Abstract
Beta interferon (IFN-β) expression is triggered by double-stranded RNA, a common intermediate in the replication of many viruses including hepatitis C virus (HCV). The recent development of cell culture-grown HCV allowed us to analyze the IFN signaling pathway following virus infection. In this study, we have examined the IFN-β signaling pathway following infection of immortalized human hepatocytes (IHH) with HCV genotype 1a (clone H77) or 2a (clone JFH1). We observed that IHH possesses a functional Toll-like receptor 3 pathway. HCV infection in IHH enhanced IFN-β and IFN-stimulated gene 56 (ISG56) promoter activities; however, poly(I-C)-induced IFN-β and ISG56 expression levels were modestly inhibited upon HCV infection. IHH infected with HCV (genotype 1a or 2a) exhibited various levels of translocation of IRF-3 into the nucleus. The upregulation of endogenous IFN-β and 2′,5′-oligoadenylate synthetase 1 mRNA expression was also observed in HCV-infected IHH. Subsequent studies suggested that HCV infection in IHH enhanced STAT1 and ISG56 protein expression. A functional antiviral response of HCV-infected IHH was observed by the growth-inhibitory role in vesicular stomatitis virus. Together, our results suggested that HCV infection in IHH induces the IFN signaling pathway, which corroborates observations from natural HCV infection in humans.
Hepatitis C virus (HCV) infection is the major cause of chronic liver disease, cirrhosis, and hepatocellular carcinoma. HCV infection affects approximately 3.2 million people in the United States (1, 17, 23). The currently approved treatment for HCV infection is pegylated alpha interferon (IFN-α) in combination with ribavirin. This leads to the clearance of HCV in 50% and 80% of the cases of HCV genotypes 1 and 2, respectively. Type I IFNs are crucial components of the innate immune response to virus attack. The host response is triggered when a pathogen-associated molecular pattern presented by the infecting virus is recognized and engaged by specific pathogen-associated molecular pattern receptor factors expressed in the host cell, initiating signals that ultimately induce the expression of antiviral effecter genes. In hepatocytes (the primary target cells of HCV infection), independent pathways of retinoic acid-inducible gene I (RIG-I) and Toll-like receptor 3 (TLR-3) signaling comprise two major pathways of host defense triggered by double-stranded RNA (dsRNA) (13). IFN-α and IFN-β are rapidly synthesized after virus infection, rapidly triggering intracellular signaling events. The subsequent expression of IFN-stimulated genes (ISGs) is central to these antiviral responses. ISG factor 3 (ISGF3) assembles and translocates from the cytoplasm to the nucleus upon IFN stimulation. ISGF3 is a multisubunit transcription factor that interacts with the IFN-stimulated response element present in the promoters of ISGs (31). ISGF3 consists of hetero-oligomers of signal transducers and activators of transcription 1 (STAT1), STAT2, and IFN-regulatory factor 9 (IRF-9). Homodimers of STAT1α and heterodimers of STAT1 and STAT2 are also activated, and IRF-9 is indispensable for their formation. They bind to inverted repeat elements in the promoters of ISGs to induce transcription (34). Oligonucleotide microarray studies have suggested that about 300 genes are induced in response to type I IFNs in fibrosarcoma cells (8).
How HCV establishes chronic infection is poorly understood. HCV genotype 2a (clone JFH1) infection does not induce IFN-β or ISG expression and prevents poly(I-C)-induced IRF-3 nuclear translocation in Huh-7 cells (7). Several HCV proteins are suggested to interact in the IFN signaling pathway. HCV NS3/4A serine protease blocks phosphorylation and the effecter action of IRF-3, a key cellular antiviral signaling molecule (10). RIG-I has been shown to bind to the secondary structured HCV RNA efficiently to confer IFN-β induction (33). HCV NS2 and NS3/4A proteins are potent inhibitors of host cell cytokine/chemokine gene expression (21). On the other hand, the replication of an HCV subgenomic replicon stimulated the activation of the IFN-β promoter and the production of IFN in human hepatoma cells (9, 12). Furthermore, many ISGs were transcriptionally elevated in chronic HCV infection (5). It is not well understood how IFN-β expression and downstream ISG expression are enhanced during HCV infection. A difference may exist between poly(I-C) or Sendai virus (SenV)- and HCV-induced IFN signaling. In this study, we have investigated the IFN signaling pathway following HCV genotype 1a (clone H77) infection of immortalized human hepatocytes (IHH). Our results demonstrate that HCV infection of IHH enhances IFN-β and STAT1 expression and inhibits vesicular stomatitis virus (VSV) growth.
MATERIALS AND METHODS
Cell lines.
The generation of IHH by the transfection of HCV core was described previously (32). IHH were adopted in Dulbecco's modified Eagle's medium (DMEM) (Cambrex, Walkersville, MD) containing 10% fetal bovine serum, 200 U/ml of penicillin G, and 200 μg/ml of streptomycin at 37°C in an atmosphere of 5% CO2.
Generation of cell culture-grown HCV and infection of IHH.
HCV genotype 1a (clone H77) was grown in IHH as recently described (19). Virus growth was measured from cell culture supernatants filtered through a 0.45-μm cellulose acetate membrane (Nalgene, Rochester, NY) by fluorescent focus-forming assay from serial dilutions. The HCV titer was calculated as ∼105 focus-forming units/ml. HCV genotype 2a (clone JFH1) was grown in Huh-7.5 cells as previously described (19). For infection, IHH were incubated with HCV (multiplicity of infection [MOI] of 0.02) for adsorption. After 5 h of adsorption, DMEM supplemented with 2.5% heat-inactivated fetal bovine serum was added.
Immunofluorescence.
IHH were mock infected or infected with or HCV. At 72 h postinfection, cells were washed and fixed with 3.7% formaldehyde, followed by blocking with 3% bovine serum albumin. Cells were incubated with an HCV NS4-specific fluorescein isothiocyanate-conjugated monoclonal antibody (Biodesign International, Saco, ME) for genotype 1a or anti-HCV NS3 for genotype 2a and an IRF-3-specific antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h. Cells were washed and incubated with either anti-mouse immunoglobulin secondary antibody conjugated with Alexa 488 or anti-rabbit immunoglobulin secondary antibody conjugated with Alexa 568 (Molecular Probes, Eugene, OR) for 1 h at room temperature. Nuclear staining was performed with TO-PRO-3-iodide (Molecular Probes). Finally, cells were washed and mounted for confocal microscopy (Bio-Rad 1024; Bio-Rad Laboratories, Hercules, CA), and the images were superimposed digitally to allow fine comparisons (3).
Luciferase assay.
IHH were infected with HCV at an MOI of 0.02. After 48 h of infection, cells were transfected with plasmid DNAs (0.2 μg) encoding the firefly luciferase gene under the control of the ISG56 promoter (ISG56-luc) or IFN-β promoter (IFN-β-luc). At 30 h posttransfection, cells were lysed with reporter lysis buffer (Promega, WI), and the luciferase activity was determined using a luminometer (Optocomp II; MGM Instruments, Hamden, CT). Luciferase activity was normalized with respect to the protein concentration of the cell lysates. In a different experiment, poly(I-C) at 50 μg/ml (Amersham, Piscataway, NJ) was added directly to the medium of IHH transfected with IFN-β-luc. Alternatively, poly(I-C) at 1.0 μg/ml was complexed with Lipofectamine (Invitrogen, Carlsbad, CA) and introduced into cells by transfection. Cell extracts were prepared for measurements of luciferase activity.
RNA quantitation.
Total RNA was isolated from cells using a Purescript RNA isolation kit (Gentra Systems, Minneapolis, MN). cDNA synthesis was performed using random hexamers. The HCV 5′ nontranslated region and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) were amplified using specific primers (19). PCR amplification was performed on cDNA templates using primers specific for IFN-β (sense primer 5′-GATTCATCTAGCACTGGCTGG-3′ and antisense primer 5′-CTTCAGGTAATGCAGAATCC-3′), for 2′,5′-oligoadenylate synthetase 1 (OAS-1) amplification (sense primer 5′-AGGTGGTAAAGGGTGGCTCC-3′ and antisense primer 5′-ACAACCAGGTCAGCGTCAGAT-3′) (6), and for TLR-3 (sense primer 5′-TCACTTGCTCATTCTCCCTT-3′ and antisense primer 5′-GACCTCTCCATTCCTGGC-3′). For RNA quantitation, real-time PCR was performed using SYBR Green I (ABI PRISM 7700; Applied Biosystems, Foster City, CA). The quantity of specific mRNA was normalized to endogenous references (GAPDH) and expressed as increases (n-fold) over the corresponding mRNA levels in untreated control cells.
Immunoblot analysis.
Cells were harvested using sodium dodecyl sulfate sample buffer. Proteins were subjected to electrophoresis on an 8% polyacrylamide gel and transferred onto a nitrocellulose membrane (Bio-Rad). The membrane was probed with a monoclonal antibody to ISG56 (15), STAT1α (Santa Cruz), or actin (Santa Cruz). Proteins were visualized using an enhanced chemiluminescent ECL Western blot substrate (Pierce, Rockford, IL) scanned by an image analyzer (Amersham Molecular Dynamics, Sunnyvale, CA) to quantify the density of the protein bands using Image Quant software.
Antiviral assay.
HCV-infected (MOI of 0.02) or mock-infected IHH were used for an antiviral bioassay using VSV Indiana serotypes (22). The virus titer in the cell culture supernatant was determined by plaque assay using IHH. After 72 h of HCV infection, cells were washed twice in phosphate-buffered saline and challenged with 50 PFU/well of VSV. Virus was adsorbed onto cells for 1 h at 37°C and washed, and cells were overlaid with an equal volume of 2× Eagle's minimal essential medium (BioWhittaker, Walkersville, MD) and 1% agarose dissolved in water (22). VSV plaques were stained after 24 or 48 h by neutral red and counted the next day. In a different experiment, HCV-infected (MOI of 0.02) or mock-infected IHH were washed after 72 h and challenged with VSV (MOI of 0.2). Culture supernatants were collected at different time points and analyzed for VSV-induced cytopathic effects (CPE) by serial 10-fold dilutions in BHK cells (27). Results were obtained by staining cells with crystal violet after 24 h of incubation.
RESULTS
TLR-3 mRNA is upregulated in IHH following poly(I-C) treatment.
Previously, we reported the growth of HCV genotype 1a in IHH using SABM, highly enriched with different growth factors, and chemically denatured serum (3, 19). The use of chemically denatured fetal bovine serum helped us to be sure that advantageous viruses were not present. However, the cost and lack of consistent availability limit the use of chemically denatured serum. Here, we examined the IFN signaling pathway in IHH infected with HCV. Growth factors in SABM may influence the IFN signaling pathway. To avoid these limitations, we maintained IHH in DMEM containing 10% fetal bovine serum and examined HCV growth. Naïve IHH grown in DMEM or SABM were infected with a known MOI of HCV of 0.02. Five days postinfection, supernatants were collected, and the copy number of the HCV genome was determined as described previously (20). A similar replication of the HCV genome between 107 and 108 genome copies/ml in IHH grown in either of the cell culture media was observed (Fig. 1A). HCV infection of IHH displayed 40 to 60% infectivity, as determined by immunofluorescence using specific antibodies for HCV proteins. We have used HCV genotype 1a in IHH grown in DMEM in our subsequent experiments.
FIG. 1.
IHH enhances TLR-3 mRNA expression following exposure to poly(I-C). IHH maintained in DMEM supplemented with fetal bovine serum support HCV genotype 1a (clone H77) growth. HCV replicated at a similar level in IHH grown in DMEM or SABM (A). IHH were transfected with 0.2 μg of IFN-β-luc plasmid DNA. After 24 h of transfection, poly(I-C) was added to the culture medium (50 μg/ml) or introduced by transfection (1 μg/ml) and incubated for 16 h. Relative luciferase activity was measured and compared with that of an untreated control (B). Semiquantitative RT-PCR was performed using TLR-3- and GAPDH-specific primers from RNA of IHH exposed to poly(I-C). The sizes of the amplified bands were verified by a DNA marker (not shown). The relative levels of TLR-3 mRNA are compared with the expression level of the housekeeping gene GAPDH (C).
Huh-7 cells or its derivatives are defective in the TLR-3 or RIG-I pathway (26, 37). To examine whether IHH possess an active TLR-3 pathway, we initially characterized the poly(I-C)-induced activation of the IFN-β promoter in IHH. TLR-3 plays an important role in the activation of the IFN-β promoter following exposure to extracellular poly(I-C) (35). Transfection of poly(I-C), mimicking intracellular dsRNA generated during viral replication, induced IFN-β promoter activation in IHH. When poly(I-C) was added to the culture medium of IHH, approximately sixfold up-regulation of IFN-β promoter activity was observed (Fig. 1B). Semiquantitative reverse transcription (RT)-PCR was performed using TLR-3-specific primers from RNA isolated from untreated control IHH, poly(I-C)-transfected IHH, or the exogenous addition of poly(I-C) in IHH. An upregulation of TLR-3 expression was also observed following poly(I-C) exposure (Fig. 1C). Our results suggested that IHH possess a functional TLR-3 pathway and can recognize dsRNAs.
HCV inhibits poly(I-C)-induced IFN-β signaling.
HCV genotype 2a (clone JFH1) blocks poly(I-C)-induced IFN-β and ISG expression in Huh-7 cells lacking TLR-3 signaling (7). The human 561 gene, encoding protein ISG56, was most strongly induced in response to type I IFN or dsRNA (2, 15) and inhibits cellular protein synthesis. We examined whether HCV genotype 1a (clone H77) can modulate the activation of poly(I-C)-induced IFN-β and ISG56 promoter activities in IHH using in vitro reporter assays. We have observed that the poly(I-C)-induced enhancement of IFN-β and ISG56 promoter activities are inhibited following HCV infection of IHH (Fig. 2). Our results corroborate previous observations (7) that HCV genotype 2a (clone JFH1) infection fails to induce IFN-β and ISG56 promoter activities in Huh-7 cells following exposure to synthetic dsRNA. However, HCV genotype 1a (clone H77) infection in IHH upregulated IFN-β and ISG56 promoter activities in the absence of poly(I-C).
FIG. 2.
HCV infection inhibits poly(I-C)-induced IFN-β and ISG56 promoter activities in IHH. Hepatocytes were infected with HCV genotype 1a (clone H77) at an MOI of 0.02. After 48 h of infection, cells were transfected with either the IFN-β-luc reporter plasmid (A) or the ISG56-luc reporter plasmid (B). Thirty-six hours later, cells were transfected with or without poly(I-C). Cell extracts were prepared after 18 h, and luciferase activity was measured. Results are presented as means along with standard errors from six different experiments.
Translocation of IRF-3 in HCV-infected IHH.
SenV or Newcastle disease virus infection of cells causes the redistribution of IRF-3 from the cytoplasm to the nucleus, which is consistent with the virus-induced activation of IRF-3 and IFN-β production. Basal activation of IRF-3 correlates in general with the expression of the IRF-3-dependent ISG56 gene (34). Translocation of SenV-induced IRF-3 was blocked by HCV NS3/4A in Huh-7 cells (10, 11, 18, 28). HCV genotype 2a (clone JFH1) also blocked poly(I-C)-induced nuclear translocation of IRF-3 in Huh-7 cells (7). In this study, we examined the subcellular localization of IRF-3 in IHH infected with HCV genotype 1a (clone H77) or 2a (clone JFH1) by immunofluorescence (Fig. 3). Seventy-two hours postinfection, cells were fixed and stained with anti-IRF-3 and anti-HCV antibodies. Cells were also stained with secondary antibody only (in the absence of primary antibody) as a negative control, and we did not observe detectable fluorescence. In mock-infected cells, IRF-3 localized exclusively in the cytoplasm (Fig. 3, first row), which is indicative of an inactive state of IRF-3. Cells transfected with poly(I-C) as a positive control displayed nuclear localization of IRF-3 (Fig. 3, second row), a sign of IRF-3 activation. When cells were infected with HCV (Fig. 3, third and fifth rows) or when poly(I-C) was introduced by transfection into HCV-infected cells (fourth row), a predominant nuclear localization of IRF-3 was observed. This is in agreement with results of IFN-β and ISG56 reporter assays (Fig. 2). We did not see an additional activation of IFN-β or ISG56 promoter activities in the presence of both HCV and poly(I-C). In fact, a modest inhibition of the poly(I-C)-induced upregulation of IFN-β or ISG56 promoter activities in HCV-infected IHH was observed. This could be because the translocation of IRF-3 into the nucleus already occurred in HCV-infected cells. Although poly(I-C) is a strong inducer, the transfection of poly(I-C) in HCV-infected cells did not further enhance IRF-3 activation. Together, these results prompted us to investigate the status of endogenous IFN expression following HCV infection.
FIG. 3.
HCV infection in IHH exhibits predominant nuclear localization of IRF-3. IHH were either mock infected or infected with HCV genotype 1a (clone H77) or 2a (clone JFH1). Seventy-two hours postinfection, cells were either transfected with poly(I-C) or left untransfected. Cells were fixed after 18 h and stained with antibodies against IRF-3 (red) and HCV NS4 for H77 or NS3 for JFH1 (green). Nuclei were visualized by staining with TO-PRO-3-iodide (blue). Arrows indicate the localization of IRF-3 in the nucleus, and arrowheads indicate various degrees of nuclear localization of IRF-3.
HCV infection induces IFN-β and OAS-1 mRNA in IHH.
OAS-1 is a major component of the antiviral pathways induced by IFNs. In the presence of dsRNA, they polymerize ATP to form 2′,5′-oligoadenylate oligomers, which, in turn, activate the latent RNase RNase L, causing mRNA degradation. Our results obtained from RT-PCR analysis suggested that the transfection of poly(I-C) into IHH induced IFN-β mRNA and ISG56 mRNA after 24 h of transfection. To examine whether HCV infection induces endogenous IFN-β mRNA expression, we infected IHH with HCV genotype 1a (clone H77) or genotype 2a (clone JFH1). Total RNA was isolated at different time points, and the expression of IFN-β mRNA and OAS-1 mRNA was examined by real-time RT-PCR. The levels of IFN-β and OAS-1 mRNA expression were enhanced at 48 h or 72 h compared to mock-infected controls (Fig. 4). Together, our results suggested that HCV infection in IHH upregulates endogenous IFN-β and OAS-1 mRNA expression.
FIG. 4.
HCV infection of IHH upregulated IFN-β mRNA and OAS-1 mRNA expression. Total cellular RNA was extracted from HCV-infected IHH at the indicated time points. Intracellular gene expression levels of IFN-β, OAS-1, and GAPDH were measured by real-time RT-PCR. The ratios of IFN-β/GAPDH and OAS-1/GAPDH from HCV H77 (A) and HCV JFH1 (B) are presented as induction (n-fold) relative to basal levels in mock-infected cells.
HCV enhances ISG56 and STAT1α protein expression.
Several studies have demonstrated that virus infection, replication, and dsRNA can activate cellular antiviral pathways and induce IFN production, which stimulates STAT1α activation and its downstream signaling molecules such as ISG56. We have examined the downstream signaling pathway of IFN-β following HCV infection in IHH. Increased expression of STAT1α and ISG56 proteins was observed after infection of IHH with HCV genotype 1a (clone H77) compared to the mock-infected control (Fig. 5). Similar results were also observed in HCV genotype 2a (clone JFH1)-infected IHH (data not shown). Together, our results suggested that the IFN signaling pathway is active in IHH following infection with HCV. Similar results were observed in clinical studies as well as from liver RNA of HCV-infected chimpanzees (5, 16, 24). We have also examined the expression of IFN-β and OAS-1 up to 10 days following HCV infection of IHH. Approximately 400-fold and 12-fold enhancements of IFN-β mRNA and OAS-1 mRNA, respectively, were observed, suggesting that HCV infection in IHH activates the IFN signaling pathway.
FIG. 5.
HCV infection enhances ISG56 and STAT1α levels in IHH. Western blot analysis was performed for ISG56 and STAT1α protein expression using specific antibodies at the indicated times after infection (A). The blot was reprobed with an antibody to actin for comparisons of equal protein loading. Densitometric analyses of STAT1α/actin (B) and ISG56/actin (C) were performed using Image Quant software (Amersham, Piscataway, Saco, ME). Results are presented together with standard errors for three independent experiments.
HCV-mediated IFN activation suppresses VSV growth.
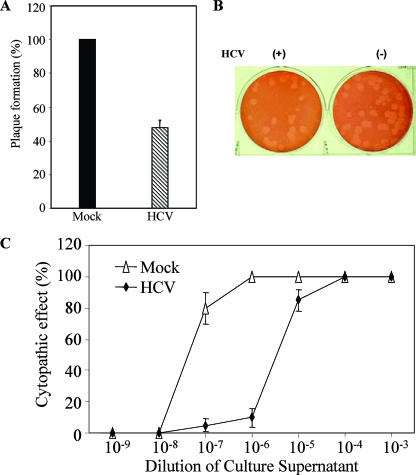
The consequence of IFN-α/β and ISG expression is the creation of an environment that is hostile to viruses (14). VSV is a negative-strand RNA virus whose growth is sensitive to the effects of the IFN pathway and which can be used to assess the antiviral status of cells (30). To evaluate the antiviral state after infection with HCV, we performed a VSV plaque assay using IHH cells. IHH were infected with HCV genotype 1a (clone H77) (MOI of 0.02), and cells were challenged with a known titer of IFN-sensitive VSV (50 PFU in IHH) after 72 h of infection. Mock-infected cells supported VSV replication as expected, while HCV-infected cells inhibited approximately 50% of VSV plaque formation at 36 h (Fig. 6A). A representative image displaying VSV plaque formation in HCV- or mock-infected IHH is shown (Fig. 6B). Similar results were observed for IHH infected with HCV genotype 2a (data not shown). In a different experiment, we examined the generation of CPE from VSV after growth in HCV- or mock-infected IHH. For this, HCV-infected IHH were washed with phosphate-buffered saline after 72 h of infection and challenged with VSV at an MOI of approximately 0.2. Supernatants were collected at 24 h, and VSV-induced CPE were measured by serial dilutions in BHK cells. Infection of IHH with HCV resulted in 50% CPE at a 100-fold-lower dilution than in mock-infected control cells (Fig. 6C). Together, our results suggested that IHH infected with HCV were inhibitory to VSV replication and corroborate data presented previously.
FIG. 6.
IHH establishes an antiviral state in response to HCV infection. IHH were infected with HCV H77 (MOI of 0.02) and incubated for 72 h. Cells were washed and challenged with a known VSV titer (50 PFU). VSV plaques were observed after 36 h and stained with neutral red for counting. Results from at least four independent assays suggested an approximately 50% reduction in VSV titers in HCV-infected IHH compared to the mock-infected control (A). Results are means of data from four independent experiments, and VSV plaque numbers from mock-infected IHH were arbitrarily assigned a value of 100%. A representative plaque assay is also shown as an illustration (B). HCV H77- or mock-infected IHH grown on a 35-mm plate were challenged with an MOI of VSV of 0.2. Cell culture supernatants were collected at 24 h, and VSV-induced CPE were compared by serial 10-fold dilutions in BHK cells (C). The results are presented as means of data from three independent experiments.
DISCUSSION
Many viruses have evolved strategies that block the effector mechanisms induced through IFN signaling pathways. Although multiple mechanisms contribute to viral persistence, the ability of the virus to evade early innate immune responses is likely to be particularly important. In this report, we have demonstrated that the infection of HCV in IHH enhances IFN-β mRNA expression and activates its downstream signaling molecules, which correlates with results from studies of intrahepatic gene expression observed during acute and chronic HCV infection in chimpanzees (4, 5). The changes due to endogenous antiviral responses of liver (e.g., induction of type I IFN-induced genes) occurred in intrahepatic gene expression as soon as HCV RNA was detectable in the serum of chimpanzees (38). Recently, Huh-7 cells or human fetal hepatocytes transfected with RNA from HCV genotype 1a (clone H77) were shown to induce type I IFN (25, 36). Our experimental findings from infection of IHH with cell culture-grown HCV are in agreement with those observations.
Viral infections activate IRF-3 through TLR-3 and/or RIG-I/MDA5 pathways. HCV NS3 protease inhibits IRF-3 phosphorylation and blocks the SenV infection-induced IRF-3 shift from the cytoplasm to the nucleus (10). The HCV genotype 1 NS3/4a protein blocks the RIG-I- and MDA5-mediated signaling pathway by cleavage of the MAVS/IPS-1 protein and by blocking downstream IFN-β activation (11, 29, 39). However, the intracellular signaling pathway(s) targeted by NS3/4A to govern IRF-3 function and HCV replication is incompletely defined. Huh-7 cells lack a functional TLR-3 pathway, and its derivative Huh-7.5 cell line is highly permissive for HCV RNA replication (26). Huh-7.5 cells have a lethal mutation in the RIG-I CARD homology domain that renders them unresponsive to structured HCV RNA or SenV-induced signaling (37). Other HCV proteins, specifically NS2 and NS4B, have been shown to regulate cytokine gene expression (21). We have observed various degrees of nuclear localization of IRF-3 in IHH infected with HCV genotype 1a or 2a, which may be sufficient for the induction of IFN signaling. The apparent discrepancy of IRF-3 localization following HCV JFH1 infection in IHH and Huh-7 cells may be due to the use of different cell types. It is also possible that HCV could utilize an alternate pathway for the enhancement of IFN-β and its downstream signaling molecules in IHH, supporting data showing that ISGs are up-regulated in patients with chronic hepatitis C (16).
We have also demonstrated that HCV infection in IHH induces IFN-β mRNA and enhances the expression of its downstream ISG56 and STAT1α proteins. IHH infected with HCV were inhibitory to VSV replication, and the antiviral effect of IFN against VSV was substantially increased upon prior infection with HCV. A similar growth-inhibitory effect was also observed in superinfection experiments with HCV and encephalomyocarditis virus (data not shown). Together, results from our study indicated that HCV infection of IHH induces the IFN signaling pathway, which corroborates data from studies of natural infections. Further studies are necessary to understand how HCV blunts the protective immune response for the establishment of chronic infection.
Acknowledgments
We thank G. C. Sen for providing the ISG56-specific antibody and the IFN-β-luc construct, M. Gale for providing ISG56-luc plasmid DNA, M. Diamond for providing encephalomyocarditis virus, and George Luo for providing us the HCV NS3 antibody.
This work was supported by research grants AI45144 (R.B.R.) and CA85486 (R.R.) from the National Institutes of Health.
Footnotes
Published ahead of print on 5 September 2007.
REFERENCES
- 1.Armstrong, G. M., A. Wasley, E. P. Simard, G. M. McQuillan, W. L. Kuhnert, and M. J. Alter. 2006. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann. Intern. Med. 144:705-714. [DOI] [PubMed] [Google Scholar]
- 2.Bandyopadhyay, S. K., G. T. Leonard, Jr., T. Bandyopadhyay, G. R. Stark, and G. C. Sen. 1995. Transcriptional induction by double-stranded RNA is mediated by interferon-stimulated response elements without activation of interferon-stimulated gene factor 3. J. Biol. Chem. 270:19624-19629. [DOI] [PubMed] [Google Scholar]
- 3.Basu, A., T. Kanda, A. Beyen, K. Saito, K. Meyer, and R. Ray. 2007. Sulfated homologues of heparin inhibit hepatitis C virus entry into mammalian cells. J. Virol. 81:3933-3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bigger, C. B., K. M. Brasky, and R. E. Lanford. 2001. DNA microarray analysis of chimpanzee liver during acute resolving hepatitis C virus infection. J. Virol. 75:7059-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bigger, C. B., B. Guerra, K. M. Brasky, G. Hubbard, M. R. Beard, B. A. Luxon, S. M. Lemon, and R. E. Lanford. 2004. Intrahepatic gene expression during chronic hepatitis C virus infection in chimpanzees. J. Virol. 78:13779-13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bridge, A. J., S. Pebernard, A. Ducraux, A. L. Nicoulaz, and R. Iggo. 2003. Induction of an interferon response by RNAi vectors in mammalian cells. Nat. Genet. 34:263-264. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, G., J. Zhong, and F. V. Chisari. 2006. Inhibition of dsRNA-induced signaling in hepatitis C virus-infected cells by NS3 protease-dependent and -independent mechanisms. Proc. Natl. Acad. Sci. USA 103:8499-8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Der, S. D., A. Zhou, B. R. Williams, and R. H. Silverman. 1998. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95:15623-15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dustin, L. B., and C. M. Rice. 2007. Flying under the radar: the immunobiology of hepatitis C. Annu. Rev. Immunol. 25:71-99. [DOI] [PubMed] [Google Scholar]
- 10.Foy, E., C. Wang, R. Sumpter, Jr., M. Ikeda, S. M. Lemon, and M. Gale, Jr. 2003. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science 300:1145-1148. [DOI] [PubMed] [Google Scholar]
- 11.Foy, E., K. Li, R. Sumpter, Jr., Y. M. Loo, C. L. Johnson, C. Wang, P. M. Fish, M. Yoneyama, T. Fujita, S. M. Lemon, and M. Gale, Jr. 2005. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc. Natl. Acad. Sci. USA 102:2986-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fredericksen, B., G. R. Akkaraju, E. Foy, C. Wang, J. Pflugheber, Z. J. Chen, and M. Gale, Jr. 2002. Activation of the interferon-β promoter during hepatitis C virus RNA replication. Viral Immunol. 15:29-40. [DOI] [PubMed] [Google Scholar]
- 13.Gale, M., Jr., and E. M. Foy. 2005. Evasion of intracellular host defense by hepatitis C virus. Nature 436:939-944. [DOI] [PubMed] [Google Scholar]
- 14.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signaling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 81:2341-2364. [DOI] [PubMed] [Google Scholar]
- 15.Guo, J., D. J. Hui, W. C. Merrick, and G. C. Sen. 2000. A new pathway of transcriptional regulation mediated by eukaryotic initiation factor 3. EMBO J. 19:6891-6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helbig, K. J., D. T.-Y. Lau, L. Semendric, H. A. J. Harley, and M. R. Beard. 2005. Analysis of ISG expression in chronic hepatitis C identifies viperin as a potential antiviral effector. Hepatology 42:702-710. [DOI] [PubMed] [Google Scholar]
- 17.Hoofnagle, J. H., and L. B. Seeff. 2006. Peginterferon and ribavirin for chronic hepatitis C. N. Engl. J. Med. 355:2444-2451. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, C. L., D. M. Owen, and M. Gale, Jr. 2007. Functional and therapeutic analysis of hepatitis C virus NS3.4A protease control of antiviral immune defense. J. Biol. Chem. 282:10792-10803. [DOI] [PubMed] [Google Scholar]
- 19.Kanda, T., A. Basu, R. Steele, T. Wakita, J. S. Ryerse, R. Ray, and R. B. Ray. 2006. Generation of infectious hepatitis C virus in immortalized human hepatocytes. J. Virol. 80:4633-4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanda, T., R. Steele, R. Ray, and R. B. Ray. 2007. Small interfering RNA targeted to hepatitis C virus 5′ nontranslated region exerts potent antiviral effect. J. Virol. 81:669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaukinen, P., M. Sillanpaa, S. Kotenko, R. Lin, J. Hiscott, K. Melen, and I. Julkunen. 2006. Hepatitis C virus NS2 and NS3/4A proteins are potent inhibitors of host cell cytokine/chemokine gene expression. Virol. J. 3:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagging, L. M., K. Meyer, R. J. Owens, and R. Ray. 1998. Functional role of hepatitis C virus chimeric glycoproteins in the infectivity of pseudotyped virus. J. Virol. 70:3539-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larrea, Z., R. Aldabe, E. Molano, C. M. Fernandez-Rodriguez, A. Ametzazurra, M. P. Civerira, and J. Prieto. 2006. Altered expression and activation of signal transducers and activators of transcription (STATs) in hepatitis C virus infection in vivo and in vitro studies. Gut 55:1188-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauer, G. M., and B. D. Walker. 2001. Hepatitis C virus infection. N. Engl. J. Med. 345:41-52. [DOI] [PubMed] [Google Scholar]
- 25.Lazaro, C. A., M. Chang, W. Tang, J. Campbell, D. G. Sullivan, D. R. Gretch, L. Corey, R. W. Coombs, and N. Fausto. 2007. Hepatitis C virus replication in transfected and serum-infected cultured human fetal hepatocytes. Am. J. Pathol. 170:478-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, K., Z. Chen, N. Kato, M. Gale, Jr., and S. M. Lemon. 2005. Distinct poly(I-C) and virus-activated signaling pathways leading to interferon-β production in hepatocytes. J. Biol. Chem. 280:16739-16747. [DOI] [PubMed] [Google Scholar]
- 27.Lin, R.-J., B.-L. Chang, H.-P. Yu, C.-L. Liao, and Y.-L. Lin. 2006. Blocking of interferon-induced Jak-Stat signaling by Japanese encephalitis virus NS5 through a protein tyrosine phosphatase-mediated mechanism. J. Virol. 80:5908-5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loo, Y. M., D. M. Owen, K. Li, A. K. Erickson, C. L. Johnson, P. M. Fish, D. S. Carney, T. Wang, H. Ishida, M. Yoneyama, T. Fujita, T. Saito, W. M. Lee, C. H. Hagedorn, D. T. Lau, S. A. Weinman, S. M. Lemon, and M. Gale, Jr. 2006. Viral and therapeutic control of IFN-beta promoter stimulator 1 during hepatitis C virus infection. Proc. Natl. Acad. Sci. USA 103:6001-6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meylan, E., J. Curran, K. Hofmann, D. Moradpour, M. Binder, R. Bartenschlager, and J. Tschopp. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437:1167-1172. [DOI] [PubMed] [Google Scholar]
- 30.Perry, S. T., and T. Compton. 2006. Kaposi's sarcoma-associated herpesvirus virions inhibit interferon responses induced by envelope glycoprotein gpK8.1. J. Virol. 80:11105-11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rani, M. R. S., and M. R. Ransohoff. 2005. Alternative and accessory pathways in the regulation of IFN-β-mediated gene expression. J. Interf. Cytok. Res. 25:788-798. [DOI] [PubMed] [Google Scholar]
- 32.Ray, R. B., K. Meyer, and R. Ray. 2000. Hepatitis C virus core protein promotes immortalization of primary human hepatocytes. Virology 271:197-204. [DOI] [PubMed] [Google Scholar]
- 33.Saito, T., R. Hirai, Y.-M. Loo, D. Owen, C. L. Johnson, S. C. Sinha, S. Akira, T. Fujita, and M. Gale, Jr. 2007. Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc. Natl. Acad. Sci. USA 104:582-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sen, G. C. 2001. Viruses and interferons. Annu. Rev. Microbiol. 55:255-281. [DOI] [PubMed] [Google Scholar]
- 35.Sen, G. C., and S. N. Sarkar. 2005. Transcriptional signaling by double-stranded RNA: role of TLR3. Cytok. Growth Factor Rev. 16:1-14. [DOI] [PubMed] [Google Scholar]
- 36.Shin, E.-C., U. Seifert, T. Kato, C. M. Rice, S. M. Feinstone, P.-M. Kloetzel, and B. Rehermann. 2006. Virus-induced type I IFN stimulates generation of immunoproteasomes at the site of infection. J. Clin. Investig. 116:3006-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sumpter, R., Jr., Y. M. Loo, E. Foy, K. Li, M. Yoneyama, T. Fujita, S. M. Lemon, and M. Gale, Jr. 2005. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 79:2689-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thimme, R., J. Bukh, H. C. Spangenberg, S. Wieland, J. Pemberton, C. Steiger, S. Govindarajan, R. H. Purcell, and F. V. Chisari. 2002. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc. Natl. Acad. Sci. USA 99:15661-15668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoneyama, M., M. Kikuchi, K. Matsumoto, T. Imaizumi, M. Miyagishi, K. Taira, E. Foy, Y. M. Loo, M. Gale, Jr., S. Akira, and S. Yonehara. 2005. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 175:2851-2858. [DOI] [PubMed] [Google Scholar]