Abstract
Defining the immune correlates of the protection against human immunodeficiency virus type 1 (HIV-1) acquisition in individuals who are exposed to HIV-1 but do not become infected may provide important direction for the creation of an HIV-1 vaccine. We have employed the simian immunodeficiency virus (SIV)/rhesus monkey model to determine whether monkeys can be repeatedly exposed to a primate lentivirus by a mucosal route and escape infection and whether virus-specific immune correlates of protection from infection can be identified in uninfected monkeys. Five of 18 rhesus monkeys exposed 18 times by intrarectal inoculation to SIVmac251 or SIVsmE660 were resistant to infection, indicating that the exposed/uninfected phenotype can be reproduced in a nonhuman primate AIDS model. However, routine peripheral blood lymphocyte gamma interferon enzyme-linked immunospot (ELISPOT), tetramer, and intracellular cytokine staining assays, as well as cytokine-augmented ELISPOT and peptide-stimulated tetramer assays, failed to define a systemic antigen-specific cellular immune correlate to this protection. Further, local cell-mediated immunity could not be demonstrated by tetramer assays of these protected monkeys, and local humoral immunity was not associated with protection against acquisition of virus in another cohort of mucosally exposed monkeys. Therefore, resistance to mucosal infection in these monkeys may not be mediated by adaptive virus-specific immune mechanisms. Rather, innate immune mechanisms or an intact epithelial barrier may be responsible for protection against mucosal infection in this population of monkeys.
Reports that cohorts of individuals exist who are repeatedly exposed to human immunodeficiency virus type 1 (HIV-1) but do not become infected provide a compelling argument that it should be possible to create an HIV vaccine that confers sterile protection against the virus (5, 14, 21). Commercial sex workers who are not infected by HIV-1 despite frequent sexual contact with HIV-1-infected partners have been described by a number of groups of investigators (5). Studies of such sex workers have led to reports that repeated mucosal exposure to HIV-1 may lead to the generation of virus-specific immunity that confers protection against acquisition of the virus (1, 2, 4, 8-14, 20, 21, 22). It has been suggested that defining the precise immune correlates of the protection against HIV-1 acquisition in these unusual cohorts may provide important direction for the creation of an effective HIV-1 vaccine.
The studies of these exposed, uninfected commercial sex workers have, however, been met with some skepticism (18). Critics of these reports have noted that it is very difficult to document a history of sexual exposure in these populations with any degree of certainty. Moreover, the virus-specific cell-mediated immunity and immunoglobulin A (IgA) antibody responses described for these individuals have been sporadic and of low frequency or low titer. Finally, many of the immunologic analyses performed on these sex workers have not been done in a blinded fashion. Therefore, there is not a consensus among investigators that exposed, uninfected individuals exist or that virus-specific immunity can explain their protection against the acquisition of HIV-1.
Nonhuman primates provide a potentially powerful model for exploring the phenomenon of individuals that are repeatedly exposed to a primate lentivirus but do not become infected. Experimental animals can be exposed to defined quantities of virus via a predetermined route of inoculation and studied intensively and on a regular schedule for evidence of virus-specific immunity. In the present study, we have employed the simian immunodeficiency virus (SIV)/rhesus monkey model to determine whether individuals can be repeatedly exposed to a primate lentivirus and escape infection. Further, we have assessed whether virus-specific immune correlates of protection from infection can be defined for these exposed, uninfected monkeys.
MATERIALS AND METHODS
Animals.
Adult rhesus monkeys (Macaca mulatta) were used in this study. All animals were maintained in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited institution in accordance with the principles of the guidelines of the National Institutes of Health and Harvard Medical School and after approval by the Animal Care and Use Committee protocols. Monkeys were screened for the presence of the Mamu-A*01 allele by using a PCR-based technique as previously described (17).
SIV challenge stocks.
The viruses employed in this study included cell-free uncloned SIVmac251 and SIVsmE660. The stock of SIVmac251 was expanded on human peripheral blood mononuclear cells (PBMC), and the stock of SIVsmE660 was expanded on rhesus monkey PBMC.
Intrarectal exposure to SIV.
Animals were placed in a sternal position with the pelvis propped up at an approximately 45° angle after being anesthetized (10 mg/kg of body weight ketamine intramuscularly [i.m.] and 0.5 mg/kg xylazine i.m.). A lubricated infant feeding catheter was inserted gently into the rectum of the animal approximately 4 to 6 inches without causing any injury. First, 5 ml of diluent (phosphate-buffered saline [PBS] with 0.5% human serum albumin) was gently flushed through the catheter, and then 1 ml of the virus was injected through the catheter, followed by a 5-ml flush with diluent. The animal was returned to its cage and kept tilted at a 45° angle until it fully recovered from anesthesia.
Plasma SIV RNA levels.
Plasma viral RNA levels were measured by an ultrasensitive branched DNA amplification assay with a detection limit of 125 copies per ml (Bayer Diagnostics, Berkeley, CA).
Antibodies.
The antibodies used in this study were purchased from BD Biosciences. All reagents were validated and titrated using rhesus monkey PBMC. The antibodies used in this study were anti-tumor necrosis factor alpha (TNF-α)-fluorescein isothiocyanate (FITC) (MAb11), anti-gamma interferon (IFN-γ)-phycoerythrin (PE)-Cy7 (B27), anti-interleukin-2 (IL-2)-allophycocyanin (APC) (MQ1-17H12), anti-MIP-1β-PE (D21-1351), anti-CD4-AmCyan (L200), anti-CD3-Pacific Blue (SP34-2), and anti-CD8α-Alexa Fluor 700 (RPA-T8).
IFN-γ ELISPOT assays.
For enzyme-linked immunospot (ELISPOT) assays, multiscreen 96-well plates were coated overnight with 100 μl per well of 5-μg/ml anti-human IFN-γ (B27; BD Pharmingen) in endotoxin-free Dulbecco's PBS (D-PBS). The plates then were washed three times with D-PBS containing 0.25% Tween 20, blocked for 2 h with D-PBS containing 5% fetal bovine serum (FBS) to remove the Tween 20, and incubated with peptide pools and 2 × 105 PBMC in triplicate in 100-μl reaction volumes. Each peptide pool was comprised of 15-amino-acid peptides overlapping by 11 amino acids. The pools covered the entire SIVmac239 Gag protein and the HIV-1 89.6P (KB9) Env protein. Each peptide in a pool was present at a 1-μg/ml concentration. Following an 18-h incubation at 37°C, the plates were washed nine times with D-PBS containing 0.25% Tween 20 and washed once with distilled water. The plates then were incubated with 2 μg/ml biotinylated rabbit anti-human IFN-γ (Biosource) for 2 h at room temperature, washed six times with Coulter wash (Beckman Coulter), and incubated for 2.5 h with a 1:500 dilution of streptavidin-AP (Southern Biotechnology). After five washes with Coulter wash and one with D-PBS, the plates were developed with nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate chromogen (Pierce) and the reaction was stopped by washing the plates with tap water. The plates were air dried and read with an ELISPOT reader (Hitech Instruments) using Image-Pro Plus image-processing software (version 4.1) (Media Cybernetics, Des Moines, IA). ELISPOT assays were performed on PBMC obtained on weeks 6, 7, 21, and 27 following the first exposure to virus.
Intracellular cytokine assays.
PBMC were incubated at 37°C in a 5% CO2 environment for 6 h in the presence of RPMI 1640-10% FCS medium alone (unstimulated), a pool of 15-mer Gag peptides (5 μg/ml each peptide), or staphylococcal enterotoxin B (5 μg/ml; Sigma-Aldrich) as a positive control. All cultures contained monensin (GolgiStop; BD Biosciences) as well as 1 μg/ml anti-CD49d (BD Biosciences). The cultured cells were stained with monoclonal antibodies (MAbs) specific for cell surface molecules (CD3, CD4, CD8, CD28, and CD95) and an amine dye (Invitrogen) to discriminate live from dead cells. After being fixed with Cytofix/Cytoperm solution (BD Biosciences), cells were permeabilized and stained with antibodies specific for IFN-γ, TNF-α, and IL-2. Labeled cells were fixed in 1.5% formaldehyde-PBS. Samples were collected on an LSR II instrument (BD Biosciences) and were analyzed using FlowJo software (Tree Star). Approximately 200,000 to 1,000,000 events were collected per sample. The background level of cytokine staining varied from sample to sample but typically was <0.01% of the CD4+ T cells and <0.05% of the CD8+ T cells. The only samples considered positive were those for which the percentage of cytokine-staining cells was at least twice that of the background or in which there was a distinct population of cells brightly positive for cytokine. ICS assays were performed on PBMC obtained on weeks 21 and 27 following the first exposure to virus.
Tetramer staining.
Soluble tetrameric Mamu-A*01/p11C complex was prepared as described previously (16). One microgram of PE-labeled tetrameric Mamu-A*01/p11C complex in conjunction with FITC-labeled anti-human CD8α (Leu2a; Becton Dickinson), PerCP-Cy5.5-labeled anti-human CD4 (L200; Becton Dickinson), and APC-labeled anti-rhesus CD3 (FN18) MAbs were used to stain p11C-specific CD8+ T cells as described previously (24). Thawed PBMC were washed in RPMI 1640 medium containing 12% FBS (R12). PBMC (5 × 106) were resuspended in 100 μl of PBS and directly stained with the reagent mixture, washed in 4 ml of PBS containing 2% FBS, and fixed in 0.5 ml of PBS containing 1.5% formaldehyde. PBMC (4 × 106) in 1 ml of R12 were cultured in the presence of 1-μg/ml SIV Gag p11C (CTPYDINQM). On day 3 of culture, 1 ml of 40-U/ml human recombinant IL-2 (Hoffman-La Roche) was added. On day 12 of culture, peptide-stimulated PBMC were centrifuged over a Ficoll gradient and were washed. Peptide-stimulated PBMC (5 × 105) were resuspended in 100 μl PBS and stained with 1 μg of PE-labeled tetrameric Mamu-A*01/p11C complex in conjunction with FITC-labeled anti-human CD8α (Leu2a; Becton Dickinson) and APC-labeled anti-rhesus CD3 (FN18) MAb. The samples then were washed in 4 ml PBS containing 2% FBS and were fixed in 0.5 ml PBS containing 1.5% formaldehyde. Samples were analyzed by four-color flow cytometry on a Coulter EPICS Elite ESP system. Gated CD3+ CD8a+ T cells were examined for staining with tetrameric Mamu-A*01/p11C. Tetramer assays were performed on PBMC and colonic lymphocytes obtained 22 weeks following the third cluster of six exposures to virus.
Collection of rectal secretions.
Weck-Cel cellulose sponges (catalog no. 16185; Medtronic ENT) premoistened with 50 μl D-PBS were used as previously described (15) to absorb secretions from the rectum of each animal. Secretions were eluted from sponges by centrifugation in the presence of 100 μl 0.5% Igepal detergent in PBS containing protease inhibitors (1). After assessing blood contamination through measurement of hemoglobin with Roche Diagnostics ChemStrips 5 indicator strips, 20 μl goat serum (GS) was added to the secretion. All secretions were determined to contain insignificant blood contaminant, as the hemoglobin was either undetectable or less than 1/50,000 the amount in blood.
Measurement of SIV-specific mucosal IgA antibodies.
Total IgA, anti-SIV gp130, or anti-SIV gag and pol antibodies were measured by enzyme-linked immunosorbent assay (ELISA) using Fisherbrand high-protein-binding microtiter plates that had been coated overnight with 50 μg/well affinity-purified goat anti-monkey IgA antibody (Rockland Immunochemicals), 100 ng/well SIVmac251 rgp130 (ImmunoDiagnostics, Woburn, MA), or a 1/400 dilution (250 ng total protein per well) of SIVmac251 viral lysate (Advanced Biotechnologies Inc., Columbia, MD). The SIV lysate has been found to contain undetectable envelope protein at the 1/400 dilution used. Hence, antibodies measured with this ELISA are referred to as being SIV gag/pol-specific. The following day, plates were washed with PBS containing 0.05% Tween 20 (PBST), blocked with 5% GS in PBST, and then loaded with twofold serial dilutions of standards and secretions in 5% GS-PBST. The standard in the total IgA ELISA was a normal monkey serum containing a known amount of IgA (2). The standards in the SIV ELISAs were two preparations of IgG-depleted pooled serum from DNA/modified vaccinia virus Ankara-vaccinated macaques (3, 24) that had been found to have high titers of serum IgA antibodies against SIV lysate or gp130 after challenge. The concentration of IgA antibody in each SIV standard had been calibrated relative to the total IgA standard by coating portions of a microtiter plate with each of the relevant coating reagents and developing them as described below.
The plates were developed by consecutive treatment with biotinylated affinity-purified goat anti-monkey IgA (Rockland), avidin-labeled peroxidase, and 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (both from Sigma) as described previously (15). After absorbance values at 414 nm were recorded, IgA antibody concentrations in specimens were interpolated from standard curves using the SoftMaxPro computer program (Molecular Devices, Sunnyvale, CA). The concentration of SIV gag/pol- or gp130-specific IgA subsequently was divided by the concentration of total IgA in the secretion to obtain the specific activity. The secretion was determined to be positive for anti-SIV IgA antibody if the specific activity was greater than or equal to the mean specific activity plus 3 standard deviations measured with 20 negative control secretions obtained from SIV-naive rhesus macaques.
RESULTS
Rhesus monkey model of repeated, low-dose SIV mucosal exposure.
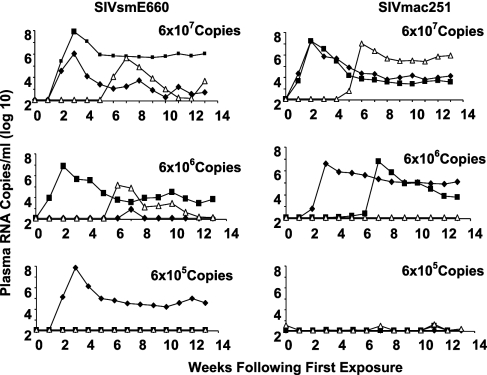
A rhesus monkey model was established to study immune correlates of natural protection against infection following repeated, low-dose mucosal exposure to SIV. Viral challenge stocks were prepared from two genetically disparate SIV isolates, SIVmac251 and SIVsmE660. Two stocks were created to allow the confirmation of any findings by using different virus challenge systems. Monkeys were exposed to virus by intrarectal inoculation through a cluster of six weekly administrations. Using three monkeys/dose/virus stock, groups of animals were exposed to 6 × 107, 6 × 106, and 6 × 105 copies of virus with each administration. The cohorts of nine SIVmac251-exposed and nine SIVsmE660-exposed monkeys then were monitored for evidence of infection by assessing plasma samples obtained on a weekly schedule for SIV gag virus RNA. As shown in Fig. 1, all three monkeys exposed to 6 × 107 copies of SIVsmE660, two of three exposed to 6 × 106 copies of SIVsmE660, and one of three exposed to 6 × 105 copies of SIVsmE660 developed persistent viremia; further, all three monkeys exposed to 6 × 107 copies of SIVmac251, two of three exposed to 6 × 106 copies of SIVmac251, and none of the monkeys exposed to 6 × 105 copies of SIVmac251 developed persistent viremia. This created a cohort of seven monkeys that were exposed by a mucosal route to highly pathogenic SIV isolates but did not develop overt evidence of infection.
FIG. 1.
Systemic infections following repeated rectal exposure of rhesus monkeys to SIVmac251 or SIVsmE660. Monkeys were exposed by intrarectal inoculation for 6 successive weeks to cell-free virus using three monkeys/dose/challenge stock and doses of 6 × 107, 6 × 106, and 6 × 105 copies of virus. Monkeys were monitored for systemic infection on a weekly basis by assessing plasma SIV gag RNA levels.
SIV-specific cellular immune responses in seven exposed, uninfected rhesus monkeys.
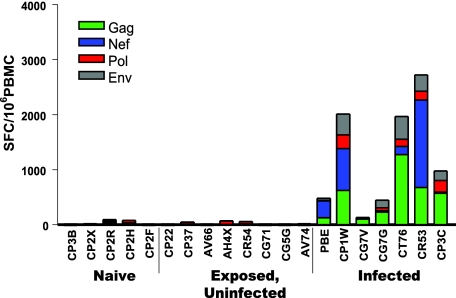
These seven exposed, uninfected rhesus monkeys then were evaluated for systemic SIV-specific cellular immunity to determine whether repeated mucosal exposure to virus can stimulate systemic immune responses that might confer protection against subsequent exposure to virus. Cellular immunity to SIV first was evaluated using a routine ELISPOT assay to assess PBMC IFN-γ responses following exposure to Env, Gag, Pol, and Nef SIVmac239 peptide pools. While PBMC of 7 of the animals in the initial cohort of 18 monkeys that became infected following mucosal exposure to virus demonstrated SIV-specific T-cell responses, PBMC of the seven exposed, uninfected monkeys demonstrated no T-cell responses (Fig. 2). Recently it has been reported that the sensitivity of ELISPOT assays can be substantially enhanced by adding cytokines to the peptide-exposed PBMC and increasing the duration of exposure of the PBMC to the viral peptides (6). PBMC of the exposed, uninfected monkeys evaluated using this highly sensitive assay also showed no evidence of virus-specific cellular immunity (data not shown).
FIG. 2.
Exposed, uninfected monkeys developed no peripheral blood T-lymphocyte IFN-γ ELISPOT responses to SIV antigens. PBMC were isolated from exposed, uninfected monkeys, naïve control monkeys, and monkeys that became infected following intrarectal inoculation. These PBMC were exposed to pools of Env, Gag, Pol, and Nef peptides and were assessed for IFN-γ spot-forming cell (SFC) responses.
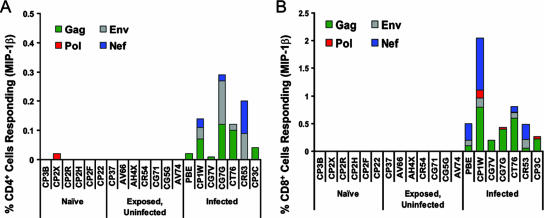
PBMC of these same monkeys also were assessed for SIV-specific cellular immunity by an intracellular cytokine staining (ICS) assay, evaluating both CD8+ and CD4+ T lymphocytes for evidence of IFN-γ, TNF-α, IL-2, and MIP-1β production following exposure to the same SIVmac239 peptide pools. Consistent with the findings of the ELISPOT study, these assays also demonstrated robust CD4+ and CD8+ T-lymphocyte responses by PBMC of the monkeys that developed overt infections, but no significant responses were detected in PBMC of the exposed, uninfected monkeys (Fig. 3 and data not shown). These findings suggested that repeated mucosal exposure to virus did not elicit systemic cellular immune responses that could be detected by routine assays.
FIG. 3.
Exposed, uninfected monkeys developed no peripheral blood T-lymphocyte ICS responses to SIV antigens. PBMC isolated from exposed, uninfected; naïve control; and SIV-infected monkeys were exposed to pools of Env, Gag, Pol, and Nef peptides and then were assessed by ICS staining to evaluate (A) MIP-1β production by CD4+ T lymphocytes and (B) MIP-1β production by CD8+ T lymphocytes.
Further exposures of uninfected rhesus monkeys to SIV.
It was possible that the initiation of a systemic infection in one of these monkeys was a stochastic event and that further mucosal exposures of these uninfected monkeys to virus would result in the initiation of additional infections. To examine that possibility, each of the seven uninfected monkeys received a second series of six weekly intrarectal exposures to the same quantity of the same SIV isolate it had received previously, and plasma viral RNA assays were monitored for evidence of the initiation of a systemic infection. These six further exposures to virus did not initiate additional infections.
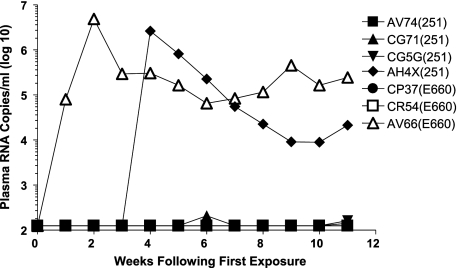
Since all of the monkeys that received mucosal exposures to 6 × 107 copies of viral RNA became infected during the initial cluster of virus inocula, we explored the possibility that the seven multiply exposed but uninfected monkeys would become infected following exposure to this larger number of viral particles. These seven animals were subjected to a further cluster of six weekly intrarectal exposures to the same virus isolate to which they were previously exposed, but all exposures were to 6 × 107 copies of viral RNA. As shown in Fig. 4, only two of these seven monkeys developed a systemic infection. Therefore, five monkeys received 18 mucosal exposures to SIVsmE660 or SIVmac251 and did not acquire a systemic infection.
FIG. 4.
Systemic infections following repeated rectal exposure of exposed, uninfected rhesus monkeys to large inocula of SIVmac251 or SIVsmE660. The monkeys that remained uninfected following 12 intrarectal exposures to SIV were exposed for 6 successive weeks by intrarectal inoculation to 6 × 107 RNA copies of cell-free virus. Monkeys were monitored for systemic infection on a weekly basis by assessing plasma SIV gag RNA levels.
Evaluation of exposed, uninfected rhesus monkeys for cellular immunity by tetramer staining.
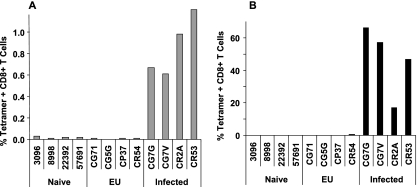
While the multiply exposed but uninfected monkeys had no evidence of systemic antiviral immunity as determined by ELISPOT and ICS assays, it remained possible that a more sensitive assay for peripheral blood cellular immunity would detect virus-specific responses in these animals. Thirteen of the 18 rhesus monkeys employed in this study were Mamu-A*01+, and 4 of the 5 remaining uninfected monkeys were Mamu-A*01+. Therefore, there was no association between resistance to mucosal infection and expression of the Mamu-A*01 allele. We were, however, able to employ a Mamu-A*01/p11C tetramer to evaluate SIV Gag epitope-specific CD8+ T-lymphocyte responses in these monkeys. We first used this highly sensitive tetramer binding assay to assess PBMC of the exposed, uninfected monkeys for evidence of systemic antiviral cellular immunity (Fig. 5A). In fact, while PBMC of Mamu-A*01+ rhesus monkeys from the original cohort of 18 animals that developed systemic infections had readily demonstrable tetramer binding CD8+ peripheral blood T lymphocytes, PBMC of the 4 Mamu-A*01+-exposed, uninfected rhesus monkeys had no tetramer binding peripheral blood CD8+ T lymphocytes.
FIG. 5.
Dominant epitope peptide/tetramer binding CD8+ T lymphocytes in the peripheral blood of the exposed, uninfected Mamu-A*01+ rhesus monkeys. PBMC were isolated from exposed, uninfected (EU) monkeys; naïve control monkeys; and monkeys that became infected following intrarectal inoculation. Freshly isolated (A) or epitope-peptide-stimulated (B) PBMC were evaluated for tetramer binding CD8+ T lymphocytes by MAb and tetramer staining followed by flow cytometric analysis.
We also have shown that the sensitivity of this tetramer binding T-lymphocyte assay can be increased by a 1-week in vitro cultivation of PBMC with the optimal epitope peptide before the cells are evaluated in a tetramer binding assay (23). This modified tetramer binding assay therefore was employed to evaluate PBMC of the exposed, uninfected Mamu-A*01+ rhesus monkeys (Fig. 5B). While tetramer binding CD8+ T lymphocytes were readily detected in epitope-peptide-stimulated PBMC from Mamu-A*01+ rhesus monkeys that had become infected, and while no tetramer binding CD8+ T lymphocytes were detected in similarly stimulated PBMC of naïve Mamu-A*01+ rhesus monkeys, no tetramer binding CD8+ T lymphocytes were detected in epitope-peptide-stimulated PBMC of the exposed, uninfected Mamu-A*01+ rhesus monkeys. Therefore, no evidence was found for systemic SIV-specific cellular immunity in the exposed, uninfected monkeys using highly sensitive tetramer binding assays.
Evaluation of rectal mucosal T lymphocytes for SIV-specific immunity.
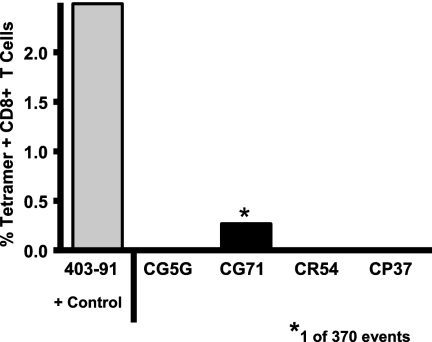
Since it is possible that virus-specific T lymphocytes are in mucosal cell populations but not in the systemic circulation, we assessed lymphocytes sampled from the distal colonic mucosa of the exposed, uninfected monkeys for evidence of SIV-specific T lymphocytes (Fig. 6). Lymphocytes were isolated from multiple colonic biopsy specimens obtained from the four Mamu-A*01+-exposed, uninfected monkeys and one Mamu-A*01+ SIV-infected monkey 22 weeks following the final mucosal exposure to virus, and these lymphocytes were assessed for Mamu-A*01/p11C tetramer binding CD8+ T cells. SIV Gag epitope-specific CD8+ T lymphocytes were demonstrated in this mucosal lymphocyte population of the SIV-infected rhesus monkey but not in the mucosal lymphocytes of the exposed, uninfected animals. Therefore, the multiply exposed, uninfected monkeys had no evidence of systemic or mucosal SIV-specific cellular immunity.
FIG. 6.
Dominant epitope peptide/tetramer binding CD8+ T lymphocytes in the distal colonic mucosa of the exposed, uninfected Mamu-A*01+ rhesus monkeys. Lymphocytes were isolated from the distal colonic mucosa of the exposed, uninfected monkeys and a control infected Mamu-A*01+ rhesus monkey. These cells were evaluated for tetramer binding CD8+ T lymphocytes by MAb and tetramer staining followed by flow cytometric analysis.
Evaluation of rectal mucosal antibodies in exposed, uninfected monkeys.
Since protection against mucosal acquisition of an SIV infection could be antibody mediated, we assessed rectal mucosal secretions for anti-SIV antibodies after repeated rectal exposure of monkeys to SIV. A new cohort of six naïve rhesus monkeys was exposed to SIVsmE660 on a weekly schedule for 6 weeks, three monkeys to 6 × 107 RNA copies and three monkeys to 6 × 106 RNA copies. Only one of the six monkeys, CK8G, developed viremia. Low-titer anti-SIV IgA antibodies were detected in rectal sponge specimens obtained from this infected monkey and from two of the exposed, uninfected monkeys, CP11 and CP2C, 1 week following the final rectal exposure to virus (Table 1). Therefore, repeated mucosal exposure to SIV elicited a local low-titer mucosal anti-SIV antibody response in some of these monkeys. However, the generation of this antibody response was not associated with protection from infection.
TABLE 1.
Levels of rectal anti-SIV IgA antibodies from multiply exposed rhesus monkeysa
| SIV group | Infection statusb | Animal no. | Amt of anti-SIV IgA (ng/ml) for:
|
Total IgA (μg/ml) | Sp act (ng antibody/μg Ig) of:
|
||
|---|---|---|---|---|---|---|---|
| gag/pol | gp130 | gag/pol | gp130 | ||||
| SME660 1:10 | + | CK8G | 24.04 | 2.68 | 54.0 | 0.445 | 0.050 |
| − | CL3G | 2.39 | 1.42 | 34.4 | 0.069 | 0.041 | |
| − | CP11 | 1.74 | ND | 7.5 | 0.232 | ND | |
| SME660 1:100 | + | CP2C | 35.34 | 0.05 | 47.8 | 0.739 | 0.315 |
| − | CP2M | ND | 0.69 | 8.0 | ND | 0.082 | |
| − | CP34 | 4.89 | 3.68 | 57.6 | 0.085 | 0.064 | |
Specimens were obtained 1 week after the final exposure to virus. ND, not determined.
Determined by branched DNA analysis of plasma specimens.
Intravenous SIV challenge of exposed, uninfected monkeys.
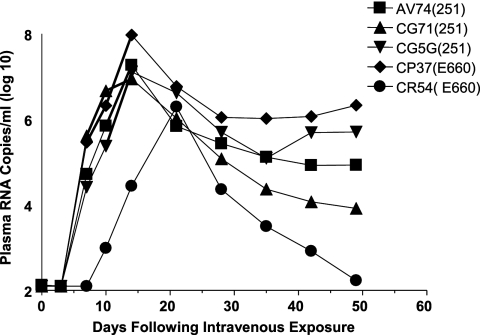
We finally assessed whether there was any evidence of protective anti-SIV immunity in the 5 rhesus monkeys from the original cohort of 18 animals that had been exposed to SIV by intrarectal administration without becoming infected. These monkeys were inoculated by the intravenous route with 2 × 105 RNA copies of the same SIV isolate to which they had previously been exposed. All five monkeys became infected, and the kinetics of this viral replication during the period of primary infection was indistinguishable from the usual kinetics of SIV replication in naïve rhesus monkeys (Fig. 7). These findings suggest that there was no potent systemic anti-SIV immunity in the exposed, uninfected animals.
FIG. 7.
Systemic infections of exposed, uninfected rhesus monkeys following a single intravenous inoculation of SIVmac251 or SIVsmE660. The five monkeys that remained uninfected following 18 intrarectal exposures to SIV were inoculated by the intravenous route with 2 × 105 RNA copies of SIVmac251 or SIVsmE660. Monkeys were monitored for systemic infection on a weekly basis by assessing plasma SIV gag RNA levels.
DISCUSSION
While an ideal animal model for HIV-1 acquisition would employ female genital mucosal exposure and very low, repeated doses of virus, we were not able to create such an experimental system. Only male macaques are available in large numbers for use in experiments, because females are needed for breeding. The availability of experimental animals therefore forced us to use rectal rather than genital mucosal exposures to virus for initiating infections. Further, in determining titers of the virus for in vivo infections (Fig. 1), we found that doses of virus of less than 6 × 106 RNA copies could only rarely initiate a mucosal infection. In fact, we found that the lowest dose of virus that could initiate an infection by the mucosal route was approximately 300 times greater than the dose needed to infect monkeys by the intravenous route. Thus, infections could not be initiated with a mucosal administration of a low dose of virus. Therefore, both practical and data-driven considerations shaped the experimental system employed in the present study.
It has been suggested that the kinetics of SIV replication and spread may differ when infections are initiated by a limiting dose of virus delivered by a mucosal route rather than by high-dose intravenous administration (7) and that the explosive virus replication that occurs following the intravenous administration of SIV creates an unrealistically difficult target for vaccine protection. In fact, intravenous administration of the SIV challenge stocks employed in the present experiment seed an infection with kinetics characterized by a peak of viral replication of 8 logs of measurable plasma viral RNA by 12 days following virus inoculation (17, 19). Interestingly, those kinetics of viral replication do not differ significantly from the kinetics of SIV replication observed in the present study in the monkeys challenged by the intrarectal route with limiting doses of virus. This observation suggests that protocols for challenging monkeys with SIV by a mucosal route do not necessarily create an infection in which virus replication may be easily aborted by a vaccine-elicited immune response.
Importantly, the observations in the present study indicate that repeated mucosal exposures to a primate lentivirus can occur without initiating an infection under certain experimental conditions. Indeed, we observed that those monkeys in which infection was not initiated following a series of 6 weekly intrarectal exposures were not infected after 12 subsequent mucosal exposures to the virus, even at a higher dose. The practical implications of this observation for vaccine trials in macaque models are considerable. If monkeys are not infected under these experimental conditions after six exposures to SIV, subsequent exposures to virus by the same route at the same dose may not initiate an infection. Importantly, the finding in the present study confirms the observation for humans that some individuals can indeed be exposed repeatedly to HIV-1 by a mucosal route without becoming infected. Nevertheless, this study also demonstrates that such a resistance to infection is not manifested by a resistance to infection initiated by an intravenous exposure to SIV. This latter finding suggests that resistance to infection may be occurring because of local mucosal factors. Since others have reported that repeated mucosal exposures to virus can eventually initiate an infection in all members of a group of macaques, the monkeys that appeared resistant to mucosal infection in the present study eventually might have been infected if repeatedly exposed to a large enough quantity of virus by mucosal administration.
The present study provides compelling evidence that the resistance to mucosally initiated infection in these monkeys may not be associated with any measurable systemic or local antigen-specific mucosal immune responses. The systemic immune assays that were monitored in these exposed, uninfected monkeys included not only the routine peripheral blood lymphocyte IFN-γ ELISPOT, tetramer, and ICS assays but also the more sensitive cytokine-augmented ELISPOT and peptide-stimulated tetramer assays. Further, this population of monkeys also was assessed for evidence of local mucosal humoral and cell-mediated immunity using ELISA and tetramer assays. The absence of any evidence for SIV-specific immunity in some of these monkeys suggests that the resistance to mucosal infection in these monkeys may not be mediated by adaptive virus-specific immune mechanisms. Innate immune mechanisms certainly may be contributing to protection from this infection. Nevertheless, variations in the epithelial barrier or local trauma also may be contributing to differences in susceptibility to mucosal infection in this cohort of monkeys. However, we observed that repeated exposure of the monkeys by the mucosal route to subinfectious doses of SIV was associated with subsequent protection against acquisition of virus administered mucosally at an infectious dose. This finding would argue that the protection may have been mediated by adaptive or innate immune mechanisms.
Acknowledgments
This work was supported in part by funds from the intramural research program of the Vaccine Research Center, NIAID, NIH, and the Harvard Medical School CFAR grant AI060354. P.A.K. was supported by grant AI058896.
Footnotes
Published ahead of print on 8 August 2007.
REFERENCES
- 1.Alimonti, J. B., J. Kimani, L. Matu, C. Wachihi, R. Kaul, F. A. Plummer, and K. R. Fowke. 2006. Characterization of CD8 T-cell responses in HIV-1-exposed seronegative commercial sex workers from Nairobi, Kenya. Immunol. Cell Biol. 84:482-485. [DOI] [PubMed] [Google Scholar]
- 2.Alimonti, J. B., S. A. Koesters, J. Kimani, L. Matu, C. Wachihi, F. A. Plummer, and K. R. Fowke. 2005. CD4+ T cell responses in HIV-exposed seronegative women are qualitatively distinct from those in HIV-infected women. J. Infect. Dis. 191:20-24. [DOI] [PubMed] [Google Scholar]
- 3.Bertley, F. M. N., P. A. Kozlowski, S.-W. Wang, J. Chappelle, J. Patel, O. Sonuyi, G. Mazzara, D. Montefiori, A. Carville, K. G. Mansfield, and A. Aldovini. 2004. Control of simian/human immunodeficiency virus viremia and disease progression after IL-2-augmented DNA-modified vaccinia virus Ankara nasal vaccination. J. Immunol. 172:3745-3755. [DOI] [PubMed] [Google Scholar]
- 4.Broliden, K., J. Hinkula, C. Devito, P. Kiama, J. Kimani, D. Trabbatoni, J. J. Bwayo, M. Clerici, F. Plummer, and R. Kaul. 2001. Functional HIV-1 specific IgA antibodies in HIV-1 exposed, persistently IgG seronegative female sex workers. Immunol. Lett. 79:29-36. [DOI] [PubMed] [Google Scholar]
- 5.Fowke, K. R., N. J. Nagelkerke, J. Kimani, J. N. Simonsen, A. O. Anzala, J. J. Bwayo, K. S. MacDonald, E. N. Ngugi, and A. F. Plummer. 1996. Resistance to HIV-1 infection among persistently seronegative prostitutes in Nairobi, Kenya. Lancet 348:1347-1351. [DOI] [PubMed] [Google Scholar]
- 6.Goonetilleke, N., S. Moore, L. Dally, N. Winstone, I. Cebere, A. Mahmoud, S. Pinheiro, G. Gillespie, D. Brown, V. Loach, J. Roberts, A. Guimaraes-Walker, P. Hayes, K. Loughran, C. Smith, J. De Bont, C. Verlinde, D. Vooijs, C. Schmidt, M. Boaz, J. Gilmour, P. Fast, L. Dorrell, T. Hanke, and A. J. McMichael. 2006. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J. Virol. 80:4717-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenier, J. L., C. J. Miller, D. Lu, P. J. Dailey, F. X. Lu, K. J. Kunstman, S. M. Wolinsky, and M. L. Marthas. 2001. Route of simian immunodeficiency virus inoculation determines the complexity but not the identity of viral variant populations that infect rhesus macaques. J. Virol. 75:3753-3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iqbal, S. M., T. B. Ball, J. Kimani, P. Kiama, P. Thottingal, J. E. Embree, K. R. Fowke, and F. A. Plummer. 2005. Elevated T cell counts and RANTES expression in the genital mucosa of HIV-1-resistant Kenyan commercial sex workers. J. Infect. Dis. 192:728-738. [DOI] [PubMed] [Google Scholar]
- 9.Kaul, R., T. Dong, F. A. Plummer, J. Kimani, T. Rostron, P. Kiama, E. Njagi, E. Irungu, B. Farah, J. Oyugi, R. Chakraborty, K. S. MacDonald, J. J. Bwayo, A. McMichael, and S. L. Rowland-Jones. 2001. CD8(+) lymphocytes respond to different HIV epitopes in seronegative and infected subjects. J. Clin. Investig. 107:1303-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaul, R., F. A. Plummer, J. Kimani, T. Dong, P. Kiama, T. Rostron, E. Njagi, K. S. MacDonald, J. J. Bwayo, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J. Immunol. 164:1602-1611. [DOI] [PubMed] [Google Scholar]
- 11.Kaul, R., S. L. Rowland-Jones, J. Kimani, T. Dong, H. B. Yang, P. Kiama, T. Rostron, E. Njagi, J. J. Bwayo, K. S. MacDonald, A. J. McMichael, and F. A. Plummer. 2001. Late seroconversion in HIV-resistant Nairobi prostitutes despite pre-existing HIV-specific CD8+ responses. J. Clin. Investig. 107:341-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaul, R., S. L. Rowland-Jones, J. Kimani, K. Fowke, T. Dong, P. Kiama, J. Rutherford, E. Njagi, F. Mwangi, T. Rostron, J. Onyango, J. Oyugi, K. S. MacDonald, J. J. Bwayo, and F. A. Plummer. 2001. New insights into HIV-1 specific cytotoxic T-lymphocyte responses in exposed, persistently seronegative Kenyan sex workers. Immunol. Lett. 79:3-13. [DOI] [PubMed] [Google Scholar]
- 13.Kaul, R., D. Trabattoni, J. J. Bwayo, D. Arienti, A. Zagliani, F. M. Mwangi, C. Kariuki, E. N. Ngugi, K. S. MacDonald, T. B. Ball, M. Clerici, and F. A. Plummer. 1999. HIV-1-specific mucosal IgA in a cohort of HIV-1-resistant Kenyan sex workers. AIDS 13:23-29. [DOI] [PubMed] [Google Scholar]
- 14.Koning, F. A., C. A. Jansen, J. Dekker, R. A. Kaslow, N. Dukers, D. van Baarle, M. Prins, and H. Schuitemaker. 2004. Correlates of resistance to HIV-1 infection in homosexual men with high-risk sexual behaviour. AIDS 18:1117-1126. [DOI] [PubMed] [Google Scholar]
- 15.Kozlowski, P. A., R. M. Lynch, R. R. Patterson, S. Cu-Uvin, T. P. Flanigan, and M. R. Neutra. 2000. Modified wick method using Weck-Cel sponges for collection of human rectal secretions and analysis of mucosal HIV antibody. J. Acquir. Immun. Defic. Syndr. 24:297-309. [DOI] [PubMed] [Google Scholar]
- 16.Kuroda, M. J., J. E. Schmitz, D. H. Barouch, A. Craiu, T. M. Allen, A. Sette, D. I. Watkins, M. A. Forman, and N. L. Letvin. 1998. Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus-infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I-peptide complex. J. Exp. Med. 187:1373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Letvin, N. L., J. R. Mascola, Y. Sun, D. A. Gorgone, A. P. Buzby, L. Xu, Z. Y. Yang, B. Chakrabarti, S. S. Rao, J. E. Schmitz, D. C. Montefiori, B. R. Barker, F. L. Bookstein, and G. J. Nabel. 2006. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 312:1530-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Letvin, N. L., and B. D. Walker. 2001. HIV versus the immune system: another apparent victory for the virus. J. Clin. Investig. 107:273-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lifson, J. D., M. A. Nowak, S. Goldstein, J. L. Rossio, A. Kinter, G. Vasquez, T. A. Wiltrout, C. Brown, D. Schneider, L. Wahl, A. L. Lloyd, J. Williams, W. R. Elkins, A. S. Fauci, and V. M. Hirsch. 1997. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J. Virol. 71:9508-9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lo Caputo, S., D. Trabattoni, F. Vichi, S. Piconi, L. Lopalco, M. L. Villa, F. Mazzotta, and M. Clerici. 2003. Mucosal and systemic HIV-1-specific immunity in HIV-1-exposed but uninfected heterosexual men. AIDS 17:531-539. [DOI] [PubMed] [Google Scholar]
- 21.Mazzoli, S., L. Lopalco, A. Salvi, D. Trabattoni, S. Lo Caputo, F. Semplici, M. Biasin, C. Bl, A. Cosma, C. Pastori, F. Meacci, F. Mazzotta, M. L. Villa, A. G. Siccardi, and M. Clerici. 1999. Human immunodeficiency virus (HIV)-specific IgA and HIV neutralizing activity in the serum of exposed seronegative partners of HIV-seropositive persons. J. Infect. Dis. 180:871-875. [DOI] [PubMed] [Google Scholar]
- 22.Schenal, M., S. Lo Caputo, F. Fasano, F. Vichi, M. Saresella, P. Pierotti, M. L. Villa, F. Mazzotta, D. Trabattoni, and M. Clerici. 2005. Distinct patterns of HIV-specific memory T lymphocytes in HIV-exposed uninfected individuals and in HIV-infected patients. AIDS 19:653-661. [DOI] [PubMed] [Google Scholar]
- 23.Seth, A., I. Ourmanov, M. J. Kuroda, J. E. Schmitz, M. W. Carroll, L. S. Wyatt, B. Moss, M. A. Forman, V. M. Hirsch, and N. L. Letvin. 1998. Recombinant modified vaccinia virus Ankara-simian immunodeficiency virus gag pol elicits cytotoxic T lymphocytes in rhesus monkeys detected by a major histocompatibility complex class I/peptide tetramer. Proc. Natl. Acad. Sci. USA 95:10112-10116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang, S.-W., P. A. Kozlowski, G. Schmelz, K. Manson, M. S. Wyand, R. Glickman, D. Montefiori, J. D. Lifson, R. P. Johnson, M. R. Neutra, and A. Aldovini. 2000. Effective induction of simian immunodeficiency virus-specific systemic and mucosal immune responses in primates by vaccination with proviral DNA producing intact but noninfectious virions. J. Virol. 74:10514-10522. [DOI] [PMC free article] [PubMed] [Google Scholar]