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 11:2090-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gewin, L., and D. A. Galloway. 2001. E box-dependent activation of telomerase by human papillomavirus type 16 E6 does not require induction of c-myc. J. Virol. 75:7198-7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gil, J., P. Kerai, M. Lleonart, D. Bernard, J. C. Cigudosa, G. Peters, A. Carnero, and D. Beach. 2005. Immortalization of primary human prostate epithelial cells by c-Myc. Cancer Res. 65:2179-2185. [DOI] [PubMed] [Google Scholar]
- 13.Glaunsinger, B. A., S. S. Lee, M. Thomas, L. Banks, and R. Javier. 2000. Interactions of the PDZ-protein MAGI-1 with adenovirus E4-ORF1 and high-risk papillomavirus E6 oncoproteins. Oncogene 19:5270-5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris, S. L., and A. J. Levine. 2005. The p53 pathway: positive and negative feedback loops. Oncogene 24:2899-2908. [DOI] [PubMed] [Google Scholar]
- 15.Hawley-Nelson, P., K. H. Vousden, N. L. Hubbert, D. R. Lowy, and J. T. Schiller. 1989. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 8:3905-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue, T., K. Oka, H. Yong-Il, K. H. Vousden, S. Kyo, P. Jing, A. Hakura, and M. Yutsudo. 1998. Dispensability of p53 degradation for tumorigenicity and decreased serum requirement of human papillomavirus type 16 E6. Mol. Carcinog. 21:215-222. [PubMed] [Google Scholar]
- 17.Jones, D. L., R. M. Alani, and K. Munger. 1997. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 11:2101-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones, D. L., D. A. Thompson, and K. Munger. 1997. Destabilization of the RB tumor suppressor protein and stabilization of p53 contribute to HPV type 16 E7-induced apoptosis. Virology 239:97-107. [DOI] [PubMed] [Google Scholar]
- 19.Kanaya, T., S. Kyo, M. Takakura, H. Ito, M. Namiki, and M. Inoue. 1998. hTERT is a critical determinant of telomerase activity in renal-cell carcinoma. Int. J. Cancer 78:539-543. [DOI] [PubMed] [Google Scholar]
- 20.Kiyono, T., S. A. Foster, J. I. Koop, J. K. McDougall, D. A. Galloway, and A. J. Klingelhutz. 1998. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396:84-88. [DOI] [PubMed] [Google Scholar]
- 21.Klingelhutz, A. J., S. A. Foster, and J. K. McDougall. 1996. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature 380:79-82. [DOI] [PubMed] [Google Scholar]
- 22.Lee, S. S., B. Glaunsinger, F. Mantovani, L. Banks, and R. T. Javier. 2000. Multi-PDZ domain protein MUPP1 is a cellular target for both adenovirus E4-ORF1 and high-risk papillomavirus type 18 E6 oncoproteins. J. Virol. 74:9680-9693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, X., H. Yuan, B. Fu, G. L. Disbrow, T. Apolinario, V. Tomaic, M. L. Kelley, C. C. Baker, J. Huibregtse, and R. Schlegel. 2005. The E6AP ubiquitin ligase is required for transactivation of the hTERT promoter by the human papillomavirus E6 oncoprotein. J. Biol. Chem. 280:10807-10816. [DOI] [PubMed] [Google Scholar]
- 24.Liu, Y., J. J. Chen, Q. Gao, S. Dalal, Y. Hong, C. P. Mansur, V. Band, and E. J. Androphy. 1999. Multiple functions of human papillomavirus type 16 E6 contribute to the immortalization of mammary epithelial cells. J. Virol. 73:7297-7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin, K., D. Trouche, C. Hagemeier, T. S. Sorensen, N. B. La Thangue, and T. Kouzarides. 1995. Stimulation of E2F1/DP1 transcriptional activity by HDM-2 oncoprotein. Nature 375:691-694. [DOI] [PubMed] [Google Scholar]
- 26.Massimi, P., N. Gammoh, M. Thomas, and L. Banks. 2004. HPV E6 specifically targets different cellular pools of its PDZ domain-containing tumour suppressor substrates for proteasome-mediated degradation. Oncogene 23:8033-8039. [DOI] [PubMed] [Google Scholar]
- 27.McMurray, H. R., and D. J. McCance. 2004. Degradation of p53, not telomerase activation, by E6 is required for bypass of crisis and immortalization by human papillomavirus type 16 E6/E7. J. Virol. 78:5698-5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meek, D. W., and U. Knippschild. 2003. Posttranslational modification of HDM-2. Mol. Cancer Res. 1:1017-1026. [PubMed] [Google Scholar]
- 29.Meyerson, M., C. M. Counter, E. N. Eaton, L. W. Ellisen, P. Steiner, S. D. Caddle, L. Ziaugra, R. L. Beijersbergen, M. J. Davidoff, Q. Liu, S. Bacchetti, D. A. Haber, and R. A. Weinberg. 1997. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell 90:785-795. [DOI] [PubMed] [Google Scholar]
- 30.Münger, K., W. C. Phelps, V. Bubb, P. M. Howley, and R. Schlegel. 1989. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J. Virol. 63:4417-4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Münger, K., B. A. Werness, N. Dyson, W. C. Phelps, E. Harlow, and P. M. Howley. 1989. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 8:4099-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura, T. M., G. B. Morin, K. B. Chapman, S. L. Weinrich, W. H. Andrews, J. Lingner, C. B. Harley, and T. R. Cech. 1997. Telomerase catalytic subunit homologs from fission yeast and human. Science 277:955-959. [DOI] [PubMed] [Google Scholar]
- 33.Nilsson, J. A., and J. L. Cleveland. 2003. Myc pathways provoking cell suicide and cancer. Oncogene 22:9007-9021. [DOI] [PubMed] [Google Scholar]
- 34.Oh, S. T., S. Kyo, and L. A. Laimins. 2001. Telomerase activation by human papillomavirus type 16 E6 protein: induction of human telomerase reverse transcriptase expression through Myc and GC-rich Sp1 binding sites. J. Virol. 75:5559-5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramakrishnan, S., U. Eppenberger, H. Mueller, Y. Shinkai, and R. Narayanan. 1998. Expression profile of the putative catalytic subunit of the telomerase gene. Cancer Res. 58:622-625. [PubMed] [Google Scholar]
- 36.Scheffner, M., J. M. Huibregtse, R. D. Vierstra, and P. M. Howley. 1993. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75:495-505. [DOI] [PubMed] [Google Scholar]
- 37.Scheffner, M., B. A. Werness, J. M. Huibregtse, A. J. Levine, and P. M. Howley. 1990. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63:1129-1136. [DOI] [PubMed] [Google Scholar]
- 38.Seavey, S. E., M. Holubar, L. J. Saucedo, and M. E. Perry. 1999. The E7 oncoprotein of human papillomavirus type 16 stabilizes p53 through a mechanism independent of p19ARF. J. Virol. 73:7590-7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sedman, S. A., N. L. Hubbert, W. C. Vass, D. R. Lowy, and J. T. Schiller. 1992. Mutant p53 can substitute for human papillomavirus type 16 E6 in immortalization of human keratinocytes but does not have E6-associated trans-activation or transforming activity. J. Virol. 66:4201-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherman, L., A. Jackman, H. Itzhaki, M. C. Stoppler, D. Koval, and R. Schlegel. 1997. Inhibition of serum- and calcium-induced differentiation of human keratinocytes by HPV16 E6 oncoprotein: role of p53 inactivation. Virology 237:296-306. [DOI] [PubMed] [Google Scholar]
- 41.Stoppler, H., M. C. Stoppler, E. Johnson, C. M. Simbulan-Rosenthal, M. E. Smulson, S. Iyer, D. S. Rosenthal, and R. Schlegel. 1998. The E7 protein of human papillomavirus type 16 sensitizes primary human keratinocytes to apoptosis. Oncogene 17:1207-1214. [DOI] [PubMed] [Google Scholar]
- 42.Takakura, M., S. Kyo, T. Kanaya, M. Tanaka, and M. Inoue. 1998. Expression of human telomerase subunits and correlation with telomerase activity in cervical cancer. Cancer Res. 58:1558-1561. [PubMed] [Google Scholar]
- 43.Veldman, T., I. Horikawa, J. C. Barrett, and R. Schlegel. 2001. Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein. J. Virol. 75:4467-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veldman, T., X. Liu, H. Yuan, and R. Schlegel. 2003. Human papillomavirus E6 and Myc proteins associate in vivo and bind to and cooperatively activate the telomerase reverse transcriptase promoter. Proc. Natl. Acad. Sci. USA 100:8211-8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, J., L. Y. Xie, S. Allan, D. Beach, and G. J. Hannon. 1998. Myc activates telomerase. Genes Dev. 12:1769-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Werness, B. A., A. J. Levine, and P. M. Howley. 1990. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248:76-79. [DOI] [PubMed] [Google Scholar]
- 47.Westbrook, T. F., D. X. Nguyen, B. R. Thrash, and D. J. McCance. 2002. E7 abolishes Raf-induced arrest via mislocalization of p21Cip1. Mol. Cell. Biol. 22:7041-7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao, Z. X., J. Chen, A. J. Levine, N. Modjtahedi, J. Xing, W. R. Sellers, and D. M. Livingston. 1995. Interaction between the retinoblastoma protein and the oncoprotein MDM2. Nature 375:694-698. [DOI] [PubMed] [Google Scholar]
- 49.zur Hausen, H. 2001. Cervical carcinoma and human papillomavirus: on the road to preventing a major human cancer. J. Natl. Cancer Inst. 93:252-253. [DOI] [PubMed] [Google Scholar]
- 50.zur Hausen, H. 1990. The role of papillomaviruses in anogenital cancer. Scand. J. Infect. Dis. Suppl. 69:107-111. [PubMed] [Google Scholar]
- 51.zur Hausen, H. 1991. Viruses in human cancers. Science 254:1167-1173. [DOI] [PubMed] [Google Scholar]