Abstract
Proliferation responses of naïve CD4+ T cells to T-cell receptor and interleukin-7 (IL-7) stimulation were evaluated by using cells from human immunodeficiency virus-positive (HIV+) donors. IL-7 enhanced responses to T-cell receptor stimulation, and the magnitude of this enhancement was similar in cells from healthy controls and from HIV+ subjects. The overall response to T-cell receptor stimulation alone or in combination with IL-7, however, was diminished among viremic HIV+ donors and occurred independent of antigen-presenting cells. Frequencies of CD127+ cells were related to the magnitudes of proliferation enhancement that were mediated by IL-7. Thus, IL-7 enhances but does not fully restore the function of naïve CD4+ T cells from HIV-infected persons.
Interleukin-7 (IL-7) plays an important role in T-cell homeostasis by modulating thymic output (1, 16, 22) and by enhancing the peripheral expansion and survival of both naïve and memory T-cell subsets (12, 18, 20, 25, 26, 31, 32). Under normal circumstances, the homeostatic maintenance of naïve CD4+ T cells is regulated by at least two types of signals that include T-cell receptor (TCR) engagement and IL-7 (10, 26, 30). In addition, IL-7 may play an important role in the conversion of effector T cells into long-term memory cells (12, 14).
Homeostasis of T cells is dysregulated in human immunodeficiency virus (HIV) infection such that there is a marked depletion of CD4+ cells and a progressive loss of naïve CD4 and CD8+ T cells (24). Although the mechanisms for these deficiencies are not fully understood, it is possible that impairments in T-cell proliferation and responsiveness to immunomodulatory cytokines could play a role. In HIV disease, IL-7 is increased in plasma (2, 5, 11, 15, 19, 21, 23) and the alpha chain of the IL-7 receptor, CD127, is less frequently expressed among T lymphocytes (2, 5, 11, 21, 23). The ability of patient T cells to respond to IL-7 stimulation may be diminished in HIV disease but may not be related to the density of CD127 expression as it is in T cells from healthy controls (4). Moreover, the responsiveness of T cells, including naïve CD4+ lymphocytes, to TCR stimulation is diminished in HIV disease (27-29). Thus, defects in responsiveness to cytokines or TCR stimulation could contribute to the perturbations in T-cell proliferation and survival in HIV disease.
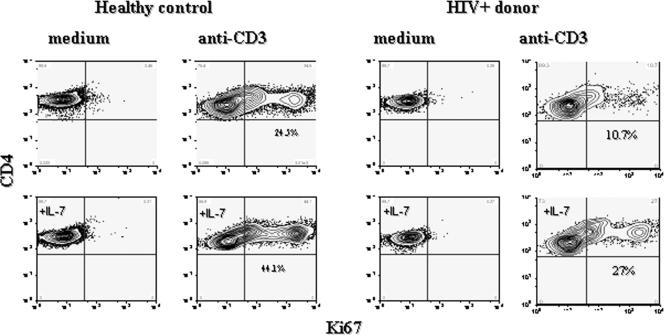
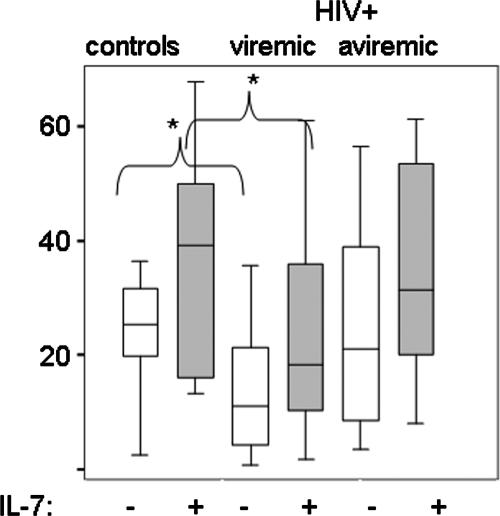
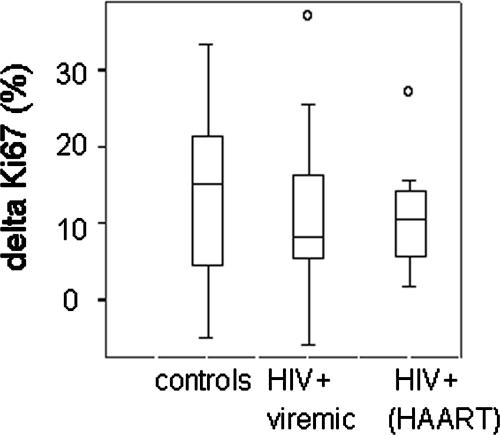
In these studies, we examined the responsiveness of naïve CD4+ T cells from viremic HIV-positive (HIV+) donors (median plasma HIV RNA level, 25,200 copies/ml [range, 1,015 to 1,000,000 copies/ml]; median CD4 cell count, 429 cells/μl [range, 41 to 950 cells/μl]; median age, 38 years [range, 22 to 64 years]; n = 25) and aviremic, highly active antiretroviral therapy (HAART)-treated HIV+ donors (plasma HIV RNA level, <400 copies/ml; median CD4 cell count, 309 cells/μl [range, 74 to 918 cells/μl]; median age, 48 years [range, 37 to 55 years]; n = 12) to the combined stimulus of recombinant IL-7 (Cytheris) plus agonistic anti-CD3 antibody. Peripheral blood mononuclear cells (PBMC) were depleted of CD45RO+ cells by magnetic bead depletion (>90% purity) and were incubated in medium alone or were stimulated with anti-CD3 antibody, IL-7, or anti-CD3 antibody plus IL-7. CD4+CD45RO−CD28+CD27+ cells were assessed for the expression of Ki67 2 days poststimulation by flow cytometric analyses. The addition of IL-7 to anti-CD3 antibody enhanced the induction of Ki67 expression in cells from both HIV+ and HIV-negative (HIV−) donors (Fig. 1 and Fig. 2). A diminished response to anti-CD3 antibody was observed among naïve CD4+ T cells from viremic HIV+ donors. In contrast, cells from aviremic HIV+ donors (all receiving antiretroviral therapy) had normal responses to anti-CD3 antibody compared to cells from healthy donors (Fig. 2). Importantly, the addition of IL-7 to the cultures significantly improved the responses to above those observed with anti-CD3 alone in HIV− and HIV+ donors, regardless of viremia (Wilcoxon signed ranks test; for each comparison, P was <0.04), and the magnitude of that enhancement, although slightly diminished in cells from HIV+ donors, was not significantly different between groups of subjects when measured as either the enhancement (n-fold; not shown) or as the change in percent Ki67+ cells above the background observed for cells stimulated with anti-CD3 alone (Fig. 3). Although IL-7 enhanced responses to TCR stimulation in HIV− subjects, the overall magnitude of the responses among cells from HIV viremic subjects did not reach the levels seen with cells from healthy donors, even in the presence of IL-7 (Fig. 2). It should be noted, however, that these functional readouts were not related to clinical indices of plasma HIV RNA level, CD4 cell count, or age when considered as continuous variables, suggesting that the functional perturbations in naïve CD4+ T cells are probably undermined by complexities extending beyond HIV replication (not shown). Together, these results suggest that TCR responsiveness is diminished in naïve CD4+ T cells from viremic HIV+ subjects, whereas responsiveness to IL-7 stimulation is relatively preserved.
FIG. 1.
IL-7 enhances the induction of Ki67 expression in naïve CD4+ T cells from healthy controls and HIV+ donors. CD45RO-depleted PBMC were incubated with anti-CD3 antibody (100 ng/ml), IL-7 (50 ng/ml), anti-CD3 antibody plus IL-7, or medium alone (RPMI with 10% fetal bovine serum). Cells were gated on CD4+CD27+CD28+ lymphocytes and examined for Ki67 expression by intracellular flow cytometry.
FIG. 2.
IL-7 responsiveness in cells from viremic and aviremic HIV+ donors. Plotted values represent the percentages of CD4+CD27+CD28+CD45RO− T cells that expressed Ki67 after a 2-day incubation with anti-CD3 or with anti-CD3 plus IL-7. Percentages of Ki67+ cells in cultures without stimulation or with IL-7 only were subtracted from the values shown. Responses of cells from healthy controls (n = 9), HIV+ subjects with plasma HIV RNA levels of >400 copies/ml (n = 25), and HIV+ subjects on HAART with suppressed viral replication (<400 copies/ml; n = 12) are shown. Statistically significant differences between cells from controls and HIV+ donors are indicated. Analyses included Kruskal-Wallis test (P = 0.002) for multigroup comparisons and Mann-Whitney U test for comparison of two groups (*, P < 0.05).
FIG. 3.
IL-7 enhances responses to anti-CD3 antibody stimulation to a similar degree in cells from HIV+ and HIV− donors. Naïve CD4+ T cells were incubated with IL-7, anti-CD3, anti-CD3 plus IL-7, or medium alone for 2 days. Background division (percent Ki67+ cells) in medium alone or IL-7 alone was first subtracted from the responses observed with cells stimulated with anti-CD3 alone or with anti-CD3 plus IL-7, respectively. The magnitude of IL-7 enhancement was then calculated by subtracting the percentage of naïve CD4+ cells that expressed Ki67+ after anti-CD3 antibody stimulation from the percentage of naïve CD4+ cells that expressed Ki67 after stimulation with anti-CD3 plus IL-7. n = 9, 25, and 12 for healthy controls, viremic subjects, and aviremic subjects, respectively.
Previous studies indicate that the frequency of CD127+ T cells, particularly memory T-cell subsets, is reduced in patients with HIV disease (5, 11, 21, 23). This could, in part, result from the modulation of receptor expression through increased exposure to IL-7 in vivo and also may reflect accumulation of CD127− effector memory cells (21). We assessed the expression of CD127 in naïve CD4+CD45RA+CD28+CD27+ and memory CD4+CD45RO+ T cells in a subset of patients and asked if the frequencies of CD127+ cells were related to the induction of Ki67 expression by anti-CD3 or by anti-CD3 plus IL-7 among naïve CD4+ T cells. We reasoned that the ability of IL-7 to enhance responses to TCR stimulation might be limited if CD127 expression was diminished among naïve CD4+ T cells from HIV+ donors. Alternatively, a defect in functional responses also could be related to increased exposure to IL-7 in vivo, as may be reflected by the absence of CD127 receptor expression on memory T-cell subsets.
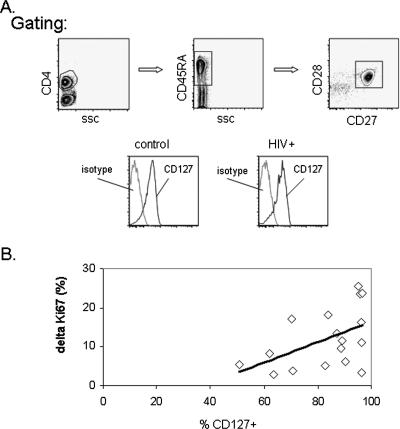
In agreement with previous studies, our results suggest that CD127 expression is relatively preserved in naïve CD4+ T cells from HIV+ donors (representative histograms in Fig. 4) (mean percentage of CD127+ cells, 87 and 83 for HIV− donors [n = 5] and HIV+ donors [n = 17], respectively; P = 0.96) but is diminished in memory CD4+ T cells from HIV+ donors (mean percentage of CD127+ cells, 83 and 59 for HIV− and HIV+ donors, respectively; P = 0.01). The frequencies of CD127+ naïve T cells were directly related to the frequencies of CD127+ memory T cells (Spearman's correlations; r = 0.711, P = 0.001; n = 18) in HIV+ subjects. This result suggests that a similar mechanism modulates the expression of CD127 in these T-cell subsets, even though the loss of CD127 expression is clearly greater among the memory T cells in HIV disease. Neither CD127 expression among naïve CD4+ T cells nor CD127 expression among memory CD4+ T cells was related to the functional response of naïve CD4+ T cells to anti-CD3 (r = 0.238 and P = 0.36 for naïve CD127 expression; r = 0.293 and P = 0.25 for memory CD127 expression) or to anti-CD3 plus IL-7 (r = 0.32 and P = 0.21 for naïve CD127 expression; r = 0.31 and P = 0.22 for memory CD127 expression). There was a relationship between the percentage of CD127+ naïve T cells and the delta Ki67 expression that resulted from the addition of IL-7 to anti-CD3-treated cultures (percentage of Ki67+ cells in cultures treated with anti-CD3 plus IL-7 minus the percentage of Ki67+ cells in cultures treated with anti-CD3 alone) (Fig. 4). This relationship was statistically significant by Pearson's correlation (r = 0.5, P = 0.041), the use of which was justified based on the normal distribution of the data. Spearman's analysis, which is independent of data distribution, indicated a similar trend that was not statistically significant (r = 0.41, P = 0.1). The mean fluorescence intensity of CD127 expression on CD4+CD45RA+CD27+CD28+ T cells was not significantly related to the delta Ki67 expression induced by IL-7 but also suggested a trend consistent with a direct relationship between these indices (r = 0.45 and P = 0.07 by Pearson's correlation; r = 0.34 and P = 0.18 by Spearman's correlation). Despite the relative preservation of IL-7 receptor in naïve CD4+ T cells from HIV+ donors, the association between the frequencies of CD127+ cells and CD4+ T-cell proliferation responses to TCR plus IL-7 suggests that subtle IL-7 receptor perturbations might contribute to functional defects of naïve CD4+ T cells in HIV-infected persons.
FIG. 4.
CD127 receptor expression is related to enhancement of proliferation by IL-7. (A) Whole blood from a healthy control and an HIV-infected person was examined by flow cytometry for expression of CD127 on CD4+CD45RA+CD27+CD28+ (naïve) T cells. The gating strategy for identifying naïve cells involved an initial gate for lymphocyte forward and side scatter (SSC) characteristics (not shown) and then sequential gates for CD4 positive, CD45RA positive and, finally, CD28+CD27+ double-positive cells. (B) Plotted values indicating the relationship between the delta Ki67 expression in naïve CD4+ T cells and the percentage of CD127+ naïve T cells that was determined by using freshly isolated whole blood. The delta Ki67 expression was calculated by subtracting the percentage of naïve CD4+ cells that expressed Ki67+ after anti-CD3 antibody stimulation from the percentage of naïve CD4+ cells that expressed Ki67 after stimulation with anti-CD3 plus IL-7.
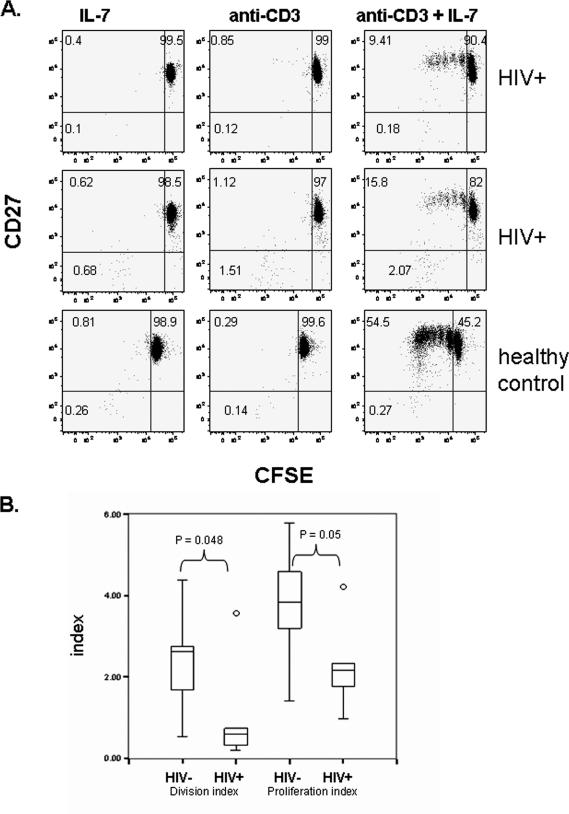
To consider the possibility that antigen-presenting cells could contribute to the diminished response of T-cells to stimulation with TCR plus IL-7, we next asked if defects in TCR-plus-IL-7 stimulation could be detected in purified naïve CD4+ T-cell populations. CD4+CD45RO− cells were negatively selected by magnetic bead depletion, achieving a purity of >90% as determined by flow cytometric analyses. Purified naïve CD4+ T cells were labeled with carboxy fluorescein succinimidyl ester (CFSE) tracking dye and incubated with IL-7, anti-CD3 antibody that was immobilized on a plate, anti-CD3 plus IL-7, or medium alone. The induction of proliferation was measured 7 days later by the dilution of CFSE tracking dye among CD4+CD27+ cells by calculating the division index (average number of cell divisions of all CD4+CD27+ cells) and the proliferation index (average number of divisions of CD4+CD27+ cells that had diluted tracking dye; Flow-Jo analysis software). These purified CD4+ T cells proliferated poorly in response to anti-CD3 antibody stimulation alone, providing functional evidence that the samples were free of antigen-presenting cell contamination (Fig. 5A). The combined treatment of anti-CD3 and IL-7 induced cellular expansion, whereas alone, neither stimulus induced cellular proliferation during the 7-day period (Fig. 5A). Responses of cells from HIV+ donors were reduced compared to those of cells from healthy donors, confirming that the defects in naïve CD4+ T-cell expansion are independent of antigen-presenting cells and not fully corrected by IL-7 (Fig. 5B).
FIG. 5.
Diminished responses to TCR plus IL-7 in purified naïve CD4+ T cells from HIV+ donors. CD4+CD45RO− cells were purified from PBMC by negative selection. Cells from HIV+ donors (n = 7) and healthy controls (n = 7) were labeled with CSFE and incubated with anti-CD3 immobilized on a plate (5 μg/ml, overnight at 4°C) plus IL-7 (10 ng/ml). CFSE dye dilution was measured among the CD4+CD27+ cells. (A) Representative histograms showing the dilution of CFSE and CD27 expression among cells incubated with anti-CD3 antibody alone, IL-7 alone, or the combination of anti-CD3 plus IL-7. Placements of quadrant gates were based on an isotype control antibody stain (for CD27 expression) and on cells that had been incubated in medium alone (for CFSE dye dilution). (B) Division indices (average number of cell divisions among CD4+CD27+ cells) and proliferation indices (average number of cell divisions among CD4+CD27+ cells that had diluted tracking dye) are shown.
IL-7 is a promising candidate for therapeutic and vaccine adjuvant applications in HIV disease. This cytokine may be especially beneficial in circumstances of immune reconstitution, since it normally plays an essential role in T-cell proliferation and survival. Here, we demonstrate that IL-7 efficiently enhances TCR-triggered naïve CD4+ T-cell expansion in cells from healthy individuals and from HIV+ donors. The mechanism of IL-7 activity is not discerned in these experiments but may involve effects on survival, such as the induction of Bcl-2 (9), or may involve the enhancement of IL-2 or IL-2 receptor expression (6, 8). In any case, our studies provide evidence that IL-7 should provide an effective therapy for the regulation of naïve CD4+ T-cell homeostasis and may be useful for vaccine adjuvant applications in HIV disease. The potential of this approach has been illustrated by recent human trials of IL-7 that demonstrated the expansion of naïve T cells in vivo after IL-7 administration to HIV-infected persons (13) and by animal studies, wherein IL-7 administration enhanced T-cell responses to immunization in mice (17).
Notably, the depletion studies and purification methods employed here did not necessarily eliminate terminally differentiated effector memory CD4+ T cells from our cultures; however, studies of CMV-specific terminally differentiated cells suggested that these cells are primarily CD27− (3), and the use of three markers to identify naïve CD4+ T cells, including the ones used here (CD27, CD28, and CD45RO) is estimated to provide 98% assurance that the cells being examined are truly naïve (7). Thus, it is likely that terminally differentiated cells were largely removed from our analyses.
Our observations provide confirmation of a significant defect in the responses of naïve CD4+ T cells to TCR triggering in HIV disease, and this defect is not fully corrected by IL-7, as shown here, or by IL-2, as we demonstrated previously (27). These deficiencies are reproduced even among naïve CD4+ T cells that are purified from professional antigen-presenting cells, indicating that the defects are intrinsic to the T cells and not a consequence of dysfunctional antigen-presenting cells. We propose that functional defects in naïve CD4+ T cells from HIV+ donors stem primarily from deficiencies in TCR signaling. Further studies that define the nature of naïve CD4+ T-cell defects in HIV disease will be required to address the underlying mechanisms.
Acknowledgments
We thank Robert Asaad for obtaining patient blood samples for these studies and Cytheris for providing the IL-7 reagent.
This work was supported by Case/UHC Center for AIDS Research (grant AI36219) and by grant AI-68636.
Footnotes
Published ahead of print on 12 September 2007.
REFERENCES
- 1.Bolotin, E., M. Smogorzewska, S. Smith, M. Widmer, and K. Weinberg. 1996. Enhancement of thymopoiesis after bone marrow transplant by in vivo interleukin-7. Blood 88:1887-1894. [PubMed] [Google Scholar]
- 2.Boulassel, M. R., M. Young, J. P. Routy, R. P. Sekaly, C. Tremblay, and D. Rouleau. 2004. Circulating levels of IL-7 but not IL-15, IGF-1, and TGF-beta are elevated during primary HIV-1 infection. HIV Clin. Trials 5:357-359. [DOI] [PubMed] [Google Scholar]
- 3.Casazza, J. P., M. R. Betts, D. A. Price, M. L. Precopio, L. E. Ruff, J. M. Brenchley, B. J. Hill, M. Roederer, D. C. Douek, and R. A. Koup. 2006. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J. Exp. Med. 203:2865-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colle, J. H., J. L. Moreau, A. Fontanet, O. Lambotte, J. F. Delfraissy, and J. Theze. 2007. The correlation between levels of IL-7Rα expression and responsiveness to IL-7 is lost in CD4 lymphocytes from HIV-infected patients. AIDS 21:101-103. [DOI] [PubMed] [Google Scholar]
- 5.Colle, J. H., J. L. Moreau, A. Fontanet, O. Lambotte, M. Joussemet, J. F. Delfraissy, and J. Theze. 2006. CD127 expression and regulation are altered in the memory CD8 T cells of HIV-infected patients—reversal by highly active anti-retroviral therapy (HAART). Clin. Exp. Immunol. 143:398-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costello, R., H. Brailly, F. Mallet, C. Mawas, and D. Olive. 1993. Interleukin-7 is a potent co-stimulus of the adhesion pathway involving CD2 and CD28 molecules. Immunology 80:451-457. [PMC free article] [PubMed] [Google Scholar]
- 7.De Rosa, S. C., L. A. Herzenberg, L. A. Herzenberg, and M. Roederer. 2001. 11-color, 13-parameter flow cytometry: identification of human naive T cells by phenotype, function, and T-cell receptor diversity. Nat. Med. 7:245-248. [DOI] [PubMed] [Google Scholar]
- 8.Gringhuis, S. I., L. F. de Leij, E. W. Verschuren, P. Borger, and E. Vellenga. 1997. Interleukin-7 upregulates the interleukin-2-gene expression in activated human T lymphocytes at the transcriptional level by enhancing the DNA binding activities of both nuclear factor of activated T cells and activator protein-1. Blood 90:2690-2700. [PubMed] [Google Scholar]
- 9.Hernandez-Caselles, T., M. Martinez-Esparza, D. Sancho, G. Rubio, and P. Aparicio. 1995. Interleukin-7 rescues human activated T lymphocytes from apoptosis induced by glucocorticoesteroids and regulates bcl-2 and CD25 expression. Hum. Immunol. 43:181-189. [DOI] [PubMed] [Google Scholar]
- 10.Kieper, W. C., J. T. Burghardt, and C. D. Surh. 2004. A role for TCR affinity in regulating naive T cell homeostasis. J. Immunol. 172:40-44. [DOI] [PubMed] [Google Scholar]
- 11.Koesters, S. A., J. B. Alimonti, C. Wachihi, L. Matu, O. Anzala, J. Kimani, J. E. Embree, F. A. Plummer, and K. R. Fowke. 2006. IL-7Rα expression on CD4+ T lymphocytes decreases with HIV disease progression and inversely correlates with immune activation. Eur. J. Immunol. 36:336-344. [DOI] [PubMed] [Google Scholar]
- 12.Kondrack, R. M., J. Harbertson, J. T. Tan, M. E. McBreen, C. D. Surh, and L. M. Bradley. 2003. Interleukin 7 regulates the survival and generation of memory CD4 cells. J. Exp. Med. 198:1797-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy, Y., L. Weis, J. P. Viard, J. Molina, C. Goujard, F. Boue, S. Delluc, C. Rouzioux, M. Morre, and J. Delfraissy. 2007. Abstr. 14th Conf. Retrovir. Opportunistic Infect., abstr. 127. Conference on Retroviruses and Opportunistic Infections, Los Angeles, CA.
- 14.Li, J., G. Huston, and S. L. Swain. 2003. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J. Exp. Med. 198:1807-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Llano, A., J. Barretina, A. Gutierrez, J. Blanco, C. Cabrera, B. Clotet, and J. A. Este. 2001. Interleukin-7 in plasma correlates with CD4 T-cell depletion and may be associated with emergence of syncytium-inducing variants in human immunodeficiency virus type 1-positive individuals. J. Virol. 75:10319-10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackall, C. L., T. J. Fry, C. Bare, P. Morgan, A. Galbraith, and R. E. Gress. 2001. IL-7 increases both thymic-dependent and thymic-independent T-cell regeneration after bone marrow transplantation. Blood 97:1491-1497. [DOI] [PubMed] [Google Scholar]
- 17.Melchionda, F., T. J. Fry, M. J. Milliron, M. A. McKirdy, Y. Tagaya, and C. L. Mackall. 2005. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J. Clin. Investig. 115:1177-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moniuszko, M., T. Fry, W. P. Tsai, M. Morre, B. Assouline, P. Cortez, M. G. Lewis, S. Cairns, C. Mackall, and G. Franchini. 2004. Recombinant interleukin-7 induces proliferation of naive macaque CD4+ and CD8+ T cells in vivo. J. Virol. 78:9740-9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Napolitano, L. A., R. M. Grant, S. G. Deeks, D. Schmidt, S. C. De Rosa, L. A. Herzenberg, B. G. Herndier, J. Andersson, and J. M. McCune. 2001. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat. Med. 7:73-79. [DOI] [PubMed] [Google Scholar]
- 20.Nugeyre, M. T., V. Monceaux, S. Beq, M. C. Cumont, R. Ho Tsong Fang, L. Chene, M. Morre, F. Barre-Sinoussi, B. Hurtrel, and N. Israel. 2003. IL-7 stimulates T cell renewal without increasing viral replication in simian immunodeficiency virus-infected macaques. J. Immunol. 171:4447-4453. [DOI] [PubMed] [Google Scholar]
- 21.Paiardini, M., B. Cervasi, H. Albrecht, A. Muthukumar, R. Dunham, S. Gordon, H. Radziewicz, G. Piedimonte, M. Magnani, M. Montroni, S. M. Kaech, A. Weintrob, J. D. Altman, D. L. Sodora, M. B. Feinberg, and G. Silvestri. 2005. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J. Immunol. 174:2900-2909. [DOI] [PubMed] [Google Scholar]
- 22.Pido-Lopez, J., N. Imami, D. Andrew, and R. Aspinall. 2002. Molecular quantitation of thymic output in mice and the effect of IL-7. Eur. J. Immunol. 32:2827-2836. [DOI] [PubMed] [Google Scholar]
- 23.Rethi, B., C. Fluur, A. Atlas, M. Krzyzowska, F. Mowafi, S. Grutzmeier, A. De Milito, R. Bellocco, K. I. Falk, E. Rajnavolgyi, and F. Chiodi. 2005. Loss of IL-7Rα is associated with CD4 T-cell depletion, high interleukin-7 levels and CD28 down-regulation in HIV infected patients. AIDS 19:2077-2086. [DOI] [PubMed] [Google Scholar]
- 24.Roederer, M., J. G. Dubs, M. T. Anderson, P. A. Raju, and L. A. Herzenberg. 1995. CD8 naive T cell counts decrease progressively in HIV-infected adults. J. Clin. Investig. 95:2061-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schluns, K. S., W. C. Kieper, S. C. Jameson, and L. Lefrancois. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1:426-432. [DOI] [PubMed] [Google Scholar]
- 26.Seddon, B., P. Tomlinson, and R. Zamoyska. 2003. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat. Immunol. 4:680-686. [DOI] [PubMed] [Google Scholar]
- 27.Sieg, S. F., D. A. Bazdar, and M. M. Lederman. 2003. Impaired TCR-mediated induction of Ki67 by naive CD4+ T cells is only occasionally corrected by exogenous IL-2 in HIV-1 infection. J. Immunol. 171:5208-5214. [DOI] [PubMed] [Google Scholar]
- 28.Sieg, S. F., C. V. Harding, and M. M. Lederman. 2001. HIV-1 infection impairs cell cycle progression of CD4+ T cells without affecting early activation responses. J. Clin. Investig. 108:757-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sieg, S. F., J. B. Mitchem, D. A. Bazdar, and M. M. Lederman. 2002. Close link between CD4+ and CD8+ T cell proliferation defects in patients with human immunodeficiency virus disease and relationship to extended periods of CD4+ lymphopenia. J. Infect. Dis. 185:1401-1416. [DOI] [PubMed] [Google Scholar]
- 30.Sprent, J., and C. D. Surh. 2003. Cytokines and T cell homeostasis. Immunol. Lett. 85:145-149. [DOI] [PubMed] [Google Scholar]
- 31.Tan, J. T., E. Dudl, E. LeRoy, R. Murray, J. Sprent, K. I. Weinberg, and C. D. Surh. 2001. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc. Natl. Acad. Sci. USA 98:8732-8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vivien, L., C. Benoist, and D. Mathis. 2001. T lymphocytes need IL-7 but not IL-4 or IL-6 to survive in vivo. Int. Immunol. 13:763-768. [DOI] [PubMed] [Google Scholar]