Abstract
The agent responsible for prion disease may exist in different forms, commonly referred to as strains, with each carrying the specific information that determines its own distinct biological properties, such as incubation period and lesion profile. Biological strain typing of ovine scrapie isolates by serial passage in conventional mice has shown some diversity in ovine prion strains. However, this biological diversity remains poorly supported by biochemical prion strain typing. The protein-only hypothesis predicts that variation between different prion strains in the same host is manifest in different conformations adopted by PrPSc. Here we have investigated the molecular properties of PrPSc associated with two principal Prnpa mouse-adapted ovine scrapie strains, namely, RML and ME7, in order to establish biochemical prion strain typing strategies that may subsequently be used to discriminate field cases of mouse-passaged ovine scrapie isolates. We used a conformation-dependent immunoassay and a conformational stability assay, together with Western blot analysis, to demonstrate that RML and ME7 PrPSc proteins show distinct biochemical and physicochemical properties. Although RML and ME7 PrPSc proteins showed similar resistance to proteolytic digestion, they differed in their glycoform profiles and levels of proteinase K (PK)-sensitive and PK-resistant isoforms. In addition, the PK-resistant core (PrP27-30) of ME7 was conformationally more stable following exposure to guanidine hydrochloride or Sarkosyl than was RML PrP27-30. Our data show that mouse-adapted ovine scrapie strains can be discriminated by their distinct conformers of PrPSc, which provides a basis to investigate their diversity at the molecular level.
The failure of proteins to fold correctly or to maintain the correctly folded state leads to a variety of pathological conditions. Prion diseases, or transmissible spongiform encephalopathies, are chronic neurodegenerative central nervous system disorders of mammals characterized by the accumulation of PrPSc, an abnormal isomer of the host protein PrPC (1, 35). During conversion of PrPC to PrPSc, a major refolding event occurs that results in a more extensive β-sheet conformation. The protein-only hypothesis predicts that the transmissible prion agent comprises solely proteinaceous material. Consequently, PrPSc is considered to be the principal, if not sole, component of the transmissible prion agent and to be responsible for conformational changes in the conversion of PrP (8, 19, 26, 36). The fact that the transmissible prion agent can exist in different forms, referred to as prion strains, has been a major argument against this view. Different prion inocula, or strains, may be characterized by their biological properties, including the disease incubation period, histopathology, and variations in the pattern of PrPSc deposition following serial passage in inbred mice (37). The existence of prion strains in hosts that express the same PrP molecule has been taken as evidence for an independently replicating information molecule or genome. However, no prion disease-specific nucleic acid has been described to date (40). In the context of the protein-only hypothesis, prion strain variation requires that PrPSc must be able to sustain distinct conformational states with the same amino acid sequence, which is faithfully replicated during the conversion of PrPC to PrPSc.
Evidence that prion strain-specific information is enciphered in the structure of PrPSc has emerged from transmission studies of natural cases of prion diseases. In fatal familial insomnia (FFI), the protease-resistant fragment of deglycosylated PrPSc is 19 kDa, whereas that in familial or sporadic Creutzfeldt-Jakob disease (CJD) is 21 kDa (30, 32). This difference in molecular size profile arises from different sites of proteolytic cleavage that are believed to occur as a consequence of distinct PrPSc conformations. These molecular size profiles of PrPSc were maintained when extracts from the brains of patients with FFI or CJD were transmitted to chimeric human-mouse PrP transgenic mice (47). Furthermore, distinct human PrPSc subtypes, distinguished by their proteinase K (PK)-resistant fragment lengths and glycoform ratios, are associated with different phenotypes of CJD (32). Biochemical properties of human PrPSc were maintained upon passage in human PrP transgenic mice and bank voles (10, 31). In sheep, classical and atypical scrapie cases are associated with distinct forms of PrPSc that show significantly different PK digestion fragments (25). These biochemical properties of sheep PrPSc were maintained when brain extracts from sheep with classical or atypical scrapie were transmitted to ovine PrP transgenic mice. Finally, the transmissible mink encephalopathy prion strains drowsy and hyper, isolated in hamsters, were distinguished by their sedimentation profiles, protease sensitivities, and molecular weight profiles of PK-resistant PrPSc (2). These biochemical and physical properties of full-length hamster PrPSc or the protease-resistant fragment of PrPSc (PrP27-30) were preserved after transmission to Syrian hamsters (3). Collectively, the results of these various transmission studies show that distinct PrPSc conformations or subtypes with the same amino acid sequence as that of PrP can exist and that strain-specific properties are maintained in the protease-resistant core of PrPSc.
The conformations of PrPSc isoforms have been studied through the use of immunoassays that recognize regions of the protein that are differentially buried or exposed depending upon the extent of denaturation of the molecule. This approach has led to the development of the conformation-dependent immunoassay (CDI), which is capable of quantifying the concentration of PrPSc without the use of PK to discriminate between PrPC and the abnormal isomer of PrP (28, 39, 48). The use of CDI has revealed that prion strains may differ from each other by the extent to which epitopes that are buried in PrPSc under native conditions become accessible upon partial denaturation. In addition, CDI has shown that PrPSc adopts both protease-resistant and -sensitive conformations and that prion strains can be discriminated by the composition of these two conformers of disease-associated PrP (39). Prion strains have also been distinguished by conformational stability of PrPSc (33, 34). Exposure of PrPSc to increasing concentrations of guanidine hydrochloride (GdnHCl) leads to a transition from the native to denatured state, measured as a loss of resistance to protease digestion. Different prion strains segregate into distinct groups based on the concentration of GdnHCl required to achieve half-maximal denaturation (27). Determination of the conformational stability of an extensive panel of synthetic and naturally occurring prions passaged in mice showed that the concentration of GdnHCl required to induce half-maximal denaturation correlated positively with the incubation time. These studies have begun to address the correlation of the biological properties of different prion strains with the biochemical properties of PrPSc. This is an important aspect of prion biology, as it begins to elucidate how a diverse population of prion strains may exist in the same host.
As with any infectious disease, knowledge of the diversity in strains is required in order to monitor the spread of disease and to predict interspecies transmission. Issues specific to ovine prion disease are the discrimination of scrapie from bovine spongiform encephalopathy (BSE) infection in sheep and the relationship between different strains of the scrapie agent and different PrP genotypes of sheep. For these reasons, it is important to establish and understand the extent of prion strain diversity in sheep. Conventional prion strain typing by bioassay in mice has shown a significant number of different ovine scrapie strains based on their biological properties following serial passage in conventional mice (5-7, 12, 16). In order to validate this suggested diversity in scrapie strains, it will be important to supplement biological strain typing of ovine scrapie isolates in mice with molecular analyses of the associated mouse-passaged ovine scrapie PrPSc. In order to establish a biochemical approach for ovine prion strain typing, we compared the biochemical and physicochemical properties of Rocky Mountain Laboratory (RML) and ME7, two principal Prnpa mouse-adapted ovine prion strains. Our data show that although RML and ME7 inocula comprise similar levels of total PrPSc, these two different prion strains are characterized by distinct PrPSc conformers that have different conformational stabilities and sensitivities to proteolytic digestion. These observations establish criteria to examine mouse-adapted ovine scrapie strains at the molecular level.
MATERIALS AND METHODS
Generation of murine recombinant PrP.
Full-length murine recombinant PrP protein (amino acid residues 23 to 231) was generated as described previously (51). PrP was verified by mass spectroscopy to confirm the correct protein sequence and the presence of a disulfide bond. Oxidized and refolded recombinant PrP was stored at −80°C.
Anti-PrP monoclonal antibodies.
The C-terminus-specific anti-PrP monoclonal antibodies A516 and V24 were generated from Prnpo/o mice immunized with ovine recombinant ARR and VRQ PrP (amino acid residues 23 to 231), respectively (51). Monoclonal antibody 245 was generated from Prnpo/o mice immunized with copper-refolded murine recombinant PrP (amino acid residues 23 to 231) (50). Monoclonal antibody 683 was generated from Prnpo/o mice immunized with a peptide of murine recombinant PrP (amino acid residues 161 to 231) (51). The specificities of the anti-PrP monoclonal antibodies were determined by epitope mapping using a panel of overlapping 15-mer peptides that spanned the entire length of mature ovine PrP. Monoclonal antibody 683 (immunoglobulin G2a [IgG2a] isotype) reacted with peptides that contained the core sequence PVDQY (residues 168 to 172). Monoclonal antibody A516 (IgG1 isotype) reacted most strongly during epitope mapping with a peptide of the sequence DRYYRENMYRYPNQV (residues 150 to 164). Monoclonal antibodies V24 and 245 did not react in the peptide mapping study, implying that their epitopes are conformational. However, V24 would appear to bind around helix 1, as its reactivity with PrP is influenced by polymorphisms in this area in ovine recombinant PrP. The reactivity of monoclonal antibody 245 is influenced by the carbohydrate residues in PrP, as it reacts with mono- and unglycosylated but not diglycosylated prion protein. Monoclonal antibody 6H4 (23), which reacts with the epitope DYEDRYYRE, was a kind gift from Prionics AG, Zürich, Switzerland. Biotinylated monoclonal antibodies were prepared for use as detector antibodies as follows. One milligram of each purified monoclonal antibody was washed four times with 50 mM sodium bicarbonate buffer (pH 9.3), using YM-30 concentrators (Fisher UK). The retentate was then labeled overnight at 4°C, using N,N-dimethylformamide (Sigma) and biotinamidocaproate N-hydroxysuccinimide ester (Sigma). Biotinylated antibodies were purified by adsorption onto YM-30 concentrator membranes followed by isolation using elution buffer (50 mM Tris-HCl [pH 7.8], 0.9% sodium chloride, 0.1% sodium azide [pH 7.8]). The immunoreactivity of biotinylated monoclonal antibodies was verified by direct enzyme-linked immunosorbent assay (ELISA), using murine recombinant PrP as the antigen, and the concentration of antibody was determined by bicinchoninic acid (BCA) assay (Pierce, Perbio Science, UK Ltd.) prior to storage at 4°C.
Passage of RML and ME7 prions.
RML and ME7 are Prnpa mouse-adapted prion strains derived from natural sheep scrapie material, although each originates from a different source. RML was derived from the scrapie-infected sheep brain homogenate SSBP/1 after transmission to goats and is passaged in CD-1 mice (9). In contrast, ME7 was isolated by passage of spleen tissue from a scrapie-infected sheep in RIII mice (a subline of the C57BL/6 strain) and has subsequently been passaged in C57BL/6 mice (13, 14, 52). We previously reported that these two prion strains show different incubation times, clinical signs, and neuropathology upon passage in conventional mice and tga20 mice that overexpress a murine Prnpa transgene (49). CD-1 Swiss or C57BL/6 wild-type mice, either male or female, were inoculated at 5 to 6 weeks of age with either RML 5.0 or ME7 brain homogenate (20 μl of a 10% homogenate) by intracerebral injection into the right parietal lobe at a depth of 4 to 5 mm. RML 5.0 and ME7 brain homogenates were diluted in phosphate-buffered saline (PBS) plus 5% bovine serum albumin (BSA) (Sigma) and in PBS, respectively. RML 5.0 was derived by passage of RML 4.1 in CD-1 Swiss mice, and ME7 was prepared at the Centre for Veterinary Science from terminally sick mice previously inoculated with ME7 obtained from the TSE Resource Centre, Institute for Animal Health, Compton, United Kingdom (supplied by the Neuropathogenesis Unit, Edinburgh, United Kingdom) (49). Inoculated mice were monitored daily for clinical signs of mouse prion disease. The diagnosis of prion disease was based upon the criteria of Dickinson et al. (15). Mice were euthanized at the point of neurological disease and dysfunction. All regulated procedures involving experimental animals were carried out under project and personal license authority issued in accordance with The Animals (Scientific Procedures) Act 1986.
Preparation of mouse brain tissue samples.
Mouse brain homogenates were prepared by two cycles of homogenization in PBS (pH 7.4) in a Bio-Rad TeSeE Precess 24 homogenizer, and individual samples were diluted to 10% in PBS prior to being tested.
Protein quantification.
The total protein concentration in mouse brain material or anti-PrP monoclonal antibody samples was measured by BCA assay (Pierce, Perbio Science, UK Ltd.). Samples were initially diluted in PBS (pH 7.4) at 1:50, 1:100, and 1:200 dilutions and included a diluent-only control. A twofold dilution series of BSA (Sigma), ranging from 800 μg/ml down to 0 μg/ml, was prepared to act as a standard. Each dilution was dispensed into triplicate wells in a 96-well flat-bottomed plate (5 μl/well). An aliquot of 95 μl of the BCA working reagent (prepared by mixing 1 part solution A and 49 parts solution B) was added to each well, and the plates were incubated for up to 30 min at 20°C. Optical density was read at 570 nm, using a Bio-Rad 680 microplate reader. Protein concentration was estimated by calculation from a standard curve, using Microplate Manager software (Bio-Rad).
Enrichment for disease-associated PrP.
Mouse brain homogenates were made to 10% (wt/vol) with PBS (pH 7.4). Samples were centrifuged at 100 × g for 1 min at 20°C to remove gross debris, and the supernatants were retained. Samples were incubated in the presence or absence of 2% (final concentration) Sarkosyl in PBS for 10 min at 37°C with shaking. Homogenates were then treated with GdnHCl or left untreated for 30 min at 20°C prior to incubation with PK (Roche, United Kingdom) at various concentrations in PBS (pH 7.4) for 30 or 60 min at 37°C with shaking. Enzymatic digestion was terminated by the addition of 1 mM (final concentration) 4-(2-aminoethyl) benzenesulfonyl fluoride (AEBSF; Sigma) in distilled water followed by incubation in the presence or absence of a 0.4% final concentration of sodium phosphotungstic acid (NaPTA) in PBS (pH 7.4) for 1 h at 37°C with shaking. For Western blot analysis, samples were prepared with or without methanol precipitation overnight at −20°C and then centrifuged as described below. All samples, other than those for Western blotting, were centrifuged at 21,000 × g for 30 min at 10°C, the supernatants were discarded, and pelleted material was retained for analysis.
CDI.
Enriched PrPSc pelleted samples were resuspended in 200 μl 0.1% Sarkosyl in PBS (pH 7.4) and 200 μl 250 mM EDTA in water (pH 8.0). Samples were thoroughly mixed and centrifuged immediately at 21,000 × g for 15 min at 10°C. The supernatants were discarded and the pellets resuspended in 0 M or 6 M GdnHCl. The 6 M GdnHCl samples were heated to 80°C for 5 min, followed by cooling to 20°C.
Capture antibody (A516 or V24) was routinely used at 0.5 μg/well in 200 μl to coat triplicate wells of Nunc Maxisorp 96-well flat-bottomed plates overnight at 4°C. Capture antibodies were diluted in coating buffer (0.01 M PBS [pH 7.4] containing 0.1% sodium azide). Excess antibody was removed, plates were washed four times with wash buffer (25× concentrated stock prepared as 500 mM Tris-HCl [pH 7.7], 154 mM NaCl, 0.5% Tween 20, and 0.1% sodium azide and diluted to a 1× working concentration with distilled water just prior to use), and wells were blocked with blocking buffer consisting of 2% (wt/vol) BSA and 0.05% sodium azide in PBS for 1.5 h at 20°C with shaking. Plates were washed four times with wash buffer. The appropriate dilutions of murine recombinant PrP protein or native murine PrP samples were diluted in assay buffer (50 mM Tris-HCl [pH 7.7], 154 mM NaCl, 0.02 mM diethylenetriaminepentaacetic acid [Sigma], 0.5% BSA [Sigma], 0.1% sodium azide, and 0.01% Tween 20) as required. Test samples were added to the wells and incubated for 1 h at 20°C with shaking. Plates were washed four times with wash buffer, and the relevant biotinylated anti-PrP detector monoclonal antibody (diluted in assay buffer) was added at 50 ng/well and incubated for 1 h at 20°C with shaking. Plates were washed four times with wash buffer, followed by incubation with europium-labeled streptavidin (Perkin-Elmer) at 50 ng/well for 1 h at 20°C with shaking. Plates were washed eight times with wash buffer, followed by the addition of enhancement solution (Perkin-Elmer). Plates were incubated for 5 min at 20°C with shaking, and the fluorescence, measured as counts per second (cps), was determined in a Victor time-resolved fluorimeter (Perkin-Elmer).
Western blot analysis.
Samples prepared by methanol precipitation overnight at −20°C were centrifuged at 12,600 × g for 30 min at 10°C, and the supernatants were discarded. Samples prepared without methanol precipitation were subsequently incubated in the presence or absence of 0.4% (final concentration) NaPTA in PBS (pH 7.4) for 30 min or 1 h at 37°C, and samples were centrifuged at 21,000 × g for 30 min at 10°C. Following centrifugation, the supernatants were discarded and pelleted material resuspended in 20 μl of 2× Laemmli sample buffer prior to loading of 25 or 50 μg of total protein per track. Other samples were centrifuged at 21,000 × g for 30 min at 10°C, the supernatants were discarded, and the pellets were resuspended in 200 μl 0.1% Sarkosyl in PBS (pH 7.4) and 200 μl 250 mM EDTA in water (pH 8.0). Samples were thoroughly mixed and centrifuged immediately at 21,000 × g for 15 min at 10°C. The supernatants were discarded, and the pellets were resuspended in 20 μl of 2× Laemmli sample buffer prior to loading of 25 or 50 μg of total protein per track. Samples were then electrophoresed through a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) minigel. Proteins were transferred to a nitrocellulose membrane by semidry blotting. Membranes were blocked with TBS-T (10 mM Tris-HCl [pH 7.8], 100 mM NaCl, 0.05% Tween 20) plus 5% nonfat milk and subsequently incubated with anti-PrP monoclonal antibody 683 (5 μg/ml) for 1 h at 20°C, followed by goat anti-mouse IgG-horseradish peroxidase (Sigma) diluted 1:2,000 for 1 h at 20°C. All dilutions of antibodies were done in 1% nonfat milk in TBS-T. PrP bands were detected by enhanced chemiluminescence (Amersham Biosciences).
Conformational stability direct ELISA.
Following enrichment of disease-associated PrP and treatment with various concentrations of GdnHCl, all samples were then diluted in PBS (pH 7.4) to give a final GdnHCl concentration of 0.1 M prior to incubation with PK at 32 μg/ml for 30 min at 37°C with shaking. Enzymatic digestion was terminated by the addition of 1 mM (final concentration) AEBSF in distilled water, followed by incubation in the presence of a 0.4% final concentration of NaPTA in PBS (pH 7.4) for 1 h at 37°C with shaking. Samples were centrifuged at 21,000 × g for 30 min at 10°C, the supernatants were discarded, and the pellets were resuspended in 200 μl 0.1% Sarkosyl in PBS (pH 7.4) and 200 μl 250 mM EDTA in water (pH 8.0). Samples were thoroughly mixed and centrifuged immediately at 21,000 × g for 15 min at 10°C. The supernatants were discarded, and the pellets were resuspended in 6 M GdnHCl and heated to 80°C for 5 min. Samples were then cooled to 20°C prior to dilution in ELISA binding buffer (0.1 M sodium bicarbonate buffer, pH 8.6). The samples (100 μl per well) were used to coat triplicate wells of 96-well flat-bottomed plates for 1 h at 20°C with shaking. Excess sample was removed, and plates were washed three times with PBS (pH 7.4). Wells were blocked with PBS containing 5% nonfat milk for 1 h at 20°C with shaking. Plates were washed three times with PBS containing 0.1% Tween 80 (PBS-T) prior to incubation with 3 μg/ml of biotinylated anti-PrP monoclonal antibody 6H4 diluted in PBS containing 1% nonfat milk for 1 h at 20°C with shaking. Plates were washed three times with PBS-T prior to incubation with avidin-alkaline phosphatase (Sigma) at a 1:2,000 dilution in PBS containing 1% nonfat milk for 1 h at 20°C with shaking. Plates were washed three times with PBS-T and once with ELISA buffer (0.05 M glycine, 0.03 M NaOH, and 0.25 mM [each] ZnCl2 and MgCl2) before the addition of the substrate p-nitrophenyl phosphate (Sigma) at 0.5 mg/ml in ELISA buffer for up to 1 h at 20°C. ELISA plates were read at 415 nm on a Bio-Rad 680 microplate reader.
RESULTS
PrPSc proteins from strains RML and ME7 show differences in glycoform profile.
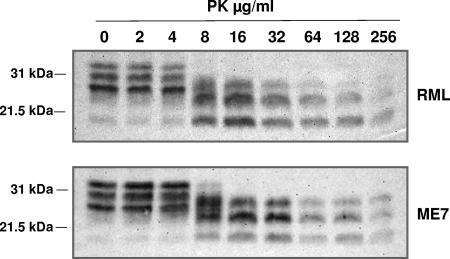
Different subtypes of PrPSc, distinguished by properties such as PK-resistant fragment length and glycoform ratio, have been associated with different prion strains or with different phenotypes of prion disease in the same species (2, 3, 10, 25, 30, 32, 34, 47). In order to investigate whether this was the case with RML and ME7, we investigated the molecular profile of PrPSc associated with each of these different prion strains by Western blot analysis. Accordingly, RML- and ME7-inoculated mouse brains isolated at terminal prion disease were homogenized and treated with or without PK prior to Western blot analysis with the anti-PrP monoclonal antibody 683. Figure 1 shows that brain homogenates from RML- and ME7-infected mice contained similar levels of total PrP and PK-resistant PrPSc at the terminal disease stage. PK-resistant PrPSc in RML- and ME7-infected mouse brains was evident after treatment with PK concentrations of ≥16 μg/ml. Both RML and ME7 were characterized by PK-resistant PrPSc fragments of similar molecular weights and with similar resistance to proteolysis over a wide range of PK enzyme concentrations. PrPSc from ME7-infected brain homogenates was characterized by an overall greater level of diglycosylated prion protein, whereas that from RML-infected brains was characterized by a predominance of the monoglycosylated form. Furthermore, this difference in glycoform profile was a consequence of prion infection, since PrPC from uninfected CD-1 and C57BL/6 mouse brains showed an indistinguishable glycoform profile that was dominated by diglycosylated PrPC (data not shown).
FIG. 1.
Western blot analysis of PK-resistant mouse PrPSc. RML and ME7 prion-infected brain homogenates were prepared as described in Materials and Methods. Brain homogenates were treated with various concentrations of PK and analyzed by SDS-PAGE and Western blotting, using anti-PrP monoclonal antibody 683. Each track was loaded with 25 μg of total protein. Molecular mass markers (kDa) are shown on the left.
Detection of RML and ME7 PrPSc by CDI.
The different biological properties of RML and ME7 may be associated with the accumulation of different subtypes or conformers of PrPSc in the brains of prion-infected mice. In order to address this, we investigated disease-associated PrP in the brains of mice inoculated with either of these two prion strains by capture-detector CDI (28, 39, 48). To do so, several anti-PrP monoclonal antibodies were tested in combination by time-resolved fluorescence immunoassay in order to establish pairs of reagents capable of efficient recognition of murine PrP. Several pairs of antibodies were chosen for use in the CDI, including monoclonal antibody A516 or V24 as the capture antibody in combination with either monoclonal antibody 245 or 6H4 as the detector reagent. Figure 2a shows the typical dose-response curve for reactivity of monoclonal antibody V24 as the capture antibody and either 245 or 6H4 as the detector reagent with murine recombinant PrP. The sensitivities of these two antibody pairs for murine recombinant PrP were comparable. Similar results were seen when the C-terminal specific monoclonal antibody A516 was used as the capture reagent (data not shown). The overall sensitivity of the capture-detector immunoassays was in the region of 50 pg/ml of murine recombinant PrP.
FIG. 2.
Detection of mouse PrP by CDI. (a) Various concentrations of mouse full-length recombinant PrP protein were captured by purified anti-PrP monoclonal antibody V24 on Nunc Maxisorp ELISA plates. Recombinant PrP was detected with 0.25 μg/ml biotinylated anti-PrP monoclonal antibody 245 (▵) or 6H4 (▴). Results shown are time-resolved fluorescence (TRF) values (mean cps ± standard deviations [SD]) for duplicate wells. (b) Various dilutions of RML-infected mouse brain homogenate were captured by purified anti-PrP monoclonal antibody A516 following treatment with (squares) or without (circles) NaPTA and with 0 M (open symbols) or 6 M (closed symbols) GdnHCl, as described in Materials and Methods. Native PrP was detected with 0.25 μg/ml biotinylated anti-PrP monoclonal antibody 245. Results shown are TRF values (mean cps ± SD) for triplicate wells. (c) Various dilutions of uninfected CD-1 mouse brain homogenate were captured by purified anti-PrP monoclonal antibody A516 following treatment with (squares) or without (circles) NaPTA and with 0 M (open symbols) or 6 M (closed symbols) GdnHCl, as described in Materials and Methods. Native PrP was detected with 0.25 μg/ml biotinylated anti-PrP monoclonal antibody 245. Results shown are TRF values (mean cps ± SD) for triplicate wells.
PrPSc was detected by the capture-detector CDI after Sarkosyl extraction of prion-infected mouse brain homogenate followed by denaturation-induced conformational changes in PrP epitope exposure to distinguish abnormal from normal PrP. We and others have previously shown that the immunoreactivity of PrPC is lost when the normal isomer of PrP is exposed to 6 M GdnHCl. This reflects the loss of surface-exposed PrP epitopes within PrPC as a consequence of chaotropic agent-induced denaturation. In contrast, the immunoreactivity of disease-associated PrP is increased upon exposure to 6 M GdnHCl, which reflects the exposure of previously buried epitopes within PrPSc that were no longer accessible in denatured PrPC. In order to allow the selective recognition of disease-associated PrP by denaturation-induced conformational changes, we made use of the fact that PrPC is soluble in detergent, whereas PrPSc is insoluble (20, 29). Accordingly, prion-infected mouse brain homogenate was extracted with Sarkosyl, with or without the use of NaPTA, and aggregated PrPSc was harvested by centrifugation at 21,000 × g. Native (0 M GdnHCl-treated) and denatured (6 M GdnHCl-treated) PrPSc forms were detected by CDI, using anti-PrP monoclonal antibody A516 or V24 as the capture agent and 245 or 6H4 as the detector, with similar efficiencies.
Figure 2b shows that significant levels of PrPSc were precipitated by Sarkosyl treatment from RML prion-infected brain samples. This was shown by the increase in fluorescence counts when PrP samples were treated with 6 M GdnHCl compared to that obtained in the absence of denaturant. This difference in counts was maintained at all the dilutions of PrPSc tested, indicating the relatively high sensitivity of this assay for the detection of disease-associated PrP. The CDI ratio (calculated as denatured/native fluorescence counts), which is a measure of conformational change, was 56.8 for RML prion-infected samples at the lowest dilution tested (1:50). The level of fluorescence for the 6 M GdnHCl-treated sample was significantly increased when Sarkosyl extraction was accompanied by NaPTA precipitation. This was accompanied by an increase in the CDI ratio to 186.9, since NaPTA precipitation did not enhance the level of fluorescence in the 0 M GdnHCl sample. In contrast to the results seen with RML prion-infected brain samples, very little, if any, aggregated PrP was detected in control brain tissue. Figure 2c shows that a low level of fluorescence was obtained, with or without the use of NaPTA, for denatured and native PrP samples obtained from uninfected CD-1 mouse brain tissue. This resulted in CDI ratios that were <1.6 at the lowest dilution of uninfected brain sample tested (1:50).
We next compared the quantities of PrPSc associated with RML and ME7 prion-infected brain homogenates by CDI, using two different anti-PrP monoclonal antibody pairs. Brain tissues from RML and ME7 prion-inoculated mice were extracted with Sarkosyl in the presence or absence of NaPTA, and the precipitated material was subjected to CDI, using anti-PrP monoclonal antibody V24 as the capture antibody and monoclonal antibody 245 or 6H4 as the detector reagent. Table 1 shows a representative set of data from three replicate experiments. Overall, monoclonal antibody 245 detected similar relative levels of disease-associated PrP in the samples derived from RML and ME7 prion-infected brains, whether or not NaPTA was used to enrich PrPSc. However, when monoclonal antibody 6H4 was used as the detector reagent, the level of PrPSc isolated from both ME7 and RML prion-infected mouse brains was increased above the level detected by monoclonal antibody 245. This was the case when PrPSc was isolated from either ME7 or RML prion-infected mouse brains, with or without the use of NaPTA. Monoclonal antibody 245 reacts with mono- and unglycosylated PrP (50), whereas monoclonal antibody 6H4 reacts with di-, mono-, and unglycosylated PrP (23). This implies that RML prions were characterized by a reduced level of diglycosylated PrPSc compared to ME7 prions.
TABLE 1.
RML and ME7 prion strains distinguished by CDI
| Detector antibody | Presence of NaPTAa | Mean PrPSc concn (ng/ml) ± SD
|
|
|---|---|---|---|
| RML | ME7 | ||
| 245 | − | 12.4 ± 0.2 | 16.3 ± 0.2 |
| + | 22.6 ± 4.9 | 24.5 ± 0.2 | |
| 6H4 | − | 42.1 ± 0.6 | 84.6 ± 1.2 |
| + | 110.3 ± 4.0 | 142.9 ± 6.4 | |
−, no NaPTA added; +, NaPTA was added to the reaction mixture.
Difference in conformational stabilities of RML and ME7 PrP27-30.
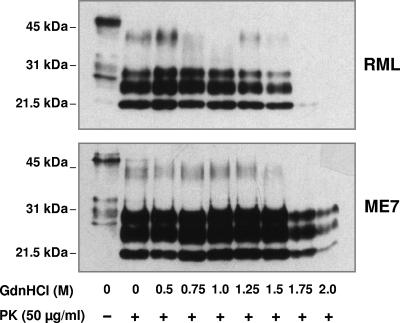
Prion strains have been characterized by the relative stability of their associated PrPSc, measured by resistance to proteolysis in the presence of increasing concentrations of GdnHCl (27, 33, 34). Accordingly, we used this approach to examine the relative stabilities of mouse-passaged RML and ME7 PrPSc. Aliquots of RML and ME7 terminally diseased brain homogenate were incubated with increasing amounts of GdnHCl for 1 h, followed by limited proteolysis with PK. The samples were subsequently treated with methanol to precipitate PK-resistant PrPSc, which was subsequently subjected to Western blotting with anti-PrP monoclonal antibody 683. Figure 3 shows that the amounts of RML and ME7 PrP27-30 remained constant following treatment with up to 1.5 M GdnHCl. Exposure to increasing amounts of GdnHCl had some effect on ME7 PrP27-30 but caused a significant increase in the sensitivity of RML PrPSc to PK-induced proteolysis, as evidenced by the reduction in PrP27-30 on the Western blot. RML PrP27-30 was completely denatured by treatment with >1.5 M GdnHCl, as evidenced by its complete proteolysis at these concentrations of denaturant. Longer exposure times of the RML blot failed to show the presence of PrP27-30 in the lanes that contained brain samples treated with 1.75 or 2.0 M GdnHCl prior to PK digestion. In contrast, significant amounts of ME7 PrP27-30 were still evident following treatment with concentrations of GdnHCl above 1.5 M.
FIG. 3.
Difference in conformational stabilities of RML and ME7 PrP27-30. RML and ME7 prion-infected mouse brain homogenates were prepared as described in Materials and Methods. Brain homogenates were treated with or without GdnHCl at the final concentrations shown, followed by incubation in the presence or absence of PK and methanol precipitation. Each track was loaded with 50 μg of total protein, and samples were analyzed by SDS-PAGE and Western blotting, using anti-PrP monoclonal antibody 683. Molecular mass markers (kDa) are shown on the left.
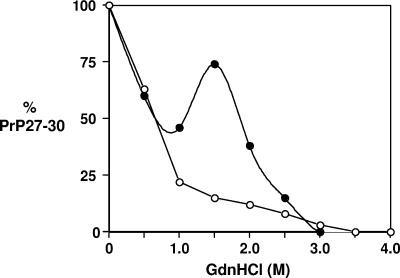
In order to quantify the relative stabilities of RML and ME7 PrPSc, we used ELISA to measure the effect of GdnHCl on the sensitivity of PrP27-30 to PK-induced proteolysis. Aliquots of RML and ME7 terminally diseased brain homogenate were incubated with increasing amounts of GdnHCl and 32 μg/ml PK in a similar manner to that already described. The samples were subsequently treated with NaPTA to precipitate PrP27-30, which was denatured with 6 M GdnHCl and subsequently subjected to direct ELISA with monoclonal antibody 6H4. Figure 4 shows that both RML and ME7 PrP27-30 proteins were fully denatured by concentrations of GdnHCl above 3.0 M. However, each prion strain was characterized by a unique denaturation curve for its associated PK-resistant core of PrPSc. A significant reduction in RML PrP27-30 occurred by treatment with >1.5 M GdnHCl. The denaturation profile of RML PrP27-30 was monophasic, which suggests a population of PrPSc molecules with uniform conformational stability. In contrast, significant levels of ME7 PrP27-30 were still evident following treatment with concentrations of GdnHCl of ≥2.0 M. Furthermore, the denaturation of ME7 PrP27-30 was biphasic, which may suggest multiple distinct populations of PrPSc conformers with different stabilities or two kinetic steps to the breakdown of PrPSc by GdnHCl. For example, one step may break PrPSc aggregates into smaller fragments and the other may denature tertiary and secondary structures.
FIG. 4.
Conformational stabilities of RML and ME7 PrP27-30 proteins, measured by direct ELISA. RML (○) and ME7 (•) prion-infected mouse brain homogenates were prepared as described in Materials and Methods. Brain homogenates were treated with various concentrations of GdnHCl, followed by 32 μg/ml of PK, prior to treatment with NaPTA. All samples were denatured using 6 M GdnHCl before inoculation onto ELISA plates. Biotinylated anti-PrP monoclonal antibody 6H4 was used as the detector antibody. Results are shown as percent PrP27-30 for triplicate wells. Data shown represent one of three experiments demonstrating similar trends.
Detection of PK-sensitive PrPSc in prion-infected brain material.
The fact that RML and ME7 were characterized by PrPSc molecules of different stabilities suggested that these prion strains may also differ in their levels of PK-sensitive and -resistant disease-associated PrP. Our isolation of RML and ME7 PrPSc without the use of PK for quantitation by CDI allowed us to investigate the levels of PK-sensitive and -resistant PrPSc conformers associated with each of these prion strains. Accordingly, RML and ME7 prion-infected mouse brain samples were subjected to Sarkosyl extraction, and precipitated PrPSc was quantified by CDI, before and after treatment of the samples with various concentrations of PK.
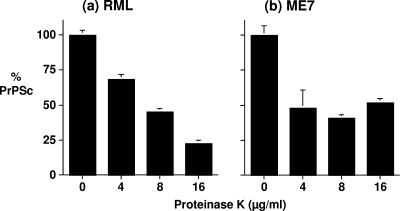
Figure 5 shows the amounts of RML and ME7 PrPSc detected by CDI after PK digestion relative to the amounts detected in the absence of proteolytic digestion. Treatment with 8 μg/ml PK reduced the levels of both RML and ME7 PrPSc to approximately 50% of the levels seen in non-PK-digested samples. No further reduction in the level of ME7 PrPSc was seen following treatment with 16 μg/ml PK. In contrast, the level of RML PrPSc was reduced to approximately 25% of the control level following exposure to 16 μg/ml PK enzyme. Collectively, these data show that although both RML and ME7 comprise PK-resistant and PK-sensitive conformers of PrPSc, RML is characterized by a greater percentage of PK-sensitive material.
FIG. 5.
Relative PK resistances of RML and ME7 PrPSc proteins, measured by CDI. RML (a) and ME7 (b) prion-infected mouse brain homogenates were prepared in Sarkosyl as described in Materials and Methods. Brain homogenates were captured by purified anti-PrP monoclonal antibody V24 following treatment with 0, 4, 8, or 16 μg/ml of PK for 30 min at 37°C. Samples were detected by CDI, using biotinylated anti-PrP monoclonal antibody 6H4. Results are shown as percentages of PrPSc in comparison with the control value ± SD for triplicate wells. Data shown represent one of three experiments demonstrating similar trends.
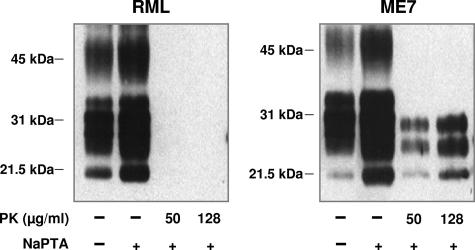
Different conformers of PK-resistant RML and ME7 PrPSc.
While RML- and ME7-infected brain homogenates contained similar levels of PK-resistant PrPSc, this did not exclude the possibility that these different prion strains were characterized by different conformers of protease-resistant PrP. To examine whether RML and ME7 PrPSc proteins were characterized by different conformers of PK-resistant PrPSc, we exposed brain homogenates infected with either of these two prion strains to PK and recovered the resultant PK-resistant material by NaPTA precipitation for analysis by Western blotting. Figure 6 shows that following digestion with PK, significant levels of PrP27-30 were recovered from ME7 brain homogenate following Sarkosyl extraction and NaPTA precipitation. In contrast, very little, if any, PrP27-30 was recovered from RML-infected brain homogenates. Similar levels of PrP27-30 were recovered from RML and ME7 brain homogenates following Sarkosyl extraction and NaPTA precipitation in the absence of prior PK digestion. These data suggest that RML PrP27-30 was rendered unstable in the presence of Sarkosyl compared to that from ME7 and, consequently, acquired a greater sensitivity to proteolytic digestion.
FIG. 6.
Western blot analysis of PK-resistant PrPSc conformers. RML and ME7 prion-infected mouse brain homogenates were prepared with Sarkosyl as described in Materials and Methods. Brain homogenates were treated with various concentrations of PK and with or without NaPTA prior to analysis by SDS-PAGE and Western blotting, using anti-PrP monoclonal antibody 683. Each track was loaded with 50 μg of total protein. Molecular mass markers (kDa) are shown on the left.
In order to confirm that this was the case, we exposed RML- and ME7-infected brain homogenates to PK in the presence of various concentrations of Sarkosyl, and the resultant PrP27-30 proteins were analyzed by Western blotting. Figure 7 shows that Sarkosyl had little effect, if any, upon the PK digestion of ME7 PrP27-30, since similar levels were evident following proteolysis in the presence or absence of detergent. In contrast, Sarkosyl had a significant effect upon RML PrP27-30, as decreasing levels of the PrPSc PK-resistant core were evident following proteolysis in the presence of increasing amounts of detergent. These experiments reinforce our view that RML PrPSc is more unstable than that associated with ME7.
FIG. 7.
Western blot analysis of Sarkosyl-treated RML and ME7 PrP27-30. RML and ME7 prion-infected mouse brain homogenates were prepared as described in Materials and Methods. Brain homogenates were treated with various concentrations of Sarkosyl in PBS, followed by treatment with or without PK prior to analysis by SDS-PAGE and Western blotting, using anti-PrP monoclonal antibody 683. Each track was loaded with 50 μg of total protein. Molecular mass markers (kDa) are shown on the left.
DISCUSSION
A feature of prion diseases is that the transmissible prion agent shows strain variation. In the context of the protein-only hypothesis, different prion strains within the same host must maintain distinct structures of PrPSc. Consequently, a major goal in prion biology is to establish how these different conformers of PrPSc may be distinguished at the molecular level and how the molecular properties of different prion strains account for their different biological properties. This is particularly relevant to scrapie disease of sheep, since several ovine prion strains have reportedly been identified by serial passage in inbred mice (5-7, 12, 16). In order to establish molecular strategies for strain typing mouse-passaged ovine prions, we have investigated the biochemical and physicochemical properties of PrPSc associated with the Prnpa mouse-adapted sheep scrapie strains RML and ME7. These two prion strains display distinct biological properties, such as different incubation times and lesion profiles. Our data presented here show that RML and ME7 prions, passaged in mice with the same PrP amino acid sequence, are characterized by distinct conformers of PrPSc. Significantly, we have identified that the PrPSc forms associated with these two mouse-adapted ovine prion strains show different glycoform ratios and that RML PrP27-30 is less stable than that of ME7 prions.
The RML and ME7 strains used here were passaged in CD-1 and C57BL/6 mice, respectively, which are both of the mouse Prnpa genotype. In control CD-1 and C57BL/6 mice, PrPC shows similar molecular masses and similar glycoform patterns, and in both mouse lines, the normal form of PrP is characterized by a predominance of diglycosylated protein. Despite this similarity of PrPC forms in CD-1 and C57BL/6 mice, propagation of RML and ME7 prions in their respective mouse lines was associated with the formation of PrPSc proteins that exhibited strain-specific glycoform patterns. Following isolation of disease-associated PrP by NaPTA precipitation, quantitatively similar levels of RML and ME7 PrPSc were detected by CDI, using anti-PrP monoclonal antibody 245, which recognizes unglycosylated and monoglycosylated PrP, as the detector. However, approximately 30% less PrPSc was detected in RML prion-infected brain homogenates than in ME7 mouse brains when the same PrPSc was measured by CDI using monoclonal antibody 6H4 as the detector reagent. Monoclonal antibody 6H4 recognizes diglycosylated PrP in addition to the unglycosylated and monoglycosylated forms of the prion protein (23), whereas monoclonal antibody 245 does not recognize the diglycosylated form (50). These data therefore indicate that RML is characterized by a lower level of diglycosylated PrPSc.
We used NaPTA to precipitate PrPSc from mouse brains infected with either the RML or ME7 prion strain. The use of NaPTA yielded a consistently increased level of PrP detected by CDI compared to that for samples analyzed without NaPTA precipitation. It follows that isolation of PrPSc with NaPTA may result in the selection of different populations of prion protein aggregates. These aggregates may differ between the RML and ME7 prion strains. This could be investigated by size fractionation of PrPSc from mouse brain homogenates infected with either prion strain in order to determine whether any differences correlate with the presence of different PrP conformers.
Differences in the conformation or oligomeric form of RML and ME7 prions may be associated with differences in the stabilities of their associated PrPSc proteins and their rates of formation and clearance. Safar et al. showed that different prion strains could be distinguished by different levels of PK-sensitive PrPSc (39). In addition, Peretz et al. have shown that prion strains may be characterized by the stability of PrP27-30, as measured by resistance to proteolytic digestion in the presence of increasing concentrations of chaotropic salt (33). The protease-resistant core of PrPSc, PrP27-30, is infectious and can initiate the faithful propagation of strains (3, 38). Significantly, transmissible mink encephalopathy prion strain properties could be preserved after transmission of either the full-length protein or PrP27-30, which indicates that strain characteristics are propagated by the protease-resistant core (3). Here we have found that RML PrP27-30 was less stable than that associated with ME7 prions. RML PrP27-30 was more sensitive to PK digestion following exposure to either GdnHCl or Sarkosyl. Since RML and ME7 exist as the same genotypic form of PrP, this difference in stability indicates a difference in conformation of PrP27-30 between these two prion strains. This conformational variation may arise as a consequence of the different glycoform profiles associated with RML and ME7 PrPSc, which is likely to influence the oligomeric arrangement of PrPSc monomers. Collectively, these observations suggest that RML prions are characterized by smaller and less stable oligomeric forms of PrPSc than those associated with ME7.
It was observed that the PrP content of ME7 prion-infected brain homogenates, after denaturation and PK digestion, displayed a biphasic curve when assessed by the direct anti-PrP ELISA. A similar biphasic response was seen for other prion strains analyzed by Legname et al. (27) in a similar manner. We postulate that the biphasic response seen with ME7 may represent multiple distinct populations of PrPSc conformers with different stabilities or two kinetic steps to the breakdown of PrPSc by GdnHCl. For example, one step may break PrPSc aggregates into smaller fragments and the other may denature secondary and tertiary structures. The PrP fractions resulting from these steps may potentially differ in their sensitivities to PK digestion. However, in our experiments reported here, we did not see a similar biphasic response when the denaturation curve for ME7 prion-infected brain homogenate was analyzed by Western blotting. We consider this variation to be due to the different protocols used for both the initial preparation of samples and the final precipitation and denaturation of PrPSc aggregates in the two different systems used to detect PrP. In the ELISA protocol used here, brain homogenates were initially treated with Sarkosyl, and in the final preparative steps, PK-digested PrPSc was concentrated by NaPTA precipitation followed by a final denaturation step with 6 M GdnHCl prior to immunodetection. The Western blot protocol utilized untreated brain homogenate samples that were methanol precipitated and boiled in the presence of 2-mercaptoethanol in the final preparative steps prior to SDS-PAGE. The mechanism by which GdnHCl denatures PrPSc and renders it sensitive to limited proteolysis is unknown. GdnHCl may disrupt tertiary or quaternary structures of PrPSc, and the rate of this process may be regulated by the size of PrPSc aggregates. Accordingly, there may be differential denaturation and proteolysis induced by GdnHCl and PK, respectively, if ME7 PrPSc aggregates are more complex than those of RML. In such a scheme, differently sized or stabilized aggregates could be selected and measured under different experimental conditions, leading to the observed biphasic response seen by ELISA but not Western blotting. This suggestion would further support our view that ME7 PrPSc is more diverse and stable than that of RML.
A large body of literature now suggests that prion strain-specific information is contained within the conformation of PrPSc (2, 3, 10, 25, 30, 32, 34, 47). However, it remains poorly understood how these changes in the conformation of PrPSc can account for their physiological effects. Studies in other biological systems, such as Saccharomyces cerevisiae, have suggested that the strain-specific properties of different prion strains correlate with physical properties of prion proteins, such as frangibility (4, 22, 45, 46). In the context of mammalian prions, it has recently been shown that smaller aggregates are more infectious than larger oligomeric forms of PrPSc (42). Consequently, it is predictable that smaller aggregates of PrPSc will be formed by prion strains that are more unstable than those that show enhanced stability. This suggestion is supported by the work of Legname et al., who measured the conformational stabilities of 30 different prion isolates from synthetic and naturally occurring PrP sources, using a conformation stability assay similar to that described here (27). When incubation times for these different prion strains were plotted against their relative stability values, a linear relationship was found, indicating that the less stable mammalian prions are more infectious, as judged by their shorter incubation times. This is presumably because unstable prions fragment more easily, giving rise to smaller aggregates of PrPSc that are more infectious than larger aggregates. Here we have shown with several different assay systems that mouse RML prions are more unstable than ME7 prions. This would suggest that the incubation times for these two prion strains in mice should be different. This is the case, since the incubation time for RML in CD-1 mice is 134 days and that for ME7 in C57BL/6 mice is 160 days (49). Furthermore, the shorter incubation time for RML than for ME7 is also seen in tga20 mice, which overexpress murine Prnpa PrP, indicating that PrPSc conformation and therefore stability are maintained at different levels of PrP expression.
Understanding how conformational variation in PrPSc can account for prion strain-specific properties will be enhanced when the structure of the abnormal form of PrP is fully elucidated. Detailed secondary structure analysis of PrPSc has been hampered because of its insolubility. Instead, structures of PrPSc have been inferred from comparative protein folding studies with other amyloidogenic proteins. Despite a diverse population of native, unmodified structures, misfolded amyloidogenic proteins adopt a fibrillar form that displays a common cross-β structure with β-strands perpendicular and β-sheet parallel to the fibril axis (17, 44). Using this framework, several models of PrPSc have been proposed whereby disease-associated PrP has a significant increase in β-sheets compared to the structure of PrPC (11, 18, 24, 41, 43). However, the mechanism of misfolding and the resultant protein folding arrangements are strongly debated. The validity of these models will be tested by their ability to show a mechanism for the generation of different prion strains with the same amino acid sequence of PrP. This may arise through prion strains adopting differential β-sheet folding within PrPSc monomers (41), which has a subsequent effect upon the higher-order structure of PrPSc aggregates. This may begin to explain why some mouse-passaged ovine scrapie strains are unable to form aggregated PrP27-30 following exposure to PK in the presence of detergent (21).
Collectively, our data presented here show that mouse-adapted ovine scrapie strains can be discriminated by their distinct conformers of PrPSc, which provides a basis to investigate their diversity at the molecular level. This type of analysis will be important in providing new and novel information with regards to establishing the true diversity of ovine prion strains and how different conformations of PrPSc may account for their different physiological effects.
Acknowledgments
This work was supported by funds from Defra. L.H. is a recipient of a Defra Ph.D. studentship.
We thank Adriano Aguzzi for the kind gift of the original RML 4.1 inoculum used to generate the RML strain used in this study. We thank Prionics for the kind gift of anti-PrP monoclonal antibody 6H4.
Footnotes
Published ahead of print on 29 August 2007.
REFERENCES
- 1.Aguzzi, A., and M. Polymenidou. 2004. Mammalian prion biology: one century of evolving concepts. Cell 116:313-327. [DOI] [PubMed] [Google Scholar]
- 2.Bessen, R. A., and R. F. Marsh. 1992. Biochemical and physical properties of the prion protein from two strains of the transmissible mink encephalopathy agent. J. Virol. 66:2096-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bessen, R. A., and R. F. Marsh. 1994. Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. J. Virol. 68:7859-7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brachmann, A., U. Baxa, and R. B. Wickner. 2005. Prion generation in vitro: amyloid of Ure2p is infectious. EMBO J. 24:3082-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruce, M. E. 2003. TSE strain variation. Br. Med. Bull. 66:99-108. [DOI] [PubMed] [Google Scholar]
- 6.Bruce, M. E., A. Boyle, S. Cousens, I. McConnell, J. Foster, W. Goldmann, and H. Fraser. 2002. Strain characterization of natural sheep scrapie and comparison with BSE. J. Gen. Virol. 83:695-704. [DOI] [PubMed] [Google Scholar]
- 7.Bruce, M. E., I. McConnell, H. Fraser, and A. G. Dickinson. 1991. The disease characteristics of different strains of scrapie in Sinc congenic mouse lines: implications for the nature of the agent and host control of pathogenesis. J. Gen. Virol. 72:595-603. [DOI] [PubMed] [Google Scholar]
- 8.Castilla, J., P. Saa, C. Hetz, and C. Soto. 2005. In vitro generation of infectious scrapie prions. Cell 121:195-206. [DOI] [PubMed] [Google Scholar]
- 9.Chandler, R. L. 1961. Encephalopathy in mice produced by inoculation with scrapie brain material. Lancet i:1378-1379. [DOI] [PubMed] [Google Scholar]
- 10.Collinge, J., K. C. Sidle, J. Meads, J. Ironside, and A. F. Hill. 1996. Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature 383:685-690. [DOI] [PubMed] [Google Scholar]
- 11.DeMarco, M. L., and V. Daggett. 2004. From conversion to aggregation: protofibril formation of the prion protein. Proc. Natl. Acad. Sci. USA 101:2293-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickinson, A. G., and H. Fraser. 1977. Scrapie pathogenesis in inbred mice: an assessment of host control and response involving many strains of agent, p. 3-14. In V. ter Meulen and M. Katz (ed.), Slow virus infections of the central nervous system. Springer-Verlag, New York, NY.
- 13.Dickinson, A. G., and V. M. Meikle. 1969. A comparison of some biological characteristics of the mouse-passaged scrapie agents, 22A and ME7. Genet. Res. 13:213-225. [DOI] [PubMed] [Google Scholar]
- 14.Dickinson, A. G., V. M. Meikle, and H. Fraser. 1969. Genetical control of the concentration of ME7 scrapie agent in the brain of mice. J. Comp. Pathol. 79:15-22. [DOI] [PubMed] [Google Scholar]
- 15.Dickinson, A. G., V. M. Meikle, and H. Fraser. 1968. Identification of a gene which controls the incubation period of some strains of scrapie agent in mice. J. Comp. Pathol. 78:293-299. [DOI] [PubMed] [Google Scholar]
- 16.Dickinson, A. G., and G. W. Outran. 1988. Genetic aspects of unconventional virus infections: the basis of the virino hypothesis, p. 63-83. In G. Buck and J. Marsh (ed.), Novel infectious agents and the central nervous system. Ciba Foundation symposium 135. John Wiley, Chichester, United Kingdom. [DOI] [PubMed]
- 17.Dobson, C. M. 2001. The structural basis of protein folding and its links with human disease. Philos. Trans. R. Soc. Lond. B 356:133-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Govaerts, C., H. Wille, S. B. Prusiner, and F. E. Cohen. 2004. Evidence for assembly of prions with left-handed beta-helices into trimers. Proc. Natl. Acad. Sci. USA 101:8342-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffith, J. S. 1967. Self-replication and scrapie. Nature 215:1043-1044. [DOI] [PubMed] [Google Scholar]
- 20.Hilmert, H., and H. Diringer. 1984. A rapid and efficient method to enrich SAF-protein from scrapie brains of hamsters. Biosci. Rep. 4:165-170. [DOI] [PubMed] [Google Scholar]
- 21.Kascsak, R. J., R. Rubenstein, P. A. Merz, R. I. Carp, H. M. Wisniewski, and H. Diringer. 1985. Biochemical differences among scrapie-associated fibrils support the biological diversity of scrapie agents. J. Gen. Virol. 66:1715-1722. [DOI] [PubMed] [Google Scholar]
- 22.King, C. Y., and R. Diaz-Avalos. 2004. Protein-only transmission of three yeast prion strains. Nature 428:319-323. [DOI] [PubMed] [Google Scholar]
- 23.Korth, C., B. Stierli, P. Streit, M. Moser, O. Schaller, R. Fischer, W. Schulz-Schaeffer, H. Kretzschmar, A. Raeber, U. Braun, F. Ehrensperger, S. Hornemann, R. Glockshuber, R. Riek, M. Billeter, K. Wuthrich, and B. Oesch. 1997. Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature 390:74-77. [DOI] [PubMed] [Google Scholar]
- 24.Langedijk, J. P., G. Fuentes, R. Boshuizen, and A. M. Bonvin. 2006. Two-rung model of a left-handed beta-helix for prions explains species barrier and strain variation in transmissible spongiform encephalopathies. J. Mol. Biol. 360:907-920. [DOI] [PubMed] [Google Scholar]
- 25.Le Dur, A., V. Beringue, O. Andreoletti, F. Reine, T. L. Lai, T. Baron, B. Bratberg, J. L. Vilotte, P. Sarradin, S. L. Benestad, and H. Laude. 2005. A newly identified type of scrapie agent can naturally infect sheep with resistant PrP genotypes. Proc. Natl. Acad. Sci. USA 102:16031-16036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Legname, G., I. V. Baskakov, H. O. Nguyen, D. Riesner, F. E. Cohen, S. J. DeArmond, and S. B. Prusiner. 2004. Synthetic mammalian prions. Science 305:673-676. [DOI] [PubMed] [Google Scholar]
- 27.Legname, G., H. O. Nguyen, D. Peretz, F. E. Cohen, S. J. DeArmond, and S. B. Prusiner. 2006. Continuum of prion protein structures enciphers a multitude of prion isolate-specified phenotypes. Proc. Natl. Acad. Sci. USA 103:19105-19110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCutcheon, S., N. Hunter, and F. Houston. 2005. Use of a new immunoassay to measure PrP Sc levels in scrapie-infected sheep brains reveals PrP genotype-specific differences. J. Immunol. Methods 298:119-128. [DOI] [PubMed] [Google Scholar]
- 29.McKinley, M. P., R. K. Meyer, L. Kenaga, F. Rahbar, R. Cotter, A. Serban, and S. B. Prusiner. 1991. Scrapie prion rod formation in vitro requires both detergent extraction and limited proteolysis. J. Virol. 65:1340-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monari, L., S. G. Chen, P. Brown, P. Parchi, R. B. Petersen, J. Mikol, F. Gray, P. Cortelli, P. Montagna, B. Ghetti, et al. 1994. Fatal familial insomnia and familial Creutzfeldt-Jakob disease: different prion proteins determined by a DNA polymorphism. Proc. Natl. Acad. Sci. USA 91:2839-2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nonno, R., M. A. Di Bari, F. Cardone, G. Vaccari, P. Fazzi, G. Dell'Omo, C. Cartoni, L. Ingrosso, A. Boyle, R. Galeno, M. Sbriccoli, H. P. Lipp, M. Bruce, M. Pocchiari, and U. Agrimi. 2006. Efficient transmission and characterization of Creutzfeldt-Jakob disease strains in bank voles. PLoS Pathog. 2:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parchi, P., R. Castellani, S. Capellari, B. Ghetti, K. Young, S. G. Chen, M. Farlow, D. W. Dickson, A. A. Sima, J. Q. Trojanowski, R. B. Petersen, and P. Gambetti. 1996. Molecular basis of phenotypic variability in sporadic Creutzfeldt-Jakob disease. Ann. Neurol. 39:767-778. [DOI] [PubMed] [Google Scholar]
- 33.Peretz, D., M. R. Scott, D. Groth, R. A. Williamson, D. R. Burton, F. E. Cohen, and S. B. Prusiner. 2001. Strain-specified relative conformational stability of the scrapie prion protein. Protein Sci. 10:854-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peretz, D., R. A. Williamson, G. Legname, Y. Matsunaga, J. Vergara, D. R. Burton, S. J. DeArmond, S. B. Prusiner, and M. R. Scott. 2002. A change in the conformation of prions accompanies the emergence of a new prion strain. Neuron 34:921-932. [DOI] [PubMed] [Google Scholar]
- 35.Prusiner, S. B. 2004. Early evidence that a protease-resistant protein is an active component of the infectious prion. Cell 116:S109. [DOI] [PubMed] [Google Scholar]
- 36.Prusiner, S. B. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216:136-144. [DOI] [PubMed] [Google Scholar]
- 37.Prusiner, S. B. 2004. Prion biology and diseases, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 38.Prusiner, S. B., M. P. McKinley, K. A. Bowman, D. C. Bolton, P. E. Bendheim, D. F. Groth, and G. G. Glenner. 1983. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell 35:349-358. [DOI] [PubMed] [Google Scholar]
- 39.Safar, J., H. Wille, V. Itri, D. Groth, H. Serban, M. Torchia, F. E. Cohen, and S. B. Prusiner. 1998. Eight prion strains have PrP(Sc) molecules with different conformations. Nat. Med. 4:1157-1165. [DOI] [PubMed] [Google Scholar]
- 40.Safar, J. G., K. Kellings, A. Serban, D. Groth, J. E. Cleaver, S. B. Prusiner, and D. Riesner. 2005. Search for a prion-specific nucleic acid. J. Virol. 79:10796-10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sawaya, M. R., S. Sambashivan, R. Nelson, M. I. Ivanova, S. A. Sievers, M. I. Apostol, M. J. Thompson, M. Balbirnie, J. J. Wiltzius, H. T. McFarlane, A. O. Madsen, C. Riekel, and D. Eisenberg. 2007. Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature 447:453-457. [DOI] [PubMed] [Google Scholar]
- 42.Silveira, J. R., G. J. Raymond, A. G. Hughson, R. E. Race, V. L. Sim, S. F. Hayes, and B. Caughey. 2005. The most infectious prion protein particles. Nature 437:257-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stork, M., A. Giese, H. A. Kretzschmar, and P. Tavan. 2005. Molecular dynamics simulations indicate a possible role of parallel beta-helices in seeded aggregation of poly-Gln. Biophys. J. 88:2442-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sunde, M., L. C. Serpell, M. Bartlam, P. E. Fraser, M. B. Pepys, and C. C. Blake. 1997. Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J. Mol. Biol. 273:729-739. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka, M., P. Chien, N. Naber, R. Cooke, and J. S. Weissman. 2004. Conformational variations in an infectious protein determine prion strain differences. Nature 428:323-328. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka, M., S. R. Collins, B. H. Toyama, and J. S. Weissman. 2006. The physical basis of how prion conformations determine strain phenotypes. Nature 442:585-589. [DOI] [PubMed] [Google Scholar]
- 47.Telling, G. C., P. Parchi, S. J. DeArmond, P. Cortelli, P. Montagna, R. Gabizon, J. Mastrianni, E. Lugaresi, P. Gambetti, and S. B. Prusiner. 1996. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 274:2079-2082. [DOI] [PubMed] [Google Scholar]
- 48.Thackray, A. M., L. Hopkins, and R. Bujdoso. 2007. Proteinase K-sensitive disease-associated ovine prion protein revealed by conformation-dependent immunoassay. Biochem. J. 401:475-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thackray, A. M., M. A. Klein, A. Aguzzi, and R. Bujdoso. 2002. Chronic subclinical prion disease induced by low-dose inoculum. J. Virol. 76:2510-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thackray, A. M., J. Y. Madec, E. Wong, R. Morgan-Warren, D. R. Brown, T. Baron, and R. Bujdoso. 2003. Detection of bovine spongiform encephalopathy, ovine scrapie prion-related protein (PrPSc) and normal PrPc by monoclonal antibodies raised to copper-refolded prion protein. Biochem. J. 370:81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thackray, A. M., S. Yang, E. Wong, T. J. Fitzmaurice, R. J. Morgan-Warren, and R. Bujdoso. 2004. Conformational variation between allelic variants of cell-surface ovine prion protein. Biochem. J. 381:221-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zlotnik, I., and J. C. Rennie. 1965. Experimental transmission of mouse passaged scrapie to goats, sheep, rats and hamsters. J. Comp. Pathol. 75:147-157. [DOI] [PubMed] [Google Scholar]