Abstract
The development of effective therapies for noroviral gastroenteritis has been hampered by the absence of a cell culture system. Recently, we reported the generation of Norwalk virus (NV) replicon-bearing cells in BHK21 and Huh-7 cells and demonstrated that alpha interferon (IFN-α) effectively inhibited the replication of NV in these cells. In continuing studies for screening potential antinoroviral agents, we tested IFN-γ and ribavirin for their effects on NV replication in the cells. Like IFN-α, IFN-γ inhibited the replication of NV in the replicon-bearing cells, showing the reduction of the NV genome and proteins in a dose-dependent manner. The effective dose for reducing 50% (ED50) of the NV genome and protein was calculated to be approximately 40 units/ml. When ribavirin was applied to the cells, it effectively reduced the NV genome and protein with the ED50 calculated as approximately 40 μM. The combination of IFN-α and ribavirin showed additive effects on the inhibition of NV replication. With the addition of guanosine to the ribavirin treatment, moderately reversed antiviral effects were observed, suggesting that the ribavirin effect may be associated with the depletion of GTP in the cells. Sequencing analysis of the conserved polymerase regions of NV in the ribavirin-treated (100 μM) and nontreated groups showed that the mutation rates were similar and indicated that ribavirin did not induce catastrophic mutations. The NV replicon-bearing cells provide an excellent tool for screening potential antinoroviral agents, and our results indicated that IFNs and ribavirin may be good therapeutic options for noroviral gastroenteritis.
Caliciviruses are positive-strand RNA viruses in the family Caliciviridae that consists of four genera, Norovirus, Sapovirus, Lagovirus, and Vesivirus (8). Caliciviruses are important pathogens in humans and animals and encompass a wide variety of pathogenicities ranging from gastroenteritis to systemic infections (8). Viruses in genera Vesivirus and Lagovirus include animal viruses, such as vesicular exanthema swine virus, feline calicivirus, and rabbit hemorrhagic disease virus. Viruses in the genera Norovirus and Sapovirus cause gastroenteritis in humans and animals and are called enteric caliciviruses (9). Recent studies estimate that human enteric caliciviruses are responsible for more than 90% of nonbacterial gastroenteritis outbreaks (6) and as many as 23 million cases of gastroenteritis in the United States each year (17). Norwalk virus (NV) is the prototype strain of the noroviruses and was associated with an outbreak of gastroenteritis in Norwalk, Ohio, in 1968 (13). Studies of the replication of human enteric caliciviruses have been severely hampered by the absence of a cell culture system (5). Among the noroviruses, only murine noroviruses, including murine norovirus 1 (MNV-1) (14) has been successfully propagated in cell culture (27). Murine noroviruses present widely in laboratory mouse colonies without apparent clinical symptoms (10, 28). Interestingly, MNV-1 has a tissue tropism of macrophage-like cells in vivo and in vitro, but it is not clear at present whether human noroviruses target such cells. Recently, we reported the generation of NV replicon-bearing cells in BHK21 and Huh-7 cells and demonstrated that alpha interferon (IFN-α) effectively inhibited the replication of NV in these cells (3). Replicon-bearing cells were generated by transfecting RNA transcripts derived from a plasmid containing the full-length NV genome and neomycin-resistant gene (neomycin phosphotransferase II [NPT II]) in the place of the VP1 region (pNV-Neo) (3). The replicon-bearing cells provide an excellent tool to study the replication of noroviruses and serve as a platform to screen potential antiviral drugs. Here, we report that IFN-γ and ribavirin also effectively inhibited the replication of NV in the replicon-bearing cells. Ribavirin (1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide) is a synthetic guanosine analogue and has shown antiviral actions against a range of DNA and RNA viruses, including hepatitis C virus (HCV) and respiratory syncytial virus (7, 24). The combination therapy of IFN-α and ribavirin is the most effective treatment for chronic HCV infections. Similar studies were done looking at the antiviral effects of IFNs, ribavirin, and other specific viral inhibitors in HCV replicon-bearing cells (1, 20). IFN-γ is synthesized by various cells, including NK cells during acute inflammations, and plays an essential role as a frontier fighter against the invading microbes before specific immune responses are elicited (23). However, many viruses have developed mechanisms against the IFN system to avoid its antiviral effects, including blocking STAT (signal transducers and activators of transcription) pathways and inhibiting the synthesis of IFNs by viral proteins or genome (23). In our previous report, using the replicon-bearing cells, we demonstrated that the NV replicon lacked such an anti-IFN mechanism, which may be a reason for the high sensitivity to IFN-α treatment (3). This is the first report that IFNs and ribavirin could potentially be excellent antiviral drugs against norovirus replication. In addition, this report confirms that NV replicon-bearing cells may be a significant tool for studying basic research and drug discovery relevant to the control of norovirus gastroenteritis.
MATERIALS AND METHODS
Cells, viruses, and reagents.
The Huh-7, HG23 (Huh-7-based NV replicon-bearing cells), G3 (BHK21-based NV replicon-bearing cells), and murine macrophage-like RAW267.4 cells were maintained in Dulbecco's minimal essential medium containing 10% fetal bovine serum and antibiotics (chlortetracycline [25 μg/ml], penicillin [250 U/ml], and streptomycin [250 μg/ml]). MNV-1 was provided by H. Virgin (Washington University, St. Louis, MO), and maintained in RAW267.4 cells. Recombinant IFN type I (human IFN-αA-IFN-αD fusion protein) and recombinant human IFN-γ were purchased from Serotec Inc. (Raleigh, NC). The polyclonal antibody specific to NV proteinase-polymerase (ProPol) was described in a previous report (3). Antibodies specific for NPT II or β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) or Cell Signaling Technology (Danvers, MA), respectively. Ribavirin and mycophenolic acid (MPA) were purchased from Sigma (St. Louis, MO) and dissolved in distilled water or dimethyl sulfoxide (DMSO), respectively.
Detection of Norwalk virus RNA and proteins. (i) Immunofluorescence assay (IFA).
The NV ProPol serum was added to methanol-fixed monolayers of cells, and the binding of antibodies was detected with fluorescein isothiocyanate (FITC)-conjugated, affinity-purified goat antibodies to guinea pig immunoglobulin G (IgG) (ICN Biomedicals, Aurora, OH) as described previously (2). We also used rabbit antiserum specific for NPT II and FITC-conjugated goat antibodies to rabbit IgG to detect the presence of the NV replicon in cells.
(ii) Western blot analysis.
Protein samples of Huh-7 and HG23 cells (subjected to various treatments or not treated) were prepared in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer containing 1% β-mercaptoethanol and sonicated for 20 s. The proteins were resolved in a 10% Novex Tris-Bis gel (Invitrogen) and transferred to a nitrocellulose membrane. The membranes were probed with guinea pig antibodies specific for the ProPol protein, and the binding of the antibodies was detected with peroxidase-conjugated, goat anti-guinea pig IgG (Sigma). In addition, membranes were probed with rabbit antiserum specific for β-actin and peroxidase-conjugated, goat anti-rabbit IgG as a loading control. Following incubation with a chemiluminescent substrate (SuperSignal West Pico chemiluminescent substrate; Pierce Biotechnology, Rockford, IL), signals were detected with X-ray film. We also used rabbit antiserum specific for NPT II and peroxidase-conjugated goat antibodies to rabbit IgG to detect the presence of the NV replicon in cells.
(iii) Real-time qRT-PCR.
The quantity of NV genome in the replicon-bearing cells was measured by real-time quantitative reverse transcription-PCR (qRT-PCR) with the One-step Platinum qRT-PCR kit (Invitrogen, Carlsbad, CA), following an established protocol with genotype I-specific primers and 6-carboxyfluorescein-labeled G1 probes (11). For quantity control of cellular RNA, qRT-PCR for β-actin was performed as described previously (25). For qRT-PCR, total RNA from cells (in six-well plates) was extracted with the RNeasy kit (QIAGEN, Valencia, CA). A standard concentration curve was generated with serial dilutions of RNA transcripts derived from pNV-Neo in each experiment to calculate the total number of genome copies present. The relative genome levels in cells subjected to various treatments were calculated after the RNA levels were normalized with those of β-actin.
Treatment of NV-harboring cells with IFN-α or IFN-γ.
The effects of IFN-α and IFN-γ on the replication of NV in the replicon-bearing cells were examined at concentrations ranging from 0.1 to 20 units/ml for IFN-α and from 1 to 200 units/ml for IFN-γ. Various concentrations of IFN-α or IFN-γ were added to 1-day-old, 80 to 90% confluent HG23 cells which were analyzed for viral protein and genome expression 24, 48, 72, or 96 h after treatment. The levels of expression of the NV protein were examined by IFA and Western blot analysis and genome expression levels by qRT-PCR as described above. Western blot analysis included the detection of β-actin as a loading control. The inhibitory effect of IFN-α or IFN-γ on the NV replicon was calculated as the concentration of IFN-α or IFN-γ that resulted in 50% reduction of NV genome (ED50) as detected by qRT-PCR. Similarly, we also applied IFN-γ to G3 cells to examine its effects on NV replication.
Effect of ribavirin on replication of NV in replicon-bearing cells.
One-day-old, 80 to 90% confluent HG23 cells were treated with various concentrations of ribavirin (0 [mock medium] to 200 μM) to examine its effects on the replication of NV. At the desired time points, the NV protein or genome were analyzed by IFA and Western blot analysis or by qRT-PCR, respectively. To examine the combined effects of ribavirin and IFN-α, 1-day-old HG23 cells were treated with 2 units/ml (ED50) of IFN-α and various concentrations of ribavirin (0 to 100 μM) with the reduction of NV protein and genome compared to that of ribavirin treatment alone. Because ribavirin causes nonspecific, cytotoxic effects, such as cytostatic action on various cells (18), we monitored the cytotoxic effects on HG23 cells using a cell cytotoxicity assay kit (Promega, Madison, WI) to calculate the maximum concentration with minimum cytotoxic effects.
Effect of ribavirin on the replication of MNV-1 in RAW267.4 cells.
Because MNV-1 can be cultured in the murine macrophage-like cell line RAW267.4, we used MNV-1 as a surrogate system to examine the effect of ribavirin on norovirus replication in cells. Confluent RAW267.4 cells in six-well plates were treated with various concentrations (0 to 200 μM) of ribavirin for 6 h before MNV-1 was added to the same medium at a multiplicity of infection (MOI) of 5. Virus-infected cells were then incubated for an additional 24 and 48 h. After the plates were frozen and thawed three times, the replication of MNV-1 in the presence of ribavirin was measured by the 50% tissue culture infective dose (TCID50) assay. The nonspecific cytotoxic effects in RAW267.4 cells by ribavirin were monitored by the method described above.
Potential mechanisms of ribavirin on the replication of NV.
Several mechanisms of action for the antiviral effects of ribavirin have been suggested, including the depletion of intracellular GTP through the inhibition of the cellular IMP dehydrogenase (IMPDH) and triggering catastrophic mutations on the virus genome. In order to examine these potential mechanisms, we performed two experiments: (i) supplementation of guanosine in the medium with ribavirin treatment, and (ii) sequence analysis of the NV genome in the presence or absence of ribavirin. First, HG23 cells were preincubated with 100 μM of guanosine for 6 h before ribavirin was added to the medium at various final concentrations (0 to 100 μM). After 24 or 48 h of the treatment, the NV protein or genome was analyzed with Western blot analysis or real-time qRT-PCR, respectively, as described above. We also tested MPA, which is a potent inhibitor of the cellular IMP dehydrogenase, on the expression of the NV genome in the replicon-bearing cells. Various concentrations of MPA, ranging from 0 (mock solvent [DMSO]) to 10 μM, were added to the medium of semiconfluent HG23 cells for 48 h, and total RNA was extracted for real-time qRT-PCR. The relative levels of expression of the NV genome affected by MPA were compared to those of the mock (DMSO) treatment. To examine whether ribavirin treatment triggered catastrophic mutagenesis, HG23 cells were incubated with ribavirin (100 μM) or without ribavirin for 48 h. After the incubation, total RNA was extracted, and RT-PCR was performed to amplify the region encoding NV Pol (region corresponding to base numbers 3695 to 4144) with primers NV-Pol-F (GATCTTGGCACTATACCGG) and NV-Pol-R (GGTATCCATTGTCTGTTC). Sequence analysis of the amplicon product was done directly or after the product was cloned into the pCR2.1 vector (Invitrogen). For sequencing of the gene in the recombinant vector, we selected 18 clones per group and analyzed mutations in the region. Sequence analysis was performed using the GenomeLab DTCS-Quick Start kit (Beckman-Coulter, Fullerton, CA). Sequences were resolved on a CEQ 8000 genetic analysis system (Beckman-Coulter).
Statistical analysis.
The effects of IFNs, ribavirin, MPA, and the combination of IFN-α and ribavirin on NV or MNV-1 replication were analyzed by Student's t test. Results were considered statistically significant when the P value was <0.05.
RESULTS
Effects of IFN-α and IFN-γ on the NV replicon.
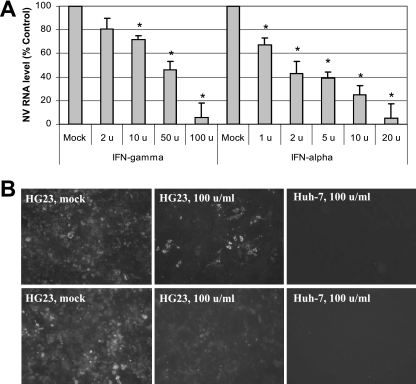
Previously, we reported that IFN-α effectively reduced the expression of NV proteins and the NV genome. The effective dose necessary for IFN-α to reduce NV protein (ProPol) and genome copies in HG23 cells to 50% of that observed in the nontreated (mock) control at 72 h was calculated to be approximately 2 units/ml. Similarly, we examined the effect of IFN-γ on the replication of NV in the cells. HG23 cells were treated with increasing concentrations of human IFN-γ (up to 200 units/ml), and its effect on NV genome and protein expression was monitored by real-time qRT-PCR and by IFA and Western blot analysis, respectively. Like IFN-α, the addition of IFN-γ to HG23 cells inhibited NV genome and protein expression in a dose-dependent manner (Fig. 1), while the cells themselves showed no toxic effects from treatment (data not shown). The presence of 100 units/ml of IFN-γ for 72 h resulted in approximately 90% clearance of the replicon proteins and RNA (Fig. 1A and B). The average reduction rates (as a percentage of the rate of the control) of the NV genome in the presence of IFN-γ were 81%, 73%, 43%, and 8% at 2, 10, 50, and 100 units/ml, respectively, for 72 h of incubation (Fig. 1A). The ED50 of IFN-γ for reducing NV protein (ProPol) and genome copies in HG23 cells at 72 h was calculated to be approximately 40 units/ml (Fig. 1). However, the recombinant human IFN-γ did not show any effects on the expression of NV proteins and genome in the BHK21-based NV replicon-bearing cells (G3 cells) (data not shown). This was also confirmed by a promoter-luciferase assay for the IFN-γ response element. When we transfected a plasmid expressing luciferase under the control of an IFN-γ response element (pGAS-luc; Clontech) into HG23 and G3 cells and incubated the cells with recombinant human IFN-γ (100 units/ml) for 18 h, we observed the increased expression of luciferase only in HG23 cells. Evaluation of NV replication was examined by measuring the expression of NPT II through IFA and Western blot analysis using an antibody against NPT II. The expression of NPT II, evaluated by IFA, correlated well with that of NV ProPol (Fig. 1B), indicating that the commercial antibody could be a good tool to study the expression of NV replication in NV replicon-bearing cells.
FIG. 1.
Effects of IFN-α and IFN-γ on NV replication in HG23 cells. (A) Effects of IFN-α and IFN-γ on the expression of NV genome. One-day-old semiconfluent NV replicon-bearing HG23 cells were incubated with various concentrations (units/ml) of IFN-α and IFN-γ for 72 h, and then total RNA was prepared for real-time qRT-PCR to detect the NV genome. The reduction of NV genome by IFNs was calculated by the comparison to that with mock (medium) treatment. Error bars represent standard deviations from at least three independent experiments. RNA levels for cells subjected to a treatment that were significantly reduced (P < 0.05) compared to the RNA level of mock-treated cells are indicated by asterisks. (B) Effect of IFN-γ on the expression of NV ProPol or neomycin phosphotransferase in HG23 cells. One-day-old semiconfluent HG23 cells were incubated with 0 (mock) or 100 units/ml of IFN-γ for 72 h. The cells were then fixed with 100% methanol and stained with antibodies to NV ProPol (top panels) or neomycin phosphotransferase II (bottom panels) and FITC-conjugated secondary antibody. The negative control includes parental Huh-7 cells treated with 100 units/ml of IFN-γ with the same staining.
Effect of ribavirin on NV replication.
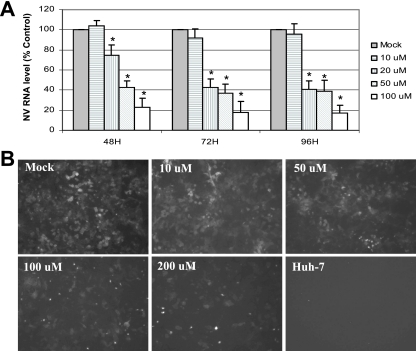
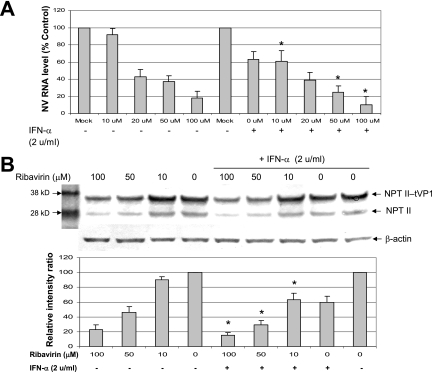
We used a known antiviral agent, ribavirin, to measure its effect on the NV replicon in HG23 cells. HG23 cells were treated with increasing concentrations of ribavirin (up to 200 μM) for up to 96 h, and its effect on NV protein expression was monitored by IFA and Western blot analysis using antibodies to NV ProPol and NPT II. In addition, a qRT-PCR assay was used to detect the NV genome in mock-treated or ribavirin-treated NV replicon-bearing cells. In HG23 cells treated with ribavirin up to 200 μM, cell viability was observed above 90% for up to 96 h. The treatment of ribavirin at concentrations above 10 μM in HG23 cells reduced the expression of NV genome in a dose-dependent manner (Fig. 2A). The reduction of NV genome was observed at 24 h of treatment and continued for 72 h. The ED50 was calculated to be approximately 40 μM after 72 h of ribavirin treatment (Fig. 2A). Like the NV genome, the expression of NV ProPol and NPT II was reduced in the presence of ribavirin in a dose-dependent manner (Fig. 2B and 3B). Because the NPT II gene was fused to the first 38 amino acids of NV VP1 in pNV-Neo (3), the expression of NPT II was observed as two forms: NPT II fused to truncated VP1 (NPT II-tVP1) and NPT II alone (Fig. 3B). The HG23 cells were incubated with ribavirin and IFN-α (2 unit/ml) to examine any enhanced effects by the cotreatment. Treatment with IFN-α alone at a concentration of 2 units/ml reduced the expression of NPT II and NV genome to about 50% (Fig. 3A and B). Ribavirin treatment showed reduction of the NV genome to 43%, 35%, and 18% of mock-treated cells at 20 μM, 50 μM, and 100 μM, respectively, while cotreatment with IFN-α (2 units/ml) at the same concentrations of ribavirin resulted in reduction to 38%, 28%, and 10% (Fig. 3A). In addition, similar reduction rates of NPT II in Western blot analysis were observed by cotreatment (Fig. 3B). These results indicated that there was an additive effect by the cotreatment in HG23 cells.
FIG. 2.
Effect of ribavirin on NV replication in HG23 cells. (A) Effect of ribavirin on the expression of the NV genome. One-day-old semiconfluent HG23 cells were incubated with various concentrations of ribavirin ranging from 0 (mock) to 100 μM for 48 h, 72 h, and 96 h, and then total RNA was prepared for real-time qRT-PCR to detect the NV genome. The reduction of NV genome by IFNs was calculated by comparison to that with mock treatment. RNA levels for cells subjected to a treatment that were significantly reduced (P < 0.05) compared to the RNA level of mock-treated cells are indicated by asterisks. Error bars represent standard deviations from at least three independent experiments. (B) Effect of ribavirin on the expression of neomycin phosphotransferase II in HG23 cells. One-day-old semiconfluent HG23 cells were incubated with various concentrations (0 [mock], 10, 50, 100, or 200 μM) of ribavirin for 72 h. The cells were then fixed with 100% methanol and stained with antibody to neomycin phosphotransferase II and FITC-conjugated secondary antibody. The negative control includes parental Huh-7 cells with the same staining.
FIG. 3.
Effect of the combination of IFN-α and ribavirin on NV replication in HG23 cells. (A) Effect of ribavirin alone or IFN-α and ribavirin on the expression of the NV genome. One-day-old semiconfluent HG23 cells were incubated with various concentrations of ribavirin ranging from 0 (mock) to 100 μM with (+) or without (−) 2 units/ml of IFN-α. After 72 h of incubation, total RNA was prepared for real-time qRT-PCR to detect the NV genome. The reduction of NV genome by the different treatments was calculated by comparison to the NV RNA level of the control (mock treated). Error bars represent standard deviations from at least three independent experiments. The positions of molecular mass markers (in kilodaltons) are shown to the left of the blot. (B) Effect of ribavirin alone or IFN-α and ribavirin on the expression of NPT II detected by Western blot analysis. One-day-old semiconfluent HG23 cells were incubated with various concentrations of ribavirin ranging from 0 (mock) to 100 μM with or without 2 units/ml of IFN-α. After 72 h of incubation, cell lysate was prepared for Western blot analysis using antibody to neomycin phosphotransferase II. The bar graph shows relative values (the value of mock-treated cells was set at 100%) of expression of NPT II (NPT II-tVP1) in cells treated with ribavirin alone or with ribavirin and IFN-α. The intensity of each band in the blots was measured by scanning and represented the ratio of β-actin to NPT II-tVP1. RNA levels or NPT II levels for cells treated with both IFN-α and ribavirin that were significantly different (P < 0.05) from the value for cells treated with ribavirin only are indicated by asterisks.
Effect of ribavirin on the growth of MNV-1.
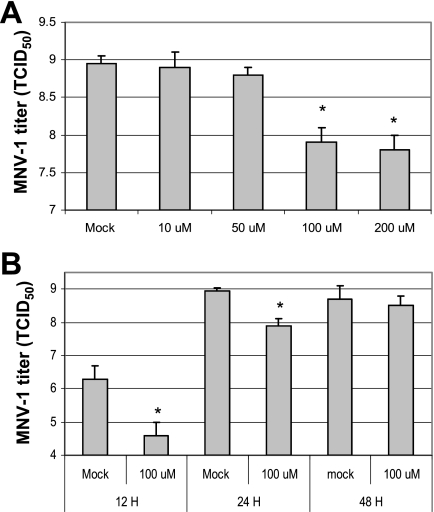
Murine norovirus 1 can be cultured in RAW267.4 cells, so we sought to observe whether ribavirin could reduce the replication of MNV-1. First, the nonspecific cytotoxicity of ribavirin in RAW267.4 cells was measured. There was minimal toxicity (over 90% of cell viability) at the concentrations below 200 μM, as determined by the methods described in Materials and Methods. Treating the cells with a ribavirin concentration above 100 μM significantly reduced the titer of MNV-1 by at least 1 log unit 24 h after the virus inoculation (Fig. 4A). However, after 48 h of treatment, the virus titers of ribavirin- and mock-treated groups were similar (Fig. 4B).
FIG. 4.
Effect of ribavirin on MNV-1 in RAW267.4 cells. (A) Confluent RAW267.4 cells in six-well plates were treated with various concentrations of ribavirin (0 to 200 μM) for 6 h before MNV-1 was added to the same medium at an MOI of 5. The virus-infected cells were incubated for an additional for 24 h, and then virus replication was measured using the TCID50 assay, after the plates had been frozen and thawed three times. (B) Cells were mock treated or treated with ribavirin (100 μM) for 6 h, and then MNV-1 (MOI of 5) was added to the same medium for 12 h, 24 h, or 48 h. Virus titers were measured by the TCID50 assay. MNV-1 titers for cells subjected to a treatment that were significantly reduced (P < 0.05) compared to the MNV-1 titer for mock-treated cells are indicated by asterisks. Error bars represent standard deviations from at least three independent experiments for panels A and B.
Potential mechanisms of antinoroviral effects by ribavirin.
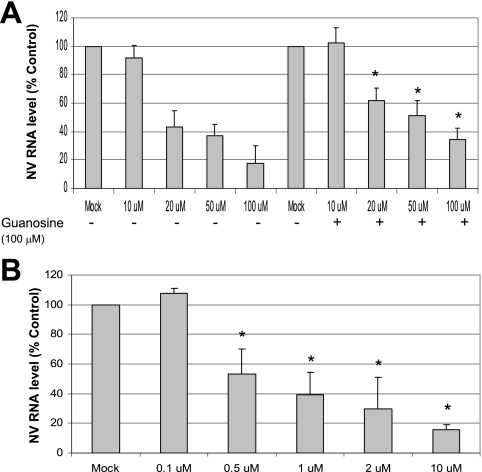
When we examined whether the replenishment of guanosine affected the antiviral effect of ribavirin, we observed that the addition of 100 μM of guanosine in the ribavirin-treated cells moderately reversed the antiviral effect of ribavirin (Fig. 5A). While the incubation of ribavirin for 48 h resulted in the reduction of the NV genome level to 90%, 42%, 37%, and 8% at 10 μM, 20 μM, 50 μM, and 100 μM, respectively, the addition of guanosine to ribavirin reduced the genome levels to 100%, 62%, 51%, and 34% at the same concentrations of ribavirin (Fig. 5A). MPA also effectively reduced the NV genome levels at concentrations above 0.5 μM (Fig. 5B) in a dose-dependent manner. Sequence analysis of the amplicon, which contained the Pol region of the NV genome, from HG23 cells treated with ribavirin (100 μM) or without ribavirin for 48 h, revealed that there was no mutation in the RT-PCR product. The sequence analysis of the NV Pol region (region corresponding to base numbers 3695 to 4144) after the amplicon was cloned into pCR2.1 indicated there were no differences in the mutation rates of the ribavirin-treated and nontreated groups. Among 18 recombinant plasmids in each group, point mutations occurred in 12 (67%) or 13 (72%) plasmids for the ribavirin-treated or nontreated groups, respectively. Additionally, the total number of mutations was 22 and 23, respectively, in the ribavirin-treated or nontreated groups.
FIG. 5.
Effect of supplementation of guanosine on NV replication in ribavirin-treated HG23 cells and effect of MPA on NV replication. (A) Effect of ribavirin alone or with guanosine (100 μM) on the NV genome in HG23 cells. One-day-old semiconfluent HG23 cells were incubated with various concentrations of ribavirin ranging from 0 (mock) to 100 μM with (+) or without (−) 100 μM guanosine. After 72 h of incubation, total RNA was prepared for real-time qRT-PCR to detect the NV genome. The reduction of NV genome by the treatments was calculated by comparison to the value for the mock-treated control. RNA levels for cells treated by guanosine and ribavirin that were significantly different (P < 0.05) from the RNA level of cells treated with ribavirin alone are indicated by asterisks. (B) Effect of MPA on NV replication. One-day-old semiconfluent HG23 cells were incubated with various concentrations of MPA ranging from 0 (mock) to 2 μM for 72 h. After the incubation, total RNA was prepared for real-time qRT-PCR to detect the NV genome. The reduction of NV genome in cells subjected to different treatments was calculated through comparison to mock-treated cells. RNA levels for cells subjected to a treatment that were significantly reduced (P < 0.05) compared to the RNA level of mock-treated cells are indicated by asterisks. Error bars represent standard deviations from at least three independent experiments for panels A and B.
DISCUSSION
Without a cell culture system, it is extremely difficult, if not impossible, to develop effective therapies for noroviral gastroenteritis. The recent development of NV replicon-bearing cells enabled us to pursue potential antinoroviral agents (3). In a previous report, we demonstrated that IFN-α was an effective inhibitor of NV replication (3), and in this report, we discovered that IFN-γ also reduced the expression of NV genome and proteins in a dose-dependent manner. Both type I (IFN-α) and II (IFN-γ) IFNs play a central role in innate immunity against virus infections before adapted immunity arises. The IFNs (type I and II) establish antiviral states of cells through interactions with IFN receptors which are expressed in all nucleated cells (23). The binding of IFNs to their cognitive receptors triggers activation of STATs and cascade events, which results in the induction of various antiviral proteins, such as RNA-dependent protein kinase and RNase L (23). However, many viruses are armed with anti-IFN mechanisms, such as those counteracting the STATs and inhibiting IFN synthesis (23). Previously, we demonstrated that NV did not have a strong anti-IFN mechanism in the replicon-bearing cells, and this may be a reason for its high sensitivity to the treatment of IFN-α or IFN-γ in this study. Interestingly, the replication of MNV-1 was shown to be sensitive to the IFN system in vivo and in vitro (14, 27), and we have observed that pretreatment with IFN-α or mouse IFN-γ at 50 units/ml each significantly reduced MNV-1 titers in RAW264.7 cells (unpublished results). Recently, Marcello et al. reported that both IFN-α and IFN-γ effectively reduced the replication of HCV in the replicon-bearing cells but by different pathways (16). They demonstrated that the anti-HCV effect of IFN-γ was independent of IFN receptors and that STAT activation and induction of potential effector genes were distinct from those of IFN-α (16). We plan to conduct similar studies to elucidate the mechanisms of the anti-NV effects of IFN-α and IFN-γ in HG23 cells.
Ribavirin is a synthetic guanosine analogue and has shown antiviral actions against a range of DNA and RNA viruses, including HCV and respiratory syncytial virus (7, 24). In this study, we found that ribavirin was also very effective at reducing NV replication in replicon-bearing cells, as the ED50 was calculated to be approximately 40 μM. Ribavirin also exhibited an inhibitory effect on MNV-1 in RAW267.4 cells. In the presence of 100 μM of ribavirin, the titer of MNV-1 dropped approximately 10-fold 24 h after virus infection. However, after 48 h of virus infection, the titers of MNV-1 in the presence and absence of ribavirin were similar. Ribavirin should be converted to the 5′-monophosphate active form to elicit antiviral activity; it is possible that virus infections disrupt the normal metabolic processes in cells and consequently interfere with the conversion. It was reported that ribavirin effectively inhibited the replication of feline calicivirus in vitro but was not effective in vivo (21, 22). It has been shown that the effects of combination treatment of IFN and ribavirin were synergistic in various viruses, including HCV in the replicon-bearing cells (12, 26). However, in this study, we found that when combined together, IFN-α and ribavirin showed only additive effects on the inhibition of NV replication in the replicon-bearing cells.
At least five distinct mechanisms of action for the antiviral activity of ribavirin have been suggested. These include (i) depletion of the intracellular GTP pool by inhibition of the cellular IMP dehydrogenase, (ii) immunomodulatory effects by enhancing the host T-cell response, (iii) induction of catastrophic mutations on the viral genome, (iv) inhibition of viral polymerase activity, and (v) inhibition of viral capping by inhibition of viral or cellular guanylyltransferase activity (7). The mechanisms of actions may be different in different viruses, and there may be more than one operating in a given virus, with one or two mechanisms being predominant (7). To elucidate potential mechanisms responsible for the antiviral activity of ribavirin in NV, we tested the first two mechanisms described above in this study. First, we replenished GTP in cells by adding guanosine to the medium in the ribavirin-treated cells. In certain viruses (yellow fever virus and human parainfluenza virus 3), the addition of guanosine in the medium efficiently reversed the antiviral effects of ribavirin (15). In this study, the addition of guanosine to the ribavirin treatment moderately reversed the antiviral effects in the cells (Fig. 5A). We also examined MPA, which is a potent noncompetitive inhibitor of IMPDH, and found that MPA effectively inhibited NV replication at concentrations above 0.5 μM. While ribavirin must be converted to the 5′-monophosphate active form to elicit antiviral activity, MPA is not required to be metabolically activated in the cells to function. The strong inhibition of NV replication by MPA suggests that the antiviral effects by ribavirin are associated with the inhibition of IMPDH. It has been suggested that ribavirin could trigger catastrophic mutations (including fetal mutations) (4). Sequencing analysis of the conserved polymerase region of NV in the ribavirin-treated (100 μM) or nontreated groups showed that both groups produced similar rates of mutation (22 and 23 mutations). Interestingly, although we did not find any mutations in RT-PCR products, we found high rates of mutations in clones treated with or without ribavirin. The NV polymerase without proofreading functions may be responsible for such high numbers of mutations. These data suggested that the antiviral effects of ribavirin on NV may not be associated with catastrophic mutations in the replicon-bearing cells.
Although norovirus infection is generally considered a self-limiting and short-term illness, recent findings demonstrated that the infection could last longer than several days or even several months, especially in immunocompromised patients (19). The treatment options for norovirus infection are limited partly because of the absence of screening systems for antiviral drugs. In this study, we demonstrated the usefulness of NV replicon-bearing cells in the development of antinoroviral therapeutics in the absence of any reliable cell culture system. The NV replicon-bearing cells may provide an excellent tool to screen potential antinoroviral agents. In current studies, we are investigating the mechanisms of IFN-mediated anti-NV effects in the replicon-bearing cells. In 1990, the U.S. Food and Drug Administration (FDA) approved IFN therapy as a treatment for chronic HCV infection. Currently, the most effective treatment for chronic HCV infection is the combination therapy of IFN-α and ribavirin. Our results indicated that IFNs and ribavirin may also be good therapeutic options for noroviral gastroenteritis.
Acknowledgments
This work was supported by NIH COBRE grant 2 P20 RR016443-07 and Animal Health Project no. KS481846.
This paper is contribution no. 07-201-J from the Kansas Agricultural Experiment Station.
Footnotes
Published ahead of print on 12 September 2007.
REFERENCES
- 1.Bartenschlager, R. 2002. Hepatitis C virus replicons: potential role for drug development. Nat. Rev. Drug Discov. 1:911-916. [DOI] [PubMed] [Google Scholar]
- 2.Chang, K. O., Y. Kim, K. Y. Green, and L. J. Saif. 2002. Cell-culture propagation of porcine enteric calicivirus mediated by intestinal contents is dependent on the cyclic AMP signaling pathway. Virology 304:302-310. [DOI] [PubMed] [Google Scholar]
- 3.Chang, K. O., S. V. Sosnovtsev, G. Belliot, A. D. King, and K. Y. Green. 2006. Stable expression of a Norwalk virus RNA replicon in a human hepatoma cell line. Virology 353:463-473. [DOI] [PubMed] [Google Scholar]
- 4.Crotty, S., D. Maag, J. J. Arnold, W. Zhong, J. Y. Lau, Z. Hong, R. Andino, and C. E. Cameron. 2000. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat. Med. 6:1375-1379. [DOI] [PubMed] [Google Scholar]
- 5.Duizer, E., K. J. Schwab, F. H. Neill, R. L. Atmar, M. P. Koopmans, and M. K. Estes. 2004. Laboratory efforts to cultivate noroviruses. J. Gen. Virol. 85:79-87. [DOI] [PubMed] [Google Scholar]
- 6.Fankhauser, R. L., J. S. Noel, S. S. Monroe, T. Ando, and R. I. Glass. 1998. Molecular epidemiology of “Norwalk-like viruses” in outbreaks of gastroenteritis in the United States. J. Infect. Dis. 178:1571-1578. [DOI] [PubMed] [Google Scholar]
- 7.Graci, J. D., and C. E. Cameron. 2006. Mechanisms of action of ribavirin against distinct viruses. Rev. Med. Virol. 16:37-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green, K. Y., T. Ando, M. S. Balayan, T. Berke, I. N. Clarke, M. K. Estes, D. O. Matson, S. Nakata, J. D. Neill, M. J. Studdert, and H. J. Thiel. 2000. Taxonomy of the caliciviruses. J. Infect. Dis. 181(Suppl. 2):S322-S330. [DOI] [PubMed] [Google Scholar]
- 9.Green, K. Y., R. M. Chanock, and A. Z. Kapikian. 2001. Human caliciviruses, p. 841-874. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 10.Hsu, C. C., L. K. Riley, H. M. Wills, and R. S. Livingston. 2006. Persistent infection with and serologic cross-reactivity of three novel murine noroviruses. Comp. Med. 56:247-251. [PubMed] [Google Scholar]
- 11.Kageyama, T., S. Kojima, M. Shinohara, K. Uchida, S. Fukushi, F. B. Hoshino, N. Takeda, and K. Katayama. 2003. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 41:1548-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanda, T., O. Yokosuka, F. Imazeki, M. Tanaka, Y. Shino, H. Shimada, T. Tomonaga, F. Nomura, K. Nagao, T. Ochiai, and H. Saisho. 2004. Inhibition of subgenomic hepatitis C virus RNA in Huh-7 cells: ribavirin induces mutagenesis in HCV RNA. J. Viral Hepat. 11:479-487. [DOI] [PubMed] [Google Scholar]
- 13.Kapikian, A. Z., R. G. Wyatt, R. Dolin, T. S. Thornhill, A. R. Kalica, and R. M. Chanock. 1972. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J. Virol. 10:1075-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karst, S. M., C. E. Wobus, M. Lay, J. Davidson, and H. W. Virgin IV. 2003. STAT1-dependent innate immunity to a Norwalk-like virus. Science 299:1575-1578. [DOI] [PubMed] [Google Scholar]
- 15.Leyssen, P., J. Balzarini, E. De Clercq, and J. Neyts. 2005. The predominant mechanism by which ribavirin exerts its antiviral activity in vitro against flaviviruses and paramyxoviruses is mediated by inhibition of IMP dehydrogenase. J. Virol. 79:1943-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcello, T., A. Grakoui, G. Barba-Spaeth, E. S. Machlin, S. V. Kotenko, M. R. MacDonald, and C. M. Rice. 2006. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology 131:1887-1898. [DOI] [PubMed] [Google Scholar]
- 17.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller, W. E., A. Maidhof, H. Taschner, and R. K. Zahn. 1977. Virazole (1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide: a cytostatic agent. Biochem. Pharmacol. 26:1071-1075. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson, M., K. O. Hedlund, M. Thorhagen, G. Larson, K. Johansen, A. Ekspong, and L. Svensson. 2003. Evolution of human calicivirus RNA in vivo: accumulation of mutations in the protruding P2 domain of the capsid leads to structural changes and possibly a new phenotype. J. Virol. 77:13117-13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pietschmann, T., and R. Bartenschlager. 2001. The hepatitis C virus replicon system and its application to molecular studies. Curr. Opin. Drug Discov. Dev. 4:657-664. [PubMed] [Google Scholar]
- 21.Povey, R. C. 1978. Effect of orally administered ribavirin on experimental feline calicivirus infection in cats. Am. J. Vet. Res. 39:1337-1341. [PubMed] [Google Scholar]
- 22.Povey, R. C. 1978. In vitro antiviral efficacy of ribavirin against feline calicivirus, feline viral rhinotracheitis virus, and canine parainfluenza virus. Am. J. Vet. Res. 39:175-178. [PubMed] [Google Scholar]
- 23.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sidwell, R. W., J. H. Huffman, G. P. Khare, L. B. Allen, J. T. Witkowski, and R. K. Robins. 1972. Broad-spectrum antiviral activity of virazole: 1-beta-d-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science 177:705-706. [DOI] [PubMed] [Google Scholar]
- 25.Spann, K. M., K.-C. Tran, B. Chi, R. L. Rabin, and P. L. Collins. 2004. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages. J. Virol. 78:4363-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanabe, Y., N. Sakamoto, N. Enomoto, M. Kurosaki, E. Ueda, S. Maekawa, T. Yamashiro, M. Nakagawa, C. H. Chen, N. Kanazawa, S. Kakinuma, and M. Watanabe. 2004. Synergistic inhibition of intracellular hepatitis C virus replication by combination of ribavirin and interferon-alpha. J. Infect. Dis. 189:1129-1139. [DOI] [PubMed] [Google Scholar]
- 27.Wobus, C. E., S. M. Karst, L. B. Thackray, K. O. Chang, S. V. Sosnovtsev, G. Belliot, A. Krug, J. M. Mackenzie, K. Y. Green, and H. W. Virgin IV. 2004. Replication of norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2:e432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wobus, C. E., L. B. Thackray, and H. W. Virgin IV. 2006. Murine norovirus: a model system to study norovirus biology and pathogenesis. J. Virol. 80:5104-5112. [DOI] [PMC free article] [PubMed] [Google Scholar]